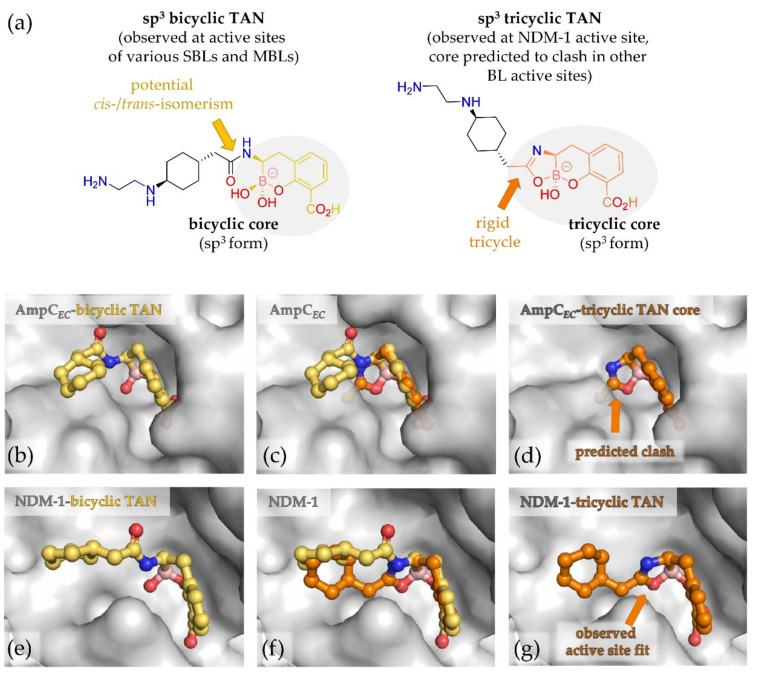
Figure 4.
Tricyclic TAN likely cannot bind at the AmpCEC active site due to a steric clash of the putative tricyclic core and side chain. (a) Structural comparison of tricyclic and bicyclic forms of TAN; (b–g) overlay of tricyclic TAN core (orange, not crystallographically observed at AmpCEC active site) with bicyclic TAN (yellow) crystallographically observed bound to active site S64 of AmpCEC (PDB ID: 6YEN) reveals a likely steric clash of the rigid tricycle in the AmpCEC active site as well as active sites of other β-lactamases (Figure S5). By contrast, both tricyclic (orange) and bicyclic (yellow) forms of TAN have been crystallographically observed at the active site of the B1 MBL NDM-1 (PDB ID: 6RMF) [24]. Note, in both structures, that the terminal amine of the side chain was disordered and therefore excluded from the model; (b) observed conformation of bicyclic TAN at the AmpCEC active site; (c) alignment of tricyclic TAN core to bicyclic TAN observed at the AmpCEC active site; (d) putative steric clash of tricyclic TAN in the AmpCEC active site based on the overlay in c). Note the apparently flexible parts of the side chain are not shown, but would make a clear steric clash with the active site; (e) observed conformation of bicyclic TAN at the NDM-1 active site; (f) overlay of tricyclic TAN and bicyclic TAN, both as observed at the NDM-1 active site; (g) observed conformation of tricyclic TAN at the NDM-1 active site.