Abstract
Procedures such as dry needling (DN) or percutaneous electrical nerve stimulation (PENS) are commonly proposed for the treatment of myofascial trigger points (MTrP). The aim of the present study is to investigate if PENS is more effective than DN in the short term in subjects with mechanical neck pain. This was an evaluator-blinded randomized controlled trial. Subjects were recruited through announcements and randomly allocated into DN or PENS groups. Pain intensity, disability, pressure pain threshold (PPT), range of motion (ROM), and side-bending strength were measured. The analyses included mixed-model analyses of variance and pairwise comparisons with Bonferroni correction. The final sample was composed of 44 subjects (22 per group). Both groups showed improvements in pain intensity (ηp2 = 0.62; p < 0.01), disability (ηp2 = 0.74; p < 0.01), PPT (ηp2 = 0.79; p < 0.01), and strength (ηp2 = 0.37; p < 0.01). The PENS group showed greater improvements in disability (mean difference, 3.27; 95% CI, 0.27–6.27) and PPT (mean difference, 0.88–1.35; p < 0.01). Mixed results were obtained for ROM. PENS seems to produce greater improvements in PPT and disability in the short term.
Keywords: neck pain, PENS, dry needling, myofascial trigger point, physiotherapy
1. Introduction
Dry needling (DN) is a therapeutic intervention in which thin needles are used to penetrate the skin to affect the underlying connective tissue and neuromuscular system without the use of any medication [1]. DN techniques can be sorted according to the depth that the needle is inserted [2]. Although in recent years it has been associated with other musculoskeletal problems, most commonly, DN is designed for the treatment of myofascial trigger points (MTrPs) [3,4]. An MTrP is a discrete, hyperirritable nodule in a taut band of skeletal muscle which is palpable and tender during physical examination [5]. A MTrP may be related to motor dysfunction, fatigue, weakness of the affected muscle, and restricted range of mobility [6]. These hyperirritable nodules can be classified as active, in which case the MTrP is clinically associated with spontaneous pain and, when palpated, reproduces a patient’s familiar pain. They may also be latent MTrPs. Latent MTrPs do not produce spontaneous pain unless they are encouraged [6,7]. DN has been shown to increase the pressure pain threshold (PPT) [8] and range of movement (ROM) [9]; to improve function [10]; and to decrease pain in patients with patellofemoral pain syndrome [10], hip osteoarthritis [11], low-back pain [12], and piriformis syndrome [13].
The possible physiological effects of DN are still not completely understood [14]. It is proposed that DN influences pain by affecting the biochemical environment of the MTrP, reducing the concentration of substance P and calcitonin gene-related peptide surrounding an MTrP [14,15] as well as increasing beta-endorphin in local tissue. In addition, DN would appear to activate different inhibitory pain pathways [16].
The dry needling technique is often non-standardized and may include adjunct treatments, such as electrotherapy. The joint application of DN and electrotherapy has been given numerous names, including electroacupuncture [17], intramuscular electrical stimulation [18], or electrical dry needling [19]. These electric stimulation techniques include the passage of an electrical current through the needle [20]. One of the most utilized techniques to treat MTrPs is percutaneous electrical nerve stimulation (PENS) [20]. PENS is the application of a low-intensity current through an acupuncture needle [19,21]. Although the neuroplasticity processes involved in its effect are not yet well understood, it is a technique of electroacupuncture used to modulate pain processing in some chronic pain conditions, such as myofascial pain syndromes and chronic tensional headache [22,23]. One of the possible mechanisms involved in its effectiveness is a decrease in the excitatory nervous system and a stimulation of the inhibitory nervous system [23,24]. The electrostimulation causes a reduction in the excitability of the motor cortex as well as a decrease in the facilitation of transmission in corticospinal neurons [25]. PENS application to MTrPs would stimulate muscle nociceptors, which activates the endogenous antinociceptive system [21,26]. Several forms of application have been described depending on where the needles are inserted [19,20,27].
Current therapeutic interventions for myofascial pain aim to disrupt the maintenance of a contracted state within the MTrP by inhibiting spinal facilitation mechanisms and by increasing blood perfusion to the muscle region [20]. Interventions for treating myofascial pain include dry needling and electrotherapeutic modalities such as PENS [9,20]. These procedures are often used in a clinical setting, and they have been recommended for myofascial pain treatment [1]. The use of DN intervention with electrical stimulation has shown to be effective in the treatment of low-back pain [28] and thoracic spine pain [29]. To our knowledge, no previous studies have investigated whether the PENS application is more effective when a pair of needle electrodes are applied into the MTrP zone in subjects with neck pain. We expect that the local application of PENS electric current to the MTrP is more effective than DN.
The aim of our study was to investigate if PENS application via insertion of a pair of needles into the MTrP of the levator scapulae muscle is more effective that DN for treating neck pain in the short term.
2. Experimental Section
2.1. Study Design
This randomized controlled trial was conducted according to the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement [30].
The protocol of the study was registered at clinicaltrials.gov (NCT03025230). Ethical approval was obtained from the Ethical Committee of the University of Alcala (Madrid, Spain, M2012/19/20130409). The study was conducted according to the Declaration of Helsinki.
2.2. Subjects
A convenience sample of subjects with neck pain was recruited through announcements at the city of Alcala. Patients were asked for their medical history to obtain information regarding some of the inclusion and exclusion criteria.
The inclusion criteria were age 18 years or older, nontraumatic unilateral mechanical neck pain of three or more months of duration, using a computer 9 or more hours per week, and presenting an active MTrP in the levator scapulae muscle on the painful side.
The exclusion criteria were radiated pain towards the arm, psychological disorders (mood and psychotic disorders such as schizophrenia, depression, and anxiety disorders), whiplash injury, previous surgical procedures in the cervical spine or shoulder, neuropathic symptoms, received physiotherapy treatment in the previous month, taking anticoagulant or antiplatelet drugs, fear of needles, and any other contraindication to dry needling procedures.
All the subjects signed a consent form before participating in the study. The following demographic data were collected: sex, age, height, weight, body mass index, symptomatic side, and hours per week of computer use.
2.3. Sample Size
The sample size calculation was conducted with the software GPower 3.0.1 (Universität Düsseldorf, Düsseldorf, Germany). It was based on the time-by-group interaction of a 2-by-4 mixed model analysis of variance (ANOVA). The effect size was estimated to be 0.25 with a correlation between repeated measures of 0.50, sphericity correction of 0.75, 90% power, and α value of 0.05. According to the sample size calculation, 36 subjects needed to be recruited. Assuming a maximum dropout rate of 20%, the final sample size was composed of 44 subjects (22 per group).
2.4. Measurements
All measurements were carried out in the Faculty of Physiotherapy of the University of Alcala. Measurements were made by a physiotherapist with more than 10 years of experience who was blinded regarding treatment group allocation. Patients were scheduled in a specific time so as not to coincide and were told not to speak with the assessor about his/her clinical status or received treatment.
Pain intensity, pressure pain threshold (PPT), side-bending strength, and range of movement were registered at baseline, posttreatment, 48 h after intervention, and at one-week follow-up. Disability was recorded at baseline and at one-week follow-up.
2.4.1. Pain and Disability
Pain intensity at the moment of measurement was measured with a visual analogue scale (VAS), where 0 represents no pain and 10 represents the worst pain imaginable. The VAS has been shown to be a valid and reliable tool (intraclass correlation coefficient (ICC) 0.71–0.99) [31,32].
Degree of disability related to the cervical spine was measured with the Neck Disability Index (NDI) questionnaire that was transculturally adapted from English into Spanish (Spain) in 2010. The NDI has shown good reliability (ICC 0.98) [33]. The questionnaire points range from 0 (no disability) to 50 (maximum degree of disability).
2.4.2. Myofascial Trigger Point Diagnosis
The presence of active MTrP in the levator scapulae muscle was tested with the patient lying on the contralateral side before the assignment to interventions, as active MTrP presence was an inclusion criterion. An active MTrP was manually diagnosed if these criteria were present: taut band, hypersensitive spot, local twitch response, and reproduction of the patient’s symptoms [34,35,36]. The levator scapulae is located at the floor of the posterior cervical triangle and commonly develops MTrPs in a central area at the angle of the neck, where the muscle emerges from beneath the anterior border of the upper trapezius muscle. The levator scapulae taut band and MTrP can be palpated with a cross-fiber flat palpation. The local twitch response, which is a local involuntary spontaneous contraction of the muscle evoked when an MTrP is mechanically stimulated, can be produced in the levator scapulae muscle by cross-fiber flat palpation perpendicular to the muscle fiber direction.
After the active MTrP was identified, the physiotherapist marked its location with a permanent and indelible marker and measured the distance from it to the mastoid apophysis with a caliper [37]. Patients were told not to clean the mark during the entire study period. In addition, the distance between the mastoid apophysis (bone reference) and MTrP was measured to ensure that follow-up measurements were all taken at the same point.
2.4.3. Range of Motion of the Cervical Spine
Range of motion of the cervical spine was measured in a sitting position with a cervical goniometer system (CROM; Performance Attainment Associates, St Paul, MN). The CROM is based on thee goniometers and a magnet system; this tool has been shown to be valid and to have good reliability in healthy and painful subjects (ICC > 0.90) [38].
The following movements were measured: flexion, extension, right and left rotation, and right and left side-bending. Three measurements were taken for each movement, and their mean was used for statistical analysis.
2.4.4. Pressure Pain Threshold
Pressure pain threshold was measured at the location of the active MTrP in the levator scapulae muscle. It was measured with a manual algometer (Wagner Force Dial, Model FDK 20, Wagner Instruments, Burien, WA, USA), which has a 1 cm2 head that records pressure in kg/cm2. This procedure has shown good reliability in patients with neck pain (ICC = 0.76–0.97) [39].
The pressure was increased by 1 kg per second, and the subject was told to indicate when their sensation changed from pressure to pain. Three measurements were taken with a 30-s rest period between them. The mean of the three measurements was used for statistical analysis.
2.4.5. Side-Bending Strength
The strength measurements were performed with a handheld dynamometer (Model microFET 2TM, Hoggan Health Industries Inc., West Jordan, UT, USA), which has been shown to have good reliability in the measurement of cervical side-bending strength (ICC = 0.87–0.95) [40].
The patient was placed lying supine with knees flexed and relaxed in a wedge. The dynamometer was placed on the temporal bone of the painful side. The patient was told to push their head towards the dynamometer and the physiotherapist resisted the movement, so the patient produced an isometric contraction for 5 seconds. Three measurements were taken with a 30-second rest between them, and their mean was used for statistical analysis.
2.5. Interventions
Subjects were randomly allocated into intervention groups based on a randomization sequence generated with the software Epidat 3.1 (Conselleria de Sanidade, Xunta de Galicia, Galicia, Spain) prior to the beginning of the study. The allocation was concealed with sequentially numbered sealed envelopes. The group allocation was revealed to the therapist after the initial assessment when the therapist opened the envelope without telling the patient what treatment he/she was going to receive. Before MTrP dry needling, the physiotherapist performed handwashing before putting on nitrile gloves to disinfect the treatment area with cotton and skin antiseptic. Participants were told to maintain their activity level and exercise practice as usual, but they were told not to use analgesic or anti-inflammatory drugs during the experimental period.
2.5.1. Dry Needling
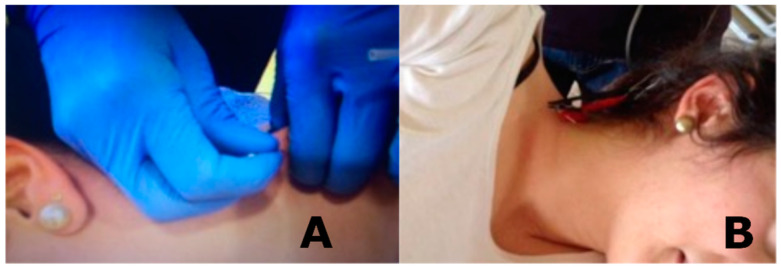
The control group was treated with deep DN (Figure 1) with a needle with a 2.5-cm length and 0.25-cm diameter. The technique was performed in the location of the active MTrP.
Figure 1.
Applied interventions: (A) dry needling and (B) percutaneous electrical nerve stimulation.
Patients were placed lying on the contralateral side. The physiotherapist firmly held the levator scapulae muscle in a pincer grasp to precisely locate the MTrP between his first and second fingers. The therapist directed the needle in an anteroposterior direction towards his finger. Then, the needle was directed upward across the muscle mass as it was held by the fingers to avoid any possibility of penetrating the lung and of causing a pneumothorax [41]. The needle was moved up and down within the muscle, using a “fast-in and fast-out” technique as described by Hong [42]. Needle insertions were repeated 8 to 10 times [43]. After the needling procedure, the therapist compressed the point of needling with cotton wool for 90 seconds.
2.5.2. Percutaneous Electrical Nerve Stimulation
The experimental group was treated with PENS (Figure 1) with the patient placed in the same position as the control group. The experimental group received the same DN protocol in the levator scapulae muscle, but two needles were inserted in the MTrP. After needle insertions were repeated 8 to 10 times, the needles were kept in the muscle. The needles were separated by 1.5 cm [7]
An electrostimulation system was connected to the needles with two crocodile electrical clamps. PENS was applied immediately after, using a portable Transcutaneous Electrical Nerve Stimulation (TENS) (Model P82&S82, Enraf-Nonius, Rotterdam, Netherlands) with an analgesic rectangular, biphasic, and asymmetric current at a 2-Hz frequency with a pulse width of 100 µs [44].
TENS intensity was selected according to the patient’s tolerance and never exceeded 3 mA to avoid tissue injury. For establishing patient’s tolerance, the patient was told before treatment started to tell the therapist when the TENS intensity was so uncomfortable that he or she could not stay at that level of intensity for 1 minute. The TENS was left connected for 20 minutes. After the PENS procedure, the therapist compressed the point of needling with cotton wool for 90 seconds.
2.6. Statistical Analysis
Data normality was evaluated with the Shapiro–Wilk test. For the descriptive analysis of quantitative variables, the mean and standard deviation (SD) were used. For categorical variables, the absolute frequencies and percentages were calculated. The homogeneity of the groups was analyzed with the Student’s t-test and the chi-squared test [45].
For the differences between groups in pain intensity, PPT, strength, flexion, and extension range of motion, 2-by-4 mixed ANOVAs were conducted with time (baseline, post-treatment, 48 h, and 1 week) as the within-subjects’ factor and treatment (DN and PENS) as the between-subjects’ factor. For the differences between groups in disability, a 2-by-2 mixed model ANOVA was conducted with time (baseline and 1 week) as the within-subjects’ factor and treatment as the between-subjects’ factor. For the differences between groups in cervical rotation and side-bending range of motion, 2-by-2-by-4 mixed ANOVAs were conducted with time and side (painful and nonpainful) as within-subjects’ factors and treatment as the between-subjects factor. Post hoc pairwise comparisons were analyzed using the Student’s t-test with Bonferroni correction [45].
The partial eta squared (ηp2) was used as an estimator of the effect size of the main effects and interactions of the ANOVAs [45].
All the analyses were conducted using the statistical software SPSS V.22 (SPSS Inc., Chicago, IL, USA). An α level of 0.05 and 95% confidence intervals (CI) were assumed for all analyses.
3. Results
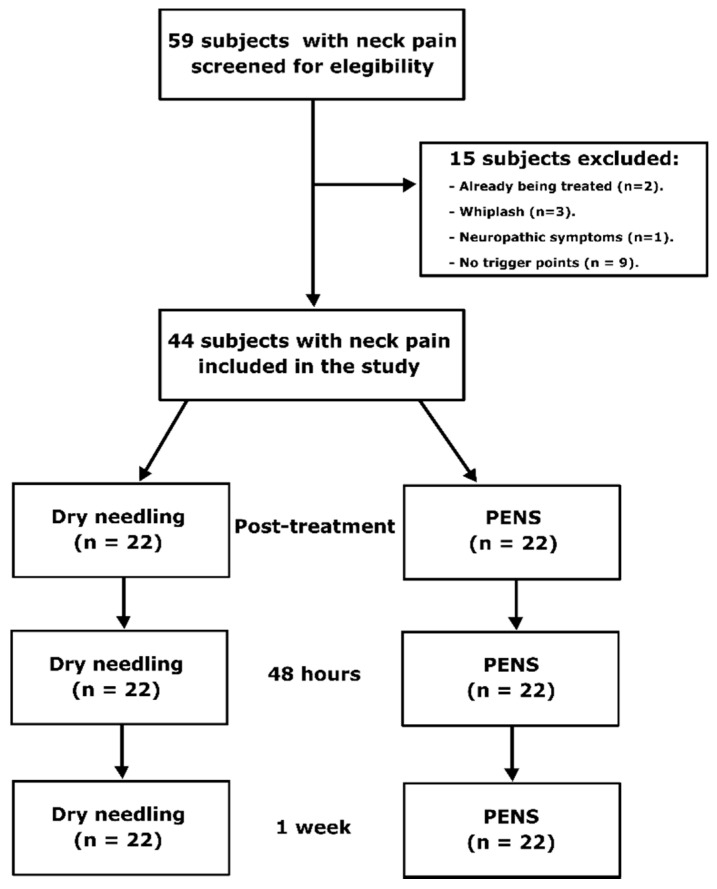
The final sample was composed of 44 subjects (Figure 2), 22 subjects in the DN group (mean age, 25.45; SD, 8.53) and 22 subjects in the PENS group (mean age, 24.14; SD, 9.39). Demographic and baseline characteristics of the subjects are presented in Table 1.
Figure 2.
Flow diagram of subjects. PENS, percutaneous electrical nerve stimulation.
Table 1.
Baseline characteristics of the subjects (n = 44).
| Characteristic, Mean (SD) | DN (n = 22) | PENS (n = 22) | p-Value |
|---|---|---|---|
| Age, years | 25.45 (8.53) | 24.14 (9.39) | 0.63 |
| Height, cm | 171.73 (8.86) | 171.59 (6.60) | 0.95 |
| Weight, kg | 65.42 (10.79) | 63.49 (8.58) | 0.51 |
| BMI, kg/m2 | 21.64 (2.67) | 21.60 (2.02) | 0.95 |
| Computer Use, hours/week | 15.77 (10.82) | 16.92 (7.84) | 0.69 |
| MTrP Distance, cm | 8.12 (1.07) | 8.08 (1.13) | 0.93 |
| VAS, cm | 5.01 (1.52) | 4.56 (1.73) | 0.36 |
| NDI | 19.64 (7.32) | 22.09 (9.75) | 0.35 |
| PPT, kg/cm2 | 1.98 (0.56) | 2.11 (0.52) | 0.43 |
| Strength, N | 145.20 (28.70) | 152.40 (32.20) | 0.44 |
| Flexion, Degrees | 44.83 (9.85) | 44.59 (11.25) | 0.94 |
| Extension, Degrees | 50.72 (9.72) | 52.81 (13.90) | 0.57 |
| Rotation, Degrees | |||
| Painful Side | 58.21 (15.46) | 61.59 (10.50) | 0.40 |
| Nonpainful Side | 52.18 (12.78) | 59.47 (14.47) | 0.08 |
| Side Bending, Degrees | |||
| Painful Side | 38.59 (5.81) | 38.50 (5.07) | 0.96 |
| Nonpainful Side | 34.30 (5.31) | 37.45 (3.65) | 0.03 |
| Sex, n (%) | 0.53 | ||
| Male | 9 (40.9) | 7 (31.8) | |
| Female | 13 (59.1) | 15 (68.2) | |
| Painful Side, n (%) | 0.07 | ||
| Right | 16 (72.7) | 10 (45.5) | |
| Left | 6 (27.3) | 12 (54.5) |
Abbreviations: SD, standard deviation; DN, dry needling; PENS, percutaneous electrical nerve stimulation; BMI, body mass index; h, hours; w, week; MTrP, myofascial trigger point; VAS, visual analogue scale; NDI, Neck Disability Index; PPT, pressure pain threshold.
3.1. Pain and Disability
The 2-by-4 mixed ANOVA for pain intensity revealed a significant main effect for time (F = 68.00; p < 0.01; ηp2 = 0.62) but not for group (F = 1.33; p = 0.26; ηp2 = 0.03). There was a nonsignificant time-by-group interaction (F = 0.37; p = 0.67; ηp2 = 0.01).
The 2-by-2 mixed ANOVA for disability revealed a significant main effect for time (F = 121.67; p < 0.01; ηp2 = 0.74) but not for group (F = 0.12; p = 0.73; ηp2 = 0.003). There was a significant time-by-group interaction (F = 4.87; p = 0.03; ηp2 = 0.10). Post hoc comparisons are presented in Table 2.
Table 2.
Pain and disability differences within- and between-group.
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| VAS, cm | ||||
| DN, Mean (SD) | 5.01 (1.52) | 3.30 (1.70) | 2.28 (1.58) | 2.26 (1.55) |
| PENS, Mean (SD) | 4.56 (1.73) | 2.62 (2.11) | 2.04 (1.69) | 1.71 (1.29) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 1.71 (1.02, 2.40) ‡ | 2.73 (1.83, 3.64) ‡ | 2.76 (1.86, 3.65) ‡ | |
| PENS | 1.94 (1.25, 2.63) ‡ | 2.52 (1.61, 3.42) ‡ | 2.85 (1.96, 3.74) ‡ | |
| Between-Group Differences, Mean (95% CI) | −0.23 (−0.94, 0.48) | 0.22 (−0.72, 1.15) | −0.09 (−1.01, 0.83) | |
| NDI | ||||
| DN, Mean (SD) | 19.64 (7.32) | - | - | 13.09 (7.45) |
| PENS, Mean (SD) | 22.09 (9.75) | - | - | 12.27 (8.20) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 6.55 (4.43, 8.66) ‡ | |||
| PENS | 9.82 (7.70, 11.94) ‡ | |||
| Between-Group Differences, Mean (95% CI) | −3.27 (−6.27, −0.27) † |
† Statistically significant (p < 0.05), ‡ Statistically significant (p < 0.01). Abbreviations: VAS, visual analogue scale; DN, dry needling; SD, standard deviation; PENS, percutaneous electrical nerve stimulation; CI, confidence interval; NDI, Neck Disability Index.
3.2. Pressure Pain Threshold
The 2-by-2 mixed ANOVA for PPT revealed a significant main effect for time (F = 154.97; p < 0.01; ηp2 = 0.79) and group (F = 60.89; p < 0.01; ηp2 = 0.59) as well as a significant time-by-group interaction (F = 31.07; p < 0.01; ηp2 = 0.43). Post hoc comparisons are presented in Table 3.
Table 3.
Pressure pain threshold and strength differences within- and between-group.
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| PPT, kg/cm2 | ||||
| DN, Mean (SD) | 1.98 (0.56) | 2.71 (0.52) | 2.72 (0.46) | 2.70 (0.52) |
| PENS, Mean (SD) | 2.11 (0.52) | 3.71 (0.46) | 3.84 (0.43) | 4.18 (0.46) |
| Within-Group Differences from Baseline, Mean (95% CI) |
||||
| DN | 0.73 (0.44, 1.01) ‡ | 0.74 (0.47, 1.01) ‡ | 0.72 (0.39, 1.04) ‡ | |
| PENS | 1.61 (1.32, 1.89) ‡ | 1.73 (1.45, 2.00) ‡ | 2.06 (1.74, 2.39) ‡ | |
| Between-Group Differences, Mean (95% CI) | 0.88 (0.58, 1.17) ‡ | 0.99 (0.71, 1.27) ‡ | 1.35 (1.01, 1.68) ‡ | |
| Strength, N | ||||
| DN, Mean (SD) | 145.30 (28.70) | 155.30 (28.80) | 163.20 (33.00) | 173.00 (37.10) |
| PENS, Mean (SD) | 152.40 (32.20) | 162.70 (32.70) | 167.10 (32.90) | 173.90 (33.60) |
| Within-Group Differences from Baseline, Mean (95% CI) |
||||
| DN | 10.00 (−2.80, 22.80) |
18.00 (6.20, 29.80) ‡ |
27.80 (15.30, 40.30) ‡ |
|
| PENS | 10.20 (−2.60, 23.00) |
14.70 (2.90, 26.50) ‡ |
21.50 (9.00, 34.00) ‡ |
|
| Between-Group Differences, Mean (95% CI) | 0.20 (−13.00, 13.40) |
−3.30 (−15.40, 8.90) |
−6.30 (−19.20, 6.60) |
† Statistically significant (p < 0.05), ‡ Statistically significant (p < 0.01). Abbreviations: PPT, pressure pain threshold; DN, dry needling; SD, standard deviation; PENS, percutaneous electrical nerve stimulation; CI, confidence interval.
3.3. Side-Bending Strength
The 2-by-4 mixed ANOVA for strength revealed a significant main effect for time (F = 24.84; p < 0.01; ηp2 = 0.37) but not for group (F = 0.29; p = 0.59; ηp2 = 0.01). There was a nonsignificant time-by-group interaction (F = 0.55; p = 0.65; ηp2 = 0.01). Post hoc comparisons are presented in Table 3.
3.4. Range of Motion of the Cervical Spine
The 2-by-4 mixed ANOVA for cervical flexion range of motion revealed a significant main effect for time (F = 9.94; p < 0.01; ηp2 = 0.19) and group (F = 4.16; p = 0.048; ηp2 = 0.09) as well as a significant time-by-group interaction (F = 3.25; p = 0.046; ηp2 = 0.07). Post hoc comparisons are presented in Table 4.
Table 4.
Cervical flexion and extension range of movement differences within- and between-group.
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| Cervical Flexion, Degrees | ||||
| DN, Mean (SD) | 44.83 (9.85) | 45.90 (8.61) | 48.36 (9.31) | 47.27 (8.41) |
| PENS, Mean (SD) | 44.59 (11.25) | 52.95 (7.50) | 54.73 (6.39) | 51.27 (7.59) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 1.08 (−4.25, 6.41) | 3.53 (−3.35, 10.32) | 2.44 (−3.74, 8.62) | |
| PENS | 8.36 (3.04, 13.69) ‡ |
10.14 (3.35, 16.92) ‡ | 6.68 (0.50, 12.86) † | |
| Between-Group Differences, Mean (95% CI) | 7.29 (1.79, 12.78) † |
6.60 (−0.39, 13.60) | 4.24 (−2.13, 10.61) | |
| Cervical Extension, Degrees | ||||
| DN, Mean (SD) | 50.72 (9.72) | 53.50 (9.86) | 57.32 (7.49) | 58.05 (7.95) |
| PENS, Mean (SD) | 52.81 (13.90) | 58.41 (10.14) | 56.64 (11.37) | 57.68 (11.26) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 2.78 (−1.09, 6.65) | 6.60 (1.00, 12.19) † | 7.33 (2.11, 12.54) ‡ | |
| PENS | 5.59 (1.72, 9.46) ‡ | 3.82 (−1.77, 9.41) | 4.86 (−0.36, 10.08) | |
| Between-Group Differences, Mean (95% CI) | 2.81 (−1.18, 6.80) | −2.78 (−8.54, 2.98) | −2.46 (−2.92, 7.84) |
† Statistically significant (p < 0.05), ‡ Statistically significant (p < 0.01). Abbreviations: DN, dry needling; SD, standard deviation; PENS, percutaneous electrical nerve stimulation; CI, confidence interval.
The 2-by-4 mixed ANOVA for cervical extension range of motion revealed a significant main effect for time (F = 10.55; p < 0.01; ηp2 = 0.20) but not for group (F = 2.44; p = 0.07; ηp2 = 0.06). There was a nonsignificant time-by-group interaction (F = 0.29; p = 0.60; ηp2 = 0.01). Post hoc comparisons are presented in Table 4.
The 2-by-2-by-4 mixed ANOVA for cervical rotation range of motion revealed a significant main effect for time (F = 17.53; p < 0.01; ηp2 = 0.57) but not for group (F = 4.07; p = 0.05; ηp2 = 0.09) or side (F = 1.19; p = 0.28; ηp2 = 0.03). There were no significant time-by-group (F = 0.09; p = 0.97; ηp2 = 0.01), time-by-side (F = 1.34; p = 0.27; ηp2 = 0.09), group-by-side (F = 0.03; p = 0.87; ηp2 = 0.001), or time-by-group-by-side (F = 1.35; p = 0.27; ηp2 = 0.09) interactions. Post hoc comparisons are presented in Table 5.
Table 5.
Cervical rotation range of movement differences within- and between-group.
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| DN, Mean (SD) | ||||
| Painful Side | 58.21 (14.46) | 64.41 (8.92) | 68.09 (9.32) | 66.50 (8.95) |
| Nonpainful Side | 52.18 (12.78) | 64.5 (8.95) | 66.27 (12.69) | 66.14 (10.67) |
| PENS, Mean (SD) | ||||
| Painful Side | 61.59 (10.50) | 69.55 (9.38) | 71.50 (8.02) | 70.55 (7.32) |
| Nonpainful Side | 59.48 (14.47) | 67.68 (9.36) | 70.41 (9.02) | 69.68 (9.52) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | ||||
| Painful Side | 6.20 (−0.84, 13.25) | 9.88 (2.15, 17.62) ‡ | 8.29 (0.59, 16.00) † | |
| Nonpainful Side | 12.32 (6.85, 17.80) ‡ | 14.10 (7.52, 20.68) ‡ | 13.96 (7.18, 20.74) ‡ | |
| PENS | ||||
| Painful Side | 7.96 (0.91, 15.00) † | 9.91 (2.18, 17.64) ‡ | 8.96 (1.25, 16.66) † | |
| Nonpainful Side | 8.21 (2.73, 13.68) ‡ | 10.93 (4.35, 17.51) ‡ | 10.21 (3.43, 16.98) ‡ | |
| Between-Group Differences, Mean (95% CI) | ||||
| Painful Side | 1.75 (−5.51, 9.01) | 0.03 (−7.95, 8.00) | 0.66 (−7.28, 8.61) | |
| Nonpainful Side | −4.12 (−9.76, 1.52) | −3.16 (−9.95, 3.62) | −3.76 (−10.74, 3.23) |
† Statistically significant (p < 0.05), ‡ Statistically significant (p < 0.01). Abbreviations: DN, dry needling; SD, standard deviation; PENS, percutaneous electrical nerve stimulation; CI, confidence interval.
The 2-by-2-by-4 mixed ANOVA for cervical side-bending range of motion revealed a significant main effect for time (F = 27.02; p < 0.01; ηp2 = 0.39) but not for group (F = 0.95; p = 0.34; ηp2 = 0.02) or side (F = 2.19; p = 0.15; ηp2 = 0.05). There were a significant time-by-side (F = 5.33; p < 0.01; ηp2 = 0.11) and time-by-group-by-side (F = 4.70; p = 0.01; ηp2 = 0.10) interactions. There were no significant time-by-group (F = 0.33; p = 0.78; ηp2 = 0.01) or group-by-side (F < 0.001; p = 0.99; ηp2 < 0.001) interactions. Post hoc comparisons are presented in Table 6.
Table 6.
Cervical side-bending range of movement differences within- and between-group.
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| DN, Mean (SD) | ||||
| Painful Side | 38.59 (5.81) | 40.09 (4.00) | 39.73 (5.68) | 40.91 (4.05) |
| Nonpainful Side | 34.30 (5.31) | 41.00 (4.92) | 40.45 (4.93) | 40.50 (5.32) |
| PENS, Mean (SD) | ||||
| Painful Side | 38.50 (5.07) | 42.27 (5.19) | 41.81 (4.15) | 41.41 (5.61) |
| Nonpainful Side | 37.45 (3.65) | 41.82 (5.24) | 40.27 (6.02) | 41.36 (5.52) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | ||||
| Painful Side | 1.50 (−0.67, 3.68) | 1.14 (−1.89, 4.16) | 2.32 (−0.43, 5.07) | |
| Nonpainful Side | 6.70 (4.15, 9.26) ‡ | 6.16 (2.96, 9.35) ‡ | 6.20 (3.16, 9.25) ‡ | |
| PENS | ||||
| Painful Side | 3.77 (1.60, 5.95) ‡ | 3.32 (0.29, 6.34) † | 2.91 (0.16, 5.66) † | |
| Nonpainful Side | 4.36 (1.81, 6.92) ‡ | 2.82 (−0.38, 6.01) | 3.91 (0.87, 6.95) ‡ | |
| Between-Group Differences, Mean (95% CI) | ||||
| Painful Side | 2.27 (0.03, 4.51) † | 2.18 (−0.94, 5.30) | 0.59 (−2.24, 3.42) | |
| Nonpainful Side | −2.34 (−4.97, 0.29) | −3.34 (−6.63, −0.05) † | −2.29 (−5.43, 0.84) |
† Statistically significant (p < 0.05), ‡ Statistically significant (p < 0.01). Abbreviations: DN, dry needling; SD, standard deviation; PENS, percutaneous electrical nerve stimulation; CI, confidence interval.
4. Discussion
4.1. Pain and Disability
Both groups showed a decrease in pain at all follow-up measurement points without differences between groups. These improvements agree with a recent meta-analysis that suggest PENS improves pain [46]. Posttreatment decreases in VAS were near the minimum clinically important difference (MCID) of 2 cm, whereas improvements in pain intensity at 48-hour and 1-week follow-up were above the MCID [47]. This nonsignificant small but greater decrease in pain intensity at 48-hour and 1-week follow-up in comparison to posttreatment measures could be due to the aggressivity of needling therapies, which could limit the therapeutic potential at posttreatment follow-up, as has been observed in previous studies [48].
Leon-Hernandez et al. [21] found that the addition of percutaneous TENS to DN significantly reduced cervical pain at posttreatment follow-up in comparison with DN alone. However, that difference was not maintained at 72 h follow-up. These differences between groups at posttreatment follow-up disagree with the results of the present study. This discrepancy could be because Leon-Hernandez et al. [21] performed the DN technique until two local twitch responses were evoked, while in the present study, needle insertions were repeated 8 to 10 times, so the dose of DN treatment in the present study could be greater. It has been observed that performance of DN until no twitch response is evoked produces more post-needling soreness in comparison to when no twitch response is evoked. However, there seems to be no difference when few twitch responses are evoked in comparison to DN until no twitch response is evoked [49]. Therefore, DN with more twitch responses produces a greater mechanical stimulus, which could increase its therapeutic potential without increasing post-needling soreness. This could explain the differences between the results of Leon-Hernandez et al. [21] and those of the present study.
The absence of between-group differences in the present study could be because of the compression applied at the end of the needling procedures. It has been shown that ischemic compression reduces post-needling soreness until 48 h after the procedure [50]. Therefore, the compression of 90 seconds applied in the present study just after the interventions could have decreased the possible potential differences between DN and PENS.
A greater improvement in the degree of disability measured with NDI in the PENS group in comparison with the DN group at one-week follow-up was found. Leon-Hernandez et al. [21] did not find a difference between groups at 72 h follow-up. It may be necessary to allow more follow-up time to appreciate the differences between electrical treatments and DN, as was shown in the present study. This improvement in disability could be explained by the greater improvements in PPT in the PENS group in comparison with the DN group observed in the present study. The decrease in muscle mechanosensitivity could cause the subject to feel less pain during active movements that contract or elongate the levator scapulae muscle during the studied week, thus diminishing their perception of neck disability. Furthermore, previous studies have found an association between neck disability and fear of movement [51]. In some cases, patients with neck pain may develop an avoidance-hypervigilance attitude, which could trigger a vicious cycle in which the patient is affected both physically and psychologically, leading to deconditioning [52]. Kinesiophobia is greater among patients with neck disability [53]. These patients believe that movement will cause reinjury and additional pain [53]. Over time, these patients can develop physical deconditioning, which limits the adequate execution of a movement or exercise and leads to more sedentary behavior [54]. In the present study, PENS showed a greater improvement in the degree of disability. It is probable that disability improvement will have major implications for a change of health-related quality of life and will enhance physical activity and work capacity in subjects with neck pain [55].
4.2. Pressure Pain Threshold
Both groups showed an increase in the pressure pain threshold in all measurement follow-ups. All the increases in PPT were above the minimum detectable change (MDC) of 0.48 kg/cm2 reported by Walton et al. [39] in subjects with neck pain.
Zaeifar et al. [48] found an increase in PPT after DN therapy but not at posttreatment follow-up. This could be because Zaeifar et al. [48] did not realize an ischemic compression after DN, which could help to increase PPT just after DN, and thus diminishing post-needling soreness, as previous studies have shown [50].
The PENS group showed a statistically significant greater increase in PPT in comparison with DN in all follow-ups. These results agree with those of Leon-Hernandez et al. [21] with percutaneous TENS. The greater improvements in the PENS group could be because electrostimulation produced a “wash-out effect” thorough muscle contractions induced by the electrical current, which could help to wash out algogenic substances, thus decreasing mechanosensitivity and increasing local PPT of the muscle [56]. However, it should be pointed out that other studies have also found an increase in PPT in distal locations with DN, suggesting a central nervous system implication in the induced hypoalgesia [57]. The absence of a distal measurement point in the present study prevents us from knowing if the between-group differences observed could be due to either a central or local component.
4.3. Strength
Improvement in side-bending strength in both groups at 48-hour and 1-week follow-ups was found but not immediately posttreatment. The absence of posttreatment improvements could be because more time is needed for changes induced by DN or PENS in the levator scapulae muscle to lead to an increase in strength. Other studies have also found an increase in cervical side-bending strength with DN [58], and a recent meta-analysis showed that DN could produce improvements in cervical strength at one-month follow-up [59].
The improvements observed at 48-hour and 1-week follow-ups were below the MDC of 3–4 kg reported by Versteegh et al. [40]. However, in that study, the strength was self-measured by the subjects, who usually are not experienced in this kind of procedure, thus decreasing the reliability of their strength measurements. In contrast, in the present study, the measures were recorded by an external physiotherapist, which could in turn lead to an increase in the reliability of the procedure, thus decreasing the MDC. However, as we did not evaluate the reliability of our procedure, we cannot ensure that the observed differences are not due to measurement errors.
There were no between-group differences at any follow-up measurement points. This could be because of the small follow-up period, so we cannot know if some differences would be observed at mid-term and long-term follow-ups.
4.4. Cervical Range of Movement
There was only a significant increase in cervical flexion range of movement in the PENS group at all follow-ups. Although the PENS group showed greater improvement in cervical flexion than the DN group at all measurement points, there was only a statistically significant between-group difference at posttreatment follow-up. Both groups increased cervical extension range of motion without differences between them. Cervical flexion has been shown to increase the mechanical stress of the nervous system compared to cervical extension [60]; thus, differences between groups in cervical flexion but not cervical extension could be because the effectiveness of PENS is mediated by responses upon the central nervous system [21,26].
Both groups showed an increase in cervical range of motion without any between-group differences. The PENS group showed an increase in cervical side-bending towards painful and nonpainful sides, whereas the DN group only showed an increase in cervical side-bending towards the nonpainful side. The levator scapulae muscle elongates with contralateral side-bending [61]. The DN group had less side-bending motion towards the nonpainful side compared to the painful side at baseline, and the motion towards the painful side was within the normal range [62]. It could be that most effectiveness of DN is local to the muscle which, along with the baseline differences, could explain why the DN group only improved towards the nonpainful side. Furthermore, both groups had raw values of side-bending within the normal range at all follow-ups, thus making it difficult to draw conclusions about the superiority of one treatment over another. Although there was also a between-side difference in rotation range of motion at the baseline in the DN group, according to previous data, both sides had less range of motion than healthy people [62], so this absence of an upper limit constraint could have prevented the same between-side differences results being obtained in cervical side-bending.
Some of these results agree with those obtained by Leon-Hernandez et al. [21]. However, the inconsistencies discussed above within the results of this study precludes us from knowing if PENS produces greater improvements than DN in cervical range of motion.
4.5. Limitations
The principal limitation of the current study is the small follow-up time, which could have contributed to the absence of between-group differences in some outcomes. It would be interesting to conduct future research with longer follow-up periods to clarify if these differences might exist.
The inclusion of a compression after the needling procedures in both groups could have also conditioned the results observed, hiding some between-group differences. Furthermore, two needles were used in the PENS group and only one in the DN group, which may be another factor that could limit the conclusions of the study about the effectiveness of PENS in comparison with DN.
Cervical side-bending is produced by other muscles than the levator scapulae, and there is no way to isolate the contraction of the levator scapulae muscle to measure its strength. This precludes us from knowing if the treatments produced an improvement in the capability of the levator scapulae muscle to produce strength.
Finally, we did not include a placebo group so placebo effects cannot be discarded. All treatments were delivered in a context that includes social and physical cues, verbal suggestions, and treatment history. This context is actively interpreted by the subject and can elicit expectations, memories, and emotions that can influence health-related outcomes [63]. A large part of the overall therapeutic response to treatments may be due to the treatment context rather than the specific treatment itself. In this study, the application time of the PENS treatment was greater than that of the DN group. This time difference could lead to greater positive expectations in patients who received the PENS treatment, and thus, the differences in effectiveness may have been because of a greater placebo effect [64]. Finally, although a recent meta-analysis has showed that PENS is better than sham PENS for decreasing pain intensity [46], we cannot exclude some placebo effect in our study results.
5. Conclusions
Percutaneous electrical nerve stimulation seems to produce greater improvements in mechanosensitivity and disability than DN at short-term follow-up. However, there seem to be no between-group differences regarding pain intensity and side-bending strength and there were inconsistencies regarding range of motion. More research is needed with mid-term and long-term follow-ups to evaluate the possible differences between these treatments over time.
Acknowledgments
The authors would like to thank all the subjects who participated in the study.
Author Contributions
Conceptualization, S.G.-d.-M., D.P.-M., and T.G.-I.; methodology, S.G.-d.-M., D.P.-M., T.L.-S., B.S.-d.-V., and L.G.-M.; software, T.G.-I. and R.F.-M.; validation, S.G.-d.-M., D.P.-M., and T.G.-I.; formal analysis, R.F.-M. and T.G.-I.; investigation, S.G.-d.-M., T.L.-S., B.S.-d.-V., and L.G.-M.; resources, D.P.-M. and T.G.-I.; data curation, T.L.-S., B.S.-d.-V., and L.G.-M.; writing—original draft preparation, S.G.-d.-M., D.P.-M., and R.F.-M.; writing—review and editing, D.P.-M. and R.F.-M.; visualization, R.F.-M.; supervision, D.P.-M. and T.G.-I.; project administration, S.G.-d.-M., D.P.-M., T.L.-S., B.S.-d.-V., and L.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gattie E., Cleland J.A., Snodgrass S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2017;47:133–149. doi: 10.2519/jospt.2017.7096. [DOI] [PubMed] [Google Scholar]
- 2.Griswold D., Wilhelm M., Donaldson M., Learman K., Cleland J. The effectiveness of superficial versus deep dry needling or acupuncture for reducing pain and disability in individuals with spine-related painful conditions: A systematic review with meta-analysis. J. Man. Manip. Ther. 2019;27:128–140. doi: 10.1080/10669817.2019.1589030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunning J., Butts R., Young I., Mourad F., Galante V., Bliton P., Tanner M., Fernández-De-Las-Peñas C. Periosteal Electrical Dry Needling as an Adjunct to Exercise and Manual Therapy for Knee Osteoarthritis. Clin. J. Pain. 2018;34:1149–1158. doi: 10.1097/AJP.0000000000000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krey D., Borchers J., McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys. Sportsmed. 2015;43:80–86. doi: 10.1080/00913847.2015.1004296. [DOI] [PubMed] [Google Scholar]
- 5.Shah J.P., Thaker N., Heimur J., Aredo J.V., Sikdar S., Gerber L. Myofascial trigger points then and now: A historical and scientific perspective. PM R. 2015;7:746–761. doi: 10.1016/j.pmrj.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings M., Baldry P. Regional myofascial pain: Diagnosis and management. Best Pract. Res. Clin. Rheumatol. 2007;21:367–387. doi: 10.1016/j.berh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Niddam D.M., Chan R.C., Lee S.H., Yeh T.C., Hsieh J.C. Central modulation of pain evoked from myofascial trigger point. Clin. J. Pain. 2007;23:440–448. doi: 10.1097/AJP.0b013e318058accb. [DOI] [PubMed] [Google Scholar]
- 8.Tesch R.D.S., Macedo L.C.D.S.P., Fernandes F.S., De Goffredo Filho G.S., Goes C.P.D.Q.F. Effectiveness of dry needling on the local pressure pain threshold in patients with masticatory myofascial pain. Systematic review and preliminary clinical trial. Cranio J. Craniomandib. Pract. 2019 doi: 10.1080/08869634.2019.1588518. [DOI] [PubMed] [Google Scholar]
- 9.Núñez-Cortés R., Cruz-Montecinos C., Vásquez-Rosel Á., Paredes-Molina O., Cuesta-Vargas A. Dry needling combined with physical therapy in patients with chronic postsurgical pain following total knee arthroplasty: A case series. J. Orthop. Sports Phys. Ther. 2017;47:209–216. doi: 10.2519/jospt.2017.7089. [DOI] [PubMed] [Google Scholar]
- 10.Zarei H., Bervis S., Piroozi S., Motealleh A. Added Value of Gluteus Medius and Quadratus Lumborum Dry Needling in Improving Knee Pain and Function in Female Athletes With Patellofemoral Pain Syndrome: A Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2020;101:265–274. doi: 10.1016/j.apmr.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Ceballos-Laita L., Jiménez-del-Barrio S., Marín-Zurdo J., Moreno-Calvo A., Marín-Boné J., Albarova-Corral M.I., Estébanez-de-Miguel E. Effects of dry needling in HIP muscles in patients with HIP osteoarthritis: A randomized controlled trial. Musculoskelet. Sci. Pract. 2019;43:76–82. doi: 10.1016/j.msksp.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Huang Q.M., Liu Q.G., Thitham N., Li L.H., Ma Y.T., Zhao J.M. Evidence for Dry Needling in the Management of Myofascial Trigger Points Associated With Low Back Pain: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018;99:144–152.e2. doi: 10.1016/j.apmr.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Tabatabaiee A., Takamjani I.E., Sarrafzadeh J., Salehi R., Ahmadi M. Ultrasound-guided dry needling decreases pain in patients with piriformis syndrome. Muscle Nerve. 2019;60:558–565. doi: 10.1002/mus.26671. [DOI] [PubMed] [Google Scholar]
- 14.Cagnie B., Dewitte V., Barbe T., Timmermans F., Delrue N., Meeus M. Physiologic effects of dry needling. Curr. Pain Headache Rep. 2013;17:348. doi: 10.1007/s11916-013-0348-5. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh Y.-L., Yang S.-A., Yang C.-C., Chou L.-W. Dry Needling at Myofascial Trigger Spots of Rabbit Skeletal Muscles Modulates the Biochemicals Associated With Pain, Inflammation, and Hypoxia. Evid. Based Complement. Altern. Med. 2012;2012:342165. doi: 10.1155/2012/342165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae Y., Chang D.S., Lee S.H., Jung W.M., Lee I.S., Jackson S., Kong J., Lee H., Park H.J., Lee H., et al. Inserting needles into the body: A meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain. 2013;14:215–222. doi: 10.1016/j.jpain.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Aranha M.F.M., Müller C.E.E., Gavião M.B.D. Pain intensity and cervical range of motion in women with myofascial pain treated with acupuncture and electroacupuncture: A double-blinded, randomized clinical trial. Braz. J. Phys. Ther. 2015;19:34–43. doi: 10.1590/bjpt-rbf.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollis S., McClure P. Intramuscular electrical stimulation for muscle activation of the tibialis anterior after surgical repair: A case report. J. Orthop. Sports Phys. Ther. 2017;47:965–969. doi: 10.2519/jospt.2017.7368. [DOI] [PubMed] [Google Scholar]
- 19.Dunning J., Butts R., Henry N., Mourad F., Brannon A., Rodriguez H., Young I., Arias-Buría J.L., Fernándezde-Las-Peñas C. Electrical dry needling as an adjunct to exercise, manual therapy and ultrasound for plantar fasciitis: A multi-center randomized clinical trial. PLoS ONE. 2018;13:e0205405. doi: 10.1371/journal.pone.0205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed S., Haddad C., Subramaniam S., Khattab S., Kumbhare D. The Effect of Electric Stimulation Techniques on Pain and Tenderness at the Myofascial Trigger Point: A Systematic Review. Pain Med. 2019;20:1774–1788. doi: 10.1093/pm/pny278. [DOI] [PubMed] [Google Scholar]
- 21.León-Hernández J.V., Martín-Pintado-Zugasti A., Frutos L.G., Alguacil-Diego I.M., De La Llave-Rincón A.I., Fernandez-Carnero J. Immediate and short-term effects of the combination of dry needling and percutaneous TENS on post-needling soreness in patients with chronic myofascial neck pain. Braz. J. Phys. Ther. 2016;20:422–431. doi: 10.1590/bjpt-rbf.2014.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couto C., De Souza I.C.C., Torres I.L.S., Fregni F., Caumo W. Paraspinal stimulation combined with trigger point needling and needle rotation for the treatment of myofascial pain: A randomized sham-controlled clinical trial. Clin. J. Pain. 2014;30:214–223. doi: 10.1097/AJP.0b013e3182934b8d. [DOI] [PubMed] [Google Scholar]
- 23.Chassot M., Dussan-Sarria J.A., Sehn F.C., Deitos A., de Souza A., Vercelino R., Torres I.S., Fregni F., Caumo W. Electroacupuncture analgesia is associated with increased serum brain-derived neurotrophic factor in chronic tension-type headache: A randomized, sham controlled, crossover trial. BMC Complement. Altern. Med. 2015;15:144. doi: 10.1186/s12906-015-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitch M., Brown R., Macefield V.G. Intramuscular stimulation of tibialis anterior in human subjects: The effects of discharge variability on force production and fatigue. Physiol. Rep. 2017;5:e13326. doi: 10.14814/phy2.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botelho L., Angoleri L., Zortea M., Deitos A., Brietzke A., Torres I.L.S., Fregni F., Caumo W. Insights about the neuroplasticity state on the effect of intramuscular electrical stimulation in pain and disability associated with chronic myofascial pain syndrome (MPS): A double-blind, randomized, sham-controlled trial. Front. Hum. Neurosci. 2018;12:388. doi: 10.3389/fnhum.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da Graca-Tarragó M., Deitos A., Brietzke A.P., Torres I.L.S., Stefani L.C., Fregni F., Caumo W. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med. (U.S.) 2016;17:877–891. doi: 10.1111/pme.12930. [DOI] [PubMed] [Google Scholar]
- 27.Aranha M.F.M., Alves M.C., Bérzin F., Gavião M.B.D. Eficácia da eletroacupuntura para dor miofascial do músculo trapézio: Uma série de casos. Rev. Bras. Fisioter. 2011;15:371–379. doi: 10.1590/S1413-35552011005000022. [DOI] [PubMed] [Google Scholar]
- 28.Rainey C.E. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: A case report. Int. J. Sports Phys. Ther. 2013;8:145–161. [PMC free article] [PubMed] [Google Scholar]
- 29.Rock J.M., Rainey C.E. Treatment of nonspecific thoracic spine pain with trigger point dry needling and intramuscular electrical stimulation: A case series. Int. J. Sports Phys. Ther. 2014;9:699–711. [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:698–702. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawker G.A., Mian S., Kendzerska T., French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res. 2011;63:S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 32.Kahl C., Cleland J.A. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: An overview of psychometric properties. Phys. Ther. Rev. 2005;10:123–128. doi: 10.1179/108331905X55776. [DOI] [Google Scholar]
- 33.Andrade Ortega J.A., Delgado Martínez A.D., Ruiz R.A. Validation of the Spanish version of the Neck Disability Index. Spine (Phila. Pa. 1976). 2010;35:E114–E118. doi: 10.1097/BRS.0b013e3181afea5d. [DOI] [PubMed] [Google Scholar]
- 34.Rathbone A.T.L., Grosman-Rimon L., Kumbhare D.A. Interrater Agreement of Manual Palpation for Identification of Myofascial Trigger Points: A Systematic Review and Meta-Analysis. Clin. J. Pain. 2017;33:715–729. doi: 10.1097/AJP.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 35.Fernández-de-las-Peñas C., Dommerholt J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A delphi study. Pain Med. (U.S.) 2018;19:142–150. doi: 10.1093/pm/pnx207. [DOI] [PubMed] [Google Scholar]
- 36.Simons D., Travell J. Travell, Simons & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. 3th ed. Wolters Kluwer; Philadelpia, PA, USA: 2019. [Google Scholar]
- 37.Shadmehr A., Bagheri H., Ansari N.N., Sarafraz H. The reliability measurements of lateral scapular slide test at three different degrees of shoulder joint abduction. Br. J. Sports Med. 2010;44:289–293. doi: 10.1136/bjsm.2008.050872. [DOI] [PubMed] [Google Scholar]
- 38.Williams M.A., McCarthy C.J., Chorti A., Cooke M.W., Gates S. A Systematic Review of Reliability and Validity Studies of Methods for Measuring Active andPassive Cervical Range of Motion. J. Manip. Physiol. Ther. 2010;33:138–155. doi: 10.1016/j.jmpt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Walton D., MacDermid J., Nielson W., Teasell R., Chiasson M., Brown L. Reliability, Standard Error, and Minimum Detectable Change of Clinical Pressure Pain Threshold Testing in People With and Without Acute Neck Pain. J. Orthop. Sport. Phys. Ther. 2011;41:644–650. doi: 10.2519/jospt.2011.3666. [DOI] [PubMed] [Google Scholar]
- 40.Versteegh T.H., Beaudet D., Greenbaum M., Hellyer L., Tritton A., Walton D. Evaluating the reliability of a novel neck-strength assessment protocol for healthy adults using self- generated resistance with a hand-held dynamometer. Physiother. Canada. 2015;67:58–64. doi: 10.3138/ptc.2013-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCutcheon L., Yelland M. Iatrogenic pneumothorax: Safety concerns when using acupuncture or dry needling in the thoracic region. Phys. Ther. Rev. 2011;16:126–132. doi: 10.1179/1743288X11Y.0000000012. [DOI] [Google Scholar]
- 42.Hong C.Z. Lidocaine injection versus dry needling to myofascial trigger point: The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994;73:256–263. doi: 10.1097/00002060-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Pecos-Martín D., Montañez-Aguilera F.J., Gallego-Izquierdo T., Urraca-Gesto A., Gómez-Conesa A., Romero-Franco N., Plaza-Manzano G. Effectiveness of dry needling on the lower trapezius in patients with mechanical neck pain: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015;96:775–781. doi: 10.1016/j.apmr.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Sluka K.A., Vance C.G.T., Lisi T.L. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J. Neurochem. 2005;95:1794–1801. doi: 10.1111/j.1471-4159.2005.03511.x. [DOI] [PubMed] [Google Scholar]
- 45.Field A.P. Discovering Statistics Using IBM SPSS Statistics. 5th ed. SAGE Publications Ltd.; London, UK: 2018. [Google Scholar]
- 46.Plaza-Manzano G., Gómez-Chiguano G.F., Cleland J.A., Arías-Buría J.L., Fernández-de-las-Peñas C., Navarro-Santana M.J. Effectiveness of percutaneous electrical nerve stimulation for musculoskeletal pain: A systematic review and meta-analysis. Eur. J. Pain (U.K.) 2020 doi: 10.1002/ejp.1559. [DOI] [PubMed] [Google Scholar]
- 47.MacDowall A., Skeppholm M., Robinson Y., Olerud C. Validation of the visual analog scale in the cervical spine. J. Neurosurg. Spine. 2018;28:227–235. doi: 10.3171/2017.5.SPINE1732. [DOI] [PubMed] [Google Scholar]
- 48.Ziaeifar M., Arab A.M., Nourbakhsh M.R. Clinical Effectiveness of Dry Needling Immediately After Application on Myofascial Trigger Point in Upper Trapezius Muscle. J. Chiropr. Med. 2016;15:252–258. doi: 10.1016/j.jcm.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martín-Pintado-Zugasti A., Fernández-Carnero J., León-Hernández J.V., Calvo-Lobo C., Beltran-Alacreu H., Alguacil-Diego I., Gallego-Izquierdo T., Pecos-Martin D. Postneedling Soreness and Tenderness After Different Dosages of Dry Needling of an Active Myofascial Trigger Point in Patients With Neck Pain: A Randomized Controlled Trial. PM R. 2018;10:1311–1320. doi: 10.1016/j.pmrj.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Martín-Pintado-Zugasti A., Pecos-Martin D., Rodríguez-Fernández Á.L., Alguacil-Diego I.M., Portillo-Aceituno A., Gallego-Izquierdo T., Fernandez-Carnero J. Ischemic Compression After Dry Needling of a Latent Myofascial Trigger Point Reduces Postneedling Soreness Intensity and Duration. PM R. 2015;7:1026–1034. doi: 10.1016/j.pmrj.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Vlaeyen J.W.S., Linton S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 52.Leeuw M., Goossens M.E.J.B., Linton S.J., Crombez G., Boersma K., Vlaeyen J.W.S. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J. Behav. Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 53.Demirbüken I., Özgül B., Kuru Çolak T., Aydoʇdu O., Sari Z., Yurdalan S.U. Kinesiophobia in relation to physical activity in chronic neck pain. J. Back Musculoskelet. Rehabil. 2016;29:41–47. doi: 10.3233/BMR-150594. [DOI] [PubMed] [Google Scholar]
- 54.Luque-Suarez A., Martinez-Calderon J., Falla D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: A systematic review. Br. J. Sports Med. 2019;53:554–559. doi: 10.1136/bjsports-2017-098673. [DOI] [PubMed] [Google Scholar]
- 55.Beltran-Alacreu H., López-de-Uralde-Villanueva I., Calvo-Lobo C., La Touche R., Cano-de-la-cuerda R., Gil-Martínez A., Fernández-Ayuso D., Fernández-Carnero J. Prediction models of health-related quality of life in different neck pain conditions: A cross-sectional study. Patient Prefer. Adherence. 2018;12:657–666. doi: 10.2147/PPA.S162702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah J.P., Phillips T.M., Danoff J.V., Gerber L.H. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J. Appl. Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 57.Mejuto-Vázquez M.J., Salom-Moreno J., Ortega-Santiago R., Truyols-Domínguez S., Fernández-De-Las-peñas C. Short- Term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2014;44:252–260. doi: 10.2519/jospt.2014.5108. [DOI] [PubMed] [Google Scholar]
- 58.Cerezo-Téllez E., Torres-Lacomba M., Fuentes-Gallardo I., Perez-Muñoz M., Mayoral-Del-Moral O., Lluch-Girbés E., Prieto-Valiente L., Falla D. Effectiveness of dry needling for chronic nonspecific neck pain: A randomized, single-blinded, clinical trial. Pain. 2016;157:1905–1917. doi: 10.1097/j.pain.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 59.Mansfield C.J., Vanetten L., Willy R., Di Stasi S., Magnussen R., Briggs M. The effects of needling therapies on muscle force production: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2019;49:154–170. doi: 10.2519/jospt.2019.8270. [DOI] [PubMed] [Google Scholar]
- 60.Johnson E.K., Chiarello C.M. The slump test: The effects of head and lower extremity position on knee extension. J. Orthop. Sports Phys. Ther. 1997;26:310–317. doi: 10.2519/jospt.1997.26.6.310. [DOI] [PubMed] [Google Scholar]
- 61.Ackland D.C., Merritt J.S., Pandy M.G. Moment arms of the human neck muscles in flexion, bending and rotation. J. Biomech. 2011;44:475–486. doi: 10.1016/j.jbiomech.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 62.Swinkels R.A.H.M., Swinkels-Meewisse I.E.J.C.M. Normal values for cervical range of motion. Spine. 2014;39:362–367. doi: 10.1097/BRS.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 63.Wager T.D., Atlas L.Y. The neuroscience of placebo effects: Connecting context, learning and health. Nat. Rev. Neurosci. 2015;16:403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malfliet A., Lluch Girbés E., Pecos-Martin D., Gallego-Izquierdo T., Valera-Calero A. The Influence of Treatment Expectations on Clinical Outcomes and Cortisol Levels in Patients With Chronic Neck Pain: An Experimental Study. Pain Pract. 2019;19:370–381. doi: 10.1111/papr.12749. [DOI] [PubMed] [Google Scholar]