Abstract
The sensitization to grass pollen is a known problem in European countries. Phl p 5 is an important allergen recognized by the majority of grass sensitized individuals. In this study, we evaluated daily variation in airborne Poaceae pollen and Phl p 5 allergen concentrations to determine whether airborne pollen concentrations alone are sufficient to reflect the actual allergenic potential of the air. The relationships between the mentioned pollen and allergen concentrations and associated environmental variables were also examined. The airborne particles were collected during the Poaceae flowering season in Bratislava in 2019. Pollen sampling was performed using a Hirst-type sampler, while a cyclone sampler was used for the aeroallergen capturing. Allergenic molecules were quantified by ELISA assay. The associations between pollen and allergen concentrations showed that these two variables are positively correlated; however, the correlation was not significant. We observed the concurrent occurrence of airborne pollen and allergen peaks on the same day. Nevertheless, during some days of the pollen season, the allergen concentrations did not correspond to the airborne pollen values. Moreover, the days with low pollen concentration but high pollen potency and vice versa were observed. The effect of selected environmental variables on daily pollen and allergen concentrations was evaluated through Spearman's correlation analysis. Of all meteorological variables considered, air temperature, precipitation, and relative air humidity were significantly correlated with airborne pollen and/or allergen concentrations. The association with air temperature was positive, while the negative association was observed with precipitation and relative air humidity. Among the atmospheric pollutants, O3 and PM10 were significantly and positively associated with both pollen and allergen concentrations, whereas CO and PM2.5 were significantly and positively associated only with pollen concentration.
Keywords: Grass pollen, Aeroallergens, Meteorological parameters, Atmospheric pollutants, Bratislava, Central Europe, Atmospheric science, Environmental analysis, Environmental health, Environmental pollution, Microbiology, Environmental science
Grass pollen; Aeroallergens; Meteorological parameters; Atmospheric pollutants; Bratislava; Central Europe, Atmospheric Science; Environmental Analysis; Environmental Health; Environmental Pollution; Microbiology, Environmental Science
1. Introduction
The Poaceae family is a large and nearly ubiquitous family of monocotyledonous flowering plants known as grasses. This family includes the cereal grasses, bamboos and the grasses of natural grassland and cultivated lawns and pasture. With around 12 000 species (Christenhusz and Byng, 2016) Poaceae is the fifth-largest plant family. In Bratislava, the most significant grass pollen sources are Arrhenatherum elatius, Lolium perenne, Bromus hordeaceus, B. sterilis, Cynodon dactylon, Dactylis glomerata, Elytrigia repens, Festuca rubra, Hordeum murinum, Poa pratensis, and Poa annua, which are the dominant species in urban lawns and at the edges of untreated habitats (Feráková and Jarolímek, 2011). Other grass pollen sources are cereals especially Secale cereale and Zea mays that are grown in the outskirts of the city. In Central Europe the species of Poaceae family bloom from the end of April up to the end of August (Ščevková et al., 2010).
Pollen grains of grasses are among the most important airborne allergens in Europe (D'Amato et al., 2007). The most common symptoms associated with sensitivity to Poaceae pollen are rhinoconjunctivitis and bronchial asthma (Djunkanovic et al., 1996). Grass pollen is responsible for most respiratory allergic reactions in the pollen-sensitive population of Bratislava (Hrubiško, 1998; Ščevková et al., 2015). Based on the skin prick testing of patients suffering from respiratory allergy during grass pollen season, Hrubiško (1998) identified the most allergenic grass-pollen types in Bratislava to be: Lolium perenne, Agrostis alba, Secale cereale, Alopecurus pratensis, Holcus lanatus, Phleum pratense, Arrhenatherum elatius, Bromus erectus, Elytrigia repens, and Dactylis glomerata. In this context, however, it is important to stress that almost all grass-pollen types show a very high degree of cross-reactivity (Martin et al., 1987; Aalberse, 1992).
Studies on allergen exposure mostly rely on airborne pollen concentrations derived from volumetric pollen traps, but research has shown that allergy symptoms may arise even at low pollen concentrations (D'Amato et al., 2007; Buters et al., 2015). Due to their size (~35–65 μm in diameter), intact pollen grains have difficulty in entering into the lower airways and cannot, thus, trigger severe allergic responses. Besides intact pollen grains, smaller particles, e.g., fragments of broken grass pollen and free allergens (0.6–2.5 μm) can be, also found in the atmosphere (Wang et al., 2012). These particles are small enough to reach lower airways, thus inducing allergic asthma symptoms. It is therefore important to additionally investigate aeroallergen concentrations. At present 13 different groups of allergens related to grass pollen are known which induce IgE responses (Andersson and Lidholm, 2003), of which group 5 allergens are one of the major allergens since 65–85% of patients allergic to grass pollen are sensitive to these allergens (Harabina et al., 2008).
This study aims to assess the correlation between Poaceae pollen and Phl p 5 allergen concentrations in the atmosphere of Bratislava and to evaluate the impact of weather-related and air pollution-related conditions on both.
2. Materials and methods
2.1. Study area
The data evaluated in this study were collected in Bratislava (Figure 1) which is located in the south-western part of Slovakia on the boundary between the Malé Karpaty Mountains and the Pannonian Lowland. Bratislava, with around 430,000 inhabitants is surrounded by farmland areas (mainly cereal fields and vineyards), deciduous Carpathian forests, and remnants of alluvial forests. The city is characterized by a temperate continental climate with warm summers (21.2 °C in July – the highest mean monthly temperature), cold winters (0.1 °C in January – the lowest mean monthly temperature), and an annual rainfall of 667.2 mm (1983–2019 average, data from the Meteorological Observatory of the Department of Astronomy, Physics of the Earth and Meteorology of the Comenius University in Bratislava).
Figure 1.
Localization of Bratislava in Slovakia.
2.2. Airborne pollen and Phl p 5 allergen monitoring
Aerobiological data, including airborne grass pollen concentrations, were recorded in the atmosphere of Bratislava from March to October 2019 by using Burkard 7-day volumetric pollen trap (Burkard Manufacturing Co Ltd.) (Hirst, 1952). The sampler was located on the roof of the Comenius University Science Park (48˚08′58″N, 17˚04′24″ E, 167 m a. s. l.), in the north-west part of the city at 18 m a.g.l. The monitoring station is surrounded by small alluvial forests and urban vegetation (e.g. lawns, ornamental trees, and shrubs). The botanical garden is located in the vicinity. The methodology used was performed according to the standard method adopted by the British Aerobiology Federation (1995). The air is pulled into the sampler through a 2 × 14 mm slit at a rate of 10 l/min and flowed over a rotating drum covered with adhesive tape. The exposed tape was changed weekly at 10:00 (local time – UTC +2 h in the summer, UTC +1 h in the winter). The tape was divided into segments 48 mm long, each corresponding to the 24-h period. Each segment was mounted on microscopic slides in gelatin-glycerin and stained with basic fuchsin. Poaceae pollen grains were counted in 12 transversal traverses per slide under a light microscope at 400 × magnification. After the pollen counting in each sampling area, a specific correction factor for the used microscope was applied. Pollen concentration was expressed as a number of pollen grains per cubic meter of air (pollen/m3).
For the aeroallergen (Phl p 5) quantification, a multi-vial cyclone sampler (Burkard Manufacturing Co Ltd.) was used. The sampler was located next to the pollen trap on the roof of the Comenius University Science Park. This is a low volume sampler with an airflow rate of 16 l/min. The air samples were collected directly into a 1.5 ml Eppendorf vials (one vial represents 24 h exposure, from 10:00 to 10:00 of the next day, but is described as a “daily average” throughout). Airborne samples were collected dry and stored at −20 °C until extraction. Aeroallergens were sampled during the main Poaceae pollen season (MPS) (considering the data from previous years), plus two weeks before and after MPS, trying to find possible aeroallergens outside the blooming period. The MPS was established according to the method by Peel et al. (2014), which define the start (end) of the MPS as the first (last) day with a daily average equal to or greater than 10 pollen/m3. The final data set included 62 days from 11 May to 11 July 2019.
To calculate the daily potency of airborne Poaceae pollen (allergen release per pollen), the daily value of noted Phl p 5 was divided by the mean daily Poaceae pollen concentration and expressed as pg Phl p 5/pollen (Celenk, 2019; Grewling et al., 2020).
2.3. Extraction and quantification of aeroallergen Phl p 5
Samples collected by Cyclone sampler were extracted according to Plaza et al. (2016). Samples in 1.5 ml Eppendorf tubes were centrifuged for 3 min at 2000 g and then re-suspended in 120 μl of extraction buffer containing 50 mM phosphate buffer (pH 7.4), 150 mM NaCl, 125 mM ammonium bicarbonate, 3 mM/l EDTA and 0.005% Tween 20. Extraction was carried at room temperature (RT) with constant stirring for 2 h. The samples were pelleted by centrifugation for 10 min at 2000 g and stored at −20 °C until further analysis.
Airborne allergen Phl p 5 was quantified by double-sandwich ELISA (Enzyme-Linked ImmunoSorbent Assay). Microtiter plates were coated overnight at 4 °C with 100 μl of monoclonal antibodies 1D11 (Indoor Biotechnologies, US) diluted in PBS to the final concentration of 1.5 μg/ml. Following day, the coated wells were blocked with 100 μl of 1% BSA in PBS-T 30 min at RT. Then, 100 μl of purified allergen or extracted airborne sample (30 μl of the extract with 70 μl of PBS) in duplicates were applied to the well and incubated 1 h at 37 °C. Purified allergen rPhl p 5 (Indoor Biotechnologies, US) in serial 2-fold dilutions from 3.125 ng/ml – 50 ng/ml were used to generate the standard curve. Afterward, 100 μl of biotinylated anti-Phlp5mAb Bo1 antibodies (Indoor Biotechnologies, US) diluted 1:1000 in 1% BSA + PBS-T were added to each well and afterward incubated with 100 μl of streptavidin-peroxidase (0.25 mg/ml diluted 1:1000 in 1% BSA + PBS-T, Merck, US). Both incubations were performed 1 h at 37 °C. The plate was washed between all steps 3 times with PBS-T (PBS with 0.05% Tween 20). After final washing, the wells were incubated with developing substrate OPD (Merck, US) for 30 min at RT in dark. The absorbance was measured at 450 nm in HiPo Microplate Photometer MPP-96 (Biosan, Latvia). If the plate could not be read immediately, we added 50 μl of 3 M HCl or 3 M H2SO4 solution per 100 ml of solution. Stopped reactions were measured at 492 nm.
Daily concentrations of allergen were interpolated from the standard curve and expressed in nanograms per milliliter. To compare the pollen and allergen values, the data were transformed to picograms per cubic meter of air (pg/m3) following the volume sampled by the sampler.
2.4. Meteorological and air pollution data
To analyze the effect of meteorological parameters on daily pollen and aeroallergen concentrations, following meteorological variables were taken into consideration: T – mean daily surface air temperature (°C), RH – mean daily relative air humidity (%), WS – mean daily wind speed (m/s), AP – mean daily atmospheric pressure (hPa), and P − total daily precipitation (mm). Meteorological data were recorded at the Meteorological Observatory of the Department of Astronomy, Physics of the Earth and Meteorology of the Comenius University in Bratislava (48˚09′04″ N, 17˚04′14″ E, 182 m a. s. l.) situated 400 m NW of the sampling site. To analyze the effect of atmospheric pollutants on pollen and aeroallergen concentrations, the following daily parameters were analyzed: PM10 − particulate matter ≤ 10 μm (μg/m3), PM2.5 − particulate matter ≤ 2.5 μm (μg/m3), O3 − ozone (μg/m3), NO2 − nitrogen dioxide (μg/m3) and CO − carbon monoxide (μg/m3). The air pollution data were obtained from the database of the Slovak Hydrometeorological Institute (SHMÚ), which operates four air quality monitoring stations throughout Bratislava. The data used in the analysis were calculated as an average of data obtained from all four monitoring stations. The temporal resolution of meteorological and air pollution data was adjusted to the pollen and allergen collection time (10:00–10:00).
2.5. Data analysis
Nonparametric Spearman's correlation test was performed to establish the relationship between 1) airborne Poaceae pollen and Phl p 5 aeroallergen concentrations, 2) pollen potency and environmental variables, and 3) concentrations of airborne Poaceae pollen and Phl p 5 aeroallergen and selected meteorological and air pollution time series since the data were neither normally nor log-normally distributed. The statistical software R (version 3.6.1) was used for this purpose.
3. Results
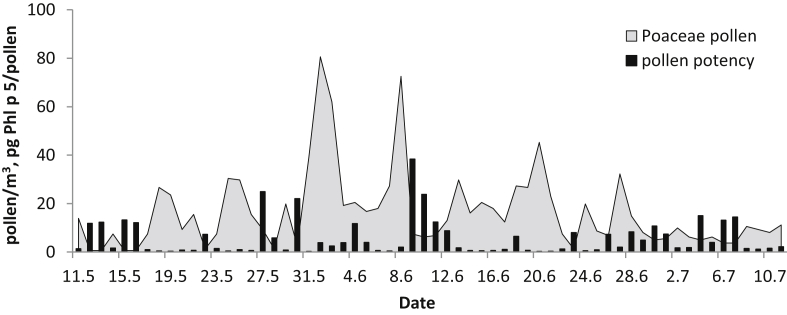
Airborne Poaceae pollen and Phl p 5 allergen concentrations over the MPS, which lasted in the atmosphere of Bratislava from 11 May to 11 July, are depicted in Figure 2. The characteristics associated with the analyzed period are shown in Table 1. Poaceae pollen and Phl p 5 allergen were recorded every day during the MPS, i.e., 62 days. During the studied period, the aeroallergen content varied between 9.5 and 305.1 pg/m3, whereas pollen concentrations varied between 4 and 81 pollen/m3. On 1 June, both pollen and allergen peak values were reached. The daily mean pollen/allergen concentrations higher than 30 pollen/m3 and 100 pg/m3 with high allergenic risks were reached during 6 and 10 days, respectively (Figure 3). The Seasonal Pollen/Allergen Integral (obtained by summing the average daily pollen/allergen concentrations over the MPS) amounted to 1,015 pollen grains and 3,119 pg of allergen during the study period (Table 1). The Seasonal Pollen Integral in 2019 was 34.6% lower than the 17-year average (years 2002–2018; data obtained from the Pollen dataset stored at the Department of Botany, Comenius University in Bratislava).
Figure 2.
Poaceae pollen (grey area) and Phl p 5 allergen (black line) concentrations, precipitation (bars) and discrepancy days (black triangles) in the air of Bratislava over the study period.
Table 1.
Characteristics of 2019 Poaceae pollen and Phl p 5 allergen seasons.
| Characteristics | |
|---|---|
| Pollen | |
| Pollen season start | 11 May |
| Pollen season end | 11 July |
| Season length (days) | 62 |
| Seasonal Pollen Integral (pollen/m3) | 1,015 |
| Peak value (pollen/m3) | 81 |
| Peak day | 1 June |
| Phl p 5 allergen | |
| Peak value (pg/m3) | 305.1 |
| Peak day | 1 June |
| Seasonal Allergen Integral (pg/m3) | 3,119 |
| Pollen potency (pg/pollen) | 5.7 |
Figure 3.
Number of days of Poaceae MPS with the pollen and aeroallergen concentrations placed in exponential classes.
The mean pollen potency (allergen release per pollen) reached 5.7 pg Phl p 5/pollen. We recorded days (27 May, 30 May, 9 June, 10 June) with low pollen concentration but high pollen potency (24.9, 22.0, 38.3, 23.8 pg Phl p 5/pollen, respectively) (Figure 4). On the other hand, we recorded days (1 June, 8 June, 20 June) when the pollen concentration was high but pollen potency was low (3.8, 2.0, 0.2 pg Phl p 5/pollen, respectively). Overall, the potency of Poaceae pollen during the second pollen peak (8 June) was 48% lower than during the first peak (1 June) and during the third peak (20 June) was 90.9% lower than during the second peak (Figure 4). Based on the results of the correlation analysis, no significant association between pollen potency and meteorological or air pollution values was observed.
Figure 4.
Airborne Poaceae pollen concentrations and pollen potency in Bratislava over the study period.
We observed a positive degree of association between the pollen and allergen levels; however, the correlation was not significant (rs = 0.201). It is worth noting that the main peak for allergen activity occurred exactly on the same day as pollen peak. However, during some days of the pollen season, the allergen concentrations were much higher than pollen values (e.g. 27 May, 4 June, 9–12 June, 18 June, 28 June) (Figure 2).
The May–August period in 2019 was characterized by a very high amount of rain, i.e. 117.4 mm (41.7% higher than the 36-year average from 1983 to 2018) and the air temperature was 0.8 °C higher than the 36-year average. May was colder with more amount of rain than the long-term average, while June was warmer and drier. The May mean temperature was 3.3 °C lower, and the amount of rain was 73.4% higher than the 36-year average, reaching 12.6 °C and 260.6 mm. On the other hand, the June mean air temperature was 4.1 °C higher, and the amount of rain was 73.4% lower than the 36-year average, reaching 23.3 °C and 18.4 mm.
Table 2 shows the results of Spearman's correlation analysis, which was performed to identify the environmental variables which the most influenced airborne Poaceae pollen and Phl p 5 allergen concentrations. The results revealed that of meteorological parameters considered, air temperature, precipitation, and relative air humidity, were important determinants of airborne pollen and/or allergen concentrations. The association with temperature was positive, whereas the association with precipitation and relative air humidity was negative. Important atmospheric washes induced by the rainfall were observed as precipitations were followed by reduced pollen and allergen levels (Figure 2). Among the atmospheric pollutants, O3 and PM10 were significantly and positively associated with both pollen and allergen concentrations during the studied period. PM2.5 and CO were significantly associated only with pollen concentration and the correlation was positive.
Table 2.
Spearman's correlation coefficients between the concentrations of Poaceae pollen and Phl p 5 aeroallergen, and the main meteorological parameters and atmospheric pollutants recorded in Bratislava in 2019.
| Variables | pollen | allergen |
|---|---|---|
| Meteorological parameters (N = 62) | ||
| Temperature (°C) | 0.222 | 0.450∗∗∗ |
| Precipitation (mm) | −0.437∗∗∗ | −0.353∗∗ |
| Relative humidity (%) | −0.155 | −0.524∗∗∗ |
| Wind speed (m/s) | −0.141 | −0.094 |
| Air pressure (hPa) | −0.074 | 0.149 |
| Atmospheric pollutants (N = 62) | ||
| O3 (μg/m3) | 0.254∗ | 0.397∗∗ |
| PM10 (μg/m3) | 0.349∗∗ | 0.375∗∗ |
| PM2.5 (μg/m3) | 0.341∗∗ | 0.222 |
| CO (μg/m3) | 0.287∗ | −0.096 |
| NO2 (μg/m3) | 0.053 | −0.172 |
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
4. Discussion
In Europe, the number of pollen allergic sufferers is constantly increasing, especially in urban areas (D'Amato et al., 2007). Despite this, the number of Poaceae pollen in the atmosphere of Bratislava has, similarly to in Spain (Jato et al., 2009) or Turkey (Acar et al., 2017) descending trend (Ščevková et al., 2010), presumably due to changes in agricultural and land-use practices. However, the low Poaceae SPIn in Bratislava in 2019 (36.7% lower than the 19-year average from 2002 to 2018) may be due to specific meteorological conditions recorded (rainier than the long-term average during the May–August period), which enhanced pollen removing from the air by both rain-out and wash-out mechanism.
Except for intact pollen grains, pollen allergens may also be carried in a micro aerosol suspension smaller than pollen grains (Solomon et al., 1983). Therefore sever respiratory allergic symptoms can occur even when the concentrations of intact pollen grains are low in the air (D'Amato et al., 2010). Our study demonstrates that the daily levels of Poaceae pollen correlated positively with Phl p 5 allergen, but not significantly, probably due to several discrepancy episodes. Similarly to other researchers (Rodríguez-Rajo et al., 2011; González-Parrado et al., 2014) who have compared airborne pollen concentrations with allergen concentrations, we recorded days with low pollen but high aeroallergen concentration and vice versa. The quantity of pollen grains in the atmosphere depends on many factors that affect their release from anthers and airborne dispersal. Apart from the biological characteristics of the source plants and aerodynamic features of pollen grains, meteorological factors belong to the most significant variables that affect the variation in airborne pollen concentration (Plaza et al., 2016). In general higher Poaceae pollen concentrations are associated with bright sunny days without precipitation (Bartková-Ščevková, 2003). On the other hand, higher aeroallergen concentrations can be associated with an increase in atmospheric pollutants (D'Amato et al., 2007) or humidity before storm episodes (Buters et al., 2015). Besides, the discordance between the daily pollen and allergen concentrations could be explained by long-distance transport processes (Moreno-Grau et al., 2016). Due to their small size, both small allergen carriers and free allergens of grass pollen origin can remain airborne longer than intact pollen grains.
Pollen grains vary in the amount of allergen they release. In the present study, the mean pollen potency reached 5.7 pg Phl p 5/pollen. It is in line with the result obtained by Buters et al. (2015), who noted the grass pollen potency variation ranging from 1.5 to 5.9 Phl p 5/pollen in different European countries. This variation is likely to depend on grass species spectrum, distribution, ecological requirements, and its adaptation to the microclimate conditions in the given geographical area. Our study also showed that the natural potency of airborne Poaceae pollen can considerably differ between days, ranging from 0.2 to 38.3 pg Phl p 5/pollen. Besides, more events with high-potency pollen were observed when pollen concentrations were low. This could explain why even low airborne pollen concentrations can result in respiratory allergy. The daily variation in pollen potency may be, similarly to fungal spore potency, affected by environmental variables such as temperature, precipitation and atmospheric pollution (Grewling et al., 2019). Based on the result of correlation analysis, however, no significant effects of weather or air pollution factors on daily variations in Poaceae pollen potency were observed in Bratislava.
This study showed a clear relationship between meteorological variables and pollen or allergen concentration. This is, however, in contrast with the results obtained by other researchers (De Linares et al., 2010; Plaza et al., 2016), who noted no correlation between aeroallergen concentration and weather parameters. The present study found precipitation to be a significant factor explaining the variability in grass pollen and Phl p 5 allergen concentrations during the MPS period, confirming the ability of rain to remove pollen grains from the atmosphere. Similarly, several researchers (Rodríguez-Rajo et al., 2011; Fernández-González et al., 2011) pointed out that the precipitation cleans pollen grains from the atmosphere, however, provoking the liberation and dispersion of aeroallergens. Moreover, Solomon et al. (1983) noted the release of allergenic molecules due to precipitation resulting from the osmotic shock and evaporation during the following hours leads to the rapid dispersion of small allergen-carrying particles in the air. Nevertheless, the negative correlation between precipitation and Phl p 5 aeroallergen concentration was observed in the present study. On the other hand, relative air humidity can also play a significant role in the allergen release. Due to high atmospheric humidity, Poaceae pollen grains with a delicate and thin pollen wall (Buters et al., 2015) can be easily burst and release free fraction of allergenic molecules in ambient air (D'Amato et al., 2010). By contrast, we observed, similarly to Alan et al. (2018) that airborne Phl p 5 allergen concentrations significantly decreased with increasing relative air humidity, presumably as a result of increased sedimentation velocity of humid pollen allergens.
Air temperature is considered as a meteorological factor responsible for anther opening, pollen release and atmospheric pollen dispersal (Fernández-González et al., 2011). Even though the significant positive correlation between airborne Poaceae pollen and the air temperature was observed by other researchers (Bartková-Ščevková, 2003; Plaza et al., 2016), no significant association between air temperature and pollen levels was observed in the present study. On the other hand, D'Amato et al. (2007) assumed that higher air temperature, similarly to the recent climate change impact, results in increased allergen production by pollen grains. Furthermore, Rodríguez-Rajo et al. (2011) pointed out that the specific microclimatic conditions in the cities, especially the urban heat island effect, could induce higher expression of allergenic molecules in the pollen of plants. Similarly to mentioned researchers we observed significant and positive association between the concentrations of Phl p 5 aeroallergen and air temperature in the present study.
Urban residents experience more respiratory allergies than rural residents (D'Amato et al., 2007) mainly due to the interaction between chemical air pollutants and pollen grains. Air pollutants might damage the pollen cell wall, facilitating allergen release into the environment (Motta et al., 2006; Ghiani et al., 2012; Plaza et al., 2016) or modify pollen allergenicity (D'Amato et al., 2007). Moreover, air pollution is considered a stress factor for plants that increases the synthesis of some allergens in pollen grains (Suárez-Cervera et al., 2008; Aina et al., 2010). However, to ascertain the effect of the atmospheric pollutants on the allergen synthesis in the pollen grains, it is essential to take into consideration the values of contaminants from the previous period of flowering.
Atmospheric pollutants considered in the present study were also associated with pollen and allergen concentrations. The results of the regression analysis showed that ozone exhibited significant and positive associations with Poaceae pollen and Phl p 5 allergen concentration during the MPS period as a result of the atmospheric temperature influence. The tropospheric ozone is a photochemical reaction product and the increased solar radiation accompanied by elevated air temperatures has a key role in its concentration (Stathopoulou et al., 2008). The elevated values of both ozone and pollen concentrations in the air of Bratislava were recorded on warm sunny days during the grass pollen season. A similar result was obtained by Puc (2011) who observed a positive correlation between Poaceae pollen concentrations and ozone levels in the atmosphere of Szczecin (Poland). By contrast, Albertine et al. (2014) pointed out that elevated O3 did not affect the amount of Phleum pratense pollen produced. On the other hand, in vivo exposure to O3 has been shown to increase the group 5 allergen content of the pollen in Lolium perenne (Masuch et al., 1997; Schoene et al., 2004). Ozone has also been shown to disrupt the cell membrane of grass pollen and thus increase allergen exposure by releasing cytoplasmic allergen-containing granules from within pollen (Motta et al., 2006; Rogerieux et al., 2007). While our study and studies mentioned above suggest that there could be an increase in airborne grass pollen allergen concentration at rising O3 levels, Albertine et al. (2014) observed no significant correlation between Phl p 5 allergen and ozone concentrations.
In Bratislava, the high concentration of CO (primary atmospheric pollutant) is attributed to increased CO emission from vehicles. We recorded a significant and positive correlation between Poaceae pollen and CO concentration in the present study. The positive effect of CO resulting from incomplete combustion of carbon materials on allergic rhinitis presence was confirmed by several researchers (Lee et al., 2003; Kim et al., 2016), however, to our knowledge, studies concerning the relationship between CO and pollen or aeroallergen concentrations are scarce. Contrary to our result, Cuinica et al. (2013) noted a significant negative effect of CO concentrations on the protein content of Betula pendula pollen even though when exposed to levels that can be considered safe for human health protection. However, more studies are needed to understand the mechanisms of the associations between CO and airborne pollen or allergen levels in the urban environment.
Particulate matters (PM) are considered as one of the most abundant air pollutants in urban areas with high levels of vehicle traffic. PM particles could bind with airborne pollen grains altering their morphology (Risse et al., 2000). Moreover, PM particles, because of their intrinsic electrostatic properties and porous surfaces, readily adhere to free airborne pollen allergens and act as their carriers (Knox et al., 1997). The presence of these particles on the surface of bioaerosols may change the dispersal pattern of bioaerosols in ambient air by altering the particle aerodynamic properties (Adhikari et al., 2006). Contrary to the results observed by Sousa et al. (2008) who pointed out that PM did not correlate well with bioaerosols because of the low contribution of the bioaerosols to the particulate matter we observed significant and positive correlation between Poaceae pollen or Phl p 5 allergen concentrations and PM10 and/or PM2.5 levels in the present study.
5. Conclusions
The study demonstrated that the relationship between airborne Poaceae pollen and Phl p 5 allergen is irregular and an increase in pollen concentration does not always result in an increase in allergen concentration. Besides, pollen potency displayed marked day-to-day variation, since pollen concentrations do not always reflect the allergen concentrations. However, the concurrent occurrence of airborne pollen and allergen peaks were observed.
Results of Spearman's correlation analysis showed that for Poaceae pollen, the significant and positive correlation was observed, except for NO2, for all atmospheric pollutants. Whereas, for meteorological parameters, the only significant correlation was observed with precipitation. However, the correlation was negative. The concentration of Phl p 5 allergen was positively associated with air temperature, ozone and PM10, while the negative association with precipitation and relative air humidity was observed.
From the respiratory allergy diseases epidemiology point of view, simultaneous pollen and allergen quantification is important to determine the aeroallergen exposure.
Declarations
Author contribution statement
Jana Ščevková: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zuzana Vašková, Jozef Dušička: Performed the experiments.
Regina Sepšiová: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Jozef Kováč: Analyzed and interpreted the data.
Funding statement
This study was supported by the Operation Program of Research and Innovation for the project: Advancing University Capacity and Competence in Research, Development and Innovation, ITMS2014+: 313021X329, co-financed by the European Regional Development Fund. This study was also supported by Grant Agency VEGA (Bratislava), Grant Nos.2/0054/18, 1/0061/20 and 1/0434/21.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge both the Division of Meteorology and Climatology (Faculty of Mathematics, Physics and Informatics of Comenius University in Bratislava) for providing meteorological data and the Slovak Hydrometeorological Institute (SHMÚ) for providing air pollution data used in this paper.
References
- Aalberse E.C. Clinically significant cross-reactivities among allergens. Int. Arch. Allergy Immunol. 1992;99:261–264. doi: 10.1159/000236261. [DOI] [PubMed] [Google Scholar]
- Acar A., Alan Ş., Kaplan A., Baysal E.Ö., Doğan C., Pinar N.M. General trends in atmospheric pollen concentration in the high populated city of Ancara, Turkey. Karaelmas Fen Ve Mühendis Derg. 2017;7:40–46. [Google Scholar]
- Adhikari A., Reponen T., Grinshpun S.A., Martuzevicius D., LeMasters L. Correlation of ambient inhalable bioaerosols with particulate matter and ozone: a two-year study. Environ. Pollut. 2006;140:16–28. doi: 10.1016/j.envpol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Aina R., Asero R., Ghiani A., Marconi G., Albertini E., Citterio S. Exposure to cadmium-contaminated soils increases allergenicity of Poa annua L. pollen. Allergy. 2010;65:1313–1321. doi: 10.1111/j.1398-9995.2010.02364.x. [DOI] [PubMed] [Google Scholar]
- Alan Ş., Şahin A.A., Sarişahin T., Şahin S., Kaplan A., Pinar N.M. The effect of geographical and climatic properties on grass pollen and Phl p 5 allergen release. Int. J. Biometeorol. 2018;62:1325–1337. doi: 10.1007/s00484-018-1536-0. [DOI] [PubMed] [Google Scholar]
- Albertine J.M., Manning W.J., DaCosta M., Stinson K.A., Muilenberg M.L., ChA Rogers. Projected carbon dioxide to increase grass pollen and allergen exposure despite higher ozone levels. PloS One. 2014;9 doi: 10.1371/journal.pone.0111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K., Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int. Arch. Allergy Immunol. 2003;130:87–107. doi: 10.1159/000069013. [DOI] [PubMed] [Google Scholar]
- Bartková-Ščevková J. The influence of temperature, relative humidity and rainfall on the occurrence of pollen allergens (Betula, Poaceae, Ambrosia artemisiifolia) in the atmosphere of Bratislava (Slovakia) Int. J. Biometeorol. 2003;48:1–5. doi: 10.1007/s00484-003-0166-2. [DOI] [PubMed] [Google Scholar]
- British Aerobiology Federation . Rotherham: National Pollen and Hayfever Bureau; 1995. Airborne Pollen and Spores. A Guide to Trapping and Counting. [Google Scholar]
- Buters J., Prank M., Sofiev M., Pusch G., Albertini R., Annesi-Maesano I., Antunes C., Behrendt H., Berger U., Brandao R., Celenk S., Galan C., Grewling L., Jackowiak B., Kennedy R., Rantio-Lehtimäki A., Reese G., Sauliene I., Smith M., Thibaudon M., Weber B., Cecchi L. Variation of the group 5 grass pollen allergen content of airborne pollen in relation to geographic location and time in season. J. Allergy Clin. Immunol. 2015;136:87–95. doi: 10.1016/j.jaci.2015.01.049. [DOI] [PubMed] [Google Scholar]
- Celenk S. Detection of reactive allergens in long-distance transported pollen grains: evidence from Ambrosia. Atmos. Environ. 2019;209:212–219. [Google Scholar]
- Christenhusz M.J.M., Byng J.W. The number of known plant species in the world and its annual increase. Phytotaxa. 2016;261:201–217. [Google Scholar]
- Cuinica L.G., Abreu I., Gomes C.R., Da Silva J.C.G.E. Exposure of Betula pendula Roth pollen to atmospheric pollutants CO, O3 and SO2. Grana. 2013;52:299–304. [Google Scholar]
- D'Amato G., Cecchi L., Bonini S., Nunes C., Annesi-Maesano I., Behrendt H., Liccardi G., Popov T., van Cauwenberge P. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- D'Amato G., Cecchi L., D'Amato M., Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Investig. Allergol. Clin. Immunol. 2010;20:95–102. [PubMed] [Google Scholar]
- De Linares C., Díaz de la Guardia C., Nieto-Lugilde D., Alba F. Airborne study of grass allergen (Lol p 1) in different-sized particles. Int. Arch. Allergy Immunol. 2010;152:49–57. doi: 10.1159/000260083. [DOI] [PubMed] [Google Scholar]
- Djunkanovic R., Feather I., Gratziou C., Walls A., Peroni D., Bradding P., Judd M., Howarth P.H., Holgate S.T. Effect of natural allergen exposure during the grass pollen season on airways inflammatory cells and asthma symptoms. Thorax. 1996;51:575–581. doi: 10.1136/thx.51.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feráková V., Jarolímek I. Bratislava. In: Kelcey J.G., Müller N., editors. Plants and Habitats of European Cities. Springer; New York: 2011. pp. 79–129. [Google Scholar]
- Fernández-González D., Rodriguez Rajo F.J., Gonzalez Parrado Z., Valencia Barrera R., Jato V., Grau S.M. Differences in atmospheric emissions of Poaceae pollen and Lol p 1 allergen. Aerobiologia. 2011;27:301–309. [Google Scholar]
- Ghiani A., Aina R., Asero R., Bellotto E., Citterio S. Ragweed pollen collected along high-traffic roads shows a higher allergenicity than pollen sampled in vegetated areas. Allergy. 2012;67:887–894. doi: 10.1111/j.1398-9995.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- González-Parrado Z., Fernández-González D., Camazón-Izquierdo B., Valencia-Barrera R.M., Vega-Maray A.M., Asturias J.A., Monsalve R.I., Mandrioli P. Molecular aerobiology – Plantago allergen Pla l 1 in the atmosphere. Ann. Agric. Environ. Med. 2014;21:282–289. doi: 10.5604/1232-1966.1108592. [DOI] [PubMed] [Google Scholar]
- Grewling Ł., Bogawski P., Ł Kostecki, Nowak M., Szymańska A., Frączak A. Atmospheric exposure to the major Artemisia pollen allergen (Art v 1): seasonality, impact of weather, and clinical implications. Sci. Total Environ. 2020;713:136611. doi: 10.1016/j.scitotenv.2020.136611. [DOI] [PubMed] [Google Scholar]
- Grewling Ł., Nowak M., Szymańska A., Ł Kostecki, Bogawski P. Temporal variability in the allergenicity of airborne Alternaria spores. Med. Mycol. 2019;57:403–411. doi: 10.1093/mmy/myy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harabina M., Peltre G., van Ree R., Moingeon P. Grass pollen allergens. Clin. Exp. Allergy Rev. 2008;8:7–11. [Google Scholar]
- Hirst J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952;39:257–265. [Google Scholar]
- Hrubiško M. Pollinosis – an actual problem also in XXI. Century. Part III: sequence and cross-reactivity of tree, grass and plant allergens by their clinical significance. Klinická Imunológia a Alergológia. 1998;2:9–17. (in Slovak) [Google Scholar]
- Jato V., Rodríguez-Rajo F.J., Seijo M.C., Aira M.J. Poaceae pollen in Galicia (NW Spain): characterization and recent trends in atmospheric pollen season. Int. J. Biometeorol. 2009;53:333–344. doi: 10.1007/s00484-009-0220-9. [DOI] [PubMed] [Google Scholar]
- Kim J., Han Y., Seo S.C., Lee J.Y., Choi J., Kim K.H., Woo S.Y., Kim E.H., Kwon H.J., Cheong H.K., Oh I., Ahn K. Association of carbon monoxide levels with allergic diseases in children. Allergy Asthma Proc. 2016;37:e1–7. doi: 10.2500/aap.2016.37.3918. [DOI] [PubMed] [Google Scholar]
- Knox R.B., Suphioglu C., Taylor P., Desai R., Watson H.C., Peng J.L., Bursill L.A. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin. Exp. Allergy. 1997;27:246e251. [PubMed] [Google Scholar]
- Lee Y.L., Shawz C.K., Su H.J., Lai J.S., Ko Y.C., Huang S.L., Sung F.C., Guo Y.L. Climate, traffic-related air pollutants and allergic rhinitis prevalence in middle-school children in Taiwan. Eur. Respir. J. 2003;21:964–970. doi: 10.1183/09031936.03.00094602. [DOI] [PubMed] [Google Scholar]
- Martin B.G., Mansfield L.E., Nelson H.S. Cross-allergenicity among the grasses. Ann. Allergy. 1987;59:149–154. [PubMed] [Google Scholar]
- Masuch G., Franz J.T., Shcoene K., Musken H., KCh Bergmann. Ozone increases group 5 allergen content of Lolium perenne. Allergy. 1997;52:874–875. doi: 10.1111/j.1398-9995.1997.tb02163.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Grau S., Aira M.J., Elvira-Rendueles B., Fernández-González M., Fernández-González D., García-Sánchez A., Martínez-García M.J., Moreno J.M., Negral L., Vara A., Rodríguz-Rajo F.J. Assessment of the Olea pollen and its major allergen Ole e 1 concentrations in the bioaerosol of two biogeographical areas. Atmos. Environ. 2016;145:264–271. [Google Scholar]
- Motta A.C., Marliere M., Peltre G., Sterenberg P., Lacroix G. Traffic-related air pollutants induce the release of allergen-containing cytoplasmic granules from grass pollen. Int. Arch. Allergy Immunol. 2006;139:294–298. doi: 10.1159/000091600. [DOI] [PubMed] [Google Scholar]
- Peel R.G., Ørby P.V., Skjøth C.A., Kennedy R., Schlünssen V., Smith M., Sommer J., Hertel O. Seasonal variation in diurnal atmospheric grass pollen concentration profiles. Biogeosciences. 2014;11:821–832. [Google Scholar]
- Plaza M.P., Alcázar P., Hernández-Ceballos M.A., Galán C. Mismatch in aeroallergens and airborne grass pollen concentrations. Atmos. Environ. 2016;144:361–369. [Google Scholar]
- Puc M. Threat of allergenic airborne grass pollen in Szczecin, NW Poland: the dynamics of pollen seasons, effect of meteorological variables and air pollution. Aerobiologia. 2011;27:191–202. doi: 10.1007/s10453-010-9188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risse U., Tomczok J., Huss-Marp J., Darsow U., Behrendt H. Health-relevant interaction between airborne particulate matter and aeroallergens (pollen) J. Aerosol Sci. 2000;31 [Google Scholar]
- Rodríguez-Rajo F., Jato V., Gonzalez-Parrado Z., Elvira-Rendueles B., Moreno-Grau S., Vega-Maray A., Fernandez-Gonzalez D., Asturias J.A., Suarez-Cervera M. The combination of airborne pollen and allergen quantification to reliably assess the real pollinosis risk in different bioclimatic areas. Aerobiologia. 2011;27:1–12. [Google Scholar]
- Rogerieux F., Godfrin D., Senechal H., Motta A.C., Marliere M., Peltre G., Lacroix G. Modifications of Phleum pratense grass pollen allergens following artificial exposure to gaseous air pollutants (O3, NO2, SO2) Int. Arch. Allergy Immunol. 2007;143:127–134. doi: 10.1159/000099079. [DOI] [PubMed] [Google Scholar]
- Ščevková J., Dušička J., Chrenová J., Mičieta K. Annual pollen spectrum variations in the air of Bratislava (Slovakia): years 2002-2009. Aerobiologia. 2010;26:277–287. [Google Scholar]
- Ščevková J., Dušička J., Hrubiško M., Mičieta K. Influence of airborne pollen counts and length of pollen season of selected allergenic plants on the concentration of sIgE antibodies on the population of Bratislava, Slovakia. Ann. Agric. Environ. Med. 2015;22:451–455. doi: 10.5604/12321966.1167712. [DOI] [PubMed] [Google Scholar]
- Schoene K., Franz J.T., Masuch G. The effect of ozone on pollen development in Lolium perenne L. Environ. Pollut. 2004;131:347–354. doi: 10.1016/j.envpol.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Solomon W., Burge H., Muilenberg M. Allergen carriage by atmospheric aerosol. 1. Ragweed pollen determinants in smaller micronic fractions. J. Allergy Clin. Immunol. 1983;72:443–447. doi: 10.1016/0091-6749(83)90579-1. [DOI] [PubMed] [Google Scholar]
- Sousa S.I.V., Martins F.G., Pereira M.C., Alvim-Ferraz M.C.M., Ribeiro H., Oliveira M., Albreu I. Influence of atmospheric ozone, PM10 and meteorological factors on the concentration of airborne pollen and fungal spores. Atmos. Environ. 2008;42:7452–7464. [Google Scholar]
- Stathopoulou E., Mihalakakou G., Santamouris M., Bagiorgas H.S. On the impact of temperature on tropospheric ozone concentration levels in urban environments. J. Earth Syst. Sci. 2008;117:227–236. [Google Scholar]
- Suárez-Cervera M., Castells T., Vega-Maray A., Civantos E., del Pozo V., Fernández-González D., Moreno-Grau S., Moral A., López-Iglesias C., Lahoz C., Seoane-Camba J.A. Effects of air pollution on Cup a 3 allergen in Cupressus arizonica pollen grains. Ann. Allergy Asthma Immunol. 2008;101:57–66. doi: 10.1016/S1081-1206(10)60836-8. [DOI] [PubMed] [Google Scholar]
- Wang Q., Nakamura S., Lu S., Xiu G., Nakajima D., Suzuki M., Sakamoto K., Miwa M. Release behaviour of small sized daughter allergens from Cryptomeria japonica pollen grains during urban rainfall event. Aerobiologia. 2012;28:71–81. [Google Scholar]