Abstract
Stress proteins (SPs) including heat-shock proteins (HSPs), RNA chaperones, and ER associated stress proteins are molecular chaperones essential for cellular homeostasis. The major functions of HSPs include chaperoning misfolded or unfolded polypeptides, protecting cells from toxic stress, and presenting immune and inflammatory cytokines. Regarded as a double-edged sword, HSPs also cooperate with numerous viruses and cancer cells to promote their survival. RNA chaperones are a group of heterogeneous nuclear ribonucleoproteins (hnRNPs), which are essential factors for manipulating both the functions and metabolisms of pre-mRNAs/hnRNAs transcribed by RNA polymerase II. hnRNPs involve in a large number of cellular processes, including chromatin remodelling, transcription regulation, RNP assembly and stabilization, RNA export, virus replication, histone-like nucleoid structuring, and even intracellular immunity. Dysregulation of stress proteins is associated with many human diseases including human cancer, cardiovascular diseases, neurodegenerative diseases (e.g., Parkinson’s diseases, Alzheimer disease), stroke and infectious diseases. In this review, we summarized the biologic function of stress proteins, and current progress on their mechanisms related to virus reproduction and diseases caused by virus infections. As SPs also attract a great interest as potential antiviral targets (e.g., COVID-19), we also discuss the present progress and challenges in this area of HSP-based drug development, as well as with compounds already under clinical evaluation.
Subject terms: Molecular biology, Drug regulation
Overview of stress proteins
Stress proteins (SPs) are a diverse group of proteins that are synthesized at increased levels when cells are exposed to either intracellular or extracellular stressful stimuli. They exhibit protective effects against stresses. Stress proteins include heat shock proteins (HSPs), RNA chaperone protein (RNPs), and proteins mainly function in the endoplasmic reticulum (ER): peptidyl-propyl isomerases, protein disulfide isomerases (PDIs) and the lectin-binding chaperone system.1 SPs are ubiquitously expressed in all kind of cells, triggering signal cascades for neutralizing and eradicating the stresses occurring both intracellularly (e.g., pathogen invasion) and extracellularly (e.g., starvation, stimulation by cytokines/chemokines or hormones). Responses triggered by SPs can either activate pathways to promote cell survival or initiate cell death (i.e., apoptosis, necrosis, pyroptosis or autophagic cell death) for eliminating the damaged cells to protect a particular organ/tissue under given conditions. It is widely noted that the dysregulation of stress proteins is associated with a variety of human diseases, including cardiovascular diseases, neurodegenerative diseases (e.g., Parkinson’s diseases, Alzheimer disease), stroke, human cancers and infectious diseases. In this review, we focus on their functions and update findings involved in infectious diseases, particularly, the diseases caused by viral infections.
Heat shock proteins
In 1962, an Italian geneticist Ritossa inadvertently elevated the incubation temperature of Drosophila larvae and discovered an increased gene transcription of unknown proteins. He nominated these protein as HSPs.2 Further studies have revealed a large number of HSPs, which form a big family and are ubiquitously expressed in cells. Based on the molecular weight, HSPs are classified into different families, including HSP100s, HSP90s, HSP70s, HSP60s, HSP40s, and some small HSPs (15–40 kDa).3–5 HSPs belong to the largest family of chaperones. The HSP expression is rapidly induced when cells meet physiological or environmental attacks such as starvation, high temperature, hypoxia or hyperoxia, pathogen invasion, malnutrition and exposure to chemicals or UV, etc. They form a network to promote or stabilize the correct folding of substrate protein to gain its functional/active conformation (Fig. 1), although they may not associate with the substrate protein in the final structure.3,6 HSPs are important factors in regulating cell survival, differentiation and cell death. Accumulating evidence shows that some HSPs participate in not only innate cellular immunity but also antigen presentation in adaptive immune response.7,8 HSPs also serve as potential biomarkers for some diseases. It has been shown that the increase of Hsp70 in plasma is associated with heart failure,9 and the elevated Hsp27 level in human peripheral blood mononuclear cells is related with coronary artery disease (CAD).10
Fig. 1.

The general chaperone cycle of Heat shock proteins. Initially, unfolded client protein bound to the HSP70-HSP40 chaperones interacts with the HSP90. ATP binding to HSP90 induces the client proteins transfer from HSP70 to HSP90. Later, the conformation of HSP70-HSP40 chaperones will be released. Finally, the hydrolysis of ATP induces additional conformation changes leading to the client protein release
Hsp90s
Hsp90, an abundant chaperone in all eukaryotic cells, controls a variety of critical signalling pathways in eukaryotic cells.5,6,11 Hsp90 is an ATP-dependent chaperone with different isoforms, including 1) Hsp90α (HSP90AA1, or HSPC1), Hsp90α-A2 (HSPAA2, or HSPC2) and Hsp90β (HSPAB1, or HSPC3) locate in cytoplasm. 2) Glucose regulated protein Grp94 (HSPC4 or GP96) locates in the ER. 3) TRAP1 (HSPC5) locates in mitochondria.12 Among them, Hsp90α and Hsp90β account for the greatest proportion in humans. Hsp90 contains three regions: an ATPase-dependent hydrolytic domain in the N-terminal region, a middle linker region, and a dimerization domain in the C-terminal region.5 Like other HSPs, Hsp90 binds non-native substrate peptide to prevent its aggregation and degradation. When Hsp90 binds ATP, a transient dimerization of the N-terminal domain allows the substrate peptide binding to Hsp90. Then the ATP hydrolysis and energy release lead to a conformational change of the N-terminal domain that facilitates the correct folding of the substrate petite (Fig. 1).5,6 Besides, Hsp90 is also involved in telomere maintenance, apoptosis, and cell cycle progression, etc.6,13 It is well known that Hsp90 not only interacts and contributes to RNA polymerase assembly and nuclear import of some (−) ssRNA viruses (e.g., PB2 of influenza virus), but plays crucial roles in the folding process of viral capsid proteins and virion assemblies as well.14
Cochaperones of Hsp90
Cochaperones of Hsp90 regulate hsp90 functions at many aspects. CDC37 (also called p50) delivers kinase to Hsp90 and inhibits its ATPase activity; Carboxyl terminus of Hsp70-interacting protein (CHIP) functions as E3 ubiquitin ligase; Hsc70/Hsp90-organizing protein (HOP, also called STI1) inhibits dimerization of the N-terminal domain; and the activator of Hsp90 ATPase 1 (AHA) and p23 participates the maturation of substrate peptides.11,15
Hsp70s
HSP70 is a subfamily of HSPs’ superfamily with ~70 kDa molecular weight. It accounts for the majority of molecular chaperones in cells.11 The members of the Hsp70 family mainly include: (1) Hsp72 (HSPA1A), Hsp70-2 (HSPA2), Hsp70B’ (HSPA6) and Hsc70 (HSPA8) are commonly located in the cytosol; (2) Grp75 (HSPA9) is located in mitochondria; (3) Grp78 (HSPA5) is associated with the ER.16 Hsp70 consists of two domains: a 44-kDa nucleotide-binding domain (NBD) which can be divided into four subdomains (IA, IB, IIA, and IIB) in the N-terminal region and a 28-kDa substrate-binding domain (SBD) composed of C-terminal α-helical (SBDα) and N-terminal β-sheet (SBDβ) subdomains in the C-terminal region.17,18 As a critical component of cellular protein surveillance, the ATP-dependent molecular chaperone protects cells from damage caused by stress and takes part in a number of folding processes, including folding of newly synthesized polypeptides, recognition and refolding of misfolded or aggregated proteins, solubilization or degradation of proteins, transporting proteins, assembly or disassembly of oligomeric protein complexes, and the regulation of certain natively folded proteins.5,13,19,20
The functions of Hsp70 are not limited to host protein folding. Its functions are considerable during viral infection. The members of Hsp70s exhibit quite different roles in the course of virus life cycle. For example, Hsp72, Hsp70B’ and Hsc70 participate in the HCV viral entry, virion assembly and translation of the viral genome. Grp78 in ER is associated with the homeostasis of viral proteins and prevents the overload of viral proteins in host cells. In hepatocytes, the elevated Grp78 stimulates innate immunity to restrict or eliminate hepatitis B virus (B) replication.21 Grp75 interacts with the NS5A protein of HCV in mitochondria.22 Accumulating evidence shows that Hsp70 interacts with viral components of Human cytomegalovirus, Rabies virus, Respiratory syncytial virus, Human papillomavirus, Herpes simplex virus.
Chaperone cycle of Hsp70
The chaperone cycle is mediated by the N-terminal NBD, which regulates the binding of Hsp70 with substrates through switch of two states. The first state is the ATP- bound state with low affinity for substrate binding, i.e., a high association and dissociation rate of the substrate peptides to the SBD. The second state is ATP hydrolysis that switches to the ADP-bound and nucleotide-free state. At this state, the substrate exchange rates are low while the affinity to substrates is high. The chaperone activity of Hsp70 mostly relies on ATP hydrolysis. The basal ATPase of Hsp70 is normally low unless it is stimulated by the substrate peptide itself. It takes 20–30 min for a molecule of ATP to be hydrolysed per Hsp70 molecule at 30 °C. As a result, it is necessary for some cochaperones to encounter with Hsp70 ATP to induce ATP hydrolysis and help the increase of the affinity for substrate peptides.19,23
Cochaperones of Hsp70
The most crucial cochaperones of Hsp70 are members of the J- domain proteins (JDPs) family and nucleotide exchange factors (NEFs) family. Previous studies focused on the function of Hsp70 machinery and led to the development of a “canonical model” for its mode of action. The model contains two steps. First, the unfolded peptide substrates bind JDPs of the Hsp40 family; then the substrates are delivered to Hsp70 that stimulate the Hsp70’s ATPase activity. Simultaneously, JDPs prevent the aggregation of unfolded proteins. Second, NEFs work as substrate release factors that assure the substrate to fold into the correct and active conformation. In this way, the cochaperones strongly facilitate the function of Hsp70. Therefore, Hsp70 generally does not work individually but cooperates with cochaperones.23,24
Hsp60s
Hsp60s are large cylindrical oligomers with two back-to-back rings.19 The non-native proteins of the central cavity in each ring are folded into the native protein through an ATP- dependent process.25,26 Hsp60s are classified into two subfamilies. Group I is mainly in prokaryotes, while group II appears in eukaryotic cytosol and some archaea.27,28
The most well-studied one in Group I is the GroEL– GroES chaperonin system in the cytosol of bacteria. GroEL is an about 57 kDa protein with two rings arranged back-to-back; and 7 subunits form a tetradecamer structure. GroES is the cochaperone of GroEL.11 The two rings-tetradecamer structure appears in two forms include asymmetric (1 GroEL: 1 GroES) and symmetric (1 GroEL: 2 GroES) complexes, which are described as “bullet”14,29,30 and “American football”31 shaped respectively. The GroEL-GroES chaperonin undergoes allosteric regulation dependent on ATP and which completes the protein folding function. The polypeptide binds to the hydrophobic sites of one of the seven subunits of GroEL and changes conformation upon ATP binging and hydrolysis. With the help of cochaperone GroES, the polypeptide finishes its folding process.32 In contrast to the GroEL–GroES system, the mammalian homolog Hsp60/Hsp10 system is less studied. Hsp60 is thought to be imported into the mitochondria and converted into its mature form with a molecular size of 58 kDa.33–35 Hsp60 exhibits important roles in facilitating protein folding, transportation and proteostasis in mitochondria.36,37
Group II chaperonins include the archaeal thermosome and eukaryotic CCT (chaperonin-containing TCP1, or called TriC), which are oligomers with eight to nine subunits with molecular weight 57–61 kDa in each ring. Compared to group I, group II chaperonins show different allosteric movements by ATP binding.11,19
HSP60 family is known to participate in viral life cycle at various stages from viral attachment to the replication of the viral genome. Hsp60s are essential for host cell immunity regulation. Some viral proteins require Hsp60 for its translocation within host cells. PB2 is a subunit of Influenza A viral RNA polymerase, which mostly locates in the nucleus but also appears in mitochondria.38 When the virus infects the host cells, PB2 is responsible for maintaining the function of mitochondria. PB2 interacts with mitochondrial antiviral signaling protein (MAVS) to downregulate intracellular immune response by decreasing the level of IFNβ so that the invading virus can easily escape from the defence of host cells.39 Hsp60 takes the role of transporting PB2 from cytosol to mitochondria in the host cells. Besides, Hsp60 shows great regulatory functions on innate immunity by inducing pro-inflammatory cytokine release, such as TNF-a, IL-6, and IL-1b, etc.40
Small heat shock proteins
Small heat shock proteins (sHSPs) are a group of small proteins with a low molecular weight ranging from ~15 to 40 kDa.4 There are 10 members in the sHSP family and some are ubiquitous including Hsp27 (HSPB1), HSPB5 (αB-crystallin, or αBC), Hsp20 (HSPB6), and Hsp22 (HSPB8), while the others are tissue-specific including HSPB2 (myotonic dystrophy protein kinase, or MKBP), HSPB3, HSPB4 (αA-crystallin, or αAC), HSPB7 (cvHsp), HSPB9, and HSPB10 (sperm outer dense fiber protein, or ODF).41 sHSPs can exist as monomers, dimers or even large multimeric complexes in the cells.42 The structure of sHSPs is different from the other HSPs due to their less conserved sequences. The basic structure of sHSPs is a conserved α-crystallin domain (ACD) flanked by two non-conserved domains including the N-terminal sequence (NTS) and the C-terminal sequence (CTS). Among these domains, ACD becomes the characteristic of different sHSPs.43,44
sHSPs play crucial roles in several physiological processes regarding stress tolerance, apoptosis, aging, and longevity.45–48 The phosphorylation together with the N-terminal WDPF motif helps sHSPs to form homo- or hetero-oligomers.49,50 The oligomerization is the hallmark of sHSPs for supporting their quite different activities. Phosphorylation favors small oligomer formation while dephosphorylation provokes a shift toward large oligomer formation.51 Oligomerization dynamics is crucial for chaperone activity because it gives rise to the possibility to format different homo- and hetero-oligomers, each with different binding properties to chaperone a broad range of substrates.41,52 For instance, the phosphorylated species are required for actin dynamics. Small phosphorylated dimers/tetramers bind F-actin to regulate actin polymerization.53
Among different sHSPs, Hsp27 has been broadly studied. Hsp27 exists in all tissues though it is known to mainly express in cardiac, skeletal and smooth muscles.54 Its importance has been demonstrated in cell differentiation, cell survival, cellular innate immunity, viral protein translation, and intracellular virus transport, etc.55–57 Same as the other sHSPs, Hsp27 shares a highly conserved α-crystallin domain.41,58,59 Hsp27 is capable of oligomerization and phosphorylation. There are three serine residues 15, 78, and 82 can be phosphorylated by different kinases including p90Rsk, PKG, MAPKAP kinases, etc.55 The phosphorylation of Hsp27 is a reversible process. The dephosphorylation contributes to the formation of large size oligomers.60,61 Hsp27 can not only form large homo oligomers up to 800 kDa; but it can also cooperate with other sHSPs (e.g., Hsp20) to form heteromeric structures.56,58 Hsp27 is upregulated and activated upon infections.62,63 The elevated Hsp27 activity promotes either cytoskeletal stability or cell motility,64,65 and prevents apoptosis.66 Hsp27 is required for IL-1- induced expression of the pro-inflammatory mediators IL-6, IL-8, and cyclooxygenase-2.67–69 Hsp27 is also linked to various signalling pathways involved in the development, differentiation, and cell growth.70,71 The long-term and high-level expression of Hsp27 stimulated by variety of stresses (such as HBV or EBV infections) enhances carcinogenesis, cell survival, stemness of cancer cells, cancer metastasis, tumour formation and drug resistance.
Transcriptional regulation of the HSPs
Heat shock factors (HSFs) display great contributions in regulating the transcriptional activation of hsp genes. In all invertebrate animals, only HSF1 is responsible for the transcriptional activation. In vertebrates, four members of HSF family (HSF1-4) regulate HSP expression.72 Among them, HSF1 is the most critical one. The fibroblasts from hsf1−/− mice undergo apoptosis upon heat stress because of no hsp transcription.73 Upon stress conditions, the originally monomeric HSF1 in the cytoplasm could trimerize and translocate into the nuclei to promote the hsp expression by binding on the heat shock elements (HSE) in the promoter region.74
Protein disulfide isomerase
Protein disulfide isomerase (PDI) is a multifunctional oxidoreductase and chaperone that catalyses the formation, isomerization and reduction of disulfide bonds in the endoplasmic reticulum (ER). During disulfide bond formation, cysteine residues at the CGHC active site of PDI accept two electrons from the cysteine residues in polypeptide substrates, leading to the reduction of PDI and oxidation of the substrate. Then PDI transfers the electrons to an acceptor to start another cycle of disulfide bond formation.75 In addition to PDI’s catalytic function as a thiol-disulfide isomerase, it also exhibits molecular chaperone properties for glycosylated protein quality control.76 ERp57 (PDIA3, Grp58) is possibly the most thoroughly studied PDI family member that shares a similar structure consisting of four domains (namely a-b-b’-a’) and possesses two localization sequence—an ER retention signal (QDEL), and a nuclear localization signal (KPKKKKK). Unlike other PDI family members that directly bind the substrates for their reductase or isomerase activities, the b domains of ERp57 have a high affinity to associate with calreticulin (CRT) and calnexin (CNX), which would help to recognize and recruit polypeptide segments of the glycoproteins.77 If the protein is not correctly folded, UDP-glucose:glycoprotein glucosyltransferase (UGGT) would be recruited to reglycosylate the proteins, allowing them to be recognized and re-associated by ERp57/CRT/CNX complex.76,78,79 Considering the essential roles of PDIs in the oxidative folding and chaperone-mediated protein quality control, they are now linked to a growing range of diseases including those are caused by virus infection.
RNA chaperones
Proteins that interact non-specifically with RNA and resolve the non-functional inhibitory structures are usually referred to as RNA chaperones, which have distinct roles without common sequences or motifs.80,81 They participate in a large number of cellular processes, including chromatin remodelling, transcription regulation, RNP assembly and stabilization, RNA export, histone-like nucleoid structuring, intracellular immunity, and viral RNA replication and translation. RNA molecules mostly rely on well-defined 3D structures to fulfill their functions. However, the process of RNA folding is very complicated.82 The multitude of possible RNA base-pairings together with the high stability of RNA duplexes would give rise to a large number of alternative secondary and tertiary structures that are thermodynamically as stable as the functional, native structure.83 RNA chaperones promote RNA folding by accelerating the escape from kinetic folding traps and prevent RNAs from being trapped in non-functional conformations.84–86 So far, no protein has been characterized whose primary function is to resolve non-specifically misfolded RNAs in cells.80,81
HnRNPs are a group of heterogeneous nuclear ribonucleoproteins. They are essential factors for manipulating both the functions and metabolisms of pre-mRNAs/hnRNAs transcribed by RNA polymerase II. More than 20 hnRNPs have been identified to date. hnRNPs contain common RNA binding motifs like arginine glycine boxes (RGG boxes), RNA recognition motifs (RRMs), hnRNP K homology (KH)-domains and zinc finger (ZF)-domains (KHZF domain).87 Well-defined functions of this family include transcription regulation, pre-mRNA splicing, 3′-end formation, mRNA packaging, RNA transport, translational regulation, RNA silencing, DNA repair, and telomere biogenesis. They also have the ability to shuttle between nucleus and cytoplasm, therefore could transiently help to form RNP complexes in nucleus and also participate in RNA metabolism in cytoplasm.88 A large collection of hnRNPs are involved in virus activities, most of which were first identified using viral RNA–protein binding assays, followed by functional assays.89
The importance of stress proteins
One of the main functions of stress proteins is to maintain cellular homeostasis. Under pressure, stress proteins are hyperactive to release the pressure. Hsp27, Hsp70 and hsp90 accumulate to a very high level in quite a few types of cancer cells.90–92 Although the mechanism underlying the increase has not been fully understood, it suggests that the fast increased HSPs respond to the folding stresses. With tumor progression, the accumulating oncogenic proteins need powerful protein folding ability. Under long-term stresses, HSPs participate or promote carcinogenesis, cell survival, anti-apoptosis, angiogenesis, cancer cell stemness, invasion and metastasis.93 However, HSPs and RNA chaperones are downregulated in nearly all age-related neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and several polyglutamine diseases such as Huntington’s disease and different forms of spinocerebellar ataxias94,95 (Fig. 2). Downregulated RNA chaperones lead to disorder of RNA metabolism;94 while the attenuated HSPs result in a failure of the protein quality control (PQC) system to adequately handle the folding or timely degradation of proteins in neurologic disease.95 Both mechanisms cause protein aggregates, the hallmark of age-related neurodegenerative diseases. In this review, instead of paying much attention to these topics, we would only focus on the main biological functions and target values of stress proteins in human diseases caused by pathogen infections, particularly by virus infections because the well-developed antibiotics have already controlled the bacterial infection very well since 1950s.
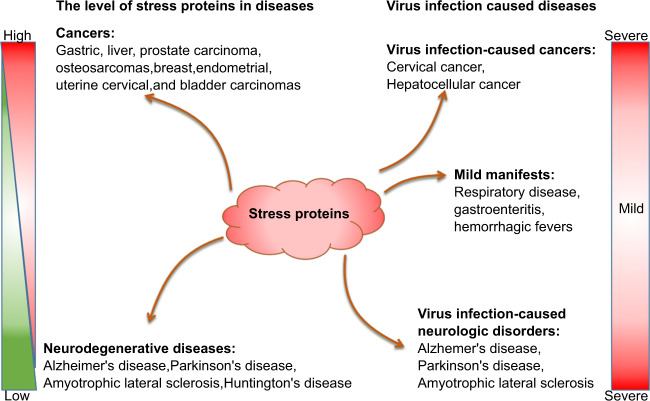
Fig. 2.
Diseases caused by dysfunction of stress proteins and their dysregulation by virus infections. The left panel shows the expression level of stress proteins in diverse human diseases, including cancers and neurodegenerative diseases. The expression level of stress proteins is significantly elevated in cancer cells but dramatically decreased in neurodegenerative disease. The right panel shows the virus infection-caused diseases from mild to severe which include cancers and neurodegenerative diseases. Stress proteins contribute to these diseases
Viruses infection causes a variety of diseases that are highly associated with dysregulation of stress proteins (Fig. 2), e.g., respiratory symptoms,96,97 gastroenteritis,98–101 Hemorrhagic fevers.102,103 Critically, it has been demonstrated that neurodegenerative diseases as well as neurobehavioral disorders are the consequences of loss of neurons and axons in the central nervous system with ageing. It is evidenced that these diseases are possibly caused by chronic neuropathic viral infections.104 For example, HIV infection can cause neurocognitive disorder. To date, increasing evidence shows that systemic viral infections are often associated with some neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, autism spectrum disorders.104,105 Over 20% of cancer cases are attributable to viral infection worldwide. The viruses associated with the greatest number of cancer cases are the human papillomaviruses (HPVs) and hepatitis viruses (HBV and HCV). HPV infection causes cervical cancer and several other epithelial malignancies, and HBV/HCV infections are responsible for the majority of hepatocellular carcinoma. Other oncoviruses include Epstein-Barr virus (EBV), Kaposi’s sarcoma-associated Herpes virus (KSHV), human T-cell leukemia virus (HTLV-I), and Merkel cell polyomavirus (MCPyV), and HIV.106 Regarding these diseases caused by the virus infections, a lot of fundamental questions have not been fully addressed. In this review, we pay special attention to the impact of stress proteins responding to virus infections on both virus reproduction and pathogenesis of diseases.
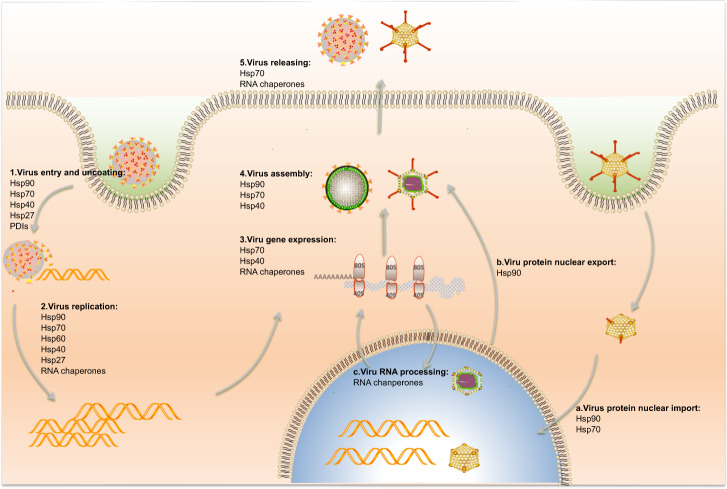
Stress proteins are involved in many steps of virus infection process, including virus entry, uncoating, replication, gene expression, as well as virus assembly and releasing as steps 1–5 shown (Fig. 3) Some viruses replicate in the nucleus, while stress proteins take part in the virus protein and/or RNA nuclear import/export processes as setp a–c shown (Fig. 3). The summary of the relationship between stress proteins, virus infection as well as host cell response is listed in Table 1.
Fig. 3.
Stress proteins participate in diverse steps of the virus life cycle. Five major steps of the virus life cycle are labeled as steps 1–5: virus entry and uncoating, virus replication, gene expression, assembly and release. Most viruses only undergo these steps in cytoplasm especially RNA viruses like EV-A71, DENV, JEV etc. While some viruses (such as influenza, SV40, HBV etc) also enter into nucleus of host cells. They may undergo other steps in their life cycle which are labelled as a–c: virus nucleus import, nucleus export, and virus RNA processing. During the process of virus infection, Hsp90, Hsp70, Hsp60, Hsp40, Hsp27, and PDIs participate in the virus entry and uncoating steps. Hsp90, Hsp70, Hsp60, Hsp40, Hsp27 and RNA chaperones take part in the virus replication step. Hsp70, Hsp40 and RNA chaperones are required in virus gene expression step. Hsp90, Hsp70 and Hsp40 assist virus assembly. Hsp70 and RNA chaperones contribute to the virus release. While Hsp90 is also important in virus nucleus import and export. Hsp70 plays a role in the virus nucleus import. And RNA chaperones play a major role in virus RNA processing, including replication, initiation of translation, stabilization and decay
Table 1.
Stress proteins participate in different steps of diverse viruses replication processes
| Chaperone family | Selected Members | Function in viral infection | Related viruses |
|---|---|---|---|
| Hsp90 | Virus entry | RNA virus: EV-A71,107 DENV,110 JEV108 | |
| DNA virus: HSV,131 HBV132 | |||
| Virus replication | RNA virus: Influenza,118 Paramyxoviruses:112,113 VSV, HPIV-2, HPIV-3, SV41 or RSV; CHIKV,114 HCV115 | ||
| DNA virus: HSV,144,145 EBV,147,163 HBV135 | |||
| Virus Protein Maturation and Virus Assembly | RNA virus: HCV,123 Picornaviruses, Poliovirus, Rhinovirus and Coxsackievirus,124 Noroviruses,125 Influenza126 | ||
| DNA virus: HBV169,170 | |||
| Virus gene expression | DNA virus: HSV,150 HCMV,151,152 VZV, EBV, KSHV,153 EBV146,164 | ||
| Virus-induced Tumorigenesis | DNA virus: EBV175,176 | ||
| Immunity modulation | DNA virus: EBV177 | ||
| Cellular transformation | RT virus: HTLV153 | ||
| Hsp70 |
GRP78 Hsc70 HSP70 HSP72 |
Virus entry | RNA virus: CAV-9,186 EV-A71,188 DENV,110,189 JEV,191 ZIKV190,197 |
| DNA virus: SV40,234 AdV,237 HSV,241 polyomavirus254 | |||
| RT virus: HTLV-1,258,259 HIV-1260 | |||
| Virus replication | RNA virus: MuV,201 CDV,203,204 HCV,206 RSV,209 EBOV,212,213 Influenza217 | ||
| Virus gene expression | RNA virus: Coxsackievirus B3,218 EV-A71,219 Influenza A222–226 | ||
| DNA virus: Polyomavirus, JCV, HCMV,245,246 HBV247–249 | |||
| Assembly | RNA virus: Reovirus,227 Poliovirus,228 Coxsackievirus B1,228 Influenza229 | ||
| DNA virus: Polyomavirus254 | |||
| Virus release | RNA virus: HCV232,233 | ||
| Cellular transformation | DNA virus: SV40,264–267 HPV,275 AdV278 | ||
| Cell survival and apoptosis | DNA virus: EBV282 | ||
| Immunity modulation | DNA virus: HBV294 | ||
| Hsp60 | Virus replication | DNA virus: HBV325,326 | |
| Immunity modulation | RNA virus: JEV,317 Influenza,38,39,318 DENV319 | ||
| DNA virus: HBV331,335 | |||
| Apoptosis regulation | RNA virus: HCV,320–322 Rotavirus323 | ||
| DNA virus: HBV329,330 | |||
| Genome integration | RT virus: HIV336,338 | ||
| Hsp40 |
Hdj2 Hsp40/DnaJB1 Hsp40/DnaJA1 Hsp40/p58IPK DNAJC14 DNAJA3 Hdj1 hTid1 DnaJB6 |
Virus entry | RT virus: HIV374,375 |
| Virus replication | RNA virus: JEV,339 Influenza340,343,344 | ||
| DNA virus: HPV,355–357 HSV,359,360 HBV 361,362 | |||
| Virus Gene expression | RNA virus: Influenza225,346 | ||
| RT virus: HIV377–379 | |||
| Protein maturation | RNA virus: YFV350 | ||
| Immunity modulation | RNA virus: HFDV354 | ||
| Cellular transformation | DNA virus: HBV,363–366 HPV275 | ||
| Small chaperone | Hsp27 | Viral replication | RNA virus: EV-A71,384 Swine fever virus (CSFV),388 Porcine epidemic diarrhoea virus (PEDV)389 |
| DNA virus: Adenovirus,390 HSV-1,397 RRV,397 PCV2,399 EBV386 | |||
| PDIs |
PDI ERdJ5 ERp57 |
Virus entry and uncoating | RNA virus: Newcastle disease Virus,402 DEGV402–404 |
| DNA virus: SV40413 | |||
| RT virus: HIV407,410 | |||
| Virus translation | RNA virus: EV-A71415 | ||
| Oxidative stress and ER stress | RNA virus: Influenza,419 HCV,421 EMCV,422 RSV,423 JEV424 | ||
| DNA virus: HBV,420 HPV425 | |||
| RT virus: HIV418 | |||
| RNA chaperone |
hnRNPA1 hnRNPA2 hnRNP A/B hnRNP A2/B1 hnRNPC hnRNPD hnRNPE hnRNPI/PTB hnRNPH hnRNPK hnRNPL hnRNPM |
Virus replication | RNA virus: JEV,435 Coronavirus,436 Poliovirus,438 HCV437 |
| DNA virus: HCMV488 | |||
| RNA transcription | DNA virus: HPV18,425,489 HBV,490HSV492 | ||
| RT virus: HIV514 | |||
| RNA polyadenylation | RT virus: Rous sarcoma virus485,486 | ||
| DNA virus: HPV,497,498 EBV501 | |||
| Virus RNA splicing | RNA virus: Influenza A virus (IAV)449 | ||
| DNA virus: HPV502 | |||
| RT virus: HIV529 | |||
| Virus RNA export | RNA virus: Influenza484 | ||
| RT virus: HIV,556 Kaposi’s sarcoma-associated herpesvirus (KSHV),560,561 HTLV-1562 | |||
| Virus translation | RNA virus: HCV,452–454 HRV-2,89 EMCV,456 calicivirus,457 EV-A71,122,471 Coxsackievirus B3(CVB3),474 NiV, DENV476 | ||
| DNA virus: HPV18508 | |||
| RT virus: HIV,563 KSHV564 | |||
| Cellular transformation | DNA virus: EBV510 | ||
| Immunity modulation | DNA virus: HSV512 |
The function of HSP90 family in virus infection
In this section, we would review and present new findings on HSP90 family proteins in the life cycles of different viruses including RNA virus, DNA virus and retrovirus. In addition, we also discuss their functions in virus-induced cellular response.
The function of HSP90 family in RNA virus infection
Virus entry
In the case of RNA virus, Hsp90 is critical for the entry of enterovirus A71 (EV-A71),107 Japanese encephalitis virus (JEV) and Dengue virus (DENV).108 EV-A71 entry is significantly blocked when cells are pre-treated with Hsp90 inhibitors or Hsp90-specific siRNAs.107,109 Since both DENV virus and JEV belong to the flavivirus, the entry of the these two viruses differently utilize Hsp90 with the support of Hsp70s in both neuroblastoma and microglial cells.95,108 DENV depends much more on Hsp90 to enter into cells as compared with JEV since anti-Hsp90 antibodies or Hsp90 inhibitors block the majority of DENV entry110 but only a small portion of JEV entry.111 Additionally, both Hsp90 and Hsp70 are associated with membrane lipid rafts in response to DENV infection.110 However, in DENV infected liver cells (HepG2), neither Hsp90 nor Hsp70 works as the receptor to enable DENV internalization,110 indicating alternative entry mechanisms in different cell types. It is likely that the receptor functions of Hsp90 and Hsp70 are replaced by other unknow molecules.
Virus replication
Hsp90 protein facilitates virus replication in several aspects. First, Hsp90 works as a classic chaperone protein to stabilize virus proteins. Hsp90 stabilizes paramyxoviruses polymerase and L protein, as well as assists virus replication. Inhibition of Hsp90 could hamper virus replication and shorten the half-life of L protein in vesicular stomatitis virus (VSV), human parainfluenza viruses-2 (HPIV-2), human parainfluenza viruses-3 (HPIV-3), simian virus 41 (SV41), or respiratory syncytial virus (RSV) infection cells.112,113 Similarly, Hsp90 is shown to maintain the stability of chikungunya virus (CHIKV) non-structural proteins (nsPs) including nsP3 (a protein essential for RNA synthesis) and nsP4 (RNA-dependent RNA polymerase, RdRp);114 and the protease of HCV nonstructural protein NS3.115 Second, Hsp90 modulates virus polymerase activity to enhance virus replication. Taking HCV as an example, Hsp90 indirectly modulates the HCV polymerase NS5 activity by maintaining the stability of kinase phosphoinositide dependent kinase l (PDK1), an upstream kinase of NS5 phosphorylation kinase PRK2.116 Besides, mediated by FKBP8, Hsp90 forms a complex with NS5 and directly regulates NS5 activity.117 Hsp90 inhibitors suppress viral replication by disrupting the Hsp90-NS5 complex formation.117 Third, Hsp90 manipulates the proper location of virus polymerases. During influenza virus infection, Hsp90 interacts with the viral RdRp subunits polymerase basic protein-1 (PB1) and -2 (PB2) to form a complex, and then co-translocates into nucleus.14,118 In this process, Hsp90 maintains the stability of PB1 and PB2. After entry into nucleus, Hsp90 dissociates from the Hsp90/PB1/PB2 complex and forms a new functional complex with polymerase acidic protein (PA).119 Extending study shows that the state of Hsp90 acetylation is strictly regulated by histone deacetylases 6/8 (HDAC6/8) in the influenza RdRp nuclear import stage. HDAC6/8 inhibitors efficiently limit the polymerase nuclear import and suppress virus replication.120 In the course of influenza infections, Hsp90 expression is stimulated through mTOR/p70S6K signalling.121
Our recent studies show that Hsp90 also exhibits significant importance on EV-A71 replication through PIM1 signalling (unpublished). It has been shown that EV-A71 infection elevated both the mRNA and protein levels of PIM1.122 The elevated PIM promoted EV-A71 replication while knockdown of PIM1 decreased EV-A71 replication. Knockdown of Hsp90β decreases 60% of virus replication 12h post infection (p.i.), while the secreted virions decrease by approximately 80%, indicating the crucial roles of Hsp90β in both virus replication and secretion (Fig. 4a–c). Other researchers reported that Hsp90β is responsible for EV-A71 assembly which may be the reason that Hsp90β attenuates the secretion of EV-A71 virions in our study.107,109,122 Notably, Hsp90β contributes to virus replication more than that of secretion. Our data also shows that knockdown or knockout of Hsp90β decreased the proteins expression level of EV-A71 structure protein (Fig. 4d, e). And Hsp90 inhibitors 17-AAG, Geldanamycin (GA) and VER50588 all dramatically inhibit EV-A71 protein expression (Fig. 4f). Among them, VER50588 display the strongest inhibitory effect which has not been reported before. We predicted that Hsp90β is a potential target of PIM1 (Fig. 4g). To address this hypothesis, we conducted experiments by overexpression PIM1 and knockdown of PIM1. Accordingly, the phosphorylated status of Hsp90β is increased and decreased (Fig. 4h,i). More importantly, knockout of Hsp90β by CRISPR/Cas9-mediated gene editing almost completely abolishes the effects of PIM1 signaling on EV-A71 replication (Fig. 4j, k).
Fig. 4.
Pim1 signaling promotes EV-A71 replication through Hsp90β phosphorylation(unpublished data). a RD cells were treated with scramble/Hsp90β siRNA for 24h, the effects of Hsp90β knockdown were determined by RT-qPCR assay. The results are shown as the means, and ±error indicates the standard deviation (SD). Data are obtained from triplicate experiments. *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. b, c RD cells were treated with scramble /Hsp90β siRNA for 4h, then infected with EV-A71 at MOI of 1 for indicated time. The intracellular (b) and extracellular (c) viral RNA levels were detected by RT-qPCR assay. The results are shown as the means, and ±error indicates the standard deviation (SD). Data are obtained from triplicate experiments. *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. d, e knockdown of Hsp90β by siRNA or knockout by CRIPSR/Cas9 mediated gene editing in RD cells, and then cells were infected with EV-A71 at MOI of 1 for indicated time. The protein level of EV-A71 was determined by western blot. f RD cells were treated with Hsp90 inhibitors at different concentrations and infected with EV-A71 at MOI of 0.01 for 48h. The protein level of EV-A71 was determined by western blot. g Pim1-protein interaction network was predicted using online GENEMANIA program. h Pim 1 was overexpressed in 293T cells and cell lysate was collected for immunoprecipitation assay. The phosphorylation status of Hsp90 was detected by western blot. i Pim1 was overexpressed/knocked down in 293T cells. And cell lysate was collected for native page analysis. j WT RD cells and Hsp90β knockout (Hsp90β-KO) cells were pretreated with 2 μM PIM1 inhibitor SGI-1776 for 2 h, then the cells were infected cells with EV-A71 at MOI of 0.01 for 48h. Intracellular viral RNA level was determined by RT-qPCR. The results are shown as the means, and ±error indicates the standard deviation (SD). Data are obtained from triplicate experiments. *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. k Pim1 was overexpressed in WT/Hsp90β -knockout RD cells, and infected with EV-A71 at MOI of 1 for 12 h. The protein level of EV-A71 was determined by western blot
Viral protein maturation, virion assembly, and release
During the viral protein expression and maturation, Hsp90 works as a classic chaperone to monitor the proper folding of viral proteins. Hsp90 modulates the maturation of HCV nonstructural protein 2/3 (NS2/3) kinase.123 HCV NS2/3 is cleaved into two separate proteins right after translation, a key step of NS2/3 protein maturation. Hsp90 strictly regulates the proper folding of newly synthesized NS2/3 protein.123 Hsp90 and its co-chaperone p23 form a complex to assist the proper folding of capsid precursor polyprotein P1 of poliovirus, rhinovirus, and coxsackievirus;124 while the inhibitor GA reduces the maturation of P1, leading to immature P1 degradation in proteasome.124 During the virion assembly, Hsp90 interacts with capsid VP1 protein of noroviruses and the termini of the murine norovirus 1 genome.124,125 This interaction not only stabilizes VP1, but ensures the viral genome to be encapsulated into capsids as well.124 Hsp90 interacts with and stabilizes influenza neuraminidase (NA), a major surface glycoprotein involving in virion release.126 More importantly, it emphasizes the Hsp90-NA complex formation on promoting cell survival, leading to more virus production.126
The function of HSP90 family in DNA virus infection
Virus entry
The entry of DNA viruses includes steps of crossing over cell membrane and nuclear import. Hsp90 is mainly shown the ability to assist the nuclear import of virus. The nuclear transport of many viruses depends on the microtubules (MT) and MT-dependent molecular motor dynein/dynactin complex.127 Virus strictly modulates the status of tubulin acetylation, a critical event for the transportation of viral components.128–130 In HSV-infected cells, Hsp90 co-localizes with acetylated tubulin and capsid protein VP5.131 Hsp90 inhibitors disrupt its binding to the acetylated tubulin, thereby inhibiting the nuclear transport of HSV capsid protein.131 During HBV infection, the glucocorticoid receptor shows a strong possibility to enhance the nuclear import of HBV.132 Hsp90 facilitates glucocorticoid receptor redistribution from the cytoplasm to the nucleus.133
Virus replication
During DNA retroviral replication, Hsp90 mainly contributes to regulating and maintaining the reverse-transcriptase (RT) activity. Taking hepatitis B virus (HBV) as an example, the reverse transcription is an essential step to generate viral genomic DNA in type VII viruses. The beginning of reverse transcription is the recognition and interaction of RT with an RNA signal (the packaging signal, ε) on the pre-genomic RNA.134 It was identified that Hsp90 is an essential host factor that facilitates Duck hepatitis B virus (DHBV) replication by interacting with viral RT.135 Treating with Hsp90 inhibitors or monoclonal antibodies (mAb) sufficiently block RT-ε binding.135 Hu et al. demonstrated that RT-ε interaction depends on Hsp90’s ATP hydrolysis activity.136 Two independent regions in the terminal protein (TP) and the RT domains of polymerase separately bind with Hsp90 at the N-Terminal and C-Terminal fragments, and both domains are essential for ribonucleoprotein (RNP) and protein priming.137,138 Although a model is established to show how Hsp90 bridges the two separate RT domains of polymerase together to enable the formation of an RNP complex with the HBV RNA;137 there are still some fundamental questions to be addressed. Firstly, whether the Hsp90 chaperones or Hsp70 chaperones are essential for the RT- ε interaction. Stahl et al. believed that Hsp70 chaperones are much more important for the RT–ε interaction. They proposed that Hsp90/Hop complex affect the quantity, not the quality of RT activity.139 While Hu et al. stated that Hsp90 is critical for the RT activity.137,140,141 Secondly, Stahl et al. believed that the stimulated RT activity is independent of Hsp90 ATPase activity;139 while Hu et al. reported that RT-ε interaction mediated by Hsp90 and its chaperone partner p23 is ATP-dependent.137,141 Lastly, another study showed that Hsp90 helps HBV RT priming rather than maintaining the HBV RT/ε RNA complex.142 This is controversial to Hu’s findings, i.e., Hsp90 function is required not only to establish but also to maintain the RT in a state for RNA binding.141 Grp94, another HSP90 family member, is shown a critical regulator in stabilizing and activating RT, allowing its preferential binding to the pregenomic RNA during HBV replication.143
The replication of most DNA viruses occurs in the nucleus, where virions form in replication centres. Therefore, the proper location of viral proteins is quite important for virus replication. Hsp90 is also found to regulate the location of virus DNA polymerase in virus-infected cells. After treated with Hsp90 inhibitors, HSV polymerases is mislocalized from the nucleus to the cytoplasm and subsequently degraded in a proteasome-dependent manner.144,145 Similar to HSV, the nuclear translocation of DNA polymerase of EBV also requires Hsp90.146,147 During the polymerase transportation, the polymerase catalytic subunit BALF5 forms a complex with BMRF1 in the assistance of Hsp90β. Hsp90 inhibitors effectively block the translocation of viral DNA polymerase.146,147
Virus gene expression
Hsp90 is important for virus gene expression both at the transcription and translation levels. The transcription of HSV immediate-early α (IEα) genes is initiated by the transcription factor complex, which is composed of octamer-binding transcription factor 1 (Oct-1), host cell factor 1 (HCF-1) and viral protein 16 (VP16).148,149 In the complex, VP16 is the major virus-encoded transcriptional activator that controls the efficiency and level of viral gene transcription. In the transcription process, Hsp90α is shown to maintain the stability of VP16 by keeping it from degradation in a macroautophagy-mediated manner.150 Similarly, Hsp90 also regulates the transcription of human cytomegalovirus (HCMV) immediate-early genes through activating Akt and NF-κB signalling pathways, which are critical for major immediate early genes transcription.151,152
At the translation level, Hsp90 promotes the translation of conserved herpesvirus protein kinases (CHPKs), including herpes simplex virus type 1 and 2 (HSV-1, HSV-2), varicella-zoster virus (VZV), EBV, KSHV.153 CHPKs play important roles in multiple processes, including gene expression,154–156 viral DNA replication,156–158 capsid nuclear egress,159,160 and DNA damage responses.161,162 The translation of EBV nuclear antigen 1 (EBNA1) protein is also manipulated by Hsp90.163,164 EBNA1 is critical for cellular transformation, tumorigenesis, and the maintenance of viral episomes.165–167 The EBNA1 translation is strictly regulated by Hsp90 through the Gly-Ala repeat domain to keep EBNA1 at a relatively low level.168 It has been demonstrated that Hsp90 inhibitors block the translation of EBNA1; and mutation of Gly-Ala repeat domain abrogates the inhibition of EBNA1 translation.163,164 Hsp90 does not interact with EBNA1directly, a bridge protein may be involved in this process. Inhibiting EBNA1 expression strongly suppresses both EBV-induced primary B cell transformation in vitro and lymphoproliferative disease in SCID mice in vivo.163,164
Virus assembly
Only a few papers reported the function of Hsp90 in DNA virus assembly. The activated Hsp90 is needed for HBV assembly.169,170 Hsp90 enhances the affinity of core protein dimers for capsid formation and prevents capsid dissociation.169 Besides, the reactive oxygen species (ROS)-promoted HBV capsid assembly also requires an active form of Hsp90.170
Virus-induced tumorigenesis
As described before, EBV is the causative regent of several tumors, including Burkitt’s lymphoma171 and Nasopharyngeal carcinoma (NPC).172 The latent membrane protein 1 (LMP1) is regarded as an oncoprotein that promotes tumor metastasis and invasiveness through inducing the expression of matrix metalloproteinase 9 (MMP9), mimicking the tumor necrosis factor receptor (TNFR) superfamily proteins, and activating the NF-kB, MAPK, PI3K/Akt and JAK/STAT signal transduction pathways.173,174 Hsp90 seems positively promoting cell growth in EBV-positive nasopharyngeal carcinoma cells and EBV-infected T and NK cells.175,176 Hsp90 inhibitors, AT13387 and BIIB021, potently inhibit cell growth and induce apoptosis by impeding LMP1 function through activating its downstream signaling pathways described above.175,176
Immunity modulation
We have discussed how Hsp90 is hijacked by DNA viruses to promote viral replication above. Under certain conditions, Hsp90 exhibits antiviral activities by promoting cell immunity. In the acute infection stage, Hsp90 is induced to express in the cell surface of EBV-infected B cells. It has a strong ability to expand gamma delta T cells (γδ T cells) population.177 The γδ T cells have potent antiviral ability in the acute phage when a host is infected by HIV, influenza, Sendai, coxsackie, vaccinia, VSV, or HSV-1. The γδ T cells work as both early sentinels of the immune system by providing immediate protection and as bridging elements between innate immunity and adaptive immunity.178 Since Hsp90 can work as an immune sensor and assist antigen presentation, it may function in the same way in EBV infection.177,179–182
The function of HSP90 family on cell transformation during retrovirus infection
Several HSPs functions as oncoproteins to promote cellular transformation. Hsp90 participates in the HTLV-1-induced cellular transformation. The tax protein of HTLV-1 controls viral replication and induces T lymphocyte transformation.183 NF-κB pathway is one of the main targets essential for this process;184 while Hsp90 acts as an important partner of tax by binding with and maintaining its stability in nucleus.153,185 Hsp90 inhibitors (e.g.,17-DMAG) or Hsp90-depletion by siRNAs cause tax degradation in proteasome, inhibition of NF-κB signalling, and activation of the long terminal repeat (LTR) of HTLV-1.153,185 Oral treatment with Hsp90 inhibitor 17-DMAG significantly suppresses the aggressive infiltration into multiple organs in ATL mice.153,185
The function of HSP70 family in virus infection
Functions of HSP70 family in RNA virus infection
Virus entry
Viruses in different families (e.g., Picornaviridae, Flaviviridae, and Reoviridae) take advantage of HSP70 family proteins for their entry into host cells. In the case of Picornaviridae, for example, the coxsackievirus A9 (CAV-9) uses Hsp70 homolog Grp78 for its entry.186 It shows that antibodies against Grp78 block 50% of virus binding. Integrin αvβ3 is another famous virus receptor.187 When cells are simultaneously treated with Grp78 and integrin αvβ3 antibodies, virus binding is blocked completely. Therefore, Grp78 functions as a co-receptor of CAV-9. Besides, Grp78 can interact with major histocompatibility complex (MHC) I molecules on the host cell membrane after infection of CAV-9. MHC I molecules help virus internalization into mammalian cells.186 In the course of EV-A71 infection, Hsp70 is dramatically upregulated and interacts with EV-A71 on the cell surface. Hsp70 antibody significantly inhibits virus binding to the cell surface.188 Besides enteroviruses, many viruses of Flaviviridae family also require Hsp70 to entry into host cells. By affinity chromatography assay, Hsp70 is discovered to form a complex with Hsp90 and DENV receptor that facilitates viral entry.110,189 Hsp70 interacts with DNEV envelope protein (E protein) and plays a significant role in virus attachment.190 Similarly, antibodies against Hsp70 and Hsp90 significantly inhibit DENV infection.110,189 The same mechanism is also observed in JEV infection.191 Hsp70 is enriched in the lipid raft and colocalized with the E protein in JEV-infected Huh7 cells.192 The depletion of cholesterol disrupts the enrichment and colocalization of the E protein and Hsp70 to a raft membrane. Eventually, it decreases JEV entry without any effects on virus attachment.192 These results suggest that Hsp70 works as a receptor of JEV; and lipid rafts serve as an organizing centre to facilitate JEV entry. At the late stage of JEV entry, Hsc70 (isoform D) is upregulated in C6/36 cells upon JEV infection. However, it seems that Hsc70 is not required for virus attachment to the cell membrane but needed for virus penetration into the host cells. It is suggested that Hsc70 holds an intense involvement in clathrin-mediated endocytosis at the late stage of viral entry, which helps JEV to penetrate into host cells.193 Recently, it is reported that Grp78 is also required for JEV both in the attachment and entry steps.194 Antibody targeting the N-terminal of Grp78 significantly prevents virus attaching to host cells whereas antibody targeting the C-terminus fails to block the attachment. Knockdown of Grp78 also inhibits JEV internalization. The colocalization and interaction between Grp78 and JEV envelope protein provide solid evidence to show the importance of Grp78 in the process of virus attachment and entry.194 Interestingly, Grp78 is secreted out of host cells after JEV infection, and the secreted Grp78 cooperates with JEV to promote virus infection.195 Recent studies show that Grp78 is a receptor of SARS-COV, MERS-CoV, and SARS-CoV-2 viruses.196 ZIKV infection is positively regulated by Hsp70 at multiple stages.197 Hsp70 inhibitors impair virus entry, RNA replication, and capsid assembly of different ZIKV strains in diverse cell lines.190,197 Rotavirus infection also needs the assistance of membrane-resident Hsc70.198 Hsc70-specific monoclonal antibodies inhibit virus internalization and infection without effect on virus attachment.198 Further evidence shows that the whole virus particle and a short domain (or a peptide) in the C-terminal region of VP5 is sufficient to bind to Hsc70.199 The ATPase domain of Hsc70 is proved to be necessary for its interaction with VP5 and induction of virion conformation change for the entry.200
Virus replication
HSP70 family proteins participate viral replication by employing different mechanisms. First, HSP70 family proteins facilitate the formation of virus replication complex and/or maintain the stability of complex proteins. In some cases, HSP70 family proteins directly interact with viral polymerase to enhance viral replication. For example, during the Mumps virus (MuV) infection, the expression level of Hsp72 is increased. The C-terminal region of Hsp72 interacts with the N-terminal region of P protein, which is an essential component of RdRp complex. Knockdown of Hsp72 results in accumulated ubiquitinated P protein as well as increased cell apoptosis.201 Besides, Hsp70 is also reported to regulate L protein, another MuV polymerase component. Hsp70 cooperates with Hsp90 to regulate L protein levels. Hsp90 inhibitor, 17-AAG, reduces the L protein level through promoting degradation via the C terminus of Hsp70-interacting protein (CHIP) -mediated proteasomal pathway. Hsp70 inhibitor VER155008 together with 17-AAG enhances L protein degradation. Therefore, Hsp90 and Hsp70 together regulate the stability of L protein and ensure the proper virus replication complex (VRC) formation.202 In the case of canine distemper virus (CDV) infection, the increased Hsp70 results in an elevated expression of light nucleocapsid (NC-L) variant, which displays polymerase activity.203,204 A more direct evidence is that Hsp70 facilitates viral RNA production in cell-free transcriptional assays.204 Furthermore, it is demonstrated that Hsp70 interacts with and regulates NC polymerase activity dependent on the Hsp70 ATP activity; because Hsp70 antibody significantly inhibits NC polymerase activity and supplementation of purified recombinant Hsp70 enhances both the basal and stress-induced NC polymerase activity.205
The other members of Hsp70s also regulate VRC formation. Hsp72 physically interacts with several replication proteins of Flavivirus including NS5A, NS3, and NS5B (RdRp). For example, Hsp72 participates the VRC formation of HCV.206 Downregulating Hsp72 leads to a decreased number of VRC in HCV-infected cells, while overexpression of Hsp72 raises the number of VRC.206 Hsc70 is associated with VRC by binding on the 3’ polyU/UC motif of HCV RNA genome.207 HCV accumulation and virion production are significantly suppressed when cells are treated with Hsp70 or Hsc70 inhibitors.208 Similar result is reported in the case of RSV infection. Ectopic expression of RSV nucleocapsid protein (N protein) and phosphoprotein (P protein) are detected to interact with Hsp70 in 293T cells.209 The N protein is responsible for interacting with the viral RNA, and P protein interacts with N protein and with the RdRp L to form the nucleocapsid. In RSV-infected cells, Hsp70 redistributes into lipid-raft membranes and colocalizes with virus N protein and lipid raft marker GM1.210 Although Hsp70 inhibitors suppress RSV polymerase activity;210 it only disrupts viral gene expression but do not affect RNA polymerization.211 Therefore, more detailed studies are needed to understand the functions of Hsp70 in modulating RSV gene expression and replication.
In term of Ebola virus (EBOV) replication, the mechanism of Hsp70 involved is much more complicated. By using immunoprecipitation and mass spectrometry assays, it has been identified that the N protein interacts with Hsp70, NEF, BAG2, and the Hsp70 co-chaperone DNAJA2.212 The N protein recognizes and binds to the viral RNA genome to establish a steady N protein–RNA complex structure (RNP). This complex further interacts with viral proteins VP30, VP35 and RdRp L, to finally form VRC. Here, Hsp70 functions to maintain the stability of N protein and helps to facilitate VRC formation.212,213 In addition, Hsp70 is also co-purified with L polymerase in insect cells.214 Besides, Hsc70 interacts with the terminal non-coding regions of the EBOV genome. Disruption of the interaction by mutating the binding site potently inhibits the minigenome replication of EBOV.215
Another mechanism that Hsp70 employs to support virus replication is to modulate nuclear import of polymerase or nuclear capsid. Some RNA viruses also replicate in the nuclear such as CDV and influenza virus. Therefore, nuclear transportation becomes a critical step for their replication. Upon CDV infection, Hsp70 is shown a strong contribution to viral replication by interacting with and promoting the translocation of the nucleocapsid particles from the cytosol to nucleus.216 Similarly, during influenza infection, Hsp70 interacts with PB2 or PB1 monomers and PB2/PB1 heterodimer in HeLa and HEK293T cells, and sequentially translocates into the nucleus with PB2 monomers or PB2/PB1 heterodimers.217 If Hsp70 and PB2/PB1 polymerases are retained in the cytosol, the polymerase activity reduces dramatically. The shuttling of Hsp70 between nuclear and cytoplasmic compartments underlies the modulatory effect of Hsp70 on influenza virus replication.217
Virus gene expression
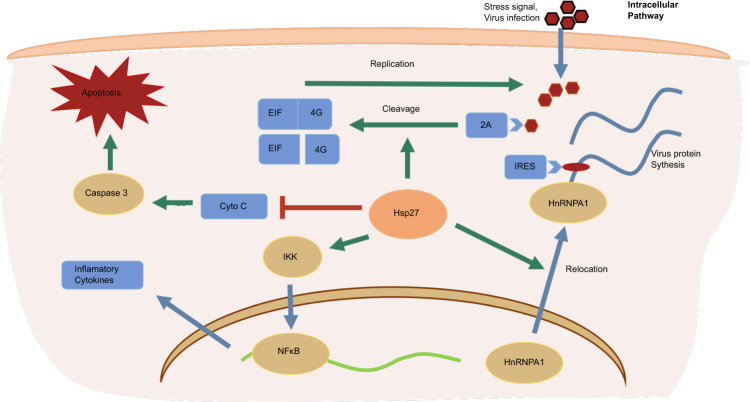
Besides viral entry and replication, Hsp70 also contributes to viral protein translation. Positive single-stranded RNA viruses (e.g., SARS-CoV-2, HCV, ZIKA, EV-A71, etc) use the internal ribosome entry site (IRES) to initiate the translation of their own proteins but inhibit the host cellular cap-dependent translation through regulation or cleavage of eukaryotic translation initiation /elongation factors (EIFs/EEFs). In the case of coxsackievirus B3 (CVA B3) infection, Hsp70 is upregulated to enhance the initiation and elongation of viral translation.218 In the translation initiation step, Hsp70 upregulates IRES-acting factor lupus autoantigen protein expression and activates eIF4E binding protein 1 (EIF4EBP1), a cap-dependent translation suppressor. In the elongation step, Hsp70 activates the Akt-mammalian target of rapamycin complex 1 (mTORC1) signal cascade, leading to activation of EEF2 via kinase p70S6K- and Cdc2-mediated phosphorylation and inactivation of EEF2 kinase (EF2K).218 Hsc70 enhances the IRES activity in EV-A71 infected cells. Hsc70 interacts with 2A protease of EV-A71 to enhance EIF4G cleavage that impairs host cell cap-dependent translation but enhances viral IRES-mediated translation.219 Therefore, Hsc70 may serve as an antiviral target against EV-A71 and HCV infections. Some viruses do not have IRES sequence, and virus replication produces plenty of dsRNAs which trigger the activation of protein kinase-RNA-activated (PKR)- EIF2a signalling cascade that shuts down global translation in cells and releases stresses.220,221 To circumvent the PKR-mediated block to viral proliferation, influenza A virus induces the cellular tetratricopeptide repeat (TPR) - domains containing JDP protein, p58IPK. Influenza virus downregulates PKR in an Hsp70-dependent way.222–226 In uninfected and unstressed cells, p58IPK activity is clogged with Hdj1 by forming a complex.225,226 During influenza A infection, the amount of Hdj1 in p58IPK-Hdj1 complex decreases to an undetectable level. These findings suggest that the activation of p58IPK appears to be a sequel to the Hsp70-mediated release of Hdj1 from the p58IPK-Hdj1 complex, which allows the monomeric p58IPK to inhibit PKR.225 In the Hsp40 chaperone part, we would discuss more details about the regulation of PKR signaling by Influenza virus.
Virion assembly
Hsp70 is reported to assist some viruses’ assembly. During morphogenesis of the double-stranded RNA Reovirus, Hsp70 contributes to the assembly of trimeric sigma 1 protein, which is responsible for the interaction with host cell receptor.227 While the N-terminal segment of the sigma 1 protein folds and trimerizes cotranslationally in an Hsp70-independent manner, a post-translational fold of the C-terminal globular domain is dependent on Hsp70. In this process, Hsp70 binds cotranslationally to the region downstream the N-terminal α-helical coiled-coil, which presumably helps to inhibit unwanted interaction and misfolding. Trimerization of the C-terminal domain of the sigma 1 protein is coupled to the ATP-dependent release of Hsp70 from the ribosome.227 Besides, in HeLa cells infected by poliovirus or coxsackievirus B1, Hsp70 is detected to interact with the capsid precursor P1.228 In the complex, P1 is mainly newly synthesized and has a longer half-life than that of total P1. The Hsp70-P1 complex is regarded as an assembly intermediate of picornaviruses.228
Interestingly, some researchers demonstrate that Hsp70 inhibits influenza virus replication by blocking nuclear export of viral ribonucleoprotein complex (vRNP), and subsequent viral morphogenesis via disassociating M1 from vRNP.229,230
Virus release
The evidence of HSPs on virus releasing is limited. Both Hsp70 and Hsc70 can interact with NS5A protein; although they play different roles in HCV infection.231 Silencing of Hsp70 decreases viral protein expression, but the virus protein level is not affected.232,233 Instead, interfering Hsc70 reduces extracellular virion production.232,233 Moreover, Hsc70 is embedded in the viral capsid. And co-localization between Hsc70 and core and E2 structural proteins of HCV has been found in lipid droplets. Therefore, Hsp70 and Hsc70 may regulate HCV infection release at different steps.
The function of HSP70 family proteins in DNA virus infection
Virus entry and genome releasing
Instead of virus attachment and entry into the host cells, here we talked about virus entry into the cytosol from ER. The cytosol entry from ER is a key step in SV40 infections. Hsc70 is reported to be essential in this step. Hsc70 interacts with and is regulated by SGTA.234 Further studies show that HSP70 superfamily member Hsp105 forms a subunit in the Hsc70-SGTA complex to facilitate SV40 cytosol entry.235 In addition, Grp78/Bip also plays a role in SV40 cytosol entry. Grp78 interacts with SV40 capsid protein in a DNAJB11-dependent manner to help SV40 disassemble and enter into the cytosol.236
Hsp70 is also needed for the genome release of some DNA viruses. Such a process is described in Adenovirus infection. After the release of the virion from endocytic vesicles into the cytoplasm, Hsp70 and Hsc70 immediately attach to the hexon protein, one of the major Adenovirus coat proteins.237 Hsp70/Hsc70 and its co-chaperone Bag3 interact with the penton base protein, the viral capsid constituent responsible for virus internalization.238,239 The intact nucleocapsid is transported to nucleus through the typical NLS-dependent nuclear import machinery.240 The nucleocapsid anchors to the nuclear pore through its hexon protein by interacting with components of the pore complex. Then viral DNA is transferred into the nucleus in a Hsp70-dependent manner but leaving hexon outside the nucleus because the purified hexon, instead of viral DNA, enter the nucleus in a Hsp70-independent manner.240 A possible explanation is that the intact nucleocapsid is too large to pass through the nuclear pore complex, while the disassembly of nucleocapsid facilitates the entry of viral DNA into nucleus. However, more solid evidence is needed for such an explanation.238 Other examples for the contribution of Hsc70 in viral genome release in host nucleus are HSV and Polyomavirus. In HSV-infected cells, the translocation of Hsc70 from the cytosol to nucleus is triggered by the immediate-early viral protein ICP0. Hsc70 is colocalized with the components of the 26S proteasome and virus UL6 portal protein, which provides the conduit for DNA entry and exit from the capsid. UL6 is highly ubiquitinated in the nucleus, indicating that Hsc70 may be responsible for the correct folding and degradation of UL6 in the ubiquitin-proteasome pathway, though there was no direct evidence that UL6 is a substrate of Hsc70.241 It is also believed that Hsc70 contributes to Polyomavirus genome nuclear import through its interaction with all viral capsid proteins VP1, VP2 and VP3 both in vitro and in vivo. Hsc70 translocates from the cytosol to the nucleus accompanied by the translocation of capsid proteins upon infection with Polyomavirus.254
Gene expression and protein maturation
Most viruses manipulate the cellular transcription and translation machinery and shut off host protein synthesis, so that they can take advantage of these machineries and recruit initiation and elongation factors for the expression of viral proteins. Some host factors exploited by viruses interact closely with components of Hsp70 complex. Therefore, the chaperone system is highly important for viral gene expression. Several transcription initiation factors are well known to physically interact with the Hsp70 co-chaperone Bag1 in vitro. Bag1 stimulates general transcription activity in an Hsp70-dependent manner.242–244 The stimulation of global transcription is detected in cells upon infections by either human polyomavirus, John Cunningham virus (JCV) or HCMV.245,246 However, the detailed molecular mechanism of the general transcriptional activation by viruses is to be further investigated.
The typical example of Hsp70 system in regulating the maturation of viral proteins is shown in HBV large envelope protein (LHBsAg).247–249 The mature LHBsAg has a unique dual transmembrane topology. Initially, the C-terminal of LHBsAg co-translationally resides in ER, while the N-terminus is resident in the cytosol and later is translocated into ER for post-tranreported that the expression of Grp78 is stimulatedslation. To ensure the correct topology, Hsp70 system strictly regulates the post translocation of N-terminal of LHBsAg. During the translation, Hsc70 interacts with the N-terminal of LHBsAg at amino acids 63 to 107 and suppresses LHBsAg translocation into ER.248,249 Hsc70 cochaperones Hip and Bag1 also regulate the activity of Hsc70 in an antagonistic way.250,251 Overexpressed Hip promotes Hsc70 activity resulting in more cytosol retention of LHBsAg N-terminal;250 while Bag1 overexpression could inhibit Hsc70 activity to promote nuclear translocation.251 In the post-translation process, Grp78 binds with LHBsAg and Hsc70 to facilitate the ER translocation of HBV large surface antigen.247,248,252,253 The function of Grp78 is regulated by both positive regulator ER-localized DnaJ-domain-containing protein 4 (ERdj4) and negative regulator BiP-associated protein (BAP).253 Increased BAP destabilizes LHBsAg/BiP complex.253 Together, Hsp70 chaperone system is crucial in modulating the sophisticated topogenesis of HBV envelope protein.247
Virus assembly
A lot of DNA viruses normally assemble in the nuclei of infected cells. Taking Polyomavirus as an example, all capsid proteins are synthesized in the cytosol, whereas subsequent assembly of virions only takes place in the nucleus. During Polyomavirus infection, the constitutive form of Hsp70 and Hsc70 are coimmunoprecipitated with all three viral capsid proteins (VP1, VP2, and VP3). Hsp70 is translocated from cytoplasm to the nucleus in the late stage of infection, coincident with localization change of the viral capsid proteins.254 In vitro studies show that Hsp70 functions to keep proper assembly of Polyomavirus.255 The prokaryotic Hsp70 chaperone DnaK also interacts with recombinant VP1 at the C-terminal domain where it links pentamers in an assembled capsid.255 When DnaK binds to VP1, it inhibits VP1 assembly, which is induced by calcium in vitro. However, combining the Hsp70 chaperone system including DnaK, DnaJ and GRpE with VP1 together is sufficient to assemble VP1 into uniform capsids in the presence of ATP alone without calcium.255
The function of HSP70 family in retrovirus infection
Human T lymphotropic virus type 1 (HTLV-1) is a well-investigated example of retrovirus that interacts with Hsp70 proteins. During HTLV-1 infection, syncytium formation is a key factor for cell-to-cell virus transfer. The syncytium formation is subject to close cell-to-cell interactions.256,257 Cell membrane-resident Hsc70 is necessary in this process as Hsc70-specific monoclonal antibodies eliminates the syncytium formation and HTLV-1 infectivity. The same outcome presents when cell is treated with a peptide derived from the HTLV-1 glycoprotein gp46, which binds to Hsc70.258,259 Interestingly, although Hsc70 enhances the syncytium formation, it has no effect on virus entry.259
Hsp70 also plays an important role in the post-entry steps. During human immunodeficiency virus type 1 (HIV-1) infection, viral protein R (Vpr) stimulates interaction between the viral preintegration complex and karyopherin-alpha to facilitate viral nuclear import. Hsp70 functionally overlaps with Vpr in this process.260 When Vpr is deficient, Hsp70 could rescue virus nuclear import by interacting with karyopherin-alpha at the N-terminal that also binds Vpr. Interestingly, some researchers argue for the antiviral role of Hsp70 because Hsp70 and Vpr share the same substrate. It seems that Hsp70 would compete and inhibit Vpr function. Since HIV-1 needs Vpr to manipulate cell cycle and apoptosis, Hsp70 neutralizes the function of Vpr in HIV-1 infection.261,262 Recently, HIV is observed to package Hsp70 as part of virion core. The virion-incorporated Hsp70 ATPase activity and correct conformation of Hsp70-virion are essential for HIV infection, since inhibition of Hsp70 ATPase activity interrupts the Hsp70-virion core association and diminishes virus infectivity.263
The effects of HSP70s on host cells upon DNA virus infection
Cellular transformation
DNA viruses those do not encode polymerase in their genome are dependent on host DNA replication machinery. To replicate in quiescent cells, the virus has to reinitiate cell cycle thereby transforming the host cells. Some mechanisms have evolved in enabling viruses to overcome the restriction points of cell cycle. The best-investigated example is SV40. The large and small T antigen (TAg) are central for the SV40 transformation ability. At their N terminus, both types of TAg contain the J-domain, the signature motif of an Hsp70 co-chaperone. Mutation or deletion of the J-domain disrupts the functional association of TAg with Hsp70s. The ability of TAg to transform mammalian cells is subsequently obliterated.264 Also, TAg sequesters the retinoblastoma family proteins (pRb, p107, and p130) and liberates members of the E2F family of transcription factors in a Hsc70-ATP hydrolysis-dependent manner.265,266 The free E2F family proteins subsequently trigger the expression of the S-phase genes leading to DNA replication.267 The above observations are interpreted in the following four steps. Firstly, the large TAg combines with pRB-E2F complex. Subsequently, the pRB-E2F-TAg complex associates with Hsc70 in the presence of ATP as Hsc70 ATP-bound form presents high substrate association rate to facilitate TAg-Hsc70 complex formation. The third step is that the J-domain of TAg in the TAg-Hsc70 complex stimulates ATP hydrolysis. This process is dependent on the active site of Hsc70 and does not occur at the T-antigen binding site. Meanwhile, Hsc70 transfers to the high-affinity conformation allowing the trap of the substrate protein pRB or E2F. In the last step, Hsc70 induces the conformation of the substrate protein in the complex which assists the dissociation of pRB and E2F. After ADP dissociation and rebinding of ATP to Hsc70, E2F and the pRB-TAg complex is released from the Hsc70–substrate complex.268–271 A second strategy of TAg to transactivate E2F transcription is independent of the disruption of the pRB-E2F complex that also involves the J-domain and Hsp70 protein.271–274 TAg functions like Bag1 to assiste the assembly of a transcription initiation complex on the respective promoters in the presence of Hsc70. Alternatively, TAg could induce Hsc70 to disassemble an inhibitory silencer complex or to assist with remodelling the chromatin (Fig. 5).
Fig. 5.

Model for the participation of Hsc70 in the TAg induced disassembling of pRBand E2F. Firstly, TAg combines with pRB-E2F complex to facilitate T-antigen-Hsc70 complex formation. Then TAg-Hsc70 complex stimulates ATP hydrolysis, and Hsc70 transfers to the high-affinity conformation allowing the trap of the substrate protein pRB or E2F. Finally, Hsc70 induces the conformation of the substrate protein in the complex which assists the dissociation of pRB and E2F to initiate cell cycle reprogram
HPV and Adenovirus have similar transforming activities by disrupting pRB-E2F complexes. Although neither E7 (HPV) nor E1A (adenovirus) protein contains a J-domain, both proteins could transform cells in a way similar to that described for SV40 TAg. E7 interacts with tumor suppressor hTid-1.275 The C terminus of E7, which mediates the interaction with hTid-1, is essential for the physical disruption of the pRB-E2F complex though it is not necessary for direct interaction with pRB.276,277 These observations suggest that the interaction with hTid-1 is involved in the disruption of the pRB-E2F complex, providing E7 with the J-domain necessary to recruit Hsc70 for the complex dissociation in analogy to the function of SV40 TAg. Alternatively, the binding of E7 to hTid-1 could transform cells through inhibition of the assumed tumor suppressor function of hTid-1. E1A directly interacts with Hsc70 to disrupt the pRB-E2F complex.278
In conclusion, most double-stranded DNA viruses depend on Hsp70 chaperones for the reprogramming of the host cells to re-enter the cell cycle.
Cell survival and apoptosis
Since Hsp70 systems are essential modulators for cell survival under stress conditions, the induction of Hsp70 protein facilitates virus infection by keeping the cell alive until mature viruses are ready to leave. This is the main reason why the disruption of apoptotic pathway is a quite common phenomenon in viral infections.279–281 In the early stage of viral life cycle, viral reproduction is simply vulnerable to cell death. Naturally, viruses are evolved in manipulating cellular apoptosis. In EBV-infected cells, nuclear oncoprotein EBNA3A helps Hsp70 nuclear translocation and Hsp70 chaperone complex formation to immortalize B cells through inhibiting apoptosis.282 In contrast, apoptosis is beneficial for virus spreading when virions are finally assembled.283–290 Virions are found in apoptotic bodies and subsequently engulfed by phagocytic cells. It has been suggested that virus can infect neighbour cells without being detected by the host immune system.291,292 On the other hand, the decrease of Hsp70 mRNA level may lead to the timed induction of apoptosis at the late stage of adenovirus infections.
Innate immunity
Hsp70 has been previously described to influence innate immunity and inflammatory responses.293 It has been believed that hepatocyte is devoid of innate immunities. Ma et al. reported that the expression of Grp78 is stimulated by HBV replication. The elevated Grp78 protein in turn activated innate immune response by induction of IFNβ expression.294 Further studies showed that Hsp70 greatly contributes to cellular innate immunity in response to either virus or bacterial infections. In the course of bacterial infections, the elevated intracellular levels of Hsp70 protect cells from LPS-mediated inflammation. Through its interaction with TNF Receptor Associated Factor 6 (TRAF6), Hsp70 can inhibit its ubiquitination and thereby block the activation of transcription factor NF-kB.295,296 Weiss et al. has presented more details on this mechanism. Hsp70 binds with IKK, leading to disturbing the function and stability of NF-κB/IkBα/IKK complex and further impairing IkBα phosphorylation.297 The disturbance of this complex also affects IkBα proteasomal degradation and nuclear translocation of NF-κB complex.298 The inhibition of NF-κB signalling has great therapeutic significance because it can prevent massive tissue damage medicated by the excessive inflammation response.
A more recent study provides another approach for Hsp70 to regulate inflammation. Pierre et al. described an NF-κB independent pathway that Hsp70 could impact NLRP3/ASC inflammasome formation through its association with NLRP3.299 Aside from the anti-inflammation function of intracellular Hsp70, the secreted extracellular Hsp70 binds to dendritic cells and macrophages before being recognized by its binding elements, most of which are innate immune receptors.293,300
Toll-like receptors (TLRs) detect virus invasion and immediately trigger intracellular innate antiviral response.301 They belong to type I integral membrane glycoproteins of IL-1 receptor (IL-1R) superfamily.302 It has been suggested that TLR2/TLR4 involved in the initiation of innate immunity by extracellular Hsp70. Hsp70 utilizes both TLR2 and TLR4 to transduce its proinflammatory signal in a CD14-dependent manner to promote proinflammatory cytokine production via MyD88/IRAK/NF-κB axis signalling cascade.8,301 Another study clearly demonstrated the function of TLR4 and its direct interaction with Hsp70.303
After ligand binding, TLRs dimerize and undergo a conformational change required for recruiting downstream signaling molecules, including the adaptor molecule myeloid differentiation primary-response protein 88 (MyD88), IL-1R-associated kinases (IRAKs), TGFβ-activated kinase (TAK1), TAK1-binding protein 1 (TAB1), TAB2 and TNF-receptor-associated factor 6 (TRAF6).304–307 Many other innate immunity-related signaling pathways would then be activated, for example, phosphorylation of NF-κB via TAK1/IKK activation. MAPKs p38, JNK, and ERK pathway are also activated, then subsequently activate CREB and AP-1 transcription factors. Both AP-1 and NF-κB activate proinflammatory cytokine expression, including TNFα, IL-6, IL-1β, and a number of other cytokines and chemokines.308–311
The functions of HSP60 family in virus infection
The effects of HSP60s on host cells upon RNA virus infection
Immunity modulation
Few studies show the function of Hsp60s in the life cycle of RNA virus; however, the function of Hsp60 in regulating host immunity has been widely studied.312–316 The majority of studies show that Hsp60 works as an activator of immune response. In the case of JEV infection, Hsp60 facilitates virus-induced inflammation by promoting IL-1β production via increasing NLRP3 inflammasome activity and NFκB phosphorylation.317 However, under certain conditions, viruses also utilize Hsp60 to evade host cell immune response. The genome-wide RNA interference (RNAi) screen identifies the interaction of Hsp60 with PB2 of influenza virus.318 Hsp60 helps PB2 translocation from the cytosol into mitochondria.38,39 Then the mitochondrial PB2 interacts with and modulates the activity of mitochondrial antiviral signaling protein (MAVS) to suppress interferon β (IFNβ) production.38 Therefore, Hsp60 determines the effect of PB2 on both mitochondrial stability and the level of IFN-β production.38,39 Besides, DENV infection also elevates Hsp60 expression. Silencing of Hsp60 results in an increase of IFN-α production and decrease of virus reproduction in macrophages.319 However, the detailed mechanism remains elusive.
Apoptosis regulation
Viruses use different mechanisms to modulate apoptosis. In the case of HCV infection, ROS production is regarded as the major contributor of HCC although it also promotes cell apoptosis.320 Study shows that the N-terminal domain of HCV core protein can induce ROS production by interacting with Hsp60 and inhibiting the normal function of Hsp60 in releasing protein stress.321,322 While another RNA virus, Rotavirus SA11, tries its best to delay the early apoptosis through modulating Hsp60 stability.323 Hsp60 helps to translocate NSP4 protein of Rotavirus into mitochondria from cytosol and induces apoptosis.323 To yield more viruses, Rotavirus infection increases the Hsp60 phosphorylation at Tyr228 by activated Src kinase that leads to the ubiquitin-mediated proteasomal degradation.323 Even though virus postpones apoptosis via Hsp60, the main function of Hsp60 is to refold proteins in mitochondria.323,324
The function of HSP60 family in DNA virus infection
Virus replication
In the previous sections, we have discussed that Hsp90 is important for RT-ε RNA complex formation in HBV infection. However, it has been shown that Hsp60 directly regulates RT activity before the RT-ε RNA complex formation.325,326 HBV replication is markedly suppressed when Hsp60 is knocked down by specific siRNAs.325 Hsp60 transiently interacts with RT to activate RT in an ATP-dependent manner.326 Upon RT activation, Hsp60 immediately dissociates from the Hsp60/RT complex without being encapsidated into viral nucleocapsid.326 More detailed research shows that at least one of two RT fragments, residues 1–199 of terminal protein (TP) domain and 680–842 of Rnase H (RH), is necessary for Hsp60 binding.327 The TP domain is also responsible for the binding of Hsp90 in the RT-ε RNA priming step.138
Apoptosis regulation
Similar to HCV infection, HBV infection also induces strong apoptosis which is thought mainly contributed by HBx protein.328 Studies show that overexpression of Hsp60 facilitates HBx-induced apoptosis.329,330 The interaction of HBx and Hsp60 has been observed and confirmed by different methods including affinity purification, mass spectrometry and co-immunoprecipitation.329 Hsp60 binds on a small domain (residues 88–117) of HBx.329 Furthermore, Hsp60 also forms a complex with HBx and Hsp70 in the mitochondria.330 However, the mechanism of how Hsp60 enhances the apoptosis remains unclear during HBV infections.
Immunity modulation
Hsp60 is involved in both the innate and adaptive immune response.314 Here, we focus on how HBV harnesses Hsp60 to evade host immune response. Numerous studies show that HBV employs active means to escape innate immune response and induce immunosuppression.331 Among these strategies, HBV infection increases the population of CD4+CD25+ T regulatory cells (Tregs) which can produce an amount of IL-10 and TGF-β.332 IL-10 is also called cytokine synthesis inhibitory factor (CSIF) displaying anti-inflammatory properties.333 It influences both the first and the second line of immune defence.334 HBV infection increases serum sHsp60 level and makes use of Hsp60 to activate CD4+CD25+ regulatory T via TLR2/MyD88/IL10 signaling.335
The function of HSP60 family in retrovirus infection
Although the role of Hsp60 in DNA/RNA virus infection process has been widely studied; only limited knowledge has been obtained in retrovirus infection. Hsp60 is encapsidated into HIV particles,336 but we have no idea about its function. The integrase catalyzes the integration of HIV-1 pro-viral DNA in the host genome.337 A small portion of Hsp60 colocalizes and interacts with the viral integrase (IN).338 Hsp60-Hsp10 complex maintains the integrase at an active form and stimulates its activity in an ATP-dependent manner.338 The integration is a critical step for successful infection of HIV-1.
The function of HSP40 (Hsp70 co-chaperones) in virus infection
The function of HSP40 family in RNA virus infection
Virus replication
Hsp40s regulate RNA viral replication by modulating polymerase activity, replication complex and nuclear transportation. In JEV-infected cells, Hsp40/DnaJ homolog Hdj2 interacts and colocalizes with NS5 protein, an RdRp essential for viral RNA genome replication.339 Overexpressed Hdj2 promotes JEV replication significantly. However, how Hdj2 modulates NS5 activity remains elusive.339 Influenza virus also takes advantage of Hsp40 to promote replication by assisting VRC to relocate into nucleus. The replication of influenza virus occurs in the nucleus of host cells; therefore, nuclear trafficking of viral ribonucleoprotein (vRNP) complex is required. The vRNP is composed of viral RNA (vRNA), polymerase heterotrimer (PA, PB1, PB2) and nucleoprotein (NP).340 NP has a key function of interacting with importins through its nuclear localization signals.341,342 Hsp40s have two strategies to help vRNP transport into nucleus. First, Hsp40/DnaJB1 interacts with NP at the early stage of infection and ensures efficient association between NP and importin alpha.343 This interaction is mediated by the J domain of Hsp40 and the N-terminal region of NP.343 Another strategy is that Hsp40/DnaJA1 binds with the PB2 and PA polymerase subunits, then co-translocates into nucleus with PB1-PA complex.344 Besides, Hsp40/DnaJA1 enhances viral RNA synthesis both in vivo and in vitro.344 Different from DnaJB1, DnaJA1 mainly depends on its C-terminal substrate-binding domain instead of typical J domain to manage viral RNA synthesis.344 In the replication process, Hsp70 is reported to enhance polymerase nuclear translocation.217 Thus it is proposed that DnaJA1 cooperates with Hsp70 and assist RNA polymerase nuclear import.217,343
Virus gene expression
Viral RNA replication produces plenty of double-stranded RNA (dsRNA) molecules. Host cell detects these dsRNA and activates interferon-induced protein kinase (PKR) to restrict viral replication by phosphorylating eukaryotic initiation factor eIF2α and preventing protein synthesis.220,221,345 However, in order to escape from the antiviral response of host cells, the influenza virus smartly blocks the activation of PKR/eIF-2α. Influenza virus NS1 directly binds the N-terminal of PKR and inhibits PKR activation.225 Besides, NP protein also interacts with Hsp40, leading to dissociation of Hsp40 and p58IPK.346 It is reported that type III Hsp40/p58IPK is an inhibitor of PKR,222–224,226,347 while Hsp40 is the inhibitor of p58IPK.225,348 Therefore, the dissociation of Hsp40 results in activation of p58IPK. Subsequently, p58IPK inhibits the activity of PKR/eIF-2α. As a result, influenza virus releases the inhibition of protein synthesis. However, in the late stage of infection, influenza virus M2, Hsp40 and p58IPK form a stable complex that would lead to PKR activation, ER-stress-induced cell death and virion release.349
Protein maturation
Flavivirus genome encodes a large polyprotein which is later cleaved into several mature structures and non-structure proteins. The mature proteins then form VRCs. At the beginning, HSP40 family protein DNAJC14 participates in the VRC formation of flavivirus. During yellow fever virus (YFV) infection, DNAJC14 is recruited to non-structural protein clustering sites with NS3 and NS5 to form VRC.350 However, either knockdown or overexpression of DNAJC14 inhibits YFV and HCV replication.350,351 Later, it has been demonstrated that DNAJC14 overexpression affects YFV polyprotein processing and alters VRC assembly. Overexpression of DNAJC14 alters the cleavage sites of NS3/4A and NS4A/2K and gives rise to abnormal NS3 to NS3-4A ratios, suggesting that the chaperone activity of DNAJC14 modulates NS3/4A/2K cleavage that ensures appropriate expression level of NS3 and NS4A. The inhibition of VRC formation upon ectopic expressing DNAJC14 is caused by chaperone dysregulation.351,352
Immunity modulation
Hsp40s sometimes act to help the virus evade host immunity, while sometimes it exhibits antiviral activity by increasing host immune response under certain conditions. It is reported that the expression of DNAJB1/Hsp40 and Hsp70 is induced by PolyI:C stimulation.353 Hsp40 cooperates with Hsp70 to suppress the MDA5/MAVS pathway though interacting with MDA5 and inhibiting MDA5 multimer formation.353 However, DNAJA3 shows its ability to suppress virus replication. During HFDV infection, VP1 is able to suppress the type I interferon signaling via suppression of phosphorylation, dimerization, and nuclear translocation of IRF3.354 However, DNAJA3 induces lysosomal degradation of VP1 protein. Therefore, DNAJA3 indirectly stimulates the immune response of host cells.354
The function of HSP40 family in DNA virus infection
Virus replication
Evidence suggests that Hsp40s regulate the initiation of DNA virus replication. In the case of HPV replication, it starts with the recognition of protein E2 on the origin (Ori) sequence and recruitments of replication initiator E1, which displays ATPase and helicase activities.355–357 Hsp40 (Hdj1 and Hdj2) and Hsp70 enhance E1 binding on the Ori independently and additively. Hsp40 directly binds with E1 and remains in the E1-ori complex, whereas Hsp70 transiently interacts with E1 in an ATP-dependent manner.355 Subsequent study reveals an additional role of Hdj2 in facilitating E1 helicase function by replacing E2 in the E1/E2/Ori complex.358 The stable association of E2 to Ori flanking the E1 binding site may act as a DNA clamp to prevent DNA unwinding. Similarly, Hsp40 (hTid1) also has similar functions to Hdj1 and Hdj2 with independent chaperone activity. Hsp40 interacts with HSV-1 replication initiator protein and helicase protein UL9, thereby promotes their binding to the replication origin.359,360 This provides another example of the involvement of J-proteins in the replication process of eukaryotic DNA viruses.
Except for enhancing HBV replication, a possible negative role of Hsp40 has also been reported. The core protein is a key component of viral capsid and essential for virion assembly, while HBx is a multifunctional virulence factor implicated in viral replication and hepatocarcinogenesis in human. A yeast 2-hybrid approach is used to identify interactions between the core protein and two Hsp40s, Hdj1 and hTid1.361,362 Individual expression of each Hsp40 in hepatocytes transfected with a replication-competent HBV construct shows an inhibitory effect on both viral replication and capsid formation. Further studies reveal that both core and HBx proteins are destabilized by co-expression with Hdj1 or hTid1; because they are targeted for enhanced proteolytic degradation. Except inhibiting HBV replication, Hdj1 also facilitates proteasome-mediated degradation of HBx.361
Virus induced cellular transformation
As mentioned above, some viruses can induce cell transformation and tumorigenesis. Here, we discuss the role of Hsp40 proteins on virus-induced cellular transformation. Hsp40 may have a negative role in HBV-induced cell transformation. It has been demonstrated that HBx is the major factor for induction of hepatocyte transformation.363–366 Nevertheless, overexpression of Hsp40s (Hdj1 and hTid1) significantly enhance the proteasome-mediated degradation of HBx. Another study suggests that hTid1 interacts with E7 oncoprotein and promotes the cellular transformation by HPV16;275 because the binding sequence of E7 shares high similarity to the TAg oncoproteins of SV40.
The function of HSP40 family in retrovirus infection
Nuclear entry of viral pre-integration complex
HIV can efficiently infect nondividing cells.367 This requires active transport of the viral pre-integration complex (PIC) into the nucleus without breaking down nuclear membrane. Some components of PIC implicated in regulating nuclear import include the central DNA flap and viral proteins IN, MA, and Vpr of HIV-1 (or Vpx of HIV-2).288,368–372 Hsp40/DnaJB6 interacts and enhances the nuclear localization of Vpx as well as promotes the nuclear import of viral PIC.373 Similarly, DnaJB6 also promotes Vpr nuclear localization during HIV-1 infection;374,375 particularly, the long isoform of DnaJB6 is extremely important in this process.375 The expression level of DNAJB6 S/L isoform is regulated by the polyadenylation factor CstF64. High level of CstF64 favors DNAJB6-S isoform production, whereas a low level of CstF64 enhances DNAJB6-L isoform production.374
Regulation of viral gene expression
A notable example of Hsp40 involvement in regulating retrovirus gene expression is its importance in enhancing the gene expression during HIV-1 infection.376 Nef protein, an important viral protein associated with pathogenesis and disease progression, stimulates Hsp40 expression by enhancing Hsp40 promoter activity via HSF1 transcription factor.377–379 Hsp40 and Nef co-translocate into nucleus where they become a part of CDK9-associated transcription complex to enhance long terminal repeat (LTR) mediated gene expression.376,380 The binding of HSF1 on HIV-1 LTR promoter induces viral gene expression directly.380 Interestingly, Hsp70 seems to act contrary to Hsp40, which also presents in the Nef-Hsp40 complex. Different from Hsp40, Hsp70 suppresses viral replication and gene expression; while Hsp40 rescues the Hsp70-downreguled viral gene expression. Hsp70 inhibits CDK9 phosphorylation, an essential event for high-affinity binding of HIV-1 transactivator of transcription-positive transcription elongation factor b complex for transactivating response RNA.381 It is also reported that some other Hsp40 family proteins negatively regulate HIV replication. Hsp40A1, B1, B6 and C5 (but not C3) are able to limit HIV-1 production, while they have no effect on viral gene expression upon infection by adenovirus, HSV-1 or vaccinia virus. The conserved DNAJ domain is suggested to be responsible for the inhibiting HIV-1 reproduction. The Hsp70/Hsp40 complex specifically recognizes and inhibits the Rev translation or accelerates its degradation, leading to inhibiting viral gene expression.382
The function of sHSPs in virus infection
Small heat shock proteins are the most upregulated proteins identified in host cells under stress conditions, for example, when cells are exposed to elevated ROS level, abnormally high temperature, or pathogen invasions.383 In most cases, sHSPs are responsible for recognizing misfolded proteins and transferring them to other ATP-dependent chaperones for proper folding, or proteasomes or autophagosomes for degradation.49,384 Hsp27 is one of the most ubiquitously expressed sHSPs with the highest level in skeletal, smooth, and cardiac muscles.59,385 Like all other sHSPs, Hsp27 shares a highly conserved α-crystallin domain, or so-called C-terminal domain. It contains 6-8 β-strands, forming 2 β-sheets as intermolecular interaction sites.41,59 Because of the importance of Hsp27, in this section, we mainly focus on the function of Hsp27 in virus infection.
Hsp27 has been shown particular importance in viral infections. Rajaiya et al. suggested that the association of Hsp27 with p38 or NFκB/p65 plays key roles in controlling the expression of pro-inflammatory mediators in virus-infected cells.64 Fukagawa et al. suggested that the phosphorylated Hsp27 is upregulated through the PI3K/Akt pathway upon EBV infection.386 We would discuss the special roles of Hsp27 in different viral infections in the following sections.
The function of Hsp27 in RNA virus infection
Hsp27 is upregulated during EV-A71 infection by proteomics analysis. Knockout of Hsp27 results in the suppression of virus replication and protein expression level, while their restoration appears after Hsp27 is restored. Also, Hsp27 enhances viral IRES activity by promoting 2Apro - mediated EIF4G cleavage.57 Interestingly, the nuclear translocation of Hsp27 from cytosol is reversely correlated with the relocalization of RNA chaperone hnRNPA1 from nucleus to the cytosol for initiating viral protein translation upon EV-A71 infection. However, knockout of Hsp27 blocks hnRNPA1 cytosol relocolization, indicating a fundamental role of Hsp27 in regulating the import/export of nuclear proteins during virus infection (Fig. 6).383 Hsp27 is rapid upregulated at the early stage (4 hours post infection) of coronavirus infection,387 suggesting an important role in virus early replication and possibly a good target for treating SARS-CoV-2 infection. Swine fever virus (CSFV) is a member of the family Flaviviridae. Hsp27 is found to bind with NS5A, which is a non-structural protein in response to viral replication and assembly. Hsp27 depletion enhances the virus replication while the replication is suppressed by ectopic expression of Hsp27 through activating NF-κB signaling in PK-15 cells.388 Porcine epidemic diarrhoea virus (PEDV) infection causes high fatality in swine. The Hsp27 is obviously upregulated in PEDV infected MARC-145 cells.389 However, the virus titer is declined by ectopic expression of Hsp27. Hsp27 could activate NF-κB signalling and enhance the transcription of IFNβ and downstream interferon stimulated genes (ISGs).389
Fig. 6.
The controversial function of Hsp27 during virus infection. Hsp27 would enhance 2A protein-mediated eIF4G cleavage, which would in turn promote IRES function and viral genome replication. It correlates with hnRNPA1 translocation from host cell nuclei to cytosol, and the consequential virus protein translation. Hsp27 is also able to suppress apoptosis via caspase 3 inhibition. On the other hand, Hsp27 could help activating IKK complex, modulating NF-κB and AP-1 to induce the expression of pro-inflammatory cytokines
The function of Hsp27 in DNA virus infection
Pro-inflammatory cytokines play crucial roles in early antiviral infections. Adenovirus infection causes a wide variety of diseases.390 The internalization of Adenovirus leads to the activation of ERK1/2, which could in turn trans-activate NF-κB and AP-1 to induce pro-inflammatory cytokine IL-8 expression in different experimental systems.391–393 A study shows that downregulation of Hsp27 leads to an increased release of IL-8 from keratinocytes stimulated with UV or TNFα. The increase of IL-8 is suppressed by NF-κB inhibitor and correlated with an enhanced IκB-α degradation and phosphorylated IκB-α accumulation in Hsp27-depleted cells. In addition, Hsp27 is shown to be associated with the IκB kinase (IKK) complex. Because the synthesis of prostanoid PGE2 and IL-8 is regulated by NF-κB, it could be a likely mechanism of Hsp27 in modulating inflammatory cytokine production.70 Further studies also show that Hsp27 is of particular importance in the cyclooxygenase-2 and IL-6 expression via activating p38 MAPK/MK2 signalling and the consequential stabilization of cyclooxygenase-2 and IL-6 mRNAs.68
Aside from its involvement in pro-inflammatory response regulation, researches have also found the antioxidant role of Hsp27 in regulating stress caused by ROS.49 It is commonly agreed that Hsp27 regulates enzyme activities by upholding glutathione in reduced form, such as glutathione reductase and glucose-6-phosphate dehydrogenase.394 More recent evidence correlates the level of sHSPs with the intracellular content of iron, a catalyzer of hydroxyl radical generation. Hsp27 also exhibits an important role in oxidized protein degradation machinery.395,396 However, there is also controversial evidence indicating the involvement of Hsp27 in accumulation of oxidized proteins that benefits herpesvirus replication. Experimental models are set up using two distantly related herpesviruses Rhesus Rhadinovirus (RRV) and HSV-1. They are close relative of Kaposi’s sarcoma-associated herpesvirus (KSHV). The oxidized proteins are accumulated during these viral infections. Results show the removal of only a part of oxidized proteins in a proteasome-dependent manner, while some others resisting degradation.397 Oxidized proteins resisting proteolysis become sequestered in foci within the nucleus and coincided with Hsp27-enriched foci; although they are not associated with virus-induced chaperone enriched domains (VICE). Furthermore, the accumulation of oxidized proteins is more pronounced in Hsp27-depleted cells.398 One possible explanation is that Hsp27 buffers the toxic effects of those defective proteins undergoing proteolysis through aggregation in the nucleus. The roles of Hsp27 are most likely not mutually exclusive during virus infection.
Hsp27 also contributes to virus replication. Porcine circovirus type 2 (PCV2) is a single-stranded DNA virus that causes the postweaning multisystemic wasting syndrome (PMWS) in pigs. The phosphorylated Hsp27 is upregulated in the nucleus in PCV2-infected PK-15 cells. Hsp27 inhibitors such as SB203580 suppress PCV2 replication. The same result appears upon Hsp27 knockdown. In contrast, ectopic expression of Hsp27 promotes viral replication.399 Moreover, the phosphorylation of Hsp27 is also upregulated in EBV-positive cells, as well as the phosphorylated (activated) Akt levels. When EBV-positive cells are treated with PI3K inhibitors, the phosphorylated Hsp27 level is decreased, suggesting that the phosphorylation of Hsp27 is upregulated through the PI3K/Akt signalling pathway upon EBV infection.386 However, studies also show that Hsp27 can both positively and negatively regulate the virus replication depending on the virus/cell types. Tong et al. reported that Hsp27 works as an antiviral protein against HBV replication through enhancing IFN production in hepatocytes. The Hsp27 expression level is increased in both HBV-infected human liver tissues and HBV-producing HepG2.2.15 cells.400
The function of PDIs in virus infection
The function of PDIs on virus entry and uncoating
The entry of some viruses into eukaryotic cells is governed by redox-regulated processes. One example is newcastle disease virus (NDV), a bird virus in the family of paramyxoviruses. This negative-sense, single-stranded RNA paramyxovirus gains entry to its host cell through large conformational changes in its fusogenic F-protein, which involves thiol/disulfide exchange.401 Overexpression of PDI and ERdJ5 (a PDI family reductase with an extra J domain) leads to an increase of viral membrane fusion, indicating a route whereby virus can take advantage of the PDI family to gain access to host cells.402 In endothelial cells surface, PDI possibly reduces β1 and β3 integrins allowing DENV entry.403,404 Also, thiol blockers and PDI inhibitors decrease the entry of rotavirus in MA104 cells, indicating the involvement of thiols for infectivity.405
The entry of HIV is regulated by PDIs. The envelope of HIV becomes unhinged by PDI for entry. Ryser et al. firstly reported that cleavage of two disulfide bonds in the gp120 surface component of the HIV-1 envelope is required for virion entry into CD4+ cells. In this process, the PDI on cell surface is responsible for this effect.406 PDI inhibitors sufficiently prevent the reduction and block the cleavage of surface-bound disulfide conjugate, thereby prevent infection at the level of HIV-1 entry.407 Now, there are numerous studies on how envelope binds on host cells. Results from different groups have demonstrated that gp120 moves laterally along the membrane surface until it collides with a patch of PDI in a domain of the membrane that distinguishes from a typical lipid raft. PDI reduces two disulfide bonds in gp120, producing conformation changes that likely stabilize the binding of gp120 to CD4 and expose the V3 loop for subsequent binding to the chemokine coreceptor. Following this, gp41 undergoes rearrangement into its fusogenic intermediates and entry occurs.408,409 During the entry, Galectin-9 binds PDI to regulate the redox environment on the cell surface and enhance HIV entry.410 Taken together, the reports from these three groups have profound implications for our understanding of the HIV virion surface structure and viral entry.
The other examples are the nonenveloped Polyomavirus (Py). Py particles contain a layer of coat protein VP1. This single protein, arranged as 72 pentamers, forms the shell surrounding the viral genome.411 After internalization, Py penetrates the ER membrane to gain access to the cytosol and then the nucleus for viral genome transcription and replication. PDIs isomerize or reduce the virus disulfide bonds to generate a membrane transport-competent intermediate for host cell membrane penetration.412 For example, SV40 entry involves caveolar/lipid raft-mediated endocytosis, vesicular transport to ER and translocation into the cytosol. ERp57 isomerizes the interchain disulfides connecting VP1 for virus uncoating.413 The further study demonstrates that ERp57 and PDI operate in concert with ERp29 to unfold the VP1 C-terminal arm. PDI and ERp72 reduce Py, while ERp57 principally isomerizes the virus in vitro. Mutagenesis study subsequently identified that the residues C11 and C15 of VP1 are important for infection, suggesting a role for these residues during isomerization.414
PDIs regulate viral protein translation
A little evidence shows that PDIs are involved in viral protein translation. Some positive single-stranded RNA (+ssRNA) virus depends on IRES-mediated translation for viral protein synthesis. EV-A71 infection is potently inhibited by an active compound Oblongifolin M (OM), which is isolated from herb Garcinia oblongifolia. Further studies show that OM suppresses the viral IRES-mediated translation of polypeptide via suppressing ERp57, and ectopic expression of ERp57 increases the IRES activity and partially rescues the decreased viral replication caused by OM treatment.415 The detailed mechanism how ERp57 downregulates IRES activity would be further investigated.
PDIs regulate viral activities by influencing oxidative stress and ER stress
Several studies have demonstrated the implication of redox balance disruption in the establishment of viral infection and the progression of virus-induced diseases. And accumulated ROS in turn may modulate the viral replication and cellular response that also contribute to viral pathogenesis.416,417 Virus-induced oxidative stress has been reported during HIV,418 influenza virus,419 HBV,420 HCV,421 encephalomyocarditis virus (EMCV),422 respiratory syncytial virus (RSV),423 and JEV424 infections.
Considering its thioredoxin-like sites, ERp57 has been thought a main player in the mechanisms of cell protection against oxidative stress, regardless of its subcellular location. Redox proteomics analysis of HPV positive tissues shows that the expression level of ERp57 and GST is positively correlated with tissue redox status, suggesting its potential association with viral-induced oxidative DNA and protein damages.425
ER stress is another consequence caused by virus activities. Viral infection would lead to exploitation of the ER membrane, accumulation of misfolded proteins, and imbalance of calcium concentration. Influenza A virus (IAV), HBV, JEV, DENV, and ZIKA virus all hijack host cell process to enhance viral pathogenesis, such as facilitating viral folding and trafficking, affecting receptor interaction, and modulating host immune responses.426–428 Therefore, as a major factor in ER stress response, ERp57 would be critical for viral protein glycosylation and maturation, which may in turn affect virus release and infection (Fig. 7).
Fig. 7.
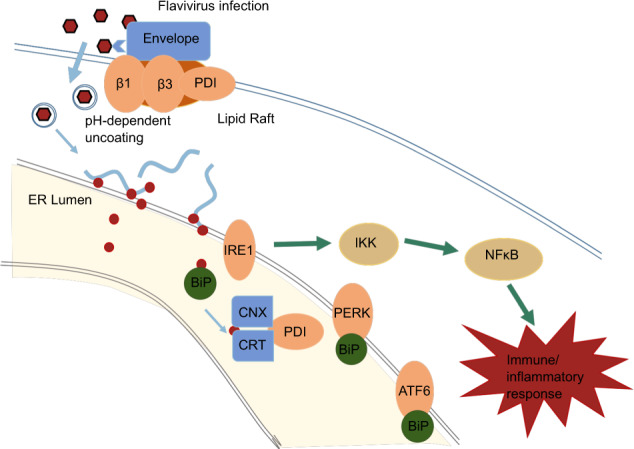
An example of PDI function and ER stress response during Flavivirus infection. Flavivirus entry into endothelial cells could be suppressed by silencing of PDI. Studies also show that PDI co-localizes with cell surface lipid rafts along with flavivirus envelope protein, leading to the activation of cell surface integrins (b1 and b3), which has direct implications in aiding the virus entering host cells. Viral RNA is then released and located around ER for translation. The increased protein synthesis may disturb ER homeostasis, leading to unfolded protein response through GRp78/BiP dissociation with PERK and ATF6 to activate PERK, ATF6, and IRE1. Downstream responses include activation of NF-κB and other signal pathways, followed by immune and inflammatory responses
The function of RNA chaperones in virus infection
The Functions of RNA chaperones in RNA virus infection
The life cycle of most RNA viruses is completed in the cytoplasm of host cells. To complete the life cycle, virus is often able to induce redistribution of many host cell proteins; particularly, those proteins involved in RNA metabolism and functional regulation of viral RNAs. hnRNPs, such as hnRNP A1,429 hnRNP C1/C2,430 hnRNP D431 and hnRNP I,432 are mainly resident in the nuclear but quite often shuttle to cytoplasm for stabilizing the viral RNA and initiating cap-independent translation.
Viruses employ various means to redistribute hnRNPs. For instance, the EBOV inhibits the nuclear import of hnRNP C1/C2. VP24 binds to NPI-1 subfamily karyopherin-alpha nuclear import proteins at the C-terminal region (amino acids 424–457) and prevents their interaction with tyrosine-phosphorylated STAT1 (pSTAT1) and hnRNP C1/C2. The inhibition results in cytoplasm retention of pSTAT1 and hnRNP C1/C2.430 Alternatively, virus may redistribute hnRNPs by promoting nuclear export. Rae1 is an mRNA export factor. VSV infection induces hnRNP A1 cytoplasm redistribution in a Rae1-dependent manner; and the redistributed hnRNPA1 is responsible for VSV-induced apoptosis.431 While the cytoplasm translocation of AUF1 is dependent on 2A protease (2Apro) and virus RNA replication.433,434 Cytoplasmic translocation of hnRNPA2 is induced by core protein. HnRNP A2 binds to negative-stranded RNA of JEV and facilitates virus replication.435 However, the detailed mechanism of how 2Apro and core protein induce the redistribution remains unknown.
Virus replication
It has been well documented that hnRNPs greatly contribute to the virus replication. For example, hnRNP I/PTB and hnRNP C participate replication of different viruses, e.g., coronavirus,436 HCV437 and poliovirus.438
The role of hnRNP I/PTBs in viral RNA synthesis seems to vary among different viruses. PTBs bind UCUAA pentanucleotide repeats at IRES and 3′-UTR. PTBs modulate coronavirus RNA synthesis.436 However, the function of hnRNP I/PTB is to some degree complicated in HCV RNA replication. It selectively interacts with the 3′ end of the HCV genomic RNA. The upstream SL2 and SL3 stem-loop structures are essential for hnRNP-I/PTB I binding whereas the most 3′-terminal stem-loop SL1 is not needed. HnRNP I/PTB I and hnRNP C specifically bind the pyrimidine-rich region of in the 3′NTR of HCV RNA genome.437 Possibly, hnRNP I/PTB I is recruited for helping to initiate viral negative-strand (−) RNA genome replication, to stabilize RNA genome, and/or to regulate the encapsidation of genomic RNA.439,440 Up to date, the detailed function of hnRNP I/PTB I binding on 3′UTR has not been elucidated. Some studies showed that PTBs have an inhibitory effect on the synthesis of HCV RNA genome,441 while Aizaki et al. reported that PTBs are required for efficient HCV RNA replication.442 It has been postulated that they suppress the initiation of viral genome RNA replication but enhance IRES-mediated translation or facilitate the replication–translation switch. It is shown that HuR can compete hnRNP I/PTB I binding on 3′ UTR of the viral RNA to facilitate La binding to the 3′ UTR; while La protein is critical for HCV genome replication.443–445
Although hnRNP C has many similarities with hnRNP I and both bind with the 3′UTR of the HCV RNA genome to facilitate viral RNA replication;437 more details demonstrate that only hnRNP C binds the 3′-ends of viral RNAs with both negative and positive polarities.446 Besides, hnRNP C also binds at the 5′- end on negative-strand RNA of poliovirus to facilitate the synthesis of positive-strand RNA;438 while miR-555 decreases the expression of hnRNP C thereby inhibits poliovirus replication.447,448
Virus RNA splicing
The main function of hnRNPs is modulating RNA process and metabolism, including splicing, stabilization and transportation. It has been documented that hnRNP K helps the RNA splicing of IAV. IAV is a major human pathogen with a genome comprised of eight negative-stranded RNA segments. Two viral RNA segments, NS1 and M, undergo alternative splicing and yield several proteins including NS1, NS2, M1, and M2 proteins. Two of the influenza virus RNA segments generate spliced products: NS segment codes for non-structural protein NS1 and nuclear export protein NEP/NS2; M segment encodes the matrix protein (M1) and ion channel (M2). NS1-BP properly splices the viral M1 mRNA segment to yield the M2 mRNA without affecting the splicing of M4 mRNA or NS mRNA segments. In this process, hnRNP K works as a mediator to bridge the interaction of NS1-BP (binding protein) and M mRNA. Lack of neither NS1-BP nor hnRNP K ensures the proper splicing of M mRNA.449,450 Further studies show that NS1-BP and hnRNP K bind M mRNA downstream of the M2 5′ splice site (5′ss). NS1-BP binds most proximal to the 5′ss, partially overlapping the U1 snRNP binding site, while hnRNP K binds further downstream and promotes U1 snRNP recruitment. Mutation of either or both the hnRNP K and NS1-BP-binding sites results in M segment mis-splicing and attenuated IAV replication.451
Virus translation
Virus RNA translation often harnesses host cellular proteins. Most hnRNPs positively regulate virus translation. Positive-sense single-stranded RNA viruses normally contain an IRES sequence that drives viral protein translation independence of the cellular cap-dependent translation. hnRNPs bind with the IRES of different viruses to assist their translation. During HCV virus infection, hnRNP I, hnRNP L, hnRNP D,452–454 hnRNP A1 and hnRNP K455 all participate the translation of HCV viral protein by binding with 5′UTR sequence. hnRNP I (PTB3), is one of the polypyrimidine tract-binding proteins (PTBs) that has been reported to bind viral RNA recurrently. They could bind the IRES of picornaviruses, including cardio-aphthovirus group (including EMCV, EV-A71) and entero-rhinovirus group (poliovirus and HRV-2).89,456 PTBs also bind HCV UTR regions,441 calicivirus RNA457 and coronavirus RNAs.436 For picornavirus and HCV, it is proposed that PTBs could help IRES folding into a translation-competent structure.458 Although unlike canonical transient interactions shared by RNA chaperones, it is not clear whether PTBs are eliminated after the IRES has folded properly.459 PTB binds the 5’ UTR of HCV RNA genome to initiate translation. In translation assays, PTB antibody efficiently blocks the IRES-mediated translation in vitro.460 Three RRM motifs of PTB monomer directly bind with IRES of FMDV to stabilize IRES structure and enhance eIF4G entry to IRES.459 hnRNP L is capable of binding the 3′ border of the IRES.461–463 hnRNP L binds with single stranded-HCV RNA when preannealing single-stranded RNA with miR-122.463 HnRNP D, also referred to as AU-rich element RNA-binding protein 1 (AUF1), shuttles between the cytosol and nucleus. It is found that hnRNP D could also interact with the stem-loop II of HCV 5′ UTR, and its overexpression enhances HCV IRES-dependent translation.452 PIM1 inhibitors induce hnRNP D relocalization from nucleus to cytosol so that it binds the IRES and inhibit EV-A71 protein translation.122 Similarly, hnRNP K interacts with SL1 located in the 5′ -UTR of HCV genome where is bound with miR-122 binding site.464,465 miR-122 is required for HCV replication. It binds at a conserved sequence in the 5′ -UTR and increases the stability of HCV RNA.466–468 It is also reported that residues 25–91, a hydrophilic region near the N terminus of HCV core protein, binds to proline-rich domains of hnRNP K and negatively regulates the effect on human thymidine kinase transcription.469 However, its function on viral replication is not well addressed.
While hnRNP A1 binds with both 5′- and 3′-UTR of HCV RNA, they form a complex with NS5b and septin 6 to assist viral protein translation. The C-terminal of hnRNP A1 and N-terminal of septin-6 are required in the translation process. Since hnRNP A1 has many homologous such as hnRNP A/B, hnRNP A2/B1, and hnRNP A2; all of them may substitute for hnRNP A1 in regulating IRES-mediated translation.470 The enteroviruses’ IRES sequence also interacts with hnRNPs. hnRNP A1 specifically binds on the 5′-UTR of EV-A71 and enhances IRES-dependent translation.471 Apigenin, a dietary flavonoid, interacts with hnRNPs and interferes with their RNA editing activity.472 The binding of hnRNP A1 with viral RNA is significantly blocked when the cells are infected with EV-A71 upon treating with Apigenin, leading to marked suppression of IRES-mediated translation. It is noted that hnRNP A1 redistribution is not affected in this experiment. Recent studies show that hnRNP A1 cytoplasmic translocation is strictly regulated by some signalling or stress proteins, such as MINK/p38 MAPK pathway473 and Hsp27.57 P38 inhibitors or Hsp27 knockout dramatically block hnRNP A1 translocating from nucleus into cytosol, leading to inhibition of virus replication.57,473
Except for promoting viral protein translation by binding with the viral IRES sequence, hnRNPs can directly bind with virus proteins to facilitate virus replication. Upon CVB3 infection, 3Cpro binds and cleaves hnRNP M to facilitate virus replication.474 However, hnRNP M does not affect IRES activity and RNA stability.475 hnRNP K is required for DENV replication.476 The core protein of DNEV interacts with hnRNP K to release the inhibitory effects of hnRNP K on transcriptional activator C/EBPb.477,478 Other studies demonstrate that hnRNP C1/C2 interact with viral RNA and NS1 protein of DNEV and facilitate virus reproduction.479–482 hnRNP D binds on both 5′- and 3′-UTR of enteroviruses and inhibits translation without affecting RNA decay.434 On the other hand, virus also applies strategy to release the inhibitory effects of hnRNP D via protease 3CD cleavage.434 The cytoplasm translocation of hnRNP D is dependent on the expression of 2Apro and viral RNA replication.433,434 hnRNP D also inhibits nucleocapsid expression by interacting with an AU-rich sequence (nt 41 to 60) in the 3′ UTR of NIV.483
Virus RNA export
hnRNP A2/B1 interacts with NS1 of influenza virus, leading to decrease of the viral NS1 RNA/protein levels as well as NS1 RNA nuclear export.484
RNA polyadenylation
The gag gene of Rous sarcoma virus contains a cis-acting negative regulator of splicing (NRS) element. The general function of NRS is usually manifested by binding serine/arginine-rich (SR) protein hnRNP H and U1/U11 snRNPs, resulting in inhibition of splicing by acting as a decoy 5′ splice site. Evidenced by in vitro polyadenylation analysis, a new function of NRS element is revealed that it is required for 3′ LTR polyadenylation. In this process, hnRNP H binds on NRS element and promotes polyadenylation; however, mutation of the binding sites of U1 and U11 snRNPs to the NRS does not affect polyadenylation.485,486 An opposite result shows that polyadenylation stimulatory activity of NRS is dependent of U1 and/or U11 snRNP as well as SR proteins; while hnRNP H seems not participating in the splicing control or Rous sarcoma virus polyadenylation.487
The functions of RNA chaperones in regulating viral activities of DNA viruses
Virus replication
The transient lytic DNA replication of HCMV relies on the cis-acting element oriLyt, six viral-encoded core proteins, the proposed DNA replication initiator protein UL84, IE2, IRS1 and the gene products from the UL112/113 loci. Here it is reported that hnRNP K is sufficient to interact with UL84 protein of HCMV thereby promotes viral replication. The interaction is further enhanced by another two virus proteins UL44 and IE2.488
Gene expression
hnRNPs regulate DNA virus gene expression at both transcriptional and post-transcription levels either positively or negatively.
At the transcription level, hnRNP D0B and hnRNP A/B are capable of binding with cis-acting AAGTATGCA core element of HPV18 late promoter to suppress the late genes L1 and L2, which are components of virus capsid proteins.425,489 hnRNP K binds enhancer II (ENII) to promote HBV replication. Ectopic expression of hnRNP K augments HBV replication; while hnRNP K knockdown significantly decreases HBV viral load.490 Further study reveals that APOBEC3B forms a super complex with hnRNP K that alters the ENII binding activity via conformational change, therefore suppresses the S gene promoter activity.491 In the course of HSV infection, the immediate early protein IE63 (ICP27), hnRNP K and casein kinase 2 (CK2) together form a big complex. CK2 is capable of phosphorylating hnRNP K and ICP27. The phosphorylated ICP27 is responsible for its nucleocytoplasmic translocation and interaction with hnRNP K. Up to date, the function of the complex formation is not well elucidated.492,493 It may affect ICP27 to recruit the cellular RNA polymerase II for the transcription of certain late genes.494–496
At the post-transcription level, hnRNPs regulate the polyadenylation, splicing, and translation during DNA virus infections. HPV genome can be divided into an early region and a late region, followed by the proximal early (pAE) and the distal late (pAL) polyadenylation signals, respectively.497,498 The virion production mainly depends on the differentiation-dependent induction of L1 and L2 late genes. It has been shown that hnRNP H downregulates the late gene L2 at an early stage by interacting with the multiple GGG motifs located 174 nucleotides downstream of the early polyadenylation signal pAE. The hnRNP H binding promotes polyadenylation at early polyadenylation signal and inhibits the L2 mRNA production, since L2 mRNA production need read-through into the late region and polyadenylation of the late transcripts at the pAL.499 While at the late infection stage, hnRNP H is captured by L1 to release the inhibitory effect on L2.499,500 This process is called late gene autoregulation which enables rapid viral capsid protein production.500 Another example for hnRNPs modulating the polyadenylation is that SM polymerase (pol) mRNA of EBV early protein is cleaved and polyadenylated inefficiently.501 Under certain conditions, EBV early protein SM may harness hnRNP A1 and hnRNP C to help the processing of polymerase mRNA.
Viral RNA splicing is also modulated by hnRNPs. hnRNP A1 negatively regulates the expression of HPV late genes by affecting RNA splicing. It directly binds with the late regulatory element (LRE) in differentiated HPV16-infected cells.502 hnRNP A1 binds with splicing silencer element to suppress the use of the 3′ splice site located immediately upstream of AUG at Late 1 (L1) mRNA. hnRNP A1 inhibits the splicing of late mRNAs through the splicing silencer sequence and prevents the premature expression of L1 gene.503–505 On the other hand, there is another 17 nucleotides resident immediately downstream of the splice site which counteracts the effect of hnRNP A1-binding splicing silencers.506 hnRNP I also interferes with the splicing inhibitory elements locating at the upstream and downstream of major late 5′ splice site SD3632, thereby activates the late gene expressions.507
At the translation level, hnRNP I and hnRNP K inhibit the translation of HPV late gene L2 via binding on a specific cis-acting element in the 3′ end of L2 mRNA;508 whereas the inhibition of L2 translation can be disrupted by c-Src-mediated phosphorylation of hnRNP K at multiple tyrosine residues.509
Cellular transformation
hnRNPs also exhibit some other functions during virus infections. AUF1 works as a major component of C promoter binding factor 2 (CBF2) to interact with EBNA2 and mediate EBNA2 targeting to the latency C promoter (Cp) of EBV, thereby inducing B-cell immortalization and viral latency in humans.510 AUF1 also binds with the EBER1 noncoding RNA of EBV. In EBV-positive cells, EBER1 is abundant; therefore it may compete with AUF1-interacting targets in the host cells.511 Both EBNA2 and EBER1 are proposed to facilitate cell transformation.
Immunity modulation
Aside from affecting virus life cycle directly, hnRNPs are able to regulate viral activities through modulating immune response. During HSV-1 infection, hnRNP A/B form a complex with viral DNA, followed by homodimerization and demethylation. These events result in translocating the complex to cytosol and activating natural immunity through type I interferon signalling. Moreover, the complex promotes N6-methyladenosine modification and cGAS-STING-related mRNAs translocation upon infection by DNA viruses, further enhancing the immune response for virus elimination.512
The function of RNA chaperones in regulating the viral activities of retroviruses
As we have mentioned above, hnRNP A1 can bind to RNA/proteins and participate intracellular nucleo-cytoplasmic transportation,513 as well as alternative splicing of mRNA in eukaryotes. During retrovirus infection, hnRNPs participate in viral RNA transcription,514 splicing,515–517 translocation516 and translation.
Transcription
In HIV-infected cells, viral nef protein is required for high-level viral replication.518 It is reported that Nef, Eed, kinase Lck and nPKC subfamily (PKCδ/θ) form a complex NAKC (Nef-associated kinase complex) responsible for promoting viral replication.519 Later on, it is found that hnRNP K is also a partner of the complex. It bridges the interaction of Nef, Eed and the kinases. The hnRNP K-nucleated complex activates ERK1/2 and results in suppressing HIV promoter, enabling suboptimal amounts of Tat and transcription factors (e.g., NF-kB) for initiation of transcription.514
Post-transcription
HIV-1 takes advantage of alternative splicing to generate doses of messenger RNAs to encode the various viral proteins. It is known that over 40 message RNAs are created by alternative splicing from a single pre-mRNA.520,521 Alteration of splicing patterns dramatically affects the infectivity and pathogenesis of HIV‐1.521,522
The splicing of HIV mRNA is mainly controlled at the early stages of spliceosome assembly on pre-mRNA by a stepwise association of the small nuclear ribonucleoprotein particles (snRNPs) U1, U2, and U5·U4/U6. The early steps include the recognition of the 5′ splice site and the branch point sequence by U1 and U2 snRNPs, respectively.523 RS splicing factors, which contain a domain rich in arginines and serines (RS domain), assist these steps. U2AF, one of the splicing factors which consists of two subunits U2AF65 and U2AF35, interacts with the polypyrimidine tract and the 3′ splice site, respectively.524 Then the interaction mediates the association of U2 snRNP with the branch point sequence.525–527 SR splicing factors are essential for virtually every step of spliceosome assembly, including early recognition of splice sites, recruitment of basic splicing factors to the pre-mRNA, and formation of bridging contacts with other RS domain-containing splicing factors.528
Prior to forming spliceosomes, the pre-mRNA is packed with hnRNPs.529 It has been documented that pre-mRNAs bind with different subsets of hnRNPs, indicating sequence specificity at some degrees. A direct role of hnRNPs in constitutive splicing has not been observed; although it seems that the binding of hnRNPs exhibits an unspecific nature. It is widely believed that hnRNPs employ crucial functions of modelling pre-mRNA structure and initiating recognition of splice sites.530
The cis-acting sequence elements of cellular and viral pre-mRNAs undergoing alternative splicing regulate the process either positively or negatively. They bind trans-splicing factor machinery together and form splicing complex. The majority of trans-acting factors are either hnRNPs or SR family proteins. These proteins also regulate alternative RNA splicing either positively or negatively. SR proteins often regulate splicing in a positive way while some hnRNPs mainly do the job in a negative way.528,530
Alternative incomplete splicing of HIV-1 genomic mRNA produces more than 40 unique viral mRNA species within an HIV-1-infected cell through highly accurate regulation by cis exon splicing silence elements (ESS) and trans hnRNPs. The production of Tat, Rev and Vpr proteins is highly controlled because they play key roles in HIV-1 multiplication. ESS2 is the first identified ESS in the HIV-1 genome that locates downstream of 3′ ss A3 within exon 4 and specifically represses tat mRNA splicing.531 ESS2 is mapped to a 10 nt core sequence CUAGACUAGA.532 ESS3, which locates at Exon 7, represses splicing at 3′ ss A7.533 Fine structure mutagenesis indicated that ESS3 is bipartite; and each sub-elements [AGAUCC (ESS3a) and UUAG (ESS3b)] inhibits splicing independently.534 A third ESS (AUAGUUAGUCCUAGG, ESSV), which locates downstream of 3’ ss A2 in exon 3 within the vif coding sequence, represses splicing at 3′ ss A2.535
To modulate the expression of Tat protein, several hnRNPs participate the splicing process by interacting with these ESS, intron splicing silencer (ISS) elements directly or interfering enhancer splicing element (ESE) activity. For example, hnRNP A1 and hnRNP K synergistically bind on ESS2 and inhibit the utilization of A3 splicing site.536,537 The UAG triplets in ESS2 is required for hnRNP A1 binding, and several hnRNP A1-binding sites are also found at SLS3. The C-terminal Gly domain of hnRNP A1 is important for the binding. SR proteins SC35 and SRp40, the strong activators of site A3, have similar binding sites to hnRNP A1. Hence, they may compete with each other to bind ESS2 and ESE2.536 Another study shows that hnRNP H displays an inhibitory effect on splicing. hnRNP H binds to ESS2 and competes with U2AF35 for binding to the exon sequence flanking 3′-splice site A3. This binding results in the inhibition of splicing at the 3′-splice site A3.538 hnRNP A1 also inhibit tat splicing by binding with ISS, which is independent of exon splicing silencer (ESS2) and blocks the entry of U2 snRNP but not U2AF65.539–541 The UP1 domain of hnRNP A1 is responsible for the ISS binding, while the RGG domain of hnRNP A1 is not needed for the alternative splicing activity.542,543 In addition to ESS2 and ISS elements that are regulated by hnRNP A1, blocking the binding of SR protein SC35 on ESE is another strategy for the virus to inhibit the Tat expression at the early infection stage. hnRNP A/B proteins bind the ESE locating at tat exon 2 to repress splicing, whereas SC35 binds the ESE to activate splicing. The binding sites of hnRNP A1 and SC35 are overlapping within the juxtaposed ESE/ESS9. It seems that hnRNP A1 inhibits the splicing of the upstream intron by binding to the ESS and directly masking the SC35 binding site.544,545 Similarly, hnRNP A1 is also reported to compete with ASF/SF2 at ESE3/(GAA)3. Therefore, the ratio of ASF/SF2 to hnRNP A1 determines the utilization of ESE3/(GAA)3 for activation or repression at site A7.515,541,546
The expression of other HIV proteins is also regulated by hnRNPs via modulating splicing. hnRNP A/B proteins inhibit the splicing at 3′ splice site A2 which is used to generate vpr mRNA. hnRNP A/B proteins bind with ESSV with a consensus sequence PyUAG. The splicing at splice site A2 is increased when the three PyUAG motifs within ESSV are mutated, leading to increased vpr mRNA levels and reduced skipping of the noncoding exon flanked by A2 and D3.547 Others reported that hnRNP D also binds at ESSV and functions as an inhibitor of splicing.548 The binding of hnRNP A/B proteins at ESSV blocks U2AF65 binding to the PPT of the repressed 3′ splice site and inhibits the splicing efficiency of 3′ splice site.549
Interestingly, hnRNP H is found to positively regulate the exon 6D splicing of HIV mRNA. It interacts with the sequence CGGA and enables U1 snRNP assembly onto exon 6D.550,551 Further study shows that hnRNP H family proteins function as a splicing enhancer through enhancing the ATP-dependent spliceosomal complex formation.552
RNA translocation
After transcription and splicing, lots of spliced and unspliced RNA molecules of HIV exist in the nucleus. Translocation of RNA into the cytoplasm is dependent on the Rev protein. On one hand, some hnRNPs facilitate RNA translocation. In HIV-1 genome, two sequences similar to the hnRNP A2 response element (A2RE) function as cis-acting RNA trafficking sequence that binds to the trans-acting trafficking factor hnRNP A2. The binding mediates a specific RNA trafficking pathway characterized extensively in oligodendrocytes. A2RE-1 locates within the major homology region of gag gene; while A2RE-2 locates at a region overlapped between vpr and tat coding sequence. hnRNP A2 binds to both A2REs in vitro to form a complex, which is necessary for RNA transportation in oligodendrocytes in vivo. A2RE-mediated RNA transport requires both microtubule and hnRNP A2. If gag and vpr RNAs containing A2RE-1 and A2RE-2 respectively are differentially labelled, it is observed that they are co-assembled into the same RNA trafficking granules and cotransported to the periphery of the cell. Although the tat RNA contains A2RE-2, it is not transported as efficiently as vpr RNA.553–555 hnRNP D also has a similar function in assisting RNA translocation.556 On the other hand, hnRNP A2B1, hnRNP C and hnRNP U can retain the HIV-1 genomic RNA in nucleus. Depletion of hnRNP A2B1 results in cytoplasmic redistribution of the virus RNA genome in the absence of rev.557,558 An N-terminal fragment of the hnRNP U specifically targets the 3′ long terminal repeat (3′LTR) and blocks the cytoplasmic accumulation of mRNAs, thereby affecting HIV gene expression.559
Besides HIV, the regulatory protein ORF57 of Kaposi’s sarcoma-associated herpesvirus (KSHV) also interacts with hnRNP K. CK2 can phosphorylate ORF57 and promote ORF57-hnRNP K interaction. The ORF57-hnRNPK-CK2 complex may be important for RNA export of KSHV since ORF57 is responsible for the nuclear export of viral mRNAs.560,561 hnRNP A1 interferes with the binding of Rex to rex-response element (XRE). The Rex protein of HTLV-1 mediates the nuclear export of unspliced and incompletely spliced viral mRNAs. This process partly depends on the binding of Rex to rex-regulatory sequences XRE.562
Viral protein translation
HnRNP A1 is induced to redistribute into the cytoplasm in the late infection stage of HIV and enhances the IRES-mediated translation.563 hnRNP D helps the redistribution of HIV mRNA into the cytoplasm, and p45 and p42 isoforms increase viral Gag protein synthesis while p40 and p37 suppressed this process.556 hnRNP I interacts with a novel IRES within a latently expressed gene (vCyclin) of KSHV and enhances vFLIP expression.564
Challenges of targeting stress proteins for antiviral therapy
HSPs, ER stress and human diseases caused by virus infections
The network of HSPs and their co-chaperones is essential for cells to maintain protein homeostasis, including nascent protein folding, protein translocation across membranes, and protein complex formation. Many stress stimuli can disrupt protein homeostasis such as thermal stress, nutrient starvation, chemical toxicity, oxidative stress, hypoxia, inflammation, and virus infection.565–568 Stresses can lead to protein misfolding and aggregation that need to be resolved quickly to prevent cell and tissue damage.568 HSPs and their partners may facilitate protein degradation when cells cannot cope with massive damaged proteins.
Many lines of evidence suggest HSPs as major factors for protein surveillance to protect host cells against virus infections. It was observed that Hsp70/Hsp90 expression increased dramatically in HSV-infected cells.569 Overexpression of Hsp70 inhibits the translocation of viral capsid into nucleoli during flavivirus infections. Although the interaction has not yet been investigated in natural infection, the in vitro findings have suggested that Hsp70 may act as a negative regulator of viral capsid protein to protect host cells against WNV infection by abolishing cytotoxic effects induced by the viral capsid.570 Studies from ours and other groups show that almost all HSP subfamily members (Hsp90s, Hsc70, Hsp70, Grp78, Erp57, Hsp27) are highly responsible to enterovirus infection and play key roles in all stages of virus life cycle. Not surprisingly, demonstrated that all most all HSPs (Hsp90s, Hsp70s, Hsp60s, Hsp40s and Hsp27) participate coronavirus infection,145,196,387,571 suggesting good target for anti-COVID-19 drug development.
As discussed in above sections, HSPs exhibit immuno-modulatory effects on both innate and adaptive immune responses.572–574 HSPs are capable of regulating not only intracellular innate immunity, but extracellular innate and adaptive immunity as well. HSPs released from tumor cells can bind to surface receptors on antigen presenting cells (APCs) and elicit tumor-specific killers by means of antigen cross-presentation.575,576 For example, Hsp27 positively modulates NF-κB phosphorylation, increases IFNβ transcription and downstream antiviral interferon-induced genes (ISGs). PEDV infection downregulates Hsp27 expression to escape host antiviral surveillance.389
Additionally, extracellular HSPs can also regulate cytokine production by dendritic cells (DCs), underscoring a connection between innate and adaptive immune responses modulated by HSPs.577 The purified or recombinant Hsp70 can stimulate the production of pro‐inflammatory cytokines and C–C chemokines in monocytes, macrophages and DCs, and upregulate MHC and costimulatory molecules in DCs.578 Binding of extracellular Hsp72 to human monocytes and dendritic cells can induce the production of the pro-inflammatory cytokines including TNF-α, IL-1β, IL-6 and IL-12 and IFN-γ.579 In the process of above HSP-induced cytokine production, HSPs act as internal stimulus of the CD14/TLR (TLR2 and TLR4) complex signal transduction pathways that further activates NF‐κB and MAPKs signalling.580,581
Although the main functions of HSPs are to protect cells upon stresses, they are often hijacked by many viruses to achieve successful infections. Recent study has demonstrated that DENV NS3 protein acts as a viral suppressor of RNA silencing by interacting with Hsc70, thereby interrupting host antiviral system by RNAi pathway and subsequently enhancing DENV replication.582 EV-A71 takes advantage of HSPs (Hsp90, HSc70, ERp57, and Hsp27) to enhance 2Apro-mediated eIF4G cleavage that is favour viral protein translation and blocks host protein translation.57,219,415
Viruses also initiate ER stress in host cells after infection. They have to manipulate UPR activation leading to cell survival, rather than inflammation induction, autophagy and apoptosis. DENV modulates UPR activation in a sequential manner to prolong the viral life cycle by allowing cellular adaptation to cope with the infection-induced ER stress. UPR is transiently induced by PERK pathway resulting in phosphorylation of eIF2α and subsequently translational attenuation in the early phase of infection. This transient event allows viral protein synthesis and accumulation that finally trigger UPR by IRE1-XBP1 (X-box binding protein 1) axis in the mid-phase of infection. This results in the increased expression of Grp78 to facilitate protein folding and also the increased expression of GADD34 (growth arrest and DNA damage 34), which dephosphorylates eIF2ɑ, and thus allowing protein translation to be continued. The increased Grp78 also prevents cellular apoptosis-mediated by CHOP (pro-apoptotic protein during stress persistence). Finally, the increased viral proteins transiently trigger ATF6 arm of UPR to provide the active spliced XBP1 for sustaining UPR activation in the late phase.583
JEV infection requires Hsp70s in particular stages of its life cycle for survival and establishment of infection, including viral entry, replication,194 and maturation.195 Cell-surface Hsp70 directly interacts with JEV envelope protein.192 Antibodies against Hsp70 or 90 significantly block virus entry.111 It is also observed that Hsp70 colocalizes and directly interacts with JEV replicase complex. In addition, Hsp70 also interacts with NS3, NS5 and viral dsRNA that stabilizes VRC formation during JEV infection.194,584
ER stress and antiviral
In mammalian cells, the ER stress is sensed and mediated by three ER transmembrane receptors: pancreatic ER kinase (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6). In resting cells, these three sensors are maintained in inactive states via interactions with the ER resident chaperone Grp78. When unfolded or misfolded proteins accumulate in the ER lumen, Grp78 dissociates from these three transducers, resulting in activation and initiation of the UPR.
Viruses also take advantage of or revolt ER stress response by different means for favour its life cycle at specific stage(s) of infection. The ER stress response constitutes a cellular process triggered by a variety of conditions disturbing protein folding in the ER. Eukaryotic cells have developed an evolutionarily conserved adaptive mechanism, i.e., the ER stress and UPR, aiming to clear unfolded/misfolded, and excessive amount of protein production; thereby restoring ER homeostasis. In cases ER stress cannot be resolved, UPR would be triggered and lead to cell death. ER stress and UPR could be observed in a large amount of virus infections, as most of viruses require host ER machinery for viral protein synthesis and modification. Virus infection usually causes ER stress. When large amount of misfolded/unfolded proteins or excessively expressed viral proteins accumulate inside ER lumen, ER stress response is triggered as indicated by fast elevated expression of ER chaperone proteins, including Grp78/Bip, Grp94, calnexin and calreticulin. It is well documented that ER stress response is triggered by a number of viruses, including influenza virus, infectious pancreatic necrosis virus (IPNV), Tula virus (TULV), PEDV, Bovine viral diarrhea virus (BVDV), DENV, JEV, HBV, HCV, Hepatitis E virus (HEV), HSV-1, canine distemper virus (CDV), RSV, simian virus 5 (SV5), CVB3, and HIV-1, etc.
In some cases, ER stress plays a role in virus pathogenesis. For instance, JEV, BVDV, TULV, severe acute respiratory syndrome coronavirus (SARS-CoV) and WNV have all been shown to induce apoptosis through UPR.585–589 Oxidative stress is mediated by ER stress during HCV infection.21 Certain viruses modulate ER stress response or preferentially activate different pathways of UPR. HCV infection activates the ATF6 pathway while blocks the IRE1 pathway. Instead, HBV infection stimulates both ATF6 and IRE1 signalling but has no effects on PERK signalling although both virus infect the same kind of hepatocytes.590–592 HSV-1 develops a virulence factor enabling dephosphorylation of eIF2α, one of the downstream effectors of the PERK pathway.593
Some consequences of UPR are beneficial for viral life cycle. For example, ATF6-induced expression of chaperone proteins may help viral protein folding and prevent protein aggregation. The PERK-eIF2α-activated ATF4 may help re-establish cell metabolism and resume protein translation. The IRE1-XBP1 pathway may facilitate virus replication by enhancing ER protein-folding ability and ER membrane biosynthesis.594 The activated ATF6 promotes the replication of ASFV and Lymphocytic choromeningitis virus (LCMV).595,596 DENV envelope protein directly interacts with Grp78, which provides a scaffold for its association with two other chaperones calnexin and calreticulin. This complex significantly improves the proper folding and stability of DENV E protein, thereby leading to increase of virus production.597 Other than DENV, Grp78 is also demonstrated to promote HCMV, JEV and RGNNV infections.195,598,599 The IRE1-XBP1 pathway is activated by IAV to facilitate its replication.600 However, other UPR outcomes are detrimental for virus replication. The PERK-eIF2α-mediated global translation attenuation is known as an antiviral response to restrict viral replication, such as infection by DENV or WNV.583,601 The activated PKR phosphorylates eIF2α at the ribosomal interface, which in turn causes a general inhibition of protein synthesis and blockage of VSV replication.602 The IRE1-XBP1(s)-mediated ER-associated protein degradation (ERAD) pathway reduces intracellular HBV particles by degrading its envelope proteins.603
Another approach of ER stress contributing to virus pathogenesis goes through modulating host cell immune responses. VSV, HCV and SARS-CoV are able to inhibit the type I IFN signaling pathway by activating the PERK signalling, which leads to the phosphorylation-dependent ubiquitination and subsequent degradation of IFNAR1, thereby promoting immune evasion and virus pathogenesis.604,605 WNV has also been reported to induce ER stress and inhibit type I IFN signaling pathway for escaping from the host immune response.601 US11 protein of HCMV activates UPR to facilitate the degradation of class I major histocompatibility complex (MHC1), leading to immune evasion.606 Moreover, ER stress is responsible for viral pathogenesis by interconnecting with the inflammatory responses. For example, HCV induces inflammatory responses by activating IRE1, which interacts with TRAF2 to phosphorylate JNK, leading to activation of inflammation mediators.607 NS4B and NS5A proteins of HCV activate NF-κB via ER stress-elicited calcium depletion and ROS production.604 The X protein (HBx) of HBV induces the expression of cyclo-oxygenase 2 (COS2), a key mediator of inflammation, through PERK-eIF2α-activated ATF4.608
RNA chaperons and human diseases caused by viral infections
hnRNPs impact mRNA metabolism including transcript synthesis, processing and degradation as well as translation.432 The function of hnRNPs is determined or modulated by cellular localization. The mechanisms that regulate the nucleo-cytoplasmic shuttling are, therefore, of extreme importance. Most hnRNPs possess a conventional nuclear localization signal (NLS) and are predominantly present in the nucleus during steady state. They are able to translocate in the cytosol upon post-translational stimulation or by the recruitment of other hnRNPs.609 Post-translational modifications like methylation, phosphorylation, ubiquitination and sumoylation are reported to affect biological activity and subcellular localization of hnRNPs.610 As stated in the above sections, virus always employs different strategies to redistribute hnRNPs in host cells.430,431,433,434
How dysregulation of hnRNPs causing human diseases has gained an increasing interest. The expression level of hnRNPs is altered in many types of diseases, including varieties of human cancers and neurodegenerative diseases (e.g., spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), and fronto-temporal lobe dementia). In this section, we mainly focus on the relationship between hnRNPs and diseases caused by virus infections.
Enteroviruses are common pathogens that cause human diseases worldwide. Although most enteroviral infections are subclinical, they may cause a wide spectrum of diseases including mild upper respiratory illness (common cold), febrile rash (hand, foot, and mouth disease and herpangina), aseptic meningitis, heart failture, pleurodynia, encephalitis, acute flaccid paralysis (paralytic poliomyelitis) and neonatal sepsis-like disease.293,611 Besides, several studies showed that enterovirus sequences could be detected in neuronal cell bodies of the spinal cord of 60–88% amyotrophic lateral sclerosis (ALS) patients, suggesting that persistent enterovirus infection may have a relationship with ALS.295,297,299 Enterovirus infections are supposed to cause or increase the risk of ALS; because the replication and translation of poliovirus, EV-A71, and coxsackievirus redistribute numerous hnRNPs (such as hnRNP A1) into the cytoplasm, the same localization during ALS pathogenesis. For example, hnRNP K, hnRNP A1, hnRNP M and hnRNP D shuttle into cytoplasm to assist virus replication or translation.300,455,472,612–614 Dislocated hnRNP A1 and loss of splicing function have been regarded as a toxic mechanism in ALS pathogenesis.301
HSV infection is one of the most common causes of infectious disease in humans.302 HSV infection often causes watery blisters in the skin or mucous membranes of the mouth, lips, nose, or genitals.615 As neurotropic and neuroinvasive viruses, HSV-1 and -2 persist in the infected individuals through hiding in the neuron cells from immune surveillance. When the carrier’s immunity compromised, HSV is reactivated that causes new sores. More seriously, accumulating evidence shows that HSV infection is associated with the pathogenesis of Alzheimer’s disease.105,616,617 During HSV infection, hnRNP K positively promotes HSV replication;618 while hnRNP A2/B1 negatively regulates HSV replication by triggering immunity response.512
HBV, HCV, EBV, HPV and HIV are oncogenic viruses that account for over 20% of human cancers. HCV or HBV infection leads to a wide spectrum of liver disease ranging from acute hepatitis (including fulminant hepatic failure) to chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC).619,620 HDV can only infect people who are already infected with HBV. The coinfection of HDV and HBV increases the risk of liver cirrhosis and liver cancer.621,622 Except for damaging the liver, 10% of HBV infected patients also have other symptoms outside the liver, such as serum-sickness–like syndrome, membranous glomerulonephritis, acute necrotizing vasculitis (polyarteritis nodosa) and papular acrodermatitis of childhood (Gianotti–Crosti syndrome).623 Multiple steps of HCV reproduction need the presence of hnRNPs. The RNA replication of HCV needs the presence of hnRNP I, hnRNP C;437,442,446 while virus RNA translation requires hnRNP A1, hnRNP D, hnRNP I, hnRNP K and hnRNP L.452,460,462–465 Besides, hnRNP A2, hnRNP L, hnRNP U and hnRNP K are highly expressed in HCC tissue. They promote liver cancer progression.8,304,305,624
EBV has been implicated in several diseases, including infectious mononucleosis,625 Burkitt’s lymphoma,626 Hodgkin’s lymphoma,627 nasopharyngeal carcinoma,628 multiple sclerosis629 and lymphomatoid granulomatosis.630 Other diseases associated with EBV infections include Gianotti–Crosti syndrome, erythema multiforme, acute genital ulcers, oral hairy leukoplakia,631 and disorders related to α-synuclein aggregation (e.g. multiple system atrophy, dementia with Lewy bodies, and Parkinson’s disease).632 During EBV infection, hnRNP A1 and hnRNP C assist the polyadenylation of EBV RNA. hnRNP D takes part in cellular transformation-induced by EBV.510
HPVs are a group of DNA viruses with more than 150 members that infect cutaneous and mucosal epithelia. The acute infection of HPV causes benign cutaneous lesions such as non-genital and genital warts, or flat cervical condylomas.633 About 15 HPV subtypes that infect genital tract are capable of inducing malignant tumors, most commonly in the cervix.308,309 These cancer-associated HPVs are grouped into high-risk types, while those not associated with cervical cancer are regarded as low-risk types.307 Most infections by low-risk type HPVs are asymptomatic, except for infection by HPV6 and HPV11 that cause most cases of genital warts (condyloma acuminatum).309 HPV-related cancers are the result of long-term persistent infection with high-risk types. HPV16 and HPV18 are the most common carcinogenic types which account for a larger proportion of cervical cancers, squamous cell cancers and adenocarcinomas in all regions.311 HPVs are also implicated in the development of a variable proportion of vulvar, vaginal, penile, and oropharyngeal cancers, anal cancer,634 head and neck cancers,635–637 lung cancer and skin cancers. hnRNP A1 is involved in splicing of HPV RNA; while hnRNP H plays a role in the polyadenylation of HPV. hnRNP K and hnRNP I are required for viral protein translation.
Besides acquired immunodeficiency syndrome (AIDS), HIV infection is also able to cause human cancers because HIV infection impairs immunity system. People with AIDS are not only more easily infected with bacteria, viruses, fungi, and parasites;638 but at high risk for developing various viral-induced cancers as well, including Burkitt’s lymphoma, Kaposi’s sarcoma, primary central nervous system lymphoma, and cervical cancer.639 hnRNP A1, hnRNP D, hnRNP H regulate the splicing of HIV RNA.538,548,550,551,556,563 hnRNP K assists HIV transcription. And some other hnRNPs participate in HIV life cycle, including hnRNP A2, HnRNP A/B, hnRNP A2B1, hnRNP U, hnRNP C, hnRNP I and hnRNP K.515,532–537,544–559,564
Potential therapeutic value by targeting stress proteins
Interest in targeting stress proteins in various diseases is increasing overtime. In this part, we will discuss the potential therapeutic value by targeting stress proteins.
First, targeting stress proteins have a wide spectrum of antiviral ability. For example, Hsp90 inhibitors, which are originally developed for anticancer, have been demonstrated to possess antiviral activity in cultured cells against poliovirus, rhinovirus, EV-A71,107,109 coxsackievirus,124 RSV,112,113 VSV, paramyxoviruses (HPIV2, HPIV3, SV5, SV41), influenza virus,126 CHIKV,114 HCV,115,124 and HSV,131,144,145,148,149 HBV,135,169 EBV,146,147,163,164 HCMV151,152 and HTLV.153,185 Depleting Hsp60 by siRNA functionally suppresses infections by influenza virus,38,39 DENV,319 HBV,325,329,331 HCV,321,322 Rotavirus,323 and HIV.338 Targeting hnRNP A1 is able to inhibit the reproduction of HIV,539–541,563 HPV,503–505 HCV,471 EV-A71472 and HTLV-1.562 Inhibition of hnRNP C could be a strategy to combat viral infections by HCV,438 poliovirus,447,448 DENV,479–482 EBV501 and HIV557,558 reproduction. Targeting HnRNP D blocks HCV and HIV replication.452–454,548,556 Dysfunction of hnRNP H effectively suppresses infections by EBV,499,500 HIV538,550–552 or RSV.485–487 Targeting PTB/hnRNP I is able to impair the reproduction of HCV,442,460 picornavirus,89,456,459 HPV507 and HIV557,558 Depriving of hnRNP K inhibits both DNA and RNA viruses’ reproduction, including influenza virus,449,450 EV-A71,455 DENV,476 VSV,479–482 HCMV,488 HBV,490 HSV,492,493 HPV,508 HIV514,536,537 and KSHV.560,561 hnRNP L and hnRNP M are important to HCV and enterovirus replication.461–463,475 Therefore, stress proteins are particularly attractive as antivirals targets for those lacking therapies and newly emerging viral diseases.
Second, under disease situations, the cellular need of stress proteins is usually stronger than that in normal conditions which enable specific selectivity of those drugs targeting stress proteins. For example, mutant p53 relies much more on Hsp90 function than wild type p53. Hsp90 inhibitor GM can easily disturb the association of mutant p53 with Hsp90, resulting in mutant p53 degradation while not affecting wild type p53.310 Additionally, stress proteins derived from stressed cells display higher affinity to clients and inhibitors. Tumor cell-derived Hsp90 exhibits a 100-fold higher binding affinity for 17-AAG than that from normal cells, since tumor cell-derived Hsp90 complexes with activating co-chaperones p23 and HOP exhibit increased ATPase activity and possess higher affinity to Hsp90 inhibitors. In contrast, Hsp90 in normal cells exists as an uncomplexed species with low ATPase activity and low affinity to Hsp90 inhibitors.294 Further study demonstrates that the difference is caused by a distinctive portion of Hsp90 forming complexes in cancer cells with oncogenic partners, such as Bcr-Abl-Hsp90 complex in mutant B-RAF-Hsp90 in SkMel28 melanoma cells, K562 chronic myeloid leukemia cells, Her3-Hsp90 and Raf1-Hsp90 complex in MDA-MB-468 breast cancer cells. Hsp90 inhibitor PU-H71 selectively binds to specific Hsp90-oncoprotein networks in these cancer cells.570 These characters of cancer cells enable stress proteins to exhibit stronger biological activity and to be discriminated by their inhibition under stress as compared with normal conditions.
Similarly, inhibitors of stress proteins show high prospect for antiviral. Hsp70 inhibitor JC40 inhibits pan-flavivirus (DENV2 and DENV4) replication in MDDCs, with negligible toxicity to host cells.640 These experiments highlight the feasibility of using HSP inhibitors therapeutically in humans.
Third, it is not observed drug resistance using HSP inhibitors for antiviral. For instance, Hsp90 inhibitors are refractory to develop drug resistance. This is clearly demonstrated in RSV infection.113 When RSV is repeatedly treated with Hsp90 inhibitors, no drug resistance was observed either in extensive passaging of the virus in cultured cells or in mice undergoing long-term treatment with Hsp90 inhibitors. Similar result is also observed in DENV infections.640 Treatment of DENV up to 10 passages with Hsp70 inhibitor JC40 does not cause any drug resistance. The lack of viral drug resistance to Hsp90 and Hsp70 inhibitors suggests that such an antiviral approach may be particularly useful for treating chronic viral infections in which drug resistance is most frequently observed.
The three features of stress proteins inhibitors make them extremely powerful antiviral agents, suggesting great application potential for treating the diseases caused by virus infections, such as COVID-19.
Challenges and perspectives: target-based drug development
Targeting Hsp90 for antivirus drug development
Hsp90 is thought to be the most abundant and evolutionarily conserved heat shock protein. A unique pocket in N-terminal region of Hsp90 is required for binding with ATP and co-chaperones.641 Hsp90 forms a flexible dimer by interaction of C-terminal domains. The formation and dissociation of compact dimers involving N- terminal domains are important for the molecular chaperone activity.642 The middle domain of Hsp90 tends to recruit and facilitate unfolded client proteins to assemble. The C-terminal domain of Hsp90 possesses a highly conserved peptide sequence for interacting with co-chaperones.643 Over 20 co-chaperones selectively interact with Hsp90 to regulate ATPase activity and recruit client proteins to assemble a big complex under certain conditions.642–645 It has been shown that more than 300 client proteins require Hsp90 and co-chaperones for folding and maturation.646 The mechanism of selectivity may rely on the direct interaction of co-chaperones with specific clients. Therefore, most Hsp90 inhibitors achieve their inhibitory effects by suppressing the ATPase activity or disrupting the interaction between Hsp90 and its co-chaperones.
Expression of Hsp90 and its client proteins are increased during viral infection and in most cancer cells. Hsp90 as an effective anti-cancer drug target has already grabbed attention; and a series of Hsp90 inhibitors as potential drugs have been intensively investigated in the laboratories, preclinical and clinical scenarios. The successful use of Hsp90 inhibitors in cancer therapy makes it much easier to apply them for treating virus infections.
As described above, we have comprehensively summarized the functions of Hsp90 and its client proteins in a diversity of virus reproduction processes. The potential of Hsp90 inhibitors has been well demonstrated to protect cultured cells against infections by EV-A71,107,109 poliovirus, rhinovirus, coxsackievirus,124 paramyxoviruses (HPIV2, HPIV3, SV5, SV41),VSV, RSV,112,113 influenza virus,126 CHIKV,114 HCV,115,124 HSV,131,144,145,148,149 HBV,135,169 EBV,146,147,163,164 HCMV,151,152 and HTLV.153,185 Notably, administration of Hsp90 inhibitors to infected animals exhibits little toxicity while potently suppresses the replication of poliovirus,107,109 EBV,163,164 HBV,135 CHIKV114 and HCV.647 These experiments highlight the feasibility of using these inhibitors therapeutically in clinic.
Here we would briefly enumerate the present Hsp90 inhibitors and their potential for antiviral therapies. Most Hsp90 inhibitors hamper Hsp90 function by competitively binding to the ATP binding site of HSP90, blocking the interaction with co-chaperones, or modulating acetylation. By doing so, we also try to address the possibility of repurposing Hsp90 inhibitors as candidate antiviral drugs (Table 2).
Table 2.
Hsp90 inhibitors
| Inhibition type | Subtype | Inhibitor and references |
|---|---|---|
| Targeting Hsp90 ATPase Activity | Ansamycins | Geldanamycin (GM)649 |
| Tanespimycin (17-AAG)650 | ||
| Alvespimycin (17-DMAG)651 | ||
| Retaspimycin hydrochloride (IPI-504)652 | ||
| Non-ansamycins | Luminespib (AUY922)653 | |
| Ganetespib (STA-9090) | ||
| BIIB021 | ||
| Onalespib (AT13387)656 | ||
| SNX-5422 (PF-04929113) | ||
| Blocking Hsp90 C-terminal ATPase activity | Novobiocin658,659 | |
| Deguelin660 | ||
| Epigallocatechin gallate (ECGC)662 | ||
| Disrupting Hsp90 and Its Co-chaperones | Targeting Hsp90-Cdc37 complex | Celastrol663–667 |
| Aferin A | ||
| Sulforaphane | ||
| Kongensin A | ||
| Platycodin D | ||
| Pep-1668 | ||
| Targeting Hsp90-Hop-Hsp70 complex | Six active compounds669 | |
| Blocking Deacetylation of Hsp90 | Vorinostat (SAHA)670 | |
| LAQ824671 | ||
| Romidepsin672 | ||
| Hsp90 cleavage | Enzymatic cleavage | Histone deacetylase inhibitors675 |
| Proteasome inhibitors676 | ||
| Non-enzymatic cleavage | Ascorbate/Menadione674 | |
| Oxidative stress (H2O2) |
Inhibitors targeting Hsp90 ATPase activity
Some Hsp90 inhibitors competitively bind to the ATP pocket in the N-terminus of Hsp90, leading to blocking of ATP hydrolysis and the closure of N-terminus of Hsp90 dimer. In addition, another ATP binding site has been found in the C-terminus of Hsp90.648 Recently, some natural products and their derivatives have been reported to competitively bind to the ATP pocket in the C-terminus of Hsp90. Generally, inhibitors of Hsp90 ATPase are classified into three types: (i) ansamycins, (ii) non-ansamycins and (iii) those block Hsp90 C-terminal ATPase activity. Ansamycins are antibiotics that possess the benzoquinone as structure core. These antibiotics include geldanamycin (GA), herbimycin A, and the macbecins. They inhibit Hsp90 activity and degrade its client proteins.649 GA competitively binds to the ATP pocket in the N-terminus and inhibits the ATPase activity.649 It has shown potent antiviral activity against coronavirus infection in the culture cells,145 indicating a good potential for treating COVID-19. Tanespimycin (17-allylamino-17-demethoxygeldanamycin, 17-AAG) is an analogue of GA.650,651 Alvespimycin (17-Dimethylaminoethylamino-17-demethoxygeldanamycin, 17-DMAG) is an analogue of 17-AAG, with high solubility in water. In addition, retaspimycin hydrochloride (IPI-504) is a more water-soluble derivative of 17-AAG.652 Non-ansamycin inhibitors of Hsp90 include Luminespib,653 BIIB021,654 Ganetespib,655 Onalespib,656 SNX-5422,657 etc.
Another type of Hsp90 inhibitors binds to the C-terminal ATP pocket rather than N-terminal ATP pocket. Novobiocin blocks the C-terminal ATPase activity.658,659 In in vitro and in vivo assays, treatment with novobiocin strongly reduces the expression of Hsp90-dependent client proteins, such as Raf-1 and p60v-src. A natural rotenoid, deguelin, is suggested to bind to the ATP pocket in the C-terminus without affecting the ATP pocket in the N-terminus.660 Treating with deguelin leads to reduced Hsp90 clients, such as Cdk4, Akt and MEK1/2.661 Like deguelin, epigallocatechin gallate (ECGC) is reported to bind to the ATP pocket in the C-terminus of Hsp90 without affecting the N-terminal ATP pocket.662 Compared with inhibition of the N-terminal ATP pocket of Hsp90, inhibitors targeting the C-terminal ATP pocket lead to a stability of Hsp70 and a negative instability of client proteins of Hsp90. However, the potential of these C-terminal inhibitors and their molecular mechanisms remain elusive. The structural features of the C-terminal domain of Hsp90 need to be further analysed. Such analysis may provide important hints on how to design effective Hsp90 inhibitors without HSR induction.
Inhibitors of Hsp90’s Co-chaperones
As the functions of the Hsp90 chaperone machinery are highly dependent on the associated co-chaperones, it is possible to selectively inhibit downstream signaling of Hsp90 by disrupting certain protein–protein interactions. Inhibitors targeting the interactions may present an alternative approach to prevent the toxicity induced by other Hsp90 inhibitors. As a result, great efforts are put into disrupting the interaction between Hsp90 and its co-chaperones and are rewarded recently in this area. Here we briefly present the advance in Hsp90-Cdc37 complex and Hsp90-Hop-Hsp70 ternary complex.
Human Cdc37 is a well-studied co-chaperone of Hsp90. The middle domain and C-terminus of Cdc37 are critical for the interaction with Hsp90. Currently, most of the reported agents for manipulating the Hsp90-Cdc37 interaction are natural products and their derivatives. These agents include celastrol, aferin A, sulforaphane, kongensin A and platycodin D. They are capable of dissociating the Hsp90-Cdc37 complex.663–667 In addition, a peptide (Pep-1, Ac-KHFGMLRRWDD-NH2) is developed and shows strong inhibitory effects on the formation of Hsp90-Cdc37 complex.668 Another well-known Hsp90/co-chaperone complex is Hsp90-Hop-Hsp70 ternary complex, which facilitates the transfer of unfolded client proteins from Hsp70 to Hsp90. Notably, six active compounds have been identified after screening the NCGC compound library.669 These compounds possess similar structural cores and have no effect on Hsp70 expression.
Studies on Hsp90-co-chaperone inhibitors are limited. Great effort should be paid on the efficacy and toxicity for further drug design and clinical studies.
Hsp90 inhibitors blocking deacetylation of Hsp90
The chaperone function of Hsp90 is also controlled by posttranslational modification (PTM). The well-studied PTMs in Hsp90 are acetylation and deacetylation in the M-domain of Hsp90. Vorinostat (suberoylanilide hydroxamic acid, SAHA) dissociates Her2/ErbB2 from Hsp90 via acetylation of Hsp90 that leads to degradation of Her2/ErbB2.670 LAQ824 induces Hsp90 acetylation that reduces Hsp90 client protein levels.671 Romidepsin is involved in the dissociation of mutant p53 and Raf-1 from Hsp90 via acetylation of Hsp90.672 In addition, the nuclear import of IAV polymerase needs the deacetylation of Hsp90 which is strictly regulated by histone deacetylases 6/8 (HDAC6/8).120 HDAC6/8 inhibitors efficiently limit polymerase nuclear import and suppress virus replication.120 Therefore, Hsp90 inhibitors that block Hsp90 deacetylation would potentially be used to treat influenza A virus infection. Their potential usage for combating other viruses (such as SARS-CoV-2) needs to be extensively explored.
Novel class of Hsp90 inhibitors for Hsp90 cleavage
Recently, Hsp90 cleavage has been observed under various stimuli such as UV irradiation,673 ascorbate/menadione,674 HDAC inhibitors,675 proteasome inhibitors676 etc. Therefore, Hsp90 cleavage is considered as another mechanism. Hsp90 cleavage induced by these inhibitors can be classified into two types: enzymatic cleavage and non-enzymatic cleavage.674,676 The enzymatic cleavage produces a 55 kDa fragment of Hsp90 via caspase 10 activation, while the non-enzymatic cleavage utilizes reactive oxygen species (ROS) to chemically degrade Hsp90 to an ~70 kDa fragment. Additionally, some substances have been reported to present Hsp90 cleavage activity, but the mechanism remains unclear.
Targeting Hsp70s for drug development
One kind of antiviral drugs are currently designed based on different properties of HSPs. Considering Hsp70 and Hsc70 are potential targets for HCV, they load the DCs with Hsp70 for a prolonged potent antigen- and tumor-specific T cell responses directed against multiple epitopes.677 Hsp70 inhibitors (such as quercetin, VER155008 and JC40) also show great potential for treating Flavivirus and Enterovirus infections.202,208,640 Inhibitors blocking the interaction between Grp78 and spike protein of coronavirus would be a useful approach for combating the infection by SARS-CoV, MERS-CoV, and SARS-CoV-2 infections.196,387,571 However, the efficacy and toxicity of these inhibitors are not yet well investigated in clinic studies.
Targeting Hsp60s for drug development
As described above, Hsp60s play critical roles in different diseases including autoimmune diseases, human cancers and virus infection-induced diseases. Studies show that silencing of Hsp60s by siRNA significantly decrease influenza virus,38,39 DENV319 and HBV325,331 reproduction by activating immunity response; and is capable of releasing HCV321,322, Rotavirus,323 HBV329 and HIV338 infection-induced pathogenesis. Therefore, small molecule regents targeting Hsp60s are potentially useful as therapeutics in these diseases.678,679 Currently, the known Hsp60 inhibitors are either from natural products or synthetic compounds (Table 3). Mechanistically, they can be grouped as two types. Type I inhibitors block ATP binding and hydrolysis. Type II inhibitors covalently interact with certain cysteine residues of Hsp60. However, the detailed binding sites have not been identified. The natural products of Hsp60 inhibitors include mizoribine, epolactaene, myrtucommulone A, Stephacidin B. Mizoribine is an imidazole nucleoside antibiotics isolated from Eupenicillium brefeldianum.680 It directly binds to Hsp60 and inhibits the chaperone activity of the Hsp60-Hsp10 complex.33 Epolactaene is isolated from the fungal strain Penicillium sp. BM 1689-P.681 Its derivate tert-butyl ester ETB has similar activity as epolactaene.682 They inhibit Hsp60-Hsp10’s chaperoning activity by covalent and selective bound to Hsp60 at residue of Cys442, close to the ATP binding pocket.683,684 Interestingly, ETB does not inhibit the ATPase activity of Hsp60,685 suggesting that the covalent interaction between ETB and Cys442 may allosterically modulate Hsp60-Hsp10’s chaperoning activity without interfering with its ATPase activity. Myrtucommulone A (MC) binds to Hsp60 and inhibits the refolding activity of the Hsp60-Hsp10 complex.686 MC is a non-prenylated acylphloroglucinol with multiple reported bioactivities, including antibacterial,687,688 antioxidant,689 anti-inflammatory,690,691 and anti-tumor properties.692,693 In addition to these natural products, several other natural products are also reported to interact with Hsp60 without direct proof. Stephacidin B is isolated from Aspergillus ochraceus WC76466 while Avrainvillamide is isolated from Aspergillus sp. CNC358.694,695 Interestingly, dimeric stephacidin B is converted into monomeric avrainvillamide in tissue culture media which implies avrainvillamide is the actual active species.696 Pull down assay only shows Hsp60 may be a putative target.696 Further validation studies have yet to be performed.
Table 3.
Hsp60 inhibitors
| Types | Inhibitors | Mechanism and references |
|---|---|---|
| Natural Products | Mizoribine | To inhibit chaperone activity of the Hsp60-Hsp10 complex; to block ATP binding and hydrolysis33 |
| Epolactaene | To inhibit chaperone activity of the Hsp60-Hsp10 complex683 | |
| Myrtucommulone A (MC) | To inhibit chaperone activity of the Hsp60-Hsp10 complex686 | |
| Stephacidin B | Undetermined694 | |
| Avrainvillamide | Undetermined695 | |
| Synthetic Compounds | O-carboranyl-phenoxyacetanilide | To inhibit chaperone activity of the Hsp60-Hsp10 complex; to block ATP binding and hydrolysis685 |
| Gold porphyrins | To inhibit chaperone activity of the Hsp60-Hsp10 complex |
Besides the natural products identified above as potential Hsp60 inhibitors, quite a few synthetic molecules have also been discovered to be able to modulate Hsp60 activity. O-carboranyl-phenoxyacetanilide is firstly identified as an HIF-1α inhibitor.697 Later it is found that O-carboranylphenoxyacetanilide selectively and directly binds to Hsp60 and inhibits Hsp60-Hsp10’s ATPase activity and refolding activity.685 Hsp60 can interact with HIF-1α, suggesting that inhibition of HIF-1α activity by carboranylphenoxyacetanilide is possibly through targeting Hsp60.685 The other class of synthetic compounds identified to inhibit Hsp60 is gold porphyrins.698 It directly binds to Hsp60 and inhibits the refolding activity of the Hsp60-Hsp10 complex.
Although several Hsp60 inhibitors potently suppress virus reproduction, a lot of work remains to be done to verify if they can serve as drugs for treating diseases caused by virus infections.
Targeting hnRNPs for antivirus drug development
Drugs targeting RNA chaperones may have a wide spectrum of antivirus capability. hnRNP A1 participates the reproduction of HIV,539–541,563 HPV,503–505 HCV,471 EV-A71472 and HTLV-1.562 Inhibition of hnRNP C suppresses the replication of HCV,438 poliovirus,447,448 DENV,479–482 EBV,501 and HIV.557,558 hnRNP D is required for HCV452–454 and HIV548,556 replication. HnRNP H is involved in multiplication of EBV,499,500 HIV,538,550–552 and RSV485–487 infections. PTB/hnRNP I depletion is able to impair the life cycle of HCV,442,460 picornavirus,89,456,459 HPV507 and HIV.557,558 Depriving of hnRNP K reduces the reproduction of influenza,449,450 EV-A71,455 DENV476 VSV,479–482 HCMV,488 HBV,490 HSV,492,493 HPV,508 HIV,514,536,537 and KSHV.560,561 HnRNP L and hnRNP M participate in HCV and enterovirus replication.461–463,475 Of note, the relationship between HIV and RNA chaperones is very strong. Most hnRNPs participates in HIV life cycle, including hnRNPA1,563 hnRNPA2,553–555 hnRNP A/B,544,545,547 hnRNP A2B1,557,558 hnRNP U,559 hnRNP C, hnRNP D,548,556 hnRNP H,538,550,551 hnRNP I557,558,564 and hnRNP K.514,536,537
However, the available regents developed targeting hnRNPs as antiviral drugs are limited. To date, only three agents targeting hnRNPs have been reported. Apigenin is a flavonoid abundant in fruits and vegetables. It targets hnRNP A2 and blocks hnRNP A2 dimerization thereafter affects the alternative splicing of key mRNAs.699 Apigenin efficiently inhibits the interaction of hnRNP A1 and A2 with EV-A71 RNA thereby inhibits EV-A71 RNA translation.472 Quercetin is also a flavonoid abundantly present in plants. It binds to the C-terminal region of hnRNP A1, impairing the ability of hnRNP A1 to shuttle between the nucleus and cytoplasm and ultimately resulting in its cytoplasmic retention.700 In addition, Quercetin down-regulates hnRNP A1 expression.701 The antiviral ability of Quercetin has been verified in some viruses such as inhibiting influenza entry,702 EV-A71,703 and HCV,208 JEV and DENV reproduction.704 However, in these researches, they explain the antivirus mechanism of Quercetin is by inhibiting Hsp70 expression208 or inhibiting virus proteases703 without mentioning the function of hnRNP A1. Another compound VPC-80051 targets RNA binding motif of hnRNP A1 and alters hnRNP A1 splicing activity.705 Its antiviral ability remains to be further investigated.
Targeting ER stress pathways for drug development
During the past few years, the enlarging role of ER stress-mediated pathways is becoming quite an attractive topic for broad-spectrum antiviral therapy, especially in the field of virus reproduction and pathogenesis, considerable progress has also been made for potential antiviral agents development.
Among pathways involved in ER stress, the three UPR pathways are the most thoroughly studied, thus antiviral targets related with these pathways are considered as the first class. Extensive investigation for antiviral development has been put int to the PERK-eIF2α pathway.
A small chemical compound named salubrinal, identified by Boyce and his colleagues, specifically inhibits the formation of PP1/GADD34 complex as well as suppresses HSV γ134.5-mediated eIF2α dephosphorylation. It could also significantly reduce HSV replication.706 Their research suggested that Salubrinal was able to attenuate PP1/GADD34-dependent eIF2α dephosphorylation, which would in turn inhibit DENV replication.707 A glucose analog 2-Deoxy-D-Glucose is responsible for the activation and phosphorylation of eIF2α, leading to suppression of herpesvirus (KSHV) replication. This agent is a potential candidate for anti-herpesvirus, and hopefully is tested in clinical therapies.708
Other than eIF2α, tremendous efforts have also been made in developing drugs to target IRE1-XBP1 pathway. 3,5-dibromosalicyladehyde is an endoribonuclease that specifically interacts with IRE1 and blocks the downstream IAV replication.600 WP1130 is another IRE1-XBP1 inhibitor with a broad-spectrum antiviral effects against multiple viruses, namely MNV-1, LCV and so on.709
ER stress-mediated apoptosis signaling is another noteworthy pathway for drug development and selection. Rana catesbeiana ribonuclease (RC-RNase) is reported to be efficient in suppression of JEV replication via activation of caspase-3, caspase-8 and caspase-9 pathways.710 Dubble-stranded RNA activated caspase oligomerizer (DRACO) is reported to have broad anti-viral effects in clinical therapy. DRACO selectively activates apoptosis in cells infected with dsDNA viruses but has no harmful effect on normal healthy cells. Over 15 different kind of viruses can be eradicated by DRACO, including HIV and Dengue Virus.711 Drugs targeting other signal transducers are also investigated, for example JNK, JAK, Bcl-2 and CHOP. A promising agent Vaticanol B can protect host cells from ER stress-induced apoptosis.712,713
Some researchers choose to focus on viral proteins that could trigger ER-stress, including non- envelope glycoproteins of SFV, precursor glycoprotein of LCMV, glycoproteins TULV, several non-structural protein of SARS-CoV, multiple non-structural proteins of flavivirus family, envelope protein of PEDV, E1, E2 and non-structral protein of HCV, surface protein of MHV, ICP0 glycoprotein B of HSV1, structural protein 4 of CHIKV. Viral proteins that are responsible for UPR activation and apoptosis induction are given special attentions such as HCV (E1, E2, core), HCMV (pUL37x1, pUL38) and SV5 V protein. Great advances have been made in this area, current progress includes, Clemizol for HCV NS4B, vaccine vectors for HSV-1 γ134, and Norakin for IAV hemagglutinin A.714,715
Due to their potential side effects on normal cell functions, the usage of inhibitors targeting stress proteins are very cautious in clinic settings; and the gone with wind infection manner of some virus (e.g., SARS-CoV) limits the opportunity of clinic studies, giving rise to additional challenges for developing stress protein-based drugs against the common and special infectious diseases such as severe acute respiratory symptom (SARS) caused by coronavirus.
Acknowledgements
We thank Prof. Robert Weiss at Cornell University College of Veterinary Medicine for critical reading and comments in preparation of this manuscript. The work was partially supported by grants from The Science Technology and Innovation Committee of Shenzhen Municipality [JCYJ20170818100531426, JCYJ20180507181627057]; grants from National Science Foundation of China [81671995]; the general research grant of Hong Kong [11100215]; and Strategic funds from City University of Hong Kong.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Qianya Wan, Dan Song
References
- 1.Daniel NH, Maurizio M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2006;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 2.Ritossa F. A. new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- 3.Hendrick JP HF. Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 4.Carra S, et al. The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones. 2017;22:601–611. doi: 10.1007/s12192-017-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Chen M, Zhou J, Zhang X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy. Int. J. Oncol. 2014;45:18–30. doi: 10.3892/ijo.2014.2399. [DOI] [PubMed] [Google Scholar]
- 6.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 7.Murshid A, Gong J, Calderwood S. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012;3:1–10. doi: 10.3389/fimmu.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 9.Zongshi L, et al. Heat shock protein 70 acts as a potential biomarker for early diagnosis of heart failure. PLoS ONE. 2013;8:e67964. doi: 10.1371/journal.pone.0067964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abaspour AR, et al. HSP27 expression in the human peripheral blood mononuclear cells as an early prognostic biomarker in coronary artery disease patients. Diabetes Metab. Syndr. 2019;13:1791–1795. doi: 10.1016/j.dsx.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoter A, El-Sabban ME, Naim HY. The HSP90 family: structure,regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018;19:1–33. doi: 10.3390/ijms19092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Naito T, Momose F, Kawaguchi A, Nagata K. Involvement of Hsp90 in assembly and nuclear import of influenza virus RNA polymerase subunits. J. Virol. 2007;81:1339–1349. doi: 10.1128/JVI.01917-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schopf FH, Biebl MM, Buchner J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017;18:345–360. doi: 10.1038/nrm.2017.20. [DOI] [PubMed] [Google Scholar]
- 16.Stetler RA, et al. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radons J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016;21:379–404. doi: 10.1007/s12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer MP. Gymnastics of molecular chaperones. Mol. Cell. 2010;39:321–331. doi: 10.1016/j.molcel.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SK, De Los Rios P, Christen P, Lustig A, Goloubinoff P. The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat. Chem. Biol. 2010;6:914–920. doi: 10.1038/nchembio.455. [DOI] [PubMed] [Google Scholar]
- 21.Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005;13:159–163. doi: 10.1016/j.tim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Khachatoorian R, French SW. Chaperones in hepatitis C virus infection. World J. Hepatol. 2016;8:9–35. doi: 10.4254/wjh.v8.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer MP. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 2013;38:507–514. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Kampinga HH, Craig EA. The Hsp70 chaperone machinery: J-proteins as drivers of functional specificity. Nat. Rev. Nol Cell Biol. 2010;11:579. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: physiology and mechanism. Annu. Rev. Cell. Dev. Biol. 2007;23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555. [DOI] [PubMed] [Google Scholar]
- 26.Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 27.Spiess C, Meyer AS, Reissmann S, Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 2004;14:598–604. doi: 10.1016/j.tcb.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutsche I, Essen L-O, Baumeister W. Group II chaperonins: new TRiC(k)s and turns of a protein folding machine. J. Mol. Biol. 1999;293:295–312. doi: 10.1006/jmbi.1999.3008. [DOI] [PubMed] [Google Scholar]
- 29.Ishi N, Taguchi H, Sumi M, Yoshida M. Structure of holo-chaperonin studied with electron microscopy Oligomeric opal0 on top of two layers of cpn60 rings with two stripes each. FEBS Lett. 1992;299:169–174. doi: 10.1016/0014-5793(92)80240-h. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida M., I. N., Muneyuki E., Taguchi H. A chaperonin from a thermophilic bacterium, thermus thermophilus. in Molecular Chaperones.(Springer, 1993). [DOI] [PubMed]
- 31.Grallert H, Buchner J. Review: a structural view of the GroE chaperone cycle. J. Struct. Biol. 2001;135:95–103. doi: 10.1006/jsbi.2001.4387. [DOI] [PubMed] [Google Scholar]
- 32.Ishii N. GroEL and the GroEL-GroES Complex. Macromol. Protein Complexes. 2017;83:483–504. doi: 10.1007/978-3-319-46503-6_17. [DOI] [PubMed] [Google Scholar]
- 33.Itoh H, Komatsuda A, Wakui H, Miura AB, Tashima Y. Mammalian HSP60 is a major target for an immunosuppressant mizoribine. J. Biol. Chem. 1999;274:35147–35151. doi: 10.1074/jbc.274.49.35147. [DOI] [PubMed] [Google Scholar]
- 34.Itoh H, et al. Mammalian HSP60 is quickly sorted into the mitochondria under conditions of dehydration. Eur. J. Biochem. 2002;269:5931–5938. doi: 10.1046/j.1432-1033.2002.03317.x. [DOI] [PubMed] [Google Scholar]
- 35.Itoh H, et al. Mammalian 60-kDa stress protein (Chaperonin Homolog) J. Biol. Chem. 1995;270:13429–13435. doi: 10.1074/jbc.270.22.13429. [DOI] [PubMed] [Google Scholar]
- 36.Ishida R, et al. Physicochemical properties of the mammalian molecular chaperone HSP60. Int. J. Mol. Sci. 2018;19:489. doi: 10.3390/ijms19020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang H, et al. Down-regulation of HSP60 suppresses the proliferation of glioblastoma cells via the ROS/AMPK/mTOR pathway. Sci. Rep. 2016;6:28388. doi: 10.1038/srep28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graef KM, et al. The PB2 subunit of the influenza virus RNA polymerase affects virulence by interacting with the mitochondrial antiviral signaling protein and inhibiting expression of beta interferon. J. Virol. 2010;84:8433–8445. doi: 10.1128/JVI.00879-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fislová T, Thomas B, Graef KM, Fodor E. Association of the influenza virus RNA polymerase subunit PB2 with the host chaperonin CCT. J. Virol. 2010;84:8691–8699. doi: 10.1128/JVI.00813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habich C, Burkart V. Heat shock protein 60: regulatory role on innate immune cells. Cell. Mol. Life Sci. 2007;64:742–751. doi: 10.1007/s00018-007-6413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrido C, Paul C, Seigneuric R, Kampinga H. The small heat shock proteins family: the long forgotten chaperones. Int J. Mol. Cell Biol. 2012;44:1588–1592. doi: 10.1016/j.biocel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Montfort van RL, Basha E, Friedrich KL, Friedrich C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct.Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- 43.Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 2015;427:1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh MK, Sharma B, Tiwari PK. The small heat shock protein Hsp27: Present understanding and future prospects. J. Therm. Biol. 2017;69:149–154. doi: 10.1016/j.jtherbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 45.MacRae TH. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell. Mol. Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shashidharamurthy R, Koteiche HA, Dong J, Mchaourab HS. Mechanism of chaperone function in small heat shock proteins. J. Biol. Chem. 2005;280:5281–5289. doi: 10.1074/jbc.M407236200. [DOI] [PubMed] [Google Scholar]
- 47.Hsu A-L. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 48.Bruey J-M, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 49.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 50.Benesch JL, Ayoub M, Robinson CV, Aquilina JA. Small heat shock protein activity is regulated by variable oligomeric substructure. J. Biol. Chem. 2008;283:28513–28517. doi: 10.1074/jbc.M804729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parcellier A, et al. Small heat shock proteins HSP27 and αB-crystallin: cytoprotective and oncogenic functions. Antioxid. redox Signal. 2005;7:404–413. doi: 10.1089/ars.2005.7.404. [DOI] [PubMed] [Google Scholar]
- 52.Garrido C, et al. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 53.Guay J, et al. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J. Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 54.Sugiyama Y, et al. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J. Biol. Chem. 2000;275:1095–1104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- 55.Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell. Mol. Life Sci. 2009;66:3289–3307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferns G, Shams S, Shafi S. Heat shock protein 27: its potential role in vascular disease. Int. J. Exp. Pathol. 2006;87:253–274. doi: 10.1111/j.1365-2613.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dan X, et al. Hsp27 responds to and facilitates enterovirus A71 replication by enhancing viral internal ribosome entry site-mediated translation. J. Virol. 2019;93:e02322–02318. doi: 10.1128/JVI.02322-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB. Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20) Biochim. Biophys. Acta. 2009;1794:486–495. doi: 10.1016/j.bbapap.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol. Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 60.Shin KD, et al. Blocking tumor cell migration and invasion with Biphenyl Isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J. Bio Chem. 2005;280:41439–41448. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]
- 61.Gobbo J, Gaucher-Di-Stasio C, Weidmann S, Guzzo J, Garrido C. Quantification of HSP27 and HSP70 molecular chaperone activities. Methods Mol. Biol. 2011;787:137–143. doi: 10.1007/978-1-61779-295-3_11. [DOI] [PubMed] [Google Scholar]
- 62.Rajaiya J, Xiao J, Rajala RV, Chodosh J. Human adenovirus type 19 infection of corneal cells induces p38 MAPK-dependent interleukin-8 expression. Virol. J. 2008;5:17. doi: 10.1186/1743-422X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh D, McCann KL, Imani F. MAPK and heat shock protein 27 activation are associated with respiratory syncytial virus induction of human bronchial epithelial monolayer disruption. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L436–L445. doi: 10.1152/ajplung.00097.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajaiya J, Yousuf MA, Singh G, Stanish H, Chodosh J. Heat shock protein 27 mediated signaling in viral infection. Biochemistry. 2012;51:5695–5702. doi: 10.1021/bi3007127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schafer C, Clapp P, Welsh MJ, Benndorf R, Williams JA. HSP27 expression regulates CCK-induced changes of the actin cytoskeleton in CHO-CCK-A cells. Am. J. Physiol. 1999;277:C1032–C1043. doi: 10.1152/ajpcell.1999.277.6.C1032. [DOI] [PubMed] [Google Scholar]
- 66.Rocchi P, et al. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005;65:11083–11093. doi: 10.1158/0008-5472.CAN-05-1840. [DOI] [PubMed] [Google Scholar]
- 67.Freshney NW, et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 68.Alford KA, et al. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta-activated kinase-1 (TAK1)-mediated signaling. J. Biol. Chem. 2007;282:6232–6241. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 69.Sur R, Lyte PA, Southall MD. Hsp27 regulates pro-inflammatory mediator release in keratinocytes by modulating NF-κB signaling. J. Invest. Dermatol. 2008;128:1116–1122. doi: 10.1038/sj.jid.5701157. [DOI] [PubMed] [Google Scholar]
- 70.Kindås-Mügge, Trautinger F. Increased expression of the M (r) 27,000 heat shock protein (hsp27) in in vitro differentiated normal human keratinocytes. Cell Growth Differ. 1994;5:777–781. [PubMed] [Google Scholar]
- 71.Spector NL, et al. 28-kDa mammalian heat shock protein, a novel substrate of a growth regulatory protease involved in differentiation of human leukemia cells. J. Biol. Chem. 1995;270:1003–1006. doi: 10.1074/jbc.270.3.1003. [DOI] [PubMed] [Google Scholar]
- 72.Anckar J, Sistonen L. Regulation of HSF 1 function in the heat stress response: implications in aging and disease. Annu. Rev. Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 73.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 74.Sakurai H, Enoki Y. Novel aspects of heat shock factors: DNA recognition, chromatin modulation and gene expression. FEBS J. 2010;277:4140–4149. doi: 10.1111/j.1742-4658.2010.07829.x. [DOI] [PubMed] [Google Scholar]
- 75.Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J. Cell. Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 76.Benham AM. The protein disulfide isomerase family: key players in health and disease. Antioxid. Redox Signal. 2012;16:781–789. doi: 10.1089/ars.2011.4439. [DOI] [PubMed] [Google Scholar]
- 77.Oliver JD, Roderick HL, Llewellyn DH, High S. ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol. Biol. Cell. 1999;10:2573–2582. doi: 10.1091/mbc.10.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 2009;11:2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 79.Bedard K, Szabo E, Michalak M, Opas M. Cellular functions of endoplasmic reticulum chaperones calreticulin, calnexin, and ERp57. Int. Rev. Cytol. 2005;245:91–121. doi: 10.1016/S0074-7696(05)45004-4. [DOI] [PubMed] [Google Scholar]
- 80.Waldsich C, Grossberger R, Schroeder R. RNA chaperone StpA loosens interactions of the tertiary structure in the td group I intron in vivo. Genes Dev. 2002;16:2300–2312. doi: 10.1101/gad.231302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herschlag D. RNA chaperones and the RNA folding problem. J. Biol. Chem. 1995;270:20871–20874. doi: 10.1074/jbc.270.36.20871. [DOI] [PubMed] [Google Scholar]
- 82.Russell R, Herschlag D. Probing the folding landscape of the Tetrahymena ribozyme: commitment to form the native conformation is late in the folding pathway. J. Mol. Biol. 2001;308:839–851. doi: 10.1006/jmbi.2001.4751. [DOI] [PubMed] [Google Scholar]
- 83.Treiber DK, Williamson JR. Exposing the kinetic traps in RNA folding. Curr. Opin. Struct. Biol. 1999;9:339–345. doi: 10.1016/S0959-440X(99)80045-1. [DOI] [PubMed] [Google Scholar]
- 84.Schroeder R, Barta A, Semrad K. Strategies for RNA folding and assembly. Nat. Rev. Mol. Cell Biol. 2004;5:908–919. doi: 10.1038/nrm1497. [DOI] [PubMed] [Google Scholar]
- 85.Tsuchihashi Z, Khosla M, Herschlag D. Protein enhancement of hammerhead ribozyme catalysis. Science. 1993;262:99–102. doi: 10.1126/science.7692597. [DOI] [PubMed] [Google Scholar]
- 86.Herschlag D, Khosla M, Tsuchihashi Z, Karpel R, An RNA. chaperone activity of non‐specific RNA binding proteins in hammerhead ribozyme catalysis. EMBO J. 1994;13:2913–2924. doi: 10.1002/j.1460-2075.1994.tb06586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bomsztyk K, Denisenko O, Ostrowski J, hnRNP K. hnRNP K: one protein multiple processes. Bioessays. 2004;26:629–638. doi: 10.1002/bies.20048. [DOI] [PubMed] [Google Scholar]
- 88.Marfatia KA, Crafton EB, Green DM, Corbett AH. Domain analysis of the Saccharomyces cerevisiae heterogeneous nuclear ribonucleoprotein, Nab2p. Dissecting the requirements for Nab2p-facilitated poly(A) RNA export. J. Biol. Chem. 2003;278:6731–6740. doi: 10.1074/jbc.M207571200. [DOI] [PubMed] [Google Scholar]
- 89.Zuniga S, Sola I, Cruz JL, Enjuanes L. Role of RNA chaperones in virus replication. Virus Res. 2009;139:253–266. doi: 10.1016/j.virusres.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daniel RC, Stuart KC. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–113. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol. Med. 2004;10:47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Calderwood SK, Gong J. Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem. Sci. 2016;41:311–323. doi: 10.1016/j.tibs.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calderwood, S. K. Heat shock proteins and cancer: intracellular chaperones or extracellular signalling ligands? Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 10.1098/rstb.2016.0524 (2018). [DOI] [PMC free article] [PubMed]
- 94.Nussbacher JK, Tabet R, Yeo GW, Lagier-Tourenne C. Disruption of RNA metabolism in neurological diseases and emerging therapeutic interventions. Neuron. 2019;102:294–320. doi: 10.1016/j.neuron.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kampinga HH, Bergink S. Heat shock proteins as potential targets for protective strategies in neurodegeneration. Lancet Neurol. 2016;15:748–759. doi: 10.1016/S1474-4422(16)00099-5. [DOI] [PubMed] [Google Scholar]
- 96.Walter JM, Wunderink RG. Severe respiratory viral infections: new evidence and changing paradigms. Infect. Dis. Clin. North Am. 2017;31:455–474. doi: 10.1016/j.idc.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manjarrez-Zavala, M. E., Rosete-Olvera, D. P., Gutiérrez-González, L. H., Ocadiz-Delgad, R. & Cabello-Gutiérrez, C. Pathogenesis of Viral Respiratory Infection. Respiratory Disease and Infection—A New Insight, IntechOpen, London (2013)
- 98.Munnink BBO, Hoek Lvd. Viruses causing gastroenteritis: the known, the new and those beyond. Viruses. 2016;8:42. doi: 10.3390/v8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siebrasse EA, et al. Identification of MW polyomavirus, a novel polyomavirus in human stool. J. Virol. 2012;86:10321. doi: 10.1128/JVI.01210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J. Clin. Microbiol. 2007;45:2144. doi: 10.1128/JCM.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kapoor A, et al. A highly prevalent and genetically diversified Picornaviridae genus in South Asian children. Proc. Natl Acad. Sci. USA. 2008;105:20482–20487. doi: 10.1073/pnas.0807979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Messaoudi I, Basler CF. Immunological features underlying viral hemorrhagic fevers. Curr. Opin. Immunol. 2015;36:38. doi: 10.1016/j.coi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paessler S, Walker DH. Pathogenesis of the viral hemorrhagic fevers. Annu Rev. Pathol. 2013;8:411–440. doi: 10.1146/annurev-pathol-020712-164041. [DOI] [PubMed] [Google Scholar]
- 104.Karim S, et al. The role of viruses in neurodegenerative and neurobehavioral diseases. CNS Neurol. Disord. Drug Targets. 2014;13:1213–1223. doi: 10.2174/187152731307141015122638. [DOI] [PubMed] [Google Scholar]
- 105.Balin BJ, Hudson AP. Herpes viruses and Alzheimer’s disease: new evidence in the debate. Lancet Neurol. 2018;17:839–841. doi: 10.1016/S1474-4422(18)30316-8. [DOI] [PubMed] [Google Scholar]
- 106.Schiller JT, Lowy DR. Virus infection and human cancer: an overview. Recent Results Cancer Res. 2014;193:1–10. doi: 10.1007/978-3-642-38965-8_1. [DOI] [PubMed] [Google Scholar]
- 107.Tsou Y-L, et al. Heat shock protein 90: role in enterovirus 71 entry and assembly and potential target for therapy. PLoS ONE. 2013;8:e77133. doi: 10.1371/journal.pone.0077133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cabrera-Hernandez A, Thepparit C, Suksanpaisan L, Smith DR. Dengue virus entry into liver (HepG2) cells is independent of hsp90 and hsp70. J. Med. Virol. 2007;79:386–392. doi: 10.1002/jmv.20786. [DOI] [PubMed] [Google Scholar]
- 109.Wang RYL, et al. Heat shock protein-90-beta facilitates enterovirus 71 viral particles assembly. Virology. 2013;443:236–247. doi: 10.1016/j.virol.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Reyes-Del Valle J, Chávez-Salinas S, Medina F, Del Angel RM. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J. Virol. 2005;79:4557–4567. doi: 10.1128/JVI.79.8.4557-4567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thongtan T, et al. Characterization of putative Japanese encephalitis virus receptor molecules on microglial cells. J. Med. Virol. 2012;84:615–623. doi: 10.1002/jmv.23248. [DOI] [PubMed] [Google Scholar]
- 112.Connor JH, McKenzie MO, Parks GD, Lyles DS. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology. 2007;362:109–119. doi: 10.1016/j.virol.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Geller R, Andino R, Frydman J. Hsp90 inhibitors exhibit resistance-free antiviral activity against respiratory syncytial virus. PLoS ONE. 2013;8:e56762. doi: 10.1371/journal.pone.0056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rathore APS, et al. Chikungunya virus nsP3 & nsP4 interacts with HSP-90 to promote virus replication: HSP-90 inhibitors reduce CHIKV infection and inflammation in vivo. Antivir. Res. 2014;103:7–16. doi: 10.1016/j.antiviral.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 115.Ujino S, Yamaguchi S, Shimotohno K, Takaku H. Heat-shock protein 90 is essential for stabilization of the hepatitis C virus nonstructural protein NS3. J. Biol. Chem. 2009;284:6841–6846. doi: 10.1074/jbc.M806452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim M-G, Moon J-S, Kim E-J, Lee S-H, Oh J-W. Destabilization of PDK1 by Hsp90 inactivation suppresses hepatitis C virus replication through inhibition of PRK2-mediated viral RNA polymerase phosphorylation. Biochem. Biophys. Res. Commun. 2012;421:112–118. doi: 10.1016/j.bbrc.2012.03.126. [DOI] [PubMed] [Google Scholar]
- 117.Okamoto T, et al. Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90. EMBO J. 2006;25:5015–5025. doi: 10.1038/sj.emboj.7601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Momose F, et al. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J. Bio Chem. 2002;277:45306–45314. doi: 10.1074/jbc.M206822200. [DOI] [PubMed] [Google Scholar]
- 119.Chase G, et al. Hsp90 inhibitors reduce influenza virus replication in cell culture. Virology. 2008;377:431–439. doi: 10.1016/j.virol.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 120.Panella S, et al. MC1568 inhibits HDAC6/8 activity and influenza A virus replication in lung epithelial cells: role of Hsp90 acetylation. Future Med. Chem. 2016;8:2017–2031. doi: 10.4155/fmc-2016-0073. [DOI] [PubMed] [Google Scholar]
- 121.Liu G, et al. Autophagy is involved in regulating influenza A virus RNA and protein synthesis associated with both modulation of Hsp90 induction and mTOR/p70S6K signaling pathway. Int J. Biochem. Cell Biol. 2016;72:100–108. doi: 10.1016/j.biocel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 122.Zhou F, et al. Pim1 impacts enterovirus A71 replication and represents a potential target in antiviral therapy. iScience. 2019;19:715. doi: 10.1016/j.isci.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Waxman L, Whitney M, Pollok BA, Kuo LC, Darke PL. Host cell factor requirement for hepatitis C virus enzyme maturation. Proc. Natl Acad. Sci. USA. 2001;98:13931–13935. doi: 10.1073/pnas.241510898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Geller R, Vignuzzi M, Andino R, Frydman J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 2007;21:195–205. doi: 10.1101/gad.1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vashist S, et al. Molecular chaperone Hsp90 is a therapeutic target for noroviruses. J. Virol. 2015;89:6352–6363. doi: 10.1128/JVI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kumar P, Gaur P, Kumari R, Lal SK. Influenza A virus neuraminidase protein interacts with Hsp90, to stabilize itself and enhance cell survival. J. Cell. Biochem. 2019;120:6449–6458. doi: 10.1002/jcb.27935. [DOI] [PubMed] [Google Scholar]
- 127.Ploubidou A, Way M. Viral transport and the cytoskeleton. Curr. Opin. Cell Biol. 2001;13:97–105. doi: 10.1016/S0955-0674(00)00180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kannan H, Fan S, Patel D, Bossis I, Zhang Y-J. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009;83:6375–6382. doi: 10.1128/JVI.02571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perdiz D, Mackeh R, Poüs C, Baillet A. The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell. Signal. 2011;23:763–771. doi: 10.1016/j.cellsig.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 130.Xuan C, et al. Regulation of microtubule assembly and stability by the transactivator of transcription protein of Jembrana disease virus. J. Biol. Chem. 2007;282:28800–28806. doi: 10.1074/jbc.M702823200. [DOI] [PubMed] [Google Scholar]
- 131.Zhong M, et al. Heat-shock protein 90 promotes nuclear transport of herpes simplex virus 1 capsid protein by interacting with acetylated tubulin. PLoS ONE. 2014;9:e99425. doi: 10.1371/journal.pone.0099425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chou CK, Wang LH, Lin HM, Chi CW. Glucocorticoid stimulates hepatitis B viral gene expression in cultured human hepatoma cells. Hepatol. 1992;16:13–18. doi: 10.1002/hep.1840160104. [DOI] [PubMed] [Google Scholar]
- 133.Czar MJ, Galigniana MD, Silverstein AM, Pratt WB. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x. [DOI] [PubMed] [Google Scholar]
- 134.Beck J, Nassal M. Hepatitis B virus replication. World J. Gastroenterol. 2007;13:48–64. doi: 10.3748/wjg.v13.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu J, Seeger C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc. Natl Acad. Sci. USA. 1996;93:1060–1064. doi: 10.1073/pnas.93.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu J, Flores D, Toft D, Wang X, Nguyen D. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J. Virol. 2004;78:13122–13131. doi: 10.1128/JVI.78.23.13122-13131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu J, Anselmo D. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: posttranslational activation by Hsp90. J. Virol. 2000;74:11447–11455. doi: 10.1128/jvi.74.24.11447-11455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cho G, Suh SW, Jung G. HBV polymerase interacts independently with N-terminal and C-terminal fragments of Hsp90beta. Biochem. Biophys. Res. Commun. 2000;274:203–211. doi: 10.1006/bbrc.2000.3119. [DOI] [PubMed] [Google Scholar]
- 139.Stahl M, Retzlaff M, Nassal M, Beck J. Chaperone activation of the hepadnaviral reverse transcriptase for template RNA binding is established by the Hsp70 and stimulated by the Hsp90 system. Nucleic Acids Res. 2007;35:6124–6136. doi: 10.1093/nar/gkm628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beck J, Nassal M. Efficient Hsp90-independent in vitro activation by Hsc70 and Hsp40 of duck hepatitis B virus reverse transcriptase, an assumed Hsp90 client protein. J. Biol. Chem. 2003;278:36128–36138. doi: 10.1074/jbc.M301069200. [DOI] [PubMed] [Google Scholar]
- 141.Hu J, Toft DO, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gyoo Park S, Kyung Rho J, Jung G. Hsp90 makes the human HBV Pol competent for in vitro priming rather than maintaining the human HBV Pol/pregenomic RNA complex. Arch. Biochem. Biophys. 2002;401:99–107. doi: 10.1016/S0003-9861(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 143.Kim SS, Shin HJ, Cho YH, Rho HM. Expression of stable hepatitis B viral polymerase associated with GRP94 in E. coli. Arch. Virol. 2000;145:1305–1320. doi: 10.1007/s007050070092. [DOI] [PubMed] [Google Scholar]
- 144.Burch AD, Weller SK. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J. Virol. 2005;79:10740–10749. doi: 10.1128/JVI.79.16.10740-10749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y-H, Tao P-Z, Liu Y-Z, Jiang J-D. Geldanamycin, a ligand of heat shock protein 90, inhibits the replication of herpes simplex virus type 1 in vitro. Antimicrob. Agents Chemother. 2004;48:867–872. doi: 10.1128/AAC.48.3.867-872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun X, Kenney SC. Hsp90 inhibitors: a potential treatment for latent EBV infection? Cell cycle. 2010;9:1665–1666. doi: 10.4161/cc.9.9.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawashima D, et al. Nuclear transport of Epstein-Barr virus DNA polymerase is dependent on the BMRF1 polymerase processivity factor and molecular chaperone Hsp90. J. Virol. 2013;87:6482–6491. doi: 10.1128/JVI.03428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wysocka J, Herr W. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 2003;28:294–304. doi: 10.1016/S0968-0004(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 149.Amici C, et al. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J. Biol. Chem. 2006;281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, et al. Heat-shock protein 90α is involved in maintaining the stability of VP16 and VP16-mediated transactivation of α genes from herpes simplex virus-1. Expert Rev. Mol. Med. 2018;24:65. doi: 10.1186/s10020-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Basha W, et al. Geldanamycin, a potent and specific inhibitor of Hsp90, inhibits gene expression and replication of human cytomegalovirus. Antivir. Chem. Chemother. 2005;16:135–146. doi: 10.1177/095632020501600206. [DOI] [PubMed] [Google Scholar]
- 152.Lv Y, et al. Curcumin inhibits human cytomegalovirus by downregulating heat shock protein 90. Mol. Med. Report. 2015;12:4789–4793. doi: 10.3892/mmr.2015.3983. [DOI] [PubMed] [Google Scholar]
- 153.Ikebe E, et al. A novel HSP90 inhibitor, 17-DMAG, induces Tax down-regulation and its oral administration to ATL-model mice intervenes against the infiltration property of the ATL-like lymphocytes and provides extended survival period. Retrovirology. 2014;11:P44. [Google Scholar]
- 154.Purves FC, Ogle WO, Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl Acad. Sci. USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Long MC, Leong V, Schaffer PA, Spencer CA, Rice SA. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J. Virol. 1999;73:5593–5604. doi: 10.1128/jvi.73.7.5593-5604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gershburg E, Raffa S, Torrisi MR, Pagano JS. Epstein-Barr virus-encoded protein kinase (BGLF4) is involved in production of infectious virus. J. Virol. 2007;81:5407–5412. doi: 10.1128/JVI.02398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krosky PM, et al. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 2003;77:7720–7727. doi: 10.1128/JVI.77.14.7720-7727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl Acad. Sci. USA. 2001;98:1895–1900. doi: 10.1073/pnas.98.4.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krosky PM, Baek M-C, Coen DM. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 2003;77:905–914. doi: 10.1128/JVI.77.2.905-914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hamirally S, et al. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Path. 2009;5:e1000275. doi: 10.1371/journal.ppat.1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li R, et al. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe. 2011;10:390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li R, et al. SUMO binding by the Epstein-Barr virus protein kinase BGLF4 is crucial for BGLF4 function. J. Virol. 2012;86:5412–5421. doi: 10.1128/JVI.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun X, et al. Hsp90 inhibitors block outgrowth of EBV-infected malignant cells in vitro and in vivo through an EBNA1-dependent mechanism. Proc. Natl Acad. Sci. USA. 2010;107:3146–3151. doi: 10.1073/pnas.0910717107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shatzer A, et al. Ganetespib, an HSP90 inhibitor, kills Epstein-Barr virus (EBV)-infected B and T cells and reduces the percentage of EBV-infected cells in the blood. Leuk. Lymphoma. 2017;58:923–931. doi: 10.1080/10428194.2016.1213823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kapoor P, Lavoie BD, Frappier L. EBP2 plays a key role in Epstein-Barr virus mitotic segregation and is regulated by aurora family kinases. Mol. Cell. Biol. 2005;25:4934–4945. doi: 10.1128/MCB.25.12.4934-4945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt’s lymphomas. Proc. Natl Acad. Sci. USA. 2003;100:14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saridakis V, et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol. Cell. 2005;18:25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 168.Yin Y, Manoury B, Fåhraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 2003;301:1371–1374. doi: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 169.Shim HY, Quan X, Yi Y-S, Jung G. Heat shock protein 90 facilitates formation of the HBV capsid via interacting with the HBV core protein dimers. Virology. 2011;410:161–169. doi: 10.1016/j.virol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 170.Kim YS, Seo HW, Jung G. Reactive oxygen species promote heat shock protein 90-mediated HBV capsid assembly. Biochem. Biophys. Res. Commun. 2015;457:328–333. doi: 10.1016/j.bbrc.2014.12.110. [DOI] [PubMed] [Google Scholar]
- 171.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from burkitt’s lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 172.Raab-Traub N. Epstein–Barr virus in the pathogenesis of NPC. Semin. Cancer Biol. 2002;12:431–441. doi: 10.1016/s1044579x0200086x. [DOI] [PubMed] [Google Scholar]
- 173.Moorthy RK, Thorley-Lawson DA. All three domains of the Epstein-Barr virus-encoded latent membrane protein LMP-1 are required for transformation of rat-1 fibroblasts. J. Virol. 1993;67:1638–1646. doi: 10.1128/jvi.67.3.1638-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wakisaka N, Pagano JS. Epstein-Barr virus induces invasion and metastasis factors. Anticancer Res. 2003;23:2133–2138. [PubMed] [Google Scholar]
- 175.Chan KC, et al. A novel Hsp90 inhibitor AT13387 induces senescence in EBV-positive nasopharyngeal carcinoma cells and suppresses tumor formation. Mol. Cancer. 2013;12:128. doi: 10.1186/1476-4598-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Suzuki M, et al. The heat shock protein 90 inhibitor BIIB021 suppresses the growth of T and natural killer cell lymphomas. Front. Microbiol. 2015;6:280. doi: 10.3389/fmicb.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kotsiopriftis M, Tanner JE, Alfieri C. Heat shock protein 90 expression in Epstein-Barr virus-infected B cells promotes gammadelta T-cell proliferation in vitro. J. Virol. 2005;79:7255–7261. doi: 10.1128/JVI.79.11.7255-7261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sciammas R, Bluestone JA. TCRgammadelta cells and viruses. Microb. Infect. 1999;1:203–212. doi: 10.1016/s1286-4579(99)80035-5. [DOI] [PubMed] [Google Scholar]
- 179.Imai T, et al. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc. Natl Acad. Sci. USA. 2011;108:16363–16368. doi: 10.1073/pnas.1108372108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kurotaki T, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J. Immunol. 2007;179:1803–1813. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 181.Ye Z, Ting JP-Y. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr. Opin. Immunol. 2008;20:3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 182.Shirasu K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 2009;60:139–164. doi: 10.1146/annurev.arplant.59.032607.092906. [DOI] [PubMed] [Google Scholar]
- 183.Grassmann R, Aboud M, Jeang K-T. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 184.Sun S-C, Yamaoka S. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene. 2005;24:5952–5964. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- 185.Gao L, Harhaj EW. HSP90 protects the human T-cell leukemia virus type 1 (HTLV-1) tax oncoprotein from proteasomal degradation to support NF-κB activation and HTLV-1 replication. J. Virol. 2013;87:13640–13654. doi: 10.1128/JVI.02006-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Triantafilou K, Fradelizi D, Wilson K, Triantafilou M. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J. Virol. 2002;76:633–643. doi: 10.1128/JVI.76.2.633-643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Williams CH, et al. Integrin alpha v beta 6 is an RGD-dependent receptor for coxsackievirus A9. J. Virol. 2004;78:6967–6973. doi: 10.1128/JVI.78.13.6967-6973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu T, et al. Heat shock protein 70 as a supplementary receptor facilitates enterovirus 71 infections in vitro. Microb. Pathog. 2019;128:106–111. doi: 10.1016/j.micpath.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 189.Vega-Almeida TO, Salas-Benito M, De Nova-Ocampo MA, Del Angel RM, Salas-Benito JS. Surface proteins of C6/36 cells involved in dengue virus 4 binding and entry. Arch. Virol. 2013;158:1189–1207. doi: 10.1007/s00705-012-1596-0. [DOI] [PubMed] [Google Scholar]
- 190.Taguwa S, et al. Zika virus dependence on host hsp70 provides a protective strategy against infection and disease. Cell Rep. 2019;26:906–920.e903. doi: 10.1016/j.celrep.2018.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Das S, Laxminarayana SV, Chandra N, Ravi V, Desai A. Heat shock protein 70 on Neuro2a cells is a putative receptor for Japanese encephalitis virus. Virology. 2009;385:47–57. doi: 10.1016/j.virol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 192.Zhu Y-Z, et al. Association of heat-shock protein 70 with lipid rafts is required for Japanese encephalitis virus infection in Huh7 cells. J. Gen. Virol. 2012;93:61–71. doi: 10.1099/vir.0.034637-0. [DOI] [PubMed] [Google Scholar]
- 193.Chuang C-K, Yang T-H, Chen T-H, Yang C-F, Chen W-J. Heat shock cognate protein 70 isoform D is required for clathrin-dependent endocytosis of Japanese encephalitis virus in C6/36 cells. J. Gen. Virol. 2015;96:793–803. doi: 10.1099/jgv.0.000015. [DOI] [PubMed] [Google Scholar]
- 194.Nain M, et al. GRP78 is an important host factor for Japanese Encephalitis virus entry and replication in mammalian cells. J. Virol. 2017;91:e02274–02216. doi: 10.1128/JVI.02274-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu Y-P, et al. Japanese encephalitis virus co-opts the ER-stress response protein GRP78 for viral infectivity. Virol. J. 2011;8:128. doi: 10.1186/1743-422X-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Elfiky, A. A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 10.1186/1477-5956-10-24 (2020) [DOI] [PMC free article] [PubMed]
- 197.Pujhari S, et al. Heat shock protein 70 (Hsp70) mediates Zika virus entry, replication, and egress from host cells. Emerg. Microbes Infect. 2019;8:8–16. doi: 10.1080/22221751.2018.1557988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guerrero CA, et al. Heat shock cognate protein 70 is involved in rotavirus cell entry. J. Virol. 2002;76:4096–4102. doi: 10.1128/JVI.76.8.4096-4102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zárate S, et al. Interaction of rotaviruses with Hsc70 during cell entry is mediated by VP5. J. Virol. 2003;77:7254–7260. doi: 10.1128/JVI.77.13.7254-7260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pérez-Vargas J, Romero P, López S, Arias CF. The peptide-binding and ATPase domains of recombinant hsc70 are required to interact with rotavirus and reduce its infectivity. J. Virol. 2006;80:3322–3331. doi: 10.1128/JVI.80.7.3322-3331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Katoh H, et al. Heat shock protein 70 regulates degradation of the mumps virus phosphoprotein via the ubiquitin-proteasome pathway. J. Virol. 2015;89:3188–3199. doi: 10.1128/JVI.03343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Katoh H, et al. Heat shock protein 90 ensures efficient Mumps virus replication by assisting withFang viral polymerase complex formation. J. Virol. 2017;91:e02220–02216. doi: 10.1128/JVI.02220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vasconcelos DY, Cai XH, Oglesbee MJ. Constitutive overexpression of the major inducible 70 kDa heat shock protein mediates large plaque formation by measles virus. J. Gen. Virol. 1998;79:2239–2247. doi: 10.1099/0022-1317-79-9-2239. [DOI] [PubMed] [Google Scholar]
- 204.Oglesbee MJ, Kenney H, Kenney T, Krakowka S. Enhanced production of morbillivirus gene-specific RNAs following induction of the cellular stress response in stable persistent infection. Virology. 1993;192:556–567. doi: 10.1006/viro.1993.1072. [DOI] [PubMed] [Google Scholar]
- 205.Oglesbee MJ, Liu Z, Kenney H, Brooks CL. The highly inducible member of the 70 kDa family of heat shock proteins increases canine distemper virus polymerase activity. J. Gen. Virol. 1996;77:2125–2135. doi: 10.1099/0022-1317-77-9-2125. [DOI] [PubMed] [Google Scholar]
- 206.Chen Y-J, et al. Heat shock protein 72 is associated with the hepatitis C virus replicase complex and enhances viral RNA replication. J. Bio Chem. 2010;285:28183–28190. doi: 10.1074/jbc.M110.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang Y, et al. Tylophorine analogs allosterically regulates heat shock Cognate Protein 70 and inhibits Hepatitis C virus replication. Sci. Rep. 2017;7:10037–10037. doi: 10.1038/s41598-017-08815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gonzalez O, et al. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatol. 2009;50:1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Oliveira AP, et al. Human respiratory syncytial virus N, P and M protein interactions in HEK-293T cells. Virus Res. 2013;177:108–112. doi: 10.1016/j.virusres.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 210.Brown G, et al. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology. 2005;338:69–80. doi: 10.1016/j.virol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 211.Munday DC, et al. Interactome analysis of the human respiratory syncytial virus RNA polymerase complex identifies protein chaperones as important cofactors that promote L-protein stability and RNA synthesis. J. Virol. 2015;89:917–930. doi: 10.1128/JVI.01783-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.García-Dorival I, et al. Elucidation of the cellular interactome of Ebola virus nucleoprotein and identification of therapeutic targets. J. Proteome Res. 2016;15:4290–4303. doi: 10.1021/acs.jproteome.6b00337. [DOI] [PubMed] [Google Scholar]
- 213.Nelson EV, et al. An RNA polymerase II-driven Ebola virus minigenome system as an advanced tool for antiviral drug screening. Antivir. Res. 2017;146:21–27. doi: 10.1016/j.antiviral.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tchesnokov EP, Raeisimakiani P, Ngure M, Marchant D, Götte M. Recombinant RNA-dependent RNA polymerase complex of Ebola virus. Sci. Rep. 2018;8:3970. doi: 10.1038/s41598-018-22328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sztuba-Solinska J, et al. A small stem-loop structure of the Ebola virus trailer is essential for replication and interacts with heat-shock protein A8. Nucleic Acids Res. 2016;44:9831–9846. doi: 10.1093/nar/gkw825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Oglesbee M, Krakowka S. Cellular stress response induces selective intranuclear trafficking and accumulation of morbillivirus major core protein. Lab. Invest. 1993;68:109–117. [PubMed] [Google Scholar]
- 217.Manzoor R, et al. Heat shock protein 70 modulates influenza A virus polymerase activity. J. Biol. Chem. 2014;289:7599–7614. doi: 10.1074/jbc.M113.507798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang, F. et al. Heat shock protein 70 promotes coxsackievirus B3 translation initiation and elongation via Akt-mTORC1 pathway depending on activation of p70S6K and Cdc2. Cell. Microbiol. 19, 10.1111/cmi.12725 (2017). [DOI] [PubMed]
- 219.Dong Q, et al. Hsc70 regulates the IRES activity and serves as an antiviral target of enterovirus A71 infection. Antivir. Res. 2018;150:39–46. doi: 10.1016/j.antiviral.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 220.Hovanessian AG, Galabru J. The double-stranded RNA-dependent protein kinase is also activated by heparin. Eur. J. Biochem. 1987;167:467–473. doi: 10.1111/j.1432-1033.1987.tb13360.x. [DOI] [PubMed] [Google Scholar]
- 221.Gale J, Michael J, Korth MJ, Katze MG. Repression of the PKR protein kinase by the hepatitis C virus NS5A protein: a potential mechanism of interferon resistance. Clin. Diagn. Virol. 1998;10:157–162. doi: 10.1016/s0928-0197(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 222.Lee TG, Tang N, Thompson S, Miller J, Katze MG. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol. Cell. Biol. 1994;14:2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lee TG, Tomita J, Hovanessian AG, Katze MG. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc. Natl Acad. Sci. USA. 1990;87:6208–6212. doi: 10.1073/pnas.87.16.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee TG, Tomita J, Hovanessian AG, Katze MG. Characterization and regulation of the 58,000-dalton cellular inhibitor of the interferon-induced, dsRNA-activated protein kinase. J. Biol. Chem. 1992;267:14238–14243. [PubMed] [Google Scholar]
- 225.Melville MW, Hansen WJ, Freeman BC, Welch WJ, Katze MG. The molecular chaperone hsp40 regulates the activity of P58IPK, the cellular inhibitor of PKR. Proc. Natl Acad. Sci. USA. 1997;94:97–102. doi: 10.1073/pnas.94.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Melville MW, et al. The cellular inhibitor of the PKR protein kinase, P58(IPK), is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J. Bio Chem. 1999;274:3797–3803. doi: 10.1074/jbc.274.6.3797. [DOI] [PubMed] [Google Scholar]
- 227.Leone G, et al. C-terminal trimerization, but not N-terminal trimerization, of the reovirus cell attachment protein Is a posttranslational and Hsp70/ATP-dependent process. J. Biol. Chem. 1996;271:8466–8471. doi: 10.1074/jbc.271.14.8466. [DOI] [PubMed] [Google Scholar]
- 228.Macejak DG, Sarnow P. Association of heat shock protein 70 with enterovirus capsid precursor P1 in infected human cells. J. Virol. 1992;66:1520–1527. doi: 10.1128/jvi.66.3.1520-1527.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hirayama E, Atagi H, Hiraki A, Kim J. Heat shock protein 70 is related to thermal inhibition of nuclear export of the influenza virus ribonucleoprotein complex. J. Virol. 2004;78:1263–1270. doi: 10.1128/JVI.78.3.1263-1270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li G, Zhang J, Tong X, Liu W, Ye X. Heat shock protein 70 inhibits the activity of Influenza A virus ribonucleoprotein and blocks the replication of virus in vitro and in vivo. PLoS ONE. 2011;6:e16546. doi: 10.1371/journal.pone.0016546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen Y-C, et al. Polo-like kinase 1 is involved in hepatitis C virus replication by hyperphosphorylating NS5A. J. Virol. 2010;84:7983–7993. doi: 10.1128/JVI.00068-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Khachatoorian R, et al. The NS5A-binding heat shock proteins HSC70 and HSP70 play distinct roles in the hepatitis C viral life cycle. Virology. 2014;454-455:118–127. doi: 10.1016/j.virol.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Khachatoorian R, et al. Structural characterization of the HSP70 interaction domain of the hepatitis C viral protein NS5A. Virology. 2015;475:46–55. doi: 10.1016/j.virol.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dupzyk A, Williams JM, Bagchi P, Inoue T, Tsai B. SGTA-dependent regulation of Hsc70 promotes cytosol entry of Simian Virus 40 from the endoplasmic reticulum. J. Virol. 2017;91:e00232–00217. doi: 10.1128/JVI.00232-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ravindran MS, Bagchi P, Inoue T, Tsai B. A non-enveloped virus hijacks host disaggregation machinery to translocate across the endoplasmic reticulum membrane. PLoS Pathog. 2015;11:e1005086. doi: 10.1371/journal.ppat.1005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Goodwin EC, et al. BiP and multiple DNAJ molecular chaperones in the endoplasmic reticulum are required for efficient simian virus 40 infection. Bio. 2011;2:e00101–e00111. doi: 10.1128/mBio.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Niewiarowska J, D’Halluin JC, Belin MT. Adenovirus capsid proteins interact with HSP70 proteins after penetration in human or rodent cells. Exp. Cell Res. 1992;201:408–416. doi: 10.1016/0014-4827(92)90290-o. [DOI] [PubMed] [Google Scholar]
- 238.Chroboczek J, Gout E, Favier AL, Galinier R. Novel partner proteins of adenovirus penton. Curr. Top. Microbiol. Immunol. 2003;272:37–55. doi: 10.1007/978-3-662-05597-7_2. [DOI] [PubMed] [Google Scholar]
- 239.Gout E, Gutkowska M, Takayama S, Reed JC, Chroboczek J. Co-chaperone BAG3 and adenovirus penton base protein partnership. J. Cell. Biochem. 2010;111:699–708. doi: 10.1002/jcb.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Saphire ACS, Guan T, Schirmer EC, Nemerow GR, Gerace L. Nuclear Import of Adenovirus DNA in Vitro Involves the Nuclear Protein Import Pathway and hsc70. J. Biol. Chem. 2000;275:4298–4304. doi: 10.1074/jbc.275.6.4298. [DOI] [PubMed] [Google Scholar]
- 241.Burch AD, Weller SK. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J. Virol. 2004;78:7175–7185. doi: 10.1128/JVI.78.13.7175-7185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Niyaz Y, Frenz I, Petersen G, Gehring U. Transcriptional stimulation by the DNA binding protein Hap46/BAG-1M involves hsp70/hsc70 molecular chaperones. Nucleic Acids Res. 2003;31:2209–2216. doi: 10.1093/nar/gkg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Niyaz Y, Zeiner M, Gehring U. Transcriptional activation by the human Hsp70-associating protein Hap50. J. Cell Sci. 2001;114:1839–1845. doi: 10.1242/jcs.114.10.1839. [DOI] [PubMed] [Google Scholar]
- 244.Zeiner M, Niyaz Y, Gehring U. The hsp70-associating protein Hap46 binds to DNA and stimulates transcription. Proc. Natl Acad. Sci. USA. 1999;96:10194–10199. doi: 10.1073/pnas.96.18.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pater A, Kumar KU, Pater MM, Devireddy LR. BAG-1, a novel Bcl-2-interacting protein, activates expression of human JC virus. J. Gen. Virol. 2000;81:351–357. doi: 10.1099/0022-1317-81-2-351. [DOI] [PubMed] [Google Scholar]
- 246.Takahashi N, et al. BAG-1M, an isoform of Bcl-2-interacting protein BAG-1, enhances gene expression driven by CMV promoter. Biochem. Biophys. Res. Commun. 2001;286:807–814. doi: 10.1006/bbrc.2001.5473. [DOI] [PubMed] [Google Scholar]
- 247.Lambert C, Prange R. Chaperone action in the posttranslational topological reorientation of the hepatitis B virus large envelope protein: Implications for translocational regulation. Proc. Natl Acad. Sci. USA. 2003;100:5199–5204. doi: 10.1073/pnas.0930813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Löffler-Mary H, Werr M, Prange R. Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology. 1997;235:144–152. doi: 10.1006/viro.1997.8689. [DOI] [PubMed] [Google Scholar]
- 249.Prange R, Werr M, Löffler-Mary H. Chaperones involved in hepatitis B virus morphogenesis. Biol. Chem. 1999;380:305–314. doi: 10.1515/BC.1999.042. [DOI] [PubMed] [Google Scholar]
- 250.Gassler CS, Wiederkehr T, Brehmer D, Bukau B, Mayer MP. Bag-1M accelerates nucleotide release for human Hsc70 and Hsp70 and can act concentration-dependent as positive and negative cofactor. J. Bio Chem. 2001;276:32538–32544. doi: 10.1074/jbc.M105328200. [DOI] [PubMed] [Google Scholar]
- 251.Kanelakis KC, et al. hsp70 interacting protein Hip does not affect glucocorticoid receptor folding by the hsp90-based chaperone machinery except to oppose the effect of BAG-1. Biochemistry. 2000;39:14314–14321. doi: 10.1021/bi001671c. [DOI] [PubMed] [Google Scholar]
- 252.Cho D-Y, Yang G-H, Ryu CJ, Hong HJ. Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo. J. Virol. 2003;77:2784–2788. doi: 10.1128/JVI.77.4.2784-2788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Awe K, Lambert C, Prange R. Mammalian BiP controls posttranslational ER translocation of the hepatitis B virus large envelope protein. FEBS Lett. 2008;582:3179–3184. doi: 10.1016/j.febslet.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 254.Cripe TP, Delos SE, Estes PA, Garcea RL. In vivo and in vitro association of hsc70 with polyomavirus capsid proteins. J. Virol. 1995;69:7807–7813. doi: 10.1128/jvi.69.12.7807-7813.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chromy LR, Pipas JM, Garcea RL. Chaperone-mediated in vitro assembly of Polyomavirus capsids. Proc. Natl Acad. Sci. USA. 2003;100:10477–10482. doi: 10.1073/pnas.1832245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Clapham P, Nagy K, Cheingsong-Popov R, Exley M, Weiss RA. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983;222:1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- 257.Hoshino H, Shimoyama M, Miwa M, Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc. Natl Acad. Sci. USA. 1983;80:7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda Y. 71-kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J. Virol. 1998;72:535–541. doi: 10.1128/jvi.72.1.535-541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fang D, et al. Heat shock cognate protein 70 is a cell fusion-enhancing factor but not an entry factor for human T-cell lymphotropic virus type I. Biochem. Biophys. Res. Commun. 1999;261:357–363. doi: 10.1006/bbrc.1999.1028. [DOI] [PubMed] [Google Scholar]
- 260.Agostini I, et al. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp. Cell Res. 2000;259:398–403. doi: 10.1006/excr.2000.4992. [DOI] [PubMed] [Google Scholar]
- 261.Bukrinsky M, Zhao Y. Heat-shock proteins reverse the G2 arrest caused by HIV-1 viral protein R. DNA Cell Biol. 2004;23:223–225. doi: 10.1089/104454904773819806. [DOI] [PubMed] [Google Scholar]
- 262.Iordanskiy S, et al. Heat shock protein 70 protects cells from cell cycle arrest and apoptosis induced by human immunodeficiency virus type 1 viral protein R. J. Virol. 2004;78:9697–9704. doi: 10.1128/JVI.78.18.9697-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gurer C, Höglund A, Höglund S, Luban J. ATPgammaS disrupts human immunodeficiency virus type 1 virion core integrity. J. Virol. 2005;79:5557–5567. doi: 10.1128/JVI.79.9.5557-5567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Srinivasan A, et al. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol. Cell. Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sullivan CS, Cantalupo P, Pipas JM. The molecular chaperone activity of simian virus 40 large T antigen is required to disrupt Rb-E2F family complexes by an ATP-dependent mechanism. Mol. Cell. Biol. 2000;20:6233–6243. doi: 10.1128/mcb.20.17.6233-6243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sullivan CS, Gilbert SP, Pipas JM. ATP-dependent simian virus 40 T-antigen-Hsc70 complex formation. J. Virol. 2001;75:1601–1610. doi: 10.1128/JVI.75.4.1601-1610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 268.Laufen T, et al. Mechanism of regulation of hsp70 chaperones by DnaJ cochaperones. Proc. Natl Acad. Sci. USA. 1999;96:5452–5457. doi: 10.1073/pnas.96.10.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mayer MP, Laufen T, Paal K, McCarty JS, Bukau B. Investigation of the interaction between DnaK and DnaJ by surface plasmon resonance spectroscopy. J. Mol. Biol. 1999;289:1131–1144. doi: 10.1006/jmbi.1999.2844. [DOI] [PubMed] [Google Scholar]
- 270.Schirmbeck R, et al. Priming polyvalent immunity by DNA vaccines expressing chimeric antigens with a stress protein-capturing, viral J-domain. FASEB J. 2002;16:1108–1110. doi: 10.1096/fj.01-0993fje. [DOI] [PubMed] [Google Scholar]
- 271.Chao HH, Buchmann AM, DeCaprio JA. Loss of p19(ARF) eliminates the requirement for the pRB-binding motif in simian virus 40 large T antigen-mediated transformation. Mol. Cell. Biol. 2000;20:7624–7633. doi: 10.1128/mcb.20.20.7624-7633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Harris KF, Christensen JB, Radany EH, Imperiale MJ. Novel mechanisms of E2F induction by BK virus large-T antigen: requirement of both the pRb-binding and the J domains. Mol. Cell. Biol. 1998;18:1746–1756. doi: 10.1128/mcb.18.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sheng Q, Love TM, Schaffhausen B. J domain-independent regulation of the Rb family by polyomavirus large T antigen. J. Virol. 2000;74:5280–5290. doi: 10.1128/jvi.74.11.5280-5290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zalvide J, Stubdal H, DeCaprio JA. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family proteins. Mol. Cell. Biol. 1998;18:1408–1415. doi: 10.1128/mcb.18.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Schilling B, De-Medina T, Syken J, Vidal M, Münger K. A novel human DnaJ protein, hTid-1, a homolog of the Drosophila tumor suppressor protein Tid56, can interact with the human papillomavirus type 16 E7 oncoprotein. Virology. 1998;247:74–85. doi: 10.1006/viro.1998.9220. [DOI] [PubMed] [Google Scholar]
- 276.Patrick DR, Oliff A, Heimbrook DC. Identification of a novel retinoblastoma gene product binding site on human papillomavirus type 16 E7 protein. J. Bio Chem. 1994;269:6842–6850. [PubMed] [Google Scholar]
- 277.Wu EW, Clemens KE, Heck DV, Münger K. The human papillomavirus E7 oncoprotein and the cellular transcription factor E2F bind to separate sites on the retinoblastoma tumor suppressor protein. J. Virol. 1993;67:2402–2407. doi: 10.1128/jvi.67.4.2402-2407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.White E, Spector D, Welch W. Differential distribution of the adenovirus E1A proteins and colocalization of E1A with the 70-kilodalton cellular heat shock protein in infected cells. J. Virol. 1988;62:4153–4166. doi: 10.1128/jvi.62.11.4153-4166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 280.Hardwick JM. Apoptosis in viral pathogenesis. Cell Death Differ. 2001;8:109–110. doi: 10.1038/sj.cdd.4400820. [DOI] [PubMed] [Google Scholar]
- 281.Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu. Rev. Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 282.Young P, Anderton E, Paschos K, White R, Allday MJ. Epstein-Barr virus nuclear antigen (EBNA) 3A induces the expression of and interacts with a subset of chaperones and co-chaperones. J. Gen. Virol. 2008;89:866–877. doi: 10.1099/vir.0.83414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Best SM, Wolfinbarger JB, Bloom ME. Caspase activation is required for permissive replication of Aleutian mink disease parvovirus in vitro. Virology. 2002;292:224–234. doi: 10.1006/viro.2001.1238. [DOI] [PubMed] [Google Scholar]
- 284.Anderson JR. The mechanisms of direct, virus-induced destruction of neurons. Curr. Top. Microbiol. Immunol. 2001;253:15–33. doi: 10.1007/978-3-662-10356-2_2. [DOI] [PubMed] [Google Scholar]
- 285.Goh PY, et al. The hepatitis C virus core protein interacts with NS5A and activates its caspase-mediated proteolytic cleavage. Virology. 2001;290:224–236. doi: 10.1006/viro.2001.1195. [DOI] [PubMed] [Google Scholar]
- 286.Henke A, et al. Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein siva. J. Virol. 2000;74:4284–4290. doi: 10.1128/jvi.74.9.4284-4290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Henke A, et al. The apoptotic capability of coxsackievirus B3 is influenced by the efficient interaction between the capsid protein VP2 and the proapoptotic host protein Siva. Virology. 2001;289:15–22. doi: 10.1006/viro.2001.1082. [DOI] [PubMed] [Google Scholar]
- 288.Muthumani K, et al. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Bio Chem. 2002;277:37820–37831. doi: 10.1074/jbc.M205313200. [DOI] [PubMed] [Google Scholar]
- 289.Muthumani K, et al. Adenovirus encoding HIV-1 Vpr activates caspase 9 and induces apoptotic cell death in both p53 positive and negative human tumor cell lines. Oncogene. 2002;21:4613–4625. doi: 10.1038/sj.onc.1205549. [DOI] [PubMed] [Google Scholar]
- 290.Schultz-Cherry S, Dybdahl-Sissoko N, Neumann G, Kawaoka Y, Hinshaw VS. Influenza virus ns1 protein induces apoptosis in cultured cells. J. Virol. 2001;75:7875–7881. doi: 10.1128/JVI.75.17.7875-7881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fazakerley JK. Neurovirology and developmental neurobiology. Adv. Virus Res. 2001;56:73–124. doi: 10.1016/s0065-3527(01)56005-4. [DOI] [PubMed] [Google Scholar]
- 292.Fazakerley JK, Allsopp TE. Programmed cell death in virus infections of the nervous system. Curr. Top. Microbiol. Immunol. 2001;253:95–119. doi: 10.1007/978-3-662-10356-2_5. [DOI] [PubMed] [Google Scholar]
- 293.Zhang R, et al. Ts-Hsp70 induces protective immunity against Trichinella spiralis infection in mouse by activating dendritic cells through TLR2 and TLR4. PLoS Negl. Trop. Dis. 2018;12:e0006502–e0006502. doi: 10.1371/journal.pntd.0006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kuzyk MA, et al. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol. Cell Proteomics. 2009;8:1860–1877. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chen H, et al. Hsp70 inhibits lipopolysaccharide-induced NF-κB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 2006;580:3145–3152. doi: 10.1016/j.febslet.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 296.Kumada K, Fuse N, Tamura T, Okamori C, Kurata S. HSP70/DNAJA3 chaperone/cochaperone regulates NF-κB activity in immune responses. Biochem Biophys. Res. Commun. 2019;513:947–951. doi: 10.1016/j.bbrc.2019.04.077. [DOI] [PubMed] [Google Scholar]
- 297.Bromberg Z, Weiss Y. The role of the membrane-initiated heat shock response in cancer. Front Mol. Biosci. 2016;3:12–12. doi: 10.3389/fmolb.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Weiss YG, et al. Enhanced heat shock protein 70 expression alters proteasomal degradation of IkappaB kinase in experimental acute respiratory distress syndrome. Crit. Care Med. 2007;35:2128–2138. doi: 10.1097/01.ccm.0000278915.78030.74. [DOI] [PubMed] [Google Scholar]
- 299.Martine P, et al. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell Death Dis. 2019;10:256–256. doi: 10.1038/s41419-019-1491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lehner T, et al. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem. Soc. Trans. 2004;32:629–632. doi: 10.1042/BST0320629. [DOI] [PubMed] [Google Scholar]
- 301.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptidembearing and peptide-negative preparations act as chaperokines. Cell CS. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 303.Fang H, et al. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 2011;286:30393–30400. doi: 10.1074/jbc.M111.266528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Satoh, T. & Akira, S. Toll-like receptor signaling and its inducible proteins. Microbiol Spectr.10.1128/microbiolspec.MCHD-0040-2016 (2016). [DOI] [PubMed]
- 305.Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107:391–397. doi: 10.1111/cas.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wi SM, et al. TAK1-ECSIT-TRAF6 complex plays a key role in the TLR4 signal to activate NF-kappaB. J. Biol. Chem. 2014;289:35205–35214. doi: 10.1074/jbc.M114.597187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Xing Y, et al. Cutting Edge: TRAF6 MEdiates TLR/IL-1R signaling-induced nontranscriptional priming of the NLRP3 inflammasome. J. Immunol. 2017;199:1561–1566. doi: 10.4049/jimmunol.1700175. [DOI] [PubMed] [Google Scholar]
- 308.Al-Huseini LM, et al. Heme oxygenase-1 regulates dendritic cell function through modulation of p38 MAPK-CREB/ATF1 signaling. J. Bio Chem. 2014;289:16442–16451. doi: 10.1074/jbc.M113.532069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pathak SK, et al. Activated apoptotic cells induce dendritic cell maturation via engagement of Toll-like receptor 4 (TLR4), dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN), and beta2 integrins. J. Biol. Chem. 2012;287:13731–13742. doi: 10.1074/jbc.M111.336545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Chen Y, et al. IL-1β induction of MUC5AC gene expression is mediated by CREB and NF-κB and repressed by dexamethasone. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L797–L807. doi: 10.1152/ajplung.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wong CP, Bray TM, Ho E. Induction of proinflammatory response in prostate cancer epithelial cells by activated macrophages. Cancer Lett. 2009;276:38–46. doi: 10.1016/j.canlet.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in Atherosclerosis. Annu. Rev. Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 313.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr. Top. Microbiol. Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- 314.Quintana FJ, Cohen IR. The HSP60 immune system network. Trends Immunol. 2011;32:89–95. doi: 10.1016/j.it.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 315.Tsan M-F, Gao B. Heat shock protein and innate immunity. Cell. Mol. Immunol. 2004;1:274–279. [PubMed] [Google Scholar]
- 316.Deniset JF, Pierce GN. Heat shock proteins: mediators of atherosclerotic development. Curr. Drug Targets. 2015;16:816–826. doi: 10.2174/1389450116666150416115423. [DOI] [PubMed] [Google Scholar]
- 317.Swaroop S, Mahadevan A, Shankar SK, Adlakha YK, Basu A. HSP60 critically regulates endogenous IL-1β production in activated microglia by stimulating NLRP3 inflammasome pathway. J. Neuroinflammation. 2018;15:177. doi: 10.1186/s12974-018-1214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Karlas A, et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463:818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 319.Padwad YS, et al. RNA interference mediated silencing of Hsp60 gene in human monocytic myeloma cell line U937 revealed decreased dengue virus multiplication. Immunobiology. 2009;214:422–429. doi: 10.1016/j.imbio.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 320.Farinati F, et al. Hepatitis C virus: from oxygen free radicals to hepatocellular carcinoma. J. Viral Hepat. 2007;14:821–829. doi: 10.1111/j.1365-2893.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 321.Okuda M, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterol. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 322.Kang S-M, et al. Interaction of hepatitis C virus core protein with Hsp60 triggers the production of reactive oxygen species and enhances TNF-alpha-mediated apoptosis. Cancer Lett. 2009;279:230–237. doi: 10.1016/j.canlet.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 323.Chattopadhyay S, et al. Tyrosine phosphorylation modulates mitochondrial chaperonin Hsp60 and delays rotavirus NSP4-mediated apoptotic signaling in host cells. Cell. Microbiol. 2017;19:1–16. doi: 10.1111/cmi.12670. [DOI] [PubMed] [Google Scholar]
- 324.Wyżewski Z, et al. Cooperation between heat shock proteins in organizing of proteins spatial structure. Postepy Hig. Med. Dosw. 2014;68:793–807. doi: 10.5604/17322693.1108406. [DOI] [PubMed] [Google Scholar]
- 325.Park SG, Lee SM, Jung G. Antisense oligodeoxynucleotides targeted against molecular chaperonin Hsp60 block human hepatitis B virus replication. J. Bio Chem. 2003;278:39851–39857. doi: 10.1074/jbc.M301618200. [DOI] [PubMed] [Google Scholar]
- 326.Park SG, Jung G. Human Hepatitis B virus polymerase interacts with the molecular chaperonin Hsp60. J. Virol. 2001;75:6962–6968. doi: 10.1128/JVI.75.15.6962-6968.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Park SG, Lim SO, Jung G. Binding site analysis of human HBV Pol for molecular chaperonin, Hsp60. Virology. 2002;298:116–123. doi: 10.1006/viro.2002.1496. [DOI] [PubMed] [Google Scholar]
- 328.Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J. Gastroenterol. 2001;36:651–660. doi: 10.1007/s005350170027. [DOI] [PubMed] [Google Scholar]
- 329.Tanaka Y, et al. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem. Biophys. Res. Commun. 2004;318:461–469. doi: 10.1016/j.bbrc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 330.Zhang SM, et al. HBx protein of hepatitis B virus (HBV) can form complex with mitochondrial HSP60 and HSP70. Arch. Virol. 2005;150:1579–1590. doi: 10.1007/s00705-005-0521-1. [DOI] [PubMed] [Google Scholar]
- 331.Busca A, Kumar A. Innate immune responses in hepatitis B virus (HBV) infection. Virol. J. 2014;11:22. doi: 10.1186/1743-422X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Xu D, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with Hepatitis B. J. Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 333.Porrini A. Interferon effects on interleukin-10 secretion Mononuclear cell response to interleukin-10 is normal in multiple sclerosis patients. J. Neuroimmunol. 1995;61:27–34. doi: 10.1016/0165-5728(95)00070-i. [DOI] [PubMed] [Google Scholar]
- 334.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthrit. Res. Ther. 2013;15:S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kondo Y, et al. Hepatitis B virus replication could enhance regulatory T cell activity by producing soluble heat shock protein 60 from hepatocytes. J. Infect. Dis. 2010;202:202–213. doi: 10.1086/653496. [DOI] [PubMed] [Google Scholar]
- 336.Bartz SR, et al. An Hsp60 related protein is associated with purified HIV and SIV. J. Med. Primatol. 1994;23:151–154. doi: 10.1111/j.1600-0684.1994.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 337.Asante-Appiah E, Skalka AM. Molecular mechanisms in retrovirus DNA integration. Antivir. Res. 1997;36:139–156. doi: 10.1016/s0166-3542(97)00046-6. [DOI] [PubMed] [Google Scholar]
- 338.Parissi V, et al. Functional interactions of human immunodeficiency virus type 1 integrase with human and yeast HSP60. J. Virol. 2001;75:11344–11353. doi: 10.1128/JVI.75.23.11344-11353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wang RY-L, et al. DnaJ homolog Hdj2 facilitates Japanese encephalitis virus replication. Virol. J. 2011;8:471. doi: 10.1186/1743-422X-8-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Compans RW, Content J, Duesberg PH. Structure of the ribonucleoprotein of influenza virus. J. Virol. 1972;10:795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Weber F, Kochs G, Gruber S, Haller O. A classical bipartite nuclear localization signal on Thogoto and Influenza A virus nucleoproteins. Virology. 1998;250:9–18. doi: 10.1006/viro.1998.9329. [DOI] [PubMed] [Google Scholar]
- 342.Cros JF, García-Sastre A, Palese P. An unconventional NLS is critical for the nuclear import of the influenza A virus Nucleoprotein and Ribonucleoprotein. Traffic. 2005;6:205–213. doi: 10.1111/j.1600-0854.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 343.Batra J, et al. Human Heat shock protein 40 (Hsp40/DnaJB1) promotes influenza A virus replication by assisting nuclear import of viral ribonucleoproteins. Sci. Rep. 2016;6:19063. doi: 10.1038/srep19063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Cao M, et al. DnaJA1/Hsp40 is co-opted by influenza A virus to enhance its viral RNA polymerase activity. J. Virol. 2014;88:14078–14089. doi: 10.1128/JVI.02475-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Meurs E, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 346.Sharma K, et al. Influenza A virus nucleoprotein exploits Hsp40 to inhibit PKR activation. PLoS ONE. 2011;6:e20215. doi: 10.1371/journal.pone.0020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J. Bio Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- 348.Tan SL, Katze MG. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J. Interferon Cytokine Res. 1998;18:757–766. doi: 10.1089/jir.1998.18.757. [DOI] [PubMed] [Google Scholar]
- 349.Guan Z, et al. Interaction of Hsp40 with influenza virus M2 protein: implications for PKR signaling pathway. Protein cell. 2010;1:944–955. doi: 10.1007/s13238-010-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yi Z, Yuan Z, Rice CM, MacDonald MR. Flavivirus replication complex assembly revealed by DNAJC14 functional mapping. J. Virol. 2012;86:11815–11832. doi: 10.1128/JVI.01022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yi Z, et al. Identification and characterization of the host protein DNAJC14 as a broadly active flavivirus replication modulator. PLoS Path. 2011;7:e1001255. doi: 10.1371/journal.ppat.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Bozzacco L, et al. Chaperone-assisted protein folding is critical for yellow fever virus NS3/4A cleavage and replication. J. Virol. 2016;90:3212–3228. doi: 10.1128/JVI.03077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Takashima K, Oshiumi H, Matsumoto M, Seya T. DNAJB1/HSP40 suppresses melanoma differentiation-associated Gene 5-Mitochondrial Antiviral signaling protein function in conjunction with HSP70. J. Innate Immun. 2018;10:44–55. doi: 10.1159/000480740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zhang W, et al. Cellular DNAJA3, a novel VP1-interacting protein, inhibits Foot-and-Mouth Disease virus replication by inducing lysosomal degradation of VP1 and attenuating its antagonistic role in the beta interferon signaling pathway. J. Virol. 2019;93:e00588–00519. doi: 10.1128/JVI.00588-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Liu JS, et al. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J. Bio Chem. 1998;273:30704–30712. doi: 10.1074/jbc.273.46.30704. [DOI] [PubMed] [Google Scholar]
- 356.Mohr IJ, et al. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- 357.Kuo SR, Liu JS, Broker TR, Chow LT. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J. Bio Chem. 1994;269:24058–24065. [PubMed] [Google Scholar]
- 358.Lin BY, Makhov AM, Griffith JD, Broker TR, Chow LT. Chaperone proteins abrogate inhibition of the human papillomavirus (HPV) E1 replicative helicase by the HPV E2 protein. Mol. Cell. Biol. 2002;22:6592–6604. doi: 10.1128/MCB.22.18.6592-6604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Eom C-Y, Lehman IR. The human DnaJ protein, hTid-1, enhances binding of a multimer of the herpes simplex virus type 1 UL9 protein to oris, an origin of viral DNA replication. Proc. Natl Acad. Sci. USA. 2002;99:1894–1898. doi: 10.1073/pnas.042689499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Tanguy Le Gac N, Boehmer PE. Activation of the herpes simplex virus type-1 origin-binding protein (UL9) by heat shock proteins. J. Bio Chem. 2002;277:5660–5666. doi: 10.1074/jbc.M108316200. [DOI] [PubMed] [Google Scholar]
- 361.Sohn S-Y, Kim J-H, Baek K-W, Ryu W-S, Ahn B-Y. Turnover of hepatitis B virus X protein is facilitated by Hdj1, a human Hsp40/DnaJ protein. Biochem. Biophys. Res. Commun. 2006;347:764–768. doi: 10.1016/j.bbrc.2006.06.158. [DOI] [PubMed] [Google Scholar]
- 362.Sohn S-Y, Kim S-B, Kim J, Ahn B-Y. Negative regulation of hepatitis B virus replication by cellular Hsp40/DnaJ proteins through destabilization of viral core and X proteins. J. Gen. Virol. 2006;87:1883–1891. doi: 10.1099/vir.0.81684-0. [DOI] [PubMed] [Google Scholar]
- 363.Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2011;26:144–152. doi: 10.1111/j.1440-1746.2010.06546.x. [DOI] [PubMed] [Google Scholar]
- 364.Koike K, et al. High-level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology. 1994;19:810–819. [PubMed] [Google Scholar]
- 365.Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189–205. doi: 10.1016/s1359-6101(00)00034-4. [DOI] [PubMed] [Google Scholar]
- 366.Feitelson MA, Zhu M, Duan LX, London WT. Hepatitis B. x antigen and p53 are associated in vitro and in liver tissues from patients with primary hepatocellular carcinoma. Oncogene. 1993;8:1109–1117. [PubMed] [Google Scholar]
- 367.Yamashita M, Emerman M. Retroviral infection of non-dividing cells: Old and new perspectives. Virology. 2006;344:88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 368.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl Acad. Sci. USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Bukrinsky MI, et al. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zennou V, et al. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 371.Mahalingam S, et al. Functional analysis of the simian immunodeficiency virus Vpx protein: identification of packaging determinants and a novel nuclear targeting domain. J. Virol. 2001;75:362–374. doi: 10.1128/JVI.75.1.362-374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Singhal PK, Kumar PR, Rao MRKS, Kyasani M, Mahalingam S. Simian immunodeficiency virus Vpx is imported into the nucleus via importin alpha-dependent and -independent pathways. J. Virol. 2006;80:526–536. doi: 10.1128/JVI.80.1.526-536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Cheng X, Belshan M, Ratner L. Hsp40 facilitates nuclear import of the human immunodeficiency virus type 2 Vpx-mediated preintegration complex. J. Virol. 2008;82:1229–1237. doi: 10.1128/JVI.00540-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ko S-H, et al. Interference of DNAJB6/MRJ isoform switch by morpholino inhibits replication of HIV-1 and RSV. Mol Ther Nucleic Acids. 2019;14:251–261. doi: 10.1016/j.omtn.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Chiang Y-P, et al. Large isoform of mammalian relative of dnaJ is a major determinant of human susceptibility to HIV-1 infection. EBioMedicine. 2014;1:126–132. doi: 10.1016/j.ebiom.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kumar M, Mitra D. Heat shock protein 40 is necessary for human immunodeficiency virus-1 Nef-mediated enhancement of viral gene expression and replication. J. Bio Chem. 2005;280:40041–40050. doi: 10.1074/jbc.M508904200. [DOI] [PubMed] [Google Scholar]
- 377.Saksela K. HIV-1 Nef and host cell protein kinases. Front. Biosci. 1997;2:d606–d618. doi: 10.2741/a217. [DOI] [PubMed] [Google Scholar]
- 378.Simmons A, Aluvihare V, McMichael A. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity. 2001;14:763–777. doi: 10.1016/s1074-7613(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 379.Doms RW. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 2000;14:2677–2688. doi: 10.1101/gad.833300. [DOI] [PubMed] [Google Scholar]
- 380.Rawat P, Mitra D. Cellular heat shock factor 1 positively regulates human immunodeficiency virus-1 gene expression and replication by two distinct pathways. Nucleic Acids Res. 2011;39:5879–5892. doi: 10.1093/nar/gkr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Kumar M, et al. Reciprocal regulation of human immunodeficiency virus-1 gene expression and replication by heat shock proteins 40 and 70. J. Mol. Biol. 2011;410:944–958. doi: 10.1016/j.jmb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 382.Urano E, Morikawa Y, Komano J. Novel role of HSP40/DNAJ in the regulation of HIV-1 replication. J. Acquir. Immune Defic. Syndr. 2013;64:154–162. doi: 10.1097/QAI.0b013e31829a2ef8. [DOI] [PubMed] [Google Scholar]
- 383.Jäättelä M. Heat shock proteins as cellular lifeguards. Ann. Med. 1999;31:261–271. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- 384.Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- 385.Sugiyama Y, et al. Muscle develops a dpecific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J. Biol. Chem. 2000;275:1095–1104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- 386.Fukagawa Y, et al. Epstein-Barr virus upregulates phosphorylated heat shock protein 27 kDa in carcinoma cells using the phosphoinositide 3-kinase/Akt pathway. Electrophoresis. 2008;29:3192–3200. doi: 10.1002/elps.200800086. [DOI] [PubMed] [Google Scholar]
- 387.Cao Z, et al. Proteomics analysis of differentially expressed proteins in chicken trachea and kidney after infection with the highly virulent and attenuated coronavirus infectious bronchitis virus in vivo. Proteome Sci. 2012;10:24. doi: 10.1186/1477-5956-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ling S, et al. Cellular Hsp27 interacts with classical swine fever virus NS5A protein and negatively regulates viral replication by the NF-κB signaling pathway. Virology. 2018;518:202–209. doi: 10.1016/j.virol.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 389.Sun M, et al. Down-regulating heat shock protein 27 is involved in porcine epidemic diarrhea virus escaping from host antiviral mechanism. Vet. Microbiol. 2017;205:6–13. doi: 10.1016/j.vetmic.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 390.Lindquist S. The heat-shock response. Annu. Rev. Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 391.Alcorn M, Booth J, Coggeshall K, Metcalf J. Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2. J. Virol. 2001;75:6450–6459. doi: 10.1128/JVI.75.14.6450-6459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Neff L, et al. NF‐κB and the MAP kinases/AP‐1 pathways are both involved in interleukin‐6 and interleukin‐8 expression in fibroblast‐like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell. Microbiol. 2001;3:703–712. doi: 10.1046/j.1462-5822.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- 393.Bian Z-M, Elner VM, Yoshida A, Kunkel SL, Elner SG. Signaling pathways for glycated human serum albumin-induced IL-8 and MCP-1 secretion in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2001;42:1660–1668. [PubMed] [Google Scholar]
- 394.Preville X, et al. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp. Cell Res. 1999;247:61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- 395.Lanneau D, et al. Heat shock proteins: essential proteins for apoptosis regulation. J. Cell. Mol. Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Lanneau D, de Thonel A, Maurel S, Didelot C, Garrido C. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1:53–60. doi: 10.4161/pri.1.1.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J. Biochem. Cell Biol. 2012;44:1622–1631. doi: 10.1016/j.biocel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 398.Mathew SS, Della Selva MP, Burch AD. Modification and reorganization of the cytoprotective cellular chaperone Hsp27 during herpes simplex virus type 1 infection. J. Virol. 2009;83:9304–9312. doi: 10.1128/JVI.01826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Liu J, et al. Heat shock protein 27 is involved in PCV2 infection in PK-15 cells. Virus Res. 2014;189:235–242. doi: 10.1016/j.virusres.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 400.Tong S-W, et al. HSPB1 is an intracellular antiviral factor against hepatitis B virus. J. Cell. Biochem. 2013;114:162–173. doi: 10.1002/jcb.24313. [DOI] [PubMed] [Google Scholar]
- 401.Jain S, McGinnes LW, Morrison TG. Role of thiol/disulfide exchange in newcastle disease virus entry. J. Virol. 2009;83:241–249. doi: 10.1128/JVI.01407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Jain S, McGinnes LW, Morrison TG. Overexpression of thiol/disulfide isomerases enhances membrane fusion directed by the Newcastle disease virus fusion protein. J. Virol. 2008;82:12039–12048. doi: 10.1128/JVI.01406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wan SW, et al. Endothelial cell surface expression of protein disulfide isomerase activates beta1 and beta3 integrins and facilitates dengue virus infection. J. Cell. Biochem. 2012;113:1681–1691. doi: 10.1002/jcb.24037. [DOI] [PubMed] [Google Scholar]
- 404.Diwaker D, Mishra KP, Ganju L, Singh SB. Protein disulfide isomerase mediates dengue virus entry in association with lipid rafts. Viral Immunol. 2015;28:153–160. doi: 10.1089/vim.2014.0095. [DOI] [PubMed] [Google Scholar]
- 405.Calderon MN, Guerrero CA, Acosta O, Lopez S, Arias CF. Inhibiting rotavirus infection by membrane-impermeant thiol/disulfide exchange blockers and antibodies against protein disulfide isomerase. Intervirology. 2012;55:451–464. doi: 10.1159/000335262. [DOI] [PubMed] [Google Scholar]
- 406.Ryser HJ, Levy EM, Mandel R, DiSciullo GJ. Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc. Natl Acad. Sci. USA. 1994;91:4559–4563. doi: 10.1073/pnas.91.10.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gallina A, et al. Inhibitors of protein-disulfide isomerase prevent cleavage of disulfide bonds in receptor-bound glycoprotein 120 and prevent HIV-1 entry. J. Biol. Chem. 2002;277:50579–50588. doi: 10.1074/jbc.M204547200. [DOI] [PubMed] [Google Scholar]
- 408.Joe Fenouillet,E, Courageot Barbouch,R, Miquelis R. The catalytic activity of protein disulfide isomerase is involved in human immunodeficiency virus envelope–mediated membrane fusion after CD4 cell binding. J. Infect. Dis. 2001;183:744–752. doi: 10.1086/318823. [DOI] [PubMed] [Google Scholar]
- 409.Barbouche R, Miquelis R, Jones IM, Fenouillet E. Protein-disulfide isomerase-mediated reduction of two disulfide bonds of HIV envelope glycoprotein 120 occurs post-CXCR4 binding and is required for fusion. J. Biol. Chem. 2003;278:3131–3136. doi: 10.1074/jbc.M205467200. [DOI] [PubMed] [Google Scholar]
- 410.Bia S, Hongb PW, Leea B, Bauma LG. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc. Natl Acad. Sci. USA. 2010;108:10650–10655. doi: 10.1073/pnas.1017954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Stehle T, Yan Y, Benjamin TL, Harrison SC. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature. 1994;369:160. doi: 10.1038/369160a0. [DOI] [PubMed] [Google Scholar]
- 412.Inoue T, Tsai B. How viruses use the endoplasmic reticulum for entry, replication, and assembly. Cold Spring Harb. Perspect. Biol. 2013;5:a013250. doi: 10.1101/cshperspect.a013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Schelhaas M, et al. Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131:516–529. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 414.Walczak CP, Tsai B. A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J. Virol. 2011;85:2386–2396. doi: 10.1128/JVI.01855-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wang M, et al. Oblongifolin M, an active compound isolated from a Chinese medical herb Garcinia oblongifolia, potently inhibits enterovirus 71 reproduction through downregulation of ERp57. Oncotarget. 2015;7:8797–8808. doi: 10.18632/oncotarget.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Randow F, MacMicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science. 2013;340:701–706. doi: 10.1126/science.1233028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Akaike T. Role of free radicals in viral pathogenesis and mutation. Rev. Med. Virol. 2001;11:87–101. doi: 10.1002/rmv.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Dobmeyer TS, et al. Ex vivo induction of apoptosis in lymphocytes is mediated by oxidative stress: role for lymphocyte loss in HIV infection. Free Radic. Biol. Med. 1997;22:775–785. doi: 10.1016/s0891-5849(96)00403-0. [DOI] [PubMed] [Google Scholar]
- 419.Knobil K, Choi AM, Weigand GW, Jacoby DB. Role of oxidants in influenza virus-induced gene expression. Am. J. Physiol. 1998;274:L134–L142. doi: 10.1152/ajplung.1998.274.1.L134. [DOI] [PubMed] [Google Scholar]
- 420.Acar A, et al. Investigation of oxidative stress and antioxidant defense in patients with hepatitis B virus infection and the effect of interferon-alpha plus lamivudine combination therapy on oxidative stress. Mikrobiyol. Bul. 2009;43:411–423. [PubMed] [Google Scholar]
- 421.Korenaga M, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J. Biol. Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 422.Ano Y, et al. Oxidative damage to neurons caused by the induction of microglial NADPH oxidase in encephalomyocarditis virus infection. Neurosci. Lett. 2010;469:39–43. doi: 10.1016/j.neulet.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 423.Olagnier D, et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. 2014;10:e1004566. doi: 10.1371/journal.ppat.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Liao SL, Raung SL, Chen CJ. Japanese encephalitis virus stimulates superoxide dismutase activity in rat glial cultures. Neurosci. Lett. 2002;324:133–136. doi: 10.1016/s0304-3940(02)00236-7. [DOI] [PubMed] [Google Scholar]
- 425.De Marco F, et al. Oxidative stress in HPV-driven viral carcinogenesis: redox proteomics analysis of HPV-16 dysplastic and neoplastic tissues. PLoS ONE. 2012;7:e34366. doi: 10.1371/journal.pone.0034366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Tatu U, Hammond C, Helenius A. Folding and oligomerization of influenza hemagglutinin in the ER and the intermediate compartment. EMBO J. 1995;14:1340–1348. doi: 10.1002/j.1460-2075.1995.tb07120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Zai J, et al. N-glycosylation of the premembrane protein of Japanese encephalitis virus is critical for folding of the envelope protein and assembly of virus-like particles. Acta Virol. 2013;57:27–33. doi: 10.4149/av_2013_01_27. [DOI] [PubMed] [Google Scholar]
- 428.Dubuisson J, Rice CM. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 1996;70:778–786. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Herschlag D, Khosla M, Tsuchihashi Z, Karpel RL. An RNA chaperone activity of non-specific RNA binding proteins in hammerhead ribozyme catalysis. EMBO J. 1994;13:2913–2924. doi: 10.1002/j.1460-2075.1994.tb06586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Shabman RS, Gulcicek EE, Stone KL, Basler CF. The Ebola virus VP24 protein prevents hnRNP C1/C2 binding to karyopherin α1 and partially alters its nuclear import. J. Infect Dis. 2011;204:S904–S910. doi: 10.1093/infdis/jir323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Pettit Kneller EL, Connor JH, Lyles D. S. hnRNPs Relocalize to the cytoplasm following infection with vesicular stomatitis virus. J. Virol. 2009;83:770–780. doi: 10.1128/JVI.01279-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Dreyfuss G, Matunis MJ, Pinol-Roma S, Burd C. G. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 433.Ullmer W, Semler BL. Direct and indirect effects on viral translation and RNA replication are required for AUF1 restriction of enterovirus infections in human cells. mBio. 2018;9:e01669–01618. doi: 10.1128/mBio.01669-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Cathcart AL, Rozovics JM, Semler BL. Cellular mRNA decay protein AUF1 negatively regulates enterovirus and human rhinovirus infections. J. Virol. 2013;87:10423–10434. doi: 10.1128/JVI.01049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Katoh H, et al. Heterogeneous nuclear ribonucleoprotein A2 participates in the replication of Japanese encephalitis virus through an interaction with viral proteins and RNA. J. Virol. 2011;85:10976–10988. doi: 10.1128/JVI.00846-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Shi ST, Lai MM. Viral and cellular proteins involved in coronavirus replication. Curr. Top. Microbiol. Immunol. 2005;287:95–131. doi: 10.1007/3-540-26765-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Gontarek R, et al. hnRNP C and polypyrimidine tract-binding protein specifically interact with the pyrimidine-rich region within the 3’NTR of the HCV RNA genome. Nucleic Acids Res. 1999;27:1457–1463. doi: 10.1093/nar/27.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Ertel KJ, Brunner JE, Semler BL. Mechanistic consequences of hnRNP C binding to both RNA termini of poliovirus negative-strand RNA intermediates. J. Virol. 2010;84:4229–4242. doi: 10.1128/JVI.02198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Clerte C, Hall KB. Characterization of multimeric complexes formed by the human PTB1 protein on RNA. RNA. 2006;12:457–475. doi: 10.1261/rna.2178406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Chung RT, Kaplan LM. Heterogeneous nuclear ribonucleoprotein I (hnRNP-I/PTB) selectively binds the conserved 3′ terminus of Hepatitis C viral RNA. Biochem. Biophys. Res. Commun. 1999;254:351–362. doi: 10.1006/bbrc.1998.9949. [DOI] [PubMed] [Google Scholar]
- 441.Domitrovich AM, Diebel KW, Ali N, Sarker S, Siddiqui A. Role of La autoantigen and polypyrimidine tract-binding protein in HCV replication. Virology. 2005;335:72–86. doi: 10.1016/j.virol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 442.Aizaki H, Choi KS, Liu M, Li YJ, Lai MM. Polypyrimidine-tract-binding protein is a component of the HCV RNA replication complex and necessary for RNA synthesis. J. Biomed. Sci. 2006;13:469–480. doi: 10.1007/s11373-006-9088-4. [DOI] [PubMed] [Google Scholar]
- 443.Shwetha S, et al. HuR displaces polypyrimidine tract binding protein to facilitate La binding to the 3′ untranslated region and enhances Hepatitis C virus replication. J. Virol. 2015;89:11356–11371. doi: 10.1128/JVI.01714-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Naushad Ali AS. The La antigen binds 5′ noncoding region of the hepatitis C virus RNA in the context of the initiator AUG codon and stimulates internal ribosome entry site-mediated translation. Proc. Natl Acad. Sci. USA. 1997;94:2249–2254. doi: 10.1073/pnas.94.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Pudi R, Abhiman S, Srinivasan N, Das S. Hepatitis C virus internal ribosome entry site-mediated translation is stimulated by specific interaction of independent regions of human La autoantigen. J. Biol. Chem. 2003;278:12231–12240. doi: 10.1074/jbc.M210287200. [DOI] [PubMed] [Google Scholar]
- 446.Spångberg K, Wiklund L, Schwartz S. HuR, a protein implicated in oncogene and growth factor mRNA decay, binds to the 3′ ends of Hepatitis C virus RNA of both polarities. Virology. 2000;274:378–390. doi: 10.1006/viro.2000.0461. [DOI] [PubMed] [Google Scholar]
- 447.Brunner JE, Ertel KJ, Rozovics JM, Semler BL. Delayed kinetics of poliovirus RNA synthesis in a human cell line with reduced levels of hnRNP C proteins. Virology. 2010;400:240–247. doi: 10.1016/j.virol.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Perwitasari O, et al. MicroRNA-555 has potent antiviral properties against poliovirus. J. Gen. Virol. 2016;97:659–668. doi: 10.1099/jgv.0.000372. [DOI] [PubMed] [Google Scholar]
- 449.Tsai P-L, et al. Cellular RNA binding proteins NS1-BP and hnRNP K regulate influenza A virus RNA splicing. PLoS Pathog. 2013;9:e1003460. doi: 10.1371/journal.ppat.1003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Wolff T, O’Neill RE, Palese P. NS1-binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J. Virol. 1998;72:7170–7180. doi: 10.1128/jvi.72.9.7170-7180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Thompson MG, et al. Co-regulatory activity of hnRNP K and NS1-BP in influenza and human mRNA splicing. Nat. Commun. 2018;9:2407. doi: 10.1038/s41467-018-04779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Paek KY, Kim CS, Park SM, Kim JH, Jang SK. RNA-binding protein hnRNP D modulates internal ribosome entry site-dependent translation of hepatitis C virus RNA. J. Virol. 2008;82:12082–12093. doi: 10.1128/JVI.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Bokinsky G, et al. Two distinct binding modes of a protein cofactor with its target RNA. J. Mol. Biol. 2006;361:771–784. doi: 10.1016/j.jmb.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Williams MC, et al. Mechanism for nucleic acid chaperone activity of HIV-1 nucleocapsid protein revealed by single molecule stretching. Proc. Natl Acad. Sci. USA. 2001;98:6121–6126. doi: 10.1073/pnas.101033198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Lin J-Y, et al. Heterogeneous nuclear ribonuclear protein K interacts with the enterovirus 71 5′ untranslated region and participates in virus replication. J. Gen. Virol. 2008;89:2540–2549. doi: 10.1099/vir.0.2008/003673-0. [DOI] [PubMed] [Google Scholar]
- 456.Florez PM, Sessions OM, Wagner EJ, Gromeier M, Garcia-Blanco MA. The polypyrimidine tract binding protein is required for efficient picornavirus gene expression and propagation. J. Virol. 2005;79:6172–6179. doi: 10.1128/JVI.79.10.6172-6179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Karakasiliotis I, Chaudhry Y, Roberts LO, Goodfellow IG. Feline calicivirus replication: requirement for polypyrimidine tract-binding protein is temperature-dependent. J. Gen. Virol. 2006;87:3339–3347. doi: 10.1099/vir.0.82153-0. [DOI] [PubMed] [Google Scholar]
- 458.Anderson EC, Hunt SL, Jackson RJ. Internal initiation of translation from the human rhinovirus-2 internal ribosome entry site requires the binding of Unr to two distinct sites on the 5′ untranslated region. J. Gen. Virol. 2007;88:3043–3052. doi: 10.1099/vir.0.82463-0. [DOI] [PubMed] [Google Scholar]
- 459.Song Y, et al. Evidence for an RNA chaperone function of polypyrimidine tract-binding protein in picornavirus translation. RNA. 2005;11:1809–1824. doi: 10.1261/rna.7430405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Naushad Ali AS. Interaction of polypyrimidine tract-binding protein with the 5’ noncoding region of the Hepatitis C virus RNA genome and its functional requirement in internal initiation of translation. J. Virol. 1995;69:6367–6375. doi: 10.1128/jvi.69.10.6367-6375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Hwang B, Lim JH, Hahm B, Jang SK, Lee S-W. hnRNP L is required for the translation mediated by HCV IRES. Biochem. Biophys. Res. Commun. 2009;378:584–588. doi: 10.1016/j.bbrc.2008.11.091. [DOI] [PubMed] [Google Scholar]
- 462.Hahm B, Kim YK, Kim JH, Kim TY, Jang SK. Heterogeneous Nuclear Ribonucleoprotein L interacts with the 3 border of the internal ribosomal entry site of Hepatitis C virus. J. Virol. 1998;72:8782–8788. doi: 10.1128/jvi.72.11.8782-8788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Li Y, Masaki T, Shimakami T, Lemon SM. hnRNP L and NF90 interact with hepatitis C virus 5′-terminal untranslated RNA and promote efficient replication. J. Virol. 2014;88:7199–7209. doi: 10.1128/JVI.00225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Fan B, et al. A human proteome microarray identifies that the heterogeneous nuclear ribonucleoprotein K (hnRNP K) recognizes the 5′ terminal sequence of the Hepatitis C virus RNA. Mol. Cell. Proteom. 2014;13:84–92. doi: 10.1074/mcp.M113.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Fan B, et al. Heterogeneous ribonucleoprotein K (hnRNP K) binds miR-122, a mature liver-specific microRNA required for Hepatitis C virus replication. Mol Cell Proteomics. 2015;14:2878–2886. doi: 10.1074/mcp.M115.050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of Hepatitis C virus RNA abundance by a liver-specific microRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 467.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 468.Jopling CL, Schutz S, Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe. 2008;4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Hsieh TY, et al. Hepatitis C virus core protein interacts with heterogeneous nuclear ribonucleoprotein K. J. Bio Chem. 1998;273:17651–17659. doi: 10.1074/jbc.273.28.17651. [DOI] [PubMed] [Google Scholar]
- 470.Kim CS, Seol SK, Song O-K, Park JH, Jang SK. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J. Virol. 2007;81:3852–3865. doi: 10.1128/JVI.01311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Lin J-Y, et al. hnRNP A1 interacts with the 5’ untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication. J. Virol. 2009;83:6106–6114. doi: 10.1128/JVI.02476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Zhang W, et al. Apigenin inhibits enterovirus-71 infection by disrupting viral RNA association with trans-acting factors. PLoS ONE. 2014;9:e110429. doi: 10.1371/journal.pone.0110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Leong SY, Ong BKT, Chu JJH. The role of Misshapen NCK-related kinase (MINK), a novel Ste20 family kinase, in the IRES-mediated protein translation of human enterovirus 71. PLoS Pathog. 2015;11:e1004686. doi: 10.1371/journal.ppat.1004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Jagdeo JM, et al. N-terminomics TAILS identifies host cell substrates of poliovirus and Coxsackievirus B3 3C proteinases that modulate virus infection. J. Virol. 2018;92:e02211–e02217. doi: 10.1128/JVI.02211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Jagdeo JM, et al. Heterogeneous nuclear ribonucleoprotein M facilitates enterovirus infection. J. Virol. 2015;89:7064–7078. doi: 10.1128/JVI.02977-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Brunetti JE, Scolaro LA, Castilla V. The heterogeneous nuclear ribonucleoprotein K (hnRNP K) is a host factor required for dengue virus and Junín virus multiplication. Virus Res. 2015;203:84–91. doi: 10.1016/j.virusres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 477.Chang C-J, et al. The heterogeneous nuclear ribonucleoprotein K (hnRNP K) interacts with dengue virus core protein. DNA Cell Biol. 2001;20:569–577. doi: 10.1089/104454901317094981. [DOI] [PubMed] [Google Scholar]
- 478.Miau L-H, Chang C-J, Shen B-J, Tsai W-H, Lee S-C. Identification of Heterogeneous Nuclear Ribonucleoprotein K (hnRNP K) as a Repressor of C/EBP-mediated Gene Activation. J. Biol. Chem. 1998;273:10784–10791. doi: 10.1074/jbc.273.17.10784. [DOI] [PubMed] [Google Scholar]
- 479.Dechtawewat T, et al. Role of human heterogeneous nuclear ribonucleoprotein C1/C2 in dengue virus replication. Virol. J. 2015;12:14. doi: 10.1186/s12985-014-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Kanlaya R, Pattanakitsakul S-n, Sinchaikul S, Chen S-T, Thongboonkerd V. Vimentin interacts with heterogeneous nuclear ribonucleoproteins and dengue nonstructural protein 1 and is important for viral replication and release. Mol. Biosyst. 2010;6:795. doi: 10.1039/b923864f. [DOI] [PubMed] [Google Scholar]
- 481.Noisakran S, et al. Identification of human hnRNP C1/C2 as a dengue virus NS1-interacting protein. Biochem. Biophys. Res. Commun. 2008;372:67–72. doi: 10.1016/j.bbrc.2008.04.165. [DOI] [PubMed] [Google Scholar]
- 482.Dinh PX, Das A, Franco R, Pattnaik AK. Heterogeneous nuclear ribonucleoprotein K supports vesicular stomatitis virus replication by regulating cell survival and cellular gene expression. J. Virol. 2013;87:10059–10069. doi: 10.1128/JVI.01257-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Hino K, et al. Downregulation of Nipah virus N mRNA occurs through interaction between its 3′ untranslated region and hnRNP D. J. Virol. 2013;87:6582–6588. doi: 10.1128/JVI.02495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Wang Y, Zhou J, Du Y. hnRNP A2/B1 interacts with influenza A viral protein NS1 and inhibits virus replication potentially through suppressing NS1 RNA/protein levels and NS1 mRNA nuclear export. Virology. 2014;449:53–61. doi: 10.1016/j.virol.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Wilusz JE, Beemon KL. The negative regulator of splicing element of Rous sarcoma virus promotes polyadenylation. J. Virol. 2006;80:9634–9640. doi: 10.1128/JVI.00845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Fogel BL, McNally MT. A cellular protein, hnRNP H, binds to the negative regulator of splicing element from Rous sarcoma virus. J. Biol. Chem. 2000;275:32371–32378. doi: 10.1074/jbc.M005000200. [DOI] [PubMed] [Google Scholar]
- 487.Fogel BL, McNally LM, McNally MT. Efficient polyadenylation of Rous sarcoma virus RNA requires the negative regulator of splicing element. Nucleic Acids Res. 2002;30:810–817. doi: 10.1093/nar/30.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Kagele D, Rossetto CC, Elorza M, Pari GS. Analysis of the interactions of viral and cellular factors with human cytomegalovirus lytic origin of replication. Virol. 2011;424:106–114. doi: 10.1016/j.virol.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Wang X, et al. Viral DNA replication orientation and hnRNPs regulate transcription of the human papillomavirus 18 late promoter. mBio. 2017;8:e00713–e00717. doi: 10.1128/mBio.00713-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Ng LFP, et al. Host heterogeneous ribonucleoprotein K (hnRNP K) as a potential target to suppress Hepatitis B virus replication. PLoS Med. 2005;2:e163. doi: 10.1371/journal.pmed.0020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Zhang W, et al. Cytidine deaminase APOBEC3B interacts with heterogeneous nuclear ribonucleoprotein K and suppresses hepatitis B virus expression. Cell. Microbiol. 2007;10:112–121. doi: 10.1111/j.1462-5822.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 492.Wadd S, et al. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Bio Chem. 1999;274:28991–28998. doi: 10.1074/jbc.274.41.28991. [DOI] [PubMed] [Google Scholar]
- 493.Koffa MD, Kean J, Zachos G, Rice SA, Clements JB. CK2 protein kinase is stimulated and redistributed by functional herpes simplex virus ICP27 protein. J. Virol. 2003;77:4315–4325. doi: 10.1128/JVI.77.7.4315-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Jenkins HL, Spencer CA. RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells. J. Virol. 2001;75:9872–9884. doi: 10.1128/JVI.75.20.9872-9884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Zhou C, Knipe DM. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 2002;76:5893–5904. doi: 10.1128/JVI.76.12.5893-5904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Jean S, LeVan KM, Song B, Levine M, Knipe DM. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (gamma 2) genes in infected cells. Virology. 2001;283:273–284. doi: 10.1006/viro.2001.0902. [DOI] [PubMed] [Google Scholar]
- 497.Zheng Z-M, Baker CC. Papillomavirus Genome Structure, Expression, and Post-Transcriptional Regulation. Front. Biosci. 2006;11:2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Billakanti, S. R., Calef, C. E., Farmer, A. D., Halpern, A. L. & Myers, G. L. Human papillomaviruses: a compilation and analysis of nucleic acid and amino acid sequences. Theoretical Biology and Biophysics Group T-10, Los Alamos National Laboratory (1994).
- 499.Oberg D, Fay J, Lambkin H, Schwartz S. A downstream polyadenylation element in human papillomavirus type 16 L2 encodes multiple GGG motifs and interacts with hnRNP H. J. Virol. 2005;79:9254–9269. doi: 10.1128/JVI.79.14.9254-9269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Zheng Z-Z, et al. Specific interaction between hnRNP H and HPV16 L1 proteins: Implications for late gene auto-regulation enabling rapid viral capsid protein production. Biochem. Biophys. Res. Commun. 2013;430:1047–1053. doi: 10.1016/j.bbrc.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 501.Key, S. C., Yoshizaki, T., & Pagano, J. S. The Epstein-Barr Virus (EBV) SM protein enhances Pre-mRNA processing of the EBV DNA polymerase transcript. J. Virol. 72, 8485–8492 (1998) [DOI] [PMC free article] [PubMed]
- 502.Cheunim T, Zhang J, Milligan SG, McPhillips MG, Graham SV. The alternative splicing factor hnRNP A1 is up-regulated during virus-infected epithelial cell differentiation and binds the human papillomavirus type 16 late regulatory element. Virus Res. 2008;131:189–198. doi: 10.1016/j.virusres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Zhao X, et al. A 57-nucleotide upstream early polyadenylation element in human papillomavirus type 16 interacts with hFip1, CstF-64, hnRNP C1/C2, and polypyrimidine tract binding protein. J. Virol. 2005;79:4270–4288. doi: 10.1128/JVI.79.7.4270-4288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Zhao X, Rush M, Schwartz S. Identification of an hnRNP A1-dependent splicing silencer in the human papillomavirus type 16 L1 coding region that prevents premature expression of the late L1 gene. J. Virol. 2004;78:10888–10905. doi: 10.1128/JVI.78.20.10888-10905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Zhao X, Schwartz S. Inhibition of HPV-16 L1 expression from L1 cDNAs correlates with the presence of hnRNP A1 binding sites in the L1 coding region. Virus Genes. 2008;36:45–53. doi: 10.1007/s11262-007-0174-0. [DOI] [PubMed] [Google Scholar]
- 506.Zhao X, Fay J, Lambkin H, Schwartz S. Identification of a 17-nucleotide splicing enhancer in HPV-16 L1 that counteracts the effect of multiple hnRNP A1-binding splicing silencers. Virology. 2007;369:351–363. doi: 10.1016/j.virol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 507.Somberg M, Zhao X, Fröhlich M, Evander M, Schwartz S. Polypyrimidine tract binding protein induces human papillomavirus type 16 late gene expression by interfering with splicing inhibitory elements at the major late 5’ splice site, SD3632. J. Virol. 2008;82:3665–3678. doi: 10.1128/JVI.02140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Collier B, Goobar-Larsson L, Sokolowski M, Schwartz S. Translational inhibition in vitro of human papillomavirus type 16 L2 mRNA mediated through interaction with heterogenous ribonucleoprotein K and poly(rC)-binding proteins 1 and 2. J. Bio Chem. 1998;273:22648–22656. doi: 10.1074/jbc.273.35.22648. [DOI] [PubMed] [Google Scholar]
- 509.Ostareck-Lederer A, et al. c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol. Cell. Biol. 2002;22:4535–4543. doi: 10.1128/MCB.22.13.4535-4543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Fuentes-Pananá EM, Peng R, Brewer G, Tan J, Ling PD. Regulation of the Epstein-Barr virus C promoter by AUF1 and the cyclic AMP/protein kinase A signaling pathway. J. Virol. 2000;74:8166–8175. doi: 10.1128/jvi.74.17.8166-8175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Lee N, Pimienta G, Steitz JA. AUF1/hnRNP D is a novel protein partner of the EBER1 noncoding RNA of Epstein-Barr virus. RNA. 2012;18:2073–2082. doi: 10.1261/rna.034900.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Wang L, Wen M, Cao X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. 2019;365:eaav0758. doi: 10.1126/science.aav0758. [DOI] [PubMed] [Google Scholar]
- 513.Cartegni L, et al. hnRNP A1 selectively interacts through its Gly-rich domain with different RNA-binding proteins. J. Mol. Biol. 1996;259:337–348. doi: 10.1006/jmbi.1996.0324. [DOI] [PubMed] [Google Scholar]
- 514.Wolf D, et al. HIV Nef enhances tat-mediated viral transcription through a hnRNP-K-nucleated signaling complex. Cell Host Microbe. 2008;4:398–408. doi: 10.1016/j.chom.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 515.Okunola HL, Krainer AR. Cooperative-binding and splicing-repressive properties of hnRNP A1. Mol. Cell. Biol. 2009;29:5620–5631. doi: 10.1128/MCB.01678-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Lévesque K, et al. Trafficking of HIV‐1 RNA is mediated by heterogeneous nuclear ribonucleoprotein A2 expression and impacts on viral assembly. Traffic. 2006;7:1177–1193. doi: 10.1111/j.1600-0854.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 517.Percipalle P, Raju CS, Fukuda N. Actin-associated hnRNP proteins as transacting factors in the control of mRNA transport and localization. RNA Biol. 2009;6:171–174. doi: 10.4161/rna.6.2.8195. [DOI] [PubMed] [Google Scholar]
- 518.Deacon NJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 519.Wolf D, et al. Novel (n)PKC kinases phosphorylate Nef for increased HIV transcription, replication and perinuclear targeting. Virology. 2008;370:45–54. doi: 10.1016/j.virol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 520.Stefan Schawartz BarbaraK, Felber DonnaM, Benko Eva-MariaFenyo, Pavlakis GN. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J. Virol. 1990;64:2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Purcell DF, Martin MA. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J. Virol. 1993;67:6365–6378. doi: 10.1128/jvi.67.11.6365-6378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Michael P, et al. A naturally arising mutation of a potential silencer of exon splicing in human immunodeficiency virus type 1 induces dominant aberrant splicing and arrests virus production. J. Virol. 1997;71:8542–8551. doi: 10.1128/jvi.71.11.8542-8551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Berget SM. Exon recognition in vertebrate splicing. J. Biol. Chem. 1995;270:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 524.Moore MJ. Intron recognition comes of AGe. Nat. Struct. Biol. 2000;7:14–16. doi: 10.1038/71207. [DOI] [PubMed] [Google Scholar]
- 525.Zamore PD, Green MR. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc. Natl Acad. Sci. USA. 1989;86:9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Valcarcel J, Gaur RK, Singh R, Green MR. Interaction of U2AF65 RS region with pre-mRNA of branch point and promotion base pairing with U2 snRNA. Science. 1996;273:1706–1709. doi: 10.1126/science.273.5282.1706. [DOI] [PubMed] [Google Scholar]
- 527.Gozani O, Potasgkin J, Reed R. A potential role for U2AF-SAP 155 Interactions in recruiting U2 snRNP to the branch site. Mol. Cell. Biol. 1998;18:4752–4760. doi: 10.1128/mcb.18.8.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Krecic AM, Swanson MS. hnRNP complexes: composition, structure, and function. Curr. Opin. Cell Biol. 1999;11:363–371. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 530.Smith CWJ, Valcárcel J. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biol. Sci. 2000;25:381–388. doi: 10.1016/s0968-0004(00)01604-2. [DOI] [PubMed] [Google Scholar]
- 531.Erkelenz S, et al. Balanced splicing at the Tat-specific HIV-1 3’ss A3 is critical for HIV-1 replication. Retrovirology. 2015;12:29. doi: 10.1186/s12977-015-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Si Z, Amendt BA, Stoltzfus CM. Splicing efficiency of human immunodeficiency virus type 1 tat RNA is determined by both a suboptimal 3’ splice site and a 10 nucleotide exon splicing silencer element located within tat exon 2. Nucleic Acids Res. 1997;25:861–867. doi: 10.1093/nar/25.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Staffa A, Cochrane. A. The tat/rev intron of human immunodeficiency virus type 1 is inefficiently spliced because of suboptimal signals in the 3′ splice site. J. Virol. 1994;68:3071–3079. doi: 10.1128/jvi.68.5.3071-3079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Sertznig H, Hillebrand F, Erkelenz S, Schaal H, Widera M. Behind the scenes of HIV-1 replication: Alternative splicing as the dependency factor on the quiet. Virology. 2018;516:176–188. doi: 10.1016/j.virol.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 535.Madsen JM, Stoltzfus CM. An exonic splicing silencer downstream of the 3’ splice site A2 is required for efficient human immunodeficiency virus type 1 replication. J. Virol. 2005;79:10478–10486. doi: 10.1128/JVI.79.16.10478-10486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Hallay H, et al. Biochemical and NMR study on the competition between proteins SC35, SRp40, and heterogeneous nuclear ribonucleoprotein A1 at the HIV-1 Tat exon 2 splicing site. J. Biol. Chem. 2006;281:37159–37174. doi: 10.1074/jbc.M603864200. [DOI] [PubMed] [Google Scholar]
- 537.Marchand V, et al. RNA Biology Identification of protein partners of the human immunodeficiency virus 1 tat/rev exon 3 leads to the discovery of a new HIV-1 splicing regulator, protein hnRNP K. RNA Biol. 2011;325:325–342. doi: 10.4161/rna.8.2.13984. [DOI] [PubMed] [Google Scholar]
- 538.Jacquenet S, et al. A second exon splicing silencer within human immunodeficiency virus type 1 tat exon 2 represses splicing of Tat mRNA and binds protein hnRNP H. J. Biol. Chem. 2001;276:40464–40475. doi: 10.1074/jbc.M104070200. [DOI] [PubMed] [Google Scholar]
- 539.Asai K, Platt C, Cochrane A. Control of HIV-1 env RNA splicing and transport: investigating the role of hnRNP A1 in exon splicing silencer (ESS3a) function. Virology. 2003;314:229–242. doi: 10.1016/s0042-6822(03)00400-8. [DOI] [PubMed] [Google Scholar]
- 540.Tange TO, Damgaard CK, Guth S, Valcárcel J, Kjems J. The hnRNP A1 protein regulates HIV-1 tat splicing via a novel intron silencer element. EMBO J. 2001;20:5748–5758. doi: 10.1093/emboj/20.20.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Damgaard CK, Tange TS, Kjems J. hnRNP A1 controls HIV-1 mRNA splicing through cooperative binding to intron and exon splicing silencers in the context of a conserved secondary structure. RNA. 2002;8:1401–1415. doi: 10.1017/s1355838202023075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Jain N, Morgan CE, Rife BD, Salemi M, Tolbert BS. Solution structure of the HIV-1 intron splicing silencer and its interactions with the UP1 domain of heterogeneous nuclear ribonucleoprotein (hnRNP) A1. J. Bio Chem. 2016;291:2331–2344. doi: 10.1074/jbc.M115.674564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Jean-Philippe J, Paz S, Lu ML, Caputi M. A truncated hnRNP A1 isoform, lacking the RGG-box RNA binding domain, can efficiently regulate HIV-1 splicing and replication. Biochim. Biophys. Acta. 2014;1839:251–258. doi: 10.1016/j.bbagrm.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Zahler AM, Damgaard CK, Kjems J, Caputi M. SC35 and heterogeneous nuclear ribonucleoprotein A/B proteins bind to a juxtaposed exonic splicing enhancer/exonic splicing silencer element to regulate HIV-1 tat exon 2 splicing. J. Bio Chem. 2004;279:10077–10084. doi: 10.1074/jbc.M312743200. [DOI] [PubMed] [Google Scholar]
- 545.Del Gatto-Konczak F, Olive M, Gesnel MC, Breathnach R. hnRNP A1 recruited to an exon in vivo can function as an exon splicing silencer. Mol. Cell. Biol. 1999;19:251–260. doi: 10.1128/mcb.19.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Marchand V, et al. A janus splicing regulatory element modulates HIV-1 tat and rev mRNA production by coordination of hnRNP A1 cooperative binding. J. Mol. Biol. 2002;323:629–652. doi: 10.1016/s0022-2836(02)00967-1. [DOI] [PubMed] [Google Scholar]
- 547.Bilodeau PS, et al. RNA splicing at human immunodeficiency virus type 1 3’ splice site A2 is regulated by binding of hnRNP A/B proteins to an exonic splicing silencer element. J. Virol. 2001;75:8487–8497. doi: 10.1128/JVI.75.18.8487-8497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Hillebrand F, et al. Differential hnRNP D isoform incorporation may confer plasticity to the ESSV-mediated repressive state across HIV-1 exon 3. Biochim. Biophys. Acta. 2017;1860:205–217. doi: 10.1016/j.bbagrm.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 549.Domsic JK, Wang Y, Mayeda A, Krainer AR, Stoltzfus CM. Human immunodeficiency virus type 1 hnRNP A/B-dependent exonic splicing silencer ESSV antagonizes binding of U2AF65 to viral polypyrimidine tracts. Mol. Cell. Biol. 2003;23:8762–8772. doi: 10.1128/MCB.23.23.8762-8772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Massimo C, Alan MZ. SR proteins and hnRNP H regulate the splicing of the HIV tev-specific exon 6D. EMBO J. 2002;21:845–855. doi: 10.1093/emboj/21.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Jablonski JA, Buratti E, Stuani C, Caputi M. The secondary structure of the human immunodeficiency virus type 1 transcript modulates viral splicing and infectivity. J. Virol. 2008;82:8038–8050. doi: 10.1128/JVI.00721-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Schaub MC, Lopez SR, Caputi M. Members of the heterogeneous nuclear ribonucleoprotein H family activate splicing of an HIV-1 splicing substrate by promoting formation of ATP-dependent spliceosomal complexes. J. Bio Chem. 2007;282:13617–13626. doi: 10.1074/jbc.M700774200. [DOI] [PubMed] [Google Scholar]
- 553.Mouland AJ, et al. RNA trafficking signals in human immunodeficiency virus type 1. Mol. Cell. Biol. 2001;21:2133–2143. doi: 10.1128/MCB.21.6.2133-2143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Lévesque K, et al. Trafficking of HIV-1 RNA is mediated by heterogeneous nuclear ribonucleoprotein A2 expression and impacts on viral assembly. Traffic. 2006;7:1177–1193. doi: 10.1111/j.1600-0854.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 555.Bériault V, et al. A late role for the association of hnRNP A2 with the HIV-1 hnRNP A2 response elements in genomic RNA, Gag, and Vpr localization. J. Bio Chem. 2004;279:44141–44153. doi: 10.1074/jbc.M404691200. [DOI] [PubMed] [Google Scholar]
- 556.Lund N, et al. Differential effects of hnRNP D/AUF1 isoforms on HIV-1 gene expression. Nucleic Acids Res. 2001;40:3663–3675. doi: 10.1093/nar/gkr1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Gordon H, et al. Depletion of hnRNP A2/B1 overrides the nuclear retention of the HIV-1 genomic RNA. RNA Biol. 2013;10:1714–1725. doi: 10.4161/rna.26542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Suh D, et al. Mapping of determinants required for the function of the HIV-1 env nuclear retention sequence. Virology. 2003;310:85–99. doi: 10.1016/s0042-6822(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 559.Valente ST, Goff SP. Inhibition of HIV-1 Gene Expression by a Fragment of hnRNP U. Mol. Cell. 2006;23:597–605. doi: 10.1016/j.molcel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 560.Cullen BR. Nuclear mRNA export: insights from virology. Trends Biochem. Sci. 2003;28:419–424. doi: 10.1016/S0968-0004(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 561.Malik P, Clements JB. Protein kinase CK2 phosphorylation regulates the interaction of Kaposi’s sarcoma-associated herpesvirus regulatory protein ORF57 with its multifunctional partner hnRNP K. Nucleic Acids Res. 2004;32:5553–5569. doi: 10.1093/nar/gkh876. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 562.Dodon MD, Hamaia S, Martin J, Gazzolo L. Heterogeneous nuclear ribonucleoprotein A1 interferes with the binding of the human T cell leukemia virus type 1 rex regulatory protein to its response element. J. Bio Chem. 2002;277:18744–18752. doi: 10.1074/jbc.M109087200. [DOI] [PubMed] [Google Scholar]
- 563.Monette A, Ajamian L, López-Lastra M, Mouland AJ. Human immunodeficiency virus type 1 (HIV-1) induces the cytoplasmic retention of heterogeneous nuclear ribonucleoprotein A1 by disrupting nuclear import: implications for HIV-1 gene expression. J. Bio Chem. 2009;284:31350–31362. doi: 10.1074/jbc.M109.048736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Bieleski L, Hindley C, Talbot SJ. A polypyrimidine tract facilitates the expression of Kaposi’s sarcoma-associated herpesvirus vFLIP through an internal ribosome entry site. J. Gen. Virol. 2004;85:615–620. doi: 10.1099/vir.0.19733-0. [DOI] [PubMed] [Google Scholar]
- 565.Meng L, Hunt C, Yaglom JA, Gabai VL, Sherman MY. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene. 2011;30:2836. doi: 10.1038/onc.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.McConnell KW, et al. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J. Immunol. 2011;186:3718–3725. doi: 10.4049/jimmunol.1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Rérole A-L, et al. Peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res. 2011;71:484–495. doi: 10.1158/0008-5472.CAN-10-1443. [DOI] [PubMed] [Google Scholar]
- 568.Wang AM, et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat. Chem. Biol. 2013;9:112. doi: 10.1038/nchembio.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Bagheri JP, Underwood AC, Walro DG. Monitering heat shock protein 70 and heat shock protein 90 during Herpes Simplex virus type 1 infection. Ohio J. Sci. 2018;118:A46–A46. [Google Scholar]
- 570.Oh W-k, Song J. Hsp70 functions as a negative regulator of West Nile virus capsid protein through direct interaction. Biochem. Biophys. Res. Commun. 2006;347:994–1000. doi: 10.1016/j.bbrc.2006.06.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Nanda SK, Johnson RF, Liu Q, Leibowitz JL. Mitochondrial HSP70, HSP40, and HSP60 bind to the 3′ untranslated region of the Murine hepatitis virus genome. Arch. Virol. 2004;149:93–111. doi: 10.1007/s00705-003-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Fong JJ, et al. Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec-5 and Siglec-14. EMBO J. 2015;34:2775–2788. doi: 10.15252/embj.201591407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Pockley AG, Henderson B, Multhoff G. Extracellular cell stress proteins as biomarkers of human disease. Biochem. Soc. Trans. 2014;42:1744–1751. doi: 10.1042/BST20140205. [DOI] [PubMed] [Google Scholar]
- 574.Henderson B, Pockley AG. Molecular chaperones and protein-folding catalysts as intercellular signaling regulators in immunity and inflammation. J. Leukoc. Biol. 2010;88:445–462. doi: 10.1189/jlb.1209779. [DOI] [PubMed] [Google Scholar]
- 575.Salimu J, et al. Cross-presentation of the oncofetal tumor antigen 5T4 from irradiated prostate cancer cells–a key role for heat-shock protein 70 and receptor CD91. Cancer Immunol. Res. 2015;3:678–688. doi: 10.1158/2326-6066.CIR-14-0079. [DOI] [PubMed] [Google Scholar]
- 576.Zhu H, et al. Membrane-bound heat shock proteins facilitate the uptake of dying cells and cross-presentation of cellular antigen. Apoptosis. 2016;21:96–109. doi: 10.1007/s10495-015-1187-0. [DOI] [PubMed] [Google Scholar]
- 577.Chen T, Cao X. Stress for maintaining memory: HSP70 as a mobile messenger for innate and adaptive immunity. Eur. J. Immunol. 2010;40:1541–1544. doi: 10.1002/eji.201040616. [DOI] [PubMed] [Google Scholar]
- 578.Wan T, et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103:1747–1754. doi: 10.1182/blood-2003-08-2828. [DOI] [PubMed] [Google Scholar]
- 579.Kaur, P. & Asea, A. A. A. in Chaperokine Activity of Heat Shock Proteins (eds Alexzander A. A. Asea & Punit Kaur), 3–22 (Springer International Publishing, 2019).
- 580.Vabulas RM, et al. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 581.Bulut Y, et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J. Immo. 2002;168:1435–1440. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- 582.Kakumani PK, et al. Association of HADHA with human RNA silencing machinery. Biochem. Biophys. Res. Commun. 2015;466:481–485. doi: 10.1016/j.bbrc.2015.09.055. [DOI] [PubMed] [Google Scholar]
- 583.Peña J, Harris E. Dengue virus modulates the unfolded protein response in a time-dependent manner. J. Bio Chem. 2011;286:14226–14236. doi: 10.1074/jbc.M111.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Ye J, et al. Heat shock protein 70 is associated with replicase complex of Japanese encephalitis virus and positively regulates viral genome replication. PLoS ONE. 2013;8:e75188–e75188. doi: 10.1371/journal.pone.0075188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Sharma M, et al. Japanese encephalitis virus activates autophagy through XBP1 and ATF6 ER stress sensors in neuronal cells. J. Gen. Virol. 2017;98:1027–1039. doi: 10.1099/jgv.0.000792. [DOI] [PubMed] [Google Scholar]
- 586.Fu Q, et al. Bovine viral diarrhea virus infection induces autophagy in MDBK cells. J. Microbiol. 2014;52:619–625. doi: 10.1007/s12275-014-3479-4. [DOI] [PubMed] [Google Scholar]
- 587.Li S, Kong L, Yu X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit. Rev. Microbiol. 2015;41:150–164. doi: 10.3109/1040841X.2013.813899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Fung T, Torres J, Liu D. The emerging roles of viroporins in ER stress response and autophagy induction during virus infection. Viruses. 2015;7:2834–2857. doi: 10.3390/v7062749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Blázquez A-B, Escribano-Romero E, Merino-Ramos T, Saiz J-C, Martín-Acebes MA. Stress responses in flavivirus-infected cells: activation of unfolded protein response and autophagy. Front. Microbiol. 2014;5:266. doi: 10.3389/fmicb.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Tardif KD, Mori K, Kaufman RJ, Siddiqui A. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J. Biol. Chem. 2004;279:17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 591.Ma Y, et al. Glucose-regulated protein 78 is an intracellular antiviral factor against hepatitis B virus. Mol. Cell. Proteom. 2009;8:2582–2594. doi: 10.1074/mcp.M900180-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Li B, et al. Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res. 2007;124:44–49. doi: 10.1016/j.virusres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 593.Cheng G, Feng Z, He B. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2alpha dephosphorylation by the gamma(1)34.5 protein. J. Virol. 2005;79:1379–1388. doi: 10.1128/JVI.79.3.1379-1388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Ron D, Hampton RY. Membrane biogenesis and the unfolded protein response. J. Cell Biol. 2004;167:23–25. doi: 10.1083/jcb.200408117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Galindo I, et al. The ATF6 branch of unfolded protein response and apoptosis are activated to promote African swine fever virus infection. Cell Death Dis. 2012;3:e341. doi: 10.1038/cddis.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Pasqual G, Burri DJ, Pasquato A, de la Torre JC, Kunz S. Role of the host cell’s unfolded protein response in arenavirus infection. J. Virol. 2011;85:1662–1670. doi: 10.1128/JVI.01782-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Limjindaporn T, et al. Interaction of dengue virus envelope protein with endoplasmic reticulum-resident chaperones facilitates dengue virus production. Biochem. Biophys. Res. Commun. 2009;379:196–200. doi: 10.1016/j.bbrc.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 598.Buchkovich NJ, et al. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J. Virol. 2008;82:31–39. doi: 10.1128/JVI.01881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Su Y-C, Wu J-L, Hong J-R. Betanodavirus up-regulates chaperone GRP78 via ER stress: roles of GRP78 in viral replication and host mitochondria-mediated cell death. Apoptosis. 2011;16:272–287. doi: 10.1007/s10495-010-0565-x. [DOI] [PubMed] [Google Scholar]
- 600.Hassan IH, et al. Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 2012;287:4679–4689. doi: 10.1074/jbc.M111.284695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Ambrose RL, Mackenzie JM. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion. J. Virol. 2011;85:2723–2732. doi: 10.1128/JVI.02050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Baltzis D, et al. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 2004;78:12747–12761. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Lazar C, Macovei A, Petrescu S, Branza-Nichita N. Activation of ERAD pathway by human hepatitis B virus modulates viral and subviral particle production. PLoS ONE. 2012;7:e34169. doi: 10.1371/journal.pone.0034169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Li S, et al. Hepatitis C virus NS4B induces unfolded protein response and endoplasmic reticulum overload response-dependent NF-κB activation. Virology. 2009;391:257–264. doi: 10.1016/j.virol.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 605.Minakshi R, et al. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS ONE. 2009;4:e8342. doi: 10.1371/journal.pone.0008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Tirosh B, et al. Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class I major histocompatibility complex products. J. Virol. 2005;79:2768–2779. doi: 10.1128/JVI.79.5.2768-2779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Merquiol E, et al. HCV causes chronic endoplasmic reticulum stress leading to adaptation and interference with the unfolded protein response. PLoS ONE. 2011;6:e24660. doi: 10.1371/journal.pone.0024660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Cho HK, Cheong KJ, Kim HY, Cheong J. Endoplasmic reticulum stress induced by hepatitis B virus X protein enhances cyclo-oxygenase 2 expression via activating transcription factor 4. Biochem. J. 2011;435:431–439. doi: 10.1042/BJ20102071. [DOI] [PubMed] [Google Scholar]
- 609.Han SP, Tang YH, Smith R. Functional diversity of the hnRNPs: past, present and perspectives. Biochem. J. 2010;430:379–392. doi: 10.1042/BJ20100396. [DOI] [PubMed] [Google Scholar]
- 610.Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: focus on hnRNP E1’s multifunctional regulatory roles. RNA. 2010;16:1449–1462. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Tang JW, Holmes CW. Acute and chronic disease caused by enteroviruses. Virulence. 2017;8:1062–1065. doi: 10.1080/21505594.2017.1308620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Tolbert M, et al. HnRNP A1 alters the structure of a conserved enterovirus IRES domain to stimulate viral translation. J. Mol. Biol. 2017;429:2841–2858. doi: 10.1016/j.jmb.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Lin, J.-Y. et al. Heterogeneous nuclear ribonuclear protein K interacts with the enterovirus 71 59 untranslated region and participates in virus replication. J. Gen. Virol.10.1099/vir.0.2008/003673-0 (2019). [DOI] [PubMed]
- 614.Li L, et al. Molecular mechanism of action of hnRNP K and RTN3 in the replication of enterovirus 71. Chin. J. Virol. 2015;31:197–200. [PubMed] [Google Scholar]
- 615.Hill, M. Sherris Medical Microbiology, 6e. 562 edn, 555 (McGraw-Hill Education, Inc., 2014).
- 616.Tzeng N-S, et al. Anti-herpetic medications and reduced risk of dementia in patients with Herpes Simplex virus infections-a nationwide, population-Based cohort study in Taiwan. Neurotherap. 2018;15:417–429. doi: 10.1007/s13311-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Wozniak MA, Frost AL, Preston CM, Itzhaki RF. Antivirals reduce the formation of key Alzheimer’s disease molecules in cell cultures acutely infected with herpes simplex virus type 1. PLoS ONE. 2011;6:e25152. doi: 10.1371/journal.pone.0025152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Schmidt T, Striebinger H, Haas J, Bailer SM. The heterogeneous nuclear ribonucleoprotein K is important for Herpes simplex virus-1 propagation. FEBS Lett. 2010;584:4361–4365. doi: 10.1016/j.febslet.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 619.Hollinger FB, L. T. Hepatitis B virus. in Fields virology. 3036 edn, 2971 (Lippincott Williams & Wilkins, 2001).
- 620.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 621.Oliveri F, Brunetto MR, Actis GC, Bonino F. Pathobiology of chronic hepatitis virus infection and hepatocellular carcinoma (HCC) tal. J. Gastroenterol. Hepatol. 1991;23:498–502. [PubMed] [Google Scholar]
- 622.Taylor JM. Hepatitis delta virus. Virology. 2006;344:71–76. doi: 10.1016/j.virol.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 623.Trepo C, Guillevin L. Polyarteritis nodosa and extrahepatic manifestations of HBV infection: the case against autoimmune intervention in pathogenesis. J. Autoimmun. 2001;16:269–274. doi: 10.1006/jaut.2000.0502. [DOI] [PubMed] [Google Scholar]
- 624.Yau W-Y, et al. Autoantibody recognition of an N-terminal epitope of hnRNP L marks the risk for developing HBV-related hepatocellular carcinoma. J. Proteom. 2013;94:346–358. doi: 10.1016/j.jprot.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 625.Weiss LM, O’Malley D. Benign lymphadenopathies. Mod. Pathol. 2013;26:S88–S96. doi: 10.1038/modpathol.2012.176. [DOI] [PubMed] [Google Scholar]
- 626.Geng L, Wang X. Epstein-Barr Virus-associated lymphoproliferative disorders: experimental and clinical developments. Int. J. Clin. Exp. Med. 2015;8:14656–14671. [PMC free article] [PubMed] [Google Scholar]
- 627.Gandhi MK, Tellam JT, Khanna R. Epstein-Barr virus-associated Hodgkin’s lymphoma. Br. J. Haematol. 2004;125:267–281. doi: 10.1111/j.1365-2141.2004.04902.x. [DOI] [PubMed] [Google Scholar]
- 628.Dogan S, et al. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head. Neck. 2014;36:511–516. doi: 10.1002/hed.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Ascherio A, Munger KL. Epstein-barr virus infection and multiple sclerosis: a review. J. Neuroimmune Pharmacol. 2010;5:271–277. doi: 10.1007/s11481-010-9201-3. [DOI] [PubMed] [Google Scholar]
- 630.Tagliavini E, et al. Lymphomatoid granulomatosis: a practical review for pathologists dealing with this rare pulmonary lymphoproliferative process. Pathologica. 2013;105:111–116. [PubMed] [Google Scholar]
- 631.Di Lernia V, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int. J. Dermatol. 2013;52:1177–1184. doi: 10.1111/j.1365-4632.2012.05855.x. [DOI] [PubMed] [Google Scholar]
- 632.Woulfe J, Hoogendoorn H, Tarnopolsky M, Muñoz DG. Monoclonal antibodies against Epstein-Barr virus cross-react with alpha-synuclein in human brain. Neurology. 2000;55:1398–1401. doi: 10.1212/wnl.55.9.1398. [DOI] [PubMed] [Google Scholar]
- 633.Greer CE, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J. Clin. Microbiol. 1995;33:2058–2063. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 635.Van Dyne EA, et al. Trends in human Papillomavirus–associated cancers—United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018;67:918–924. doi: 10.15585/mmwr.mm6733a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Ault, K. A. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect. Dis. Obstet. Gynecol. 10.1155/IDOG/2006/40470 (2006). [DOI] [PMC free article] [PubMed]
- 637.D’Souza G, et al. Case–control study of human Papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 638.Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa. Clin. Infect. Dis. 2003;36:652–662. doi: 10.1086/367655. [DOI] [PubMed] [Google Scholar]
- 639.Vogel M, et al. The treatment of patients with HIV. Dtsch Arztebl Int. 2010;107:507–515. doi: 10.3238/arztebl.2010.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Taguwa S, et al. Defining Hsp70 subnetworks in dengue virus replication reveals key vulnerability in Flavivirus infection. Cell. 2015;163:1108–1123. doi: 10.1016/j.cell.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Mayer MP, Prodromou C, Frydman J. The Hsp90 mosaic: a picture emerges. Nat. Struct. Mol. Biol. 2009;16:2–6. doi: 10.1038/nsmb0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Röhl A, Rohrberg J, Buchner J. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem. Sci. 2013;38:253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 643.Scheufler C, et al. Structure of TPR domain–peptide complexes: critical elements in the assembly of the Hsp70–Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 644.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93:211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Prodromou C. The ‘active life’ of Hsp90 complexes. Biochim. Biophys. Acta. 2012;1823:614–623. doi: 10.1016/j.bbamcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Chrisostomos P, Laurence HP. Structure and functional relationships of Hsp90. Curr. Cancer Drug Targets. 2003;3:301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- 647.Beran RKF, et al. Cellular growth kinetics distinguish a cyclophilin inhibitor from an HSP90 inhibitor as a selective inhibitor of hepatitis C virus. PLoS ONE. 2012;7:e30286. doi: 10.1371/journal.pone.0030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Alison D, Brian SJB. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide- binding pocket. Curr. Med. Chem. 2008;15:2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.James PG, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds Geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 650.Karkoulis PK, Stravopodis DJ, Margaritis LH, Voutsinas GE. 17-Allylamino-17-demethoxygeldanamycin induces downregulation of critical Hsp90 protein clients and results in cell cycle arrest and apoptosis of human urinary bladder cancer cells. BMC Cancer. 2010;10:481. doi: 10.1186/1471-2407-10-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Hostein I, Robertson D, DiStefano F, Workman P, Andrew Clarke P. Inhibition of signal transduction by the Hsp90 inhibitor 17-Allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003. [PubMed] [Google Scholar]
- 652.Leow CC, et al. Antitumor efficacy of IPI-504, a selective heat shock protein 90 inhibitor against human epidermal growth factor receptor 2-positive human xenograft models as a single agent and in combination with trastuzumab or lapatinib. Mol. Cancer Ther. 2009;8:2131–2141. doi: 10.1158/1535-7163.MCT-08-1038. [DOI] [PubMed] [Google Scholar]
- 653.Eccles SA, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 654.Lundgren K, et al. BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Mol. Cancer Ther. 2009;8:921. doi: 10.1158/1535-7163.MCT-08-0758. [DOI] [PubMed] [Google Scholar]
- 655.Ying W, et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol. Cancer Ther. 2012;11:475. doi: 10.1158/1535-7163.MCT-11-0755. [DOI] [PubMed] [Google Scholar]
- 656.Graham B, et al. The heat shock protein 90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Scie. 2012;103:522–527. doi: 10.1111/j.1349-7006.2011.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Chandarlapaty S, et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase–dependent cancers. Clinl Cancer Res. 2008;14:240. doi: 10.1158/1078-0432.CCR-07-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 659.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 660.Chang D-J, et al. Design, synthesis, and biological evaluation of novel deguelin-based heat shock protein 90 (HSP90) inhibitors targeting proliferation and angiogenesis. J. Med. Chem. 2012;55:10863–10884. doi: 10.1021/jm301488q. [DOI] [PubMed] [Google Scholar]
- 661.Oh SH, et al. Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J. Natl Cancer Inst. 2007;99:949–961. doi: 10.1093/jnci/djm007. [DOI] [PubMed] [Google Scholar]
- 662.Yin Z, Henry EC, Gasiewicz TA. Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry. 2009;48:336–345. doi: 10.1021/bi801637q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Li T, et al. Novel Hsp90 inhibitor platycodin D disrupts Hsp90/Cdc37 complex and enhances the anticancer effect of mTOR inhibitor. Toxicol. Appl. Pharmacol. 2017;330:65–73. doi: 10.1016/j.taap.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 664.Li Y, et al. Sulforaphane inhibits pancreatic cancer through disrupting Hsp90–p50Cdc37 complex and direct interactions with amino acids residues of Hsp90. J. Nutr. Biochem. 2012;23:1617–1626. doi: 10.1016/j.jnutbio.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Yu Y, et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 2010;79:542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Zhang T, et al. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol. Cancer Ther. 2008;7:162. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 667.Li D, et al. Natural product kongensin A is a non-canonical HSP90 inhibitor that blocks RIP3-dependent necroptosis. Cell Chem. Biol. 2016;23:257–266. doi: 10.1016/j.chembiol.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 668.Wang L, et al. Discovery and identification of Cdc37-derived peptides targeting the Hsp90–Cdc37 protein–protein interaction. RSC Adv. 2015;5:96138–96145. [Google Scholar]
- 669.Li T, Jiang H-L, Tong Y-G, Lu J-J. Targeting the Hsp90-Cdc37-client protein interaction to disrupt Hsp90 chaperone machinery. J. Hematol. Oncol. 2018;11:59–59. doi: 10.1186/s13045-018-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Bali P, et al. Activity of suberoylanilide hydroxamic acid against human breast cancer cells with amplification of Her-2. Clin. Cancer Res. 2005;11:6382. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- 671.Chen L, et al. Chemical ablation of androgen receptor in prostate cancer cells by the histone deacetylase inhibitor LAQ824. Mol. Cancer Ther. 2005;4:1311. doi: 10.1158/1535-7163.MCT-04-0287. [DOI] [PubMed] [Google Scholar]
- 672.Yu X, et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 Expression in lung cancer cells by depsipeptide FR901228. J. Natl Cancer Ist. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 673.Chen H, Xia Y, Fang D, Hawke D, Lu Z. Caspase-10-mediated heat shock protein 90β cleavage promotes UVB irradiation-induced cell apoptosis. Mol. Cell. Biol. 2009;29:3657. doi: 10.1128/MCB.01640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Beck R, et al. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem. Pharmacol. 2009;77:375–383. doi: 10.1016/j.bcp.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 675.Park S, et al. Suberoylanilide hydroxamic acid induces ROS-mediated cleavage of HSP90 in leukemia cells. Cell Stress Chaperones. 2015;20:149–157. doi: 10.1007/s12192-014-0533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Park S, Park J-A, Yoo H, Park H-B, Lee Y. Proteasome inhibitor-induced cleavage of HSP90 is mediated by ROS generation and caspase 10-activation in human leukemic cells. Redox Biol. 2017;13:470–476. doi: 10.1016/j.redox.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Maeda Y, et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol., Immunother. 2015;64:1047–1056. doi: 10.1007/s00262-015-1709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Cappello F, et al. Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opin. Ther. Targets. 2014;18:185–208. doi: 10.1517/14728222.2014.856417. [DOI] [PubMed] [Google Scholar]
- 679.Hiroyuki N, Hidemitsu M. HSP60 as a drug target. Curr. Pharm. Des. 2013;19:441–451. [PubMed] [Google Scholar]
- 680.Mizuno K TM, Takada M, Hayashi M, Atsumi K. Studies on bredinin. I. isolation,characterization and biological properties. J. Antibiot. (Tokyo). 1974;27:775–782. doi: 10.7164/antibiotics.27.775. [DOI] [PubMed] [Google Scholar]
- 681.Hideaki Kakeya IT, Okada Gen, Isono Kiyoshi, Osada. Hiroyuki. Epolactaene, a novel neuritogenic compound in human neuroblastoma cells, produced by a marine fungus. J. Antibio. 1995;48:733–735. doi: 10.7164/antibiotics.48.733. [DOI] [PubMed] [Google Scholar]
- 682.Nagumo Y, et al. Structure–activity relationships of epolactaene derivatives: structural requirements for inhibition of Hsp60 chaperone activity. Bioorg. Med. Chem. Lett. 2004;14:4425–4429. doi: 10.1016/j.bmcl.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 683.Nagumo Y, et al. Epolactaene binds human Hsp60 Cys442 resulting in the inhibition of chaperone activity. Biochem. J. 2005;387:835–840. doi: 10.1042/BJ20041355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Nisemblat S, Yaniv O, Parnas A, Frolow F, Azem A. Crystal structure of the human mitochondrial chaperonin symmetrical football complex. Proc. Nat. Aca Sci. 2015;112:6044. doi: 10.1073/pnas.1411718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Ban HS, Shimizu K, Minegishi H, Nakamura H. Identification of HSP60 as a primary target of o-carboranylphenoxyacetanilide, an HIF-1α inhibitor. J. Am. Chem. Soc. 2010;132:11870–11871. doi: 10.1021/ja104739t. [DOI] [PubMed] [Google Scholar]
- 686.Wiechmann K, et al. Mitochondrial chaperonin HSP60 is the apoptosis-related target for myrtucommulone. Cell Chem. Biol. 2017;24:614–623.e616. doi: 10.1016/j.chembiol.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 687.Rotstein A, Lifshitz A, Kashman Y. Isolation and antibacterial activity of acylphloroglucinols from myrtus communis. Antimicrob. Agents Chemother. 1974;6:539. doi: 10.1128/aac.6.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Appendino G, et al. Oligomeric acylphloroglucinols from myrtle (myrtus communis) J. Nat. Prod. 2002;65:334–338. doi: 10.1021/np010441b. [DOI] [PubMed] [Google Scholar]
- 689.Rosa A, et al. Antioxidant activity of oligomeric acylphloroglucinols from Myrtus communis L. Free Radic. Res. 2003;37:1013–1019. doi: 10.1080/10715760310001595739. [DOI] [PubMed] [Google Scholar]
- 690.Rossi A, et al. Myrtucommulone from myrtus communis, exhibits potent anti-inflammatory effectiveness in vivo. J. Pharmacol. Exp. Ther. 2009;329:76. doi: 10.1124/jpet.108.143214. [DOI] [PubMed] [Google Scholar]
- 691.Feißt C, Franke L, Appendino G, Werz O. Identification of molecular targets of the oligomeric nonprenylated acylphloroglucinols from and myrtle (myrtus communis) and their implication as anti-inflammatory compounds. J. Pharmacol. Exp. Ther. 2005;315:389. doi: 10.1124/jpet.105.090720. [DOI] [PubMed] [Google Scholar]
- 692.Tretiakova I, et al. Myrtucommulone from Myrtus communis induces apoptosis in cancer cells via the mitochondrial pathway involving caspase-9. Apoptosis. 2008;13:119–131. doi: 10.1007/s10495-007-0150-0. [DOI] [PubMed] [Google Scholar]
- 693.Cindy G, et al. Dual induction of mitochondrial apoptosis and senescence in chronic myelogenous leukemia by myrtucommulone A. Anticancer Agents Med. Chem. 2015;15:363–373. doi: 10.2174/1871520614666141202143757. [DOI] [PubMed] [Google Scholar]
- 694.Qian-Cutrone J, et al. Stephacidin A and B: two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]
- 695.Fenical, W., Jensen, P. R., and Cheng, X. C. Avrainvillamide, a cytotoxic marine natural product, and derivatives thereof. Patent US6066635. (2000).
- 696.Wulff JE, Herzon SB, Siegrist R, Myers AG. Evidence for the rapid conversion of stephacidin B into the electrophilic monomer avrainvillamide in cell culture. J. Am. Chem. Soc. 2007;129:4898–4899. doi: 10.1021/ja0690971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Shimizu K, et al. Boron-containing phenoxyacetanilide derivatives as hypoxia-inducible factor (HIF)-1α inhibitors. Bioorg. Med. Chem. Lett. 2010;20:1453–1456. doi: 10.1016/j.bmcl.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 698.Hu D, et al. Anticancer Gold(III) porphyrins target mitochondrial chaperone Hsp60. Angew. Chem. Int. Ed. Engl. 2016;55:1387–1391. doi: 10.1002/anie.201509612. [DOI] [PubMed] [Google Scholar]
- 699.Arango D, et al. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Pro Nat. Aca Sci. 2013;110:E2153. doi: 10.1073/pnas.1303726110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Ko CC, et al. Chemical proteomics identifies heterogeneous nuclear ribonucleoprotein (hnRNP) A1 as the molecular target of quercetin in its anti-cancer effects in PC-3 cells. J. Biol. Chem. 2014;289:22078–22089. doi: 10.1074/jbc.M114.553248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Tummala R, Lou W, Gao AC, Nadiminty N. Quercetin targets hnRNPA1 to overcome enzalutamide resistance in prostate cancer cells. Mol. Cancer Ther. 2017;16:2770–2779. doi: 10.1158/1535-7163.MCT-17-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Wu W, et al. Quercetin as an antiviral agent inhibits influenza A virus (IAV) Entry. Viruses. 2016;8:1–12. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Yao C, et al. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018;15:116–116. doi: 10.1186/s12985-018-1023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Johari, J., Kianmehr, A., Mustafa, R. M., Abubakar, S. & Zandi, K. Antiviral activity of baicalein and quercetin against the Japanese Encephalitis virus. Int. J. Mol. Sci. 13, 10.3390/ijms131216785 (2012). [DOI] [PMC free article] [PubMed]
- 705.Carabet, A. L. et al. Computer-aided discovery of small molecules targeting the RNA splicing activity of hnRNP A1 in castration-resistant prostate cancer. Molecules.24, 10.3390/molecules24040763 (2019). [DOI] [PMC free article] [PubMed]
- 706.Boyce M, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 707.Umareddy I, et al. Dengue virus serotype infection specifies the activation of the unfolded protein response. Virol. J. 2007;4:91–91. doi: 10.1186/1743-422X-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Leung HJ, et al. Activation of the unfolded protein response by 2-deoxy-D-glucose inhibits Kaposi’s sarcoma-associated herpesvirus replication and gene expression. Antimicrob. Agents Chemother. 2012;56:5794–5803. doi: 10.1128/AAC.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Perry JW, et al. Antiviral activity of a small molecule deubiquitinase inhibitor occurs via induction of the unfolded protein response. PLoS Pathog. 2012;8:e1002783. doi: 10.1371/journal.ppat.1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Lee YH, Wei CW, Wang JJ, Chiou CT. Rana catesbeiana ribonuclease inhibits Japanese encephalitis virus (JEV) replication and enhances apoptosis of JEV-infected BHK-21 cells. Antivir. Res. 2011;89:193–198. doi: 10.1016/j.antiviral.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 711.Rider TH, et al. Broad-spectrum antiviral therapeutics. PLoS One. 2011;6:e22572. doi: 10.1371/journal.pone.0022572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Tabata Y, et al. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am. J. Phys. Cellphys. 2007;293:C411–C418. doi: 10.1152/ajpcell.00095.2007. [DOI] [PubMed] [Google Scholar]
- 713.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Einav S, Dvory-Sobol D-S, Gehrig E, Glenn JS. The hepatitis C virus (HCV) NS4B RNA binding inhibitor clemizole is highly synergistic with HCV protease inhibitors. J. Infec Dis. 2010;202:65–74. doi: 10.1086/653080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Shah A, Parker J, Shimamura M, Cassady K. Spontaneous and engineered compensatory HSV mutants that counteract the host antiviral PKR response. Viruses. 2009;1:510–522. doi: 10.3390/v1030510. [DOI] [PMC free article] [PubMed] [Google Scholar]