Abstract
Study Objectives
Formation and maintenance of fear-extinction memories are disrupted in post-traumatic stress disorder (PTSD) and anxiety disorders. Sleep contributes to emotional memory consolidation and emotion regulation. Insomnia disorder (ID) is characterized by persistent sleep disturbance as well as rapid eye movement (REM) sleep abnormalities and often precedes or develops in parallel with PTSD and anxiety disorders. Here, we explore the impact of chronic poor sleep and sleep immediately following fear conditioning and extinction learning on preservation of extinction memories.
Methods
Twenty-four ID age- and sex-matched to 24 healthy, good sleeper controls (GS) completed up to 2 weeks of habitual sleep monitoring with daily sleep–wake diaries and actigraphy, and then participated in a two-session fear conditioning, extinction learning and extinction recall procedure. Fear Conditioning and Extinction Learning occurred during session 1, followed by Extinction Recall approximately 24 hours later. Skin-conductance responses (SCR) and shock expectancies were recorded throughout all experimental phases to evaluate associative learning and memory. Overnight sleep between sessions 1 and 2 was recorded using ambulatory polysomnography.
Results
ID showed greater physiological reactivity during Fear Conditioning. REM sleep physiology was associated with poorer extinction memory in ID but better extinction memory in GS.
Conclusion
REM sleep physiology may differentially support emotional memory retention and expression in ID and GS. In the former, REM may enhance retention of fear memories, while in the later, REM may enhance the expression of extinction memories.
Keywords: insomnia, fear conditioning, fear extinction, REM sleep, emotion, sleep and memory, post-traumatic stress disorder, sleep and learning, sleep and psychiatric conditions
Statement of Significance.
Incident insomnia disorder exhibits bidirectional risk with incident emotional disorders such as post-traumatic stress disorder (PTSD). Individuals with PTSD show deficits in fear extinction memory. Fear extinction learning and memory are important mechanisms supporting our ability to overcome fears, both in everyday life and during psychotherapies, such as exposure therapy, that treat PTSD. However, to date, evidence is limited as to how insomnia influences fear extinction memory or how intervening sleep, and rapid eye movement (REM) sleep in particular, influence the consolidation of extinction memory. Understanding how REM sleep interacts with fear extinction memory in insomnia may provide a mechanistic link by which those with poor sleep sometimes go on to develop PTSD.
Introduction
Mounting evidence supports the hypothesis that emotional disorders, such as post-traumatic stress disorder (PTSD), may be disorders of emotional learning and memory [1–3]. Fear conditioning protocols are hypothesized to probe emotional learning and memory processes similar to those evoked during and after a traumatic experience [4]. In brief, Fear Conditioning is a form of associative learning that occurs when an emotionally neutral stimulus occurs in close temporal proximity to an inherently aversive experience (unconditioned stimulus or “US”). Repeated pairings with the US result in the neutral stimulus becoming capable, on its own, of evoking a “conditioned response” (CR), or a fear state similar to that evoked by the US. The previously neutral stimulus thus becomes a “conditioned stimulus” (CS). Conditioned Fear is the memory of this CS–US contingency (i.e. remembering that the once neutral stimulus now predicts an aversive outcome). Extinction Learning occurs when the CS is subsequently presented repeatedly without the US resulting in a diminishment of the CR. However, rather than erasing the CS−US association, extinction learning represents the formation of a new Extinction Memory that competitively inhibits the memory of the CS−US contingency when the CS is subsequently encountered [4–7]. As with other types of memory, Extinction Memory must be encoded, consolidated and retrieved in order to be expressed at a later time. Thus, the facilitative effects of sleep on memory consolidation may extend also to extinction [8].
In healthy, good sleeping individuals (GS), sleep facilitates generalization of extinction memory to previously un-extinguished stimuli [9, 10] and promotes the retention of habituation to aversive stimuli [11]. Similarly, sleep promotes consolidation of extinction memory for phobic fears [10, 12]. Rapid eye movement (REM) sleep occurring between extinction learning and recall correlates with greater retention of both extinction memories [13] and safety learning (learning to discriminate between similar stimuli that either predict an aversive outcome or not) [14]. Further, REM sleep deprivation has been observed to impair memory for extinction in humans [15] and the rat [16]. For a review, see ref. [8].
Extinction memory processing is abnormal in PTSD [2, 17–20] as well as in anxiety-related [21] and other psychiatric disorders [22, 23]. For example, individuals with PTSD show poorer extinction memory than trauma-exposed, healthy controls [17, 18]. Such abnormalities may be related to comorbid sleep disturbance [1], including insomnia. Notably, disrupted sleep, and insomnia specifically, are established risk factors for the development of PTSD [24–26], although the relationship between insomnia and PTSD is clearly bidirectional [27, 28].
In chronic insomnia, evidence is mounting that abnormalities in emotion regulation exist beyond those associated with sleep loss alone [29–34]. One key marker influencing emotion regulatory processes in insomnia may be hyperarousal which has been observed both in central and peripheral physiology [35–38] as well as in cognitive traits [39, 40]. Additionally, REM sleep abnormalities have been observed in chronic insomnia [41] in which REM sleep stability and continuity may be compromised [42, 43]. Similar abnormalities, such as REM sleep fragmentation, have been observed following a trauma in those who go on to develop PTSD [26, 44], and thus may serve as an overlapping biomarker in insomnia and PTSD. We have previously hypothesized that impairments in REM-sleep-dependent processing of emotional memories, including those for fear extinction, may contribute to the development of PTSD [1].
With the same participants comprising the current study, we recently completed the first neuroimaging study of fear conditioning and extinction in insomnia disorder (ID) without comorbid medical or psychiatric disorders [45]. During fear conditioning, both ID and GS activated fear-related structures. Across Extinction Learning, ID demonstrated little change whereas GS activated both fear and extinction-related areas, including the hippocampus, insula, dorsal anterior cingulate cortex (dACC), and ventromedial prefrontal cortex (vmPFC). During Extinction Recall 24 hours later, whereas GS now demonstrated limited activation, ID activated regions similar to those previously activated in GS (vmPFC, dACC, insula). Because, across Extinction Learning, GS but not ID activated both fear and extinction-related networks whereas, at Extinction Recall, ID engaged similar regions but GS no longer did so, individuals with ID may show a delayed acquisition of fear extinction memories.
In the current study, we compared psychophysiological and self-report measures of fear conditioning, extinction learning and extinction memory between ID and GS participants. In addition, we investigated associations between Extinction Memory and polysomnograph (PSG)-recorded REM sleep physiology as REM is the sleep stage most often associated with emotional memory consolidation in GS [46–48]. We hypothesized that: (1) hyperarousal characteristic of ID would be reflected by overall elevation of sympathetic reactivity compared to GS; (2) in ID compared to GS, fear conditioning would be enhanced and/or extinction learning and memory would be impaired as reflected in physiological expression and subjective awareness of fear conditioning, extinction learning, and extinction memory; and (3) there will exist associations of extinction recall with REM-sleep predictors including REM latency, percentage, continuity, density and theta spectral power. If REM sleep physiology were to thus influence extinction memory and do so differentially in ID and GS, this would suggest one potential mechanism by which sleep disruption, and specifically pre-existing ID, might increase vulnerability to trauma-related disorders. A subsample of the psychophysiological (skin conductance response; SCR) data reported here were previously published in our fMRI study [45]. However, in this prior report, psychophysiological findings mirrored specific blood oxygen level dependent (BOLD) contrasts and each experimental phase was not comprehensively analyzed as is reported here.
Methods
Participants
A total of 24 ID and 24 age and sex-matched healthy GS participants, who were right-handed (determined by the Edinburgh Handedness Inventory [49]) were recruited from the community (demographic characteristics are presented in Table 1). All participants completed an initial telephone screening in which they were given a full description of the study procedures and screened for exclusion criteria (detailed in Supplementary Materials). Prior to all study procedures, participants provided written informed consent. All study procedures were in accord with the Declaration of Helsinki and approved by the Partners Healthcare Institutional Review Board. Participants were compensated for their participation.
Table 1.
Group comparisons of demographic and sleep variables. Significant values have been bolded for clarity
| Insomnia disorder (N = 24) | SD | Good sleepers (N = 24) | SD | t | df | p | U | p’ | Effect size (Cohen’s d) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Sex | 18 F, 6 M | - | 18 F, 6 M | - | - | - | - | - | - | - |
| Age† | 30.38 | 13.60 | 30.79 | 13.71 | −0.11 | 46.00 | 0.92 | 271.50 | 0.73 | −0.03 |
| Self-reported habitual sleep | ||||||||||
| PSQI | 10.44 | 2.41 | 1.46 | 1.06 | 16.41 | 29.97 | 0.00 | 4.82 | ||
| ISI† | 18.91 | 3.92 | 1.04 | 1.30 | 20.79 | 26.61 | 0.00 | 0.00 | 0.00 | 6.12 |
| ESS | 8.79 | 5.76 | 4.42 | 3.24 | 3.24 | 36.26 | 0.00 | 0.94 | ||
| MEQ | 49.46 | 9.57 | 52.67 | 7.55 | −1.29 | 46.00 | 0.20 | −0.37 | ||
| Sleep–Wake Diaries | ||||||||||
| TST (min) | 412.94 | 68.82 | 453.37 | 44.31 | −2.35 | 35.33 | 0.03 | −0.70 | ||
| SOL (min)† | 34.86 | 17.00 | 11.56 | 6.26 | 6.06 | 26.17 | 0.00 | 31.00 | 0.00 | 1.82 |
| SE (%)† | 85.64 | 7.12 | 96.09 | 2.10 | −6.61 | 24.49 | 0.00 | 19.00 | 0.00 | −1.99 |
| Sleep Midpoint (mins past midnight) | 238.81 | 58.61 | 217.52 | 59.08 | 1.24 | 45.00 | 0.22 | 0.36 | ||
| Actigraphy | ||||||||||
| TST (min) | 386.76 | 60.56 | 392.62 | 37.88 | −0.39 | 37.16 | 0.70 | −0.12 | ||
| SOL (min)† | 22.15 | 15.69 | 13.46 | 9.78 | 2.20 | 35.18 | 0.03 | 166.00 | 0.07 | 0.66 |
| SE (%) | 82.22 | 4.36 | 84.96 | 5.54 | −1.85 | 43.00 | 0.07 | −0.55 | ||
| Sleep Midpoint (mins past midnight) | 233.60 | 57.10 | 214.84 | 59.98 | 1.06 | 42.00 | 0.29 | 0.32 | ||
| Consolidation PSG | ||||||||||
| TST (min) | 391.65 | 71.73 | 384.98 | 86.63 | 0.28 | 43.00 | 0.78 | 0.08 | ||
| NREM1 (%) | 8.60 | 3.56 | 7.26 | 3.35 | 1.29 | 42.00 | 0.20 | 0.39 | ||
| NREM2 (%) | 52.23 | 7.81 | 53.27 | 9.26 | −0.41 | 43.00 | 0.69 | −0.12 | ||
| NREM3 (%) | 20.96 | 8.63 | 18.52 | 9.93 | 0.88 | 43.00 | 0.38 | 0.26 | ||
| REM (%) | 17.24 | 6.44 | 20.94 | 7.38 | −1.80 | 43.00 | 0.08 | −0.54 | ||
| REM Latency (min) | 113.78 | 47.27 | 86.91 | 44.00 | 1.95 | 42.00 | 0.06 | 0.59 | ||
| Average REM Segment Length (mins) | 7.88 | 3.00 | 7.90 | 2.86 | −0.03 | 42.00 | 0.98 | −0.01 | ||
| REMD† | 5.93 | 3.76 | 6.88 | 3.08 | −0.92 | 42.00 | 0.37 | 187.00 | 0.20 | −0.28 |
| Relative frontal REM theta power | 8.29 | 3.38 | 9.82 | 2.37 | −1.64 | 36 | 0.11 | 0.52 | ||
| Relative central REM theta power† | 10.02 | 4.12 | 12.58 | 2.96 | −2.17 | 34 | 0.04 | 103.00 | .07 | 0.71 |
ESS, Epworth Sleepiness Scale; ISI, Insomnia Severity Index; MEQ, Morningness-Eveningness Questionnaire; NREM, Non-Rapid Eye Movement Sleep; PSQI, Pittsburgh Sleep Quality Index; REM, Rapid Eye Movement Sleep; REMD, rapid eye movement density; SE, sleep efficiency; SOL, sleep onset latency; TST, total sleep time.
†Values non-normally distributed; U, Mann–Whitney U test statistic; p’, significance value for Mann–Whitney U test. Bold values are the p-values for the various tests.
Psychological assessment
The Pittsburgh Structured Clinical Interview for Sleep Disorders (PSCI-SD; a widely used [50, 51], unpublished in-house instrument) and the Insomnia Severity Index [52] conferred ID diagnosis. Using participant responses on the PSCI-SD, we were able to determine that ID participants met criteria for DSM-5 Insomnia Disorder and ICSD-3 Chronic Insomnia Disorder, but no other sleep disorder. ID participants met or exceed the ISI threshold for clinical insomnia (ISI ≥ 15) with the exception of two who met criteria for subthreshold insomnia (ISI = 13). Of subjects exceeding the clinical insomnia threshold, seven met or exceeded criteria for severe clinical insomnia (ISI ≥ 22). Additionally, all ID participants exceeded the threshold for clinical sleep disturbance (≥ 5) on the Pittsburgh Sleep Quality Index (PSQI) [53] (Table 1). GS participants showed no evidence of insomnia or other sleep disorders on the PSCI-SD, no evidence of clinical insomnia on the ISI (all < 4) and no evidence of clinical sleep disturbance on the PSQI (all < 3) (Table 1). The Structured Clinical Interview for DSM-IV for Non-Patients (SCID 1/NP) [54] was administered by a trained clinical psychologist (N.L.) and determined current and lifetime history of DSM-IV-TR Axis I psychiatric disorders. Participants with any current psychiatric disorders (other than insomnia disorder for ID participants) were excluded. To confirm that participants were free from psychoactive substances, a urine toxicology screen (Medimpex United, Inc.) was performed to test for 11 substances of abuse.
Habitual sleep assessment
For up to 14 days following the psychological assessment, each participant’s sleep was continuously monitored with wrist actigraphy (Actiwatch 2, Philips Respironics, Bend, OR; see Supplementary Materials for actigraphy acquisition and analysis methods). Additionally, participants completed the Morningness-Eveningness Questionnaire [55], Epworth Sleepiness Scale [56] and twice daily (morning at awakening and evening prior to lights out) made entries on the Evening/Morning Sleep Questionnaire (EMSQ) sleep diary (see Supplementary Materials for questionnaire content and analysis details) [57, 58]. During this 14-day assessment period, participants were required to abstain from alcohol and recreational drugs and, on the day before and during the two scanning days, were required to refrain from caffeine and daytime napping (see Supplementary Materials for compliance data from these 3 days).
Participants remained eligible if they exhibited no clinically referable sleep disturbances (obstructive sleep apnea [OSA] or periodic limb movement disorder [PLMD]) during a Diagnostic/Acclimation ambulatory PSG recording. Those in the ID group maintained eligibility if they reported a combined EMSQ sleep onset latency (SOL) and wake time after sleep onset of >45 minutes on at least 3 days per 7-day period. Participants who maintained eligibility throughout the assessment period completed a second night of ambulatory PSG (“Baseline” night) and then reported to the laboratory between 16:00 and 22:00 the following day to complete the Fear Conditioning and Extinction Learning phases of the experimental protocol in the MRI scanner. Directly following the scan, PSG was again applied to participants who were then monitored for a second night with ambulatory PSG (“Consolidation” night). Participants returned to the lab approximately 24 hours later between 16:00 and 22:00 and completed the Extinction Memory phase of the experimental protocol in the same scanner (see Supplementary Materials for description of fMRI procedures, Supplementary Figure 1 for the experimental study schedule, and Supplementary Figure 2 for the Fear Conditioning, Extinction Learning and Extinction Recall protocol).
Fear conditioning, extinction learning, and extinction recall procedures
Fear conditioning, extinction learning, and extinction memory were probed using an extensively validated 2-day protocol that consisted of four phases (Habituation, Fear Conditioning, Extinction Learning, and Extinction Recall; see Supplementary Figure 2) [18, 21, 59–65]. During each phase, computer-generated images of a desk lamp, superimposed on one of two backdrop contexts (Conditioning Context = office or Extinction Context = conference room, see Supplementary Figure 2), switched to one of three different colors (blue, red, or yellow). During the first 3 seconds of image presentation, contexts appeared with lamps turned off, after which time the lamp turned on in one of the three colors and remained illuminated for 6 additional seconds. Stimulus presentation, stimulus onset/offset event marks and administration of finger shocks were controlled using Superlab 4.5 (Cedrus Corp., San Pedro, CA).
During the Habituation phase, all possible combinations of lamp color and context were presented across six trials to minimize novelty effects on later learning. Directly following Habituation, during the Fear Conditioning phase, participants underwent fear conditioning to two of three colored lamps in the Conditioning Context. Each conditioned stimulus (CS+) was presented eight times with partial reinforcement (five of eight presentations or 63%) by a 0.5-second finger shock presented at stimulus offset. The remaining colored lamp, which was never paired with a shock (CS-), was interspersed among the CS+s for a total of 16 presentations. Pseudorandom arrangement of CSs was such that no more than two CS+s or CS-s occurred in a row. Immediately following Fear Conditioning, the Extinction Learning phase began in which one of the two previously established CS+s (CS+E) was presented 16 times, without the finger shock, in the Extinction Context. Additionally, 16 presentations of the CS- were interspersed throughout the trial, again with no more than 2 consecutive CS+s or CS-s. The second (omitted) CS+ remained conditioned but un-extinguished (CS+U). During the Extinction Recall phase, which occurred 24 hours after Extinction Learning, each CS+ (CS+E and CS+U) was presented 8 times in addition to 16 interspersed CS- presentations without any USs. Fear Conditioning and Extinction Recall trials adhered to a block design in which each CS+ was presented eight times with eight corresponding CS-s (i.e. during Fear Conditioning, the first and second CS+, and during Extinction Recall, the CS+E and the CS+U were never presented in the same block). To avoid order effects, eight different task versions counterbalanced the order in which the two CS+s were presented during Fear Conditioning and Extinction Recall. Colors were also counterbalanced. ID and GS were presented these versions with equal frequency.
Immediately following each phase except Habituation, participants verbally reported shock expectancy for the first two and last two presentations of each CS (i.e. colored light) presented in the phase on a scale from 1 (“not expecting a shock at all”) to 5 (“expecting a shock very much”).
Skin conductance monitoring and finger shock protocol
Prior to entering the scanner, MRI-safe 11-mm, Ag/AgCl skin conductance monitoring electrodes were attached to the participant’s left palm. Skin conductance level was continuously monitored at 37.5 Hz using the MP150 system with Acqknowledge 4.3 (BIOPAC Systems, Inc., Goleta, CA) software. Additionally, stimulating electrodes, which delivered finger shocks, were attached to the participant’s right index and middle fingers. Finger shock level was established by administering increasing intensities of shock in six increments (0.8–4.0 mA) using the Coulbourn Transcutaneous Aversive Finger Stimulator (Coulbourn Instruments, Allentown, PA). Participants selected a level of shock that they perceived to be “highly annoying but not painful” [66].
SCR was calculated for each trial as the mean skin conductance level in microSiemens (µS) during the last 2 seconds of context presentation subtracted from the maximum skin conductance level during the 6 seconds of CS presentation. SCRs were square-root transformed and, if the untransformed SCR was negative, the negative sign was retained after calculating the square root of the SCR’s absolute value [66]. “Non-conditioners” were defined as those who exhibited less than 2 non-square-root transformed SCR responses to either of the two CS+s (in any combination) that were equal to or exceeding 0.05 µS during the Fear Conditioning phase. In total, seven ID and six GS nonconditioners were excluded from SCR analyses based on these criteria (final N = 35).
PSG recording and scoring
Participant sleep was monitored for a total of three nights (Diagnostic/Acclimation, Baseline, and Consolidation) with the Somté PSG (Compumedics, Charlotte, NC) ambulatory polysomnography device (sampled rate: 250 Hz, high- and low-pass filter 0.3 and 35 Hz, respectively, notch filter 60 Hz). A standard montage of surface electrodes, referenced to contralateral mastoid electrodes (M1 and M2), recorded frontal (F3 and F4), central (C3 and C4), and occipital (O1 and O2) electroencephalogram (EEG), left and right electrooculogram (EOG), submental electromyogram (EMG), and electrocardiogram (ECG) from right clavicle and left fifth intercostal space leads. On the Diagnostic/Acclimation night, pulse-oximetry, respiration, nasal cannula, and tibialis sensors were added to screen for OSA and PLMD. All sleep records were staged and assessed for the presence of any clinically significant OSA and PLMD by the same experienced, registered PSG technologist (K.G.) who remained blind to participants’ diagnostic group. Sleep was staged in 30-second epochs as either NREM stages 1–3 or REM, following standard American Academy of Sleep Medicine criteria [67].
Outcome variables
Three primary outcome variables were measured in each study phase as follows:
(1) Sympathetic reactivity was indexed by SCR, a pure index of sympathetic activation [68]. These data were reduced for analyses as follows: For Fear Conditioning, SCRs to corresponding presentations of the two CS+s were first averaged (i.e. average of SCRs to first presentation of CS+1 and the first presentation of CS+2, average of SCRs to the second presentation of CS+1 and the second presentation of CS+2, etc.). The two CS-s associated with the two CS+s were similarly averaged. For Extinction Learning, SCRs to successive pairs of CS+Es were first averaged (i.e. average of SCRs to the first and second CS+E, average of SCRs to the third and fourth CS+E, etc.). SCRs to successive pairs of CS-s were similarly averaged. For Extinction Recall, SCRs to CS+s were averaged (collapsed) across CS+E and CS+U, and SCRs to CS-s were similarly averaged across the CS-s associated with the CS+E and CS+U, respectively.
(2) Physiologically expressed fear conditioning, extinction learning, and extinction memory were indexed using differential SCR (SCRd) calculated by subtracting from the SCR to each CS+ the SCR from its closest temporally corresponding CS-. This measure represents specific learned reactivity to the reinforced CS+ adjusted for nonspecific reactivity to the nonreinforced CS-. SCRd data for Fear Conditioning and Extinction Learning phases were reduced in the same manner as SCR except that a single value represented each CS+ and its corresponding CS-. For Extinction Recall, only SCRs to the first four CS+Es and the first four CS+Us were analyzed in order to measure recall of extinction from session 1, but not the new extinction learning occurring during this phase in session 2.
In order to examine the relationship between extinction recall and REM sleep variables we computed a unitary index, Differential Extinction Retention Index (dERI), using the following formula: dERI = Average of the first 4 CS+E presentations at Extinction Recall phase/maximum SCRd to a CS+ during the Fear Conditioning phase. Therefore, lower dERI values indicated greater extinction memory (i.e. lower SCRd to the extinguished stimulus [CS+E]).
(3) Subjective awareness of fear conditioning, extinction learning, and extinction memory was indexed using the self-reported shock expectancy ratings provided at the end of each experimental phase. During the Fear Conditioning phase, the two different CS+s had not yet been differentiated into CS+E and CS+U (which occurred during Extinction Learning); therefore, their expectancies were averaged. In order to examine the relationship between subjective extinction recall and REM sleep variables, we computed a unitary index, Subjective Extinction Retention Index (sERI) calculated using the following formula: sERI = 100% – [(expectancy to the first two CS+E in Extinction Recall/mean expectancy for the last two of each CS+ during Fear Conditioning) × 100]. Larger sERI indicated greater extinction memory.
Associations of extinction recall with five REM-sleep predictors
(1) REM percent was computed as total REM time as a percentage of total sleep time.
(2) REM latency (REML) was computed as the amount of time between the first epoch scored as any sleep stage and the first epoch scored as REM.
(3) Relative REM theta power (REM θ): Quantitative EEG analysis was performed on scored sleep records using custom written Matlab scripts (The MathWorks Inc., Natick, MA) within the EEGLAB toolbox (http://sccn.ucsd.edu/eeglab/). EEG artifacts generated by muscle, cardiac and eye-blink interference were visually identified and excluded on an epoch-by-epoch basis by a research technician (S.G.) blind to study group. Matlab’s power spectral density and bandpower functions employing FFT (Hanning window, 512 samples, 2 seconds, spectral resolution = 0.5 Hz) were then used to obtain spectral band power in the theta range (4.0–7.0 Hz) during REM sleep from frontal (F3, F4) and central (C3, C4) derivations. An estimate of theta power for each 30-second epoch of REM was calculated by averaging 15, two-second, consecutive segments. Relative theta power was computed by normalizing power in the theta band to total power.
(4) REM segment length (REMSL) is inversely related to REM sleep fragmentation. REM sleep fragmentation has been hypothesized to be elevated in insomnia [42, 43, 69] as well as in trauma-exposed individuals who later develop PTSD [26, 44]. REMSL was computed as the average length of continuous REM segments (i.e. continuous REM epochs that contained no more than 30 seconds of wake or NREM sleep intrusions) across the night [26, 44].
(5) Rapid eye-movement density (REMD) was computed as the number of rapid eye movements per minute during REM sleep. REMD is reported to be elevated in PTSD [70, 71], a condition for which insomnia may constitute a particular risk [1, 24]. REMD was determined using the open-source Yetton et al. [72] machine learning algorithm (Open Science Framework; https://osf.io/fd837/) that has been validated in nonclinical samples [72].
These five REM features were interpreted as follows; (1) Greater REM% is indicative of greater proportional amounts of REM sleep. (2) Greater REML is indicative of lesser physiological pressure to enter REM. (3) Greater relative REM θ power is interpreted as greater relative contribution of a putative emotion regulatory spectral component to REM sleep [73, 74]. (4) Greater REMSL is indicative of greater consolidation of REM bouts. (5) Greater REMD reflects greater brain activation during REM.
Statistical analyses
Normality of descriptive data was determined using Shapiro-Wilk tests (Supplementary Materials). Group differences in demographic and sleep metrics were determined using, when appropriate, parametric and nonparametric tests.
Sympathetic reactivity (SCR) for Fear Conditioning, Extinction Learning, and Extinction Recall phases were analyzed using linear mixed-effects models with Group (ID vs. GS) and Type (CS+ vs. CS-) as fixed effects [75–77].
Physiologically expressed learning and memory (SCRd) for Fear Conditioning and Extinction Learning phases were also analyzed using linear mixed-effects models with only Group (ID vs. GS) as a fixed effect. Physiologically expressed extinction memory during the Extinction Recall phase used a linear mixed-effects model with Group (ID vs. GS) and Type (CS+E vs. CS+U) as fixed effects.
For all linear mixed-effects models, in order to account for covariance in SCR reactivities (or SCRd memory events), we specified a first-order autoregressive (AR1) covariance matrix for residual errors in the model. Therefore, residual errors for within-subject SCR estimates are correlated, but treated independently between subjects [77]. No random-effects were specified in any models as they were not theoretically expected.
For shock expectancies, mixed ANOVAs for each phase included the between subject factor Group as well as two within-subject factors, Order (ratings for first two presentations, ratings for last two presentations) and CS Type. For Fear Conditioning, CS Type included CS- and averaged ratings for CS+1 and CS+2; for Extinction Learning, CS Type included CS- and CS+E ratings; and for Extinction Recall, CS Type included CS-, CS+E, and CS+U but only ratings for first two of each were analyzed.
Because dERI and sERI were not normally distributed (details in Supplementary Materials), their correlations with the five REM-sleep predictors were performed using nonparametric (Spearman’s Rho) tests. Correlations were performed separately in the ID and GS groups. Intercorrelations among the five REM-sleep predictors were performed using simple regression. Participants were excluded whose dERI or sERI exceeded ± 3 SD from the group mean.
Results
Demographics and descriptive statistics
Demographics and descriptive statistics for ID and GS are displayed in Table 1. Compared with GS, ID reported significantly greater subjective retrospective sleep disturbance as measured by the PSQI, insomnia severity as measured by the ISI, and daytime sleepiness as measured by the ESS. On sleep diaries, compared to GS, ID reported significantly lower TST, greater SOL, and lower SE. No significant group differences were seen in demographic or objective sleep measures.
Sympathetic reactivity
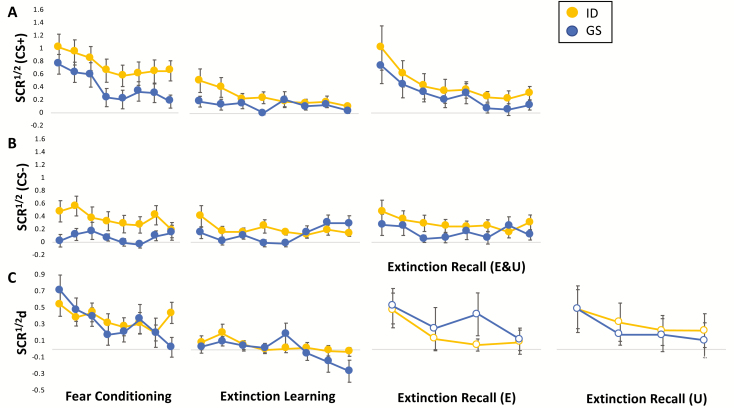
For the Fear Conditioning phase, mixed-effects analyses revealed a main effect of Group (β [SE] = 0.263 [0.126], t(52) = 2.095, p = 0.041, CI = [0.011, 0.516]; ID greater) and CS Type (β [SE] = 0.239 [0.064], t(501) = 3.721, p < 0.001; CS+ greater; Figure 1, A and B, left-most panels). For the Extinction Learning phase, mixed-effects analyses revealed no main effect of CS Type (β [SE] = 0.013 [0.043], t(198) = 0.316, p = 0.753, CI = [−0.098, 0.071]) and the main effect of Group approached, but did not reach, significance (β [SE] = 0.089 [0.045], t(122.69) = 1.962, p = 0.052, CI = [−0.0008, 0.179]). During Extinction Recall, mixed-effects analyses revealed no main effects of Group (β [SE] = 0.152 [0.139], t(67) = 1.095, p = 0.277, CI = [−0.125, 0.429] or Type (β [SE] = 0.187 (0.101), t(283) = 1.847, p = 0.066, CI = [−0.012, 0.386].
Figure 1.
Reactivity (measured by square-root transformed skin conductance responses [SCR1/2]) and learning and memory (measured by differential SCR1/2 [SCRd; SCR1/2 to CS- subtracted from temporally corresponding SCR1/2 to CS+]) curves for Insomnia Disorder (ID) and Good Sleeper (GS) groups across Fear Conditioning, Extinction Learning, and Extinction Recall phases. (A) Trial-wise reactivity for sequentially paired CS+s (i.e. average of the first CS+1 and first CS+2, average of the second CS+1 and second CS+2, … etc.) across all study phases. (B) Trial-wise reactivity for sequentially paired CS-s (i.e. average of the first CS-1 and first CS-2, average of the second CS-1 and second CS-2, … etc.) across all study phases. (C) Trial-wise learning and memory for SCRd across all study phases. Error bars represent standard error of the mean.
Physiologically expressed fear conditioning, extinction learning, and extinction memory
Mixed-effects analyses revealed no main effect of Group for Fear Conditioning (β [SE] = 0.007 [0.128], t(42) = 0.060, p = 0.952, CI = [−0.266, 0.251] or Extinction Learning (β [SE] = 0.043 [0.058], t(104) = 0.743, p = 0.459, CI = [−0.159, 0.072]. For Extinction Recall, mixed-effects analyses revealed no main effect of Group (β [SE] = 0.026 [0.156], t(48) = 0.170, p = 0.866, CI = [−0.287, 0.341] or CS type (i.e. CS+E vs. CS+U; β [SE] = 0.168 [0.119], t(188) = 1.409, p = 0.161, CI = [−0.067, 0.403]).
Subjective awareness of fear conditioning, extinction learning, and extinction memory
During Fear Conditioning, there was a significant Order (first two presentations vs. last two presentations) × CS type (CS+ vs. CS-) interaction [F(1,46) = 54.21, p = 0.0001, pη 2 = 0.541] in which subjective shock expectancy significantly increased for CS+ and significantly decreased for CS- (both p = 0.0001) from the first two to the last two trials (Figure 2). However, there was no main effect of Group (p = 0.46) or interaction of Group with Order (p = 0.11) or CS Type (p = 0.14).
Figure 2.
Subjective shock expectancy across Fear Conditioning, Extinction Learning, and Extinction Recall phases. Bars represent subjective shock expectancy for the first two presentations (“Pre”) or last two presentations (“Post”) of study stimuli (i.e. colored lights) at each study phase. Error bars represent standard error of the mean. *p < 0.001.
Successful Extinction Learning was demonstrated by an Order × CS Type interaction [F(1,46) = 22.12, p = 0.0001, pη 2 = 0.325] which indicated that shock expectancy for the CS+ decreased more than for the CS- from the first two to the last two trials during Extinction Learning (Figure 2). However, there was no main effect of Group (p = 0.82) or interaction of Group with Order (p = 0.79) or CS Type (p = 0.51).
At Extinction Recall, there was a significant CS Type main effect [F(2,84) = 19.96, p = 0.0001, pη 2 = 0.322] in which shock expectancy to the CS- was significantly lower than to the CS+E or CS+U (both p = 0.0001) but expectancy of shocks to the CS+U was not significantly higher than to the CS+E (p = 0.083) (Figure 2). However, there was no main effect of Group (p = 0.24) or interaction of Group with CS Type (p = 0.56). Additionally, no Group differences were observed for sERI (Median(ID)= 33.3, Median(GS) = 25, Mann–Whitney U = 231, n(ID) = n(GS) = 22, p = 0.80, two-tailed).
Associations of extinction recall with five REM-sleep predictors
Among ID, dERI was positively correlated with REM% and REMSL and negatively correlated with REML (Figure 3, A–C). Thus, in ID, greater physiologically measured extinction memory (i.e. lesser dERI) was associated with lesser REM%, less pressure to enter REM and shorter (less consolidated) REM bouts. In contrast, among GS, dERI was negatively correlated with REMSL and REM θ (Figure 3, A and D). Thus, in GS, greater physiologically measured extinction memory (i.e. lesser dERI) was associated with longer (more consolidated) REM bouts and with greater contribution by a putative emotion regulatory spectral component of REM sleep. These correlations remained significant when correcting for multiple comparisons (Bonferroni correction, two dependent variables, corrected α = 0.025). No REM sleep correlations were observed with sERI in either group (all ps > 0.08). Low collinearity was observed among these five REM sleep measures (all r’s < 0.6), with the exception of REML and REM% in ID (r = −0.713).
Figure 3.
Relationships between psychophysiologically measured extinction recall (dERI) and Consolidation night (A) REM sleep percentage, (B) REM sleep latency (in minutes), (C) average REM sleep segment length (in minutes), and (D) relative REM sleep frontal theta spectral power (4–7 Hz). Note that a more positive dERI values signifies poorer extinction memory. Nonparametric correlation coefficients (Spearman’s Rho, ρ) and significance levels (p) are presented next to group abbreviations, colored to correspond with scatter plot points and trend lines (ID, gold; GS, blue).
Discussion
The present findings support our first hypothesis by showing that sympathetic reactivity was higher during Fear Conditioning for ID compared to GS. This may reflect a state of hyperarousal that is believed to characterize chronic insomnia [35–38]. However, our second hypothesis that, compared to GS, ID would exhibit greater fear conditioning but lesser extinction learning and recall, was not supported. Fear conditioning, extinction learning and extinction recall did not differ between groups when measured physiologically (SCRd) or subjectively using shock expectancy. This was not completely surprising as a recent report [78] demonstrated that acquisition of fear conditioning was similar in ID and GS whether measured physiologically (SCRd) or subjectively (shock expectancy). Our findings best support our third hypothesis, that greater REM quality during the night between Extinction Learning and Extinction Recall phases would impact extinction recall, although in an unpredicted manner. Whereas, in GS, greater REM sleep continuity (greater average REMSL) and REM theta power predicted greater extinction memory, in ID, greater REM percentage and continuity as well as faster entry into REM sleep predicted lesser extinction memory. Convergent findings link aspects of REM to consolidation of both emotional memory in general [79] and extinction memory in particular [8]. Additionally, REM sleep in the interval intervening between extinction learning and extinction recall has been implicated in the retention of learned extinction in good sleeping adults [15, 80–82]. Such observations may point to the importance of nonfragmented REM sleep in the normal consolidation process of extinction memories as suggested by our observation in GS (Figure 3C). However, since a REM fragmentation/consolidation variable was absent from prior studies, future studies are needed to examine the relationship of this variable to extinction recall. Notably, however, REM fragmentation following trauma is predictive of later posttraumatic symptoms [26, 44] thus such fragmentation might impede naturally occurring extinction [1].
It remains unclear why REM sleep was negatively associated with extinction memory recall in ID, although a recent series of reports by Wassing and colleagues (2019) [33, 83, 84] suggests that regulation of emotional reactivity is deficient in insomnia disorder across both acute and chronic poor sleep. One explanation might be that chronic poor sleep, by disrupting consolidation of extinction memory, creates a bias toward consolidating the less fragile fear memory as sleep supports both types of emotional memory [8, 85]. In fact, in studies that probed fear conditioning alone, REM sleep has been shown to positively correlate with retention and expression of fear memories [86, 87]. Alternatively, REM sleep might function properly in both healthy, good sleepers and those with insomnia, though the memory traces prioritized may be different. In the case of the present study, it is possible that fear traces are more salient for those with insomnia and thus are prioritized for strengthening over REM sleep. Conversely, REM sleep in healthy, good sleepers may have prioritized extinction memory as has been observed in previous reports [85]. It is important to note that extinction inhibits rather than erases fear [4]. Hence each confrontation with a conditioned and then extinguished stimulus sets up a competition for the expression of these two forms of emotional memory, a competition in which poorer sleep may favor fear expression. Lastly, it has been recently suggested that REM instability characterizes insomnia [42]. Although such instability was not apparent in our measures of REM fragmentation, deficits in REM quality, undetected in our data, may have nonetheless degraded the ability of REM to retain extinction over fear in ID.
Limitations
We acknowledge the following limitations in the current study. First, SCR shows strong generic habituation over repeated presentation of stimuli regardless of concurrent fear learning. Nonetheless a vast literature has shown that SCR can be used to successfully demonstrate differential fear conditioning and the phenomenon of habituation of SCR to the CS+ during acquisition of fear conditioning is usually reported (e.g. Refs. [88–95]). Second, use of ambulatory PSG also limited experimental control over equipment malfunction as well as participants’ sleep period (e.g. “lights off” time) and sleeping environment. However, sleep in a naturalistic environment may have greater ecological validity when studying insomnia: the familiar bedroom context where repeated failures to initiate sleep occur may produce conditioned arousal which serves to maintain certain symptoms of the condition [96]. Third, the rate of nonconditioning (13/48 = 27%) in our study is at the upper end of nonconditioning rates described in previous studies (see ref. [89] for review). In this regard, we cannot discount the impact that the high magnetic field of the MR environment might have had on the skin conductance signal, despite following procedures used in several other studies in psychiatric patient populations under similar conditions [18, 21–23, 97]. Fourth, given variability in scan start times (16:00–22:00) we cannot discount the potential impact of circadian factors and are limited by the absence of objective circadian markers. However, 72% (all but 13) finished Extinction Learning before 21:00 during their first scan and 80% (all but 7) finished Extinction Recall before 19:00 during their second scan. Assuming melatonin onset occurs around 2 hours before sleep onset [98], memory processes in the majority of participants would not have been influenced by high levels of melatonin or concomitant circadian physiology. Fifth, our method of automated rapid eye movement detection employs a classifier that was trained on healthy, good sleepers, therefore we cannot rule out the possibility that rapid eye movements were either under-detected or over-detected in our ID participants. However, to our knowledge, no evidence to date suggests differences in morphological characteristics of rapid eye movements between healthy, good sleepers and those with insomnia. Lastly, we report results from small samples. A recent study by Ackermann et al. [99] sought sleep stage correlations with overnight consolidation of emotional and neutral declarative memory in 929 healthy adults and found neither positive correlation with REM% (but a slight negative correlation), nor did it find correlations with any other sleep stage. This finding highlights the elevated risk of false positive results in under-powered correlational studies, a pervasive problem in many areas of neuroscience [100]. Acknowledging that multiple simple regressions inflate risk of Type-1 error, non-normality in distribution of our extinction memory indices contraindicated use of multiple regression models. Additionally, the nature of our extinction memory indices (e.g. including negative and fractional values) made standard methods of normalization (e.g. log transformation) problematic. Nonetheless, while acknowledging the possibility of false positive correlations, the consistency with which REM predicted decreased extinction memory in ID across REM indices argues against findings being entirely due to chance (especially given low collinearity observed between REM measures).
Conclusion
Taken together, these results suggest a greater general sympathetic reactivity to fear-related stimuli in insomnia that possibly reflects the putative hyperarousal in persons with this condition [13]. In addition, compared with good sleepers, results suggest that REM sleep may have different effects on emotional processing in those with insomnia. Specifically, despite its association with maintaining and potentially promoting expression of extinction memory in GS, REM sleep, in ID, may preferentially consolidate or favor expression of fear memory.
Supplementary Material
Acknowledgments
Special thanks to Benjamin D. Yetton for his design and support with implementing REM counting and density measures. Thanks to Mark Vangel, PhD for statistical advice.
Funding
This project was supported by the National Institute of Mental Health grants 1R21MH101567 and R01MH109638 to E.P.S. and the 2016 Harvard Mind/Brain/Behavior Interfaculty Initiative (E.P.S., Co-PI).
Conflict of interest statement. None declared.
References
- 1. Pace-Schott EF, et al.. Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol Mood Anxiety Disord. 2015;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zuj DV, et al.. The centrality of fear extinction in linking risk factors to PTSD: a narrative review. Neurosci Biobehav Rev. 2016;69:15–35. [DOI] [PubMed] [Google Scholar]
- 3. Elzinga BM, et al.. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Milad MR, et al. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hermans D, et al.. Extinction in human fear conditioning. Biol Psychiatry. 2006;60(4):361–368. [DOI] [PubMed] [Google Scholar]
- 6. Quirk GJ, et al.. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herry C, et al.. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31(4):599–612. [DOI] [PubMed] [Google Scholar]
- 8. Pace-Schott EF, et al.. Effects of sleep on memory for conditioned fear and fear extinction. Psychol Bull. 2015;141(4):835–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pace-Schott EF, et al.. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 10. Pace-Schott EF, et al.. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res. 2012;46(8):1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pace-Schott EF, et al.. Napping promotes inter-session habituation to emotional stimuli. Neurobiol Learn Mem. 2011;95(1):24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kleim B, et al.. Sleep enhances exposure therapy. Psychol Med. 2014:44(7):1511–1519. [DOI] [PubMed] [Google Scholar]
- 13. Spoormaker VI, et al.. The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J Psychiatr Res. 2010;44(16):1121–1128. [DOI] [PubMed] [Google Scholar]
- 14. Marshall AJ, et al.. Fear conditioning, safety learning, and sleep in humans. J Neurosci. 2014;34(35):11754–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spoormaker VI, et al.. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp. 2012;33(10):2362–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu J, et al.. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144(4):1186–1192. [DOI] [PubMed] [Google Scholar]
- 17. Milad MR, et al.. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42(7):515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milad MR, et al.. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garfinkel SN, et al.. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34(40):13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rougemont-Bücking A, et al.. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17(4):227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milad MR, et al.. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70(6):608–618; quiz 554. [DOI] [PubMed] [Google Scholar]
- 22. Holt DJ, et al.. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69(9):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spencer AE, et al.. Abnormal fear circuitry in Attention Deficit Hyperactivity Disorder: a controlled magnetic resonance imaging study. Psychiatry Res Neuroimaging. 2017;262:55–62. [DOI] [PubMed] [Google Scholar]
- 24. Gehrman P, et al.. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright KM, et al.. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240–1258. [DOI] [PubMed] [Google Scholar]
- 26. Mellman TA, et al.. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696–1701. [DOI] [PubMed] [Google Scholar]
- 27. Geng F, et al.. Bidirectional associations between insomnia, PTSD and depressive symptoms among adolescent earthquake survivors: a longitudinal multiwave cohort study. Sleep 2019; 42(11). doi: 10.1093/sleep/zsz162 [DOI] [PubMed] [Google Scholar]
- 28. Babson KA, et al.. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baglioni C, et al.. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. [DOI] [PubMed] [Google Scholar]
- 30. Kyle SD, et al.. Altered emotion perception in insomnia disorder. Sleep. 2014;37(4):775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crönlein T, et al.. Impaired recognition of facially expressed emotions in different groups of patients with sleep disorders. PLoS One. 2016;11(4):e0152754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wassing R, et al.. Slow dissolving of emotional distress contributes to hyperarousal. Proc Natl Acad Sci USA. 2016;113(9):2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wassing R, et al.. Overnight worsening of emotional distress indicates maladaptive sleep in insomnia. Sleep 2019;42(4). doi: 10.1093/sleep/zsy268 [DOI] [PubMed] [Google Scholar]
- 34. Kales A, et al.. Personality patterns in insomnia. Theoretical implications. Arch Gen Psychiatry. 1976;33(9):1128–1124. [DOI] [PubMed] [Google Scholar]
- 35. Bonnet MH, et al.. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):9–15. [DOI] [PubMed] [Google Scholar]
- 36. Nofzinger EA, et al.. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. [DOI] [PubMed] [Google Scholar]
- 37. Riemann D, et al.. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. [DOI] [PubMed] [Google Scholar]
- 38. Kay DB, et al.. Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. 2017;7(3):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernández-Mendoza J, et al.. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72(4): 397–403. [DOI] [PubMed] [Google Scholar]
- 40. Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–893. [DOI] [PubMed] [Google Scholar]
- 41. Feige B, et al.. The microstructure of sleep in primary insomnia: an overview and extension. Int J Psychophysiol. 2013;89(2):171–180. [DOI] [PubMed] [Google Scholar]
- 42. Riemann D, et al.. REM sleep instability–a new pathway for insomnia? Pharmacopsychiatry. 2012;45(5):167–176. [DOI] [PubMed] [Google Scholar]
- 43. Feige B, et al.. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17(2):180–190. [DOI] [PubMed] [Google Scholar]
- 44. Mellman TA, et al.. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20(5):893–901. [DOI] [PubMed] [Google Scholar]
- 45. Seo J, et al.. Delayed fear extinction in individuals with insomnia disorder. Sleep 2018;41(8). doi: 10.1093/sleep/zsy095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishida M, et al.. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19(5):1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wagner U, et al.. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van der Helm E, et al.. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21(23):2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1): 97–113. [DOI] [PubMed] [Google Scholar]
- 50. Insana SP, et al.. Validation of the Pittsburgh Sleep Quality Index Addendum for posttraumatic stress disorder (PSQI-A) in U.S. male military veterans. J Trauma Stress. 2013;26(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stocker RPJ, et al.. Effects of sleep loss on subjective complaints and objective neurocognitive performance as measured by the immediate post-concussion assessment and cognitive testing. Arch Clin Neuropsychol. 2017;32(3):349–368. [DOI] [PubMed] [Google Scholar]
- 52. Bastien CH, et al.. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 53. Buysse DJ, et al.. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 54. First MB, et al.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders–Non-Patient Edition (SCID-I/NP). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2007. [Google Scholar]
- 55. Horne JA, et al.. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 56. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 57. Pace-Schott EF, et al.. Nightcap measurement of sleep quality in self-described good and poor sleepers. Sleep. 1994;17(8):688–692. [DOI] [PubMed] [Google Scholar]
- 58. Pace-Schott EF, et al.. Sleep quality deteriorates over a binge–abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl). 2005;179(4):873–883. [DOI] [PubMed] [Google Scholar]
- 59. Milad MR, et al.. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62(10):1191–1194. [DOI] [PubMed] [Google Scholar]
- 60. Milad MR, et al.. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. [DOI] [PubMed] [Google Scholar]
- 61. Zeidan MA, et al.. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70(10):920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Linnman C, et al.. Resting cerebral metabolism correlates with skin conductance and functional brain activation during fear conditioning. Biol Psychol. 2012;89(2):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Linnman C, et al.. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry. 2012;169(4):415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Linnman C, et al.. An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol Mood Anxiety Disord. 2011;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Linnman C, et al.. Unconditioned responses and functional fear networks in human classical conditioning. Behav Brain Res. 2011;221(1):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Orr SP, et al.. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109(2):290–298. [PubMed] [Google Scholar]
- 67. Iber C, et al.. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 68. Dawson ME, et al.. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, eds. Handbook of Psychophysiology. 3rd ed New York, NY: Cambridge University Press; 2007: 159–181. [Google Scholar]
- 69. Pérusse AD, et al.. REM sleep as a potential indicator of hyperarousal in psychophysiological and paradoxical insomnia sufferers. Int J Psychophysiol. 2015;95(3):372–378. [DOI] [PubMed] [Google Scholar]
- 70. Kobayashi I, et al.. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. [DOI] [PubMed] [Google Scholar]
- 71. Habukawa M, et al.. Differences in rapid eye movement (REM) sleep abnormalities between posttraumatic stress disorder (PTSD) and major depressive disorder patients: REM interruption correlated with nightmare complaints in PTSD. Sleep Med. 2018;43:34–39. [DOI] [PubMed] [Google Scholar]
- 72. Yetton BD, et al.. Automatic detection of rapid eye movements (REMs): a machine learning approach. J Neurosci Methods. 2016;259:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hutchison IC, et al.. The role of REM sleep theta activity in emotional memory. Front Psychol. 2015;6:1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cowdin N, et al.. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014;232(5):1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McCulloch CE, et al.. Generalized, Linear and Mixed Models. New York, NY: John Wiley and Sons, 2000. [Google Scholar]
- 76. Pinheiro JC, et al.. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer; 2000. [Google Scholar]
- 77. SPSS. Linear mixed effects modeling in SPSS: An introduction to the MIXED procedure. SPSS Technical Report https://www.spss.ch/upload/1126184451_Linear Mixed Effects Modeling in SPSS.pdf: SPSS, Inc., 2005.
- 78. Kuhn M, et al.. Declarative virtual water maze learning and emotional fear conditioning in primary insomnia. J Sleep Res. 2018;27(6):e12693. [DOI] [PubMed] [Google Scholar]
- 79. Tempesta D, et al.. Sleep and emotional processing. Sleep Med Rev. 2018;40:183–195. [DOI] [PubMed] [Google Scholar]
- 80. Menz MM, et al.. REM sleep is causal to successful consolidation of dangerous and safety stimuli and reduces return of fear after extinction. J Neurosci. 2016;36(7):2148–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Straus LD, et al.. Sleep deprivation disrupts recall of conditioned fear extinction. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pace-Schott EF, et al.. Interactions of time of day and sleep with between-session habituation and extinction memory in young adult males. Exp Brain Res. 2014;232(5):1443–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wassing R, et al.. (2019).. Haunted by the past: old emotions remain salient in insomnia disorder. Brain. 142(6):1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wassing R, et al.. (2019).. Restless REM sleep impedes overnight amygdala adaptation. Current Biology. 29(14):2351–2358. [DOI] [PubMed] [Google Scholar]
- 85. Gobin CM, et al.. Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. J Sleep Res. 2015;24(5):535–542. [DOI] [PubMed] [Google Scholar]
- 86. Menz MM, et al.. The role of sleep and sleep deprivation in consolidating fear memories. Neuroimage. 2013;75:87–96. [DOI] [PubMed] [Google Scholar]
- 87. Spoormaker VI, et al.. Ventromedial prefrontal cortex activity and rapid eye movement sleep are associated with subsequent fear expression in human subjects. Exp Brain Res. 2014;232(5):1547–1554. [DOI] [PubMed] [Google Scholar]
- 88. Geller DA, et al.. Fear conditioning and extinction in pediatric obsessive-compulsive disorder. Ann Clin Psychiatry. 2017;29(1):17–26. [PMC free article] [PubMed] [Google Scholar]
- 89. Alexandra Kredlow M, et al.. Who is studied in de novo fear conditioning paradigms? An examination of demographic and stimulus characteristics predicting fear learning. Int J Psychophysiol. 2018;130:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marin MF, et al.. Absence of conditioned responding in humans: a bad measure or individual differences? Psychophysiology. 2020;57(1):e13350. [DOI] [PubMed] [Google Scholar]
- 91. Milad MR, et al.. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42(4):456–464. [DOI] [PubMed] [Google Scholar]
- 92. Orr SP, et al.. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biol Psychol. 2006;73(3):262–271. [DOI] [PubMed] [Google Scholar]
- 93. Peri T, et al.. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47(6):512–519. [DOI] [PubMed] [Google Scholar]
- 94. Pineles SL, et al.. An alternative scoring method for skin conductance responding in a differential fear conditioning paradigm with a long-duration conditioned stimulus. Psychophysiology. 2009;46(5):984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pace-Schott EF, et al.. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. J Psychiatr Res. 2013;47(11):1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Perlis ML, et al.. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6(3):179–188. [DOI] [PubMed] [Google Scholar]
- 97. Milad MR, et al.. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118(2):389–394. [DOI] [PubMed] [Google Scholar]
- 98. Zawilska JB, et al.. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009;61(3):383–410. [DOI] [PubMed] [Google Scholar]
- 99. Ackermann S, et al.. No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep 2015;38(6):951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Button KS, et al.. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.