Abstract
We designed a placebo controlled, double-blind, randomized, dose-finding phase II study on OMT-28 in the maintenance of sinus rhythm after electrical cardioversion (DCC) in patients with persistent atrial fibrillation (PROMISE-AF). OMT-28 is a first-in-class, synthetic analog of 17,18-epoxyeicosatetetraenoic acid, a bioactive lipid mediator generated by cytochrome P450 enzymes from the omega-3 fatty acid eicosapentaenoic acid. OMT-28 improves Ca2+-handling and mitochondrial function in cardiomyocytes and reduces pro-inflammatory signaling. This unique mode of action may provide a novel approach to target key mechanism contributing to AF pathophysiology. In a recent phase I study, OMT-28 was safe and well tolerated and showed favorable pharmacokinetics. The PROMISE-AF study (NCT03906799) is designed to assess the efficacy (primary objective), safety, and population pharmacokinetics (secondary objectives) of three different doses of OMT-28, administered once daily, versus placebo until the end of the follow-up period. Recruitment started in March 2019 and the study will include a total of 120 patients. The primary efficacy endpoint is the AF burden (% time with any AF), evaluated over a 13-week treatment period after DCC. AF burden is calculated based on continuous ECG monitoring using an insertable cardiac monitor (ICM). The primary efficacy analysis will be conducted on the modified intention-to-treat (mITT) population, whereas the safety analysis will be done on the safety population. Although ICMs have been used in other interventional studies to assess arrhythmia, PROMISE-AF will be the first study to assess antiarrhythmic efficacy and safety of a novel rhythm-stabilizing drug after DCC by using ICMs.
Keywords: OMT-28, Omega-3 epoxyeicosanoid, Atrial fibrillation, Phase II
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting worldwide more than 30 million patients [1], [2]. AF is associated with an increased risk for stroke, heart failure and dementia, increased morbidity and mortality in affected patients and causes significant health costs [1], [2].
AF is a clinical condition which may progress from paroxysmal (duration < 7 days, self-terminating), to persistent (duration > 7 days, requires medical intervention for termination), and permanent AF (despite treatment no restoration of normal sinus rhythm or not treated) [3]. The pathogenesis is multifactorial and includes risk factors like coronary artery disease, valvular heart disease, hypertension, heart failure, diabetes, sleep apnea, obesity, inflammation, and activation of the autonomic nervous system [4].
Manifestation of electrical (e.g., ion channel dysfunction, conduction slowing) and structural (e.g., interstitial fibrosis, cellular hypertrophy, mitochondrial dysfunction) remodeling are key elements of the substrate that promotes the progression of AF to more persistent forms and contributes to the development of an atrial cardiomyopathy that supports the persistence of AF [5], [6], [7], [8], [9].
1.1. Standard of care and unmet medical need for novel therapies
Current AF treatment comprises prevention of thromboembolism and stroke, risk factor management as well as antiarrhythmic therapy by drugs, electrical cardioversion, and catheter ablation [10]. Effective long-term rhythm control improves quality of life and can reduce associated health risk factors. Despite recent advances in interventional ablation therapy [11], antiarrhythmic drugs (AADs) remain the cornerstone treatment option for the majority of AF patients worldwide, although AADs are only moderately effective and possess substantial cardiac and non-cardiac toxicity. Thus, there is an unmet need for novel AADs with improved efficacy and safety profiles [12]. The expected increase in AF prevalence with the aging of the population increases the pressure to develop improved AADs because invasive ablation procedures cannot be applied to this large patient population. In addition, the improvement of AF detection methods, e.g., the use of (insertable) cardiac monitors (ICMs) for continuous 24/7 surveillance of patients or emerging wearable, home-based AF sensors [13], enhances continuously the understanding of the disease and calls for improvement of AADs available. Compared to the electrocardiogram (ECG)-based assessment of the AF phenotypes, continuous and long-term AF monitoring offers improved opportunities to define and quantify the real AF burden, the relationship of AF burden to cardiovascular and neurological outcomes, and the success rate of AF treatments [14], [15].
Currently marketed AADs follow the classic concepts of selective channel and multichannel block. However, low long-term efficacy and the risk of proarrhythmia along with non-cardiac adverse events are relevant disadvantages limiting the clinical benefit for AF patients [16], [17], [18]. Conceptually, novel AADs should target the multifactorial pathogenesis of AF and ideally prevent the development and halt the progression of the AF-promoting atrial cardiomyopathy. Several molecular mechanisms contributing to AF-promoting electrical and structural remodeling have been identified as potential molecular targets for novel therapeutic approaches [19] including alterations in intracellular Ca2+-handling [9], [20], [21], mitochondrial dysfunction [22], oxidative stress [23], and inflammatory signaling [24], [25]. A new paradigm in the pharmacotherapy treatment of AF should consider the patients’ individual arrhythmia mechanism and co-morbidities with the aim to increase both therapeutic efficacy and safety [26].
1.2. OMT-28 to reinforce endogenous mechanisms of cardioprotection and antiarrhythmia
OMT-28 is a metabolically robust synthetic analog of 17,18-epoxyeicosatetraenoic acid (17,18-EEQ) and has been developed to mimic the antiarrhythmic and cardioprotective mechanisms of endogenous omega-3 epoxyeicosanoids [27]. Omega-3 epoxyeicosanoids are bioactive lipid mediators generated by cytochrome P450 (CYP) enzymes from long-chain omega-3 polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). 17,18-EEQ is the main product of EPA metabolism as catalyzed by CYP2J2, the major CYP epoxygenase in the human heart [28]. Upon dietary EPA/DHA supplementation, 17,18-EEQ is the predominant epoxyeicosanoid both in rodents [28] and humans [29].
Omega-3 epoxyeicosanoids protect cardiomyocytes against Ca2+ overload [28], inflammatory injury [30], and hypoxia/reoxygenation-induced loss of cell viability and mitochondrial function [31]. Most recently, the cardioprotective effect of omega-3 epoxyeicosanoids was shown to involve inhibition of inflammatory signaling [32]. There is also evidence from animal studies indicating that reinforcing the CYP epoxygenase pathway has the potential to protect against cardiac remodeling and arrhythmia [33], [34]. In line with this notion, transgenic overexpression of CYP2J2 [35], as well as inhibition of soluble epoxide hydrolase-mediated epoxyeicosanoid degradation [36], was shown to reduce ventricular tachyarrhythmia and AF vulnerability in mice.
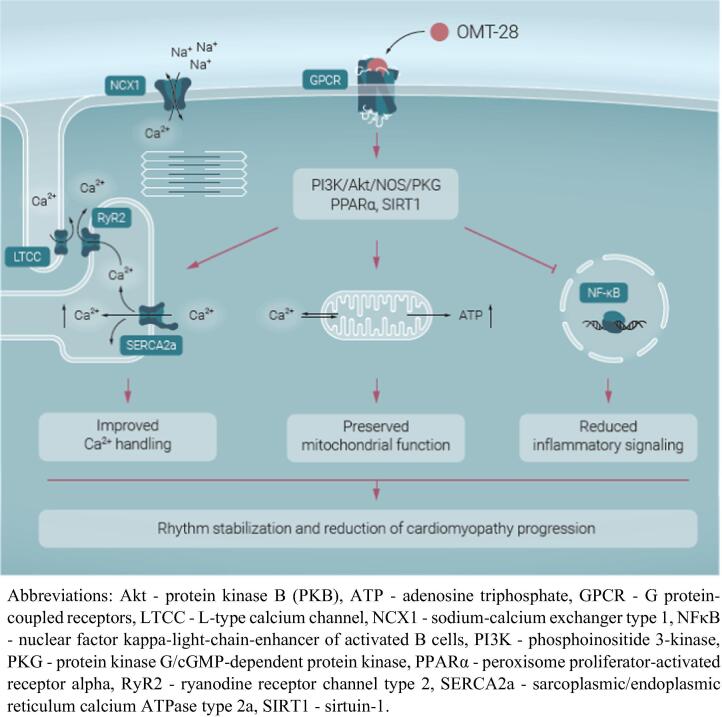
OMT-28 shares the cardioprotective and antiarrhythmic properties with its natural counterparts. Omega-3 epoxyeicosanoids and OMT-28 act via a putative Gαi protein-coupled receptor, which elicits PI3K/Akt/eNOS/PKG signaling as well as activation of sirtuin-1 and PPARα in cardiomyocytes. The net effect of this pathway consists in modulation of Ca2+-cycling, reduced electrical excitability, and protection against mitochondrial dysfunction and inflammatory injury (Fig. 1). This multimodal mode of action differentiates OMT-28 from the classical ion channel-inhibiting AADs and has the potential to simultaneously target several of the key mechanisms contributing to the evolution of the AF-promoting atrial substrate and an arrhythmia-related cardiomyopathy of the ventricles.
Fig. 1.
Putative mechanisms of antiarrhythmic actions of OMT-28.
1.3. OMT-28 – results of the phase I study
OMT-28 was developed as an orally active compound. In an already completed phase I study in 76 healthy volunteers (NCT03078738), OMT-28 was proven to be safe and well tolerated in single and multiple ascending oral doses up to 60 mg once daily for up to 14 days. OMT-28 had no detectable effect on the normal values of investigated safety laboratory parameters, as well as heart rate and blood pressure. Notably, high-resolution ECGs excluded any exposure-related increase in the QTc interval. The compound reached steady-state within 14 days of once-daily dosing and showed high oral bioavailability and favorable pharmacokinetics with a fast uptake into the plasma (tmax of 1.25–2.0 h, median) and a plasma half-life (t1/2) of 65.8–85.9 h (median), validating its suitability for oral, once daily application.
2. Methods
2.1. PROMISE-AF study design [ClinicalTrials.gov Identifier: NCT03906799]
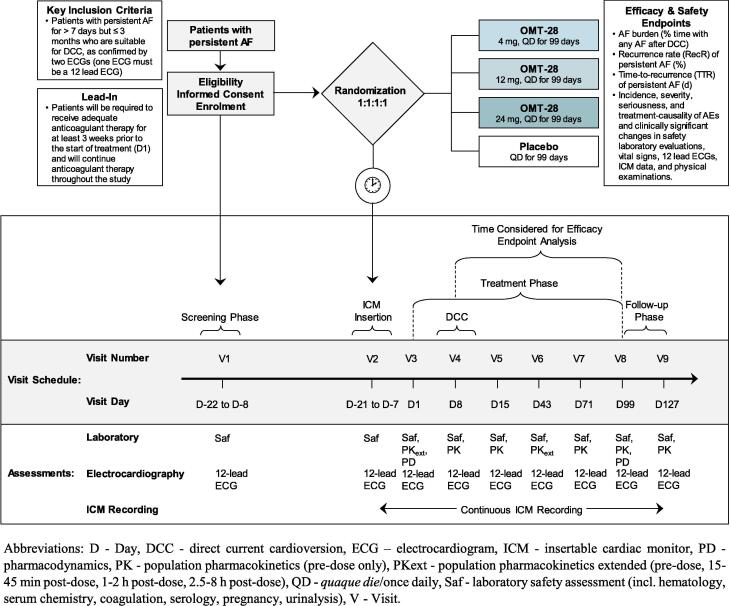
We designed a placebo-controlled, double-blind, randomized, dose-finding, multicenter phase II study in patients with persistent AF for > 7 days but ≤ 3 months who are suitable for direct current cardioversion (DCC) (Fig. 2). This study evaluates the efficacy, safety, and population pharmacokinetic (popPK) of three different doses of OMT-28 given once daily versus placebo in patients with persistent AF in maintaining sinus rhythm after DCC.
Fig. 2.
Phase II study design and flowchart.
This study will be conducted in compliance with the protocol, the ethical principles as outlined in the Declaration of Helsinki, the International Conference on Harmonization (ICH) consolidated Guideline E6 for Good Clinical Practice (CPMP/ICH/135/95), and applicable regulatory requirement(s).
2.1.1. Study population
This study will enroll patients with documented AF for > 7 days but ≤ 3 months that are eligible for DCC and anticoagulant therapy. Patients with prior or ongoing anticoagulant and/or single antiplatelet therapy are eligible as well as treatment-naïve patients. In contrast, patients with dual antiplatelet therapy are excluded because therapy also in combination with anticoagulants (i.e., triple therapy) is known to be associated with an increased risk of bleeding [37], [38], [39], [40]. Patients that have undergone surgical or catheter ablation for AF or atrial flutter are not allowed to participate in the study. However, patients with medical history of atrial flutter were not excluded because it is a common finding in AF patients and recruitment was not to be jeopardized. Nonetheless, special attention will be paid to these patients, i.e., information on incidence and duration of atrial flutter will be recorded as exploratory endpoint. Detailed inclusion and exclusion criteria are shown in Supplemental Table 1.
2.1.2. Study medication
OMT-28 will be administered orally in form of microcrystalline cellulose and anhydrous silica containing capsules of three different strengths. OMT-28 will be tested against placebo in patients receiving standard of care therapy, including anticoagulant or rate control therapy (e.g., Ca2+-channel blocker, β-blocker). Patients taking antiarrhythmic agents including dronedarone, oral amiodarone, or omega-3 fatty acids will not be included – exclusion criteria 15 and 16. If during the study, the use of above-mentioned agents is deemed necessary by the investigator, the patient must be withdrawn from the study. The first dose of study drug is administered during Visit 3 under the supervision of the investigator. The study drug is labelled according to the ICH-GCP Guideline E6 (European Commission2/3/2010).
2.1.3. Study objectives & endpoints
The primary objective of the study is to assess the efficacy of three different doses of OMT-28 administered once daily versus placebo in the maintenance of normal sinus rhythm after electrical DCC in patients with persistent AF treated with an appropriate anticoagulant therapy.
The secondary objective of the study is the assessment of (i) the safety of three different doses of OMT-28 administered once daily versus placebo after DCC in patients with persistent AF and (ii) the PK of OMT-28 by means of popPK analysis.
All primary, secondary, and exploratory study endpoints on efficacy (incl. biomarker analysis) and safety are summarized in Table 1.
Table 1.
Primary, secondary, and exploratory efficacy and safety endpoints.
| Endpoints |
|---|
| Primary Efficacy Endpoint |
|
| Secondary Efficacy Endpoints |
|
| Exploratory Efficacy Endpoints |
|
| Safety Endpoints |
|
| Pharmacokinetic and Exploratory Pharmacodynamic Endpoints |
|
2.1.3.1. Key measurement – insertable cardiac monitor
All patients will undergo ICM insertion during randomization at Visit 2. The trigger of the ICM from BIOTRONIK, BioMonitor 2-AF, will be programmed to detect the following arrhythmic events: AF, asystole, bradycardia, and high ventricular rate. The confirmation time for an AF episode is set from 6 min (standard settings) to 2 min. Further details on the study-specific programming are listed in Supplemental Table 2. Furthermore, patients will be equipped with the Remote Assistant, a device that can be used by the patient to trigger ICM recording in case of any symptoms.
Data recorded by the ICM will be transferred into the BIOTRONIK Home Monitoring® Service Center and finally into the software inSuite Cardio (Doc Cirrus), the latter allowing transfer of the data to the independent ICM Data Review Committee (ICM-DRC), comprising of qualified cardiologists who are not investigators in the study and not otherwise directly associated with the study sponsor, responsible for the evaluation of recorded episodes. The members of the committee will remain blinded to treatment throughout the evaluation process and the study. Evaluated data will be used in the final efficacy and safety analyses.
2.1.3.2. Pharmacokinetics
A popPK analysis will be performed to gain relevant PK information in patients who are representative of the target population. There will be two study visits – at the beginning of treatment and at steady state (Visit 3, Day 1 and Visit 6, Day 43 ± 3) – with one pre-dose and three post-dose blood sampling procedures (within 15–45 min post dose, 1–2 h post dose, and 2.5–8 h post-dose). At Visit 4, 5, 7, 8, and 9 there will be one pre-dose sample only.
2.1.3.3. Pharmacodynamics
Blood samples for PD assessment will be taken pre-dose at Visit 3, Day 1 and at steady state at Visit 8, Day 99. PD biomarker analysis includes the analysis of different cytokines, fatty acid profile in erythrocytes, or the endogenous CYP eicosanoid profile. Further PD analysis will be performed on proteins that are biologically related to (i) persistent AF and its progression and (ii) the known mode of action of OMT-28 and are, therefore, likely involved in further pharmacological effects. This includes biomarkers of oxidative stress, inflammation, and remodeling like growth differentiation factor 15 (GDF15), Interleukin-6 (IL-6), transforming growth factor beta-1 (TGF-β1), N-terminal pro-A/B-type natriuretic peptide (NT-proANP/NT-proBNP), tissue inhibitors of metalloproteinases metallopeptidase inhibitor 1 (TIMP-1), matrix metallopeptidase 9 (MMP-9), Galectin-3, glycated hemoglobin (HbA1c), and high-sensitivity troponin I. Measurement of C-reactive protein (CRP), total cholesterol, triglycerides, low density lipoprotein (LDL), high-density lipoprotein (HDL) and very low-density lipoprotein (VLDL) will allow to assess OMT-28-related changes in inflammation and lipid metabolism.
2.1.4. Study visit schedule
2.1.4.1. Overview
Patients will be screened for eligibility during the screening period (within 22 days prior to dosing) (Fig. 2). At latest at randomization (Visit 2), the duration of the current episode of persistent AF must be shown to be > 7 days but ≤ 3 months, as confirmed by two ECGs (one ECG must be a 12-lead ECG).
Patients will be randomized, stratified by sex in order to avoid gender-related exposure differences, in a 1:1:1:1-ratio to receive once-daily OMT-28 at a dose of 4, 12, or 24 mg or placebo. The following controls will be employed to maintain the double-blind status of the study: (i) placebo capsules will be identical in appearance to the OMT-28 capsules, (ii) investigator(s) and other members of staff involved with the study will remain blinded to the treatment randomization code during the assembly procedure, and (iii) all data will be provided to the Contract Research Organization in a blinded manner. Therefore, an Interactive Web Response System (IWRS) is implemented in the study and study medication bottles will be delivered automatically in a blinded fashion. Whenever necessary, in the opinion of the investigator, the study blind may be broken, and the patient will be withdrawn from the study. Date, time, and reason of the decision will be recorded in the patient’s source data.
At the same visit, Visit 2, patients will receive the ICM device, which will be inserted subcutaneously under local anesthesia according to local treatment guidelines.
Eligible patients with proven persistent AF must have received adequate anticoagulant therapy for at least three weeks prior to first administration of study drug (OMT-28 or placebo) on Visit 3. Patients will receive study drug for at least one week prior to DCC on Visit 4.
In total, each patient will be treated over a period of 99 days once daily with either placebo or one of three doses of OMT-28. The definition for the treatment duration goes back to an extensive literature research, which showed that the timepoint of ~13 weeks is, for most AF post-DCC trials, predictive for the 6 months outcome. A comparable study (incl. placebo-control and post-DCC treatment) from De Ferrari et al. had only a slightly longer treatment duration of 16 weeks [41]. The treatment phase is followed by an observation/follow-up phase of further 28 days (Fig. 2). No long-term follow-up is planned; however the patients are allowed to keep the ICM inserted.
Patients will be monitored for cardiac events throughout the study (Visit 2 to Visit 9) using the ICM. In addition, patients are requested to complete daily a patient diary to report subjectively perceived symptoms.
The study will include a sample size re-estimation analysis, when approximately 50% of the patients have completed the treatment phase (Visit 8).
2.1.4.2. DCC
On Visit 4 (seven days after start of study drug administration), provided that anticoagulation is adequate in the preceding four weeks, patients remaining in AF, as confirmed by 12-lead ECG, will undergo DCC (performed according to the current local institutional practices). Patients will be allowed to receive more than one direct current shock if the first attempt failed to restore sinus rhythm. A 12-lead ECG will be performed 2 h after the DCC. Only patients with a successful cardioversion (i.e., who remain in sinus rhythm for at least 2 h after the DCC) will be included in the mITT population.
All patients (including those who do not remain in sinus rhythm for at least 2 h after the DCC) will continue to receive study drug and continue to be monitored throughout the remainder of the study. Patients who were not successfully cardioverted may be replaced.
2.1.5. Sample size calculation
The study aims to demonstrate that OMT-28 compared to placebo improves at least one of the three efficacy endpoints – AF burden, recurrence rate (RecR), and time-to-recurrence (TTR) – in the 3-months treatment period (Day 8-Day 99). AF burden is defined as % time with any AF after DCC. According to the set triggers all AF episodes > 2 min duration are considered. RecR refers to persistent AF (defined as AF burden ≥ 23/24 h over 7 consecutive days) in at least one 7-day period. TTR is defined as the time (days) from the successful cardioversion to first documented recurrence of persistent AF in at least one 7-day period. Therapeutic effects of OMT-28 on RecR and/or TTR will be reflected in AF burden and no formal sample size estimation has been done for these measures. The probability of finding an expected predefined effect (statistical power) – reflected in changes of AF burden – is set to 80%. The probability of a false positive finding (significance level) is set to 5%. Statistical tests will be done with a predefined direction of difference, thus one-tailed significance level will be computed. An interim analysis will be performed if 50% of patients completed treatment phase (Visit 8). As such preliminary analysis increases the chance for false positive findings, the p-value will be adjusted for multiple testing. The O'Brien Fleming methodology of splitting alpha will be used. P-threshold for the interim analyses will be 0.005574597, leading to a remaining p-threshold of 0.044425403 for the final analysis.
The estimated sample size is the number of patients per treatment group in the mITT population, as this is the primary population for evaluation of efficacy. Since published AF burden data in a post-DCC population from an interventional study with comparable design was not available, sample size was estimated based on simulations with parameters set according to published results [42]. As base population, a random sample from a beta-distribution with shape parameters of 0.55 and 1 was drawn with 100,000 patients. From these data, a sub-population was filtered with AF burden between 2% and 95%. This population reflects the expected baseline values in the study population. From this population, 1000 samples per sample size were drawn and tested for significance. The RecR of placebo was set to 50, 60, 70, 80, or 85%. The effect size of verum compared to placebo (ΔRecR) was set - for each individual RecR placebo setting separately - to ±0, −5, −10, −20, and −30% to reflect a broad range of scenarios (e.g., RecR placebo 70%: RecR verum 70, 65, 60, 50, and 40%). As most realistic scenario, the RecR for placebo was set to 50%; the RecR for all treatment groups (verum) was set to 30%. Reduction in AF burden is the expected mean reduction of AF burden in patients with recurring AF. Atrial fibrillation burden reduction was set to 60%. This is a conservative estimate for the reduction of AF burden in the study as the “0″ for non-recurring AF will be included in the computation from study data. 1000 samples were simulated with affected/unaffected subjects according to RecR for placebo/verum and affected subjects with 60% reduced burden in simulated AF burden for verum only. For each simulation run, placebo vs. verum were compared by non-parametric tests, the rate of significant results over all simulations determined empirical power. Under these assumptions, 30 patients per study arm will give the desired statistical power. An interim analysis for sample size re-estimation will be performed once approximately 15 patients per study arm have completed the treatment phase (Visit 8) of the study to avoid an underpowered study because of imprecise estimates for study population or overoptimistic parameter estimates.
2.1.6. Study conduct & organization
2.1.6.1. Study conduct
For the purpose of study conduct, 25 study sites in 4 different countries (Bulgaria, Czech Republic, Hungary, and Ukraine) were initiated. The recruitment of the study started in March 2019 and is expected to be completed within 9 months.
2.1.6.2. Study organization
All investigators, sub-investigators, study coordinators and other involved site staff are obliged to comply with the study specifications.
An independent Data Monitoring Committee (DMC) is responsible for ensuring the safety and well-being of study patients, study integrity, and validity of study results by reviewing safety and efficacy data. Based on ongoing study data, the DMC is expected to make recommendations concerning study continuation, modification, and termination, if needed.
A quality control was implemented by installation of the independent, study-specific ICM-DRC, which will review recorded ECGs (≤6 per day per patient) in a blinded way and comment on the recorded episodes (true/false/not evaluable). Evaluated data will be considered for the final efficacy and safety analyses.
The study is funded by OMEICOS Therapeutics GmbH. The authors are solely responsible for the design and conduct of the study as well as drafting and editing of the manuscript, and its final contents.
3. Conclusions
In summary, PROMISE-AF is a clinical phase II a/b dose-finding study evaluating three doses of OMT-28 compared to placebo in patients undergoing planned electrical cardioversion for persistent AF. The use of an ICM in all patients will allow to calculate the AF burden and other standard AF efficacy endpoints based on continuous monitoring and thus provide an extensive data set for the analysis and interpretation of the study results as a guidance for further clinical development of OMT-28.
Any Potential Conflicts of Interest, Including Related Consultancies, Shareholdings and Funding Grants
Sarah Berlin, Luciana Summo, Janine Lossie, Alexander Gebauer, and Robert Fischer are employees of OMEICOS Therapeutics. Wolf Schunck is Board Director of OMEICOS Therapeutics. Andreas Goette has served as a consultant and speaker for Bayer Health Care, MSD, Sanofi Aventis, BMS/Pfizer, Daiichi Sankyo, Boehringer Ingelheim and OMEICOS Therapeutics. Dobromir Dobrev is member of Scientific Advisory Boards of OMEICOS Therapeutics, Acesion Pharma and Sanofi and received speaker’s fees for educational lectures from Boston Scientific, Novartis and Bristol-Myers Squibb. His laboratory executed research contracts for OMEICOS Therapeutics. Gerald V. Naccarelli has received the following (outside the submitted work): personal fees from Acesion, Correvio, Janssen, Milestone, OMEICOS Therapeutics, and Sanofi; grants from Janssen. Naab Al-Saady is an employee of Covance Inc. (study contract research organization). John Camm has received personal fees form Correvio, Sanofi, Acesion Pharma, OMEICOS Therapeutics, Huya, Milestone, and Incarda.
CRediT authorship contribution statement
Sarah Berlin: Writing - original draft, Visualization, Project administration. Andreas Goette: Writing - review & editing, Conceptualization, Methodology. Luciana Summo: Writing - review & editing, Project administration. Janine Lossie: Writing - review & editing. Alexander Gebauer: Writing - review & editing, Project administration, Supervision, Funding acquisition, Conceptualization, Methodology. Naab Al-Saady: Writing - review & editing. Leonardo Calo: Writing - review & editing, Conceptualization, Methodology. Gerald Naccarelli: Writing - review & editing, Conceptualization, Methodology. Wolf-Hagen Schunck: Writing - original draft, Visualization, Conceptualization, Methodology. Robert Fischer: Writing - original draft, Visualization, Funding acquisition, Conceptualization, Methodology. A. John Camm: Writing - review & editing, Conceptualization, Methodology. Dobromir Dobrev: Writing - review & editing, Conceptualization, Methodology.
Acknowledgments
Acknowledgment
We acknowledge the contributions and continuous support by.
-
•
The Clinical Advisory Board Members of OMEICOS Therapeutics GmbH: L. Caló, MD, University Cattolica, Rome (Italy); A.J. Camm, MD, FRCP, University of London and Imperial College (United Kingdom); D. Dobrev, MD, FIACS, FISHR, University of Duisburg-Essen (Germany); A. Goette, MD, FEHRA, St. Vincenz-Hospital, Paderborn (Germany); G. Naccarelli, MD, FACC, FAHA, FHRS, Pennsylvania State University, Pennsylvania (USA).
-
•
The Members of the Data Monitoring Committee (DMC): U. Tebbe, MD, B. Katgely, MD, and M. Klasser.
-
•
The Members of the Insertable Cardiac Monitor Data Review Committee (ICM-DRC): F. Günther, MD, A. Ohler, MD, and W. Haverkamp, MD, from ExCard GmbH, Berlin (Germany).
We would also like to thank A. Busjahn (HealthTwist GmbH) for his support in sample size planning, R. Sroka (Semdatex GmbH) and D. Haverkamp (ExCard GmbH) in data management.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100573.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnabel R.B., Yin X., Gona P., Larson M.G., Beiser A.S., McManus D.D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuster V., Rydén L.E., Cannom D.S., Crijns H.J., Curtis A.B., Ellenbogen K.A. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 4.Lau D.H., Nattel S., Kalman J.M., Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136(6):583–596. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 5.Andrade J., Khairy P., Dobrev D., Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014;114(9):1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 6.Goette A., Kalman J.M., Aguinaga L., Akar J., Cabrera J.A., Chen S.A. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm. 2017;14(1):e3–e40. doi: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schild L., Bukowska A., Gardemann A., Polczyk P., Keilhoff G., Tager M. Rapid pacing of embryoid bodies impairs mitochondrial ATP synthesis by a calcium-dependent mechanism - a model of in vitro differentiated cardiomyocytes to study molecular effects of tachycardia. BBA. 2006;1762(6):608–615. doi: 10.1016/j.bbadis.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukowska A., Schild L., Keilhoff G., Hirte D., Neumann M., Gardemann A. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp. Biol. Med. (Maywood) 2008;233(5):558–574. doi: 10.3181/0706-RM-155. [DOI] [PubMed] [Google Scholar]
- 9.Heijman J., Voigt N., Nattel S., Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014;114(9):1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 10.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Jr 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhof P., Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur. Heart J. 2017;38(1):20–26. doi: 10.1093/eurheartj/ehw260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijman J., Ghezelbash S., Dobrev D. Investigational antiarrhythmic agents: promising drugs in early clinical development. Expert Opin. Invest. Drugs. 2017;26(8):897–907. doi: 10.1080/13543784.2017.1353601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinhubl S.R., Waalen J., Edwards A.M., Ariniello L.M., Mehta R.R., Ebner G.S. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320(2):146–155. doi: 10.1001/jama.2018.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camm A.J. The role of continuous monitoring in atrial fibrillation management. Arrhythmia Electrophysiol. Rev. 2014;3(1):48–50. doi: 10.15420/aer.2011.3.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L.Y., Chung M.K., Allen L.A., Ezekowitz M., Furie K.L., McCabe P. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137(20):e623–e644. doi: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vamos M., Hohnloser S.H. Amiodarone and dronedarone: An update. Trends Cardiovasc. Med. 2016;26(7):597–602. doi: 10.1016/j.tcm.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Boriani G., Blomstrom-Lundqvist C., Hohnloser S.H., Bergfeldt L., Botto G.L., Capucci A. Safety and efficacy of dronedarone from clinical trials to real-world evidence: implications for its use in atrial fibrillation. Europace. 2019 doi: 10.1093/europace/euz193. [DOI] [PubMed] [Google Scholar]
- 18.L. Valembois, E. Audureau, A. Takeda, W. Jarzebowski, J. Belmin, C. Lafuente-Lafuente, Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation, Cochrane Database Syst. Rev. 9 (2019) CD005049. [DOI] [PubMed]
- 19.Heijman J., Guichard J.B., Dobrev D., Nattel S. Translational challenges in atrial fibrillation. Circ. Res. 2018;122(5):752–773. doi: 10.1161/CIRCRESAHA.117.311081. [DOI] [PubMed] [Google Scholar]
- 20.Denham N.C., Pearman C.M., Caldwell J.L., Madders G.W.P., Eisner D.A., Trafford A.W. Calcium in the pathophysiology of atrial fibrillation and heart failure. Front. Physiol. 2018;9:1380. doi: 10.3389/fphys.2018.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina C.E., Abu-Taha I.H., Wang Q., Rosello-Diez E., Kamler M., Nattel S. Profibrotic, electrical, and calcium-handling remodeling of the atria in heart failure patients with and without atrial fibrillation. Front. Physiol. 2018;9:1383. doi: 10.3389/fphys.2018.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Yang X., Li Y., Yuan M., Tian C., Yang Y. Mitochondria and the pathophysiological mechanism of atrial fibrillation. Curr. Pharm. Des. 2018;24(26):3055–3061. doi: 10.2174/1381612824666180903125300. [DOI] [PubMed] [Google Scholar]
- 23.Wolke C., Bukowska A., Goette A., Lendeckel U. Redox control of cardiac remodeling in atrial fibrillation. BBA. 2015;1850(8):1555–1565. doi: 10.1016/j.bbagen.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Yao C., Veleva T., Scott L., Jr., Cao S., Li L., Chen G. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138(20):2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott L., Jr., Li N., Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int. J. Cardiol. 2019;287:195–200. doi: 10.1016/j.ijcard.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int. J. Cardiol. Heart Vasculature. 2018;21:11–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schunck W.H., Konkel A., Fischer R., Weylandt K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018;183:177–204. doi: 10.1016/j.pharmthera.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J. Biol. Chem. 2010;285(43):32720–131733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 2014;55(6):1150–1164. doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.V. Samokhvalov, K.L. Jamieson, J. Vriend, S. Quan, J.M. Seubert, CYP-epoxygenase metabolites of docosahexaenoic acid protect HL-1 cardiac cells against LPS-induced cytotoxicity Through SIRT1, Cell Death Discov. (2015) 1. [DOI] [PMC free article] [PubMed]
- 31.Samokhvalov V., Jamieson K.L., Fedotov I., Endo T., Seubert J.M. SIRT is required for EDP-mediated protective responses toward hypoxia-reoxygenation injury in cardiac cells. Front. Pharmacol. 2016;7:124. doi: 10.3389/fphar.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darwesh A.M., Jamieson K.L., Wang C., Samokhvalov V., Seubert J.M. Cardioprotective effects of CYP-derived epoxy metabolites of docosahexaenoic acid involve limiting NLRP3 inflammasome activation (1) Can. J. Physiol. Pharmacol. 2018:1–13. doi: 10.1139/cjpp-2018-0480. [DOI] [PubMed] [Google Scholar]
- 33.Westphal C., Konkel A., Schunck W.H. CYP-eicosanoids–a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011;96(1–4):99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 34.P. Sirish, N. Li, V. Timofeyev, X.D. Zhang, L. Wang, J. Yang, et al., Molecular mechanisms and new treatment paradigm for atrial fibrillation, Circul. Arrhythmia Electrophysiol. 9(5) (2016). [DOI] [PMC free article] [PubMed]
- 35.Westphal C., Spallek B., Konkel A., Marko L., Qadri F., Degraff L.M. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0073490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu D., Li N., He Y., Timofeyev V., Lu L., Tsai H.J. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci. USA. 2006;103(49):18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanas A., García-Rodríguez L.A., Arroyo M.T. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin nonsteroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731–1738. doi: 10.1136/gut.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen M.L., Sørensen R., Clausen M.T. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch. Intern. Med. 2010;170:1433–1441. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- 39.Lopes R.D., Rao M., Simon D.N. Triple vs dual antithrombotic therapy in patients with atrial fibrillation and coronary artery disease. Am. J. Med. 2016;129:592–599e1. doi: 10.1016/j.amjmed.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Sørensen R., Hansen M.L., Abildstrom S.Z. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967–1974. doi: 10.1016/S0140-6736(09)61751-7. [DOI] [PubMed] [Google Scholar]
- 41.De Ferrari G.M., Maier L.S., Mont L., Schwartz P.J., Simonis G., Leschke M. Ranolazine in the treatment of atrial fibrillation: results of the dose-ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following An ELectricaL CardiOversion) study. Heart Rhythm. 2015;12(5):872–878. doi: 10.1016/j.hrthm.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Charitos E.I., Pürerfellner H., Glotzer T.V., Ziegler P.D. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence. JACC. 2014;63(25):2840–2848. doi: 10.1016/j.jacc.2014.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.