Highlights
-
•
CIN management is currently surveillance or surgical therapy. This review describes current research on medical therapies.
-
•
Imiquimod is the most studied with evidence of safety and efficacy.
-
•
5-Fluorouracil has also shown promise with two clinical trials showing efficacy as adjuvant and primary treatment.
-
•
Antivirals therapies have produced mixed results with cidofovir showing the most potential.
-
•
The data remains weak regarding hormonal, herbal and alternative therapies rending it difficult to draw conclusions.
Keywords: Cervical intraepithelial neoplasia (CIN) 2/3, Cervical dysplasia, Medical management, Topical therapy, Dysplasia treatment review, Excision alternatives
Abstract
Current management of Cervical Intraepithelial Neoplasia (CIN), caused by high-risk human papillomavirus (hr-HPV), is based on surveillance and surgical therapy. Procedures carry potential risks such as preterm birth, and access remains limited throughout the world. However, there are no medical therapies recommended to promote the clearance of hr-HPV infection or CIN. Ultimately, even if less efficacious than excision procedures, medical therapies have the potential to decrease cervical cancer by eliminating barriers to treatment, such as access to treatment, or serving as an adjunct to surgical treatment in both high- and low-resource settings.
This review describes current research on topical therapies with the potential for self-application for the treatment of HPV or CIN. Therapies included are immune-modulators, anti-proliferative medications, antivirals, hormones, and herbal/alternative therapies. Randomized trials of immune-modulating (imiquimod), anti-proliferative (5-fluorouracil), and anti-viral (cidofovir) therapies have had the most promising results. However, no option has sufficient clinical trial evidence to be recommended as treatment for CIN 2–3 and surgery remains the standard of care.
The research described in this review serves as a guide for the development of future trials in the burgeoning arena of topical therapies for CIN 2–3.
1. Introduction
Persistent high-risk human papillomavirus (hr-HPV) infection is a necessary, but not always sufficient precursor to cervical cancer, the leading gynecologic cancer worldwide. (Ferlay et al., 2012) It is estimated that 80% of sexually active women will contract genital HPV by 50 years of age. (Dunne et al., 2007) Persistent hr-HPV infections are the most significant risk factors for the development of high-grade cervical dysplasia (also known as Cervical Intraepithelial Neoplasia or CIN 2–3) and cancer (Koshiol et al., 2008).
Standard-of-care management for CIN 2–3 consists of surgical therapy, which ablates or excises the cervical transformation zone. (Saslow et al., 2012) Ablative procedures consist of cryotherapy, thermal ablation or laser therapy. Excisional options include a loop electrosurgical excision procedure (LEEP) or cold knife cone (CKC), which remove one to three centimeters of cervical tissue. Although surgical therapy is highly successful in the majority of people, 5–16% of people with CIN 2–3 will have a recurrence of disease within 5 years of an excisional procedure; most recurrences will be within two years. (Katki et al., 2013, College, 2013) In high-risk people, such as those living with HIV in low- or middle-income countries, the risk can be as high as 19–37% (Smith et al., 2017, Greene et al., 2017).
The overall success of excisional treatments is high, and the immediate post-procedure risks, which are few, are minor. These include bleeding, infection and pain. However, there are long-term risks to consider, particularly in people of childbearing age. People who undergo excisional procedures for cervical dysplasia have an increased risk of preterm delivery, 1.56 relative risk for loop excision to 2.70 relative risk with a cold knife cone. (Krygiou et al., 2016) A systematic review and meta-analysis which included 65,082 treated women reported that this risk increases as more cervical tissue is removed through depth of excision or repeated treatments. (Krygiou et al., 2016) There is also psychological distress associated with the need for invasive procedures, (Kola and Walsh, 2009, Rogstad, 2002) as well as significant individual and infrastructure costs. (Insinga et al., 2005, Shireman et al., 2001) The estimated cost of the common excisional procedures (cryotherapy, LEEP and cold knife cone) was estimated to cost $112, $407 and $3,739) with a projected annual cost of $75–91 million per 100,000 patients from LEEPs alone, based on 2001 US dollars. (Kleinberg et al., 2003) In a more contemporary analysis, the cost of cervical HPV-related disease is estimated to cost the US $4 billion annually, as adjusted to 2004 US dollars. The development of nonsurgical, noninvasive, and patient-controlled modes of treatment have the potential to decrease the long-term morbidity and cost of treatment.
Cervical cancer is a cancer of economic, social, and educational disparities. A medical therapy option could overcome many of the barriers to surgical therapy – desire for child-bearing, cost, health provider skill, equipment, geography, and patient fear. (Yabroff et al., 2005) Ultimately, even if less efficacious than excision procedures, medical therapies have the potential to decrease cervical cancer by eliminating barriers or as an adjunctive to surgical treatment to increase the efficiency of therapy. (Desravines et al., 2020, Maiman et al., 1999) Additionally, primary medical therapies would address a treatment bottleneck in both high- and low-resource settings, by allowing people to begin treatment immediately.
There are currently no topical therapies recommended to promote the clearance of hr-HPV infection or CIN however this is in large part due to the paucity of available data on the subject. This systematic review will discuss the published literature on topical therapies, exclusively those with the potential for patient self-application as a medical therapeutic alternative for the treatment of CIN 2/3.
2. Methods
2.1. Search methods
A medical librarian developed search strategies using a combination of keywords and MeSH and Emtree subject headings. As for the therapies, the search terms utilized were:” fluorouracil”, “5-FU”, “5-fluorouracil”, “adrucil”, “carac”, “efudix”, “efudex”, “fluoro-uracile”, “fluoro aracile”, “fluoroplex”, “fluorouracile”, “tolak”, “neofluor”, “cidofovir”, “imiquimod”, “flurorophyrimidine”, “curcumin”, “interferon-alpha”, “interferon”, “antimetabolites”, and “antineoplastic”. For disease site and status, the search terms were “cervix uteri”,”cervical”, “cervix”, “uterine”, “uterus”, “endocervix”, “endocervical”, “intra-epithelial”, “intraepithelial”, “dysplasia”, “cervical intraepithelial neoplasia”, or “uterine cervical dysplasia”. Additional keywords and Mesh terms were used for drug formulation and application method. The complete search terms included in the query are noted in Supplemental Figure 2. These were used to search PubMed via NLM, Embase via Elsevier, Web of Science, and ClinicalTrials.gov from the date of database inception to September 21, 2018, when all searches were completed.
2.2. Inclusion and exclusion
Articles were reviewed according to prospectively determined inclusion and exclusion criteria. Treatments with the potential for self-application were chosen for inclusion. In instances in which the topical therapy was applied by a medical provider, these studies were excluded. Treatments requiring or including provider-administration (injections, ablative therapies, excisional procedures, and photodynamic therapy) were excluded from the review. Animal and in-vitro studies were also excluded along with conference reports, conference abstracts, and editorials.
2.3. Data extractions
Citations were exported to Endnote and evaluated for duplicity. An initial review of all abstracts and titles were completed by two independent gynecologists that evaluated for pertinence and applicability. Any conflicts between the two reviewers were resolved by a third gynecologist. Remaining abstracts were then be reviewed in its entirety for appropriateness based on the aforementioned inclusion and exclusion criteria. All studies that were determined applicable to the topical therapies for the treatment of CIN 2/3 were utilized for the completion of this review. We followed a systematized process, based on the PRISMA reporting standards for systematic reviews. The nature of these studies were heterogenous and lacked high quality studies. Study authors determined that this information was not amenable to a quantitative synthesis and thus the results of this research are presented as an expert narrative synthesis. The final studies included in this narrative review are outlined in Table 1.
Table 1.
Summary of Topical Therapies for the treatment of Cervical Intraepithelial Neoplasia (CIN) 2/3.
| Study | Study Type | Dysplasia | Regimen | Sample Size | Diagnostic Testing | Findings |
|---|---|---|---|---|---|---|
| IMMUNE MODULATORS | ||||||
| Imiquimod | ||||||
| Lin 2012 | Prospective Cohort | CIN or VAIN | Twice weekly for 12 doses | 72 | Histology 6 months post-treatment | 51.4% with histological regression while 8.3% had progressive histology |
| Grimm 2012 | RCT | CIN 2/3 | Titrating 1–3 vaginal suppositories over 16 weeks | 24(per group) | 4 quadrant colposcopic-guided biopsies at 20 weeks | 73% regression to low grade or normal vs. 39% in the placebo group |
| Diaz-Arrastia 2001 | Prospective Case series | HSIL of vulvar, vagina or cervix | Application three times weekly for 6–16 weeks | 8(2 cervical dysplasia) | Histology by 1 month | 50% with complete resolution, 25% with regression to low grade |
| (Chen, 2013) | Retrospective case series | CIN or VAIN already treated with surgery | Twice weekly for 8 weeks | 76 | Repeat cytology/HPV 3 mos. after treatment | 76.3% with normal pap smear. No histology performed. |
| Pachman 2012 | RCT | CIN 2/3, recur or pers CIN I | 1 packet every 4 days for 5 application then ablative vs. excisional therapy | 56 | Cytology +/- histology at 4 mos then q4-6 mos. Endpoint: persis/recur CIN by 2 years | No difference dysplasia (CIN2/3 or recurrent CIN with same HPV type) between 2 groups (25% vs. 21% in control) |
| Trans retinoic Acid | ||||||
| Weiner 1986 | Prospective cohort | Mild, mod, sev dysplasia | Daily for 4 days; 2 different concentration cohorts | 42 | Histology at 5 – 18 months | 14% (2/14) and 45% (10/22) with resolution in the low and high concentration |
| Meyskens 1983 | Phase I prospective cohort | Mild or mod CIN | Escalating doses of TRA for 4 consecutive days | 35 | Clinical and colposcopic examination of side effects | No systemic effects, mild cervical inflammation at higher doses, high vaginal toxicity at 0.484% |
| Graham 1986 | Phase II prospective cohort | Mild, mod, sev dysplasia | Cervical cap for 4 days then 2-days every 3 months for one year | 25 | Cytology and Histology | 50% (10/20) histologic and cytologic repression regression of disease |
| DiSilvestro 2001 | Prospective cohort | CIN 2/3 | Gel on sponge inside cervical cap for 4, 8 or 14 days | 52 | Colposcopic directed histology 90 days after treatment | 46.9% regression. 53.1% with no change. |
| ªChen 1994 | RCT | NR | Once daily for 50 days | NR | 74.3% regression after two-courses of treatment | |
| Surwit 1982 | Prospective Cohort | CIN 2/3 | Daily applications for 4 days | 18 | Conization of cervix 4 weeks post- treatment | Reduced lesion size in 33% and complete regression in 11% |
| Meyskens 1994 | Phase III RCT | CIN 2/3 | Cervical sponge daily for 4 days then 2 days at 3 and 6 mos. | 301 | Cytology and colposcopic guided histology at 15 months | Histologic resolution in 43% vs. 27% in CIN 2 placebo group. No difference in the CIN3 group. |
| Ruffin 2004 | Prospective cohort | CIN 2/3 | 4 doses daily at different dosages | 175 | Histology at 12 weeks | Response to therapy not statistically significant in all 4 dose groups |
| Interferon | ||||||
| Krause 1987 | Prospective Cohort | CIN 3 | Interferon gel in vaginal cup for 4 consecutive weeks | 9 | Conization after 4 weeks | 22% (2/9) with complete resolution |
| Moller 1983 | Prospective Cohort | CIN 2/3, CIS | Interferon gel via cervical cap2 times per week for 6 weeks | 6 | Histology at 6 and 12 wks | 50% (3/6) with “light dysplasia” |
| Schneider 1995 | Phase II RCT | CIN 2/3 | 3 concentrations of interferon gel for 28 days via diaphragm | 33(24 therapy vs 9 laser) | Cytology and histology with HPV typing after 6 months | 42% (10/24) vs 89% (8/9) with resolution and 42% (10/24) vs 11% (1/9) with regression |
| Yliskoski 1990 | RCT | CIN or VAIN | Vaginal gelnightly for 2 weeks, multiple courses over 1 year | 19(9 interferon vs 10 placebo) | HPV genotyping at 16 months | 67% (6/9) HPV negative |
| Choo 1985 | Prospective cohort | Mod, sev dysplasia, CIS | Twice daily application of gel via vaginal applicator | 7 patients | Colposcopy – no histology | 28% (2/7) resolution, 43% (3/7) regression and 28% (2/7) with no response on colposcopy |
| Granulocyte-macrophage colony stimulation factor (GM-CSF) | ||||||
| Hubert 2010 | RCT | CIN 1 | Gel applied every 3 days for 4 doses with applicator | 26 (15 CIN 1 and 11 controls) | Plasma HPV viral load and histology at 26–30 months | Decreased HPV viral load; no clinical response based on histology. |
| ANTIPROLIVERATIVE | ||||||
| 5-Fluorouracil (5FU) | ||||||
| Maiman 1999 | RCT | CIN 2/3 | Cream applied once every 2 weeks for 6 months after excision | 101 (50 therapy and 51 controls) | Histology at up to 18 mos | 28% (14/50) vs. 47% (24/51) with recurrent CIN 2/3 |
| (Barten, 1987) | Prospective Cohort | CIN 1,3 | Cervical cap daily for 7 days | 10 | Histology at 6–12 mos | 20% (2/10) resolution, 40% (4/10) with regression to CIN1, and 40% (4/10) with persistent CIN1/CIS |
| Sillman 1981 | Prospective cohort | Vulvar, vaginal,cervical mod, sev dysplasia | Cream applied nightly for 2 weeks then once monthly | 16 | Histology | 100% (16/16) with resolution |
| Rahangdale 2014 | RCT | CIN 2 | Cream every 2 weeks for 16 weeks | 60 (31 treatment vs 29 placeb0) | Histology at 6 months | 93% (26/28) vs. 56% (15/27) with regression |
| Pride 1982 | Prospective Cohort | Vaginal/Cervical dysplasia | Nightly for 10 nights via diaphragm | 11 | Conization or hysterectomy | 55% (6/11) with resolution |
| Sidhu 1997 | RCT | CIN 1/2 | Bio-adhesive film using applicator | 104 (51 with therapy vs 53 with placebo | Histology at 6 months | 67% (32/48) and 72% (33/46) regression or resolution in the treatment and placebo group. |
| Cisplatin | ||||||
| ªNakayama 1992 | Prospective cohort | mild/mod/sev and “microinvasive ca” | Daily placement via gauze tampon for 10 days. | 12 | Histology at day 10 | 100% with resolution in CIN group (10/10) and 50% (1/2) regression in microinvasive ca group |
| Iodosteric Acid | ||||||
| †Gleeson 1992 | RCT | CIN 2/3 | Application with pessary vs. placebo for 30 nights | NR | Histology at 30 days | No clinically significant difference in the 2 groups |
| ANTIVIRALS | ||||||
| Cidofovir | ||||||
| VanPachterbeke 2009 | RCT | CIN 2,3 | Gel via cervical cap once 6 weeks prior to conization | 48 (23 treatment vs 25 placebo) | Conization | 61% (14/23) cidofovir vs 20% (5/25) placebo with resolution |
| Snoeck 2000 | Prospective Cohort | CIN 3 | Gel applied every other day for 3 applications | 15 | Histology within 1 month | 47% 7/15 with resolution, 7% (1/15) with regression to CIN I, 33% (5/15) with persistent CIN 2/3 |
| Bossens 2018 | Prospective cohort | CIN 2,3 | Gel applied once per week for 3 weeks for 5 or 10 h | 9 | N/A | No clinically significant adverse events |
| Lopinavir and Ritonavir | ||||||
| Hampson 2016 | Prospective Cohort study | HSIL | Vaginal pessary twice daily for 2 weeks | 23 | Cytology at 12 weeks | 63.6% with no dysplasia, 18.2% with low grade |
| Vidarabine | ||||||
| †Okamoto 1999 | Cohort study | CIN I and 2 | Vidarabine ointment and/or podophyllin | 21 | Cytology and Histology | 81% (17/21) with regression |
| Niwa 2003 | Cohort study | CIN and Stage IA1 cervical cancer | Regimen not described | 30 | HPV typing by PCR | 10% (1/10) with resolution of HPV infection |
| Terameprocol | ||||||
| Khanna 2007 | Phase I/II clinical trial | CIN 1 and 2/3 | Direct cervical application once weekly for 3 weeks | 7 | N/A | No serious adverse events noted |
| HORMONALS | ||||||
| Progesterone | ||||||
| Hefler 2010 | Phase II clinical trial | CIN 1 | 10 days month for 6 months | 40 | Cytology and histology | 30% regression vs 38.3% in the placebo |
| Dehydroepiandrosterone (DHEA) | ||||||
| Suh-Burgmann 2003 | Prospective Cohort study | LSIL | Daily for up to 6 months | 12 | Histology | 83% (10/13) with no dysplasia |
| HERBAL AND ALTERNATIVE REMEDIES | ||||||
| Alternative Remedies | ||||||
| Joshi 2011 | Prospective cohort | LSIL | Capsules twice daily for 12 weeks | 21 | Histology | 76% (16/21) with resolution to normal and 19% (3/21) unchanged. |
| Ahn 2003 | RCT | Cervicitis, CIN 1, 2 and 3 | 4 groups (1 topical). Ointment twice weekly for 12 weeks | 27 (topical group) | Histology | 74% (20/27) topical vs. 10% (4/39) control with regression |
| Ashrafian 2015 | RCT | CIN 1 and 2 | 3 groups (high dose, low dose, placebo) daily application for 180 days | 25 high dose vs 24 low dose vs 23 placebo | Histology | 100%, 90.5% and 61% regression in high dose, low dose and placebo |
| Shukla 2009 | RCT | HPV 16 infection +/- LSIL | Praneem tablet vaginally daily for 30 days | 20 (10 treatment vs 10 placebo) | HPV 16 PCR | Elimination of HPV 16 in 60% (6/10) |
| Swanick 2009 | Case Report | CIN 2/3 | Escharotic treatment 2x/weekly for 5 weeks | 1 | Cytology and colposcopy | NILM pap smear at 4 and 10 mos and satisfactory colposcopy at 10 mos |
| Valencia 2011 | Prospective Cohort | LSIL cytology | Oral or topical spray for 8 weeks minimum. | 62 | Treatment until improvement per cytology and colposcopy | Improvement in colposcopic appearance in 74% at 12 weeks |
| Laccetta 2015 | Prospective Cohort | ASCUS or LSIL cytology | Two cycles of daily application for 20 days | 356 (176 treatment vs 180 placebo) | Cytology and Colposcopic evaluation at 6 and 12 mos. | 83.5% vs. 60% of controls with negative pap smear. 55.4% vs. 24.7% of controls with no colpo lesions |
| Stentella 2017 | Retrospective case-control | CIN 1/2 | Carbodymethyl beta-glucan gel | 999 | Cytology, histology, colposcopic changes | Nonsignificant regression of CIN 2 |
| Stefani 2014 | Prospective Cohort | CIN 1 | Bovine colostrum containing vaginal tablets | 256 | Histology at 6 months | 75.5% regression |
| †Bottino 1991 | RCT | NR | Cream applied vaginally | 40 in dysplasia group | NR | 70% (28/40) efficacy in dysplasia patients |
ªIn these studies, only an abstract was available in English.
†In these studies, only an abstract was available for review.
RCT = randomized controlled trial; CIN = Cervical Intraepithelial Neoplasia, VAIN = Vaginal intraepithelial neoplasia, HSIL = High grade intraepithelial lesion, LSIL = Low grade intraepithelial lesion, CIS = carcinoma in situ, ASCUS = atypical squamous cells or unknown significance, mos = months, ca = carcinoma, NR = not reported.
3. Results
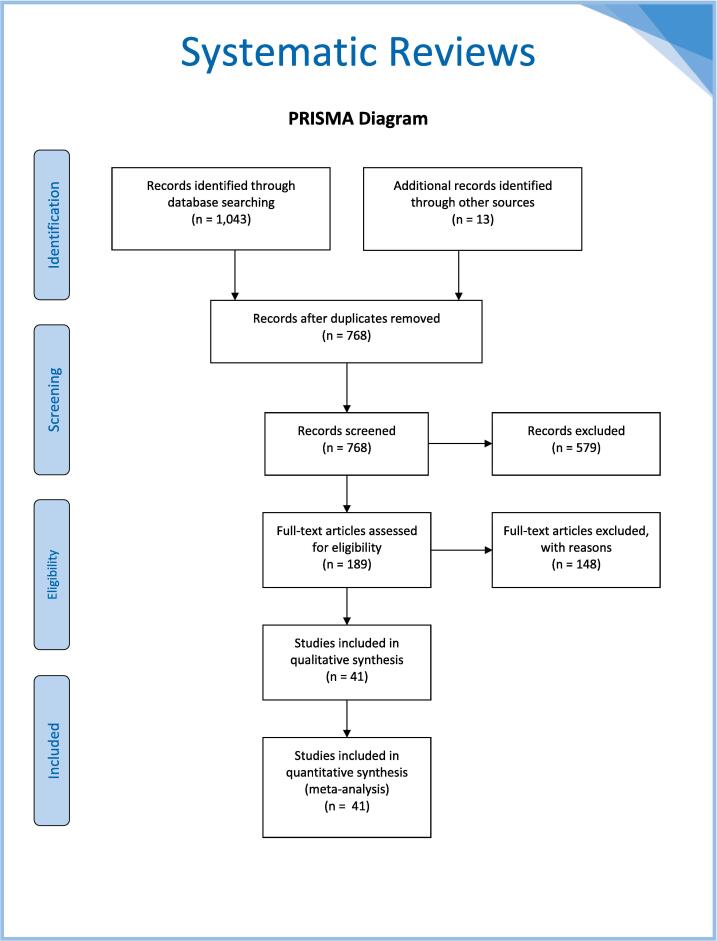
The collective database searches yielded a total of 1,043 citations, with an additional 13 citations found by searching ClinicalTrials.gov. The 1056 total citations were exported to Endnote and 288 duplicates were removed, leaving 768 unique citations found in all searches. Two reviewers (LR, ND) screened titles and abstracts for relevance, excluding 579 irrelevant studies. A third reviewer (KM) reviewed any conflicts. The full text of the remaining 189 papers were then reviewed for relevance and a further 145 studies were exclude for wrong study designs, interventions, outcomes, or patients. Ultimately, 41 studies were included in the review and data extracted. Fig. 1 provides a PRISMA diagram showing the process of study inclusion. Table 1 summarizes the types of therapies that have been studied to date.
Fig. 1.
The PRISMA diagram for database search results of topical therapies for the treatment of Cervical Intraepithelial Neoplasia (CIN) 2/3.
3.1. Immune-modulators
Imiquimod functions at the TLR7 receptor to stimulate the innate immune system and increase production of the cytokines interferon α, interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α). Imiquimod is used to treat genital warts and vulvar/vaginal dysplasia. It has also been studied as a medical therapy for high-grade squamous intraepithelial lesions of the cervix. (Grimm et al., 2012, Pachman et al., 2012, Lin et al., 2012, Chen, 2013) Imiquimod is typically used as a topical cream with concentrations from 1% to 5% applied three to seven times per week for eight to 16 weeks. Local reactions can include erythema, pruritis, excoriation, erosion, edema, scabbing, or crusting. Though used topically with minimal systemic absorption, imiquimod therapy can produce systemic reactions such as myalgia, headache, fatigue, and nausea. (Grillo-Ardila et al., 2014) A review of its use in cervical, vulvar, and vaginal dysplasia also described mild to moderate local and systemic side effects but overall it was well-tolerated. (de Witte et al., 2015) In a review of 3 studies in which imiquimod was utilized for the treatment of CIN, side effects were well tolerated by patients; only 2 out of 132 patients discontinued therapy due to side effects. (de Witte et al., 2015)
De Witt et al reviewed 3 studies and only one was a randomized controlled trial. This randomized controlled trial was conducted by Grimm et al and showed that a 16-week course of self-applied dose escalating intravaginal imiquimod treatment of CIN 2–3 led to histologic regression and HPV clearance (73% versus 39%, p < 0.05 for histologic regression and 60% versus 14%, p < 0.05 for HPV clearance). (Grimm et al., 2012) More than 90% of participants in the imiquimod arm reported both local and systemic (flu-like symptoms and fatigue) symptoms however no participants experiences high-grade side effects and only one discontinued therapy (Grimm et al., 2012).
There are active studies evaluating the use of imiquimod. The “Topical Imiquimod treatment of high-grade Cervical intraepithelial neoplasia” (TOPIC) trial was a 2015 study designed to randomize patients into an imiquimod arm and an immediate treatment arm with excision however was prematurely stopped in 2017 due to poor enrollment. The study design was then converted to a non-randomized efficacy trial entitled TOPIC-3. A related study, TOPIC-2, is a non-inferiority randomized single blinded study in women with recurrent/persistent CIN after previous excisional treatment. (van de Sande et al., 2018) The study anticipates recruiting 433 patients to establish non-inferiority to recurrent excisional procedure. It is estimated to complete in 2020.
Other immune-modulating approaches have included trans-retinoic acid or interferon with both being used to treat viral infections and cancers. Trans-retinoic acid is a metabolite of Vitamin A and plays a key role in mucosal immune responses. Interferons are cytokines produced by the immune system when triggered by viral infection or other immune processes. They have both been studied on low-grade and high-grade CIN since the 1980s with little progress in the development of a viable agent. (Weiner et al., 1986, Meyskens et al., 1983, Krause et al., 1987, Meyskens et al., 1994, Ruffin et al., 2004, Graham et al., 1986, Choo et al., 1985, Yliskoski et al., 1990, Schneider et al., 1995) A Cochrane review of 5 studies utilizing retinoids (given orally and topically) for the treatment of CIN showed that retinoids are not effective in preventing progression in any grade of CIN (Helm et al., 2013).
Studies are notable for the delivery of medication through sponges in cervical caps and vaginalettes (delivery agents soaked in gel) which may be useful in the development of self-applied therapies. (Graham et al., 1986, Surwit et al., 1982, DiSilvestro et al., 2001, Singer et al., 1993) Additionally, a new approach has been the local application of GM-CSF (granulocyte–macrophage colony-stimulating factor), a cytokine produced in response to immune stimuli, in order to increase numbers of antigen-presenting cells (APCs) in CIN lesions. A Phase 1b study of 15 people with CIN 1 reported favorable toxicity, and significant increases in APCs and cytotoxic T-lymphocyte infiltration of cervical biopsies (Hubert et al., 2010).
3.2. Anti-Proliferative therapies
5-fluorouracil (5-FU) is an anti-metabolite which has been used widely to treat malignancies such as colon cancer as well as dermatologic conditions. (Ashton et al., 1970, van de Nieuwenhof et al., 2008, Stanley, 2003) The primary mechanism of 5-FU is to block synthesis of thymidine and prevent DNA replication. For decades, observational studies have demonstrated efficacy in using topical 5-FU for treatment of HPV-related diseases (e.g., genital warts, and vulvar and vaginal dysplasia). (van de Nieuwenhof et al., 2008, Weis, 2013, Stanley, 2003) Previously, 5-FU topical treatment was limited by side effects, including burning, erythema, erosion, pain, and chronic ulceration, because standard treatment regimens required multiple daily applications. (Krebs and Helmkamp, 1991) However, limiting the application of 5-FU to biweekly dosing or decreasing the concentration of 5-FU has shown improved tolerance. (Maiman et al., 1999, Rahangdale et al., 2014)
Small proof-of-concept studies of 5-FU for treatment of CIN were initiated in the 1980s. (Silman et al., 1981, Barten, 1987) Subsequently, Maiman et al. studied intravaginal 5-FU to prevent recurrence of CIN 2–3 after excisional treatment in HIV-infected people. (Maiman et al., 1999) People who self-applied 2 g of 5% intravaginal 5-FU every two weeks for up to six months were less likely to develop a CIN 2–3 recurrence at 18 months than those in the observation arm (31% vs. 8%, p = 0.014). (Maiman et al., 1999) Rahangdale et al used a similar dose schedule (2 g every two weeks) for 16 weeks for primary treatment of CIN 2. (Rahangdale et al., 2014) (Table 1) In this study, 60 people without HIV were randomly assigned to self-applied 5-FU (8 total doses) versus observation. Those in the 5-FU group were more likely to experience disease regression (CIN 1 or normal) (93% 5-FU versus 56% control; p = 0.01). The composite outcome of histology, Pap smear, and hr-HPV results was more likely to be normal in the 5-FU group than the observation arm (RR 2.25, 95% CI 1.05–5.09). In both studies people tolerated the biweekly dosing, and although mild side effects (e.g. bleeding, irritation, and discharge) were common, no moderate or severe side effects were reported (Rahangdale et al., 2014).
A recent case series highlights the role of 5-FU in clinical practice for patients seeking a LEEP alternative in both recurrent and primary CIN 2/3. (Desravines et al., 2020) A Phase 1 study of feasibility of combination therapy with topical 5-FU with imiquimod for women with CIN 2–3 is estimated to complete in 2020 (ClinicalTrials.gov Identifier: NCT03196180).
3.3. Antivirals
Cidofovir is an acyclic nucleoside phosphonate derivative with broad-spectrum anti-DNA virus activity and an extended half-life, allowing for infrequent application. It is used as treatment for vulvar and non-genital HPV lesions as well as for viruses such as herpes, Mollusca contagiosa, and adenovirus. Studies of cidofovir gel have shown promising results for clearance of CIN with minimal toxicity. (Snoeck et al., 2000, Van Pachterbeke et al., 2009, Bossens et al., 2018) Previous dosing regimens have ranged from three times every other day to three times per week to once per week. Van Pachterbeke, et al, reported a randomized, placebo-controlled trial of 2% cidofovir gel applied three times in one week in 48 people with CIN 2–3. Subsequent cervical biopsy demonstrated that 61% cidofovir versus 20% placebo (p < 0.01) demonstrated regression or clearance of CIN 2–3 (Van Pachterbeke et al., 2009) (Table 1).
Lopinavir and ritonavir, a combination oral HIV protease inhibitor (Lopimune) has been studied as potential treatment for CIN. (Hampson et al., 2006, Lahiri et al., 2015, Batman et al., 2011) Hampson, et al conducted an exploratory study of 23 HIV-uninfected people with hr-HPV and HSIL cytology. (Hampson et al., 2016) Each person self-inserted one lopinavir/ritonavir combination capsule per vagina twice a day for two weeks. Cytology and hr-HPV testing at 12 weeks showed 82% regression in cytology results from HSIL to either low grade or no dysplasia; 78% of these were confirmed by histology. The therapy was well-tolerated with local side effects of vaginal discharge and irritation, and systemic side effects of headache, nausea, and abdominal pain. (Hampson et al., 2016)
Other antivirals being studied include Vidarabine and Terameprocol. Vidarabine, a DNA polymerase inhibitor used for treatment of HSV, has been applied successfully as an ointment in conjunction with podophyllin or compared to 5-FU for treatment of HPV infection in studies of ten to 28 people. (Niwa et al., 2003, Okamoto et al., 1999) Terameprocol (meso-tetra-o-methyl nordihydroguaiaretic acid, EM-1421) is a transcription inhibitor that selectively interferes with HPV viral genes E6 and E7. A phase 1 study of people with CIN 1–3 treated with terameprocol demonstrated safety and a maximum tolerated dose of 1% and 2% ointments (Okamoto et al., 1999, Khanna et al., 2007).
3.4. Hormonal
Hormones have been studied as topical therapy for CIN 1. Though there is growing interest in developing treatment for CIN 1, these HPV-related lesions are usually observed and not routinely treated with surgical therapy. (Saslow et al., 2012) Vaginal progesterone has previously been shown to increase the vaginal presence of Langerhans cells, which are critical to the local immune response to HPV. People with CIN 1 were treated with intravaginal micronized progesterone suppositories (400 mg) inserted daily for ten days per month for six months. The progesterone group experienced less histologic regression (30%) than the control group (38%) (HR 3.8, 95% CI 1.8–8.3). (Hefler et al., 2010) Dehydroepiandrosterone (DHEA) is an adrenal steroid with immune modulation and tumor inhibition functions. A pilot study of 12 people with CIN 1 treated with intravaginal DHEA for three months reported regression in 83% (10/12) and was well-tolerated. (Suh-Burgmann et al., 2003) No hormonal therapies have been studied as alternative therapies for CIN 2–3.
3.5. Herbal/alternative
A variety of alternative herbal or non-pharmacologic options have been studied for treatment of HPV and CIN with mixed results. A prospective randomized placebo controlled trial study of CIN 1–2 treated with self-applied diindolylmethane vaginal suppositories found that treatment groups at 100 mg/day and 200 mg/day reported regression of 91% (95% CI 70–99%) and 100% (95% CI 82–100%), respectively, compared to placebo 61% (95% CI 36–83%). (Ashrafian et al., 2015) However, the distribution of CIN 1 and CIN 2 was not described. Histologic regression was not defined, and participants with normal appearing colposcopy were not biopsied. (Table 1) A case report of use of Sanquinaria canadensis (bloodroot) used as escharotic treatment has described successful treatment of CIN 2–3 in a 20-year old (Swanick et al., 2009) A retrospective study of beta-glucan included CIN 2–3 lesions and did not show benefit (Stentella et al., 2017).
Studies of green tea extract, Azadirachta indica (neem), beta-glucan, bovine colostrum, curcuma (tumeric) and glucyrrhizinic acid (licorice root) have demonstrated tolerability and possible efficacy in treatment of HPV infections or CIN 1. (Stentella et al., 2017, Ahn et al., 2003, Joshi et al., 2011, Shukla et al., 2009, Laccetta et al., 2015, Stefani et al., 2014, Valencia et al., 2011) Ahn et al. in 2003 in an RCT found that application of a green tea cervical ointment twice weekly for 12 weeks results in the regression of 74% (20/27) of those in the treatment group versus 10% (4/39) in the control group however, the cohort contained with cervicitis, CIN 1, 2 and 3 thus limiting the inferences on efficacy that could be made from this group. (Ahn et al., 2003) The prospective study of glycyrrhizinic acid had participants using an oral or topical spray until there was improvement as noted on pap smear and colposcopy but not necessarily histology. (Valencia et al., 2011) There next two studies utilizing beta glucan with mixed results. (Stentella et al., 2017, Laccetta et al., 2015) Laccetta et al. described women with ASCUS and LSIL cytology and found a significant regression of visual colposcopic lesions (55% v. 25%) meanwhile Stentella et al. found a nonsignificant difference in a group of women with CIN 1 or 2. (Stentella et al., 2017, Laccetta et al., 2015)
A 2009 study of praneem, the seed extract of Azadirachta indica, reported high risk HPV 16 elimination in 60% (6/10) in the treatment compared to 10% (1/10) in the placebo group. (Shukla et al., 2009) A single study evaluating the effect of curcuma (tumeric) vaginal capsules in women with LSIL cytology found 76% resolution of dysplasia (16/21) however, there was no control group for comparison. (Joshi et al., 2011) A pilot study of bovine colostrum tablets in women with CIN 1 found 75% regression of lesions based on histology. Studies of herbal remedies were either small pilot, feasibility, or proof of concept studies that demonstrated possible efficacy but conclusions remain elusive.
4. Discussion
There has been a long-standing and continued interest in the development of a safe, effective topical therapy for treatment of CIN and HPV. A medical therapy option could overcome many of the barriers to surgical therapy – desire for child-bearing, cost, health provider skill, equipment, geography, and patient fear. (Yabroff et al., 2005) In this review, we have summarized the available data on immune modulators (imiquimod, transretinoic acid, interferon and GM-CSF), anti-proliferative therapy (5-Fluorouracil), antivirals (cidofovir, lopinavir/ritonavir, vidarabine and terameprocol), hormonals (progesterone, DHEA) and herbal supplements. Overall, the randomized trials of immune-modulating (imiquimod), anti-proliferative (5-FU), and anti-viral (cidofovir) therapies have had the most promising results, but there is still more need for study.
Of the immune modulators, there has been little progress with transretinoic acid and interferons for a viable agent. The metanalysis of the 5 retinoids have not found them to effective. The GM-CSF study is in its infancy as a Phase 1b study. Imiquimod shows the most promise in this group. There is no conclusive data at this time but multiple studies using imiquimod for the treatment of CIN 2–3 as primary therapy or in conjunction with HPV vaccines are ongoing, specifically the TOPIC-2 and TOPIC-3 trial. (Koeneman et al., 2016) The results of both of these studies will better inform our knowledge of imiquimod as topical therapy in primary and recurrent cervical dysplasia compared with excision therapy.
5-FU, an antiproliferative agent, has been limited by its side effect profile in the treatment of other sites. Modified regimens have shown better patient tolerance in the treatment of recurrent and primary cervical dysplasia. (Desravines et al., 2020, Maiman et al., 1999, Rahangdale et al., 2014) There is interest from patients in using this therapy as evidenced by the recent case series featuring 25 women. There is also a phase I study of feasibility using combination therapy of topical 5-FU with imiquimod. However, more research is still needed to understand the potential of this agent as a treatment for CIN 2/3.
Of the antivirals, cidofovir has demonstrated the most promising results largely based on the van Pachterbeke randomized control trial which demonstrated regression of CIN 2–3. The work on terameprocol is early with information arising from a Phase 1 trial. Vidarabine at this time has only been used in conjunction with other topical treatments. Only an exploratory analysis is available for lopinavir and ritonavir and more research is needed on this modality.
No conclusions can be made regarding utility of hormonal and herbal therapies due to study design, inclusion criteria or limited sample size. The progesterone and DHEA studies enrolled participants with low grade dysplasia (CIN 1) where standard of care is observation. This was a similar concern in the diindolylmethane, an herbal therapy, where participants had either CIN I or 2 and the distribution of disease was not described. Beta gluten is not effective and there is only a singular report of using sanquinara canadensis.
Limitations of this review include the low quality of clinical studies, few randomized controlled trials, and small sample sizes showing nonsignificant results. Regimens and inclusion criteria often varied so results were not comparable or generalizable. Strengths of this paper include that this was a standardized review using reproducible methods on a unique contemporary topic. The review highlights our current understanding of the topic in order to guide future directions for research on topical therapies for HPV or CIN.
Important aspects of a topical therapy to consider in the development of future therapies for treatment of HPV or CIN are safety, efficacy, delivery, and accessibility. Ideally, a topical therapy would be patient-controlled though either provider- or self- administered methodologies continue to be studied. All studies must have rigorous assessment of safety including monitoring for local and systemic adverse events and consideration of fetal risk with unplanned pregnancy. An observation period of six months with careful screening and follow up is considered reasonable when considering risk of progression. (Silverman et al., 2002) Sample size calculations must consider a threshold for efficacy which allows topical therapy to be comparable with current standard surgical options. Delivery methods ranged from a capsule or suppository to inserting the cream via vaginal applicator, sponge, pessary, or ring. As topical therapies are developed, the ease of administration and feasibility of the delivery method is critical to patient acceptance. A final consideration is accessibility. Any product in development will require extensive study including cost in order to achieve regulatory approval for sale in the market. This cost may make topical therapy unfeasible for high-risk, under-insured individuals who are most at risk for developing cervical cancer. Of the therapies described, imiquimod and 5-FU are already in use as topical therapies, have generic formulations, and have been studied as self-applied preparations. There may be an advantage to exploring therapies that can be procured at a lower cost for ease of scale up.
5. Conclusion
At this time, there is no option with sufficient clinical trial evidence or proven efficacy to recommend topical therapy as a primary treatment for HPV infection or CIN. Unanswered questions about topical therapies include effectiveness outside of the research setting, patient acceptability, and long-term efficacy. But there is potential that by combining differing mechanisms of action (e.g. immune modulation and anti-proliferative therapy), neoadjuvant or adjuvant approaches to surgery, and/or in conjunction with HPV vaccines, more effective and accessible treatment regimens will be created with a lower risk profile. This approach may also be useful for providing options to people desiring alternative or less-invasive management, and be readily available in settings where there are treatment delays or limited access. While surgical therapy is the gold standard and is highly effective in treating CIN 2–3, an effective topical therapy may provide an accessible additional option in our cancer prevention toolkit.
6. Contribution to authorship
ND participated in the carrying out, analysis, and writing of the paper. KM participated in the planning, carrying out, analysis, and writing of the paper. RC created the search strategies and participated in the planning, carrying out, and writing of the paper. CC participated in the analysis and writing of the paper. LR led the conception, planning, carrying out, analysis, and writing of the paper. All authors have provided substantial contribution and are in agreement with all aspects of the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2020.100608.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ahn W.S., Yoo J., Huh S. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003;12:383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- Ashrafian L, Sukhikh G, Kiselev V, et al. Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolymethane’s efficacy and safety in the treatment of CIN: implications for cervical cancer prevention. EPMA J. 2015;6:doi: 10.1186/s13167-13015-10048-13169. [DOI] [PMC free article] [PubMed]
- Ashton H., Beveridge G.W., Stevenson C.J. Topical treatment of skin tumours with 5-fluorouracil. Br. J. Dermatol. 1970;82(2):207–209. doi: 10.1111/j.1365-2133.1970.tb15018.x. [DOI] [PubMed] [Google Scholar]
- Barten G. Local treatment of cervical intraepithelial neoplasia using 5 percent 5-fluoruracil cream. Zentralbl. Gynakol. 1987;109:1510–1516. [PubMed] [Google Scholar]
- Batman G., Hampson L., Hampson I.N. Lessons from repurposing HIV drugs: a prospective novel strategy for drug design. Future Virology. 2011;6:1021–1023. [Google Scholar]
- Bossens M., Van Pachterbeke C., De Maertelaer V. Safety and tolerance of cidofovir as a 2% gel for local application in high-grade cervical intraepithelial neoplasia: A phase 1 investigation. Int. J. Clin. Pharmacol. 2018;56:134–141. doi: 10.5414/CP203126. [DOI] [PubMed] [Google Scholar]
- Chen F.P. Efficacy of imiquimod 5% cream for persistent human papillomavirus in genital intraepithelial neoplasm. Taiwanese J. Obstetrics Gynecol. 2013;52(4):475–478. doi: 10.1016/j.tjog.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Choo Y., Hsu C., Seto W.H. Intravaginal application of leukocyte interferon gel in the treatment of cervical intraepithelial neoplasia (CIN) Arch Gynecol. 1985;237:51–54. doi: 10.1007/BF02133952. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gyncologists. Management of abnormal cervical cancer screening test results and cervical cancer precursors. Practice Bulletin No. 140. Obstet Gynecol. 2013;122:1338-1367. [DOI] [PubMed]
- de Witte C.J., van de Sande A.J.M., van Beekhuizen H.J., Koeneman M.M., Kruse A.J., Gerestein C.G. Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: a review. Gynecol. Oncol. 2015;139:377–384. doi: 10.1016/j.ygyno.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Desravines N, Chibwesha CJ, Rahangdale L. Low dose 5-fluorouracil intravaginal therapy for the treatment of cervical intraepithelial neoplasia 2/3: A case series. J. Gynecol. Surg. 2020;36.
- DiSilvestro P.A., DiSilvestro J.M., Lernhardt W., Pfahl M., Mannel R.S. Treatment of cervical intraepithelial neoplasia levels 2 and 3 with adapalene, a retinoid-related molecule. J. Low Genit Tract. Dis. 2001;5:33–37. doi: 10.1046/j.1526-0976.2001.51007.x. [DOI] [PubMed] [Google Scholar]
- Dunne E.F., Unger E.R., Sternberg M. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Ervik M., et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 1International Agency for Research on Cancer. http://globocan.iarc.fr. Published 2013. Accessed Jan 9, 2014.
- Graham V., Surwit E.S., Weiner S., Meyskens F.L. Phase II trial of beta-all-transretinoic acid for cervical intraepithelial neoplasia via a collagen sponge and cervical cap. West. J. Med. 1986;145:192–195. [PMC free article] [PubMed] [Google Scholar]
- Greene SA, Nyongesa-Malava E, Richardson BA, et al. Randomized trial of LEEP vs cyrotherapy to treat CIN2/3 in HIV-infected women. Conference on Retroviruses and Opportunistic Infections; 2017; Seattle, WA.
- Grillo-Ardila C.F., Angel-Muller E., Salazar-Diaz L.C., Gaitan H.G., Ruiz-Parra A.I., Lethaby A. Imiquimod for anogenital warts in non-immunocompromised adults. Cochrane Database Syst. Rev. 2014:11. doi: 10.1002/14651858.CD010389.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C., Polterauer S., Natter C. Treatment of cervical intraepithelial neoplasia with topical imiquimod: a randomized controlled trial. Obstet. Gynecol. 2012;120(1):152–159. doi: 10.1097/AOG.0b013e31825bc6e8. [DOI] [PubMed] [Google Scholar]
- Hampson L., Kitchener H.C., Hampson I.N. Specific HIV protease inhibitors inhibit the ability of HPV 16 to degrade p53 and selectively kill E6-dependent cervical carcinoma cells in vitro. Antivir. Ther. 2006;11:813–825. [PubMed] [Google Scholar]
- Hampson L., Maranga I., Masinde M.S. A single-arm, proof-of-concept trial of lopimune (lopinavir/ritonavir) as treatment for HPV-related pre-invasive cervical disease. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0147917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefler L., Grimm C., Tempfer C., Reinthaller A. Treatment with vaginal progesterone in women with low-grade cervical dysplasia: a phase II trial. Anticancer Res. 2010;30:1257–1261. [PubMed] [Google Scholar]
- Helm C.W. et al. Retinoids for preventing the progression of cervical intra-epithelial neoplasia. Cochrane Systematic Review. 2013. [DOI] [PMC free article] [PubMed]
- Hubert P., Doyen J., Capelle X. Local applications of GM-CSF induce the recruitment of immune cells in cervical low-grade squamous intraepithelial lesions. Am. J. Reprod. Immunol. 2010;64:126–136. doi: 10.1111/j.1600-0897.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- Insinga R.P., Dasback E.J., Elbasha E.H. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US. Pharmacoeconomics. 2005;23:1107–1122. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- Joshi J.V., Paradkar P.H., Jagtap S.S., Agashe S.V., Soman G., Vaidya A.B. Chemopreventative potential and safety profile of curcuma longa extract in women with cervical low-grade squamous intraepithelial neoplasia. Asian Pac. J. Cancer Prev. 2011;12:3305–3311. [PubMed] [Google Scholar]
- Katki H.A., Schiffman M., Castle P.E. Five-year risk of recurrence after treatment of CIN 2, CIN 3, or AIS: performance of HPV nd Pap cotesting in posttreatment management. J. Low Genit. Tract. Dis. 2013;17:S78–S84. doi: 10.1097/LGT.0b013e31828543c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna N., Dalby R., Tan M., Arnold S., Stern J., Frazier N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gynecol Onc. 2007;107:554–562. doi: 10.1016/j.ygyno.2007.08.074. [DOI] [PubMed] [Google Scholar]
- Kleinberg M.J.S.J.J., Stringer J.S., Partridge E.E. A cost-effectiveness analysis of management strategies for cervical intraepithelial neoplasia grades 2 and 3. Am. J. Obstet. Gynecol. 2003;1186–1188 doi: 10.1067/mob.2003.280. [DOI] [PubMed] [Google Scholar]
- Koeneman MM, Kruse AJ, Kooreman LFS, et al. TOPical Imiquimod treatment of high-grade Cervical intraepithelial neoplasia (TOPIC trial): study protocol for a randomized controlled trial. BMC Cancer. 2016:doi: 10.1186/s12885-12016-12187-12883. [DOI] [PMC free article] [PubMed]
- Kola S., Walsh J.C. Patients' psychological reactions to colposcopy and LLETZ treatment for cervical intraepithelial neoplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;146:96–99. doi: 10.1016/j.ejogrb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Koshiol J., Lindsay L., Pimenta J.M., Poole C., Jenkins D., Smith J.S. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am. J. Epidemiol. 2008;168:123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S., Phillipsen T., Rank F., Stroyer I. Interferon and cervical dysplasia: CIN III treated wtih local interferon application. Colposcopy Gynecologic Laser Surgery. 1987;3:195–198. [Google Scholar]
- Krebs H.B., Helmkamp B.F. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirus-associated lesions. Obstet. Gynecol. 1991;78(2):205–208. [PubMed] [Google Scholar]
- Krygiou M., Athanasiou A., Paraskevaidi M. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ. 2016;354 doi: 10.1136/bmj.i3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laccetta G., Carrone A., Burratti M., Mancino P. Effect of the treatment with beta-glucan in women with cervical cytologic report of atypical squamous cells of undetermined significance (ASCUS) and low-grade intraepithelial lesions (L-SIL) Minerva Ginecol. 2015;67:113–120. [PubMed] [Google Scholar]
- Lahiri C.D., Dugan K.B., Xie X. Oral lopinavir use and human papillomavirus infection in HIV-positive women. J. Acquir. Immune Defic. Syndr. 2015;70:e63–e66. doi: 10.1097/QAI.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.T., Qiu J.T., Wang C.J. Topical imiquimod treatment for human papillomavirus infection in patients with and without cervical/vaginal intraepithelial neoplasia. Taiwanese J. Obstetr. Gynecol. 2012;51(4):533–538. doi: 10.1016/j.tjog.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Maiman M., Watts D.H., Andersen J., Clax P., Merino M., Kendall M.A. Vaginal 5-fluorouracil for high-grade cervical dysplasia in human immunodeficiency virus infection: a randomized trial. Obstet. Gynecol. 1999;94(6):954–961. doi: 10.1016/s0029-7844(99)00407-x. [DOI] [PubMed] [Google Scholar]
- Meyskens F.L., Graham V., Chvapil M., Dorr R.T., Alberts D.S., Surwit E.A. A phase I trial of beta-all-transretinoic acid delivered via a collagen sponge and a cervical cap for mild or moderate intraepithelial cervical neoplasia. J. Natl Cancer Inst. 1983;71:921–925. [PubMed] [Google Scholar]
- Meyskens F.L., Surwit E., Moon T.E. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: a randomized trial. J. Natl Cancer Inst. 1994;86:539–543. doi: 10.1093/jnci/86.7.539. [DOI] [PubMed] [Google Scholar]
- Niwa K., Tagami K., Lian Z., Gao J., Mori H., Tamaya T. Topical vidarabine of 5-fluoruracil treatment against persistant HPV in genital (pre)cancerous lesions. Oncol Reports. 2003;10:1437–1441. [PubMed] [Google Scholar]
- Okamoto A., Woodworth C.D., Yen K. Combination therapy with podophyllin and vidarabine for human papillomavirus positive cervical intraepithelial neoplasia. Oncol Rep. 1999;6:269–276. doi: 10.3892/or.6.2.269. [DOI] [PubMed] [Google Scholar]
- Pachman DR, Barton DL, Clayton AC, et al. Randomized clinical trial of imiquimod: an adjunct to treating cervical dysplasia. Am. J. Obstet. Gynecol. 2012;206(1):42 e41-47. [DOI] [PMC free article] [PubMed]
- Rahangdale L., Lippmann Q., Garcia K., Budwit D., Smith J.S., Van le L. Topical 5-fluorouracil for treatment of Cerivcal Intraepithelial Neoplasia 2: a randomized controlled trial. Am. J. Obstet. Gynecol. 2014;210:e1–e8. doi: 10.1016/j.ajog.2013.12.042. [DOI] [PubMed] [Google Scholar]
- Rogstad K.E. The psychological impact of abnormal cytology and colposcopy. Br. J. Obstet. Gynecol. 2002;109:364–368. doi: 10.1111/j.1471-0528.2002.99023.x. [DOI] [PubMed] [Google Scholar]
- Ruffin M.T., Bailey J.M., Normolle D.P. Low-dose topical delivery of all-trans retinoic acid for cervical intraepithelial neoplasia II and III. Cancer Epidemiol Biomarkers Prev. 2004;13:2148–2152. [PubMed] [Google Scholar]
- Saslow D., Solomon D., Lawson H.W. American cancer society, American society for colposcopy and cervical pathology, and american society for clinical pathology screening guidelines for prevention and early detection of cervical cancer. J. Low Genit. Tract. Dis. 2012;16:175–204. doi: 10.1097/LGT.0b013e31824ca9d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Gruber T., Kirchmayr R., Wagner D., Papendick U., Schlunk G. Efficacy trial of topically administered Interferon gamma-1beta gel in comparison to laser treatment in cervical intraepithelial neoplasia. Arch. Gynecol Obste. 1995;256:75–83. doi: 10.1007/BF00634712. [DOI] [PubMed] [Google Scholar]
- Shireman T.I., Tsevat J., Goldie S.J. Time costs associated with cervical cancer screening. Int. J. Technol. Assess. Health Care. 2001;17:146–152. doi: 10.1017/s0266462301104137. [DOI] [PubMed] [Google Scholar]
- Shukla S., Bharti A.C., Hussain S. Elimination of high-risk human papillomavirus HPV 16 infection by “Praneem” polyherbal table in women with early cervical intraepithelial lesions. J. Cancer Res. Clin. Oncol. 2009;135:1701–1709. doi: 10.1007/s00432-009-0617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silman F.H., Boyce J.G., Macasaet M.A., Nicastri A.D. 5-fluorouracil/chemosurgery for intraepithelial neoplasia of the lower genital tract. Obstet. Gynecol. 1981;58:356–360. [PubMed] [Google Scholar]
- Silverman MH, Hedley ML, Petry KU, JS W. Clinical trials in cervical intraepithelial neoplasia: balancing the need for efficacy with patient safety. J Low Genit Tract Dis. 2002;6:206-211. [DOI] [PubMed]
- Singer Z., Soos E., Feichter G. Treatment of cervical intraepithelial neoplasia associated with human pappilomavirus by interferon vaginalettes. Radiation Oncol. 1993;27:321–325. [Google Scholar]
- Smith J.S., Sanusi B., Swarts A. A randomized clinical trial comparing cervical dysplasia treatment with cryotherapy vs loop electrosurgical excision procedure in HIV-seropositive women from Johannesburg, South Africa. Am. J. Obstet. Gynecol. 2017 doi: 10.1016/j.ajog.2017.03.022. [DOI] [PubMed] [Google Scholar]
- Snoeck R., Noel J.C., Muller C., Clercq De, Bossens M. Cidofovir, a new approach for the treatment of cervix intraepithelial neoplasia III (CIN III) J. Med. Virol. 2000;60:205–209. doi: 10.1002/(sici)1096-9071(200002)60:2<205::aid-jmv16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Stanley M. Chapter 17: Genital human papillomavirus infections--current and prospective therapies. J Natl Cancer Inst Monogr. 2003(31):117-124. [DOI] [PubMed]
- Stefani C., Liverani C.A., Bianco V. Spontaneous regresson of low-grace cervical intraepithelial lesions is positively improved by topical bovine colostrom preparations (GINEDIE (R)). A multicentre, observational, Italian pilot study. Eur. Rev. Med. Pharm. Sci. 2014;18:728–733. [PubMed] [Google Scholar]
- Stentella P., Biamonti A., Carraro C. Efficacy of carboxymethyl beta-glucan in cervical intraepithelial neoplasia: a retrospective, case-control study. Minerva Ginecol. 2017;69:425–430. doi: 10.23736/S0026-4784.17.04053-9. [DOI] [PubMed] [Google Scholar]
- Suh-Burgmann E., Sivret J., Duska L.R., Del Carmen M., Seiden M.V. Long-term administration of intravaginal dehydroepiandrosterone on regression of low-grade cervical dysplasia - a pilot study. Gynecol. Obstet. Invest. 2003;55:25–31. doi: 10.1159/000068953. [DOI] [PubMed] [Google Scholar]
- Surwit E., Graham V., Droegemuller W. Evaluatiom of topically applied trans-retinoic acid in the treatment of cervical intraepithelial lesions. Am. J. Obstet. Gynecol. 1982;143:821–823. doi: 10.1016/0002-9378(82)90016-3. [DOI] [PubMed] [Google Scholar]
- Swanick S., Windstar-Hamlin K., Zwickey H. An alternative treatment for cervical intraepithelial neoplasia II, III. Integr. Cancer Ther. 2009;8:164–167. doi: 10.1177/1534735409335504. [DOI] [PubMed] [Google Scholar]
- Valencia M.H., Pacheco A.C., Quijano T.H., Giron A.V., Lopez C.V. Clinical response to glycyrrhizinic acid in genital infection dueto human papillomavirus and low-grade squamous intraepithelial lesion. Clin. Pract. 2011 doi: 10.4081/cp.2011.e93. 1(e93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Nieuwenhof H.P., van der Avoort I.A., de Hullu J.A. Review of squamous premalignant vulvar lesions. Crit. Rev. Oncol. Hematol. 2008;68(2):131–156. doi: 10.1016/j.critrevonc.2008.02.012. [DOI] [PubMed] [Google Scholar]
- van de Sande A., Koeneman M., Gerestein C., Kruse A., van Kemenade F., van Beekhuizen H. TOPical Imiquimod treatment of residual or recurrent cervical intraepithelial neoplasia (TOPIC-2 trial): a study protocol for a randomized controlled trial. BMC Cancer. 2018;18:4510–4517. doi: 10.1186/s12885-018-4510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pachterbeke C., Bucella D., Rozenberg S. Topical treatment of CIN 2+ by cidofovir: Results of a phase II, double-blind, prosective, placebo-controlled study. Gynecol Onc. 2009;115:69–74. doi: 10.1016/j.ygyno.2009.06.042. [DOI] [PubMed] [Google Scholar]
- Weiner S.A., Surwit E.A., Graham V.E., Meyskens F.L. A phase I trial of topically applied trans-retinoic acid in cervical dysplasia-clinical efficacy. Invest. New Drugs. 1986;4:241–244. doi: 10.1007/BF00179590. [DOI] [PubMed] [Google Scholar]
- Weis S.E. Current treatment options for management of anal intraepithelial neoplasia. Onco. Targets Ther. 2013;6:651–665. doi: 10.2147/OTT.S38217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabroff K.R., Lawrence W.F., King J.C. Geographic disparities in cervical cancer mortality: what are roles of risk factor prevalence, screening, and use of recommended treatment? J. Rural. Health. 2005;21:149–157. doi: 10.1111/j.1748-0361.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Yliskoski M., Cantell K., Syrjanen K., Syrjanen S. Topical treatment with human leukocyte interferon of HPV 16 infections associated with cervical and vaginal intraepithelial neoplasias. Gynecol Onc. 1990;36:353–357. doi: 10.1016/0090-8258(90)90141-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.