Abstract
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are used for treating EGFR-mutated lung cancer, and osimertinib is effective in cases that acquired T790M mutations after treatment with the first- and second-generation EGFR-TKIs. However, no study has evaluated its safety and efficacy in older patients. This phase II trial (jRCTs071180002) evaluated osimertinib in T790M mutation-positive Japanese patients who were ≥75 years old and had experienced relapse or progression after previous EGFR-TKI treatment. Our previous report that enrolled 36 patients showed the overall response rate (58.3%) and disease control rate (97.2%), while this report describes the results for the progression-free survival (PFS), overall survival (OS), and safety analyses. The median PFS was 11.9 months (95% confidence interval (CI): 7.9–17.5), and the median OS was 22.0 months (95% CI: 16.0 months–not reached). The most frequent adverse events were anemia/hypoalbuminemia (27 patients, 75.0%), thrombocytopenia (21 patients, 58.3%), and paronychia/anorexia/diarrhea/neutropenia (15 patients, 41.7%). Pneumonitis was observed in four patients (11.1%), including two patients (5.6%) with Grade 3–4 pneumonitis. These results suggest that osimertinib was relatively safe and effective for non-small cell lung cancer that acquired T790M mutations after previous EGFR-TKI treatment, even among patients who were ≥75 years old.
Keywords: non-small cell lung cancer, EGFR-TKI, T790M, osimertinib
1. Introduction
Treatment for epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) typically involves EGFR tyrosine kinase inhibitors (EGFR-TKIs). Gefitinib and erlotinib are the first-generation EGFR-TKIs that provide significant survival benefits compared with platinum-based chemotherapy in clinical trials [1,2,3,4,5,6]. Afatinib and dacomitinib are the second-generation EGFR-TKIs that provide significantly longer progression-free survival (PFS) compared to that of platinum-based chemotherapy and first-generation EGFR-TKIs, although the second-generation EGFR-TKIs did not significantly improve overall survival (OS) [7,8,9,10,11]. In addition, these drugs are associated with more severe toxicity profiles, such as skin disorders, relative to the first-generation EGFR-TKIs.
Various mechanisms are responsible for resistance to the first-generation and second-generation EGFR-TKIs, with more than one-half of the cases involving the EGFR exon 20 T790M mutation [12]. Osimertinib is a third-generation EGFR-TKI that was developed to address this issue [12], and the AURA3 study revealed that it provided significantly longer PFS compared to platinum-based chemotherapy among patients with T790M-mutated lung cancer [13]. Moreover, the FLAURA trial conducted on first-line treatment revealed that osimertinib administered as an initial treatment for EGFR-mutated cases significantly prolonged PFS and OS compared with the first-generation EGFR-TKIs, with a median OS of >3 years [14,15]. Furthermore, osimertinib is expected to have good central nervous system translocation and a limited inhibition of the wild-type EGFR, which may make it less toxic, and therefore, the first choice for EGFR-mutated NSCLC [16,17,18]. Nevertheless, additional evidence is needed to support this application based on various patient populations. We have performed a phase II study to investigate the efficacy and safety of osimertinib in elderly Japanese patients (≥75 years old) with NSCLC containing the T790M mutation who progressed or experienced a relapse while receiving the first- and second- generations of EGFR-TKI treatment. In our previous report, the response rate was the primary endpoint, and the disease control rate was the secondary endpoint [19]. This report presents the results from our final analyses of PFS, OS, and safety events, which were the additional secondary endpoints in that trial.
2. Experimental Section
2.1. Patients
The study eligibility and exclusion criteria have been previously reported [19,20]. Patients were enrolled in this study between July 2016 and May 2018 if they met the following eligibility criteria: recurrence of NSCLC after achieving stable disease or better as their best overall response after treatment with the first- and second-generation of EGFR-TKIs; harboring an EGFR mutation (activating) and being T790M-positive; aged over 75 years; performance status of ≤1 based on the Eastern Cooperative Oncology Group (ECOG) scale; adequate bone marrow function (leukocyte count 3000–12,000/µL, platelet count ≥100,000/µL, and hemoglobin level ≥9.0 g/dL), adequate hepatic function (bilirubin level ≤1.5 mg/dL, aspartate aminotransferase of ≤100 IU/L, alanine aminotransferase of ≤100 IU/L), and adequate renal function (serum creatinine ≤2.0 mg/dL); a measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1; and provision of written informed consent. The exclusion criteria were pulmonary disorders; including idiopathic pulmonary fibrosis; interstitial pneumonia; pneumoconiosis; active radiation pneumonitis and drug-induced pneumonia, active infection; symptomatic brain metastasis; uncontrollable diabetes mellitus or severe comorbidities such as heart disease or renal disease; watery diarrhea; active concomitant malignancy; pregnancy or other medical problems that could prevent compliance with the protocol. The trial protocol was registered at Japan Registry of Clinical Trials (jRCTs071180002) and was approved by the ethical review board of Clinical Research Network Fukuoka Certified Review Board (CRB7180004). All patients provided written informed consent before enrollment.
2.2. Study Design, Treatments, and Endpoints
This single-arm-multicenter study involved daily oral administration of osimertinib (80 mg/day). Osimertinib had to be started at 80 mg/day, and if adverse events (AEs) occurred, dose reduction was performed according to the dose reduction criteria. Administration of osimertinib was continued until the patient met the discontinuation criteria or disease progression. Tumor assessments were performed at baseline, every 6 weeks (± 2 weeks) for 6 months, and then every 9 weeks (± 2 weeks) until disease progression. Baseline brain imaging was performed on a similar schedule. Among patients with T790M mutations, the objective response rate (ORR) was 62% (95% confidence interval [CI]: 54–68) in the AURA extension study (201 patients). In the AURA2 study (210 patients) the ORR was 70% (95% CI: 64–77) and the median PFS was 9.9 months (95% CI: 9.5–12.3) [21,22,23]. Docetaxel is the standard treatment for elderly patients based on the Japanese guidelines, as it provided an ORR of 22.7% in a study that compared docetaxel to vinorelbine [24]. Another recent study evaluated carboplatin plus pemetrexed for elderly Japanese patients and revealed an ORR of 41.2% [25]. Based on these findings, a required sample size of 31 patients was calculated according to the normal approximation method, with an expected response rate of 60%, a threshold response rate of 35%, two-sided alpha = 0.05, and 1 – beta = 0.8. However, the target sample size was increased to 35 patients to account for potential dropout cases. The primary endpoint for the trial was the overall response rate (ORR), while the secondary endpoints were PFS, OS, disease control rate (DCR), and safety events.
2.3. Statistical Methods
The ORR was calculated as the proportion of subjects with complete response or partial response as their best treatment responses. The DCR was calculated as the proportion of subjects who achieved stable disease (or better) as their best treatment response. The PFS interval was calculated from the date of enrollment to the first instance of disease progression, death from any cause, or the last follow-up without evidence of progression (for surviving patients with no evidence of progression). The OS interval was calculated from the date of enrollment to the date of death from any cause. Adverse events were evaluated from the first drug administration to 30 days after the last drug administration and were graded based on the Japanese JCOG translation of version 4.0 of the Common Terminology Criteria for Adverse Events.
The Wilson method was used to estimate the ORR and DCR with their two-sided 95% CIs. Statistical significance was considered present when the lower limit of the estimated 95% CI was above the threshold of 35% for ORR. The Kaplan–Meier method was used to evaluate the survival curves for PFS and OS, as well as the median and annual values. The Brookmeyer and Crowley method was used to estimate the CI values for median values, and Greenwood’s formula was used to estimate the standard error for annual values.
3. Results
3.1. Patient Characteristics
The study enrolled 36 patients between July 2016 and May 2018, with 23 female patients (63.9%) with a median age of 80 years, and 19 patients (52.8%) who were ≥80 years old. The histological types were adenocarcinoma in 35 patients (97.2%) and a mixed type with small cell lung cancer in only 1 patient. Based on the 7th edition of the AJCC system for staging lung cancer, 25 cases (69.4%) were considered stage IV, 10 cases (27.8%) involved relapse after surgery, and 1 case (2.8%) was considered stage IIIB. Among the enrolled patients, 30.6% were former smokers. The EGFR gene mutations involved the exon 20 T790M mutation in all cases, as well as exon 19 deletion in 22 cases (61.1%) and the exon 21 L858R point mutation in 11 cases (30.6%). Brain metastasis was detected in 15 patients (41.7%) (Table 1).
Table 1.
Patient characteristics.
| n (%) | ||
|---|---|---|
| Sex | Male | 13 (36.1) |
| Female | 23 (63.9) | |
| Age | Median (range) | 80 (75–92) |
| ≥80 years | 52.8 % | |
| >85 years | 11.1 % | |
| PS |
0 1 |
8 (22.2) 28 (77.8) |
| Histology | Adenocarcinoma | 35 (97.2) |
| Adenocarcinoma + SCLC | 1 (2.8) | |
| Stage | IIIB | 1 (2.8) |
| IV | 25 (69.4) | |
| Relapse after surgery | 10 (27.8) | |
| EGFR mutation | T790M | 36 (100.0) |
| Exon 19 deletion | 22 (61.1) | |
| L858R | 11 (30.6) | |
| G719X | 1 (2.8) | |
| Smoking status | Ex-smoker | 11(30.6) |
| Pre-treatment | Surgery | 12 (33.3) |
| Chemotherapy EGFR-TKI Afatinib Erlotinib Gefitinib |
13 (36.1) 36 (100.0) 5 (13.9) 10 (27.8) 21 (58.3) |
|
| Radiotherapy | 10 (27.8) | |
| Thoracic drainage | 4 (11.1) | |
| Metastasis site | Lung | 18 (50.0) |
| Pleural dissemination | 12 (33.3) | |
| Brain | 15 (41.7) | |
| Bone | 12 (33.3) | |
| Liver | 8 (22.2) |
PS: performance status, SCLC: small cell lung cancer.
3.2. Efficacy
The ORR from our previous report was 58.3% (95% CI: 42.2–72.9), which included a complete response rate of 2.8% and a partial response rate of 55.6%. The stable disease rate was 38.9%, and the DCR was 97.2%. The median response duration was 54.9 weeks (95% CI: 26.9–69.1), and a waterfall plot revealed that 33 patients (91.6%) experienced tumor shrinkage, which indicated favorable antitumor activity. Sixteen patients (44.4%) continued treatment beyond progression.
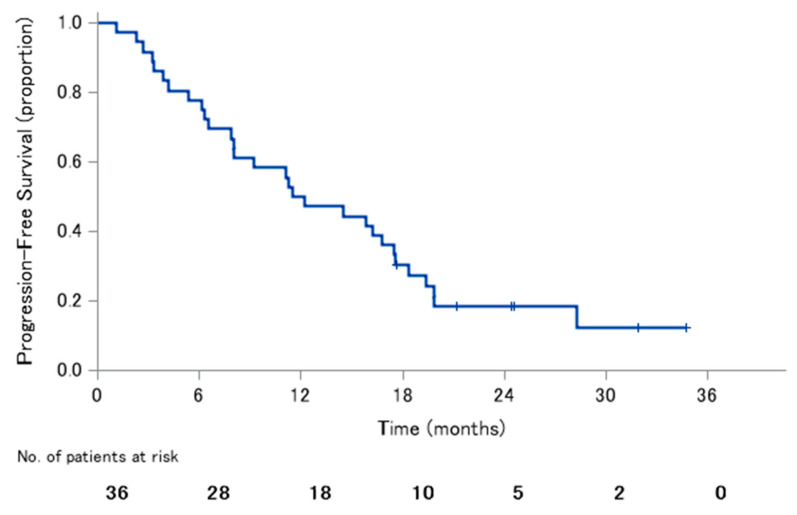
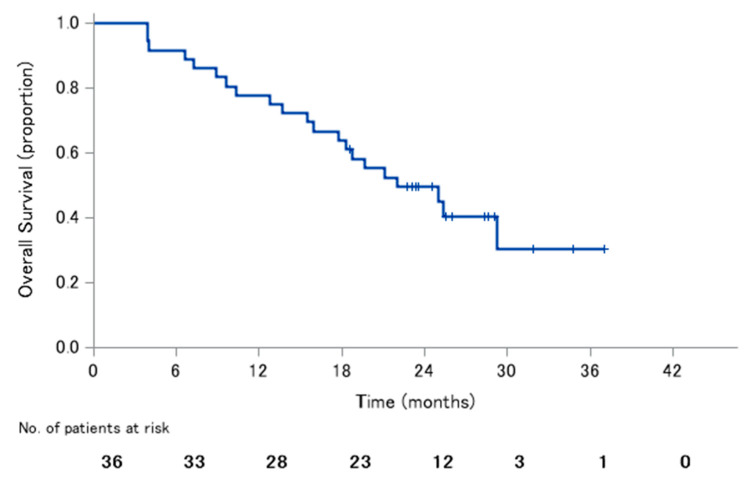
The median PFS was 11.9 months (95% CI: 7.9–17.5), with 1-year PFS rate of 50.0% and 2-year PFS rate of 18.3% (Figure 1). The median OS was 22.0 months (95% CI: 16.0–not reached), with 1-year OS rate of 77.8% and 2-year OS rate of 49.5% (Figure 2).
Figure 1.
Progression-free survival.
Figure 2.
Overall survival.
3.3. Safety
Adverse events occurred in 31 cases (86.1%), with Grade 3 or higher adverse events observed in 10 cases (27.8%). Seven patients (19.4%) required dose reductions, 10 patients (27.8%) discontinued treatment because of adverse events, and 1 patient died (2.8%). The adverse event leading to death was a pulmonary infection, although this was judged unlikely to have been caused by the osimertinib treatment. There were no death events caused by drug-induced lung injury. The most frequent adverse event was anemia/hypoalbuminemia (27 patients, 75.0%), which was followed by thrombocytopenia (21 patients, 58.3%), paronychia/anorexia/diarrhea/neutropenia (15 patients, 41.7%), leukopenia/aspartate aminotransferase increase (14 patients, 38.9%), fatigue/acneiform eruption (13 patients, 36.1%), and alanine aminotransferase increase/alkaline phosphatase increase/creatinine increase (11 patients, 30.6%). The Grade 3–4 adverse events included fatigue, anorexia, diarrhea, cardiac ejection fraction decreased, prolonged QT, leukopenia, neutropenia, and aspartate aminotransferase increase. The cases of cardiac ejection fraction were decreased and the cases of prolonged QT were different cases, and delirium and hallucinations were observed in the same patient. Pneumonitis was observed in four patients (11.1%), including two patients (5.6%) with Grade 3–4 pneumonitis (Table 2).
Table 2.
Adverse events.
| Any Grade | Grade 3–4 | |
|---|---|---|
| All adverse events > 15%, n (%) | ||
| Anemia | 27 (75.0) | 0 (0.0) |
| Hypoalbuminemia | 27 (75.0) | 0 (0.0) |
| Platelet count decreased | 21 (58.3) | 0 (0.0) |
| Neutrophil count decreased | 15 (41.7) | 1 (2.8) |
| Paronychia | 15 (41.7) | 0 (0.0) |
| Decreased appetite | 15 (41.7) | 4 (11.1) |
| Diarrhea | 15 (41.7) | 1 (2.8) |
| White blood cell decreased | 14 (38.9) | 1 (2.8) |
| Aspartate aminotransferase increased | 14 (38.9) | 2 (5.6) |
| Fatigue | 13 (36.1) | 3 (8.3) |
| Dermatitis acneiform | 13 (36.1) | 0 (0.0) |
| Alanine aminotransferase increased | 11 (30.6) | 0 (0.0) |
| Alkaline phosphatase increased | 11 (30.6) | 2 (5.6) |
| Creatinine increased | 11 (30.6) | 0 (0.0) |
| Pruritus | 8 (22.2) | 0 (0.0) |
| Mucositis oral | 8 (22.2) | 0 (0.0) |
| Grade 3–4 adverse events, n (%) | ||
| Pneumonitis | 4 (11.1) | 2 (5.6) |
| Left ventricular systolic dysfunction | 1 (2.8) | 1 (2.8) |
| Electrocardiogram QT prolongation | 1 (2.8) | 1 (2.8) |
| Delirium | 1 (2.8) | 1 (2.8) |
| Hallucination | 1 (2.8) | 1 (2.8) |
| Dyspnea | 1 (2.8) | 1 (2.8) |
| Dehydration | 1 (2.8) | 1 (2.8) |
| Lung infection | 2 (5.6) | 1 (2.8) |
| Sinusitis | 1 (2.8) | 1 (2.8) |
4. Discussion
Treatment of NSCLC has advanced dramatically after the introduction of molecularly targeted drugs, such as EGFR-TKIs for EGFR-mutated cases. The first- and second-generation of EGFR-TKIs proved to be highly effective in several studies, although the effects tended to only last for approximately 1 year [1,2,3,4,5,6,7,8,9,10,11]. Approximately one-half of the resistant cases involved a gatekeeper mutation in exon 20 (T790M), and osimertinib was developed and approved for the treatment of these cases [12,13]. The results of the FLAURA trials positioned osimertinib as a standard treatment option, and even as an initial treatment option [14,15]. However, many cases still involve treatment in the second line or later, as the T790M mutation was identified via re-biopsy in patients who received first-generation or second-generation EGFR-TKIs as their initial treatment. When the T790M mutation was identified in these cases, patients typically received osimertinib.
Aging populations are becoming increasingly common worldwide, and many lung cancer cases involve older patients [26,27]. There are concerns that older patients have a higher risk of developing adverse events, which may necessitate dose reduction or treatment discontinuation, and subsequently result in decreased efficacy. Thus, this phase II study aimed to evaluate the safety and efficacy of osimertinib in elderly patients with EGFR-mutated lung cancer involving the T790M mutation. The primary endpoint was the ORR, and our previous report found that the ORR was 58.3% (95% CI: 42.2–72.9), which fulfilled the efficacy criterion (the lower limit of the CI exceeded the threshold response rate of 35%) [19]. This report describes the secondary endpoints, which include the DCR (97.2%), median PFS (11.9 months), and median OS (22.0 months). In terms of efficacy, the pooled results from the AURA expansion and AURA2 studies revealed an ORR of 66%, a DCR of 91%, a median PFS of 9.9 months, and a median OS of 26.8 months [23]. In addition, phase 3 AURA3 studies revealed an ORR of 70.6%, a DCR of 93.2%, a median PFS of 10.1 months, and a median OS of 26.8 months [13,28]. Thus, while our ORR was lower than that shown in the previous studies, it agrees with the slightly lower ORR (61.1%) that was retrospectively observed in another sample of elderly Japanese patients [29]. Furthermore, our findings regarding PFS and OS do not appear inferior to the results from previous studies, thereby suggesting that osimertinib was effective in elderly Japanese patients. Regarding the effects based on the PS, the ORR of PS0 and PS1 was 75% and 53.6%, respectively, and the PFS was 13.7 months and 11.9 months, respectively. Since there were few cases, it was impossible to discuss the significant differences, but the PS0 group tended to be superior.
It is also important to compare the results from osimertinib treatment to those from cytotoxic anticancer drugs, which are the alternative options if osimertinib is not used for T790M-positive cases. For example, the control group for the AURA3 study received platinum plus pemetrexed, which provided an ORR of 31%, a DCR of 74%, a median PFS of 4.4 months, and a median OS of 22.5 months [13,28]. A subgroup analysis of ≥70-year-old Japanese patients from the JACAL study evaluated carboplatin plus pemetrexed and revealed an ORR of 24%, a DCR of 68%, a median PFS of 5.2 months, and a median OS of 16.8 months [30,31]. Thus, our OS findings may be comparable to the results from the entire AURA3 population, although our ORR, DCR, and PFS outcomes are comparable or even slightly better. Interestingly, 71% of the patients in the group that received platinum plus pemetrexed subsequently received additional treatment, with 60% experiencing a greater effect after crossing over to osimertinib treatment. Therefore, while the JACAL study had only included EGFR-mutated cases and did not specifically consider older patients, we believe that osimertinib may provide good outcomes among older patients with EGFR-mutated (T790M) NSCLC.
Safety is also an important consideration in this setting, given the concerns regarding the potentially higher risk of adverse events among older patients. In the AURA3 study, it appears that Japanese patients had a higher risk of paronychia, diarrhea, and skin pruritus, although no clear increase was observed among elderly patients. However, elderly patients had a clearly increased frequency of myelosuppression events, such as anemia (75% in this study vs. 8% in AURA3 study), leukopenia (38.9% in this study vs. 8% in AURA3 study), neutropenia (41.7% in this study vs. 8% in AURA3 study), and thrombocytopenia (58.3% in this study vs. 10% in AURA3 study), although the frequencies of Grade 3–4 adverse events were generally comparable. Osimertinib has also been reported to be more frequently myelosuppressed than in other EGFR-TKI in a pivotal study [13,14]. In addition, myelosuppression was reported to be stronger in the analysis of the Japanese population [32]. Although the obvious mechanism was unclear, it was suggested that racial differences might be involved. Since myelosuppression was observed more frequently in the present study than in the aforementioned analysis of the Japanese population, caution should be exercised in the elderly Japanese. Fiala et al. reported that pre-treatment hypoalbuminemia correlated with poor prognosis in advanced NSCLC patients treated with erlotinib [33]. The present study also revealed that anorexia and exhaustion were common (30–40% of cases vs. 16-18% of cases in AURA3 study, including some Grade 3–4 cases), as well as hypoalbuminemia (75% of cases vs. N/A in AURA3 study). Therefore, careful follow-up is needed for elderly patients who are receiving osimertinib. Elevated alkaline phosphatase and creatinine values were also observed, albeit not serious cases, and related follow-up testing is also important. Cardiac adverse events, such as decreased left heart ejection fraction and QT prolongation, were observed in some cases, although only one patient experienced a Grade 3–4 cardiac adverse event. Central nervous system events, such as delirium and hallucination, may be explained by the large proportion of cases with brain metastasis (41.7%), although caution should be exercised if these events present in conjunction with sinusitis and pulmonary infection. Regarding AE by PS, no clear difference was observed between PS0 and PS1.
All-grade pneumonitis was observed in 11.1% of cases, and Grade 3–4 pneumonitis was observed in 5.6% of cases. The rates after conventional EGFR-TKI treatment were 4% in the AURA3 study and 7.3% in the Japanese subset of patients, which suggests that Japanese patients may have a higher rate of pneumonitis [13,34]. The difference between our findings and the previous findings may be related to differences in the proportions of patients with a history of smoking (69.4% for the present study, 32.2% for the AURA3 study, and 31.7% for the Japanese subset of the AURA3 population). In addition, the Japanese subset of the FLAURA study population had a higher frequency of pulmonary disorders (all grades: 12%, Grade 3 or higher: 2%); it should be noted that this is a first-line trial. Other reports have also suggested that osimertinib may be associated with an increased incidence of pulmonary disorders relative to other EGFR-TKIs [32]. Nevertheless, the odds ratio for pulmonary disorders after gefitinib treatment was 1.92-fold higher among Japanese patients who were ≥55 years old, which suggests that careful follow-up is required for patients who are ≥75 years old [35].
The present study revealed all-grade AEs in 86.1%, Grade 3 or worse AEs in 27.8%, and fatal AEs in 2.8% of the patients. These rates did not appear to be substantially elevated among elderly patients, based on results from the AURA3 study and its Japanese subgroup (all-grade: 97.8% and 100%, Grade 3 or higher: 22.6% and 31.7%, and fatal AEs: 1.4% and 0%). However, AEs leading to treatment discontinuation occurred in 12 patients (33.3%) in our study, which was more common than the rates of 6.8% in the AURA3 study and 7.3% in the Japanese subgroup. For example, we observed drug-induced lung injury in four patients (11.1%), and these patients needed to stop treatment. In addition, three patients (8.3%) discontinued treatment because of Grade 4 AEs (pulmonary infection, hallucinations, and hepatic dysfunction), although those events were judged unlikely to be associated with their treatment. One patient (2.8%) required a two-step dose reduction, and two patients (5.6%) were unable to continue the treatment protocol because of a ≥4-week treatment disruption. Treatment was also stopped in one case involving Grade 3 aspiration pneumonia, one case at the attending physician’s discretion, and one case because the patient refused to continue treatment. Thus, although the safety of osimertinib outside the study protocol has not been evaluated, most of these AEs and treatment discontinuations were likely not to have been caused by a drug-induced pulmonary injury.
Most all-grade adverse events involved anorexia, fatigue, myelosuppression, and gastrointestinal symptoms. These complications were generally not serious and could be addressed using conventional management strategies. However, it is important to note that the frequency of drug-induced lung injury may increase, which highlights the importance of a careful follow-up in this population. Despite the potential need for a careful follow-up and the small sample size, which was the limitation in this study, it appears that osimertinib can be a standard treatment even for the elderly patients harboring T790M mutation.
While the present study provided encouraging data, we are conducting an additional phase II study (SPIRAL-0) to confirm the safety and efficacy of osimertinib in ≥75-year-old patients with untreated NSCLC harboring EGFR-activating mutations [36]. This may provide further information to guide the increasing use of osimertinib treatment in this setting.
Acknowledgments
We thank all of the patients who participated in this study, as well as their families. We also thank the Clinical Research Support Center Kyushu for managing the study.
Author Contributions
Conceptualization, J.U.; validation, J.U., A.N., and K.T.; formal analysis, K.Y.; investigation, O.H., C.S., T.A., N.H., T.I., T.T., M.K., Y.G., H.I., N.H., K.N., H.U., K.U., M.F. (Minoru Fukuda), Y.U., T.Y. (Toshihide Yokoyama), M.A., T.M. (Tadashi Mio), S.N., Y.C., N.T., Y.K., T.M. (Takako Mouri), T.Y. (Tadaaki Yamada) and M.F. (Masaki Fujita); data curation, K.Y.; writing—original draft preparation, A.N.; writing—review and editing, J.U.; supervision, K.T.; project administration, J.U.; funding acquisition, J.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AstraZeneca. K.K., grant number ESR-15-11419.
Conflicts of Interest
K. Takayama received grants from Chugai-Roche Co., Ono Pharmaceutical Co. and personal fees from AstraZeneca Co., MSD-Merck Co., Eli Lilly Co., Boehringer-Ingelheim Co., Daiichi Sankyo Co. and Chugai-Roche Co. outside of the submitted work. K. Yoshimura received personal fees from AstraZeneca Co. and Chugai-Roche Co. outside of the submitted work. T. Ishizuka received grants from Boehringer-Ingelheim Co., Bayer Co, MSD Co., Astellas Co., Ono Pharmaceutical Co., Pfizer Co., Eli Lilly Co, Novartis Pharma K.K., Mochida Pharmaceutical Co and personal fees from AstraZeneca Co., Boehringer-Ingelheim Co., Novartis Pharma Co., and GlaxoSmithKline Co. outside of the submitted work. M. Fukuda received grants from AstraZeneca Co. and Eli Lilly Co. outside of the submitted work. M. Fujita received grants and personal fees from AstraZeneca Co. T. Yokoyama received personal fees from AstraZeneca Co., MSD-Merck Co., Eli Lilly Co., Boehringer-Ingelheim Co., Chugai-Roche Co. outside of the submitted work. J. Uchino received grants from Eli Lilly Japan K.K. and AstraZeneca Co. that are outside of the submitted work. The other authors have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., Seto T., Satouchi M., Tada H., Hirashima T., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C., Wu Y.L., Chen G., Feng J., Liu X.Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y.L., Zhou C., Liam C.K., Wu G., Liu X., Zhong Z., Lu S., Cheng Y., Han B., Chen L., et al. First-Line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann. Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 7.Sequist L.V., Yang J.C., Yamamoto N., O’Byrne K., Hirsh V., Mok T., Geater S.L., Orlov S., Tsai C.M., Boyer M., et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y.L., Zhou C., Hu C.P., Feng J., Lu S., Huang Y., Li W., Hou M., Shi J.H., Lee K., et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 9.Park K., Tan E.H., O’Byme K., Zhang L., Boyer M., Mok T., Hirsh V., Yang J.C., Lee K.H., Lu S., et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L., Tan E.H., O’Byme K., O’Byrne K., Zhang L., Hirsh V., Boyer M., Yang J.C., Mok T., Lee K.H., et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-Lung 7 trial. Ann. Oncol. 2017;28:270–277. doi: 10.1093/annonc/mdw611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y.L., Cheng Y., Zhou X., Lee K.H., Nakagawa K., Niho S., Tsuji F., Linke R., Rosell R., Corral J., et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 12.Yu H.A., Arcila M.E., Rekhtman N., Sima C.S., Zakowski M.F., Pao W., Kris M.G., Miller V.A., Ladanyi M., Riely G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok T.S., Wu Y.L., Ahn M.J., Garassino M.C., Kim H.R., Ramalingam S.S., Shepherd F.A., He Y., Akamatsu H., Theelen W.S., et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 15.Ramalingam S.S., Vanteenkiste J., Planchard D., Cho B.C., Gray J.E., Ohe Y., Zhou C., Reungwetwattana T., Cheng Y., Chewaskulyong B., et al. Overall survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 16.Nanjo S., Ebi H., Takeuchi S., Takeuchi S., Yamada T., Mochizuki S., Okada Y., Nakada M., Murakami T., Yano S. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget. 2016;7:3847–3856. doi: 10.18632/oncotarget.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanjo S., Hata A., Okuda C., Kaji R., Okada H., Tamura D., Irie K., Okada H., Fukushima S., Katakami N. Standard-Dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br. J. Cancer. 2018;118:32–37. doi: 10.1038/bjc.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross D.A., Ashton S.E., Ghiorghlu S., Eberlein C., Nebhan C.A., Spitzler P.J., Orme J.P., Finlay M.R., Ward R.A., Mellor M.J., et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao A., Hiranuma O., Uchino J., Sakaguchi C., Kita T., Hiraoka N., Ishizuka T., Kubota Y., Kawasaki M., Goto Y., et al. Osimertinib in elderly patients with epidermal growth factor receptor T790M-positive non-small-cell lung cancer who progressed during prior treatment: A phase II trial. Oncologist. 2019;24:593-e170. doi: 10.1634/theoncologist.2019-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchino J., Nakao A., Tamiya N., Kaneko Y., Yamada T., Yoshimura K., Fujita M., Takayama K. Treatment rationale and design of the SPIRAL study: A phase II trial of osimertinib in elderly epidermal growth factor receptor T790M-positive nonsmall-cell lung cancer patients who progressed during prior EGFR-TKI treatment. Medicine. 2018;97:e11081. doi: 10.1097/MD.0000000000011081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J.C., Ahn M.J., Kim D.W., Ramalingam S.S., Sequist L.V., Su W.C., Kim S.W., Kim J.H., Planchard D., Felip E., et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J. Clin. Oncol. 2017;35:1288–1296. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
- 22.Goss G., Tsai C.M., Shepherd F.A., Bazhenova L., Lee J.S., Chang G.C., Crino L., Satouchi M., Chu Q., Hida T., et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 23.Ahn M.J., Tsai C.M., Shepherd F.A., Bazhenova L., Sequist L.V., Hida T., Yang J., Ramalingam S.S., Mitsudomi T., Jänne P.A., et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer. 2019;125:892–901. doi: 10.1002/cncr.31891. [DOI] [PubMed] [Google Scholar]
- 24.Kudoh S., Takeda K., Nakagawa K., Takada M., Katakami N., Matsui K., Shinkai T., Sawa T., Goto I., Semba H., et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: Results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904) J. Clin. Oncol. 2006;24:3657–3663. doi: 10.1200/JCO.2006.06.1044. [DOI] [PubMed] [Google Scholar]
- 25.Tamiya M., Tamiya A., Kaneda H., Nakagawa K., Yoh K., Goto K., Okamoto H., Shimokawa T., Abe T., Tanaka H., et al. A phase II study of pemetrexed plus carboplatin followed by maintenance pemetrexed as first-line chemotherapy for elderly patients with advanced non-squamous non-small cell lung cancer. Med. Oncol. 2016;33:2. doi: 10.1007/s12032-015-0715-7. [DOI] [PubMed] [Google Scholar]
- 26.Barta J.A., Zinner R.G., Unger M. Lung cancer in the older patient. Clin. Geriatr. Med. 2017;33:563–577. doi: 10.1016/j.cger.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Torre L.A., Siegel R.L., Ward E.M., Jemal A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y.L., Mok T.S., Han J., Ahn M.J., Delmonte A., Ramalingam S.S., Kim S., Shepherd F.A., Laskin J., He Y., et al. Overall Survival (OS) from the AURA3 phase III study: Osimertinib vs Platinum-Pemetrexed (PLT-PEM) in Patients (PTS) with EGFR T790M advanced Non-Small Cell Lung Cancer (NSCLC) and progression on a prior EGFR-tyrosine kinase inhibitor (TKI) Ann. Oncol. 2019;20(Suppl. 9):ix157–ix181. doi: 10.1016/j.annonc.2020.08.2100. [DOI] [PubMed] [Google Scholar]
- 29.Furuta H., Uemura T., Yoshida T., Kobara M., Yamaguchi T., Watanabe N., Shimizu J., Horio Y., Kuroda H., Sakao Y., et al. Efficacy and safety data of Osimertinib in elderly patients with NSCLC who harbor the EGFR T790M mutation after failure of initial EGFR-TKI treatment. Anticancer Res. 2018;38:5231–5237. doi: 10.21873/anticanres.12847. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto I., Aoe K., Kato T., Hosomi Y., Yokoyama A., Imamura F., Kiura K., Hirashima T., Nishio M., Nogami N., et al. Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naïve patients with advanced nonsquamous non-small-cell lung cancer. Investig. New Drugs. 2013;31:1275–1282. doi: 10.1007/s10637-013-9941-z. [DOI] [PubMed] [Google Scholar]
- 31.Nogami N., Nishio M., Okamoto I., Enatsu S., Suzukawa K., Takai H., Nakagawa K., Tamura T. Pemetrexed and carboplatin combination therapy followed by pemetrexed maintenance in Japanese patients with non-squamous non-small cell lung cancer: A subgroup analysis of elderly patients. Respir. Investig. 2019;57:27–33. doi: 10.1016/j.resinv.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Ohe Y., Imamura F., Nogami N., Okamoto I., Kurata T., Kato T., Sugawara S., Ramalingam S.S., Uchida H., Hodge R., et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn. J. Clin. Oncol. 2019;49:29–36. doi: 10.1093/jjco/hyy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiala O., Pesek M., Finek J., Racek J., Minarik M., Benesova L., Bortlicek Z., Sorejs O., Kucera R., Topolcan O. Serum albumin is a strong predictor of survival in patients with advanced-stage non-small cell lung cancer treated with erlotinib. Neoplasma. 2016;63:471–476. doi: 10.4149/318_151001N512. [DOI] [PubMed] [Google Scholar]
- 34.Akamatsu H., Katakami N., Okamoto I., Kato T., Kim Y.H., Imamura F., Shinkai M., Hodge R.A., Uchida H., Hida T. Osimertinib in Japanese patients with EGFR T790M mutation-positive advanced non-small-cell lung cancer: AURA3 trial. Cancer Sci. 2018;109:1930–1938. doi: 10.1111/cas.13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudoh S., Kato H., Nishiwaki Y., Fukuoka M., Nakata K., Ichinose Y., Tsuboi M., Yokota S., Nakagawa K., Suga M. Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case-control study. Am. J. Respir. Crit. Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 36.Chihara Y., Yamada T., Uchino J., Tamiya N., Kaneko Y., Kishimoto J., Takayama K. Rationale and design of a phase II trial of osimertinib as first-line treatment for elderly patients with epidermal growth factor receptor mutation-positive advanced non-small cell lung cancer (SPIRAL-0 study) Transl. Lung Cancer Res. 2019;8:1086–1090. doi: 10.21037/tlcr.2019.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]