Abstract
At the beginning of 2020, the national health system and medical communities are faced with unprecedented public health challenges. A novel strain of coronavirus, later identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread globally, marking another pandemic of coronaviruses. This viral disease is responsible for devastating pneumonia, named coronavirus disease of 2019 (COVID-19), and projected to persist until the end of the year. In tropical countries, however, concerns arise regarding the similarities of COVID-19 with other infectious diseases due to the same chief complaint, which is fever. One of the infectious disease of a primary concern is dengue infection, which its peak season is approaching. Others report that there are cases of serological cross-reaction of COVID-19 and dengue infection. In this comprehensive review, we underscore the importance of knowing similar clinical presentations of both diseases and emphasize why excluding COVID-19 in the differentials in the setting of a pandemic is imprudent.
Keywords: SARS-CoV-2, COVID-19, DENV, Dengue, Mimicker
Introduction
On 11 March 2020, the World Health Organization (WHO) raised the coronavirus disease of 2019 (COVID-19) status from the public health emergency of international concern to a pandemic [1]. The culprit that is responsible for COVID-19 is severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [2]. As of 25 April 2020, this disease affected almost three million people and claimed more than 187,000 lives, worldwide [3]. There is also concern regarding SARS-CoV-2 infection because it has similar symptoms with other diseases, particularly dengue infection [4].
In tropical countries, COVID-19 can be easily misdiagnosed with other more common infectious diseases, because the main presenting symptom is fever. With the dengue infection season approaching [5], healthcare professionals, primarily those who are residing in the emergency department (ED), are faced with additional challenges that COVID-19 has already possessed. In this setting, complete history taking and meticulous physical examinations are needed to be accompanied by judicious laboratory examinations. The differential diagnosis is to be kept broad enough and always include COVID-19 when someone comes into the ED with a chief complaint of fever.
Here, we discuss the similarities of findings from dengue infection with COVID-19 from the history taking, physical examinations, and diagnostic modalities, which explain the justification of why hastily excluding COVID-19 is imprudent.
Etiology
SARS-CoV-2
SAR-CoV-2 is an enveloped, positive-sense RNA virus that belongs to the β-coronavirus genus. Its diameter is about 65–125 nm, contains a single strand of RNA, and is coated by crown-like spikes on its outer surface (Table 1). It has four main structural proteins including spike (S) glycoprotein, envelope (E) glycoprotein, membrane (M) glycoprotein, nucleocapsid (N) protein, and also several non-structural proteins and multiple unique accessory proteins [6, 7]. The spike glycoprotein comprises two subunits which are responsible for the binding of the virus to the host cell receptor (S1 subunit) and the fusion of the virus to the cell membrane (S2 subunit) [6]. The nucleocapsid (N) protein is located in the endoplasmic reticulum region and bound to nucleic acid material of the virus. This N protein is responsible for the viral genome and viral replication cycle. The membrane (M) protein is the protein that gives structure to the virus and has a role in determining the shape of the virus envelope, whereas the envelope (E) protein has a role in the production and maturation of the virus [7].
Table 1.
The structure differences between SARS-CoV-2 and DENV
 |
 |
|
|---|---|---|
| Species | SARS-CoV-2 | Dengue virus |
| Family | Coronaviridae | Flaviviridae |
| Diameter | 65–125 nm | 50 nm |
| Gene material | ssRNA | ssRNA |
| Structural protein | Spike (S) glycoprotein, envelope (E) glycoprotein, membrane (M) glycoprotein, nucleocapsid (N) protein | Nucleocapsid (N) or core protein, membrane (M) glycoprotein, and envelope (E) protein |
| Characteristic findings | Crown-like spikes (corona) on its outer surface. | Non-structural protein-1 (NS-1) |
DENV
Dengue virus is one of the viral hemorrhagic fever that belongs to the Flaviviridae family. Its structure is smaller than SARS-CoV-2. Its diameter is about 50 nm and contains single-stranded RNA (Table 1). Compared to SARS-CoV-2, the dengue virus does not have spike protein but has three main structural protein genes, including nucleocapsid (N) or core protein, membrane (M) glycoprotein, and envelope (E) protein [8]. Dengue virus also has seven non-structural (NS) protein genes. One of which is NS-1, diagnostic and pathological importance in the confirmation of dengue infection [8].
Pathophysiology of SARS-CoV-2 vs. DENV Infection
Although the complete understanding of COVID-19 pathophysiology is still being unraveled every day, here we briefly explain from the current literature. The infection of SARS-CoV-2 is primarily from respiratory droplets through person to person transmissions and viral entry mainly through mucous membranes via eyes, nose, and mouth [9]. There is a vast spectrum of clinical symptoms of COVID-19, ranging from asymptomatic carriers to critically ill patients, characterized by multiorgan failure with the need for multiple life supports [9].
Based on a single-center observational cohort study, 80% of the patients who are affected with SARS-CoV-2 are mild to moderately ill, 13.8% are severely ill, and the rest of them are critically ill, defined as one of the following: respiratory failure, septic shock, or multiorgan failure [10]. According to the COVID-19 symptomatology, the clinical manifestations can be divided into three phases along its continuum; they are starting, accelerating, and recovery phases. Three of them correspond to the acquisition of the virus and subsequent viremia, secondary damage of organs and tissues, which are not exhibited by all patients, and overall clinical improvement, respectively [11].
After the virus gains entry through the mucous membrane, it is then directed into the pulmonary tissue, the type 2 pneumocyte in particular, via the angiotensin-converting enzyme 2 (ACE2) [12]. Direct pneumocyte infection and the viral cytopathic effect stimulate the innate immune system, which consists of monocytes, macrophage, and toll-like receptors, to produce various inflammatory cytokines and to stimulate the adaptive immune system [13]. Subsequently, the activated adaptive immune system is liable for markedly increased inflammatory cytokines concentration [13].
Many proposed that massive cytokines production, leading to cytokine storm syndrome (CSS), played a pivotal role behind COVID-19 pathophysiology in severe and critically ill patients [13–16]. However, others argued that COVID-19 is a disease of different entity and independent from the classic acute respiratory distress syndrome (ARDS) and CSS because interleukin-6 (IL-6) concentration is much lower in comparison to both diseases [17]. Thus, it is erroneous to say that COVID-19 is identical to ARDS or CSS. Leismann et al. proposed that COVID-19 is a disease of the blood vessels separated by three phases which ultimately ends with endothelial dysfunction [17].
Nevertheless, the number of cytokines in COVID-19 are significantly increased. Those cytokines are IL-2, IL-7, IL-10, tumor necrosis factor (TNF), granulocyte-colony stimulating factor (G-CSF), interferon gamma-induced protein 10 (IP-10; CXCL10), MCP-1 (CCL2), and MIP-1A (CCL3), which were found to be increased in intensive care unit (ICU) patients compared to non-ICU patients, but not IL-6 [16]. Other cytokines that recently were found to contribute in COVID-19 are IL-1β, IL-1ra, IL-2R, IL-6, IL-8 (CXCL8), IL-17, interferon (IFN)-γ, and granulocyte macrophage colony-stimulating factor (GM-CSF) [18–21].
Much like COVID-19, the hallmark of dengue hemorrhagic fever is endothelial dysfunction. Three principal pathophysiologies, which are T cell immunology, antibody-dependent enhancement (ADE) of the virus, and complement activation, are attributed to the resulting aberrant immunological response [22–24]. Furthermore, in dengue infection, several cytokines such as GM-CSF, IFN-γ, IL-10, IL-15, IL-8, MCP-1, IL-6, MIP-1β, and TNF-α were also increased [25]. In addition, four cytokines, which are IFN-γ, GM-CSF, IL-10, and MIP-1β, correlated significantly with disease severity and therefore, could serve as potential predictors [26].
Thus, to some extent, although COVID-19 and dengue fever initially had very different pathophysiology and target organs, both of them ultimately end in the same direction, which is endothelial dysfunction. Therefore, this might explain the similarities of clinical presentations between these two diseases.
Clinical Manifestation of COVID-19 Mimicking Dengue Infection
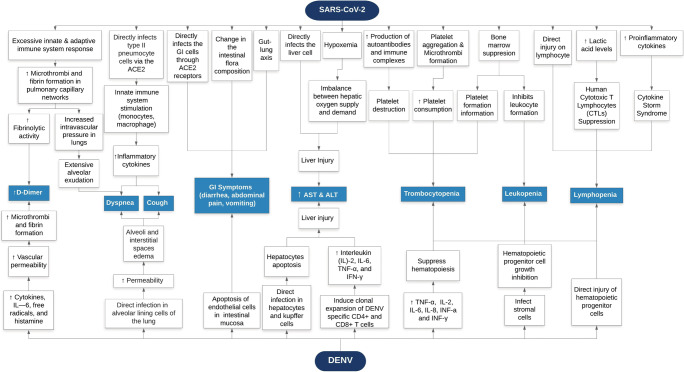
COVID-19 and dengue infection are hard to distinguish because they share similar clinical features [27]. Moreover, there were some misdiagnosed cases of dengue suspected patients that later confirmed to be SARS-CoV-2 infection [4]. Here, we present a number of COVID-19 symptoms and their similarities with dengue. In addition, we also describe similar laboratory findings of both diseases (Fig. 1).
Fig. 1.
SARS-CoV2 and DENV pathomechanisms of clinical manifestations. This flowchart diagram depicts similarities between COVID-19 and DENV clinical manifestations with their respective pathomechanisms
Constitutional Symptoms
Fever
Fever is the most common chief complaint in both dengue fever and COVID-19 patients. In two cohorts involving 515 and 154 subjects infected with dengue virus, all experienced fever [28, 29]. In comparison, based on three cohorts, the proportion of COVID-19 patients presented with fever were 92.23%, 98%, and 98.6%, respectively [30–32].
The characteristic temperature pattern of dengue fever is a high temperature with abrupt onset, sometimes accompanied by two temperature peaks or known as saddleback fever [8]. In contrast, not much is known about the characteristic temperature pattern of COVID-19. In a single-center study consisting of 11 laboratory-confirmed SARS-CoV-2 subjects, the median time of fever defervescence took 6 days (4–11.5 days), although it is worth noting that all of them are in the category of mild and moderate COVID-19 [33]. In dengue-infected patients, fever lasting more than 6 days is unusual, because, at the fourth or fifth day of dengue fever, the temperature tends to drop [8].
Interestingly, based on a retrospective cohort study of 201 hospitalized patients due to COVID-19 pneumonia, although fever was associated with a higher likelihood of ARDS development (hazard ratio (HR) of 1.77; 95% CI, 1.11–2.84), it was associated with a lower likelihood of death (HR, 0.41; 95% CI, 0.21–0.82) [34]. Meanwhile, in dengue fever, the temperature drop after the third day of illness could be a harbinger to systemic circulatory failure leading to hypovolemic shock [8].
Headache
In COVID-19, headache is an uncommon clinical presentation. In an observational cohort group involving 1099 subjects, headache only presented in 150 patients (13.6%) [35]. Moreover, in a case series involving 138 subjects, it presented in only nine patients (6.5%) [32]. Prospective cohort study also confirms this, with headache only presented in three subjects (8%) out of 41 subjects [30]. On the contrary, 45% and 95% of dengue patients had been reported to experience headache [8, 28].
Musculoskeletal Involvement
Musculoskeletal symptoms present variably in COVID-19 patients from 15 to 44% based on four descriptive studies in China [27, 30–32]. On the contrary, only 12% of dengue patients reported having myalgia [8].
Respiratory Symptoms
Cough is the most common respiratory symptom that occurs in viral infections, in addition to fever, and usually presents in the first two until the fourth day of illness. Among two COVID-19 cases which once were thought to have dengue infection due to false-positive dengue serology reported in Singapore, both patients experienced cough in the first 3 and 4 days of their illnesses [36]. This is not unusual considering that cough can also be found in patients with dengue infection on the early days of the disease, which present in 21.5% dengue patients [37]. Meanwhile, in COVID-19, 31 out of 41 (76%) patients present with cough [30].
According to a prospective study among 41 laboratory-confirmed COVID-19 patients in Wuhan, more than half of patients (55%) developed dyspnea with the median time of 8 days (5–13 days), and 29% of patients developed ARDS with the median time of 9 days (8–14 days) since the onset of the illness. In addition, 10% of these patients required invasive mechanical ventilation and another 5% experienced refractory hypoxemia [30].
In a cohort of 100 patients hospitalized due to PCR-confirmed dengue infections consisting of 43 dengue fever (DF), 42 dengue hemorrhagic fever (DHF), and 15 dengue shock syndrome (DSS) patients, respiratory manifestations were not uncommon. The incidence of ARDS, pulmonary hemorrhage, bilateral pneumonitis, and pleural effusion were 19.4% (n = 8), 21.4% (9), 9.5% (4), and 95.2% (40) in the DF group, respectively [38]. Meanwhile, in the DHF group, the incidence of aforementioned complications was 53.3% (8), 6.6% (1), 6.6% (1), and 100% (15), respectively [38].
As opposed to the SARS-CoV-2 which directly infects type II pneumocyte cells, the pathomechanism of pulmonary dysfunction and respiratory distress syndrome in dengue infection is due to the presence of dengue virus antigen in alveolar lining cells of the lung, which responsible for the increased permeability of the alveolar-capillary membrane, resulting in alveoli and interstitial spaces edema [39].
Gastrointestinal and Liver Symptoms
It is noteworthy that patients with COVID-19 can present with gastrointestinal (GI) symptoms such as diarrhea, abdominal pain, vomiting, and nausea. The same receptors, the ACE2, are used by the SARS-CoV-2 to gain entry to multiple organs, including the intestines. As expected, 3% of COVID-19 patients presented with only fever and gastrointestinal symptoms based on a multicenter cross-sectional study [31]. Diarrhea as a part of clinical manifestation of COVID-19 presents variably. Based on four descriptive studies, diarrhea presented in 2, 3, 10, and 34% of COVID-19 patient, respectively [27, 30–32]. Furthermore, a cohort study from China confirmed that RNA of SARS-CoV-2 had been detected in a stool specimen [40].
On the other hand, 6% of dengue hemorrhagic patients complained about diarrhea [8]. However, the pathomechanism of diarrhea in this subset of patients was different from SARS-CoV-2 infection as dengue virus (DENV) does not utilize ACE2. Moreover, diarrhea as a solitary clinical finding of dengue infection has never been reported.
In COVID-19, vomiting presents in only 4 to 5% of patients [31, 32, 35]. Moreover, abdominal pain has been reported in COVID-19 patients but only constitute 2% of the total patients [31, 32]. Meanwhile, vomiting is a common symptom of dengue infection and presents in 30% and 58% of patients based on a couple of studies [8, 41]. In addition, abdominal pain is also a common symptom in this disease, which presents in 17% and 25% of dengue patients [28, 41].
In respect of GI symptoms in COVID-19 patients, there are three main pathomechanisms that might explain this. First, SARS-CoV-2 directly infects the GI cells through ACE2 receptors that are expressed on them. From 73 hospitalized patients, 39 patients (53.42%), consisting of 25 male and 14 female patients, tested positive for SARS-CoV-2 RNA in their stools [40]. One of them was sent for pathological examination, which revealed SARS-CoV-2 nucleocapsid protein (NP) in the cytoplasm of gastric, duodenal and rectum glandular epithelial cells, and pathological findings of numerous infiltrating plasma cells and lymphocytes with interstitial edema [40].
Second, SARS-CoV-2 infection might cause changes in the intestinal flora composition, a component that is essential for physiological homeostasis of the human body consisting of nutritional metabolism, immunity development and maturation, and antibacterial effect, which produce these GI symptoms. Lastly, the gut-lung axis theory, which through common mucosal immunity, the respiratory system and GI system are interconnected and reciprocally influence each other, may explain the presence of GI symptoms in COVID-19 pneumonia [42, 43]. In dengue infection, GI symptoms are best explained by the apoptosis of endothelial cells of the intestinal mucosa, which is evident in fatal DHF cases [44].
Three descriptive studies from China have shown that 31–35% of COVID-19 patients had an increased aspartate aminotransferase (AST), and 24–28% COVID-19 patients had an increased alanine aminotransferase (ALT) levels [27, 30, 35]. In comparison, patients with severe COVID-19 had a higher prevalence of increases in AST and ALT levels (39.4 and 28.1%) than patients with the non-severe disease (18.2 and 19.8%) [7, 15]. Meanwhile, it was previously shown from a report of three cases that SARS-CoV-1 could cause direct infection in the liver resulting in liver injury [45]. All of them had positive RT-PCR in the liver tissues biopsy, but due to viral load suppression by antiviral treatment before the biopsy, no virus could be identified from the electron microscopy [45].
Histological findings of the liver tissue in SARS-CoV-1-infected patients revealed microvesicular fatty changes, focal hemorrhages, hepatocyte necrosis with scattered acidophilic bodies, lymphocytic and neutrophilic infiltration, but no viral inclusions were seen [45, 46]. Nevertheless, more investigations are needed pertaining to direct viral infection. In addition, pneumonia caused by SARS-CoV-2 infection can be so severe, leading to hypoxemia. Ultimately, this can cause an imbalance between hepatic oxygen supply and demand, resulting in liver injury [47].
Guan et al. propose that the mechanism of liver injury due to SARS-CoV-2 infection other than excessive inflammatory response is due to compensatory proliferation of hepatocytes derived from bile duct epithelial cells, which culminates in upregulation of ACE2 receptor [48]. Thereby mediating the viral entry to the hepatocytes. Increased AST and ALT levels also have been reported in several studies, ranging from 63 to 97% and 45–97% patients, respectively [49–55].
Dengue virus can cause liver injury through direct infection and immune mechanism. Dengue virus infects hepatocytes and Kupffer cells in the human body as a prime target cell [56–58]. Hepatocyte infection is mediated by virus envelope protein (E protein), which ultimately terminates in cellular apoptosis [59, 60]. In addition, DENV can induce clonal selection and expansion of DENV specific CD4+ and CD8+ T cells which can produce IL-2, IL-6, TNF-α, and IFN-γ culminating in a cytokine storm, which indirectly causing liver injury [61]. Furthermore, these T cells may cause liver cell damage by direct cytolytic and/or cytokine-mediated effects [56].
Cutaneous Symptoms
Cutaneous involvement in viral diseases is a common phenomenon, including COVID-19. One patient was initially mistaken with dengue infection due to skin rash presenting as petechiae and laboratory findings of thrombocytopenia. Fever was also absent in this patient, but after few days, the disease progressed and manifested as respiratory distress, then the patient was referred to the tertiary hospital, in which he was subsequently diagnosed with SARS-CoV-2 infection and was confirmed by a reverse transcriptase-polymerase chain reaction (RT-PCR) [62].
In one case series consisting of 88 patients, 18 (20.4%) developed cutaneous manifestation. Whereas eight patients developed this finding at the onset of the disease, the other ten developed after hospitalization. The most common findings were erythematous rash (14 patients), followed by widespread urticaria (three patients), and only one patient presented with chickenpox-like vesicles [63]. In contrast, in dengue fever, the most common cutaneous findings are skin flushing that blanches on pressure, petechiae, and convalescent rash described as “land of white in the sea of red” due to island of normal skin interspersed between generalized confluent petechial rash [8, 64]. The former two are cutaneous findings in the early phase (1st until the third day) of dengue fever, whereas the latter can be found in the convalescent period [8].
Hematological Abnormalities and Coagulopathy
Thrombocytopenia
Based on one retrospective, single-center study, thrombocytopenia is more prevalent (12% vs 4%) in COVID-19 patients compared to thrombocytosis [27]. Another study of COVID-19 patients involving 869 patients found that 315 (36.2%) of them developed thrombocytopenia [44]. On the contrary, a higher proportion of dengue-infected patients developed thrombocytopenia. Based on a prospective observational study involving 515 dengue patients, 358 (69.51%) experienced thrombocytopenia [28]. In another study involving 184 dengue-infected patients, all developed thrombocytopenia [65].
Currently, the mechanism of thrombocytopenia in COVID-19 remains unclear. Nevertheless, there are many hypotheses that readily explain this pathomechanism. Xu et al. proposed three possible mechanisms of thrombocytopenia in SARS-CoV-2 infections, which are platelet destruction by the immune system, increased platelet consumption, and the inhibition of platelet synthesis [66].
Destruction of platelets might be explained by the increased production of autoantibodies and immune complexes after SARS-CoV-2 infection, which acts through molecular mimicry, resulting in platelet destruction by reticuloendothelial cells [66]. Furthermore, increased platelet consumption is thought to be caused by platelet aggregation and microthrombi formation due to viral infection [66]. In addition, in the early phase of infection, the binding of SARS-CoV-2 to ACE2 on the endothelial cells is predicted to increase local angiotensin II concentration, which leads to platelet activation and enhance a prothrombotic milieu [17].
Chan et al. found that SARS-CoV-2 has 82% nucleotide similarity with SARS-CoV-1 [67]. Moreover, SARS-CoV-1 and human coronavirus-229E (HCoV-229E) are assumed to have identical antigen characteristics. Therefore, SARS-CoV-2 and HCoV-229E are speculated to have some characteristics in common. It has been known that the binding of HCoV-229E and its receptor (human aminopeptidase-N or CD-13) leads to thrombocytopenia by disrupting hematopoiesis in the bone marrow [68]. This theory speculated that SARS-CoV-2 inhibits platelet formation in the bone marrow through certain receptors leading to thrombocytopenia.
Meanwhile, two pathomechanisms are responsible for thrombocytopenia in dengue infection. The first is the peripheral destruction and aggregation of thrombocytes by various mechanisms, which we describe below. It has been shown that during dengue infection, the stimulatory effect of acute-phase plasma leads to aggregation of autologous platelets [69]. By in vitro study, Funahara et al. demonstrated that endothelial dysfunction due to dengue virus exposed the subendothelial collagen, which subsequently interacts with platelets leading to platelet aggregation and lysis, and ultimately resulting in thrombocytopenia [70].
Another mechanism is through defective virus-specific antibody that binds to human platelets, supporting a role for immune-mediated clearance of platelets in the pathogenesis of thrombocytopenia in DHF/DSS [71]. Furthermore, Boonpuchnaving et al. have demonstrated by direct immunofluorescence technique that dengue antigen, human immunoglobulin (Ig), and C3 present in 48% DHF patients. They also found that the amount of Ig and C3 are correlated with the degree of thrombocytopenia and the day of illness [72].
The second pathomechanism is decreased production of platelets. In patients with DHF, bone marrow studies showed marked hypocellularity of all hematopoietic cell lines in the early phase of the acute febrile illness [73]. Furthermore, Nakao et al. demonstrated direct injury of hematopoietic progenitor cells attributed to dengue virus type 4 (DENV4) infection and replication in bone marrow mononuclear cells [74]. Also, DENV2 indirectly inhibits hematopoietic progenitor cell growth by infecting the stromal cells that secrete the necessary cytokines for optimal growth [75]. In addition, cytokines, such as TNF-α, interleukins (IL-2, IL-6, IL-8), and interferons (IFN-α and IFN-γ) were also found to play a role in thrombocytopenia by suppressing hematopoiesis. These cytokines were also well correlated with the clinical severity of dengue infection [76–79].
Increased D-Dimer
In an observational cohort study from China, 260 out of 560 (46.4%) COVID-19 patients showed increased levels of D-Dimer (DD). Moreover, the proportion of increased DD levels were higher in ICU patient (60%) compared to 43% in non-ICU patients [35].
D-Dimer levels were elevated in dengue infected patients as well. In a prognostic study evaluating DD for predicting severe dengue/dengue hemorrhagic fever (DHF) involving 41 dengue patients (22 girls, 19 boys), high DD was significantly present (P < 0.03) in DHF group (n = 26; 87%) than in DF group (n = 4; 13%) and it predicted severe dengue with sensitivity and specificity of 90% and 67%, respectively [80]. However, a case-control study from Sudan involving 334 dengue-infected patients and 101 control subjects failed to show a significant difference in the proportion of patients with increased DD level. Moreover, the proportion is higher in DF group (n = 70; 86%) than DHF group (n = 17; 85%) [81].
Although it is not fully elucidated yet, dengue is characterized by the occurrence of a “cytokine tsunami” thought to be generated from the sequential release of cytokines, IL-6, free radicals, and histamine which causes an abrupt increase of vascular permeability that leads to the development of a leak syndrome four until 6 days after the onset of fever [82]. Furthermore, increased permeability is concurrently accompanied by thrombotic state leading to disseminated intravascular coagulation (DIC), which is characterized by the increased levels of D-Dimer, a fibrin degradation product (FDP) [83, 84].
On the contrary, two mechanisms might be responsible for the increased DD in COVID-19 patients. First, CSS associated with SARS-CoV-2 infection will have major impacts upon thrombin generation and fibrin deposition within the lung [85]. Second, others have proposed that elevated DD in COVID-19 patients was due to the pulmonary intravascular coagulopathy (PIC). The excessive innate and adaptive immune system is leading to local macrophage activation syndrome (MAS) with the net results of the excessive microthrombi formation of the extensive pulmonary capillary networks and the failing, albeit vigorous fibrinolytic activity [86].
A clearly different entity from DIC, PIC is reflected by increased DD levels, but normal to slightly increased in fibrinogen and prothrombin time/activated partial thromboplastin time (PT/APTT), although few patients develop DIC in the terminal stage of COVID-19. An elevated plasma level of D-dimer also constitutes a significant independent biomarker of poor prognosis [86]. Furthermore, hypoxemia in asymptomatic patients and dyspnea are attributed to the aforementioned processes by causing alveolar exudation [87].
Lymphopenia
In 41 hospitalized patients with laboratory-confirmed COVID-19, most of them were predominantly lymphopenic (n = 26; 63%) [30]. Moreover, lymphopenia was more common in patients admitted to the intensive care unit (ICU); 11 from 13 patients (85%), compared to non ICU care; 15 from 28 patients (54%) [30]. In a study evaluating the WHO revised criteria for the classification of clinical disease severity of dengue infection involving 184 subjects from two tertiary hospitals, lymphopenia was a common laboratory finding (n = 117; 63%) [65]. However, in patients with severe dengue, lymphopenia was more prevalent than in non-severe variants (82.5% vs 58.3%) and significantly (p = 0.005) predict the severe dengue course with an odds ratio of 3.367 (OR = 1.396–8.123, 95% CI) [65].
The pathomechanism of lymphopenia in SARS-CoV-2 infection is still unknown. However, three pathways may explain this condition. First, SARS-CoV-2 may cause direct injury because ACE2, which are crucial for viral entry, is expressed on the surface of lymphocyte [88]. Second, in the severe-critical state of COVID-19, lactic acid levels start to build up in the blood and suppress human cytotoxic T lymphocytes (CTLs) [89]. Lastly, based on SARS-CoV-1 study, it may cause lymphopenia through indirect mechanism via cytokine storm, which ultimately induces lymphocyte or monocyte apoptosis [90].
Unlike COVID-19, the pathomechanism of lymphopenia in dengue infection is well known. There are three pathways, which are direct infection of DENV to hematopoietic progenitor cells, activated dengue-specific T-cells, and marrow stromal cells infection by DENV, resulting in the release of marrow-suppressive cytokines. In addition, DENV can cause generalized bone marrow suppression leading to lymphopenia [91].
Leukopenia
From an observational cohort study, leukopenia could be observed in 25% COVID-19 patients [30]. Meanwhile, in another cohort study from Singapore, 19 of 65 (29%) COVID-19 patients experienced leukopenia [92].
In dengue-infected patients, the proportion of leukopenia is not much different from COVID-19 patients. In a prospective observational study from India, involving 515 patients, approximately 20% of them (n = 104) were experiencing leukopenia [28]. However, significantly higher numbers of leukopenic patients were seen in other descriptive studies. One cohort study from China showed that 38 of 55 (76%) dengue fever patients had leukopenia [93]. Another cohort study from Singapore had shown that 1579 of 1921 (82.2%) dengue patients had some form of leukopenia [94]. It is worth noting that in DF or DHF, the leukocyte reached its nadir at the fifth or sixth day after fever onset [93, 94].
It is possible that SARS-CoV2 causes leukopenia through the same pathomechanism of thrombocytopenia, which is bone marrow suppression. Meanwhile, leukopenia in DF and DHF is attributed to the fact that DENV causes myeloid progenitor cell destruction and inhibition. From bone marrow studies, mild hypocellularity was seen at the acute stage (less than 1 week), whereas it reverts back to normal in the convalescent stage (greater than 1 week) [93]. Furthermore, bone marrow CFU-GM that was performed within 1 week of illness showed no growth or low colony count and nearly normalized after 1 week of fever onset [93].
In a review of experimental dengue infection of volunteers, bone marrow’s histopathological studies, and long-term marrow culture (LTMC), it is concluded that dengue is responsible for cytopenias by infecting the adventitial reticular cells (ARCs) and altering cytokine productions of infected stromal cells, leading to dengue virus-induced marrow suppression. This condition is purposely to protect the marrow stem/progenitor cell compartment from subsequent damaging inflammation intended to eliminate infected cells [91].
Serological Cross-Reaction and Other Tropical Diseases
Although similarities between COVID-19 and dengue fever are remarkable in clinical presentations, we thought maybe we could circumvent these problems with serological testing. Unfortunately, it is not the case in COVID-19. There have been two case reports from Singapore reporting serological cross-reaction of patients who were thought initially to be infected with dengue virus, only to test positive of SARS-CoV-2 infection by RT-PCR of a nasopharyngeal (NP) swab. Both cases were without travel and contact history, the first patient experienced fever, whereas the second experienced fever, cough, and myalgia. Both present with dyspnea and experienced thrombocytopenia, lymphopenia, and positive dengue serology [95]. One may think cough is the differentiating symptom that can distinguish COVID-19 and dengue fever. However, both of them have normal chest X-rays [95].
In another case report from Thailand, a nurse contracted COVID-19 infection during performing a blood draw from a patient that was provisionally diagnosed with dengue infection [96]. Due to this diagnosis, the nurse does not wear appropriate personal protective equipment (PPE) for COVID-19. The patient laboratory workup shows thrombocytopenia and a false-positive dengue serology, reinforcing the diagnosis. However, he was later diagnosed with COVID-19 after 3 days of hospitalization, because he reported shortness of breath, his chest X-ray showed progressive infiltration, and NP and throat swabs that came back positive [96].
Others reported a case of a Pakistani medical student that was initially thought to have COVID-19 infection due to abrupt onset of symptoms of fever, chills, dry cough, myalgias, and diarrhea and travel history to cities with known COVID-19 transmission [97]. Consequently, he was hospitalized, contact, and droplet precautions were initiated, and appropriate PPE was given for the staff caring for the patient. His RT-PCR tests for SARS-CoV-2 were negative. He did not experience pulmonary symptoms, although his fever, cough, myalgia, and fever worsened. On the third day of hospitalization, a maculopapular rash appeared on the trunk and face and spread to extremities over 2 days. He was later diagnosed with concomitant dengue and measles infection after his laboratory workup revealed positive IgM (1.6 U/L) titer for dengue and elevated IgM for measles (137 U/L).
Therefore, when a diagnosis of an infectious disease is not yet firmly established, we believed it is judicious to take additional safety measures by using the appropriate PPE, especially in the setting of a pandemic in tropical countries, which other diseases might obscure COVID-19 diagnosis.
Conclusion
In summary, we conclude that COVID-19 and dengue infection can be difficult to distinguish because they share a similar vast spectrum of clinical features. In Tables 2 and 3, we summarize the proportions of clinical manifestations and laboratory findings in patients with COVID-19 and dengue infection. However, these results must be cautiously and thoroughly interpreted because much more is to be known about this novel disease. Thus, more data from descriptive studies and further researches are still needed. Therefore, in the light of COVID-19 pandemic, meticulous history taking pertaining to the course of illness and comprehensive physical examination has to be carried out and supported by additional laboratory workups in order to avoid these diagnostic pitfalls.
Table 2.
The proportion of clinical manifestation differences between COVID-19 and dengue patients
| No. | Clinical manifestation | COVID-19 | Dengue infection |
|---|---|---|---|
| 1. | Fever |
No specific fever patterns. Defervescence after 6 days of illness [33] |
Saddleback fever (fever with two peaks) [8] |
| 2 | Headache | 6.5–13.6% [30, 32, 35] | 45–95% [8, 28] |
| 3 | Myalgia | 15–44% [27, 30–32] | 12% [8] |
| 4 | Cough | 76% [30] | 21.5% [37] |
| 5 | Dyspnea | 55% [30] | 9.5–95.2% [38] |
| 6 | Diarrhea | 2–34% [27, 30–32] | 6% [8] |
| 7 | Abdominal pain | 2% [31, 32] | 17–25% [28, 41] |
| 8. | Vomiting | 4–5% [31, 32, 35] | 30–58% [8, 41] |
| 9 | Cutaneous manifestation | Erythematous rash, urticaria, chickenpox-like vesicles [66] | Skin flushing that blanches on pressure, petechiae, and convalescent rash [8, 64] |
Table 3.
The proportion of laboratory findings differences between COVID-19 and dengue patients
| No. | Laboratory findings | COVID-19 | Dengue infection |
|---|---|---|---|
| 1 | Thrombocytopenia | 12–36.2% [27, 44] | 69.51–100% [28, 65] |
| 2 | Leukopenia | 25–29% [30, 92] | 20–82.2% [28, 93, 94] |
| 3 | Lymphopenia | 63% [30] | 63% [65] |
| 4 | Increase AST | 31–35% [27, 30, 35] | 63–97% [49–55] |
| 5 | Increase ALT | 24–28% [27, 30, 35] | 45–97% [49–55] |
| 6 | Increased D-dimer | 46.4% [35] | 13–87% [80, 81] |
Author Contributions
JH conceived and design the study. ICSP, SL, QFB, and JH performed literature research, acquired the data, and drafting the manuscript. JH also provides revision of the manuscript. AC contributed in revision of the manuscript, supervision, and final approval of the manuscript. All authors approved the final manuscript.
Compliance with Ethical Standards
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. [cited 2020 Apr 13]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020.
- 2.Naming the coronavirus disease (COVID-19) and the virus that causes it. [cited 2020 May 12]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 3.Coronavirus disease 2019 (COVID-19) Situation Report – 96. 2020 [cited 2020 May 12]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200425-sitrep-96-covid-19.pdf.
- 4.Lorenz C, Azevedo TS, Chiaravalloti-Neto F. COVID-19 and dengue fever: A dangerous combination for the health system in Brazil. Travel Med Infect Dis. 2020 [cited 2020 Apr 25]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7144614/. [DOI] [PMC free article] [PubMed]
- 5.Campbell KM, Lin CD, Iamsirithaworn S, Scott TW. The complex relationship between weather and dengue virus transmission in Thailand. Am J Trop Med Hyg. 2013;89:1066–1080. doi: 10.4269/ajtmh.13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walls AC, Park Y-J, Tortorici MA, Wall A, AT MG, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astuti I, Ysrafil. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr. 2020 [cited 2020 May 14]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7165108/. [DOI] [PMC free article] [PubMed]
- 8.World Health Organization, editor. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. Rev. and expanded. ed. New Delhi, India: World Health Organization Regional Office for South-East Asia; 2011.
- 9.Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid-19. Solomon CG, editor. N Engl J Med. 2020 [cited 2020 Apr 25]; Available from: http://www.nejm.org/doi/10.1056/NEJMcp2009249. [DOI] [PubMed]
- 10.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020 [cited 2020 May 12]. Available from: https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19).
- 11.Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. Oxford Academic; 2020 [cited 2020 Apr 25];7. Available from: https://academic.oup.com/ofid/article/7/3/ofaa102/5810740. [DOI] [PMC free article] [PubMed]
- 12.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;105954. [DOI] [PMC free article] [PubMed]
- 14.Mehta P, DF MA, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020 [cited 2020 May 9]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7161506/. [DOI] [PMC free article] [PubMed]
- 16.Rahmati M. Cytokine-targeted therapy in severely ill COVID-19 patients: options and cautions. Eurasian J Med Oncol. 2020 [cited 2020 May 8]; Available from: https://www.ejmo.org/10.14744/ejmo.2020.72142/.
- 17.Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020 [cited 2020 May 4]; Available from: http://link.springer.com/10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed]
- 18.Li B, Feng F, Yang G, Liu A, Yang N, Jiang Q, et al. Immunoglobulin G/M and cytokines detections in continuous sera from patients with novel coronaviruses (2019-nCoV) infection. Rochester, NY: Social Science Research Network; 2020 Feb. Report No.: ID 3543609. Available from: https://papers.ssrn.com/abstract=3543609.
- 19.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020 [cited 2020 May 12]; Available from: https://academic.oup.com/nsr/advance-article/doi/10.1093/nsr/nwaa041/5804736. [DOI] [PMC free article] [PubMed]
- 20.Zheng H-Y, Zhang M, Yang C-X, Zhang N, Wang X-C, Yang X-P, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. Cold Spring Harbor Laboratory Press; 2020;2020.02.10.20021832.
- 22.Halstead SB, O’rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 23.Halstead S, O’Rourke E. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishiura H, Halstead SB. Natural History of Dengue Virus (DENV)—1 and DENV—4 Infections: Reanalysis of Classic Studies. J Infect Dis. 2007;195:1007–1013. doi: 10.1086/511825. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Liang W, Chen S, Zhu Y, Chen H, Mok CKP, et al. Serum cytokine profiles in patients with dengue fever at the acute infection phase. Dis Markers. 2018 [cited 2020 May 9];2018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5831957/. [DOI] [PMC free article] [PubMed]
- 26.Patro ARK, Mohanty S, Prusty BK, Singh DK, Gaikwad S, Saswat T, et al. Cytokine signature associated with disease severity in dengue. Viruses. 2019 [cited 2020 May 8];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6357178/. [DOI] [PMC free article] [PubMed]
- 27.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshwal R, Qureshi MI, Singh R. Clinical and Laboratory Profile of Dengue Fever. J Assoc Physicians India. 2015;63:30–32. [PubMed] [Google Scholar]
- 29.Chaloemwong J, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E, et al. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study. BMC Hematol. 2018 [cited 2020 May 13];18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6114047/. [DOI] [PMC free article] [PubMed]
- 30.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pongpirul WA, Mott JA, Woodring JV, Uyeki TM, MacArthur JR, Vachiraphan A, et al. Early release - clinical characteristics of patients hospitalized with coronavirus disease, Thailand - Volume 26, Number 7—July 2020 - Emerging Infectious Diseases journal - CDC. 2020 [cited 2020 May 8]; Available from: https://wwwnc.cdc.gov/eid/article/26/7/20-0598_article. [DOI] [PMC free article] [PubMed]
- 34.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan G, Lee CK, Lam LTM, Yan B, Chua YX, Lim AYN, et al. Covert COVID-19 and false-positive dengue serology in Singapore. Lancet Infect Dis. Elsevier; 2020 [cited 2020 Apr 25];0. Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30158-4/abstract. [DOI] [PMC free article] [PubMed]
- 37.Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18:954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed NA, El-Raoof EA, Ibraheem HA. Respiratory manifestations of dengue fever in Taiz-Yemen. Egypt J Chest Dis Tuberc. 2013;62:319–323. [Google Scholar]
- 39.Lum LCS, Thong MK, Cheah YK, Lam SK. Dengue-associated adult respiratory distress syndrome. Ann Trop Paediatr. 1995;15:335–339. doi: 10.1080/02724936.1995.11747794. [DOI] [PubMed] [Google Scholar]
- 40.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huy BV, Hoa LNM, Thuy DT, Van Kinh N, Ngan TTD, Duyet LV, et al. Epidemiological and clinical features of dengue infection in adults in the 2017 outbreak in Vietnam. BioMed Res. Int. Hindawi; 2019 [cited 2020 May 9]. p. e3085827. Available from: https://www.hindawi.com/journals/bmri/2019/3085827/. [DOI] [PMC free article] [PubMed]
- 42.Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 43.He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J. Gut-lung axis: The microbial contributions and clinical implications. Crit Rev Microbiol. 2017;43:81–95. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 44.Limonta D, Capó V, Torres G, Pérez AB, Guzmán MG. Apoptosis in tissues from fatal dengue shock syndrome. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2007;40:50–54. doi: 10.1016/j.jcv.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Chau T-N, Lee K-C, Yao H, Tsang T-Y, Chow T-C, Yeung Y-C, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatol Baltim Md. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 47.Ebert EC. Hypoxic liver injury. Mayo Clin Proc. 2006;81:1232–1236. doi: 10.4065/81.9.1232. [DOI] [PubMed] [Google Scholar]
- 48.Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, et al. [Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia]. Zhonghua Gan Zang Bing Za Zhi Zhonghua Ganzangbing Zazhi Chin J Hepatol. 2020;28:E002. [DOI] [PubMed]
- 49.Kuo CH, Tai DI, Chang-Chien CS, Lan CK, Chiou SS, Liaw YF. Liver biochemical tests and dengue fever. Am J Trop Med Hyg. 1992;47:265–270. doi: 10.4269/ajtmh.1992.47.265. [DOI] [PubMed] [Google Scholar]
- 50.de Souza LJ, Alves JG, Nogueira RMR, Gicovate Neto C, Bastos DA, Siqueira EW d S, et al. Aminotransferase changes and acute hepatitis in patients with dengue fever: analysis of 1,585 cases. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2004;8:156–163. doi: 10.1590/s1413-86702004000200006. [DOI] [PubMed] [Google Scholar]
- 51.Itha S, Kashyap R, Krishnani N, Saraswat VA, Choudhuri G, Aggarwal R. Profile of liver involvement in dengue virus infection. Natl Med J India. 2005;18:127–130. [PubMed] [Google Scholar]
- 52.Wong M, Shen E. The utility of liver function tests in dengue. Ann Acad Med Singap. 2008;37:82–83. [PubMed] [Google Scholar]
- 53.Parkash O, Almas A, Jafri SMW, Hamid S, Akhtar J, Alishah H. Severity of acute hepatitis and its outcome in patients with dengue fever in a tertiary care hospital Karachi, Pakistan (South Asia) BMC Gastroenterol. 2010;10:43. doi: 10.1186/1471-230X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trung DT, Thao LTT, Hien TT, Hung NT, Vinh NN, Hien PTD, et al. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774–780. doi: 10.4269/ajtmh.2010.10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee LK, Gan VC, Lee VJ, Tan AS, Leo YS, Lye DC. Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. PLoS Negl Trop Dis. 2012;6:e1676. doi: 10.1371/journal.pntd.0001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg. 2006;100:608–614. doi: 10.1016/j.trstmh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Marianneau P, Steffan AM, Royer C, Drouet MT, Jaeck D, Kirn A, et al. Infection of primary cultures of human Kupffer cells by Dengue virus: no viral progeny synthesis, but cytokine production is evident. J Virol. 1999;73:5201–5206. doi: 10.1128/jvi.73.6.5201-5206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thongtan T, Panyim S, Smith DR. Apoptosis in dengue virus infected liver cell lines HepG2 and Hep3B. J Med Virol. 2004;72:436–444. doi: 10.1002/jmv.20004. [DOI] [PubMed] [Google Scholar]
- 59.Couvelard A, Marianneau P, Bedel C, Drouet MT, Vachon F, Hénin D, et al. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum Pathol. 1999;30:1106–1110. doi: 10.1016/s0046-8177(99)90230-7. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Maguire T, Marks RM. Demonstration of binding of dengue virus envelope protein to target cells. J Virol. 1996;70:8765–8772. doi: 10.1128/jvi.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaturvedi UC, Elbishbishi EA, Agarwal R, Raghupathy R, Nagar R, Tandon R, et al. Sequential production of cytokines by dengue virus-infected human peripheral blood leukocyte cultures. J Med Virol. 1999;59:335–340. doi: 10.1002/(sici)1096-9071(199911)59:3<335::aid-jmv13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 62.Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82:e177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 64.Matsuura H, Kishida M, Nakata Y, Hirata K, Sasaki E, Kiura Y. Dengue rash: white islands in a sea of red. Postgrad Med J. 2019;95:676. doi: 10.1136/postgradmedj-2019-136976. [DOI] [PubMed] [Google Scholar]
- 65.Jayaratne S, Atukorale V, Gomes L, Chang T, Wijesinghe T, Fernando S, et al. Evaluation of the WHO revised criteria for classification of clinical disease severity in acute adult dengue infection. BMC Res Notes. 2012;5:645. doi: 10.1186/1756-0500-5-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020 [cited 2020 Apr 23]; Available from: http://link.springer.com/10.1007/s00277-020-04019-0 [DOI] [PMC free article] [PubMed]
- 67.Chan JF-W, Kok K-H, Zhu Z, Chu H, To KK-W, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, et al. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srichaikul T, Nimmannitya S, Sripaisarn T, Kamolsilpa M, Pulgate C. Platelet function during the acute phase of dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 1989;20:19–25. [PubMed] [Google Scholar]
- 70.Funahara Y, Ogawa K, Fujita N, Okuno Y. Three possible triggers to induce thrombocytopenia in dengue virus infection. Southeast Asian J Trop Med Public Health. 1987;18:351–355. [PubMed] [Google Scholar]
- 71.Wang S, He R, Patarapotikul J, Innis BL, Anderson R. Antibody-enhanced binding of dengue-2 virus to human platelets. Virology. 1995;213:254–257. doi: 10.1006/viro.1995.1567. [DOI] [PubMed] [Google Scholar]
- 72.Boonpucknavig S, Vuttiviroj O, Bunnag C, Bhamarapravati N, Nimmanitya S. Demonstration of dengue antibody complexes on the surface of platelets from patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 1979;28:881–884. [PubMed] [Google Scholar]
- 73.Na-Nakorn S, Suingdumrong A, Pootrakul S, Bhamarapravati N. Bone-marrow studies in Thai haemorrhagic fever*. Bull World Health Organ. 1966;35:54–55. [PMC free article] [PubMed] [Google Scholar]
- 74.Nakao S, Lai CJ, Young NS. Dengue virus, a flavivirus, propagates in human bone marrow progenitors and hematopoietic cell lines. Blood. 1989;74:1235–1240. [PubMed] [Google Scholar]
- 75.Seshi B, Kumar S, Sellers D. Human bone marrow stromal cell: coexpression of markers specific for multiple mesenchymal cell lineages. Blood Cells Mol Dis. 2000;26:234–246. doi: 10.1006/bcmd.2000.0301. [DOI] [PubMed] [Google Scholar]
- 76.Laur F, Murgue B, Deparis X, Roche C, Cassar O, Chungue E. Plasma levels of tumour necrosis factor alpha and transforming growth factor beta-1 in children with dengue 2 virus infection in French Polynesia. Trans R Soc Trop Med Hyg. 1998;92:654–656. doi: 10.1016/s0035-9203(98)90800-8. [DOI] [PubMed] [Google Scholar]
- 77.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, et al. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 78.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol Baltim Md 1950. 1998;161:6338–6346. [PubMed] [Google Scholar]
- 79.Raghupathy R, Chaturvedi UC, Al-Sayer H, Elbishbishi EA, Agarwal R, Nagar R, et al. Elevated levels of IL-8 in dengue hemorrhagic fever. J Med Virol. 1998;56:280–285. doi: 10.1002/(sici)1096-9071(199811)56:3<280::aid-jmv18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 80.Setrkraising K, Bongsebandhu-phubhakdi C, Voraphani N, Pancharoen C, Thisyakorn U, Thisyakorn C. D-dimer as an indicator of dengue severity. 2007;1:5.
- 81.Ab B, Ok S. The distinct pattern of DIC among the patients with dengue virus infection, Red Sea State. Sudan. 2013;4:4. [Google Scholar]
- 82.Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136:373. [PMC free article] [PubMed] [Google Scholar]
- 83.da Costa PSG, Ribeiro GM, Junior CS, da Costa Campos L. Severe thrombotic events associated with dengue fever, Brazil. Am J Trop Med Hyg. 2012;87:741–742. doi: 10.4269/ajtmh.2012.11-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Venugopal A. Disseminated intravascular coagulation. Indian J Anaesth. 2014;58:603–608. doi: 10.4103/0019-5049.144666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Browne P, et al. COVID-19 coagulopathy in Caucasian patients. Br J Haematol. 2020;bjh.16749. [DOI] [PMC free article] [PubMed]
- 86.McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. Elsevier; 2020 [cited 2020 May 12];0. Available from: https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(20)30121-1/abstract. [DOI] [PMC free article] [PubMed]
- 87.Belen-Apak FB, Sarıalioğlu F. Pulmonary intravascular coagulation in COVID-19: possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J Thromb Thrombolysis. 2020:1–3. [DOI] [PMC free article] [PubMed]
- 88.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 90.Chan PKS, Chen GG. Mechanisms of lymphocyte loss in SARS coronavirus infection. Hong Kong Med J Xianggang Yi Xue Za Zhi. 2008;14(Suppl 4):21–26. [PubMed] [Google Scholar]
- 91.La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol. 1995;8:249–270. doi: 10.1016/s0950-3536(05)80240-9. [DOI] [PubMed] [Google Scholar]
- 92.Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 93.Lin SF, Liu HW, Chang CS, Yen JH, Chen TP. Hematological aspects of dengue fever. Gaoxiong Yi Xue Ke Xue Za Zhi. 1989;5:12–16. [PubMed] [Google Scholar]
- 94.Thein T-L, Lye DC, Leo Y-S, Wong JGX, Hao Y, Wilder-Smith A. Severe neutropenia in dengue patients: prevalence and significance. Am J Trop Med Hyg. 2014;90:984–987. doi: 10.4269/ajtmh.14-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Covert COVID-19 and false-positive dengue serology in Singapore - The Lancet Infectious Diseases. 2020 [cited 2020 Apr 25]. Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30158-4/fulltext. [DOI] [PMC free article] [PubMed]
- 96.Prasitsirikul W, Pongpirul K, Pongpirul WA, Panitantum N, Ratnarathon AC, Hemachudha T. Nurse infected with Covid-19 from a provisional dengue patient. Emerg Microbes Infects. 2020;0:1–5. doi: 10.1080/22221751.2020.1775131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bokhari SMMA, Mahmood F, Bokhari SMSA. Case report: diagnosis of novel coronavirus disease (COVID-19) versus tropical diseases in Pakistan. Am J Trop Med Hyg. 2020;tpmd200356. [DOI] [PMC free article] [PubMed]