Abstract
Study Objectives
To explore the clinical significance of pulse wave amplitude (PWA)-drops during sleep as a biomarker for cardiometabolic disorders and describe their main characteristics in a general population sample.
Methods
Cross-sectional study of HypnoLaus cohort, in which 2162 individuals underwent clinical assessment and in-home full polysomnography. PWA-drops were derived from photoplethysmography and processed using a validated automated algorithm. Associations between PWA-drop features (index, mean duration, and mean area under the curve [AUC]) with hypertension, diabetes, and previous cardiovascular (CV) event were analyzed using multivariable-adjusted logistic regression.
Results
Two thousand one hundred forty-nine participants (59 ± 11 years, 51% women, 9.9% diabetes, 41.3% hypertension, 4.4% CV event) were included. Mean ± standard deviation (SD) of PWA-drop index, duration, and AUC during sleep were 51.0 ± 20.3 events/hour, 14.0 ± 2.7 seconds, and 527±115 %seconds, respectively. PWA-drop index was lower in women and decreased with age, while its mean duration and AUC increased in men and elderly. Overall, lower PWA-drop index, longer duration and greater AUC were associated with increased odds of hypertension, diabetes, or CV event after adjustment for confounders. Participants in the lowest quartile of mean duration-normalized PWA-drop index had a significantly higher odds ratio (OR) of hypertension (OR = 1.60 [1.19–2.16]), CV event (OR = 3.26 [1.33–8.03]), and diabetes (OR = 1.71 [1.06–2.76]) compared to those in the highest quartile. Similar results were observed for mean AUC-normalized PWA-drop index regarding hypertension (OR = 1.59 [1.19–2.13]), CV event (OR = 2.45 [1.14–5.26]) and diabetes (OR = 1.76 [1.10–2.83]).
Conclusions
PWA-drop features during sleep seem to be an interesting biomarker independently associated with cardiometabolic outcomes in the general population.
Keywords: biomarkers, diabetes, hypertension, cardiovascular medicine, epidemiology, autonomic nervous system
Statement of Significance.
This is the first study characterizing pulse wave amplitude (PWA)-drops during sleep using a simple noninvasive method (photoplethysmography) in a middle-to-old general population (HypnoLaus cohort). We found a strong and independent association of PWA-drop features (lower frequency, longer duration, and greater area under the curve) with hypertension, diabetes, and cardiovascular (CV) events. While traditional indexes derived from polysomnography are inconsistently associated with CV and metabolic outcomes, PWA-drop features seem to reflect impaired autonomic nervous system and vascular reactivity associated with these disorders. Future prospective studies are needed in order to explore the clinical value of PWA-drop characteristics as a biomarker of incident CV and metabolic diseases.
Introduction
Photoplethysmography (PPG) is a simple and low-cost noninvasive technique that assesses spontaneous oscillations in the capillary blood volume through the measurement of tissue red and infrared light absorption. PPG oscillates at the heartbeat frequency, reflecting variations in pulsatile blood volume [1]. PPG is widely available in the sleep laboratory setting as it is derived from the signal analysis of pulse oximetry, which is included in standard in-lab and ambulatory sleep recordings.
The amplitude of PPG signal is considered as a surrogate of the pulse wave amplitude (PWA). Moreover, PWA variations seem to be proportional to the sympathetic outflow directed to the vessels, thus reflecting the sympathovagal balance [2, 3]. Norepinephrine, but not isoproterenol, has been reported to induce a dose-dependent increase of PWA, suggesting that it could represent a biomarker of α-adrenergic receptor activity [4]. Conversely, after administration of epidural anesthesia with 0.5% bupivacaine, a sympathetic nerve blocker, a decrease of PWA was observed [5]. During sleep, PWA attenuations, also denominated as PWA-drops, have shown to improve, although not consistently [6] the value of portable monitoring in the diagnostic of obstructive sleep apnea (OSA) by detection of respiratory events and respiratory-related cortical arousals [7–12].
Considering the dynamics of the autonomic nervous system (ANS) during sleep and the degree and frequency of PWA-drops, which provides information about functional and structural vascular properties, the assessment of PWA may be useful to understand cardiovascular (CV) pathophysiology. The first step is, however, to describe its general profile during sleep in an unselected population before abnormal PWA variations and their association with clinical outcomes can be investigated.
Thus, this study aimed to characterize the frequency and other characteristics of PWA-drops during sleep as well as to assess their clinical correlates in a general population sample. We hypothesized that PWA-drop features during sleep would be independently associated with hypertension, diabetes, and previous CV event as these conditions are associated with impairments in ANS and vascular function.
Methods
Studied population
The HypnoLaus sleep cohort study [13], a nested study of CoLaus/PsyCoLaus [14, 15], was designed to assess the prevalence and correlates of sleep characteristics and sleep disorders in a general unselected middle-to-older-age population of Lausanne, Switzerland. Details of sampling and procedure methodologies have been described elsewhere [13, 14]. Briefly, between September 2009 and June 2013, 2162 participants underwent a complete examination, including an assessment of demographic and clinical characteristics and an overnight unattended full polysomnography (PSG; see below). All participants signed written informed consent forms and the ethical committee at University of Lausanne (Lausanne, Switzerland) approved the study protocol (approval numbers 16/03 and 33/09).
Clinical assessment
Participants from HypnoLaus study were invited to attend the outpatient clinic at the Lausanne University Hospital (CHUV, Lausanne, Switzerland). After overnight fasting, they had blood samples collected for biochemical assays, answered sleep questionnaires such as the Epworth Sleepiness Scale (ESS) [16] and the Pittsburgh Sleep Quality Index (PSQI) [17], and reported their medical history about smoking status, alcohol consumption, and use of medications. Glucose and insulin were quantified in fresh blood samples by colorimetric and immunometric assays, as previously described [14].
Anthropometric measurements were performed by trained observers with standard techniques. Bodyweight, height, and waist circumference were measured with participants standing without shoes in light clothes. Body mass index (BMI) was calculated as body mass in kg divided by the square of the patient’s height in meters.
Systolic (SBP) and diastolic (DBP) blood pressure were assessed in triplicate on the left arm at 5-minute intervals with the participant seated and resting for at least 10 minutes using a calibrated automated oscillometric sphygmomanometer (Omron HEM-907, Matsusaka, Japan) [18]. The mean of the second and third measurements was used for analysis, as recommended [19].
Polysomnography
Participants were invited to attend the Center for Investigation and Research in Sleep at the Lausanne University Hospital (CHUV, Lausanne, Switzerland) to be equipped with a polysomnographic recorder (Titanium, Embla Flaga, Reykjavik, Iceland) and undergo a full-night PSG at their home. PSG setup specifications followed the 2007 American Academy Sleep Medicine (AASM) recommendations. Physiological parameters evaluated during PSG recordings were: electroencephalogram (central, occipital, and frontal), electrooculogram (right and left eyes), surface electromyogram (chin and anterior tibialis muscles), electrocardiogram (ECG), airflow (nasal cannula), abdominal and thoracic respiratory efforts (respiratory inductance plethysmography), snoring, and body position. In addition, oxygen saturation (SpO2) and PWA were recorded by pulse oximetry, which is based on digital PPG pulses in two wavelengths (sampling rate: 32 Hz).
Sleep stage and arousal scorings were performed according to the 2007 AASM manual [20]. Respiratory events were scored according to the recommended rules of the 2012 AASM manual [21].
Heart rate variability
Measurements of the ANS activity was performed according to HRV analysis. One lead ECG from the PSG recording was imported as EDF format. Each QRS complex was validated and raw RR series were exported to the HRVanalysis software [22] and an accurate preprocessing was performed using a spline cubic interpolation to correct for non-sinusal beats, including ectopic beats and artifacts, as suggested in the HRV guidelines. HRV analysis included standard time-domain as well as frequency-domain analysis. Further details are available in the Supplementary Material.
Detection of PWA-drops
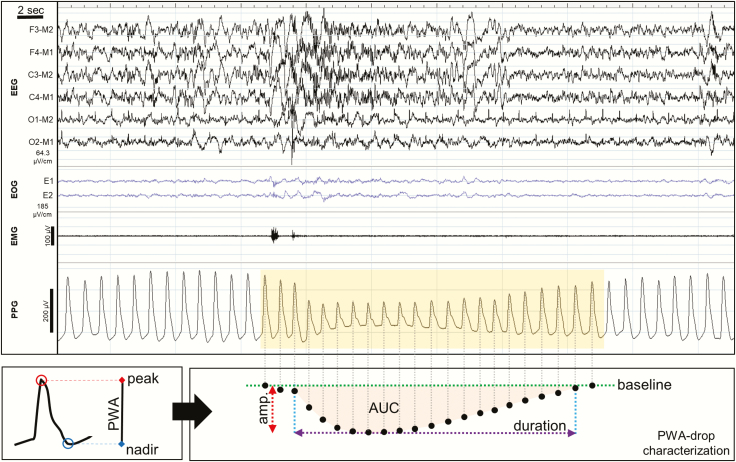
Drops in PWA were detected in the PPG signal and processed using a validated algorithm [23]. The overall performance of the algorithm reached 90.8% sensitivity, 99.6% specificity, and 95.2% precision. These values were similar also when computed separately for REM and NREM sleep. In brief, the PPG signal was smoothed and detrended before extraction of the PWA-signal, defined at each cardiac cycle as the difference between the peak and nadir values of the corresponding PPG-waveform. The time-courses of PWA-variance and first-derivative were then evaluated using a moving-window over five heartbeats. Candidate time-points for potential PWA-drops were defined as local peaks in the PWA-variance showing correspondent first-derivative negative values. For each PWA-drop candidate, an observation interval was delimited between the closest previous and subsequent PWA maxima, and the maximum percent decrease (amplitude) was computed with respect to the mean of the previous 5 PWA values extracted from stable signal tracts (i.e. tracts containing no artifacts or PWA-drops). Valid PWA-drops were required to be longer than 3 seconds but shorter than 60 seconds. Since spontaneous variations of PWA in the magnitude of 10–30% may reflect normal oscillations of the ANS in healthy subjects [7], PWA-drops with an amplitude >30% and a duration >4 heartbeats were identified (Figure 1), and their frequency, duration (seconds) and area under the curve (AUC; %seconds) were estimated.
Figure 1.
PWA-drops detection. The PWA-signal is obtained from the PPG signal by calculating, at each cardiac cycle, the difference between the maximum (peak) and minimum (nadir) value of the blood volume pulse wave (bottom-left panel). Then, PWA-drops were detected in the PWA-signal and the following parameter were computed and stored for further evaluation (bottom-right panel): amplitude (Amp.), maximum absolute signal percent decrease with respect to baseline PWA-signal (%); duration, time interval between the onset and the offset of the PWA-drop (seconds); integral of the absolute instantaneous percent decrease over the duration expressed in seconds (%seconds).
The total number of PWA-drops during sleep was divided by the number of hours of sleep to compute the PWA-drop index. The mean duration and AUC were calculated across all detected PWA-drops. Based on our preliminary findings that showed opposite effects of PWA-drop index on cardiometabolic outcomes compared to both PWA-drop mean duration and mean AUC, we elected to include two additional parameters for a better comprehension of our findings: the mean duration-normalized PWA-drop index (ratio between PWA-drop index and mean duration) and the mean AUC-normalized PWA-drop index (ratio between PWA-drop index and mean AUC). All PWA-drop features were assessed during total sleep time and for each sleep stage separately (N1, N2, N3, and REM).
The magnitude of PWA-drop features was defined according to their quartiles in the total sample. Boundaries of PWA-drop index quartiles were 0.1 to 37.0 events/hour, 37.1 to 51.2 events/hour, 51.2 to 65.0 events/hour, and 65.1 to 119.1 events/hour. For PWA-drop mean duration quartiles, they corresponded to 4.7 to 12.2 seconds, 12.2 to 13.7 seconds, 13.8 to 15.5 seconds, and 15.6 to 27.9 seconds. For PWA-drop mean AUC quartiles, they were 150 to 449 %seconds, 450 to 517 %seconds, 518 to 591 %seconds, 592 to 1108 %seconds.
Outcomes
Hypertension was defined as an SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, and/or use of anti-hypertensive medication.
Diabetes was considered positive when fasting plasma glucose levels were ≥7.0 mmol/L or there was use of antidiabetic medication [24].
-
Previous CV event was diagnosed based on all available information and established by a local adjudication committee according to international recommendations [25]. All medical records compatible with a CV event were thoroughly reviewed by a cardiologist. The definition of events was as following:
Myocardial infarction: hospital discharge diagnosis (HDD) indicating typical electrocardiogram and elevated myocardial enzymes.
Acute coronary syndromes: HDD providing evidence of typical electrocardiographic changes or coronary angiogram or history of percutaneous transluminal coronary angioplasty.
Stroke: HDD describing the occurrence of new neurologic deficits lasting more than 24 hours.
Statistical analysis
Data distribution was graphically assessed through normal Q-Q plot. Bivariate analysis included Kruskal-Wallis and analysis of variance (ANOVA) for non-normal and normal distributed variables, respectively. Descriptive statistics are represented as percentage within groups (number of participants, n) and mean ± standard deviation (SD) or median (interquartile range, IQR). Pearson’s chi-square test evaluated the association between categorical variables. Multivariable-adjusted logistic regression models assessed the association of PWA-drop features with the presence of hypertension, diabetes, and CV event. Based on univariate analysis and clinical significance, important covariates were identified and included in the model using forward selection. Multicollinearity among independent variables was avoided using variance inflation factor (VIF) <5 through linear regression analysis. Goodness-of-fit of the model was assessed through Hosmer-Lemeshow test. For both hypertension and diabetes, the models were fully adjusted for age, sex, BMI (continuous), smoking (ex-smoker, current smoker, nonsmoker), alcohol consumption (continuous), total sleep time (continuous) and apnea-hypopnea index (AHI) (continuous). For previous CV event, the model was additionally adjusted for diabetes (yes/no) and hypertension (yes/no). When testing PWA-drop mean duration or mean AUC, models were additionally adjusted for the number of PWA-drops (continuous) since there was an inverse correlation between them. Analysis of covariance (ANCOVA) was used to compare the adjusted means of PWA-drop features according to the presence of each outcome. The associations of PWA-drop features with cardiometabolic, HRV, and polysomnographic parameters were calculated using multivariable-adjusted linear regression models. Results are expressed as odds ratios (ORs) with 95% confidence intervals (CIs) for logistic regressions and as linear regression coefficient (β) ± standard error (SE) for linear regressions.
In the HypnoLaus cohort, 1.7% of participants were using alpha-blockers and 8.4% were using beta-blockers. However, as sensitivity analysis did not show any impact on the results when including or excluding those subjects, we included all individuals in the results. To avoid type 1 error, we corrected multiple testing by false discovery rate (FDR) approach. Statistical significance was considered as FDR-corrected p < 0.05 for a two-sided test. All analyses were performed using SPSS software version 25 (IBM, Chicago, IL).
Results
Studied population sample
Of 2162 participants, 13 were excluded from this study due to technical failure and/or insufficient PPG signal quality, resulting in 2149 PSG recordings available for PWA-drop analysis. The sample comprised 51% women, median (IQR) age of 57 (49–68) years old, and mean ± SD BMI of 26.2 ± 4.4 kg/m2. The prevalence of hypertension, CV event, and diabetes was 41.3% (n = 887), 4.4% (n = 88), and 9.9% (n = 212), respectively.
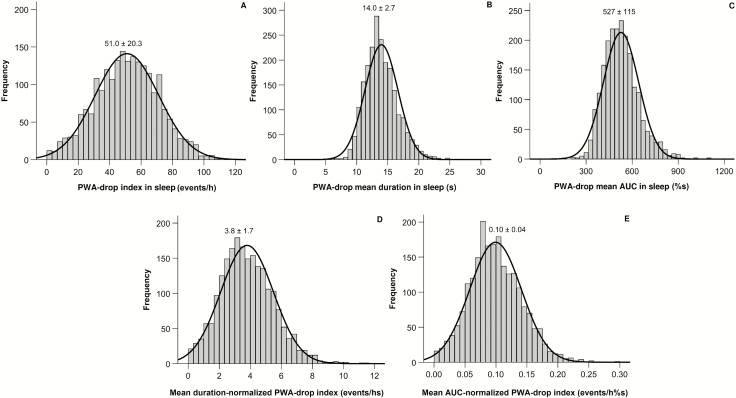
Figure 2 shows the distribution of PWA-drop features during sleep in the HypnoLaus cohort. On average, participants presented around 51 PWA-drop per hour of sleep with a mean duration of 14 seconds each and a mean AUC of 527 %seconds.
Figure 2.
Histogram showing the distribution and mean ± SD of PWA-drop features in the HypnoLaus cohort.
Characteristics of PWA-drops
As shown in Table 1, PWA-drop index was lower in women and decreased with age. PWA-drop mean duration was longer in men and increased with both age and BMI. PWA-drop mean AUC was higher in men as well as in overweight and obese subjects. Mean duration- and mean AUC-normalized PWA-drop index were lower in elderly and decreased with BMI. In addition, the mean AUC-normalized PWA-drop index was lower in men. All PWA-drop features differed significantly according to each sleep stage (Table 2). Specifically, PWA-drop index was higher during REM sleep and lower during N3, while PWA-drop mean duration and mean AUC were higher during N1 (followed by REM sleep) and lower during N3. Both mean duration- and mean AUC-normalized PWA-drop indexes were higher in REM sleep and lower in N3.
Table 1.
Characterization of PWA-drop features according to sex, age, and body mass index
| PWA-drop features | Sex | Age | Body mass index | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | p | <60 years | ≥60 years | p | Eutrophic | Overweight | Obese | p | |
| Index, event/hour | 50.0 ± 19.8 | 52.2 ± 19.8 | 0.010 | 54.5 ± 20.0 | 46.5 ± 20.0 | <0.0001 | 51.0 ± 20.2 | 51.8 ± 20.2 | 49.4 ± 20.7 | 0.165 |
| Mean duration, second | 13.6 ± 2.6 | 14.4 ± 2.6 | <0.0001 | 13.5 ± 2.6 | 14.6 ± 2.6 | <0.0001 | 13.6 ± 2.5 | 14.3 ± 2.7a | 14.3 ± 2.8a | <0.0001 |
| Mean AUC, %seconds | 502 ± 112 | 554 ± 112 | <0.0001 | 528 ± 112 | 527 ± 112 | 0.868 | 512 ± 108 | 539 ± 115a | 539 ± 126a | <0.0001 |
| Mean duration-normalized index, event/hs | 3.8 ± 1.6 | 3.8 ±1.6 | 0.576 | 4.2 ± 1.7 | 3.3 ± 1.7 | <0.0001 | 3.9 ± 1.7 | 3.8 ± 1.7 | 3.6 ± 1.7a | 0.035 |
| Mean AUC-normalized index, event/ hour %seconds | 0.10 ± 0.04 | 0.09 ± 0.04 | 0.004 | 0.11 ± 0.04 | 0.09 ± 0.04 | <0.0001 | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.09 ± 0.04a | 0.012 |
Data are presented as mean ± SD. Data were evaluated by independent T-test and ANOVA followed by Bonferroni’s post-hoc test as appropriate.
aStatistically different from eutrophic group.
Table 2.
Characterization of PWA-drops features according to sleep stages.
| PWA-drop features | Sleep stages | ||||
|---|---|---|---|---|---|
| N1 | N2 | N3 | REM | p | |
| Index, event/hour | 58.8 ± 20.3 | 49.4 ± 21.6a | 36.9 ± 23.9a,b | 64.8 ± 23.9a,b,c | <0.0001 |
| Mean duration, second | 15.7 ± 3.2 | 13.7 ± 2.7a | 12.8 ± 3.1a,b | 14.3 ± 3.2a,b,c | <0.0001 |
| Mean AUC, %min/hour | 639 ± 165 | 508 ± 118a | 447 ± 120a,b | 550 ± 163a,b,c | <0.0001 |
| Mean duration-normalized index, event/hs | 3.9 ± 1.5 | 3.8 ± 1.8a | 3.1 ± 2.1a,b | 4.8 ± 2.1a,b,c | <0.0001 |
| Mean AUC-normalized index, event/hour %seconds | 0.10 ± 0.04 | 010 ± 0.05a | 0.09 ± 0.06a,b | 0.12 ± 0.05a,b,c | <0.0001 |
Data are presented as mean ± SD. Data were evaluated by repeated measures ANOVA followed by Bonferroni’s post-hoc test as appropriate.
aStatistically different from N1.
bStatistically different from N2.
cStatistically different from N3.
Table 3 demonstrates the univariate associations between cardiometabolic outcomes and the PWA-drop features as quartiles. Participants in the lowest quartile of PWA-drop index showed a higher prevalence of hypertension, previous CV event, and diabetes. Similar results were obtained with mean duration- and mean AUC-normalized PWA-drop index quartiles. On the other hand, a higher frequency of cardiometabolic outcomes was found in the highest quartile of PWA-drop mean duration. The same pattern was observed with PWA-drop mean AUC quartiles, except for the association with previous CV event, which was not significant.
Table 3.
Association between cardiometabolic outcomes and PWA-drop features
| PWA-drop features | Hypertension | Previous CV event | Diabetes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p | No | Yes | p | No | Yes | p | |
| Index | |||||||||
| Quartile 1 | 48.4 (260) | 51.6 (277)a | 0.004 | 91.8 (459) | 8.2 (41)a | <0.0001 | 86.4 (464) | 13.6 (73)a | 0.004 |
| Quartile 2 | 59.1 (337) | 40.9 (219) | 96.2 (483) | 3.8 (19) | 89.8 (483) | 10.2 (55) | |||
| Quartile 3 | 62.8 (337) | 37.2 (200) | 97.4 (491) | 2.6 (13) | 92.2 (494) | 7.8 (42) | |||
| Quartile 4 | 64.4 (346) | 35.6 (191) | 97.1 (497) | 2.9 (15) | 92.2 (494) | 7.8 (42) | |||
| Mean duration | |||||||||
| Quartile 1 | 69.6 (373) | 30.4 (163) | <0.0001 | 97.2 (481) | 2.8 (14) | <0.0001 | 93.5 (502) | 6.5 (35) | <0.0001 |
| Quartile 2 | 65.4 (351) | 34.6 (186) | 96.8 (490) | 3.2 (16) | 91.6 (492) | 8.4 (45) | |||
| Quartile 3 | 54.6 (293) | 45.4 (244)a | 96.5 (491) | 3.5 (18) | 91.6 (492) | 8.4 (45) | |||
| Quartile 4 | 45.3 (243) | 54.7 (294)a | 92.1 (468) | 7.9 (40)a | 83.8 (449) | 16.2 (87)a | |||
| Mean AUC | |||||||||
| Quartile 1 | 61.6 (330) | 38.4 (206) | <0.0001 | 95.8 (477) | 4.2 (21) | 0.112 | 91.6 (492) | 8.4 (45) | <0.0001 |
| Quartile 2 | 63.9 (343) | 36.1 (194) | 97.0 (491) | 3.0 (15) | 90.5 (487) | 9.5 (51) | |||
| Quartile 3 | 58.7 (315) | 41.3 (222) | 95.8 (482) | 4.2 (21) | 93.7 (503) | 6.3 (34) | |||
| Quartile 4 | 50.7 (272) | 49.3 (265)a | 93.9 (480) | 6.1 (31) | 84.7 (453) | 15.3 (82)a | |||
| Mean duration-normalized index | |||||||||
| Quartile 1 | 44.9 (241) | 55.1 (296)a | <0.0001 | 92.1 (464) | 7.9 (40)a | <0.0001 | 84.9 (456) | 15.1 (81)a | <0.0001 |
| Quartile 2 | 57.3 (307) | 42.7 (229) | 95.4 (476) | 4.6 (23) | 89.6 (481) | 10.4 (56) | |||
| Quartile 3 | 62.9 (338) | 37.1 (199) | 96.4 (503) | 3.6 (18) | 92.0 (494) | 8.0 (43) | |||
| Quartile 4 | 69.6 (374) | 30.4 (163) | 98.6 (503) | 1.4 (7) | 94.0 (504) | 6.0 (32) | |||
| Mean AUC-normalized index | |||||||||
| Quartile 1 | 46.0 (248) | 54.0 (291)a | <0.0001 | 91.7 (464) | 8.3 (42)a | <0.0001 | 84.4 (455) | 15.6 (84)a | <0.0001 |
| Quartile 2 | 57.3 (307) | 42.7 (229) | 95.8 (477) | 4.2 (21) | 90.9 (487) | 9.1 (49) | |||
| Quartile 3 | 62.2 (333) | 37.8 (202) | 96.9 (493) | 3.1 (16) | 91.0 (488) | 9.0 (48) | |||
| Quartile 4 | 69.3 (372) | 30.7 (165) | 98.2 (496) | 1.8 (9) | 94.2 (505) | 5.8 (31) |
Data are presented as % (n) and analyzed by Pearson’s chi-square test.
aObserved frequency statistically different from overall frequency.
Further demographic and clinical characterization of the sample according to PWA-drop features as quartiles are represented in Supplementary Tables S1–S3. Overall, increasing PWA-drop index was associated with decreased frequency of smoking, use of antidepressants; lower SBP; and increased REM sleep and total arousal index. Increasing PWA-drop mean duration was associated with lower rate of smokers; and higher SBP, DBP, alcohol consumption, total arousal index, and AHI. Increasing PWA-drop mean AUC was associated with lower smoking and total sleep time; and higher SBP, DBP, total arousal index, AHI, and ESS score.
Means ± SD of PWA-drop features adjusted for age, sex, BMI, and AHI according to the presence of the cardiometabolic outcomes are shown in Supplementary Table S4. Participants with hypertension, diabetes or previous CV event showed a decrease in PWA-drop index and an increase in PWA-drop mean duration compared to those without each respective outcome. Greater PWA-drop mean AUC was found only in participants with hypertension compared to those without. Nonetheless, both mean duration- and mean AUC-normalized PWA-drop indexes were significantly lower in those with hypertension, previous CV event, and diabetes compared to those without.
PWA-drops features and cardiometabolic outcomes
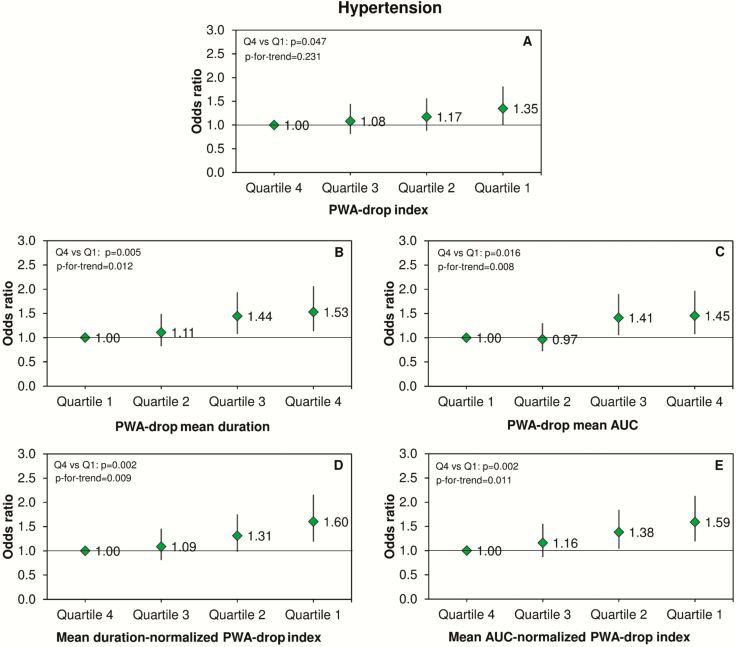
After full adjustment for potential confounders, participants in the lowest quartile of PWA-drop index (Figure 3A) and in the highest quartile of PWA-drop mean duration (Figure 3B) or PWA-drop mean AUC (Figure 3C) showed increased odds of hypertension. Mean duration- and mean AUC-normalized PWA-drop indexes also presented a significant trend across their quartiles in association with increased odds of hypertension (Figure 3, D and E).
Figure 3.
PWA-drops and hypertension. Odds ratios and 95% confidence intervals for the association between quartiles of PWA-drop features during sleep and the prevalence of hypertension. Data were analyzed using logistic regression adjusted for age, sex, body mass index, smoking, alcohol consumption, total sleep time, and apnea-hypopnea index. The number of PWA-drops was additionally adjusted in the analysis of PWA-drop mean duration and mean AUC.
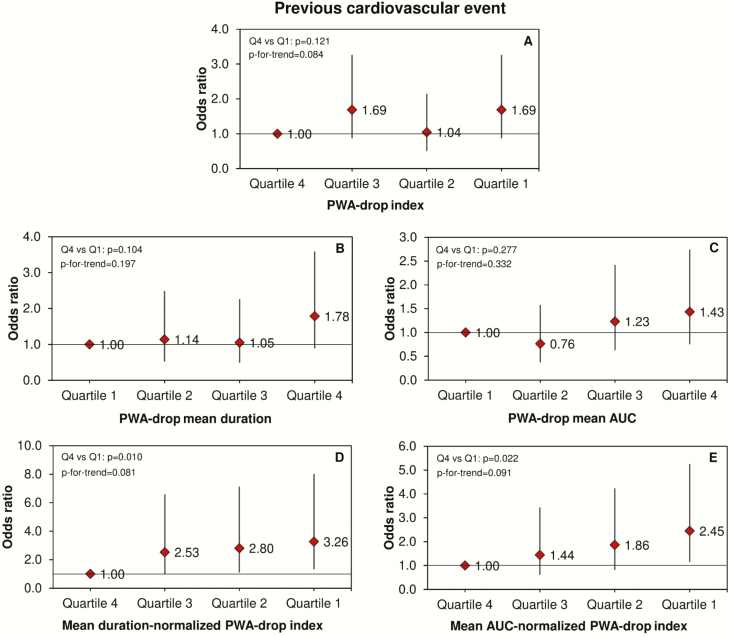
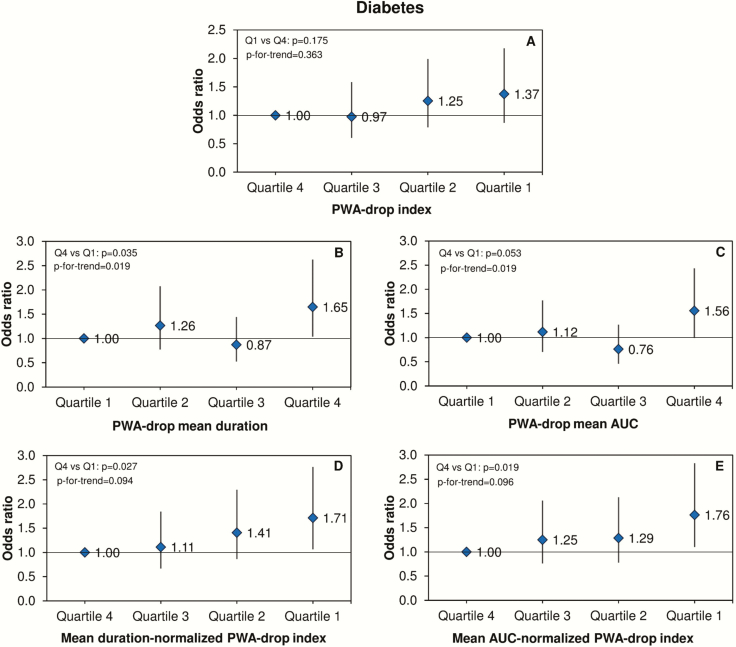
In relation to previous CV event, we also observed independent associations for both mean duration- and mean AUC-normalized PWA-drop indexes. Their lowest quartiles were independently associated with decreased odds of having a previous CV event (Figure 4, D and E). Similar results were found for diabetes (Figure 5, D and E). Additionally, increasing PWA-drop mean duration and mean AUC were independently associated with increased odds of diabetes (Figure 5, B and C).
Figure 4.
PWA-drop and cardiovascular events. Odds ratios and 95% confidence intervals for the association between quartiles of PWA-drop features during sleep and the ocurrence of previous cardiovascular event. Data were analyzed using logistic regression adjusted for age, sex, body mass index, smoking, alcohol consumption, total sleep time, apnea-hypopnea index, diabetes, and hypertension. The number of PWA-drops was additionally adjusted in the analysis of PWA-drop mean duration and mean AUC.
Figure 5.
PWA-drops and diabetes. ORs and 95% confidence intervals for the association between quartiles of PWA-drop features during sleep and the prevalence of diabetes. Data were analyzed using logistic regression adjusted for age, sex, body mass index, smoking, alcohol consumption, total sleep time, and apnea-hypopnea index. The number of PWA-drops was additionally adjusted in the analysis of PWA-drop mean duration and mean AUC.
Using PWA-drop features as continuous variables, we consistently observed the independent association of both normalized PWA-drop indexes with the cardiometabolic outcomes (Supplementary Table S5).
Supplementary Tables S6 and S7 show the independent associations of PWA-drop features with polysomnographic, cardiometabolic, and HRV-related parameters through multivariable-adjusted linear regressions. Increasing PWA-drop index, mean duration, and mean AUC were associated with higher N1, lower N3 and increased total arousal index and AHI. In addition, longer mean duration was associated with increased SBP, while greater mean AUC was associated with increased DBP. Finally, PWA-drop index was associated with increased levels of insulin and changes in heart rate variability, demonstrated by higher SD of normal-to-normal RR intervals (SDNN) in the time-domain analysis, and increased low frequency (LF), low frequency to high frequency ratio (LF/HF), and total frequency power in the frequency-domain analysis.
Discussion
This is the first study defining the frequency and characteristics of PWA-drops during sleep, and providing evidence for their association with CV risk factors and CV disease in a middle-to-old general population sample. Our findings show that lower PWA-drop index was independently associated with higher odds of hypertension, while longer PWA-drop mean duration and greater PWA-drop mean AUC were associated with increased odds of hypertension and diabetes. In turn, decreased mean duration- and mean AUC-normalized PWA-drop indexes were independently associated with increased odds of hypertension, CV event, and diabetes. Taken together, our results suggest that these new sleep-related parameters could be interesting biomarkers of cardiometabolic morbidity.
PWA-drops and sleep
A dense vascular bed rich in arterial anastomosis characterizes the skin of limb extremities and drives important changes in the blood flow rate from small arteries to the venous pool following changes in ANS activity. This area of the body is ideal to study PWA variations using PPG, especially during sleep, since the ANS activity is more stable compared to wakefulness [26]. In our study, we observed that a higher frequency of PWA-drops during sleep was associated with sympathetic dominance as shown by a higher LF/HF ratio for HRV.
Overall, in non-rapid eye movement (NREM) sleep there is an increase in the parasympathetic tone and a decrease in the sympathetic tone, leading to a reduction in both skin blood flow and the pulse volume [27–29]. In REM sleep, a high variability in the ANS activity occurs, characterized by oscillations in the parasympathetic tonus and phasic sympathetic discharges, leading to skin vasoconstriction [28]. Accordingly, in the current study, a higher frequency of PWA-drops was observed in REM sleep, while during slow-wave sleep, there were less frequent, shorter and shallower PWA-drops (Table 2), in agreement with our previous observation in a more limited sample [23].
Cortical arousals can also cause central autonomic activation, leading to increased sympathetic activity and peripheral vasoconstriction [30]. Similarly, obstructive apneas and hypopneas may generate nocturnal PWA-drops [4, 31] due to an overloaded sympathoadrenergic vascular tone that occurs during sleep [30]. This response to respiratory events can also be accentuated in the presence of an arousal [31] and have been shown to be reversed by positive airway pressure (PAP) treatment in OSA patients [32]. In agreement, our findings showed an independent association of PWA-drops index with AHI and total arousal index (Supplementary Table S6).
PWA-drops and hypertension
Increased PWA-drop mean duration was consistently and positively associated with prevalent hypertension as well as SBP even after adjustments for the main risk factors. This could indicate that subjects with hypertension have impaired endothelial function slowing the blood vessels re-dilation after a vasoconstriction, or that sympathetic activations during sleep are longer in hypertensive subjects. We could speculate that longer PWA-drops may be an early physiological sign of impaired vascular function, but a prospective analysis would be needed to identify a possible causal relationship.
A previous case-control study showed that office blood pressure and median overnight average attenuation in PWA, but not PWA-attenuation frequency, were correlated (R = 0.26 for SBP and R = 0.31 for DBP). Multivariable analysis demonstrated that this association was independent of age, sex, BMI, anti-hypertensive medication, number of PWA-attenuation episodes, AHI, oxygen desaturation index and total arousal index [33]. Each 10% increase in the median PWA attenuation during sleep was associated with 5 mmHg increase in SBP and 3 mmHg increase in DBP. Unfortunately, although our results seem to corroborate their findings, we cannot directly compare this study with our results, since they differ in many aspects such as the measurement (median PWA attenuation vs. mean PWA-drops index), sample size (n = 81 vs. n = 2149), population (14 hypertensive individuals without diabetes vs. general unselected population), and technical aspects (pulse arterial tonometry vs. pulse oximetry).
Overall, our findings suggest that individuals with hypertension may present a less reactive vascular bed during sleep in response to a given sympathetic activation, accompanied by a longer autonomic response.
PWA-drops and CV events
In the current study, there was an association between PWA-drop index and the presence of a previous CV event; however, with the inclusion of hypertension in the model, the significance tended to be borderline (p = 0.061, Supplementary Table S5). Nevertheless, using the mean duration- or mean AUC-normalized PWA-drop indexes, the results indicated an independent negative association with the occurrence of a previous CV event. As we cannot infer causality, one could speculate that CV disease may lead to diminished vascular reactivity; on the other hand, subjects with a high and fast vascular reactivity generating frequent vasoconstrictions could be protected against CV events. We observed in our cohort that aging was associated with a decrease in PWA-drop index, which also suggests that higher frequency of PWA-drop may be healthier. Another explanation is that high PWA-drop index reflects a reactive and fast-adapting ANS, as we have found independent association with increased HRV and sympathetic dominance (Supplementary Table S7). It has been shown that high HRV is associated with lower risk of CV diseases [34] and that conversely, terminally ill patients (e.g. congestive heart failure, diabetes, end-stage renal disease, etc.) have reduced HRV [35, 36].
Previous studies suggested that PPG could be used as a predictor of CV disease. Using a combined analysis algorithm based on 8 PPG-derived parameters including PWA-attenuation index (similar to PWA-drop index in our work), Sommermeyer et al. [37] could predict individual CV risk according to established risk factors in a multicenter setting of 520 subjects referred for a sleep study due to OSA symptoms [37]. The same research group applied a modified version of the algorithm in a sample of 631 patients suspected for OSA, finding a dose-response relationship between the composite biosignal risk score and the number of CV risk factors [38]. However, in these studies, we cannot isolate the contribution of each PPG component on the CV risk and compare it with our findings.
PWA-drops and diabetes
Our findings showed, for the first time, increased PWA-drop mean duration and mean AUC in subjects with diabetes, suggesting an inadequate and long vascular response to the sympathetic activation during sleep. There is evidence showing increased aortic pulse wave velocity (PWV), a marker of arterial stiffness, in both diabetic and impaired glucose tolerance patients compared to euglycemic subjects [39, 40]. Another study showed that vasoconstrictor responses to a deep breath and body cooling were significantly reduced in the fingers and toes of newly diagnosed diabetes type 2 patients, suggesting impaired endothelial function [41].
Data from animal models of diabetes have shown that chronic hyperglycemia leads to wall hypertrophy and fibrosis of the vessels through activation of the renin-angiotensin-aldosterone system and changes in the vasoelastic properties of the vessels [42]. However, other factors associated with diabetes, such as insulin resistance, advanced glycation end-products, inflammation, and oxidative stress, have to be considered in the vascular alterations early promoted by diabetes [39]. In our study, we did not find any independent relationship between PWA-drop features and glucose levels. However, higher frequency of PWA-drops was independently associated with a small increase in insulin levels (Supplementary Table S7), suggesting a protective role for diabetes. Lastly, considering the correlation found between PWA-drop index and LF, our data is in agreement with a prospective study in the Atherosclerosis Risk In Communities (ARIC) cohort, in which they found that participants with a lower LF were at a higher risk of developing diabetes. Further research is needed to determine whether PWA-drop variations are a consequence or cause of diabetes.
Limitations and strengths
This work has some limitations. Although our study suggests a link between PWA-drop features and vascular function, we did not directly assess endothelial function, as this study was a secondary analysis. Despite the novelty and complete characterization of PWA-drop features in the HypnoLaus cohort, the cross-sectional design of the study does not allow establishing a causal relationship between the findings. Nonetheless, this pioneer study, performed in a large middle-to-old age general population with well-defined objective outcomes, strongly suggests that PWA-drop characteristics could become new sleep-related biomarkers of cardiometabolic risk. Future prospective studies and randomized clinical trials exploring the effects of low PWA-drop frequency and/or long and large PWA-drops are needed to ensure the value of these physiological parameters for clinical purpose as factors associated with incident CV events. Furthermore, experimental studies aiming to correlate these sleep PWA parameters with ANS and endothelial function using pharmacological intervention (e.g. sympatholytic drugs) and vascular reactivity paradigms (e.g. reactive hyperemia), respectively, should be performed. Finally, comparison of PWA-drop features in patients with and without known vascular disease, ANS dysfunction, and pre-diabetes, before and after disease treatment, could also help to better understand their clinical significance and pathophysiology.
Conclusion
This study described PWA-drop features during sleep, which were shown to vary according to differences in sympathovagal activity related to sleep stages, age, BMI, and sex. Reductions in PWA-drop features such as mean duration-normalized or mean AUC-normalized PWA-drop indexes were independently associated with increased prevalence of hypertension, CV event, and diabetes, suggesting that they could be an interesting biomarker of these disorders.
Funding
The CoLaus study was and is supported by research grants from GlaxoSmithKline, the Université de Lausanne, and the Swiss National Science Foundation (SNF, grants 33CSCO-122661, 33CS30-139468, and 33CS30-148401).
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
We would like to thank Mehdi Tafti, Martin Preisig, Daniela Andries, Nadia Tobback, Vincent Mooser, and all other investigators of CoLaus/PsyCoLaus study, who made the HypnoLaus study possible. We are grateful to the Lausanne population who volunteered to participate in the HypnoLaus study.
References
- 1. Babchenko A, et al. Photoplethysmographic measurement of changes in total and pulsatile tissue blood volume, following sympathetic blockade. Physiol Meas. 2001;22(2):389–396. [DOI] [PubMed] [Google Scholar]
- 2. Alian AA, et al. Photoplethysmography. Best Pract Res Clin Anaesthesiol. 2014;28(4):395–406. [DOI] [PubMed] [Google Scholar]
- 3. Colombo R, et al. Pulse photoplethysmographic analysis estimates the sympathetic activity directed to heart and vessels. Anesthesiology. 2015;123(2):336–345. [DOI] [PubMed] [Google Scholar]
- 4. Grote L, et al. Finger plethysmography–a method for monitoring finger blood flow during sleep disordered breathing. Respir Physiol Neurobiol. 2003;136(2-3):141–152. [DOI] [PubMed] [Google Scholar]
- 5. Mihaljevic S, et al. Area under the curve of finger photoplethysmography as an evaluation measure for sympathetic activity during lumbar epidural anaesthesia. Turk J Anaesthesiol Reanim. 2018;46(2):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vat S, et al. Scoring criteria for portable monitor recordings: a comparison of four hypopnoea definitions in a population-based cohort. Thorax. 2015;70(11):1047–1053. [DOI] [PubMed] [Google Scholar]
- 7. Bosi M, et al. Arousal responses to respiratory events during sleep: the role of pulse wave amplitude. J Sleep Res. 2018;27(2):259–267. [DOI] [PubMed] [Google Scholar]
- 8. Karmakar C, et al. Detection of respiratory arousals using photoplethysmography (PPG) signal in sleep apnea patients. IEEE J Biomed Health Inform. 2014;18(3):1065–1073. [DOI] [PubMed] [Google Scholar]
- 9. Haba-Rubio J, et al. Obstructive sleep apnea syndrome: effect of respiratory events and arousal on pulse wave amplitude measured by photoplethysmography in NREM sleep. Sleep Breath. 2005;9(2):73–81. [DOI] [PubMed] [Google Scholar]
- 10. Delessert A, et al. Pulse wave amplitude drops during sleep are reliable surrogate markers of changes in cortical activity. Sleep. 2010;33(12):1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zacharia A, et al. Sleep apnea syndrome: improved detection of respiratory events and cortical arousals using oxymetry pulse wave amplitude during polysomnography. Sleep Breath. 2008;12(1):33–38. [DOI] [PubMed] [Google Scholar]
- 12. Adler D, et al. Pulse wave amplitude reduction: a surrogate marker of micro-arousals associated with respiratory events occurring under non-invasive ventilation? Respir Med. 2013;107(12):2053–2060. [DOI] [PubMed] [Google Scholar]
- 13. Heinzer R, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Firmann M, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Preisig M, et al. The PsyCoLaus study: methodology and characteristics of the sample of a population-based survey on psychiatric disorders and their association with genetic and cardiovascular risk factors. BMC Psychiatry. 2009;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 17. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 18. El Assaad MA, et al. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit. 2002;7(4):237–241. [DOI] [PubMed] [Google Scholar]
- 19. Weber MA, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications, 1st ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21. Berry RB, et al. ; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pichot V, et al. HRVanalysis: a free software for analyzing cardiac autonomic activity. Front Physiol. 2016;7:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Betta M, et al. Quantifying peripheral sympathetic activations during sleep by means of an automatic method for pulse wave amplitude drop detection. Sleep Med. 2020 (In press). [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cutlip DE, et al. ; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. [DOI] [PubMed] [Google Scholar]
- 26. Elsenbruch S, et al. Heart rate variability during waking and sleep in healthy males and females. Sleep. 1999;22(8):1067–1071. [DOI] [PubMed] [Google Scholar]
- 27. Noll G, et al. Skin sympathetic nerve activity and effector function during sleep in humans. Acta Physiol Scand. 1994;151(3):319–329. [DOI] [PubMed] [Google Scholar]
- 28. Khatri IM, et al. Hemodynamic changes during sleep. J Appl Physiol. 1967;22(5):867–873. [DOI] [PubMed] [Google Scholar]
- 29. Tank J, et al. Relationship between blood pressure, sleep K-complexes, and muscle sympathetic nerve activity in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R208–R214. [DOI] [PubMed] [Google Scholar]
- 30. Zou D, et al. Obstructive apneic events induce alpha-receptor mediated digital vasoconstriction. Sleep. 2004;27(3): 485–489. [DOI] [PubMed] [Google Scholar]
- 31. O’Donnell CP, et al. The effect of upper airway obstruction and arousal on peripheral arterial tonometry in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166(7): 965–971. [DOI] [PubMed] [Google Scholar]
- 32. Randerath WJ, et al. Parameters of Overnight Pulse Wave under Treatment in Obstructive Sleep Apnea. Respiration. 2016;92(3):136–143. [DOI] [PubMed] [Google Scholar]
- 33. Zou D, et al. Nocturnal pulse wave attenuation is associated with office blood pressure in a population-based cohort. Sleep Med. 2009;10(8):836–843. [DOI] [PubMed] [Google Scholar]
- 34. Tsuji H, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. [DOI] [PubMed] [Google Scholar]
- 35. Stein PK, et al. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;16(1):47–66. [DOI] [PubMed] [Google Scholar]
- 36. Liao D, et al. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes. 2002;51(12):3524–3531. [DOI] [PubMed] [Google Scholar]
- 37. Sommermeyer D, et al. The use of overnight pulse wave analysis for recognition of cardiovascular risk factors and risk: a multicentric evaluation. J Hypertens. 2014;32(2): 276–285. [DOI] [PubMed] [Google Scholar]
- 38. Sommermeyer D, et al. Detection of cardiovascular risk from a photoplethysmographic signal using a matching pursuit algorithm. Med Biol Eng Comput. 2016;54(7):1111–1121. [DOI] [PubMed] [Google Scholar]
- 39. Gajdova J, et al. Pulse wave analysis and diabetes mellitus. A systematic review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(3):223–233. [DOI] [PubMed] [Google Scholar]
- 40. Rahman S, et al. Early manifestation of macrovasculopathy in newly diagnosed never treated type II diabetic patients with no traditional CVD risk factors. Diabetes Res Clin Pract. 2008;80(2):253–258. [DOI] [PubMed] [Google Scholar]
- 41. McAuley DF, et al. Vasoconstriction to endogenous endothelin-1 is impaired in patients with type II diabetes mellitus. Clin Sci (Lond). 2000;99(3):175–179. [PubMed] [Google Scholar]
- 42. Jesmin S, et al. Role of angiotensin II in altered expression of molecules responsible for coronary matrix remodeling in insulin-resistant diabetic rats. Arterioscler Thromb Vasc Biol. 2003;23(11):2021–2026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.