Abstract
Study Objectives
In young men, sleep restriction decreases testosterone (Te) and increases afternoon cortisol (F), leading to anabolic–catabolic imbalance, insulin resistance, and other andrological health consequences. Age-related differences in the hypothalamo–pituitary–testicular/adrenal response to sleep restriction could expose older individuals to greater or lesser risk. We aimed to evaluate and compare the 24-h and time-of-day effect of sleep restriction on F, luteinizing hormone (LH), and Te in young and older men.
Methods
Thirty-five healthy men, aged 18–30 (n = 17) and 60–80 (n =18) years, underwent overnight sleep deprivation (complete nighttime wakefulness) or nighttime sleep (10 pm to 6 am) with concurrent 10-min blood sampling in a prospectively randomized crossover study. F, LH, and Te secretion were calculated by deconvolution analysis.
Results
Sleep deprivation had multiple effects on 24-h Te secretion with significant reductions in mean concentrations, basal, total and pulsatile secretion, and pulse frequency (each p < 0.05), in the absence of detectable changes in LH. These effects were most apparent in older men and differed according to age for some parameters: pulsatile Te secretion (p = 0.03) and Te pulse frequency (p = 0.02). Time-of-day analyses revealed that sleep restriction significantly reduced Te in the morning and afternoon, reduced LH in the morning in both age groups, and increased F in the afternoon in older men.
Conclusions
These data suggest a time-of-day dependent uncoupling of the regulatory control of the testicular axis and of F secretion. Future studies will need to directly verify these regulatory possibilities specifically and separately in young and older men.
Clinical Trial
Not applicable.
Keywords: human, LH, testosterone, cortisol, deconvolution, sleep
Statement of Significance.
We show for the first time in a single cohort of men that sleep loss both decreases testosterone, the main anabolic hormone, and increases afternoon cortisol, the main catabolic hormone. This combination of findings has also never been previously shown in a population that includes older men and could plausibly cause metabolic and reproductive ill-health when accumulated over decades of life. Accordingly, these findings may explain how chronic sleep loss may contribute to the metabolic and reproductive diseases that are more prevalent in older men. Age and time-of-day related differences in the network regulation of hypothalamo–pituitary–testicular, and adrenal response, to sleep loss were also unveiled, but these preliminary findings still require direct verification by interventions that manipulate hormones during the morning and late afternoon in appropriately matched cohorts of young and older men.
Introduction
The adverse cardiometabolic consequences of insufficient sleep are becoming increasingly recognized [1]. Therefore, developing countermeasures to prevent or mitigate adverse effects without requiring more sleep is needed since sleep loss is sometimes unavoidable. Understanding the mechanisms by which insufficient sleep leads to adverse cardiometabolic and/or other health outcomes would allow such targeted methods to be developed.
An emerging thesis is that hormonal imbalances of testosterone (Te) and cortisol (F) underpin some of the adverse health consequences of insufficient sleep, particularly illnesses that are considered important by men [2]. The concept is appealing because Te and F are, respectively, the main anabolic and catabolic signals in men, regulation of the hypothalamo–pituitary testicular and hypothalamo–pituitary adrenal axes are intertwined, and anabolic–catabolic imbalance is likely to impair the precise physiological processes required for metabolism and reproduction [2]. Despite this, the exact impact of sleep loss on F and Te regulation is poorly understood, particularly across the entire adult male lifespan.
Current studies utilizing repetitive blood sampling show that experimentally restricting sleep in young men under controlled laboratory conditions probably causes an overall reduction in systemic Te exposure [3–6]. An important limitation is that only two of these studies have assessed the effect of sleep restriction on systemic Te exposure across the day and night period by assessing blood Te every 15–30 min [3], or every other hour [6], for 24 h. Two other studies sampled blood every hour from 7 am to 11 pm [5], or every other hour but only for 11 h during the biological day [4]. Of these four frequently sampled studies [3–6], only one showed that sleep loss decreased overall Te concentrations [3]. Further investigation utilizing frequent blood sampling for an entire 24-h period are needed to adjudicate these discrepant findings.
Recently, it was proposed that sleep loss must occur during the second half of the biological night to cause Te to fall [5]. A major limitation is that all studies were performed in men aged <45 years and the majority of all these studies sampled blood or saliva once in the morning [7–12]. This distinction is important because age-related hypoandrogenemia is well-established and occurs as an ensemble outcome of deteriorated network regulation [13] and sleep and sleep architecture changes with age [14]. Furthermore, healthy older adults are able to better preserve attention and neuropsychological performance during sleep restriction compared with their healthy younger counterparts [15] suggesting that age, or factors associated with aging, may modulate the effect of sleep restriction. Accordingly, the effects of sleep restriction on Te and on the regulation of the testicular axis could plausibly be modulated by aging but this has not previously been studied.
In contrast to the two studies assessing the effects of sleep restriction on 24 h Te, seven studies have examined the effect of sleep restriction on systemic F exposure across the entire 24-h day by frequent blood sampling that occurred at least once every 30–60 min [3, 16–21], and two other studies where blood was sampled less frequently every other hour [6, 22]. The timing of sleep loss has again been postulated to modulate the F response to sleep restriction [23]. Findings from the seven studies with frequent sampling are consistent with the hypothesis that sleep loss during the first half of the biological night increases F the following late afternoon/early evening [3, 16–21]. Five of these studies directly demonstrated a late afternoon/early evening [3, 16–18] or early morning [21] rise in F, one did not specifically examine for it [20], and in the other, sleep loss did not occur in the first half of the biological night [19]. Another important caveat is that only two of these studies enforced total overnight sleep deprivation [6, 21].
The increase in the late afternoon/early evening F with sleep loss has important health consequences because interventional studies directly show that increased afternoon/evening F worsens insulin resistance in humans [24, 25] and rodents [26]. Increased afternoon/early evening F also underlies the increased insulin resistance of aging [25, 27], as well as impaired physical performance [28], and neurocognitive deficits [29]. Hence a secondary exploratory aim of this analysis was to examine hormone changes during a time window in the late afternoon. Additionally, age itself is associated with increased late afternoon/early evening F [30]. Accordingly, it is plausible that the effect of sleep restriction on F and F dynamics may be modulated by age. Only one study so far has examined both young and older adults, and reported that sleep deprivation increased 4 pm salivary F [31]. Age did not modulate the effect of sleep deprivation on F response, but this study was limited by the examination of F during a narrow late afternoon time window.
The present investigation was undertaken to assess the effect of sleep loss on actual pulsatile Te, luteinizing hormone (LH) and F secretion assessed in young and older men over 24 h, in the early morning and in the late afternoon. Prior studies have directly confirmed the importance of quantifying actual pulsatile hormone secretion, and the motivations and methods to do so have been extensively reviewed [32]. For example, interventional studies in humans have now directly verified that specific pulsatile delivery of F, rather than nonpulsatile delivery of the same total dose, affects cognitive and other neurological responses differentially [33]. These data culminate prior studies in rodents and in cell culture systems which show that pulsatile delivery of glucocorticoids modulate receptor and other physiological responses [34, 35]. We have provided similar evidence highlighting the importance of the pulsatile characteristics of LH and Te for the testicular axis [36, 37].
Methods
Overview
This was an investigator-initiated prospectively randomized, controlled crossover study where participants were randomized to sleep restriction (complete nighttime wakefulness) or 8 h of sleep (nighttime sleep, with sleep opportunity from 10 pm to 6 am). The two inpatient Clinical Research Unit sessions were scheduled at least 3 weeks, but not more than 2 months, apart. Participant number was powered at 90 per cent to detect a 30 per cent effect of sleep restriction on Te concentrations. Volunteers were recruited by newspaper advertisements, local posters, the Clinical Trials Center webpage, and community (general and minority) bulletin boards. The protocol was approved by the Mayo Institutional Review Board. Witnessed voluntary written consent was obtained before study enrollment.
Criteria for inclusion
Inclusion criteria were: healthy men ages 18–30 and 60–80 who provided written informed consent. A medical history, physical examination, and screening tests of hematological, renal, hepatic, metabolic, and endocrine function were normal.
Criteria for exclusion
Exclusion criteria were the recent use of any systemic medications (medically prescribed or over the counter) with the exception of replacement thyroid hormone, laxatives, antacids, thiazide diuretics, ophthalmic solutions, or skin preparations; anabolic steroid or glucocorticoid use (last 3 months); drug or alcohol abuse, psychosis, depression, mania, or severe anxiety; acute or chronic organ-system disease including cardiovascular disease, cancer (excluding localized basal cell carcinoma removed or surgically treated with no recurrence), sleep apnea, chronic obstructive pulmonary disease, hematological dyscrasias, acute or chronic inflammatory conditions, renal insufficiency, hepatic failure, and chronic infections including hepatitis and HIV; self-reported history of sexual dysfunction or sleep disorder; morbid obesity; abnormal laboratory test results, including those suggestive of hypogonadism (serum total Te < 240 ng/dL or bioavailable Te < 100 ng/mL, LH > 10 IU/L or follicle-stimulating hormone [FSH] > 20 IU/L); nightshift work or recent (10 d) >3 time-zones transmeridian travel; acute weight change (loss or gain of >2 kg in 8 weeks); unwillingness or inability to provide written informed consent due to cognitive decline, institutionalization, or imprisonment; or history or suspicion of prostatic disease (elevated prostate-specific antigen > 4.0 ng/mL, indeterminate nodule or mass, or obstructive uropathy).
Detailed protocol
Volunteers were required to maintain their usual dietary, activity, and sleeping patterns at home, and arrived at the Unit at 4 pm for admission and placement of two intravenous catheters in (contralateral) antecubital veins to allow uninterrupted blood sampling. Blood samples (2.7 mL) were withdrawn every 10 min for 24 h (145 samples per session) beginning at 6 pm on day 1, 2 h after admission, to measure LH, Te, and F. Identical meals were served under controlled feeding conditions during both conditions and were standardized in composition (8 kcal/kg of 50 per cent carbohydrate, 20 per cent protein, and 30 per cent fat) and time (5 pm, and on the following day at 8 am, 12 pm, and 6 pm). Food was not self-selected and not ad libitum. Participants were discharged approximately 26 h later, after the last blood draw and the 6 pm dinner. Due to the frequent blood sampling, participants remained reclined throughout and at least one nurse was always present in the room under standard bright light (600 lux) during all periods of wakefulness, including wakefulness at night during the nighttime wakefulness condition. Napping was avoided during all periods of wakefulness through interactive conversation. During the nighttime sleep condition, lights were switched off at 10 pm and switched back on at 6 am. For this time period, a nurse was present for blood sampling and other staff would also variously check the condition of the participant and the sampling line. Based on this visual inspection, participants slept within 20 min of lights out and remained asleep until 6 am. Apneas were not reported.
Analytical methods
Assays, LH, Te, and F, were determined in duplicate and batch-wise in each participant. LH was measured using an automated two-site monoclonal immunochemiluminescence assay with a sensitivity of 0.20 IU/L (First International Reference Preparation) and median inter- and intra-assay coefficients of variation of 5.5 per cent and 8.5 per cent, respectively [38]. Cross-reactivity with FSH, thyroid-stimulating hormone (TSH), α-subunits, or free LH β-subunits is less than 0.1 per cent. Total Te concentrations were assessed in duplicate by robotics-automated chemiluminescence assay. Sensitivity was 20 ng/dL (0.69 nmol/L), and median intra- and inter-assay coefficients of variation were 5.2 per cent and 8.3 per cent, respectively. This assay correlated at R = 0.975 with a slope 1.12 with Te measured by mass spectrometry. F was also measured in duplicate by robotics-automated chemiluminescence assay. Sensitivity was 0.2 µg/dL (5.5 nmol/L), and intra- and inter-assay coefficients of variation were 7.2–9.4 per cent and 6.3–9.4 per cent, respectively [30]. No samples were undetectable in any assay. Other screening measures were performed as previously described [38].
Deconvolution analysis
LH concentration time series were analyzed using a recently developed automated deconvolution method. Pulse detection was empirically validated using hypothalamo–pituitary sampling and simulated pulsatile time series [39]. Sensitivity and specificity both exceed 93 per cent. The MATLAB-based algorithm first detrends the data and normalizes concentrations to the unit interval (0, 1) and then the program creates multiple successively decremental potential pulse-onset time sets, each containing one fewer pulse by a smoothing process (a nonlinear adaptation of the heat-diffusion equation). A maximum-likelihood expectation (MLE) estimation method calculates all secretion and elimination parameters simultaneously conditional on each of the candidate pulse-time sets. Deconvolution parameters comprise basal secretion, secretory-burst mass (concentration units), mode (time delay in minimum to maximum value), and frequency (number of bursts per sampling duration and lambda of Weibull distribution). Pulsatile secretion is the sum of secretory-burst mass and total secretion of the sum of basal and pulsatile secretion. Basal secretion and lambda (pulse frequency) are assumed to be constant for the entire sampling duration. The fast half-life of LH was represented as 18 min, constituting 63 per cent of the decay amplitude and the slow half-life as 90 min [40]. Statistical model selection was performed using the Akaike information criterion.
Te concentration time series were analyzed similarly using fast (1.4) and slow (27) Te half-lives, with the fast half-life constituting 34 per cent of the decay amplitude, determined previously by pulsatile Te injections [41]. F concentration time series utilized a fast (2.4) and slow (56) cortisol half-lives, with the fast half-life constituting 37 per cent of the decay amplitude, previously determined by bolus cortisol infusion [42].
The above analyses were performed on the entire 24 h time series (145 data points).
Assessment of joint synchrony
Cross approximate entropy (XApEn) was applied to the time series, as a sensitive (>90 per cent) and specific (>95 per cent) measure of feedforward or feedback-conferred pattern of LH–Te and Te–LH orderliness, respectively [43, 44].
Statistical assessment
The primary hypotheses were that the effects of sleep restriction would differ according to age on parameters of hormone (Te, F, and LH) secretion. The secondary exploratory hypotheses were that these effects could differ in the morning versus the late afternoon/early evening. Accordingly, the analyses were primarily on deconvolution parameters of the entire 24 h time series, after which data were binned into consecutive 3 h windows. The secondary exploratory analyses examined the 3 h time windows separately in the morning from 6 am to 9 am (the first wake time period) and also in the late afternoon from 3 pm to 6 pm (the latest afternoon time period). These time windows were chosen a priori due to the 6 pm to 6 pm duration of blood sampling, and because 3 h time windows are still sufficiently long to allow assessment of hormone pulses and joint synchrony as previously described [30]. An early morning time window was examined because Te measurement for the assessment of hypogonadism should be performed in the early morning [2, 45]. The afternoon time window was examined because elevated F during this time causes insulin resistance which leads to type 2 diabetes and metabolic ill-health [24–26]. Hormones at both time periods were examined to allow time-of-day inferences.
Analyses were by repeated measures mixed model of sleep restriction, age group, and the interaction. A priori contrasts of the effect of sleep restriction in young and older individuals separately were directly obtained from each and every mixed model analysis. The Bonferroni-adjusted threshold, therefore, corresponds to an unadjusted p value of 0.025 for each of these two contrasts. p values of 0.05 and less were considered significant. Statistical power was 90 per cent to detect a 30 per cent difference in Te concentrations between sleep-restricted and rested conditions. Calculations were performed using proc mixed with the contrast statement to simultaneously assess the two preplanned contrasts with SAS 9.3 (SAS Institute Inc, Cary, NC).
Results
Participants
Seventeen young (aged 18–30) and 18 older (aged 60–80) men participated in two overnight Clinical Research Unit-based studies each. Demographic characteristics showed that older men were significantly shorter and obese (Table 1). As expected, total Te and insulinlike growth factor-1 (IGF-1) were lower, whereas FSH and sex hormone-binding globulin (SHGB) were higher in older men. LH and F did not differ by age. Serum levels of estradiol (E2), SHBG, LH, FSH, and Te were all within age-appropriate reference ranges. There were no dropouts; however, serum was insufficient to measure F in two older men (one during the control week, 103 samples; and another during the sleep restriction week, 24 samples). The entire time series for both were excluded from the analysis.
Table 1.
Demographic variables before randomization
| Young n = 17 mean ± SD or median (IQR) | Old n = 18 mean ± SD or median (IQR) | P-value | |
|---|---|---|---|
| Age (yr) | 24.1 ± 2.9 | 63.9 ± 4.0 | <0.001 |
| Height (m) | 180 ± 7 | 175 ± 6 | 0.04 |
| BMI (kg/m2) | 25.0 (22.9–27.5)* | 29.5 (26.4–31.7)† | 0.002 |
| Albumin (g/dL) | 4.7 ± 0.2 | 4.2 ± 0.3 | <0.001 |
| LH (U/L) | 4.1 ± 2.0 | 4.9 ± 3.0 | 0.36 |
| FSH (U/L) | 3.4 ± 1.6 | 10.3 ± 2.98 | 0.002 |
| SHBG (nmol/L) | 28.6 ± 9.8 | 38.4 ± 10.1 | 0.006 |
| T (nmol/L) | 18.9 ± 4.7 | 14.9 ± 3.6 | 0.008 |
| Cortisol (nmol/L) | 202 ± 139 | 196 ± 122 | 0.9 |
| TSH (mU/L) | 2.3 ± 1.0 | 2.9 ± 1.3 | 0.14 |
| IGF-1 (ug/dL) | 195 ± 32 | 123 ± 37 | <0.001 |
†No young men and seven older men with BMI > 30 kg/m2.
Twenty-four-hour analyses
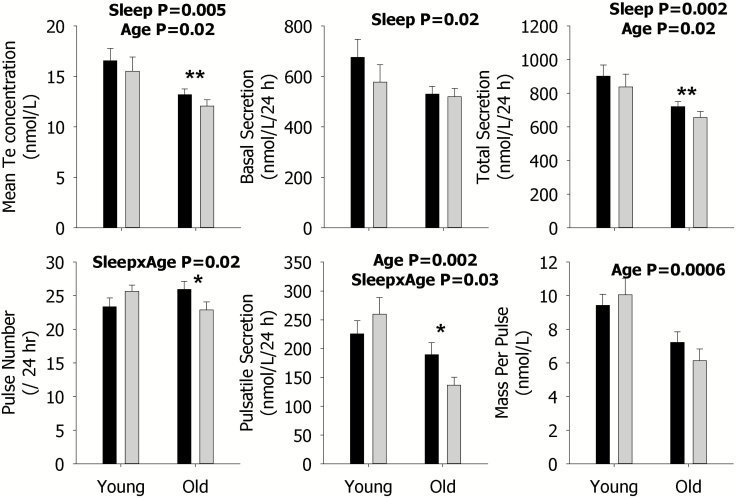
Sleep restriction reduced most components of Te secretion averaged across an entire 24 h period because there were statistically significant main effects for sleep or its interaction for mean concentration (p = 0.02), basal (p = 0.02), pulsatile (p = 0.03, interaction), and total secretion (p = 0.02) as well as pulse number (p = 0.02, interaction) (Figure 1). Furthermore, the effect of sleep restriction differed according to age for pulsatile secretion and pulse number (Figure 1). Examination of the specific contrasts, shown by asterisks, revealed that sleep restriction significantly decreased pulsatile secretion and pulse number in older individuals, as well as mean Te concentration and total secretion. Significant contrasts were not observed in younger men, as indicated by the lack of asterisks.
Figure 1.
Data are the mean ± SEM of parameters of Te secretion during rested sleep (black column) or restricted sleep (gray column) in young (left) and older (right) men within each panel. The mean concentration (left), basal secretion (middle), and total secretion (right) are shown in the upper panels; and pulse number (left), pulsatile secretion (middle), and mass per pulse (right) are illustrated in the lower panels. Statistically significant Sleep, Age, and SleepxAge effects are shown by the p-value. Statistically significant contrasts between the rested sleep and restricted sleep conditions in young or older men are shown by *p < 0.05 (Bonferroni adjusted) or **p < 0.0167 (Bonferroni adjusted). The absence of asterisks indicates that a significant contrast was not observed.
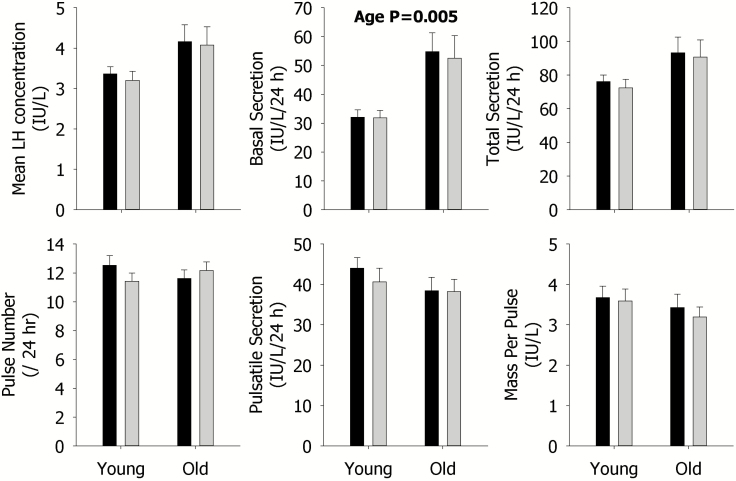
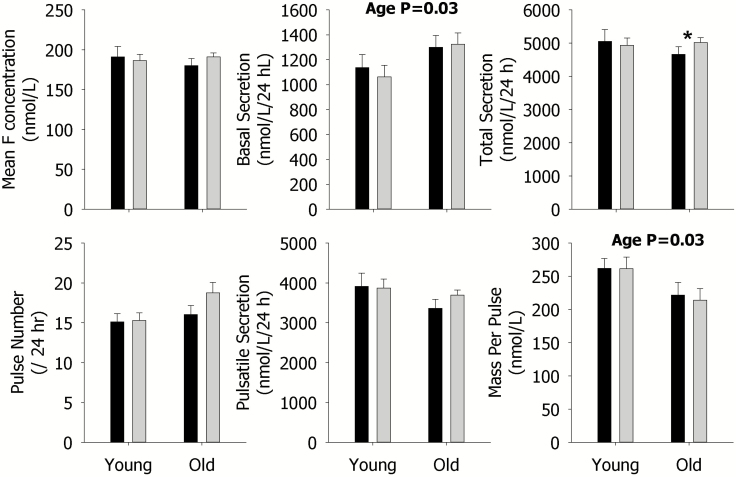
Despite many changes in Te secretion with sleep restriction, no effect on any parameter of overall 24-h LH secretion was observed (Figure 2). In older men, sleep restriction significantly increased the total secretion of F as indicated by the asterisk, but no other effects were observed (Figure 3).
Figure 2.
Data are the mean ± SEM of parameters of LH secretion during rested sleep (black column) or restricted sleep (gray column) in young (left) and older (right) men within each panel. The mean concentration (left), basal secretion (middle), and total secretion (right) are shown in the upper panels; and pulse number (left), pulsatile secretion (middle), and mass per pulse (right) are illustrated in the lower panels. Statistically significant Sleep, Age, and SleepxAge effects are shown by the p-value. Statistically significant contrasts between the rested sleep and restricted sleep conditions in young or older men are shown by *p < 0.05 (Bonferroni adjusted) or **p < 0.0167 (Bonferroni adjusted). The absence of asterisks indicates that a significant contrast was not observed.
Figure 3.
Data are the mean ± SEM of parameters of F secretion during rested sleep (black column) or restricted sleep (gray column) in young (left) and older (right) men within each panel. The mean concentration (left), basal secretion (middle), and total secretion (right) are shown in the upper panels; and pulse number (left), pulsatile secretion (middle), and mass per pulse (right) are illustrated in the lower panels. Statistically significant Sleep, Age, and SleepxAge effects are shown by the p-value. Statistically significant contrasts between the rested sleep and restricted sleep conditions in young or older men are shown by *p < 0.05 (Bonferroni adjusted) or **p < 0.0167 (Bonferroni adjusted). The absence of asterisks indicates that a significant contrast was not observed.
Older, compared with younger men, showed higher basal F (p = 0.03) and LH (p = 0.005) secretion, but lower F (p = 0.03) and Te (p = 0.0006) mass per pulse (see Figures 1–3). Older men also exhibited lower mean Te concentrations (p = 0.02), pulsatile (p = 0.002), and total (p = 0.02) secretion, compared with younger men (Figure 1). These age-related findings are expected.
Morning and late afternoon analyses
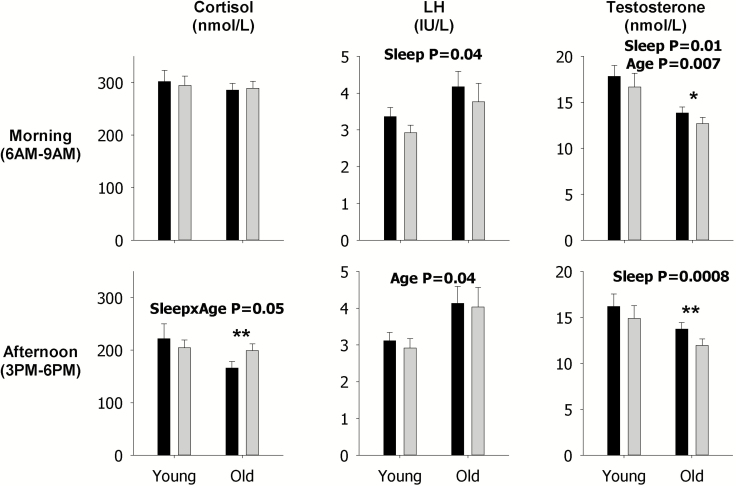
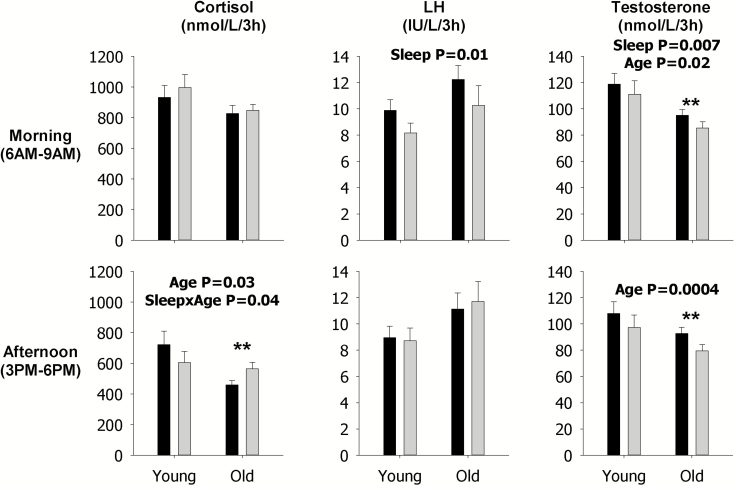
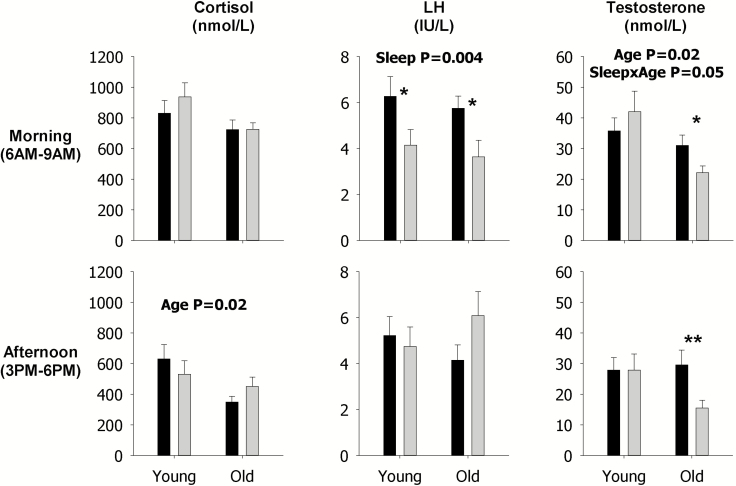
These analyses show that sleep restriction, as indicated by the p value for the sleep main effect, decreased LH mean concentration (p = 0.01, Figure 4), pulsatile secretion (p = 0.01, Figure 5), and mass per pulse (p = 0.004, Figure 6) in the morning across both young and older men, but not the late afternoon, as indicated by lack of p values for the sleep main effect in the corresponding figures since only statistically significant p values are shown in figures. Sleep restriction also decreased morning mean Te concentrations (p = 0.01, Figure 4) and pulsatile secretion (p = 0.007, Figure 5) across both young and older men, and mass per pulse (p = 0.05, interaction Figure 6) in the older men. In the late afternoon, sleep restriction significantly decreased mean concentrations of Te (p = 0.0008, Figure 4) across both young and older men, but increased mean concentrations of F (p = 0.05, interaction Figure 4), and pulsatile F secretion (p = 0.04, interaction Figure 5) in older men. Examination of the specific contrasts, shown by asterisks, confirm the latter findings in older men (see Figures 4 and 5). Significant effects of sleep restriction solely in younger men were not observed in the secretory dynamics of cortisol, LH, or Te, as indicated by the lack of asterisks (see Figures 4–6).
Figure 4.
Data are the mean ± SEM of mean concentrations of F (left), LH (middle), and Te (right) during the morning (top) and in the late afternoon (bottom). Within each panel, data are grouped by rested sleep (black column) or restricted sleep (gray column) in young (left) and older (right) men. Statistically significant Sleep, Age, and SleepxAge effects are shown by the p-value. Statistically significant contrasts between the rested sleep and restricted sleep conditions in young or older men are shown by *p < 0.05 (Bonferroni adjusted) or **p < 0.0167 (Bonferroni adjusted). The absence of asterisks indicates that a significant contrast was not observed.
Figure 5.
Data are the mean ± SEM of pulsatile secretion of F (left), LH (middle), and Te (right) during the morning (top) and in the late afternoon (bottom). Within each panel, data are grouped by rested sleep (black column) or restricted sleep (gray column) in young (left) and older (right) men. Statistically significant Sleep, Age, and SleepxAge effects are shown by the p-value. Statistically significant contrasts between the rested sleep and restricted sleep conditions in young or older men are shown by *p < 0.05 (Bonferroni adjusted) or **p < 0.0167 (Bonferroni adjusted). The absence of asterisks indicates that a significant contrast was not observed.
Figure 6.
Data are the mean ± SEM of mass per pulse of F (left), LH (middle), and Te (right) during the morning (top) and in the late afternoon (bottom). Within each panel, data are grouped by rested sleep (black column) or restricted sleep (gray column) in young (left) and older (right) men. Statistically significant Sleep, Age, and SleepxAge effects are shown by the p-value. Statistically significant contrasts between the rested sleep and restricted sleep conditions in young or older men are shown by *p < 0.05 (Bonferroni adjusted) or **p < 0.0167 (Bonferroni adjusted). The absence of asterisks indicates that a significant contrast was not observed.
Expected morning and late afternoon age contrasts in F, LH, and Te mean concentrations, pulsatile secretion, and mass per pulse were observed (Figures 4–6) and mirror those observed from 24 h analyses (Figures 1–3).
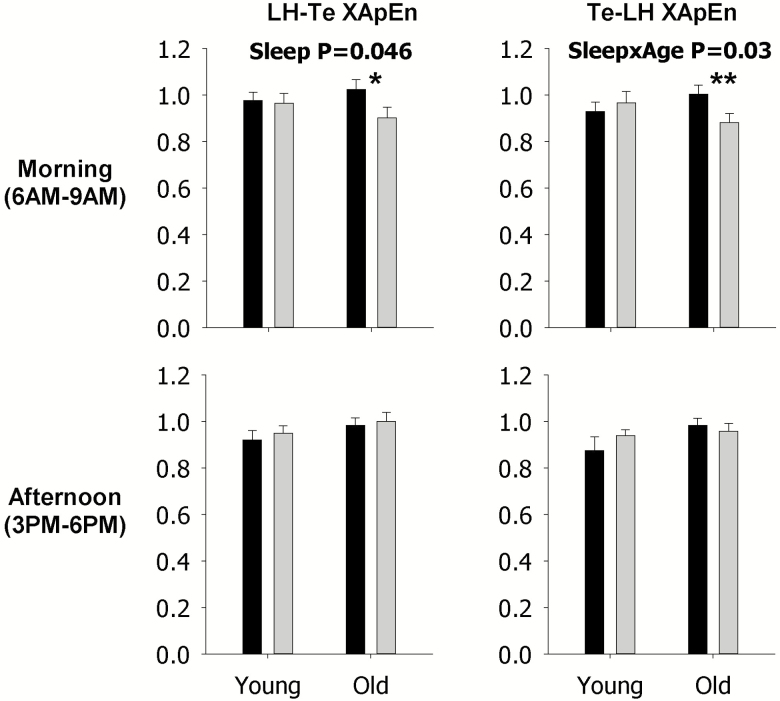
XApEn was utilized to quantify the effect of sleep restriction on feedforward (LH–Te, left) and feedback (Te–LH, right) linkages during the morning (top) and late afternoon (bottom) in young and older men (Figure 7). Sleep restriction significantly decreased both feedforward and feedback LH–Te XApEn during the morning in older men, as indicated by the asterisks. Significant effects in the late afternoon, or solely in young men, were not observed, as indicated by the lack of asterisks. Also, significant LH–Te and Te–LH linkages across the entire 24 h time period were not observed (data not shown).
Figure 7.
Data are the mean ± SEM of cross approximate entropy (XApEn) values quantified for feedforward luteinizing hormone-testosterone (LH–Te, left) and feedback testosterone-luteinizing hormone (Te–LH, right) linkages during the morning (top) and late afternoon (bottom). Within each panel, data are grouped by rested sleep (black column) or restricted sleep (gray column) in young (left) and older (right) men. Statistically significant Sleep, Age, and SleepxAge effects are shown by the p-value. Statistically significant contrasts between the rested sleep and restricted sleep conditions in young or older men are shown by *p < 0.05 (Bonferroni adjusted) or **p < 0.0167 (Bonferroni adjusted). The absence of asterisks indicates that a significant contrast was not observed.
Other analyses
In order to allow comparison with other published studies, we analyzed mean hormone concentrations from 10 pm to 6 am and from 9 am to 3 pm. There were no significant sleep deprivation effects on F, LH, and Te from 10 pm to 6 am, nor on F and LH from 9 am to 3 pm. However, sleep deprivation significantly reduced Te from 9 am to 3 pm across both young and older men (sleep main effect, p = 0.0005). The latter finding is consistent with the prior analyses showing sleep deprivation reduces 24 h Te, as well as Te measured 6 am–9 am, and 3 pm–6 pm.
Discussion
Our study helps resolve past discrepancies on the effect of sleep restriction on 24 h blood Te concentrations, since our study which sampled blood every 10 min, and the other study which sampled blood every 15–30 min [3], both show that sleep restriction decreases overall 24 h blood Te concentrations, in contrast with the other three studies that sampled blood much less frequently [4–6]. To our knowledge, our study is the first to assess actual pulsatile secretory events in LH or Te since this requires ultra high-intensity sampling (ideally at least every 10 min) to allow accurate mathematical deconvolution of serial hormone concentrations. Using this methodology, we found that sleep restriction reduced many 24 h parameters of Te secretion, in the absence of any detectable changes in LH concentration or any parameter of LH secretion. These effects were most apparent in older men and differed from younger men only for Te pulsatile secretion and Te total pulse number where a significant sleep by age interaction was detected.
Another novel finding is that the effect of sleep restriction on LH, and therefore on LH–Te feedforward and Te–LH feedback network regulation, appears to be dependent on time-of-day. Since assessment of parameters calculated over 24 h averages, and thereby blurs, potential time-of-day effects, we assessed discrete morning and late afternoon windows to sharpen specific preplanned contrasts. In this context, sleep restriction not only decreased morning mean LH concentrations, but also decreased morning pulsatile LH secretion and LH mass per pulse. This effect was specific to the morning and not observed in the late afternoon. In contrast, sleep restriction altered Te concentrations at both times of day. Changes in Te pulsatile secretion and mass per pulse with sleep restriction were most evident in older men and present at both times of the day. Furthermore, sleep restriction decreased a specific and sensitive marker of network regulation, XApEn. Both LH–Te and Te–LH XApEn decreased with sleep restriction in the morning. This indicates that sleep restriction enforced greater LH–Te and Te–LH joint synchrony in the morning, but had no such effect by the late afternoon. A parsimonious explanation of these LH secretory, Te secretory, and XApEn findings is that sleep restriction decreases LH secretion in the early morning which led to a reduction in Te secretion. By the late afternoon, LH secretion has recovered, but the Te response became uncoupled and remained low. Future studies will need to verify these regulatory possibilities by performing interventional experiments that directly assess testicular responsiveness to LH, pituitary responsiveness to gonadotropin-releasing hormone, and hypothalamo–pituitary feedback inhibition by Te at specific times during the day. At present only one study has addressed this issue and found that the early morning hypothalamo–pituitary–testicular axis response to insulin-induced hypoglycemia was not altered by complete sleep loss [9]. Although conceptually ground-breaking, the use of insulin-induced hypoglycemia in this balanced order, but non-randomized, crossover study, of 10 young men is a nonphysiological stimulus to assess gonadal axis function.
Examination of the adrenal axis revealed an increase in late afternoon F concentrations—an effect that was apparent in the older population—without affecting 24 h or morning F secretion. This finding extends prior studies in young men which support the developing hypothesis that sleep loss during the first half of the biological night specifically increases late afternoon/early evening F assessed by frequent (at least every 30–60 min) blood sampling for 24 h [3, 16–21]. Furthermore, the majority of these studies [3, 16–20], except one [21], also show no change in mean 24 h F supporting our contention that examining time-of-day specific windows is required to properly assess adrenal function. In fact, sleep loss during the first half of the biological night has been shown to increase late afternoon/early evening blood F in two other less intensively sampled studies which have sampled blood every 1–3 [46] or 2–3 [47] h for different parts of the 24 h period, as well as another two studies examining blood F collected every 20 min [48] or every other hour [4] for up to 15 h from 9 am. Other studies measuring salivary F in the late afternoon/early evening also show that sleep loss during the first half of the biological night increases afternoon/evening F [16, 31, 49–51], with only a few exceptions [11].
Since our purpose was primarily to examine the effects of sleep, we controlled for the effect of age either by examining the effect of sleep in each age group separately or by including age as a factor in a full factorial mixed model. Nevertheless, our study provides an opportunity to examine the effect of age on LH, Te, and F. In this regard, older men exhibited lower pulsatile, pulse mass, and total Te secretion that culminated in lower mean Te concentrations. Older men also displayed significantly higher basal LH secretion, but this only resulted in a trend toward higher mean LH concentration. These findings are concordant with prior studies of male gonadal aging which have consistently shown an age-associated decline in testosterone with variable changes in LH [13]. Older men also exhibited a higher basal F secretion, but a reduced mass per pulse, and ultimately no difference in mean F concentrations. However, in a much larger study of 143 adults (including 79 men) spanning seven decades of life, regression analyses were able to show a small reduction in mean 24-h F concentrations, of only 10 nmol/L per decade of life [30].
The present report joins the minority of prior studies that did not demonstrate an increase in the late afternoon/early evening F with sleep restriction in young men. However, only two of these prior studies utilized complete overnight sleep deprivation [6, 21], and neither of these studies reported a late afternoon or early evening increase in F concentrations. Hence our findings in young men are consistent with the two most comparable studies. Potential reasons for these discrepancies amongst all studies include differences in sleep restriction conditions such as the exact timing that sleep restriction occurred or the number of days of sleep restriction, different sample size among studies, as well as how and exactly when cortisol was assessed. For these reasons, it may be premature to conclude that sleep restriction has no effect on young men, only that effects were more apparent in older men.
In addition to changes to mean F concentrations, we also found that sleep restriction increased 24 h total F secretion and also increased late afternoon, but not morning, pulsatile F secretion and mass per pulse in older men. These findings extend prior work in 13 young men that reported no change in the overall F secretory dynamics with sleep restriction that included sleep loss in the first half of the biological night [48]. However, this earlier study sampled blood every 20 min for a 15-h period from 9 am to midnight and assessed pulsatile secretion of cortisol using an algorithm dependent on the precision of the hormone assay rather than mathematical deconvolution. Time-of-day contrasts were also not reported.
One limitation of our study is that we did not measure adrenocorticotropic hormone so we cannot address the detailed analysis of hypothalamo–pituitary–adrenal axis regulation. However, our assessment of actual pulsatile events in F, LH, and Te provides a more refined assessment of the effects of sleep restriction than prior studies. Our study also did not optimize sleep (e.g. through the use of an adaptation night or separate-room blood drawing) and did not quantify the amount of sleep by EEG. Although it is possible that participants were not always sleeping during periods of perceived sleep by study personnel, we did utilize a dramatic intervention—a night of total sleep loss—as the comparison group. Therefore, the effects of sleep restriction may have been greater if we had utilized sleep optimization strategies during the control night so null findings may be compromised, but significant differences are real and may be of a larger magnitude than observed.
In conclusion, our study supports the hypotheses generated by the literature that sleep restriction decreases Te and increases late afternoon F. These hormonal changes, although seemingly minor, could plausibly lead to clinically relevant ill-health when accumulated over decades of life. Our study now extends these hypotheses to older men and reports physiologically relevant pulse characteristics obtained by mathematical deconvolution. Using such an approach, in conjunction with a sensitive and specific parameter of joint hormone synchrony, we suggest that the effects of sleep restriction on the gonadal axis, and on F secretion, may also be time-of-day dependent. Future studies will need to establish any interdependencies between these gonadal and adrenal findings, directly interrogate these putative time of day and possible age-related differences in the gonadal and adrenal response to sleep restriction, and also establish consistent findings with multiple paradigms of sleep restriction that could vary by degree, duration, and pattern of sleep loss. Future studies will also need to establish whether these age-related differences are due to age or factors associated with age, such as differences in hormones, sleep patterns, body mass index, or other unknown factors.
Funding
This study was supported in part via R01 AG019695, R01 DK073148, R01 AG029362, R01 AG031763, R01 HL124211, K24 HL138632, and P30 DK050456 from the National Institutes of Health (Bethesda, MD). This study was also supported by UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS) and 60NANB10D005Z from the National Institute of Standards and Technology.
Conflict of interest statement: The authors have nothing to disclose.
Acknowledgments
This study was performed at Mayo Translational Science Center, Rochester, MN, 55905. We thank the Mayo Immunochemical Laboratory for assay assistance and the Mayo research nursing staff for implementing the protocol. Contents are solely the responsibility of the authors and do not necessarily represent the official views of any federal institution.
References
- 1. Killick R, et al. Implications of sleep restriction and recovery on metabolic outcomes. J Clin Endocrinol Metab. 2012;97(11):3876–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu PY. A clinical perspective of sleep and andrological health: assessment, treatment considerations, and future research. J Clin Endocrinol Metab. 2019;104(10):4398–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leproult R, et al. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305(21):2173–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reynolds AC, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012;7(7):e41218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmid SM, et al. Sleep timing may modulate the effect of sleep loss on testosterone. Clin Endocrinol (Oxf). 2012;77(5):749–754. [DOI] [PubMed] [Google Scholar]
- 6. Dáttilo M, et al. Effects of sleep deprivation on acute skeletal muscle recovery after exercise. Med Sci Sports Exerc. 2020;52(2):507–514. [DOI] [PubMed] [Google Scholar]
- 7. Akerstedt T, et al. Adrenocortical and gonadal steroids during sleep deprivation. Sleep. 1980;3(1):23–30. [DOI] [PubMed] [Google Scholar]
- 8. Carter JR, et al. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol. 2012;302(10):H1991–H1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jauch-Chara K, et al. Pituitary-gonadal and pituitary-thyroid axis hormone concentrations before and during a hypoglycemic clamp after sleep deprivation in healthy men. PLoS One. 2013;8(1):e54209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sauvet F, et al. Protective effects of exercise training on endothelial dysfunction induced by total sleep deprivation in healthy subjects. Int J Cardiol. 2017;232:76–85. [DOI] [PubMed] [Google Scholar]
- 11. Cote KA, et al. Sleep deprivation lowers reactive aggression and testosterone in men. Biol Psychol. 2013;92(2): 249–256. [DOI] [PubMed] [Google Scholar]
- 12. Smith I, et al. Sleep restriction and testosterone concentrations in young healthy males: randomized controlled studies of acute and chronic short sleep. Sleep Health. 2019;5(6):580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu PY, et al. An ensemble perspective of aging-related hypoandrogenemia in men. In: Winters S, Huhtaniemi I, eds. Male Hypogonadism. Cham: Humana Press; 2017: 325–348. [Google Scholar]
- 14. Li J, et al. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duffy JF, et al. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57(7):1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spiegel K, et al. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. [DOI] [PubMed] [Google Scholar]
- 17. Nedeltcheva AV, et al. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94(9):3242–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broussard JL, et al. Sleep restriction increases free fatty acids in healthy men. Diabetologia. 2015;58(4):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pejovic S, et al. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. Am J Physiol Endocrinol Metab. 2013;305(7):E890–E896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ackermann K, et al. Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiol Int. 2013;30(7):901–909. [DOI] [PubMed] [Google Scholar]
- 21. Wright KP Jr, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson NS, et al. Repeating patterns of sleep restriction and recovery: do we get used to it? Brain Behav Immun. 2016;58:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilms B, et al. Timing modulates the effect of sleep loss on glucose homeostasis. J Clin Endocrinol Metab. 2019;104(7):2801–2808. [DOI] [PubMed] [Google Scholar]
- 24. Plat L, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84(9):3082–3092. [DOI] [PubMed] [Google Scholar]
- 25. Dallman MF, et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14(4):303–347. [DOI] [PubMed] [Google Scholar]
- 26. Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34(2):271–2 92, vii. [DOI] [PubMed] [Google Scholar]
- 27. Kern W, et al. Changes in cortisol and growth hormone secretion during nocturnal sleep in the course of aging. J Gerontol A Biol Sci Med Sci. 1996;51(1):M3–M9. [DOI] [PubMed] [Google Scholar]
- 28. Gardner MP, et al. ; Halcyon Study Team Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: an individual participant meta-analysis. Psychoneuroendocrinology. 2013;38(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilpin H, et al. Atypical evening cortisol profile induces visual recognition memory deficit in healthy human subjects. Mol Brain. 2008;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roelfsema F, et al. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr Connect. 2017;6(7):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwarz J, et al. Does sleep deprivation increase the vulnerability to acute psychosocial stress in young and older adults? Psychoneuroendocrinology. 2018;96:155–165. [DOI] [PubMed] [Google Scholar]
- 32. Veldhuis JD, et al. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7): 823–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalafatakis K, et al. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc Natl Acad Sci U S A. 2018;115(17):E4091–E4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conway-Campbell BL, et al. Molecular dynamics of ultradian glucocorticoid receptor action. Mol Cell Endocrinol. 2012;348(2):383–393. [DOI] [PubMed] [Google Scholar]
- 35. Young EA, et al. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25(2):69–76. [DOI] [PubMed] [Google Scholar]
- 36. Veldhuis JD, et al. Dynamic testosterone responses to near-physiological LH pulses are determined by the time pattern of prior intravenous LH infusion. Am J Physiol Endocrinol Metab. 2012;303(6):E720–E728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roelfsema F, et al. Feedback on LH in testosterone-clamped men depends on the mode of testosterone administration and body composition. J Endocr Soc. 2019;3(1):235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Veldhuis JD, et al. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302(1):E117–E122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu PY, et al. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297(2):E538–E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Veldhuis JD, et al. Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest. 1986;77(4):1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Veldhuis JD, et al. Kinetics of removal of intravenous testosterone pulses in normal men. Eur J Endocrinol. 2010;162(4):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bright GM. Corticosteroid-binding globulin influences kinetic parameters of plasma cortisol transport and clearance. J Clin Endocrinol Metab. 1995;80(3):770–775. [DOI] [PubMed] [Google Scholar]
- 43. Pincus SM, et al. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci U S A. 1996;93(24):14100–14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veldhuis JD, et al. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):R721–R729. [DOI] [PubMed] [Google Scholar]
- 45. Bhasin S, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. [DOI] [PubMed] [Google Scholar]
- 46. Axelsson J, et al. Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/ T helper 2 balance in humans. PLoS One. 2013;8(12):e82291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gil-Lozano M, et al. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab. 2016;310(1):E41–E50. [DOI] [PubMed] [Google Scholar]
- 48. Guyon A, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99(8):2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Omisade A, et al. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99(5):651–656. [DOI] [PubMed] [Google Scholar]
- 50. Buxton OM, et al. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wolkow A, et al. The impact of sleep restriction while performing simulated physical firefighting work on cortisol and heart rate responses. Int Arch Occup Environ Health. 2016;89(3):461–475. [DOI] [PubMed] [Google Scholar]