Abstract
Salmonella typhimurium is one of the major bacteria responsible for gastroenteritis in humans caused by foodborne pathogens. As pork is one of the main routes of transmission, bioactive compounds used as feed additives may be an important strategy to control Salmonella typhimurium. The aim of this study was to assess the antimicrobial activity of several organic acids and nature identical compounds against Salmonella typhimurium ATCC®® 6994™. Moreover, the effect of sub-lethal concentrations of thymol and carvacrol in counteracting a Salmonella typhimurium in vitro infection on Caco-2 cells was evaluated, focusing on the maintenance of the epithelial barrier and the alteration of Salmonella virulence genes. The results showed a protective effect of the compounds on the integrity of the intestinal monolayer, improving transepithelial electrical resistance and bacterial translocation compared to the non-treated cells. A real-time PCR study highlighted a significant downregulation of the main virulence genes of Salmonella (hilA, prgH, invA, sipA, sipC, sipD, sopB, sopE2). These findings indicate that thymol and carvacrol could be good candidates for the control of Salmonella typhimurium in pigs.
Keywords: Salmonella typhimurium, tight junctions, epithelial barrier, virulence genes, nature identical compounds, thymol, carvacrol, antimicrobial activity, feed additives
1. Introduction
Salmonella typhimurium is a Gram-negative foodborne pathogen, reported as the second and third most common cause of human gastrointestinal infections in Europe and the United States, respectively [1,2]. Among European cases, 27.3% of the isolated strains were associated with matrices of porcine origin, confirming this species as one of the main pathways of transmission to humans [1,3]. S. typhimurium infection in pigs must not be underestimated, since it can cause enterocolitis, but it can also persist asymptomatically, making the pig an important reservoir of the pathogen, reducing productivity and average daily gain [4,5]. In the latter case, S. typhimurium colonizes the gastrointestinal tract of the pig in a persistent and chronic way and, during periods of stress, this asymptomatic colonization often flares up [6]. The most dangerous situations are during transport and at the slaughterhouse, where the spread of this pathogen is amplified and the contamination of food through fecal matter becomes the main route of transmission to humans [7,8].
The pathogenesis of Salmonella spp. is widely studied and it is known to be associated with Salmonella pathogenicity islands (SPI) [9]. SPI-1 and SPI-2, in particular, encode for two type III secretion systems (T3SS), a complex apparatus composed of structural, translocator and effector proteins that allow the entry and the maintenance of bacteria in the host intestinal cell [10]. Structural proteins are responsible for the assembly of the T3SS apparatus, formed by two rings crossing the inner and outer bacterial membrane, linked to a needle-like system that pierces the host cellular membrane [10,11]. The latter structure is crucial for the transport of the effector proteins, delivered by translocators from the bacterial cell into the host: here, effectors manage fundamental changes enabling key steps for Salmonella pathogenesis [12]. During the bacterial infection, Salmonella invade the enterocyte and survive into the host cytoplasm surrounded by large vesicles, called Salmonella-containing vacuoles (SCV) [13]. The disruption of the intestinal epithelial barrier, as a consequence of the disruption of tight junctions (TJ), is the main issue of the infection, giving rise to diarrhea-generating gastroenteritis. The Caco-2 cell monolayer system is a proven in vitro model for studying the structure and functionality of TJ, even during bacterial challenges [14,15]. In fact, most enteric pathogens are known to perturb the intestinal epithelial barrier, impacting transepithelial electrical resistance (TER) or paracellular permeability, and altering the arrangement of TJ with different mechanisms. It has been demonstrated that several effector proteins encoded by SPI-1, such as SipA, SopB, SopE, and SopE2, are responsible for alteration of these parameters [16].
Nowadays, it is crucial to use alternatives to antibiotics in the control of S. typhimurium. Nature identical compounds (NIC) have been widely studied for their antibacterial properties [8,17], which makes them suitable candidates as feed additives.
The aim of the study was to investigate the role of sub-lethal concentrations of thymol and carvacrol in counteracting a S. typhimurium infection of Caco-2 cells, focusing on the maintenance of the epithelial barrier and the alteration of Salmonella virulence genes.
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
The strain used in this study was the ATCC®® Salmonella typhimurium 6994™. S. typhimurium was conserved at −80 °C in Brain Heart Infusion broth (BHI) supplemented with 20% (v/v) glycerol, and it was recovered in BHI at 37 °C. The identification was carried out using Microbact™ 24E (Oxoid, Basingstoke, United Kingdom).
2.2. Chemicals and Test Solutions
Citric acid, sorbic acid, benzoic acid, butyric acid, hexanoic acid, thymol, vanillin, carvacrol, and eugenol were purchased from Alfa Aesar (Thermo Fisher GmbH, Kandel, Germany). Stock solutions of organic acids and NIC were prepared in BHI and BHI supplemented with 70% (v/v) ethanol, respectively. All solutions were buffered to pH 6.5 and filter-sterilized, then they were conserved at +4 °C and brought back to room temperature before each use.
2.3. Minimal Inhibitory Concentration of Bioactive Compounds
The minimal inhibitory concentrations (MIC) of organic acids and NIC were determined in triplicate using the broth microdilution method in 96-well microtiter plates. Citric acid, sorbic acid, benzoic acid, butyric acid, and hexanoic acid were tested at final concentrations ranging from 100 to 1.56 mM (2-fold dilutions), whereas thymol, vanillin, carvacrol, and eugenol from 7.5 to 0.12 mM (2-fold dilutions). An overnight bacterial culture of S. typhimurium was diluted to obtain an inoculum of 106 CFU/mL. For each bioactive compound, the MIC value was defined as the lowest concentration that resulted in null absorbance (630 nm) registered with Varioskan™ LUX Multimode Microplate Reader (Thermo fisher Scientific Inc., Waltham, MA, USA) after 24 h of incubation at 37 °C.
2.4. Time-Kill Curve of Thymol and Carvacrol
A time-kill assay was performed in order to evaluate the bactericidal effect of thymol and carvacrol against S. typhimurium over time. S. typhimurium, diluted at 106 CFU/mL in BHI pH 6.5 from an overnight culture, was incubated at 37 °C for 24 h without any substance (CTR), and either with thymol or carvacrol at 1.87 (MIC), 0.94, and 0.47 mM. Bacteria were counted at the inoculation time (0 h), every hour for the following 8 h, and then after 24 h of incubation. At each time-point, a 100 µl aliquot was collected from each tube and the samples were serially (10-fold) diluted in saline solution. Dilutions were then plated on BHI agar plates and incubated at 37 °C for 24 h.
2.5. Effect of Thymol and Carvacrol on Intestinal Caco-2 Cells Monolayer Integrity
The human colon adenocarcinoma cell line (Caco-2) was obtained from DSMZ (DSMZ-German Collection of Microorganisms and Cell Cultures, Leibniz Institute, Braunschweig, Germany). Caco-2 cells were seeded at a density of 1.5 × 105 cells/well onto 12 well transwell polyethylene terephthalate inserts (3.0 µm pore; Corning Incorporated, Corning, NY, USA) and were maintained at 37 °C, in an atmosphere containing 5% CO2 at 95% relative humidity, in DMEM supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids (NEAA), 1% penicillin/streptomycin (P/S) and 1% L-Glutamine. The medium was replaced every other day, both before and after cells reached confluence. Moreover, confluent cells were monitored measuring transepithelial electrical resistance (TER) at the same time as the change of medium, using an epithelial tissue voltohmmeter (Millicell ERS-2, Merck KGaA, Darmstadt, Germany).
Cells were used for bacterial challenge 28 days after the seeding on filters, once TER values were stable. On day 28, TER was measured right before the start of the challenge and the cellular medium was removed and substituted with the same medium without P/S. Cells were apically challenged with S. typhimurium in exponential phase at a concentration of 105 CFU/mL with EtOH in a concentration equivalent to that found in the treated groups (challenged control, CTR+), and with either thymol or carvacrol at 0.47 mM (n = 5). A group in the same conditions of CTR+, but without bacterial challenge, was identified as the non-challenged control (CTR−). In order to determine the intestinal monolayer integrity and the infection process, TER and bacterial translocation were examined during a 4 h challenge at 2 and 4 h. For bacterial translocation, a 100 µL aliquot of the basolateral medium was collected, serially (10-fold) diluted in saline solution and seeded on BHI agar plates in order to count how many bacteria translocated from the apical to the basolateral side of the intestinal monolayer.
2.6. Determination of Salmonella Virulence mRNA Expression
mRNA expression analyses were performed by extracting RNA from S. typhimurium cultures with thymol and carvacrol. In brief, 106 CFU/mL of S. typhimurium was incubated for 4 h at 37 °C with thymol or carvacrol at 0.47 mM. A control group with and without ethanol was also performed (EtOH; CTR). After incubation, the bacterial cultures were centrifuged for 5 min at 5000× g and the supernatants were discarded. The pellets were resuspended in 100 µL of TE buffer supplemented with 1 mg/mL of lysozyme and incubated for 10 min at 37 °C. NucleoSpin®® RNA Kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) was used for RNA extraction and purification in accordance with manufacturer’s instructions. RNA yield and quality were verified spectrophotometrically using A230, A260, and A280 nm measurements (μDrop™ Plate and Varioskan™ LUX, Thermo fisher Scientific Inc., Waltham, MA, USA). The extracted RNA was converted into cDNA using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to manufacturer’s instructions.
After cDNA synthesis, S. typhimurium virulence gene expression was determined with a real-time PCR, testing the genes listed in Table 1, using CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction contained 5 μL of 2× iTaq™ Universal SYBR®® Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA), 200 or 600 nM of each primer, 2 μL of 5 ng/μL cDNA, and nuclease-free water up to the final volume of 10 μL. The samples were analyzed under the following conditions: 3 min at 95 °C, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Specificity of each reaction was evaluated by melting-curve analysis with 0.5 °C/s heating rate from 55 up to 95 °C.
Table 1.
List of primers used for real-time PCR.
| Gene | Function | Sequence (5′ → 3′) | Product Length (bp) | Accession Number | Reference |
|---|---|---|---|---|---|
| hilA | Transcriptional regulator of the SPI-1 gene expression | F: CTGTACGGACAGGGCTATCG R: GCAGACTCTCGGATTGAACC |
130 | U25352.1 | [19] |
| prgH | Assembly of base ring structure of T3SS | F: CGCTGCGCAAAATGAAAGAG R: TTACGCGGCTCATCGAAATG |
177 | U21676 | [20] |
| invA | Assembly of needle-like structure of T3SS | F: TCTGGATGGTATGCCCGGTA R: TCATCGCACCGTCAAAGGAA |
140 | M90846.1 | This study |
| sipA | Actin rearrangement | F: GTCATTCGCGTGTGGATTCG R: TTCGGATGAAGCGTTGGTCA |
143 | U40013.1 | This study |
| sipC | Actin rearrangement | F: ACGGGCAGAATAGCGTCAAA R: ATACCCAGACTTTCCGTGGC |
150 | U25631.1 | This study |
| sipD | Translocation of effector proteins | F: ATTCCGCTTCTCCTCATCCG R: ACCGCGATGTTCTGTGGTAG |
107 | U40013.1 | This study |
| sopB | Invasion, generation, and maintenance of SCV | F: GATGGCGGCGAACCCTATAA R: GAAGACTACCAGGCGCACTT |
181 | AF213335.2 | This study |
| sopE2 | Actin rearrangement | F: GAACGCTTCTGAGGGTAGGG R: CGAGCATAGGCCGGATCTTT |
117 | AF200952.1 | This study |
| rpoD | Housekeeping | F: GTGAAATGGGCACTGTTGAACTG R: TTCCAGCAGATAGGTAATGGCTTC |
131 | NC_003197.2 | [21] |
mRNA expression was normalized using rpoD as housekeeping gene. After determining the threshold cycle (Ct) for each gene, the relative changes in mRNA expression of S. typhimurium grown with thymol and carvacrol compared to controls were calculated using the 2−ΔΔCt method [18].
2.7. Statistical Analysis
The data were analyzed with GraphPad Prism v. 8.4.1 (GraphPad Software, Inc., San Diego, CA, USA) and differences were considered significant at p ≤ 0.05. For cell culture and mRNA expression data, two-way ANOVA was performed, followed by the Tukey post hoc test for multiple comparisons.
3. Results
3.1. Thymol and Carvacrol Have the Lowest MIC among Tested Bioactive Compounds
The antimicrobial activity of organic acids and NIC against S. typhimurium are reported in Figure 1A,B, respectively. Citric and butyric acid did not inhibit S. typhimurium, while the MIC value of sorbic, benzoic, and hexanoic acid was defined as 100 mM. All the tested NIC were effective at lower concentrations than organic acids. In particular, vanillin inhibited S. typhimurium at 7.5 mM, eugenol at 3.75 mM, and both thymol and carvacrol at 1.87 mM.
Figure 1.
Salmonella typhimurium growth after 24 h in the presence of organic acids (A) or nature identical compounds (B). Bacterial growth is expressed as a percentage relative to the control (strain only).
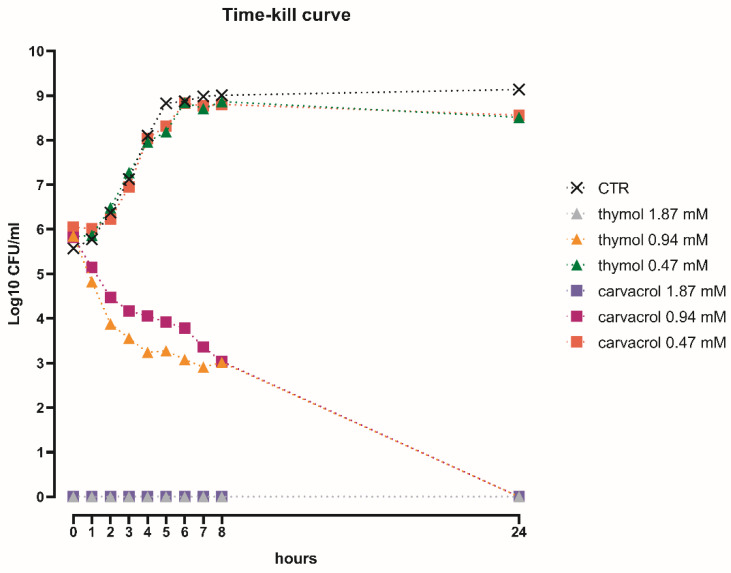
3.2. The Bactericidal Effect of Thymol and Carvacrol Is Dose-Dependent
The time-kill study highlighted a dose-dependent effect over time for both thymol and carvacrol, as shown in Figure 2. The highest concentrations tested (1.87 mM), corresponding to the MIC value defined by the microdilution method, had a strong bactericidal effect against S. typhimurium immediately after inoculation. Thymol and carvacrol at 0.94 mM gradually reduced Salmonella viability during the first 8 h, to reach a complete kill within 24 h. Finally, the two NIC at 0.47 mM did not have any direct antibacterial activity, since the growth of S. typhimurium was comparable to the control without substances.
Figure 2.
Time-kill curve of thymol or carvacrol 1.87, 0.94, and 0.47 mM against S. typhimurium.
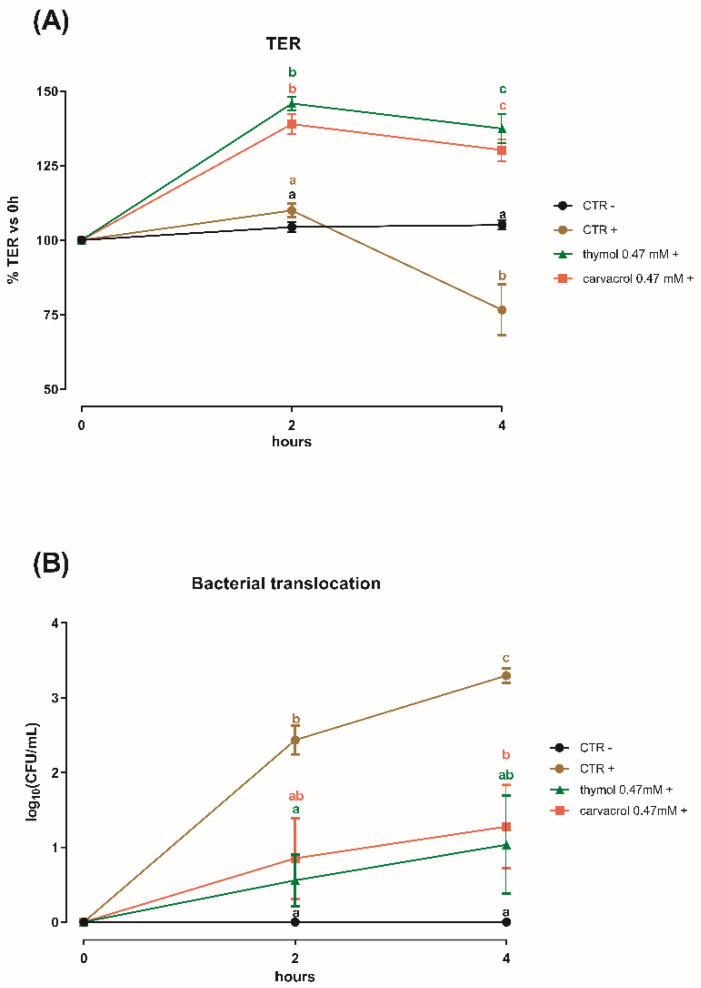
3.3. Thymol and Carvacrol at Sub-Lethal Concentrations Can Counteract a S. typhimurium Challenge on Caco-2 Cells
Results of transepithelial electrical resistance (TER) and bacterial translocation are represented in Figure 3A,B, respectively. The challenge with S. typhimurium on Caco-2 cells induced a drop in TER and an increase in bacterial translocation. After 2 h no differences were shown in the measurement of TER between the two control groups, while thymol and carvacrol increased TER by more than 30% (p < 0.0001). At 4 h post-challenge, CTR+ showed a 25% drop in TER. Cells treated with thymol or carvacrol maintained a higher TER compared to both CTR+ and CTR− (p < 0.0001).
Figure 3.
(A) Transepithelial electrical resistance (TER) and (B) bacterial translocation of Caco-2 cells cultured with ethanol (CTR−; CTR+), thymol, or carvacrol 0.47 mM post-challenge with S. typhimurium (+), or without bacterial challenge (−). Data are presented as mean ± SEM. Different letters indicate statistical significance with p < 0.05.
Differences were also observed for bacterial translocation. After 2 h, CTR+ results were significantly different compared to CTR− and the thymol treated groups (p < 0.05). At 4 h, bacteria counted in the basolateral side of both NIC treated cells were significantly lower than in the CTR+ (p < 0.05); however, thymol at 0.47 mM was comparable to the non-challenged control at both time-points.
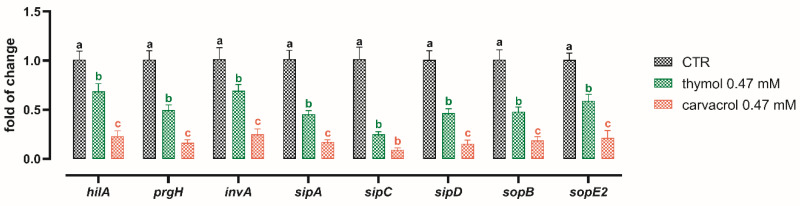
3.4. Sub-Lethal Concentrations of Thymol and Carvacrol Downregulate Virulence Genes of S. typhimurium
All of the S. typhimurium virulence genes that were investigated were downregulated by thymol and carvacrol (Figure 4). No differences were found between the two control groups (CTR and EtOH) and for this reason EtOH data are not shown. Thymol and carvacrol decreased the expression of all the genes, compared to CTR (p < 0.05). Moreover, mRNA expression was significantly lower in treatment with carvacrol than thymol, except for sipC.
Figure 4.
mRNA expression of hilA, prgH, invA, sipA, sipC, sipD, sopB, sopE2 from S. typhimurium cultures alone (CTR), and with either thymol or carvacrol 0.47 mM. Data are presented as mean ± SEM. Different letters indicate statistical significance with p < 0.05.
4. Discussion
S. typhimurium is one of the major foodborne pathogens worldwide. Since pigs are usually asymptomatic, the main concern related to swine salmonellosis is the sly transmission to humans through pork products. In order to prevent contamination of animal products at the slaughterhouse, the key is to prevent the colonization of pigs during the production cycle. In the past years, several strategies have been studied for the reduction of Salmonella prevalence in swine farms, including measures of hygiene and biosecurity, vaccine programs, and feeding practices [22]. Focusing on feeding, the main principle is the balancing of intestinal microflora in favor of beneficial bacteria, avoiding the harmful colonization of Salmonella. For this purpose, in the last decades several feed additives were adopted, as organic acids and botanicals, thanks mainly to their antimicrobial properties [23,24]. In fact, the role of organic acids in killing harmful bacteria, as well as in the alteration of Salmonella gene expression, has been investigated for many years [25]. Although herbal extracts are a relatively new approach compared to acids, the antimicrobial action of these compounds is equally powerful [26].
The approach of this study was to perform a preliminary screening of organic acids and NIC with the aim to detect the molecules with the greatest direct antimicrobial activity against S. typhimurium. This first screening revealed that NIC had stronger antimicrobial activity than organic acids, which is consistent with other reports [27,28,29], and this would offer an explanation in the theory of acid tolerance of S. typhimurium in response to low pH [30]. Then, as thymol and carvacrol were identified as the most effective molecules, they were used in a time-kill assay, with the purpose of assessing sub-inhibitory concentrations to test on cell cultures. The protective effects of thymol and carvacrol on the in vitro intestinal monolayer were verified by measuring functionality and integrity parameters (TER, bacterial translocation) of Caco-2 cells infected with S. typhimurium.
Caco-2 cells, together with other human adenocarcinoma cell lines, mimic the intestinal epithelium by polarizing on permeable filters [31]. This system is suitable for bacterial challenges that interfere with the intestinal monolayer, and it is a standardized approach for studying S. typhimurium invasivity [15]. In the model of Finlay and Falkow [15], S. typhimurium (107) was added on 10–14 day-old Caco-2 cells on 3 um filters. Authors were able to observe bacteria invading host cytoplasm enclosed in SCV by scanning and transmission electron microscopy. Moreover, consistent with our results, bacteria appeared in the basolateral medium 2 h post-challenge, while transepithelial electrical resistance (TER) dropped after 3–4 h, suggesting that our challenge worked correctly. In this study, thymol and carvacrol were able to counteract the bacterial challenge by increasing TER values even beyond the non-challenged control and maintaining the high values throughout the experiment. Transepithelial electrical resistance is an indicator of the health status and the tightness of the intestinal epithelium [32], and it is reported that many NIC have anti-inflammatory and antioxidant activity, that in turn can improve gut morphology and integrity as mediated by tight junction proteins [33]. In parallel, thymol and carvacrol also reduced bacterial translocation across the monolayer. Several studies investigated the role of carvacrol against the invasion of IPEC-J2 cells by S. typhimurium [34,35]. Inamuco et al. [34] found that carvacrol 0.5 mM significantly reduced the invasion of porcine epithelial cells, probably due to the loss of functionality of flagella. A subsequent study by Burt et al. [35] confirmed the results obtained by Inamuco et al., assuming that virulence factors could be affected by carvacrol. In a study conducted by Zhang et al. [36], thymol 0.2 mM significantly inhibited the translocation of SipA in an in vitro infection of HeLa cells. This finding indicates that the inhibition of T3SS is the primary protective effect of sub-lethal concentrations of thymol. These data are confirmed in our study, as the same concentration of thymol reduced cell invasion by reducing virulence gene expression, including SipA. Bacteria have, among their survival strategies, the flexibility of altering the mRNA expression patterns in response to environmental stress [37], as for example when cultured with non-lethal concentrations of antimicrobials. Since the mechanism of action of NIC involves the alteration of the bacterial cell membrane and induces an intracellular ATP leakage [29,38], it can be postulated that bacteria subjected to stressful sub-lethal concentrations of NIC switch their energies from motility or virulence systems to vital and essential processes, until a more favorable environment is restored [39]. Recently, it has been demonstrated that a stress induced by thymol influences the S. typhimurium proteome, downregulating genes involved in chemotaxis, motility, and virulence [20]. In addition, it is reported that these substances can affect quorum sensing of bacteria [40], which, in turn, controls virulence factor production [41,42].
It is plausible that the amelioration of TER and the reduction of bacterial translocation across Caco-2 cells observed in this study was mediated by the downregulation of a panel of several virulence genes that are involved in a complex series of reactions necessary to exploit Salmonella pathogenicity. In particular, we observed a reduction of the expression of the transcriptional activator of invasion HilA, which is the first trigger and activates genes responsible for the assembly of the T3SS structure, including prgH and invA [11]. HilA upregulation was correlated with tetracycline resistance cases [19], whereas its downregulation is attributed to several bioactive compounds such as short and medium chain fatty acids [43,44]. This would support our findings suggesting a possible cascade effect on the expression of prgH and invA. The other genes that were significantly downregulated by thymol and carvacrol are all initiators of a cascade of reactions that ultimately ends in cytoskeletal rearrangements and the disruption of TJ proteins, with a consequent increase in paracellular permeability [16,45,46]. Ultimately, this would facilitate the passage of Salmonella from the apical side to the basolateral side, thereby ending the Salmonella vicious cycle with a worsening of its pathogenicity [47].
5. Conclusions
The data from this study support a dual mechanism of action of thymol and carvacrol in ameliorating the effects associated with a S. typhimurium in vitro challenge. From the host side, thymol and carvacrol have anti-inflammatory and anti-oxidant properties that can prevent the cascade of inflammatory cytokines due to Salmonella infection, and contribute to the maintenance of the epithelial integrity, whereas from the pathogen side these molecules have direct antimicrobial properties that can inhibit the growth of Salmonella, but at the same time at sub-inhibitory concentrations they can alter a set of virulence genes that are responsible for invasion and damage to the epithelium. Since thymol and carvacrol are already widely used as feed additives, after confirming these findings also in vivo, these molecules could be helpful candidates in the control of salmonellosis in pigs.
Acknowledgments
This research was supported by a grant from Vetagro SpA.
Author Contributions
Conceptualization, E.G.; methodology, B.R. and B.T.; investigation, G.G., B.R., F.G. and A.B.; writing—original draft preparation, G.G.; writing—review and editing, E.G.; supervision, E.G and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Andrea Piva serves as a professor at the University of Bologna and is a member of the board of directors of Vetagro S.p.A. (Reggio Emilia, Italy). Ester Grilli serves as an advisor of Vetagro S.p.A.
References
- 1.EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) National Salmonella Surveillance Annual Report, 2016. US Department of Health and Human Services, CDC; Atlanta, GA, USA: 2018. [Google Scholar]
- 3.Barilli E., Bacci C., StellaVilla Z., Merialdi G., D’Incau M., Brindani F., Vismarra A. Antimicrobial resistance, biofilm synthesis and virulence genes in Salmonella isolated from pigs bred on intensive farms. Ital. J. Food Saf. 2018;7:7223. doi: 10.4081/ijfs.2018.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedorka-Cray P.J., Gray J.T., Wray C. Salmonella Infections in Pigs. In: Wray C., Wray A., editors. Salmonella in Domestic Animals. CABI Publishing; New York, NY, USA: 2000. pp. 191–208. [Google Scholar]
- 5.Farzan A., Friendship R.M. A clinical field trial to evaluate the efficacy of vaccination in controlling Salmonella infection and the association of Salmonella-shedding and weight gain in pigs. Can. J. Vet. Res. 2010;74:258–263. [PMC free article] [PubMed] [Google Scholar]
- 6.Verbrugghe E., Van Parys A., Leyman B., Boyen F., Haesebrouck F., Pasmans F. HtpG contributes to Salmonella Typhimurium intestinal persistence in pigs. Vet. Res. 2015;46:118. doi: 10.1186/s13567-015-0261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbrugghe E., Boyen F., Van Parys A., Van Deun K., Croubels S., Thompson A., Shearer N., Leyman B., Haesebrouck F., Pasmans F. Stress induced Salmonella Typhimurium recrudescence in pigs coincides with cortisol induced increased intracellular proliferation in macrophages. Vet. Res. 2011;42:118. doi: 10.1186/1297-9716-42-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grilli E., Foresti F., Tugnoli B., Fustini M., Zanoni M.G., Pasquali P., Callaway T.R., Piva A., Alborali G.L. Microencapsulated sorbic acid and pure botanicals affect Salmonella Typhimurium shedding in pigs: A close-up look from weaning to slaughter in controlled and field conditions. Foodborne Pathog. Dis. 2015;12:813–819. doi: 10.1089/fpd.2015.1953. [DOI] [PubMed] [Google Scholar]
- 9.Nieto P.A., Pardo-Roa C., Salazar-Echegarai F.J., Tobar H.E., Coronado-Arrázola I., Riedel C.A., Kalergis A.M., Bueno S.M. New insights about excisable pathogenicity islands in Salmonella and their contribution to virulence. Microbes Infect. 2016;18:302–309. doi: 10.1016/j.micinf.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Coburn B., Sekirov I., Finlay B.B. Type III Secretion Systems and Disease. Clin. Microbiol. Rev. 2007;20:535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukhan A., Kubori T., Wilson J., Galán J.E. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 2001;183:1159–1167. doi: 10.1128/JB.183.4.1159-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh Y., Saxena A., Kumar R., Kumar Saxena M. Virulence System of Salmonella with Special Reference to Salmonella enterica. In: Mascellino M.T., editor. Salmonella A Re-Emerging Pathogen. IntechOpen Limited; London, UK: 2018. pp. 41–53. [Google Scholar]
- 13.Finlay B.B., Ruschkowski S., Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J. Cell. Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 14.Cruz N., Qi L., Alvarez X., Berg R.D., Deitch E.A. The Caco-2 cell monolayer system as an in vitro model for studying bacterial-enterocyte interactions and bacterial translocation. J. Burn Care Rehabil. 1994;15:207–212. doi: 10.1097/00004630-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Finlay B.B., Falkow S. Salmonella Interactions with Polarized Human Intestinal Caco-2 Epithelial Cells. J. Infect. Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 16.Boyle E.C., Brown N.F., Finlay B.B. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell. Microbiol. 2006;8:1946–1957. doi: 10.1111/j.1462-5822.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 17.Nair D.V.T., Venkitanarayanan K., Kollanoor Johny A. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods. 2018;7:167. doi: 10.3390/foods7100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Weir E.K., Martin L.C., Poppe C., Coombes B.K., Boerlin P. Subinhibitory concentrations of tetracycline affect virulence gene expression in a multi-resistant Salmonella enterica subsp. enterica serovar Typhimurium DT104. Microbes Infect. 2008;10:901–907. doi: 10.1016/j.micinf.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Qi Y., Zhao W., Wang T., Pei F., Yue M., Li F., Liu X., Wang X., Li H. Proteomic analysis of the antimicrobial effects of sublethal concentrations of thymol on Salmonella enterica serovar Typhimurium. Appl. Microbiol. Biotechnol. 2020;104:3493–3505. doi: 10.1007/s00253-020-10390-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J.S., Corredig M., Morales-Rayas R., Hassan A., Griffiths M.W., LaPointe G. Downregulation of Salmonella Virulence Gene Expression During Invasion of Epithelial Cells Treated with Lactococcus lactis subsp. cremoris JFR1 Requires OppA. Probiotics Antimicrob. Proteins. 2019 doi: 10.1007/s12602-019-09574-1. [DOI] [PubMed] [Google Scholar]
- 22.Arguello H., Rubio P., Carvajal A. Salmonella Control Measures at Farm in Swine Production. In: Annous B., Gurtler J.B., editors. Salmonella—Distribution, Adaptation, Control Measures and Molecular Technologies. IntechOpen Limited; London, UK: 2012. pp. 99–122. [Google Scholar]
- 23.Liu Y., Espinosa C.D., Abelilla J.J., Casas G.A., Lagos L.V., Lee S.A., Kwon W.B., Mathai J.K., Navarro D.M.D.L., Jaworski N.W., et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa L.B., Luciano F.B., Miyada V.S., Gois F.D. Herbal extracts and organic acids as natural feed additives in pig diets. S. Afr. J. Anim. Sci. 2013;43:181–193. [Google Scholar]
- 25.Van Immerseel F., Russell J.B., Flythe M.D., Gantois I., Timbermont L., Pasmans F., Haesebrouck F., Ducatelle R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- 26.Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- 27.Gómez-García M., Sol C., De Nova P.J.G., Puyalto M., Mesas L., Puente H., Mencía-Ares Ó., Miranda R., Argüello H., Rubio P., et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porc. Health Manag. 2019;5:32. doi: 10.1186/s40813-019-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guillier L., Nazer A.I., Dubois-Brissonnet F. Growth response of Salmonella typhimurium in the presence of natural and synthetic antimicrobials: Estimation of MICs from three different models. J. Food Prot. 2007;70:2243–2250. doi: 10.4315/0362-028X-70.10.2243. [DOI] [PubMed] [Google Scholar]
- 29.Helander I.M., Alakomi H.-L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E.J., Gorris L.G.M., Von Wright A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 30.Bearson S., Bearson B., Foster J.W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997;147:173–180. doi: 10.1111/j.1574-6968.1997.tb10238.x. [DOI] [PubMed] [Google Scholar]
- 31.Pinto M., Robineleon S., Appay M.D., Kedinger M., Triadou N., Dussaulx E., Lacroix B., Simonassmann P., Haffen K., Fogh J., et al. Enterocyte-Like Differentiation and Polarization of the Human-Colon Carcinoma Cell-Line Caco-2 in Culture. [(accessed on 19 May 2020)];Biol. Cell. 1983 Available online: https://www.scienceopen.com/document?vid=10c4912d-182f-4361-98a7-50c52d5b3617. [Google Scholar]
- 32.Srinivasan B., Kolli A.R., Esch M.B., Abaci H.E., Shuler M.L., Hickman J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015;20:107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi B., Toschi A., Piva A., Grilli E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr. Res. Rev. 2020:1–17. doi: 10.1017/S0954422420000013. [DOI] [PubMed] [Google Scholar]
- 34.Inamuco J., Veenendaal A., Burt S., Post J., Bokhoven J., Haagsman H., Veldhuizen E. Sub-lethal levels of carvacrol reduce Salmonella Typhimurium motility and invasion of porcine epithelial cells. Vet. Microbiol. 2011;157:200–207. doi: 10.1016/j.vetmic.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Burt S.A., Adolfse S.J.M., Ahad D.S.A., Tersteeg-Zijderveld M.H.G., Jongerius-Gortemaker B.G.M., Post J.A., Brüggemann H., Santos R.R. Cinnamaldehyde, Carvacrol and Organic Acids Affect Gene Expression of Selected Oxidative Stress and Inflammation Markers in IPEC-J2 Cells Exposed to Salmonella typhimurium. Phytother. Res. 2016;30:1988–2000. doi: 10.1002/ptr.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Liu Y., Qiu J., Luo Z.-Q., Deng X. The Herbal Compound Thymol Protects Mice From Lethal Infection by Salmonella Typhimurium. Front Microbiol. 2018;9:1022. doi: 10.3389/fmicb.2018.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grantcharova N., Peters V., Monteiro C., Zakikhany K., Römling U. Bistable Expression of CsgD in Biofilm Development of Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2009;192:456–466. doi: 10.1128/JB.01826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chouhan S., Sharma K., Guleria S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan W., Yuk H.-G. Effects of Sublethal Thymol, Carvacrol, and trans-Cinnamaldehyde Adaptation on Virulence Properties of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2019;85:1–11. doi: 10.1128/AEM.00271-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazzaro F., Fratianni F., D’Acierno A., Coppola R., Ayala-Zavala F.J., Da Cruz A.G., Feo V.D. Essential Oils and Microbial Communication. In: El-Shemy H., editor. Essential Oils—Oils of Nature. IntechOpen Limited; London, UK: 2019. pp. 1–26. [Google Scholar]
- 41.Rutherford S.T., Bassler B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect Med. 2012;2:1–25. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi J., Shin D., Ryu S. Implication of Quorum Sensing in Salmonella enterica Serovar Typhimurium Virulence: The luxS Gene Is Necessary for Expression of Genes in Pathogenicity Island 1. Infect Immun. 2007;75:4885–4890. doi: 10.1128/IAI.01942-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Hautefort I., Thompson A., Hinton J.C., Immerseel F.V. Butyrate Specifically Down-Regulates Salmonella Pathogenicity Island 1 Gene Expression. Appl. Environ. Microbiol. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyen F., Haesebrouck F., Vanparys A., Volf J., Mahu M., Van Immerseel F., Rychlik I., Dewulf J., Ducatelle R., Pasmans F. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet. Microbiol. 2008;132:319–327. doi: 10.1016/j.vetmic.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Hallstrom K., McCormick B.A. Salmonella Interaction with and Passage through the Intestinal Mucosa: Through the Lens of the Organism. Front. Microbiol. 2011;2:1–10. doi: 10.3389/fmicb.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tafazoli F., Magnusson K.-E., Zheng L. Disruption of Epithelial Barrier Integrity by Salmonella enterica Serovar Typhimurium Requires Geranylgeranylated Proteins. Infect Immun. 2003;71:872–881. doi: 10.1128/IAI.71.2.872-881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Köhler H., Sakaguchi T., Hurley B.P., Kase B.A., Kase B.J., Reinecker H.-C., McCormick B.A. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G178–G187. doi: 10.1152/ajpgi.00535.2006. [DOI] [PubMed] [Google Scholar]