Abstract
Among biological agents for the treatment of atopic dermatitis (AD), dupilumab is a front-runner. Although many studies have been conducted on the real-world use of dupilumab, the sample size is often small and data is primarily on Western people. Therefore, we investigated the efficacy and safety of dupilumab in patients with moderate-to-severe AD in Korea. All patients with moderate-to-severe AD treated with dupilumab from September 2018 to June 2019 in this institution were included and analyzed by medical records. They were evaluated using the Eczema Area and Severity Index (EASI), Numerical Rating Scale (NRS), Patient Oriented Eczema Measure (POEM), and Dermatology Quality of Life Index (DLQI), respectively on admission, after two weeks (only EASI and NRS) and after 16 weeks. Laboratory tests were measured before and 16 weeks after treatment. A total of 101 patients were included. All efficacy tools showed a significant decrease after 16 weeks; EASI 77.4%, NRS 70.0%, POEM 60.7%, and DLQI 65.0%. EASI was characterized by a marked improvement of 51.5% in just two weeks. The treatment response was not significantly different according to the interval of treatment. Elevated Lactate Dehydrogenase (LDH) at 16 weeks was associated with poor treatment response. Moreover, a high eosinophil count was related to a lower change in EASI and POEM. In the correlation analysis, EASI was not correlated to DLQI before treatment. For changes after 16 weeks, POEM showed the highest correlation with DLQI. (R = 0.66, p < 0.001) In the additional analysis for factors affecting treatment response, the female gender was associated with good treatment response. (odds ratio = 5.4, p = 0.04) Adverse events from treatment included facial erythema (9.9%) and conjunctivitis (5.0%). Overall, it was confirmed that the efficacy of dupilumab in the real-world is similar to that of the existing clinical trials. We suggest that POEM is a useful tool for identifying the quality of life. The female gender was associated with a good treatment response. Both an elevated LDH and a high eosinophil count could be a therapeutic biomarker. Further research will be needed for a long-term period.
Keywords: atopic dermatitis, dupilumab, Patient Oriented Eczema Measure (POEM), female gender, lactate dehydrogenase (LDH), eosinophilia
1. Introduction
Dupilumab is the first approved biologic agent for atopic dermatitis (AD). It acts by inhibiting the differentiation of naive T-cells into T helper (Th) 2 cells and inhibits the proliferation of Th 2 cells by blocking interleukin (IL)-4 and IL-13 simultaneously [1,2,3]. IL-4 and IL-13 are involved in the upstream initiation and clonal expansion of the Th2 reaction and play an essential role in the downstream Th2 reaction as Th2 effector cytokines. Therefore, theoretically, dupilumab might be an efficacious therapeutic agent.
Many real-world studies of dupilumab have been conducted, however, most studies [4,5,6,7] have used small sample sizes, and have usually been conducted using a Western sample. Therefore, to identify the efficacy and safety of dupilumab in Korea, we evaluated the real-world use of dupilumab in patients with moderate-to-severe AD in Korea. This study included the largest sample size from a single institution in Korea, and evaluated various efficacy measures during the treatment, although it was not a clinical study. In addition, we performed correlation analysis between efficacy measures to see the correlation between them, and identified the variables affecting the treatment outcomes.
2. Methods
2.1. Study Design and Data Collection
All patients with moderate-to-severe AD treated with dupilumab between September 2018 and June 2019 were included, and their cases were retrospectively analyzed using medical records. This study was approved by the Institutional Review Board (IRB) of National Medical Center and conducted in accordance with research ethics. AD was diagnosed by a dermatologist associated with our clinic according to the revised Hanifin and Rajka criteria [8]. Inclusion criteria were age >18 years and an Eczema Area and Severity Index (EASI) score >16 to include moderate-to-severe AD. The basic treatment regimen was treatment with dupilumab alone. If patients were treated with systemic steroid or cyclosporine before treatment of dupilumab at baseline, the systemic therapy was maintained to prevent a recurrence. At each visit, the dermatologist evaluated the status of AD. In the case of worsening or slower improvement than before, concomitant systemic treatment was maintained. In the case of improvement, it was gradually tapered, and in some cases, the concomitant treatment was discontinued. The dose of dupilumab was 600 mg at first administration and 300 mg during the subsequent visit. The interval between the doses was two weeks. However, there were some cases in which the interval was three, four, or more weeks depending on the patient’s financial status or distance from hospital; dupilumab is not covered by the National Health Insurance in Korea. As representative factors for AD, EASI, Numerical Rating Scale (NRS), Patient Oriented Eczema Measure (POEM), and Dermatology Quality of Life Index (DLQI) were measured before treatment and 16 weeks after the first dose of dupilumab [9,10,11,12]. EASI and NRS were also measured two weeks after the first dose. Laboratory tests such as those for immunoglobulin E (IgE), lactate dehydrogenase (LDH), and total eosinophil count (TEC) were conducted before and 16 weeks after the first dose.
2.2. Statistical Analysis
All data processing was performed using the R 3.5.2 version (Microsoft Corp., Redmond, WA, USA). Differences between the baseline and 16 weeks post-treatment measures were analyzed using a Wilcoxon signed rank test. Correlation analyses were performed to understand the correlations between efficacy measures. Also, the Wilcoxon rank-sum test and the Kruskal–Wallis test were performed to analyze the differences in efficacy measures based on the interval between doses and administration of concomitant treatment. The differences in response to treatment based on LDH and TEC levels at baseline and 16 weeks were also analyzed. Bonferroni correction was used for post-hoc analyses. The threshold of statistical significance was set at p < 0.05.
3. Results
3.1. Demographics
In total, 101 patients were enrolled (65 men and 36 women). Of these, 34.7% of patients had a family history of allergic diseases. Additionally, 77 patients (76.2%) had allergic diseases other than AD, of which, allergic rhinitis accounted for 67.5%, allergic conjunctivitis 18.2%, and asthma 14.3%. The antigens causing the allergy had been identified in 75 patients (74.3%): 57.3% were house dust mite and 22.7% were mold. Regarding previous treatment, 89 patients (88.1%) had been treated with topical steroids and 66 patients (65.4%) with topical calcineurin inhibitors; 66 patients (65.4%) had been treated with systemic steroids; 73 patients (72.3%) had taken systemic immunosuppressants, of which, 61 (82.4%) had taken cyclosporine; and 43 patients (42.6%) had received oriental medicine or a folk remedy (Table 1).
Table 1.
Baseline demographics and history of treatment (N = 101).
| Type | Variables | Mean (Min, Max) or Count (%) |
|---|---|---|
| Demographics | Age, Years | 30.11 (18.00–50.00) |
| Gender | ||
| Male | 65 (64.36%) | |
| Female | 36 (35.64%) | |
| Occupation | ||
| Yes | 72 (71.29%) | |
| Marriage | ||
| Yes | 22 (21.78%) | |
| Disease Onset | ||
| Adult exacerbation | 13 (12.87%) | |
| Childhood | 88 (87.13%) | |
| Family History of Allergy | ||
| Yes | 35 (34.65%) | |
| Allergy History | 77 (76.24%) | |
| Allergic conjunctivitis | 14 (18.18%) | |
| Allergic rhinitis | 52 (67.53%) | |
| Asthma | 11 (14.29%) | |
| Known Allergy | 75 (74.26%) | |
| House dust mite | 43 (57.33%) | |
| Mold | 17 (22.67%) | |
| Food | 8 (10.67%) | |
| Cat | 4 (5.33%) | |
| Dog | 3 (4.00%) | |
| Ocular Disease Telated to Past Treatment | 18 (17.82%) | |
| Cataract | 6 (33.33%) | |
| Cataract (had operation) | 7 (38.89%) | |
| Corneal transplantation | 1 (5.56%) | |
| Glaucoma | 2 (11.10%) | |
| Retinal detachment | 2 (11.10%) | |
| History of treatment | Topical Treatment | |
| Topical steroid | 89 (88.12%) | |
| Topical calcineurin inhibitor | 66 (65.35%) | |
| Systemic Treatment | ||
| Systemic steroid | 66 (65.35%) | |
| Immunosuppressant | 73 (72.28%) | |
| Cyclosporine | 61 (82.43%) | |
| Methotrexate | 9 (12.16%) | |
| Mycophenolate mofetil | 2 (2.70%) | |
| Azathioprine | 1 (1.35%) | |
| Phototherapy | 10 (9.90%) | |
| Oriental medicine | 43 (42.57%) | |
| Folk remedy | 6 (5.94%) |
For the interval of dupilumab treatment, 50 patients (49.5%) maintained two weeks, and 29 patients (28.7%) took the doses at four-week intervals. There were 80 patients (79.2%) who took no concomitant therapy, while nine, seven, and five patients were on concomitant cyclosporine, methotrexate, and systemic steroid therapy, respectively (Table 2).
Table 2.
Characteristics of treatment.
| Dose Interval | N = 101 | Combined Treatment | N = 101 |
|---|---|---|---|
| 2 weeks | 50 (49.50%) | Cyclosporine | 9 (8.91%) |
| 3 weeks | 5 (4.95%) | Methotrexate | 7 (6.93%) |
| 4 weeks | 29 (28.71%) | Systemic steroid | 5 (4.95%) |
| Increase of interval | 16 (15.84%) | None (dupilumab-only) | 80 (79.21%) |
| Decrease of interval | 1 (0.99%) |
3.2. Efficacy of Treatment
3.2.1. Baseline Values of Efficacy Measures
The median baseline values before the administration of dupilumab were EASI 29.0, NRS 8.0, POEM 24.0, and DLQI 23.0 (Table 3).
Table 3.
Percent change in efficacy tools after two weeks and 16 weeks. All scores showed a significant decrease.
| Baseline | After 2 Weeks | p-Value ** | After 16 Weeks | p-Value ** | |
|---|---|---|---|---|---|
| Total group | |||||
| EASI score (min–max) | 29.0 (14.0–60.0) | 15.1 (4.3–37.5) | 7.5 (1.7–29.6) | ||
| Median percent change ± IQR * in EASI | 51.5 ± 24.9 | <0.001 | 77.4 ± 12.5 | <0.001 | |
| NRS score (min–max) | 8.0 (2.0–10.0) | 4.0 (0.0–7.0) | 3.0 (1.0–7.0) | ||
| Median percent change ± IQR * in NRS | 50.0 ± 25.8 | <0.001 | 70.0 ± 27.8 | <0.001 | |
| POEM (min-max) | 24.0 (5.0–28.0) | 9.0 (1.0–24.0) | |||
| Median percent change ± IQR * in POEM | 60.7 ± 31.4 | <0.001 | |||
| DLQI (min-max) | 23.0 (14.0–30.0) | 9.0 (0.0–25.0) | |||
| Median percent change ± IQR * in DLQI | 65.0 ± 29.1 | <0.001 | |||
| Dupilumab-only group *** | |||||
| EASI score (min-max) | 26.9 (14.0–60.0) | 14.4 (4.3–36.6) | 6.4 (1.7–29.6) | ||
| Median percent change ± IQR * in EASI |
51.7 ± 24.6 | <0.001 | 77.9 ± 12.5 | <0.001 | |
| NRS score (min-max) | 8.0 (2.0–10.0) | 3.0 (0.0–7.0) | 2.0 (1.0–7.0) | ||
| Median percent change ± IQR * in NRS | 57.1 ± 30.6 | <0.001 | 70.0 ± 17.8 | <0.001 | |
| POEM (min-max) | 24.0 (5.0–28.0) | 9.0 (1.0–20.0) | |||
| Median percent change ± IQR * in POEM | 64.4 ± 27.0 | <0.001 | |||
| DLQI (min-max) | 23.0 (14.0–30.0) | 8.0 (0.0–25.0) | |||
| Median percent change ± IQR * in DLQI | 66.1 ± 27.0 | <0.001 | |||
* The median percent change ± IQR was defined as the median of the percentage of change from baseline to 16 weeks. ** p-value calculated using the Wilcoxon signed-rank test. *** Dupilumab-only group without concomitant treatment. EASI: Eczema Area and Severity Index; NRS: Numerical Rating Scale; POEM: Patient-Oriented Eczema Measure; DLQI: Dermatology Life Quality Index; IQR: interquartile range.
3.2.2. Changes in EASI and NRS at 2 Weeks
At two weeks the decrease was 51.5% in EASI and 50.0% in NRS (p < 0.001; Table 3).
3.2.3. Change in Efficacy Measures at 16 Weeks
All measures showed a significant decrease (p < 0.001), EASI by 77.4%, NRS by 70.0%, POEM by 60.7%, and DLQI by 65.0%. Patients who were only on dupilumab therapy without any concomitant treatment also showed a significant decrease in the efficacy measures (Table 3); this was done to see the effect of dupilumab alone.
3.2.4. EASI 50 and EASI 75 at 16 Weeks
EASI 50 and EASI 75 mean the proportion of patients showing improvement of more than 50% and 75% in EASI, respectively. Two weeks after the first administration of dupilumab, the proportion of EASI 50 and EASI 75 was 51.4% and 2.8%, respectively. After 16 weeks, EASI 50 was 92.7%, and EASI 75 was 63.6%.
3.2.5. Changes in Laboratory Tests at 16 Weeks
IgE, TEC, and LDH were measured at follow-up. At 16 weeks, only LDH was significantly reduced (in 24.1% of the patients; p = 0.010). However, there was no significant change in values in the dupilumab-only treatment group (Table 4).
Table 4.
Change in laboratory test after 16weeks.
| Baseline | After 16 Weeks | p-Value ** | |
|---|---|---|---|
| Total group | |||
| Serum total IgE (min–max) | 316.5 (11.0–5000.0) | 304.0 (6.0–2648.0) | |
| Median percent change ± IQR * in total IgE |
3.48 ± 117.64 **** | 0.981 | |
| Serum TEC (min–max) | 672.0 (57.0–6066.0) | 600.0 (82.0–6536.0) | |
| Median percent change ± IQR * in TEC | 14.09 ± 84.80 **** | 0.954 | |
| Serum LDH (min–max) | 252.0 (130.0–491.0) | 218.0 (132.0–385.0) | |
| Median percent change ± IQR * in LDH | 24.14 ± 21.85 | 0.010 | |
| Dupilumab-only group *** | |||
| Serum total Ig E (min–max) | 368.0 (11.0–5000.0) | 364.0 (52.0–2648.0) | |
| Median percent change ± IQR * in total IgE | 5.01 ± 105.66 **** | 0.636 | |
| Serum TEC (min–max) | 563.0 (57.0–6066.0) | 588.0 (82.0–3840.0) | |
| Median percent change ± IQR * in TEC |
13.51 ± 77.75 **** | 0.860 | |
| Serum LDH (min–max) | 242.0 (130.0–392.0) | 215.0 (132.0–348.0) | |
| Median percent change ± IQR * in LDH | 21.27 ± 27.83 **** | 0.098 | |
* The median percent change ± IQR was defined as the median of the percentage of change from baseline to 16 weeks. ** p-value calculated using the Wilcoxon signed-rank test. *** Dupilumab-only group without concomitant treatment. **** Negative value means an increase compared with baseline. IgE: immunoglubulin E; TEC: total eosinophil count; LDH: lactate dehydrogenase; IQR: interquartile range.
3.2.6. Relationship Between LDH or Eosinophil Level and Treatment Response
To identify the variables affecting treatment response, we analyzed the relationship between elevated LDH or eosinophilia and response to treatment. We found that in cases in which LDH was persistently elevated at 16 weeks, changes in NRS, POEM, and DLQI were significantly lower than those in the normal LDH group (Table 5). We also found that patients with eosinophilia (>500) and hypereosinophilia (>1500) at baseline showed a significantly smaller change in POEM and DLQI at 16 weeks than those without eosinophilia and hypereosinophilia. Moreover, patients with eosinophilia and hypereosinophilia at 16 weeks showed significantly smaller EASI and POEM changes than those without eosinophilia and hypereosinophilia (Table 6).
Table 5.
The difference of efficacy, according to LDH. Elevated LDH at 16 weeks showed inadequate treatment response in NRS, POEM, and DLQI.
| LDH at Baseline | LDH at 16 Weeks | |||
|---|---|---|---|---|
| <250 | ≥250 | <250 | ≥250 | |
| Percent change of EASI after 16 weeks | 77.77 | 65.09 | 76.83 | 77.33 |
| p-value * | 0.095 | 0.512 | ||
| Percent change of NRS after 16 weeks | 65.71 | 62.50 | 73.21 | 37.5 |
| p-value * | 0.640 | <0.001 | ||
| Percent change of POEM after 16 weeks | 69.23 | 51.79 | 66.28 | 41.67 |
| p-value * | 0.182 | 0.014 | ||
| Percent change of DLQI after 16 weeks | 66.67 | 67.86 | 67.3 | 52.0 |
| p-value * | 0.764 | 0.021 | ||
* p-values were obtained using the Wilcoxon signed-rank test. LDH: Lactate Dehydrogenase; EASI: eczema area and severity index; NRS: numerical rating scale; POEM: patient-oriented eczema measure; DLQI: dermatology life quality index.
Table 6.
The difference of efficacy, according to TEC. High TEC at baseline, showed a lower change in POEM after 16 weeks. High TEC at 16 weeks showed a lower change in EASI and POEM after 16 weeks.
| TEC at Baseline | TEC at 16 Weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| <500 | ≥500 | <1500 | ≥1500 | <500 | ≥500 | <1500 | ≥1500 | |
| Percent change of EASI after 16 weeks | 76.96 | 76.04 | 77.78 | 73.49 | 77.67 | 69.27 | 78.00 | 73.25 |
| p-value * | 0.336 | 0.062 | 0.034 | 0.046 | ||||
| Percent change of NRS after 16 weeks | 62.50 | 70.00 | 70.00 | 62.50 | 70.00 | 66.25 | 70.00 | 66.67 |
| p-value * | 0.806 | 0.994 | 0.836 | 0.455 | ||||
| Percent change of POEM after 16 weeks | 69.23 | 53.97 | 64.71 | 37.50 | 64.71 | 33.94 | 69.51 | 53.57 |
| p-value * | 0.035 | 0.003 | 0.021 | 0.007 | ||||
| Percent change of DLQI after 16 weeks | 71.42 | 56.83 | 67.26 | 50.00 | 65.51 | 53.26 | 67.33 | 56.52 |
| p-value * | 0.157 | 0.044 | 0.087 | 0.187 | ||||
* p-values were obtained using the Wilcoxon signed-rank test. TEC: total eosinophil count; EASI: eczema area and severity index; NRS: numerical rating scale; POEM: patient-oriented eczema measure; DLQI: dermatology life quality index.
3.3. Subgroup Analysis
3.3.1. Baseline Characteristics in Subgroups
Before performing subgroup analysis, the baseline status of each subgroup was identified to be able compare results to one another. Regarding treatment interval, there were no significant differences in EASI, NRS, POEM, and DLQI between the groups. In contrast, the concomitant therapy group showed higher EASI, NRS, POEM, and DLQI levels than the dupilumab-only group (Table 7).
Table 7.
Baseline characteristics in subgroup analysis.
| According to Treatment Interval | According to Concomitant Treatment | |||
|---|---|---|---|---|
| Interval of 2 Weeks | Interval Others | Dupilumab-Only Group |
Concomitant Treatment Group |
|
| EASI score (min–max) | 26.5 (14.0–60.0) | 26.0 (14.0–60.0) | 26.9 (14.0–60.0) | 31.7 (15.9–60.0) |
| NRS score (min–max) | 8.0 (2.0–10.0) | 8.0 (2.0–10.0) | 8.0 (2.0–10.0) | 9.0 (2.0–10.0) |
| POEM (min–max) | 25.5 (5.0–28.0) | 23.5 (12.0–28.0) | 24.0 (5.0–28.0) | 25.0 (9.0–28.0) |
| DLQI (min–max) | 23.0 (14.0–30.0) | 23.0 (14.0–30.0) | 23.0 (14.0–30.0) | 26.0 (14.0–29.0) |
3.3.2. Differences in Efficacy Measures According to Dosage Interval
Most patients had a two-week interval, however, the two-week interval group’s efficacy measures were not significantly different from those of the other group with a longer interval.
3.3.3. Differences in Efficacy Measures with Concomitant Therapy
We analyzed the differences in treatment response between patients concomitantly treated with systemic steroids, cyclosporine, or methotrexate, and those treated only dupilumab as the primary therapy. The change in EASI (p = 0.046), POEM (p = 0.020), and DLQI (p = 0.031) between the two groups at 16 weeks was significantly different. Patients in the dupilumab-only group showed a larger change than the concomitant treatment group.
3.4. Correlations Between Efficacy Measures
3.4.1. Before Treatment
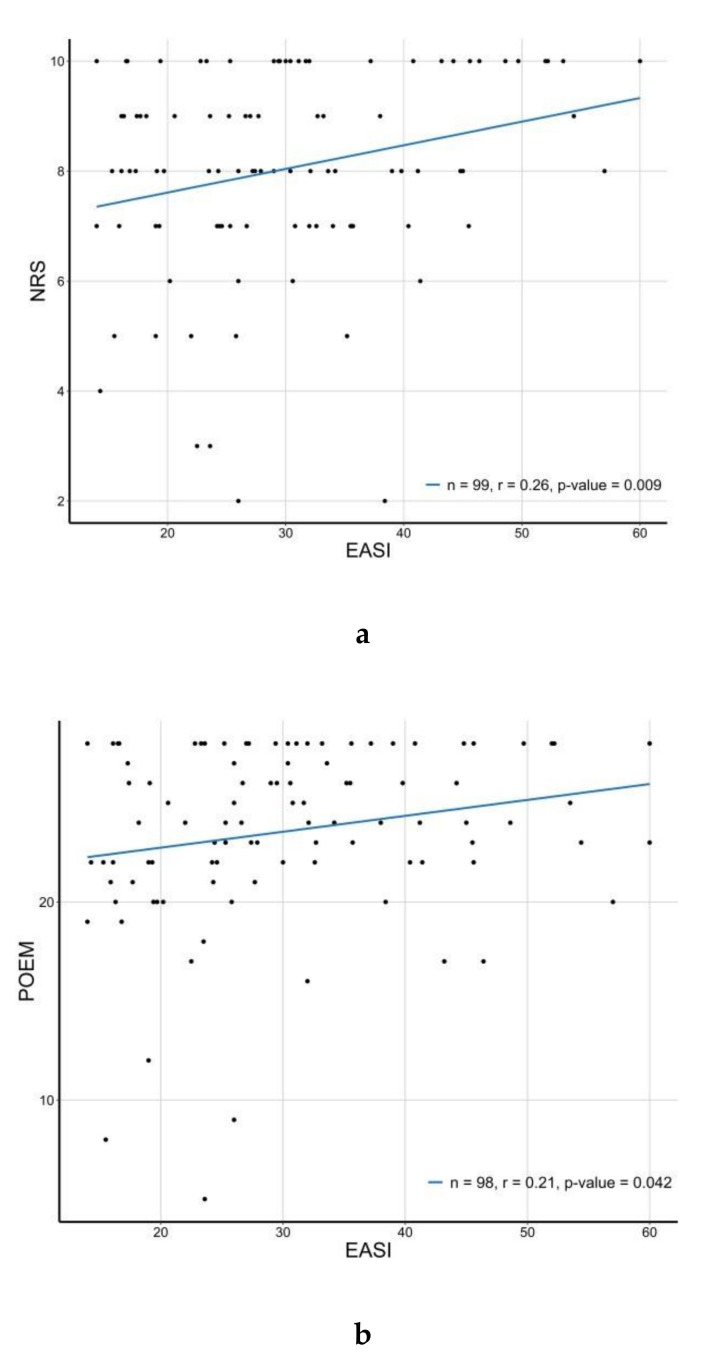
Correlation analysis was performed between the efficacy measures before the first dose of dupilumab. The results showed significant correlations between NRS and POEM (R = 0.54, p < 0.001), NRS and DLQI (R = 0.5, p < 0.001), POEM and DLQI (R = 0.45, p < 0.001), EASI and NRS (R = 0.26, p = 0.009), and EASI and POEM (R = 0.21, p = 0.042). The value of NRS was most related to that of DLQI; POEM was related, but EASI was not significantly related.
3.4.2. Changes at 16 Weeks After the Treatment of Dupilumab
Correlation analyses between the changes in the values of efficacy measures was performed at 16 weeks after the first administration of dupilumab. Significant correlations in changes were observed between POEM and DLQI (R = 0.66, p < 0.001), NRS and POEM (R = 0.46, p = 0.001), NRS and DLQI (R = 0.43, p = 0.001), EASI and POEM (R = 0.4, p = 0.003), and EASI and DLQI (R = 0.39, p = 0.005). The measures related to changes in DLQI were POEM, NRS, and EASI, in order of significance. We also analyzed the correlation between changes in efficacy measures and laboratory tests. Only TEC showed a significant correlation with DLQI (R = 0.4, p = 0.015). Among the laboratory tests, TEC and LDH showed significant correlation (R = 0.59, p = 0.033).
3.5. Adverse Events After Treatment
Ten patients (9.9%) showed aggravation of erythema or newly developed erythema of the face after administration of dupilumab; among them, one patient was diagnosed with Systemic Lupus Erythematosus (SLE).
Five patients (5.0%) developed conjunctivitis after the administration of dupilumab. They were referred to ophthalmologists and showed improvement in conjunctivitis.
4. Discussion
This retrospective real-world study of dupilumab in Korea revealed that the efficacy of dupilumab in the real world is similar to that of the existing clinical trials. In addition, we identified the characteristics of moderate-to-severe AD patients in Korea and performed analyses on the correlations between efficacy measurements and variables affecting the treatment response.
In this study, the proportion of allergic conjunctivitis and asthma was lower than that of allergic rhinitis. This result contrasts previous clinical trials [13,14]. The low proportion of AD patients with asthma is distant from allergic march [15]. Therefore, large-scale cohort studies would be necessary to accurately understand allergic comorbidities in Korea.
The mean DLQI of our patients was higher than that of patients in previous clinical trials [3,13,14], because unlike in other countries, 42.6% of Koreans AD patients in this study received oriental or folk treatment, suggesting that this population is inclined to opt for alternative treatment for atopic dermatitis. The poor therapeutic effect [16] might be considered a factor affecting the quality of life.
In the study, the different treatment intervals could be a limitation, but we were able to compare the different interval groups in the treatment responses. Despite no difference in baseline status between the groups (shown in Table 7), there was no significant difference between the efficacy measures at different intervals. It is supposed that in cases showing good response to treatment, increasing the interval to four weeks or more could be considered during treatment. However, since our conclusions are based on clinical progress without drug pharmacokinetics, planned clinical trials are necessary.
We showed that the pre-treatment status of AD was worse in the concomitant group than in the dupilumab-only group (shown in Table 7). Since AD was already more severe in the concomitant group before the administration of dupilumab, the efficacy in the concomitant group was less than in the dupilumab-only group. Accordingly, concomitant treatment should be administered parallelly, and gradual tapering is required for preventing rebound effect.
In the correlation analyses, NRS, POEM, and DLQI, which represent the patients’ subjective symptoms, showed significant correlations with each other. However, EASI, which may represent the objective symptom, was not significantly related to DLQI. This result suggests that the physical state of the lesion and the subjective symptoms might not be related. As shown in Figure 1a,b, despite a low EASI score, the subjective symptoms might be severe. It suggests that subjective symptoms should not be overlooked despite low EASI scores.
Figure 1.
Correlation between (a) Eczema Area and Severity Index (EASI) and Numerical Rating Scale (NRS) (R = 0.26, p = 0.009), and (b) EASI and Patient Oriented Eczema Measure (POEM) (R = 0.21, p = 0.042) at baseline.
Regarding the changes for 16 weeks, POEM and DLQI showed the highest correlation, but EASI and DLQI showed the lowest correlation. It is different from a previous study on psoriasis [17], where a higher Psoriasis Area and Severity Index (PASI) correlated with a more significant change in DLQI. In psoriasis, the visible lesions may have a more significant impact on the quality of life than the subjective symptoms and go through the chronic course rather than the acute stage. In contrast, subjective symptoms, such as itching, have a more significant impact on the quality of life than the visible lesions do in the case of AD. Moreover, in addition to the chronic course, the symptoms of acute-stage lesions can greatly affect the patient. Due to the highest correlation with DLQI, we infer that POEM is particularly useful in evaluating the patient’s condition, expecially as POEM is easily done in a short time.
Not all patients who use dupilumab have a good prognosis; thus, much more attention is being directed at the factors affecting therapeutic effect. To identify the variables causing differences in response to treatment, we divided the study population into two groups based on the achievement of EASI 75. The only significant parameter was gender, with an odds ratio for women achieving EASI 75 equaling 5.4 (p = 0.04). It indicates that women are more likely to achieve EASI 75. This result is consistent with previously published real-world data in France [5].
There was no significant difference in the change in LDH after 16 weeks between the groups, but the elevation of LDH after 16 weeks was related to lower treatment response as indicated by subjective efficacy measures (Table 5). Although not related to EASI, this result is consistent with a recent real-world study of dupilumab [5], where LDH was associated with a therapeutic response. Therefore, LDH could be used as a biomarker for therapeutic response.
Another possible factor is eosinophil count; the EASI 75 achieving group showed lower TEC both at baseline and 16 weeks, and the patient with high eosinophil count at 16 weeks showed a lower change in EASI (Table 6). There are no laboratory findings related to hypereosinophilia in those with high TEC. This result indicates that a high eosinophil count might affect the treatment response, and a persistently high eosinophil count might be related to an inadequate response to treatment. In a previous study [18], there were some cases of eosinophilia after treatment with dupilumab.
Regarding adverse effects, facial erythema is a major concern associated with dupilumab in other countries [19,20], but the mechanism is not well understood. Facial erythema might be related to the worsening of existing AD lesions, withdrawal of systemic treatment, concomitant allergic contact dermatitis, or the adverse effects of dupilumab.
Conjunctivitis was reported in the clinical trials and has become a constant problem. The cause of dupilumab-associated conjunctivitis, as well as the relationship between its various pathogenetic mechanisms, are currently unclear, but possible hypotheses include inhibition of IL-4 and IL-13 signaling pathways involved in the development of atopic keratoconjunctivitis. Additionally, it appears to be aggravated in people with a history of allergic conjunctivitis. For the accurate diagnosis, symptoms such as itching of the eyelids and eyes or exacerbation of a periorbital facial-skin problem are important.
5. Conclusions
This study showed the efficacy of dupilumab similar to previous clinical trials, and that the POEM is helpful to represent symptoms of AD and the quality of life. Furthermore, elevated LDH and high eosinophil count might be related to inadequate response to dupilumab treatment. There was no significant difference in treatment response at different intervals, implying that the interval can be increased during treatment. Finally, this study would be beneficial for the understanding of dupilumab treatment.
Author Contributions
D.H.J. and J.A. wrote the paper; D.H.J., H.J.J. and M.Y.P. collected and analyzed the data; S.J.H. performed statistical analysis; S.J.S. and J.A. designed this study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Trial Registration
Not applicable. We just retrospectively reviewed Electronic Medical Record (EMR).
References
- 1.Silverberg J.I., Kantor R. The Role of Interleukins 4 and/or 13 in the Pathophysiology and Treatment of Atopic Dermatitis. Dermatol. Clin. 2017;35:327–334. doi: 10.1016/j.det.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi N.A., Bennett B.L., Graham N.M.H., Pirozzi G., Stahl N., Yancopoulos G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2015;15:35–50. doi: 10.1038/nrd4624. [DOI] [PubMed] [Google Scholar]
- 3.Simpson E.L., Bieber T., Guttman-Yassky E., Beck L.A., Blauvelt A., Cork M.J., Silverberg J.I., Deleuran M., Kataoka Y., Lacour J.-P., et al. Two phase 3 Trials of Dupilumab versus Placebo in Atopic dermatitis. N. Eng. J. Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 4.De Wijs L.E.M., Bosma A.L., Erler N.S., Hollestein L.M., Gerbens L., Middelkamp-Hup M.A., Kunkeler A.C.M., Nijsten T.E., Spuls P., Hijnen D.J. Effectiveness of dupilumab treatment in 95 patients with atopic dermatitis: Daily practice data. Br. J. Dermatol. 2019;182:418–426. doi: 10.1111/bjd.18179. [DOI] [PubMed] [Google Scholar]
- 5.Olesen C., Holm J., Nørreslet L.B., Serup J., Thomsen S., Agner T. Treatment of atopic dermatitis with dupilumab: Experience from a tertiary referral centre. J. Eur. Acad. Dermatol. Venereol. 2019;33:1562–1568. doi: 10.1111/jdv.15609. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Villaverde R., Dominguez-Cruz J., Armario-Hita J.C., Martinez-Pilar L., Alcantara-Luna S., Pereyra-Rodriguez J.J. Dupilumab: Short-term effectiveness and security in real clinical practice – A retrospective multicentric study. J. Eur. Acad. Dermatol. Venereol. 2019;33:21–22. doi: 10.1111/jdv.15118. [DOI] [PubMed] [Google Scholar]
- 7.Tauber M., Apoil P.A., Richet C., Laurent J., De Bonnecaze G., Mouchon E., Cassagne M., Marguery M.C., Hegazy S., Konstantinou M.P., et al. Effect of dupilumab on atopic manifestations in patients treated for atopic dermatitis in real-life practice. Br. J. Dermatol. 2019;180:1551–1552. doi: 10.1111/bjd.17629. [DOI] [PubMed] [Google Scholar]
- 8.Hanifin J.M., Cooper K.D., Ho V.C., Kang S., Krafchik B.R., Margolis D.J., Schachner L.A., Sidbury R., Whitmore S.E., Sieck C.K., et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association “Administrative Regulations for Evidence Based Clinical Practice Guidelines”. J. Am. Acad. Dermatol. 2004;50:391–404. doi: 10.1016/j.jaad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Hanifin J.M., Thurston M., Omoto M., Cherill R., Tofte S.J., Graeber M., The Easi Evaluator Group The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. Exp. Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 10.Yosipovitch G., Reaney M., Mastey V., Eckert L., Abbé A., Nelson L., Clark M., Williams N., Chen Z., Ardeleanu M., et al. Validation of the Peak Pruritus Numerical Rating Scale. Br. J. Dermatol. 2019;181:e94. doi: 10.1111/bjd.18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spuls P., Gerbens L., Simpson E., Apfelbacher C., Chalmers J., Thomas K.S., Prinsen C.A., Von Kobyletzki L., Singh J., Williams H., et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: A Harmonising Outcome Measures for Eczema (HOME) statement. Br. J. Dermatol. 2017;176:979–984. doi: 10.1111/bjd.15179. [DOI] [PubMed] [Google Scholar]
- 12.Finlay A., Khan G. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 13.De Bruin-Weller M., Thaçi D., Smith C.H., Reich K., Cork M.J., Radin A., Zhang Q., Akinlade B., Gadkari A., Eckert L., et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: A placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ) Br. J. Dermatol. 2018;178:1083–1101. doi: 10.1111/bjd.16156. [DOI] [PubMed] [Google Scholar]
- 14.Blauvelt A., de Bruin-Weller M., Gooderham M., Cather J.C., Weisman J., Pariser D., Simpson E.L., Papp K.A., Hong H.C.-H., Rubel D., et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 15.Selene B., Zhou Z., Tao Z. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J. Clin. Cell Immunol. 2014;5:202. doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung H.J., Bae J.Y., Kim J.E., Na C.H., Park G.H., Bae Y., Shin M.K., Lee Y.B., Lee U.H., Jang Y.H., et al. Survey of disease awareness, treatment behavior and treatment satisfaction in patients with atopic dermatitis in Korea: A multicenter study. J. Dermatol. 2018;45:1172–1180. doi: 10.1111/1346-8138.14540. [DOI] [PubMed] [Google Scholar]
- 17.Revicki D.A., Willian M.K., Menter A., Saurat J.-H., Harnam N., Kaul M. Relationship between Clinical Response to Therapy and Health-Related Quality of Life Outcomes in Patients with Moderate to Severe Plaque Psoriasis. Dermatology. 2008;216:260–270. doi: 10.1159/000113150. [DOI] [PubMed] [Google Scholar]
- 18.Faiz S., Giovannelli J., Podevin C., Jachiet M., Bouaziz J.-D., Reguiai Z., Nosbaum A., Lasek A., Ferrier le Bouedec M.C., Du Thanh A., et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J. Am. Acad. Dermatol. 2019;81:143–151. doi: 10.1016/j.jaad.2019.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Dalia Y., Marchese Johnson S. Case Report: First Reported Case of Facial Rash after Dupilumab Therapy. Practical Dermatology; Wayne, PA, USA: 2018. pp. 25–26. [Google Scholar]
- 20.Albader S.S., Alharbi A.A., Alenezi R.F., Alsaif F.M. Dupilumab side effect in a patient with atopic dermatitis: A case report study. Biologics. 2019;13:79–82. doi: 10.2147/BTT.S195512. [DOI] [PMC free article] [PubMed] [Google Scholar]