Abstract
Study Objectives
To assess the microstructural architecture of non-rapid eye movement (NREM) sleep known as cyclic alternating pattern (CAP) in relation to the age, gender, self-reported sleep quality, and the degree of sleep disruption in large community-based cohort studies of older people.
Methods
We applied a high-performance automated CAP detection system to characterize CAP in 2,811 men from the Osteoporotic Fractures in Men Sleep Study (MrOS) and 426 women from the Study of Osteoporotic Fractures (SOF). CAP was assessed with respect to age and gender and correlated to obstructive apnea–hypopnea index, arousal index (AI-NREM), and periodic limb movements in sleep index. Further, we evaluated CAP across levels of self-reported sleep quality measures using analysis of covariance.
Results
Age was significantly associated with the number of CAP sequences during NREM sleep (MrOS: p = 0.013, SOF = 0.051). CAP correlated significantly with AI-NREM (MrOS: ρ = 0.30, SOF: ρ = 0.29). CAP rate, especially the A2+A3 index, was inversely related to self-reported quality of sleep, independent of age and sleep disturbance measures. Women experienced significantly fewer A1-phases compared to men, in particular, in slow-wave sleep (N3).
Conclusions
We demonstrate that automated CAP analysis of large-scale databases can lead to new findings on CAP and its subcomponents. We show that sleep disturbance indices are associated with the CAP rate. Further, the CAP rate is significantly linked to subjectively reported sleep quality, independent from traditionally scored markers of sleep fragmentation. Finally, men and women show differences in the microarchitecture of sleep as identified by CAP, despite similar macro-architecture.
Keywords: cyclic alternating pattern, deep learning, sleep fragmentation, sleep disorder breathing, sleep studies, sleep quality
Statement of Significance.
We report the prevalence of periodically occurring cortical activation phases in large population samples of older men and women. To the best of our knowledge, this effort represents the first time that cyclic alternating pattern (CAP) was scored and analyzed with a high-performance automated detection system in large community-based cohort studies. We ascertain the relationship with gender, age, self-reported sleep quality measures, and traditional polysomnographic indices of disordered sleep. Individuals experiencing a higher CAP rate, in particular, A2+A3-phases, report a lower sleep quality independent of apnea–hypopnea index, arousal index, and periodic limb movement index. In older populations, age is a significant predictor for non-rapid eye movement sleep fragmentation. We also reveal gender differences in the microarchitecture of sleep despite similar macro-architecture.
Introduction
Since Rechtschaffen and Kales [1] published their scoring guide in 1968, sleep has been traditionally divided into states of high neuronal activity and quiescence, typically known as rapid eye movement (REM) and non-REM (NREM). The latter, in turn, is partitioned into three distinct stages according to the current consensus detailed in the American Academy of Sleep Medicine (AASM) scoring manual [2]. One major drawback of these scoring rules is the neglect of short-lasting events such as K-complexes and transient power alterations in frequency bands [3]. In the AASM framework, short periods of changes in cortical activation are only captured by the arousal definition [2]. Phasic events like K-complexes and delta bursts show arousal-like characteristics but they are not regarded as arousals when not related to short-term frequency increases in an electroencephalogram (EEG) [3]. Hence, an additional sleep scoring atlas was devised including such recurring phasic events in brain activity under the name of cyclic alternating pattern (CAP) [4].
CAP analysis seeks to capture the microstructure of sleep. It focuses on short EEG amplitude increases (<60 s) that reappear periodically in NREM stages, separated by equally long time spans of lower-amplitude background activity [3]. Such short events are called activation phases because of their high neural excitability and autonomic correlates. It is believed that recurring periods of activation during sleep represent time windows that facilitate sensory input for the brain and synchronize with physiological and pathological events [5]. Hence, an increased CAP rate may occur in sleep disorders such as periodic limb movement disorder, sleep apnea syndrome, or insomnia [6]. In recent years, the role of CAP has been receiving enlarged clinical interest, but current evidence is limited to small studies focusing on particular disorders. The role and prevalence of CAP during sleep on the broader population remain still largely unknown.
In this study, we characterize for the first time CAP across large population samples. We describe the prevalence of CAP in relation to age and gender. Moreover, we explore the relationship between CAP and common disorders that have been associated with sleep fragmentation as well as self-reported sleep quality measures.
Methods
Definition of CAP
We defined CAP in agreement with Terzano et al. [4] as sequences of at least two consecutive cycles that consist of an activation phase (A-phase) followed by the period between two repetitive A-phases, called B-phase (background). A-phases represent transient, phasic events that stand out from the background, whereas B-phases are thought to embody rebound deactivation reflecting active inhibition rather than passive recovery of the stationary baseline during NREM sleep [3]. We defined A-phases or B-phases to last 2–60 s but did not limit the number of cycles per CAP sequence. In accordance with the CAP atlas, the time period between two CAP sequences was considered as non-CAP. The last A-phase prior to a non-CAP period was also defined as non-CAP as it does not form a cycle.
Typical patterns for A-phases include delta bursts, vertex sharp transients, K-complex sequences, K-alpha, polyphasic bursts, intermittent alpha, and arousals [4]. Thus, A-phases consist of either high-voltage, slow waves or low-voltage, fast waves or a combination of both. High-voltage slow waves portray synchronized EEG patterns and low-amplitude fast rhythms represent desynchrony [3]. Based on the content of these two frequency components, we subdivided A-phases into three subtypes. Subtype A1 is associated with periods where high EEG synchrony is prevalent, i.e. slow rhythms with high amplitudes. Desynchronized patterns are classified as A2 and A3 subtypes mostly occurring in time periods before and after REM sleep. They represent high-frequency rhythms with low amplitudes. As REM sleep includes mainly desynchronized A-phases located further apart than 60 s, we restricted CAP to NREM sleep in agreement with Terzano et al. [4].
Automated A-phase detection and CAP quantification
We deployed our previously developed, highly precise automated system for CAP analysis, which is described in detail in the work of Hartmann and Baumert [7]. At the core of the system is a deep learning recurrent neural network (RNN) that was trained specifically to recognize A-phases in EEG recordings. The entire system is divided into four major parts: preprocessing, feature extraction, classification, and post-processing. In the preprocessing step, the raw signal of one central EEG channel is prepared to reduce intersubject variation by removing the cardiac field and eye movement artifacts. Based on the processed signal, multiple features in the time and frequency domain are calculated such as Hjorth activity, Shannon entropy, Teager Energy Operator, band power descriptor, and differential EEG variance. The extracted feature set serves as input for the RNN classifier in the classification stage. The classifier was trained with the Fβ—score as loss function to increase the quantity of correctly detected A-phases and reduce the number of incorrectly classified periods. Finally, we post-processed the output of the A-phase detection system applying the aforementioned rules for CAP sequences. Isolated A-phases that did not form a sequence were removed from the scoring outcome. The training comprised 15 healthy participants and 24 participants with sleep disorders from a publicly available database [8]. The polysomnographic measurements in the training set including visual CAP scoring were conducted by the Sleep Disorders Center of the Ospedale Maggiore of Parma, Italy. The second-by-second A-phase inter-rater reliability between visual scoring and our system, quantified by the Cohen’s kappa coefficient, was 0.53 on a set of 16 healthy participants and 0.56 on a set of 30 participants with nocturnal frontal lobe epilepsy. The event-based inter-rater reliability between human scorers ranges between 0.42 and 0.75 [9].
Measurements recorded with a low bit rate or a low physical range were excluded because they often contain severe clipping leading to false classification results. In this study, we computed the CAP rate and subtype rate based on the following equations:
Subtypes A2 and A3 were merged into a single parameter due to their congruent nature.
We defined four consecutive sleep periods of 90-min duration each to investigate the relationship between CAP and sleep intervals during 6 h of sleep. Sleep stage scoring manually performed by trained sleep technicians during the implementation of the studies was used to identify NREM sleep. As both studies were conducted before the release of the AASM scoring manual, sleep stage scoring was performed in accordance to the criteria in the work of Rechtschaffen and Kales [1]. Sleep stages 3 and 4 were merged to a single stage (called here SWS) and sleep stage 1 was excluded due to the low occurrence in the majority of the participants.
Study samples: MrOS and SOF
For our analysis, we utilized data from two multi-center sleep cohorts: Osteoporotic Fractures in Men (MrOS) Study and Study of Osteoporotic Fractures (SOF). Both data sets were provided by the National Sleep Research Resource (available online at the National Sleep Research Resource; sleepdata.org) [10].
MrOS is a long-term cohort study designed to determine fracture risk in relation to multiple factors such as bone characteristics, lifestyle, anthropometric and neuromuscular measures, and fall propensity. In total, 5,995 men aged 65 or older were examined during a 25-month period from 2000 to 2002 followed by a second visit in 2005 [11]. The study was conducted at six clinical sites with the requirement that all participants needed to be able to walk without assistance and must not have had a bilateral hip replacement. As part of the MrOS cohort, 3,115 men were recruited for an ancillary sleep study (MrOS Sleep Study) including comprehensive overnight polysomnography (PSG), designed to identify the cardiovascular and health consequences of sleep disturbances [12]. Men who used mechanical devices or oxygen during sleep were excluded from the study. The baseline sleep exam (Visit 1) was conducted between 2003 and 2005 and a follow-up exam (Visit 2) was conducted between 2009 and 2012. We removed recordings with technically inadequate PSG or fewer than 3 h of good EEG quality resulting in 2,811 participants for Visit 1 and 933 participants for Visit 2.
SOF was designed to investigate the risk factor for hip fractures among older women [13]. Women who were community-dwelling, 65 years or older, able to walk unassisted, and had no previous bilateral hip replacement were recruited during September 1986 and October 1988 in four metropolitan areas [14]. Within the latest visit cycle between 2002 and 2004, a subset of 461 women underwent an unattended overnight 12-channel in-home PSG to evaluate the relationship of sleep disturbances to a number of health outcomes [15]. After discarding recordings with inadequate EEG quality by applying the same approach as for MrOS, 426 recordings from SOF were available for analysis.
Statistical methods
Statistical analysis was conducted using non-parametric tests based on the assumption that the CAP rate and subtype indices do not follow a normal distribution. For each statistical test, the significance level was adjusted to the number of variables under consideration using Bonferroni correction. All values are presented as median ± interquartile range (IQR).
We subdivided CAP rate data across both cohorts into quartiles to evaluate the association of CAP with the obstructive apnea–hypopnea index at 4% oxygen desaturation (OAHI), the arousal index (AI-NREM) in NREM sleep, and the periodic limb movement in sleep index (PLMSI) as clinical indicators of sleep fragmentation. The Jonckheere–Terpstra test was applied to identify a statistically significant trend between quartiles of CAP parameters and indices of disordered sleep. Spearman correlation coefficients were determined to examine the relationship between the aforementioned indices and CAP parameters.
Participants in MrOS were asked to score the quality of their sleep following PSG on a Likert scale of five items from light to deep, from short to long, and from restless to restful. In SOF, self-reported sleep quality after PSG was not measured. To investigate the effect of these measures on CAP, we applied the analysis of covariance (ANCOVA) with CAP rate, A1 index, and A2+A3 index as dependent variables, the three self-reported sleep quality measures as independent variables, and age, OAHI, AI-NREM, and PLMSI as covariates.
To explore the effect of age on CAP, multivariable regression was conducted with age, OAHI, AI-NREM, and PLMSI as independent predictors for CAP rate, A1 index, and A2+A3 index. Each multivariable regression was carried out separately in MrOS and SOF. To analyze the effect of gender on CAP, two normalized, age-matched subsets were sampled from the MrOS and SOF cohorts, respectively, comprising 220 men and women. Both subsets were restricted to participants with AHI less than 15, AI-NREM less than 25, and PLMSI less than 15. We selected the Mann–Whitney–Wilcoxon test for comparing the independent gender groups and sleep stages within both genders. To determine differences between sleep intervals within both genders, we used the Kruskal–Wallis test by ranks. Finally, the reproducibility of the applied system was tested comparing matching participants from MrOS Sleep Visit 1 and Visit 2.
Results
The median age of participants was 76 years in MrOS (baseline exam) and 82 years in SOF. Participants in MrOS had a median BMI of 26.7 kg/m2; women in SOF demonstrated a median BMI of 27.1 kg/m2. MrOS participants experienced a median OAHI of 8.1/h as well as a median AI-NREM of 22.3/h. In SOF, the median OAHI was 6.7/h, and the median AI-NREM was 19.8/h. Both cohorts show a median duration of total NREM sleep of about 5 h (MrOS: 288 min [IQR: ±69.0], SOF: 286 min [IQR: ±78.0]). The total scored sleeping time in MrOS was 357.5 min (±83.8) and in SOF was 353 min (±95.0), which results in an NREM sleep percentage of 80.5% (±8.9) in MrOS and 81.7% (±9.8) in SOF.
CAP and sleep fragmentation
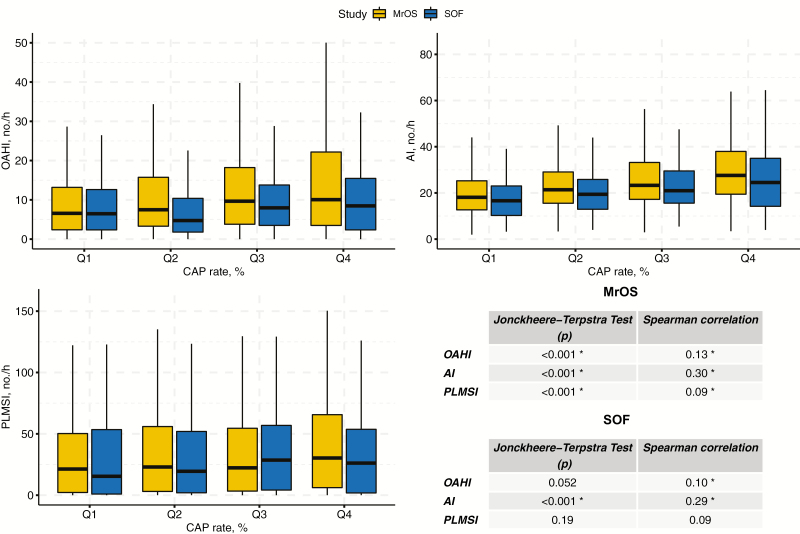
In terms of CAP, the male cohort (MrOS) displayed an overall large amount of 57.0% (±21.5) NREM sleep occupied by periodically occurring phasic events. The even older female cohort (SOF) demonstrated similar values of CAP accounting for 54.1% (±26.1) of NREM sleep. Indices of disordered sleep (OAHI, PLMSI, and AI-NREM) increased significantly with increasing CAP in MrOS (Figure 1). SOF participants showed a similar relationship, except for PLMSI, which was slightly reduced in the last quartile compared to the previous quartile but followed a similar trend overall.
Figure 1.
Indices of disordered sleep for CAP rate quartiles in MrOS and SOF. The relationship between indices of disordered sleep (OAHI, AI-NREM, and PLMSI) and CAP quartiles in Osteoporotic Fractures in Men (MrOS) Study and Study of Osteoporotic Fractures (SOF). Significance level (*p < 0.017) was adjusted according to the number of variables under consideration. OAHI, obstructive apnea–hypopnea index; AI-NREM, arousal index; PLMSI, periodic limb movement in sleep index.
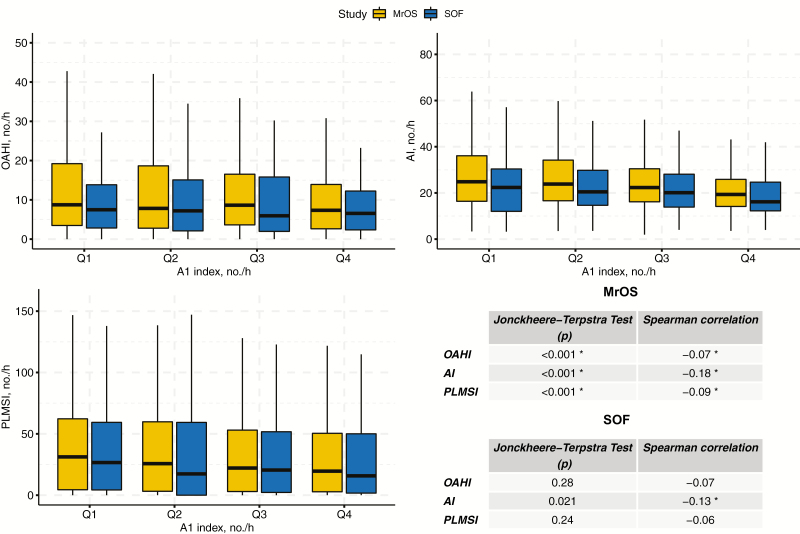
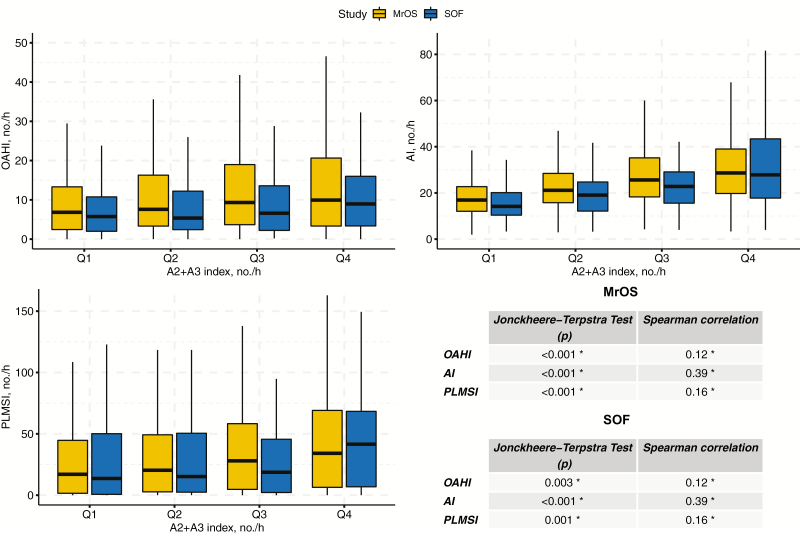
On average, 15.2 (±20.7) A1-phases occurred per hour of NREM sleep in MrOS and 13.1 (±18.5) in SOF. The A2+A3 index was substantially higher in both cohorts (46.6 [±31.1] in MrOS and 44.5 [±33.2] in SOF). The statistics on the relationship between indices of disordered sleep and A-phase subtypes are summarized in Figures 2 and 3, respectively. In MrOS, increasing A1 was associated with decreasing OAHI, AI-NREM, and PLMSI. In SOF, the same effect could be observed for AI-NREM. Conversely, in both cohorts, increasing A2+A3 was associated with higher OAHI, AI-NREM, and PLMSI.
Figure 2.
Indices of disordered sleep for A1 index quartiles in MrOS and SOF. The relationship between indices of disordered sleep (OAHI, AI-NREM, and PLMSI) and A1 index quartiles in Osteoporotic Fractures in Men (MrOS) Study and Study of Osteoporotic Fractures (SOF). Significance level (*p < 0.017) was adjusted according to the number of variables under consideration. OAHI, obstructive apnea–hypopnea index; AI-NREM, arousal index; PLMSI, periodic limb movement in sleep index.
Figure 3.
Indices of disordered sleep for A2+A3 index quartiles in MrOS and SOF. The relationship between indices of disordered sleep (OAHI, AI-NREM, and PLMSI) and A2+A3 index quartiles in Osteoporotic Fractures in Men (MrOS) Study and Study of Osteoporotic Fractures (SOF). Significance level (*p < 0.017) was adjusted according to the number of variables under consideration. OAHI, obstructive apnea–hypopnea index; AI-NREM, arousal index; PLMSI, periodic limb movement in sleep index.
CAP and self-reported sleep quality measures
Supplementary Figure S1 illustrates the results of the ANCOVA for all three self-reported sleep quality measures reported in MrOS with CAP rate, A1 index, and A2+A3 index as dependent variables and age, AI-NREM, OAHI, and PLMSI as covariates. CAP rate decreased significantly with increasing quality of sleep for all three self-reported measures (light vs deep: 58.8 ± 22.3% vs 54.6 ± 20.5%, p < 0.001; short vs long: 58.4 ± 21.4% vs 55.1 ± 20.5%, p < 0.001; restless vs restful: 59.4 ± 20.8% vs 55.6 ± 21.0%, p = 0.002). The A1 index did not vary significantly across all three sleep quality parameters (light vs deep: 12.9 ± 20.2 no./h vs 17.7 ± 21.8 no./h, p = 0.19; short vs long: 15.8 ± 20.4 no./h vs 16.4 ± 17.6 no./h, p = 0.76; restless vs restful: 15.1 ± 20.1 no./h vs 15.8 ± 21.1 no./h, p = 0.94). Similar to the CAP rate, the A2+A3 index decreased with increasing values for each self-reported measure (light vs deep: 49.0 ± 32.0 no./h vs 41.3 ± 29.0 no./h, p < 0.001; short vs long: 47.5 ± 28.2 no./h vs 44.9 ± 30.3 no./h, p < 0.001; restless vs restful: 48.8 ± 31.4 no./h vs 42.9 ± 30.0 no./h, p < 0.001). Detailed ANCOVA results including all three self-reported sleep quality measures with AI-NREM as dependent variable are listed in Supplementary Tables S1–S4.
CAP and age
Several multivariable regression models were evaluated to investigate the effect of age on CAP rate, A1 index, and A2+A3 index in MrOS and SOF, respectively (Table 1).
Table 1.
Multivariable Regression Results Predicting CAP Rate, A1 Index, and A2+A3 Index in MrOS and SOF
| MrOS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAP rate, % | A1 index, no./h | A2+A3 index, no./h | ||||||||||
| Variables | B | SE | t | p | B | SE | t | p | B | SE | t | p |
| Age, years | 0.13 | 0.05 | 2.48 | 0.013* | −0.05 | 0.05 | −0.92 | 0.36 | 0.18 | 0.08 | 2.36 | 0.019 |
| AI-NREM, no./h | 0.36 | 0.03 | 14.05 | <0.001* | −0.23 | 0.03 | −8.81 | <0.001* | 0.70 | 0.04 | 18.77 | <0.001* |
| OAHI, no./h | 0.02 | 0.02 | 0.69 | 0.49 | −0.02 | 0.03 | −0.81 | 0.42 | −0.07 | 0.04 | −1.86 | 0.063 |
| PLMSI, no./h | 0.01 | 0.01 | 1.47 | 0.14 | −0.03 | 0.01 | −3.90 | <0.001* | 0.07 | 0.01 | 6.01 | <0.001* |
| Constant | 37.02 | 3.90 | 9.49 | <0.001* | 29.79 | 4.03 | 7.39 | <0.001* | 17.57 | 5.70 | 3.08 | <0.01* |
| R 2 | 0.10 | 0.05 | 0.16 | |||||||||
| Adj. R2 | 0.10 | 0.05 | 0.16 | |||||||||
| F(4,2806) = 75.60 | p < 0.001 | F(4,2806) = 36.17 | p < 0.001 | F(4,2806) = 131.90 | p < 0.001 | |||||||
| SOF | ||||||||||||
| CAP rate, % | A1 index, no./h | A2+A3 index, no./h | ||||||||||
| Variables | B | SE | t | p | B | SE | t | p | B | SE | t | p |
| Age, years | −0.52 | 0.26 | −1.96 | 0.051 | 0.12 | 0.25 | 0.49 | 0.62 | −0.77 | 0.35 | −2.21 | 0.028 |
| AI-NREM, no./h | 0.40 | 0.07 | 5.88 | <0.001* | −0.20 | 0.06 | −3.06 | <0.01* | 0.72 | 0.09 | 7.91 | <0.001* |
| OAHI, no./h | 0.07 | 0.08 | 0.95 | 0.34 | −0.04 | 0.07 | −0.60 | 0.62 | 0.06 | 0.10 | 0.59 | 0.56 |
| PLMSI, no./h | 0.031 | 0.02 | 1.19 | 0.23 | −0.02 | 0.02 | −0.81 | 0.42 | 0.07 | 0.03 | 2.46 | 0.014* |
| Constant | 85.35 | 21.62 | 3.95 | <0.001* | 12.74 | 20.56 | 0.62 | 0.54 | 94.33 | 28.73 | 3.28 | <0.01* |
| R 2 | 0.11 | 0.03 | 0.18 | |||||||||
| Adj. R2 | 0.10 | 0.02 | 0.17 | |||||||||
| F(4,421) = 12.98 | p < 0.001 | F(4,421) = 3.62 | p < 0.01 | F(4,421) = 23.33 | p < 0.001 |
Results of each multivariable regression predicting CAP rate, A1 index, and A2+A3 index in Osteoporotic Fractures in Men (MrOS) Study and women in Study of Osteoporotic Fractures (SOF) with age as independent variable and the obstructive apnea–hypopnea index (OAHI) at 4% oxygen desaturation, the arousal index (AI-NREM) in NREM sleep, and the periodic limb movement in sleep index (PLMSI) as additional independent variables. B, an estimate of beta coefficient; SE, standard error of beta coefficient. *Significance level: p < 0.017 (adjusted to the number of variables under consideration).
Age and AI-NREM were significantly associated with CAP rate in MrOS (age: B = 0.13, p = 0.013; AI-NREM: B = 0.36, p < 0.001), whereas only AI-NREM was significantly associated with CAP rate in SOF (age: B = −0.50, p = 0.051; AI-NREM: B = 0.40, p < 0.001). Neither OAHI or PLMSI was associated with CAP in either cohort. The overall model fit for CAP rate was R2 = 0.10 for both cohorts.
Neither age nor OAHI was significantly associated with the A1 index in MrOS or SOF. In MrOS, AI-NREM and PLMSI were significantly negatively associated with the A1 index (PLMSI: B = −0.03, p < 0.001; AI-NREM: B = −0.23, p < 0.001), whereas in SOF the only significant association was for AI-NREM (AI-NREM: B = −0.20, p < 0.01). The overall model fit for the A1 index prediction was R2 = 0.05 in MrOS and R2 = 0.02 in SOF.
Finally, AI-NREM and PLMSI were significantly negatively associated with the frequency of A2+A3-phases in MrOS (PLMSI: B = −0.07, p < 0.001; AI-NREM: B = −0.70, p < 0.001) and SOF (PLMSI: B = −0.07, p = 0.014; AI-NREM: B = −0.72, p < 0.001). Age and OAHI were not associated with A2+A3 in either cohort. The overall model fit for A2+A3 index prediction was R2 = 0.16 in MrOS and R2 = 0.17 in SOF.
CAP and gender
A subset of 110 participants with identical age distribution and no severe sleep disorders (AHI <15, AI-NREM <25, and PLMSI <15) was sampled from the MrOS cohort and the SOF cohort to compare both sexes while eliminating the age influence. The statistics for each subset on sleep parameters and indices of sleep disturbance are listed in Table 2. Mann–Wilcoxon U-test shows a significantly lower A1 index in women compared to men (A1 index: p = 0.036). Men had a significantly higher percentage of stage 2 sleep compared to women, but a lower percentage of SWS (S2: p = 0.002, SWS: p < 0.001).
Table 2.
MrOS vs SOF With Identical Age Distribution
| Age, years | AI-NREM, no./h | OAHI, no./h | PLMSI, no./h | CAP rate, % | A1 index, no./h | A2+A3 index, no./h | Scored sleep time, min | Percentage of sleep in stage 2, % | Percentage of sleep in stage 3/4, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | ||||||||||
| Men in MrOS | 82.0 (±4.0) | 15.4 (±5.9) | 4.5 (±6.9) | 1.2 (±4.9) | 51.3 (±18.9) | 17.3 (±21.6) | 37.8 (±21.5) | 369.0 (±83.8) | 58.4 (±14.0) | 13.9 (±14.3) |
| Women in SOF | 82.0 (±4.0) | 14.2 (±9.3) | 4.6 (±6.3) | 1.1 (±7.4) | 48.9 (±27.1) | 13.7* (±18.8) | 38.6 (±25.0) | 356.4* (±90.0) | 52.5* (±18.6) | 20.8* (±19.0) |
Comparison between men in Osteoporotic Fractures in Men (MrOS) Study and women in the Study of Osteoporotic Fractures (SOF) using a subsample with identical age distribution. OAHI, obstructive apnea–hypopnea index; AI-NREM, arousal index; PLMSI, periodic limb movement in sleep index. *Significance level: p < 0.05.
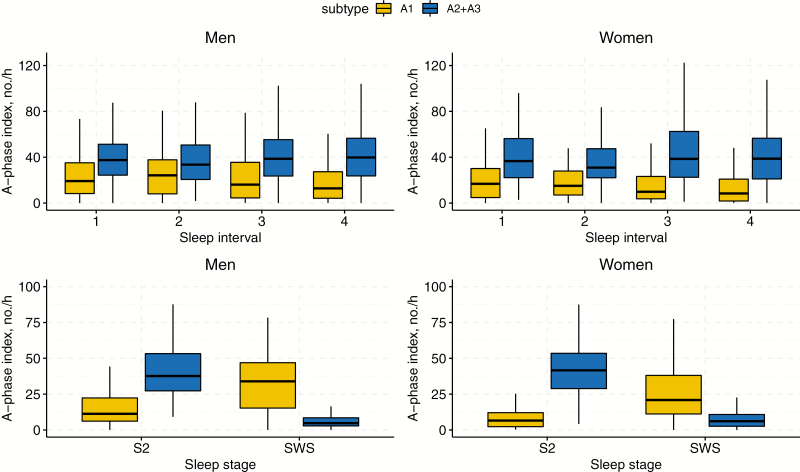
Figure 4 displays A1 and A2+A3 indices for gender groups across sleep intervals and NREM stages. Both men and women display a decrease in A1-phases throughout the night (sleep interval: p < 0.001), whereas men experienced more A1-phases compared to women (gender: p < 0.001). The A2+A3 index did not show any variations between men and women and remained constant throughout the night, (gender: p = 0.95, sleep interval: p = 0.11). Regarding sleep stages, the A1 index was significantly higher in men in both stages. The number of A1-phases increased from S2 to SWS (gender: p < 0.001, sleep stage: p < 0.001). On the contrary, both genders experienced less A2+A3-phases in SWS compared to S2 (gender: p = 0.42, sleep stage: p < 0.001).
Figure 4.
Analysis of A-phase subtypes during sleep intervals and NREM stages based on gender. Average number of A-phase subtypes per hour of NREM sleep across consecutive 90-min intervals (top), as well as NREM sleep stages (bottom) for men (left) and women (right) with identical age distribution in Osteoporotic Fractures in Men (MrOS), Study and Study of Osteoporotic Fractures (SOF), respectively.
Reproducibility test
The histograms of the CAP rate for MrOS Visit 1 and Visit 2 (mean difference: 6 years) illustrate the reproducibility of the automated system for CAP detection (Supplementary Figure S2). Both histograms demonstrate an identical distribution with a minor shift for Visit 2 (Mann–Wilcoxon U-test: p = 0.091; Spearman correlation: ρ = 0.38, p < 0.001). Detailed values for CAP parameters and traditional polysomnographic indices of sleep disturbance for matched participants in the baseline and follow-up study are tabulated in Supplementary Table S1.
Discussion
Our analysis showed that age is independently associated with the CAP rate in older populations. Multivariable regression analysis, adjusting for sleep disturbance indices such as AI-NREM, OAHI, and PLMSI, showed that CAP rate in MrOS and SOF indicated a significant or close to significant association with age. Although all participants were within a narrow age range (68–90 years), our findings are consistent with previous studies that have shown that across the entire age spectrum CAP rate follows generally a U-shaped function of age [16] and thus continuously increases with age in older populations. On the contrary, the frequency of A1-phases decreases linearly with age. The negative beta coefficients in the multivariable regression analysis for A1-phases in this study confirm this behavior. Interestingly, multivariable regression in SOF demonstrated also a negative association between CAP rate and age, while this association was positive in MrOS. This could be caused by the higher age in SOF, considering that the CAP rate diminishes at very high ages [17], or reflect gender differences in CAP rates with advanced aging.
Further, women appeared to experience fewer A1-phases per hour throughout the night. CAP rate and A2+A3 index were comparable throughout the night for both men and women, resulting in a higher A1-to-A2+A3 ratio for men. Women did not show any significant variations in indices for sleep disturbance (AI-NREM, OAHI, and PLMSI) with CAP compared to men. In S2 and SWS, men exhibited a higher number of A1-phases compared to women, whereas the A2+A3 remained approximately identical to women. Although women demonstrated a higher percentage of SWS (which is characterized by a higher A1 index), men still experienced a higher number of A1-phases throughout the night. One can speculate that older women show less periodic activity in lower EEG frequency bands than older men due to more isolated or monomorphic events. This gender difference may provide clinical insight into the contradictory observations that compared to men, women have more SWS but report more frequent concerns over sleep quality [18, 19]. Our data suggest that the microarchitecture of SWS in men and women may differ. Future research is needed to examine whether differences in the A1 index may explain gender differences in sleep quality.
Considering the number of A-phase subtypes per hour reported in this study, the A1 index tends to be lower than reported in the works of Parrino et al. [17] and Maestri et al. [20], whereas the A2+A3 indices are comparable. One reason for the lower frequency of A1-phases could be the higher age of the participants in MrOS and SOF compared to a previous study [20] as the frequency of A1-phases steadily declines with age. Another reason could be the low representation of older people in the data set used to train our automated CAP detection system.
Our data confirm the link between CAP and markers of sleep disturbances, suggesting that respiratory and leg movement events and increased arousals fragment the sleep microstructure. Across the large population samples, we observed a significant correlation between CAP and AI-NREM. Arousals have by definition a broad overlap with the characters of subtypes A2 and A3 and thereby CAP. Previous studies have shown that the majority of arousals (87%) appear within a CAP sequence [21]. Moreover, 95% of subtypes A3 and 62% of subtypes A2 meet the scoring requirements for arousals [22]. We observed only a moderate relationship between the A2+A3 index and AI-NREM, possibly because arousals were scored manually while CAP events were detected automatically using our system. Due to possibly low representations of arousals in general and overlaps with A-phases in the training set of our detection system, high variations in correlations with the consistent automated scoring of CAP events can be expected. Furthermore, our study confirms the connection between CAP and sleep pathologies such as sleep-disordered breathing or PLMS disorder, respectively. Our analysis depicts an inverse linear association between OAHI and the A1 subtype and, conversely, a positive association between OAHI for A2+A3-phases, analogous to results found in children with OSAS [23]. This shift in the subtype ratio raises sleep instability and has a severe negative impact on the NREM sleep microstructure [17]. Data from middle-aged persons with OSAS also support our findings [24]. According to Terzano et al. [24], B-phases appear to be connected to episodes of breathing cessations, whereas A-phases seem to be linked to the recovery of effective breathing. Regarding PLMS, the majority of limb movements was reported to occur with the onset of A-phases and follow the periodicity of CAP [25]. Our results demonstrate a significant increase of PLMS in people with higher CAP rates. Thus, individuals with a high CAP occurrence are more likely to experience disruptive sleep.
An additional finding of our study is the inverse relationship between CAP rate, in particular, the frequency of fast low-amplitude EEG rhythms (A2+A3), and self-reported sleep quality that is independent of clinical markers of sleep disturbances. Our results show that the CAP rate, mainly the frequency of A2+A3-phases, is reduced in older men who report good sleep quality. ANCOVA of the A1 index did not show any significant relationship with self-reported sleep quality measures, possibly due to the low occurrence of A1-phases. Our results are in line with previously reported outcomes on correlations between CAP and self-reported sleep quality, mostly quantified by means of visual analog scales (VAS). Terzano et al. [26] suggested the first time a possible relation between CAP and self-reported sleep quality in their study on the influence of increasing levels of acoustic perturbation on sleep structure. Such a negative correlation between CAP rate and self-reported sleep quality has been confirmed by larger studies in subsequent years [27–29]. We show in our analysis that a negative correlation between CAP and self-reported sleep quality is independent of age and sleep disturbance reflected in OAHI, AI-NREM, and PLMSI. Our results also show a strong relationship between AI-NREM and self-reported sleep measures although objective sleep quality measures derived from PSG have shown not to be suitable predictors for individually reported quality of sleep especially in older adults [30, 31]. Our data are in agreement with the correlation between AI and VAS reported by Terzano et al. [29].
To the best of our knowledge, this effort represents the first time that CAP was scored and analyzed in large community-based cohort studies. Commonly, the scoring of CAP is performed manually, which is a tedious and time-consuming task for the scorer, considering that one recording consists of multiple hours of sleep. The low number of sleep technicians trained in CAP scoring and the immense volume of work required have likely been barriers that prevented CAP studies with large numbers of participants in the past. Previous studies on CAP were limited to 10–50 recordings with rare exceptions such as the work of Terzano and Parrino [6] that included 400–500 persons. Here, we evaluated in total 3,237 participants (MrOS: 2,811, SOF: 426) using a high-performance automated detection algorithm that enabled in an unprecedented examination of CAP in elderly male and female populations.
A limitation of this study is the older age of the participants, precluding assessment across the full age range. Nonetheless, sleep disorders and quality are of particular relevance in older populations. Sleep fragmentation is highly prevalent among older people [32, 33]. Another limitation pertains to the accuracy of our developed automated detection system. Although the system has demonstrated outstanding performance in comparison to manual scoring as the gold standard [7], the results depend on the training data set, i.e. it may be biased to the human expert who scored the training data. Visual scoring may allow a human scorer to consider subject-specific variations, whereas our system will strictly score events based on the representations of events in the training set. On the other hand, the inter-rater concordance for manual CAP scoring is approximately 83% [9], reflecting some inconsistency in scoring compared to our system. The high reproducibility of our system is evident when comparing repeated measurements between MrOS Sleep Visit 1 and Visit 2. The statistics demonstrate an identical CAP rate distribution for Visit 1 and Visit 2 with a non-significant shift in Visit 2 due to the time gap of 6 years. Also, the automated scoring results are easily reproducible as the automated analysis of one recording takes only a few seconds, unlike manual scoring results. Furthermore, the method was implemented in MATLAB, a numerical computing environment, mostly using a built-in function that makes it easy to reproduce.
In sum, these findings confirm in large community-based cohort studies that CAP scoring serves as an important indicator for sleep quality and sleep fragmentation in older populations. Moreover, the results provide fundamental data on the variation of CAP in older adults, providing the bases for future studies of the relationship of CAP with health outcomes. Finally, automated scoring systems such as the algorithm employed in this study can assist in analyzing CAP in large populations.
Funding
This study was partly supported by a grant from the Australian Research Council (DP0663345). The NSRR is supported by grant number HL114473 from the National Heart, Lung, and Blood Institute (NHLBI), NIH. The NHLBI provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The Study of Osteoporotic Fractures (SOF) is supported by the National Institutes of Health funding. The National Institute on Aging provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720.
Nonfinancial disclosure: None.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1. Rechtschaffen A, et al. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages for Human Subjects. Washington, DC: U. S. Government Printing Office; 1968. [Google Scholar]
- 2. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 3. Terzano MG, et al.. Origin and significance of the cyclic alternating pattern (CAP). Review article. Sleep Med Rev. 2000;4(1):101–123. [DOI] [PubMed] [Google Scholar]
- 4. Terzano MG, et al.. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001;2(6):537–553. [DOI] [PubMed] [Google Scholar]
- 5. Terzano MG, et al. Chapter 8: The cyclic alternating pattern (CAP) in human sleep. In: Guilleminault CBT-H of CN, ed. Handbook of Clinical Neurophysiology. Vol 6 Amsterdam, The Netherlands: Elsevier; 2005:79–93. [Google Scholar]
- 6. Terzano MG, et al.. Clinical applications of cyclic alternating pattern. Physiol Behav. 1993;54(4):807–813. [DOI] [PubMed] [Google Scholar]
- 7. Hartmann S, et al.. Automatic A-phase detection of cyclic alternating patterns in sleep using dynamic temporal information. IEEE Trans Neural Syst Rehabil Eng. 2019;27(9): 1695–1703. [DOI] [PubMed] [Google Scholar]
- 8. Goldberger AL, et al.. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23): E215–E220. [DOI] [PubMed] [Google Scholar]
- 9. Ferri R, et al. Inter-rater reliability of sleep cyclic alternating pattern (CAP) scoring and validation of a new computer-assisted CAP scoring method. Clin Neurophysiol. 2005;116(3):696–707. [DOI] [PubMed] [Google Scholar]
- 10. Dean DA 2nd, et al.. Scaling up scientific discovery in sleep medicine: the national sleep research resource. Sleep. 2016;39(5):1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orwoll E, et al.. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. [DOI] [PubMed] [Google Scholar]
- 12. Blackwell T, et al.; Osteoporotic Fractures in Men Study Group Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59(12):2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummings SR, et al.. Appendicular bone density and age predict hip fracture in women. The study of osteoporotic fractures research group. JAMA. 1990;263(5): 665–668. [PubMed] [Google Scholar]
- 14. Claman DM, et al.; Study of Osteoporotic Fratures Research Group Prevalence and correlates of periodic limb movements in older women. J Clin Sleep Med. 2006;2(4):438–445. [PubMed] [Google Scholar]
- 15. Spira AP, et al.. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56(1):45–50. [DOI] [PubMed] [Google Scholar]
- 16. Parrino L, et al.. Cyclic alternating pattern (CAP) in normal sleep: polysomnographic parameters in different age groups. Electroencephalogr Clin Neurophysiol. 1998;107(6):439–450. [DOI] [PubMed] [Google Scholar]
- 17. Parrino L, et al.. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012;16(1):27–45. [DOI] [PubMed] [Google Scholar]
- 18. Redline S, et al.. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. [DOI] [PubMed] [Google Scholar]
- 19. Bixler EO, et al.. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res. 2009;18(2):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maestri M, et al.. Non-rapid eye movement sleep instability in mild cognitive impairment: a pilot study. Sleep Med. 2015;16(9):1139–1145. [DOI] [PubMed] [Google Scholar]
- 21. Terzano MG, et al. CAP and arousals in the structural development of sleep: an integrative perspective. Sleep Med. 2002;3(3):221–229. [DOI] [PubMed] [Google Scholar]
- 22. Smerieri A, et al. Relationship of slow and rapid EEG components of CAP to ASDA arousals in normal sleep. Sleep. 2001;24(8):881–885. [DOI] [PubMed] [Google Scholar]
- 23. Kheirandish-Gozal L, et al.. Reduced NREM sleep instability in children with sleep disordered breathing. Sleep. 2007;30(4):450–457. [DOI] [PubMed] [Google Scholar]
- 24. Terzano MG, et al.. Polysomnographic analysis of arousal responses in obstructive sleep apnea syndrome by means of the cyclic alternating pattern. J Clin Neurophysiol. 1996;13(2):145–155. [DOI] [PubMed] [Google Scholar]
- 25. Parrino L, et al.. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol. 1996;13(4):314–323. [DOI] [PubMed] [Google Scholar]
- 26. Terzano MG, et al.. Modifications of sleep structure induced by increasing levels of acoustic perturbation in normal subjects. Electroencephalogr Clin Neurophysiol. 1990;76(1):29–38. [DOI] [PubMed] [Google Scholar]
- 27. Svetnik V, et al.. Alterations in cyclic alternating pattern associated with phase advanced sleep are differentially modulated by gaboxadol and zolpidem. Sleep. 2010;33(11):1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozone M, et al.. Effects of zolpidem on cyclic alternating pattern, an objective marker of sleep instability, in Japanese patients with psychophysiological insomnia: a randomized crossover comparative study with placebo. Pharmacopsychiatry. 2008;41(3):106–114. [DOI] [PubMed] [Google Scholar]
- 29. Terzano MG, et al.. CAP variables and arousals as sleep electroencephalogram markers for primary insomnia. Clin Neurophysiol. 2003;114(9):1715–1723. [DOI] [PubMed] [Google Scholar]
- 30. Kaplan KA, et al.; Osteoporotic Fractures in Men (MrOS), Study of Osteoporotic Fractures SOF Research Groups When a gold standard isn’t so golden: lack of prediction of subjective sleep quality from sleep polysomnography. Biol Psychol. 2017;123:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buysse DJ, et al.. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991;14(4):331–338. [PubMed] [Google Scholar]
- 32. Carskadon MA, et al.. Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging. 1982;3(4):321–327. [DOI] [PubMed] [Google Scholar]
- 33. Bonnet MH, et al.. EEG arousal norms by age. J Clin Sleep Med. 2007;3(3):271–274. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.