Abstract
Edible flowers are consumed for their appearance, colours, nutritional and healthy properties, but the use is limited by the actual number of the species. Seven edible flowers of the Lamiaceae family (Ocimeae and Mentheae tribes) were investigated: Monarda didyma ‘Fireball’, Nepeta × faassenii ‘Six Hills Giant’, Ocimum basilicum ‘Blue Spice’, O. basilicum ‘Cinnamon’, Ocimum × citriodorum, Salvia discolor, and Salvia microphylla ‘Hot Lips’. Total soluble sugars, proteins, polyphenols, carotenoids, ascorbic acid and antioxidant activity were detected. The species of the Mentheae tribe contained higher sugar content than Ocimeae flowers, the opposite with regard to protein content. Ocimeae tribe flowers showed high polyphenols and carotenoids content. The Ocimeae tribe together with two specie of the Mentheae tribe showed an aroma profile dominated by sesquiterpene hydrocarbons (58.0% in S. discolor to 77.9% in Ocimum × citriodorum). Oxygenated monoterpenes prevailed in Nepeta and Monarda, also present in the essential oil of this latter species (84.5%). By contrast, Nepeta and S. discolor evidenced non-terpenes as the principal class (41.2% and 77.5%, respectively), while the oxygenated sesquiterpene was the main one in S. microphylla. The two varieties of Ocimum spp. showed oxygenated monoterpenes as the main class of volatiles.
Keywords: Salvia spp., Ocimum spp., Nepeta × faassenii, Monarda didyma, VOCs, nutraceutical properties, essential oil, health effect
1. Introduction
Lamiaceae (order Lamiales) is a family of flowering species, also known as the mint family [1]. The taxonomy rank is composed of 236 genera and 6900–7200 species, distributed all over the world [2]. Lamiaceae is divided in 12 subfamilies [3] of which Nepetoidae is one of the most clearly defined [4] and has strongly aromatic species with volatile terpenoids [1].
Lamiaceae are usually herbs, subshrubs, or shrubs. Their leaves and flowers are generally scented, and this is a distinctive feature of this family. Many Lamiaceae species produce a wide spectrum of bioactive compounds (flavonoids, terpenoids, phenolics and alkaloids), that are characterized by numerous biological activities (e.g., antioxidant, anti-inflammatory, and antibacterial properties) [5,6,7,8,9]. Therefore, several species were listed in the official Pharmacopoeias [10] and currently used in pharmaceutical, cosmetic, food and pesticides industries [11]. Furthermore, many members of the Lamiaceae family are widely cultivated as culinary herbs, such as basil, oregano, rosemary, thyme, mint, and sage [12]. Most of them produce edible flowers [13], even if their consumption is lower compared to that of the leaves, generally used as seasonings.
Edible flowers are consumed in different part of the world, since they are able to improve appearance, colour and nutritive values of meals [13,14,15]. Although Lu et al. [16] reported that 180 species, 100 genera and 97 families produce edible flowers, no official list has been published by any international organization [13,17] and only a small part of them have been studied so far [18]. Several scientific reports highlight their nutritional and healthy properties [18,19,20]. In fact, even though edible flowers are usually composed of 70% to 95% of water [18], pollen, nectar and petals can be a real source of primary metabolites [13,21], vitamins [22], and minerals [23,24]. Edible flowers are also rich in antioxidant molecules (e.g., polyphenols and pigments), useful to prevent several diseases [16,18]. Aroma and scent are further distinctive features of most of the edible flowers currently consumed [17,25]. Both are essential to entice people to purchase this product [26].
In order to improve the research on edible flowers, plants of 2 tribes of the subfamily Nepetoideae were investigated herein: three types of O. basilicum (Ob) belonging to the tribe Ocimeae and four different species of the Mentheae tribe. The Ocimum genus, with its 150 species, is widely distributed in the temperate region of the world [27,28]. Various cultivars differ in flowers’ and leaves’ morphology (colour size, shape), as well as the composition of substances like essential oils (EOs) [29]. O. basilicum var. italicum, also called Sweet Basil, is cultivated all over the world for its EO as well as being a culinary and ornamental plant [28]. EOs of O. basilicum have important biological activities and depending on environmental conditions, age of plant, agronomic techniques, and their chemotypes [29,30]. The chemotypes are based on 1–2 predominant constituents of leaf EO. According to the literature, the studied types O. basilicum ‘Blue Spice’ (Ob-BS), O. basilicum ‘Cinnamon’ (Ob-Cn) and O. × citriodorum (Ob-Ct) belong to three different chemotypes [29].
The first Menthae member includes one of the most popular genera and presumably the largest and widely distributed one within the Lamiaceae family: Salvia [31,32]. Beside their ethnobotanical importance, plants of these taxa have a commercial importance due to their culinary, nutraceutical, medicinal and fragrance uses. Within the genus, S. discolor (S. disc) and S. microphylla (S. micro) are plants initially used for ornamental purpose [33]; they are of a good nutritive intake [34] and are known for their aromatic volatile compounds and medicinal properties [35,36,37]. Nepeta with its 280 spp. is considered one of the largest genera of the Mentheae tribe as is the Salvia genus, and grows in Southern Europe and in central Asia [38]. Commonly known as “catnip” or “catmint”, these species are traditionally used in human medicine to treat many disorders especially due to the presence of nepetalactone. Moreover, Nepeta spp. are used for ornamental and, sometimes, culinary purposes [39,40]. Nepeta × faassenii (N × faas.) is a garden plant produced by crossbreeding of two Mediterranean species: N. mussinii Spreng. Ex henckel and N. nepetella L. The EOs of Nepeta species are used in food, medicine and perfume industries, and the one obtained from N × faas aerial parts is characterized by two nepetalactones and 1,8 cineole [41]. The last studied species was Monarda didyma (M. did), that belongs to the genus which encompass 18 species, endemic to North America [42]. The economic relevance of these plants is related not only to the presence of the EO rich in active compounds [42], but also to the use of leaves as a flavouring agent in the food industry [43].
The European cross-border cooperation programme between France and Italy INTERREG ALCOTRA “ANTEA” project (N° 1139) was focused on the exploitation of the edible flowers use as functional food and aimed to increase the number of the species used for this purpose. In this study, different species of Lamiaceae family have been considered: Monarda didyma ‘Fireball’, Nepeta × faassenii ‘Six Hills Giant’, Ocimum basilicum ‘Blue Spice’, Ocimum basilicum ‘Cinnamon’, Ocimum × citriodorum, Salvia discolor, Salvia microphylla ‘Hot Lips’ (Figure 1). The selection was based on the flowers’ ornamental value, ease in potting growth, prolonged flowering, and flowers’ aroma and taste (Table 1).
Figure 1.
Selected flowers belonging to Lamiaceae family: (a) Monarda didyma ‘Fireball’ (M. did), (b) Nepeta × faassenii “Six Hills Giant” (N. × faas), (c) Ocimum basilicum ‘Blue Spice’ (Ob-BS), (d) O. basilicum ‘Cinnamon’ (Ob-Cn), (e) Ocimum × citriodorum (Ob-Ct), (f) Salvia discolor (S. disc), (g) Salvia microphylla ‘Hot Lips’ (S. micro).
Table 1.
Main botanical information of the examined Lamiaceae flowers. * = The taste was evaluated by CREA (Research Centre for Vegetable and Ornamental Crops, Sanremo, IT) and CREAM (Chambre d’Agriculture des Alpes-Maritimes, Nice, FR), as one of the ANTEA project goals.
| Acronyms | Species/Hybrid | Variety/Genotype | English Name | Flowering Period | Taste * |
|---|---|---|---|---|---|
| M. did | Monarda didyma L. | Fireball | Bee balm | Jun-Aug | Sweet oregano |
| N. × faas | Nepeta × faassenii Bergmans ex Stearn | Six Hills Giant | Catmint | Mar-Nov | Strong aromatic |
| Ob-BS | Ocimum basilicum L. | Blue Spice | - | Apr-Nov | Spice |
| Ob-Cn | Ocimum basilicum L. | Cinnamon | Cinnamon basil | Apr-Nov | Cinnamon |
| Ob-Ct | Ocimum × citriodorum Vis | - | Thai lemon basil | Apr-Nov | Lemon peel |
| S. disc | Salvia discolor Kunth | - | Andean sage | Jan-Nov | Black currant and pine nut |
| S. micro |
Salvia microphylla Kunth |
Hot Lips | - | Feb-Oct | Floral and fruity |
2. Results
2.1. Bioactive Compounds
Table 2 reported the contents of total crude proteins and soluble sugars (glucose, fructose, and sucrose) in the different flowers. Sugars are an important component of flowers, since the flavor is often related to that content. The Mentheae tribe members resulted in higher sugars content than Ocimeae ones. The two sage species, Salvia microphylla Kunth (S. micro) and Salvia discolor Kunth (S. disc), characterized by a fruity taste, showed the highest content of sucrose (7.91 and 9.6 mg/g FW) and of hexoses (glucose and fructose) in comparison to Monarda didyma L. (M. did) and Nepeta × faassenii Bergmans ex Stearn (N. × faas). Within the Ocimum flowers, Ocimum × citriodorum Vis (Ob-Ct) showed the lowest content of soluble sugars (Table 2).
Table 2.
Determination of primary and secondary metabolites in the seven studied flowers of Lamiaceae family. Data are presented as means ± standard error (SE, n = 3). Abbreviations: FW = fresh weight; DW = dry weight; GAE—gallic acid equivalents; CE—± catechin equivalents; ME—malvin equivalents; sig.= significant post hoc test at p < 0.05.
| Parameters | Monarda didyma ‘Fireball’ (1) | Nepeta × faassenii ‘Six Hills Giant (2) | Ocimum basilicum ‘Blue Spice (3) | Ocimum basilicum ‘Cinnamon’ (4) | Ocimum × citriodorum (5) | Salvia discolor (6) | Salvia microphylla ‘Hot Lips’ (7) | Sig. |
|---|---|---|---|---|---|---|---|---|
| Primary metabolites | ||||||||
| D-Glucose (GLU) mg/g FW | 5.07 ± 0.16 | 4.36 ± 0.40 | 4.70 ± 0.35 | 3.49 ± 0.12 | 3.03 ± 0.11 | 5.02 ± 0.19 | 7.60 ± 0.50 | 1vs4,5,7//2vs7//3vs5,7// 4vs1,6,7//5vs1,3,6,7 6vs4,5,7//7vs1,2,3,4,5,6 |
| D-Fructose (FRU) mg/g FW | 2.19 ± 0.22 | 4.11 ± 0.46 | 3.58 ± 0.15 | 6.85 ± 0.64 | 2.10 ± 0.08 | 3.96 ± 0.21 | 2.46 ± 0.27 | 1vs2,4,6//2vs1,4,5//3vs4// 4vs1,2,3,5,6,7//5vs2,4,6// 6vs1,4,5//7vs4 |
| Sucrose (SUC) mg/g FW |
6.66 ± 0.56 | 4.46 ± 0.02 | 2.44 ± 0.29 | 1.27 ± 0.11 | 1.60 ± 0.05 | 9.60 ± 0.84 | 7.91 ± 0.43 | 1vs2,3,4,5,6//2vs1,4,5,6,7// 3vs1,6,7//4vs1,2,6,7//5vs1,2,6,7// 6vs1,2,3,4,5//7vs2,3,4,5 |
| Crude protein (% DW) | 6.79 ± 0.16 | 12.69 ± 0.25 | 16.16 ± 0.16 | 9.62 ± 0.12 | 13.81 ± 0.00 | 3.19 ± 0.31 | 6.29 ± 0.16 | 1vs2,3,4,5,6//2vs1,3,4,5,6,7// 3vs1,2,4,5,6,7//4vs1,2,3,5,6,7// 5vs1,2,3,4,6,7//6vs1,2,3,4,5,7// 7vs2,3,4,5,6 |
| Secondary metabolites | ||||||||
| Total carotenoids (TCar) μg/ g FW | 1.91 ± 0.02 | 6.92 ± 0.98 | 51.59 ± 6.48 | 68.33 ± 3.10 | 81.86 ± 1.48 | 61.34 ± 0.09 | 4.25 ± 0.53 | 1vs3,4,5,6//2vs3,4,5,6// 3vs1,2,4,5,7 4vs1,2,3,7//5vs1,2,3,6,7 6vs1,2,5,7//7vs3,4,5,6 |
| Total anthocyanins (TAnth) mg ME/g FW | 0.98 ± 0.04 | 0.09 ± 0.00 | 0.16 ± 0.00 | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.98 ± 0.08 | 0.20 ± 0.02 | 1vs2,3,4,5,7//2vs1,3,4,5,6,7 3vs1,2,4,5,6//4vs1,2,3,6,7 5vs1,2,3,6,7//6vs2,3,4,5,7 7vs1,2,4,5,6 |
| Total polyphenols (TPC) mg GAE/g FW | 4.14 ± 0.08 | 5.11 ± 0.21 | 7.42 ± 0.13 | 8.06 ± 0.18 | 7.63 ± 0.14 | 6.53 ± 0.29 | 2.41 ± 0.18 | 1vs3,4,5,6,7//2vs3,4,5,6,7 3vs1,2,7//4vs1,2,6,7//5vs1,2,7 6vs1,2,4,7//7vs1,2,3,4,5,6 |
| Ascorbic acid reduced form (ASA) mg AsA/100 g FW |
1.36 ± 0.07 | 1.77 ± 0.05 | 0.56 ± 0.03 | 0.81 ± 0.05 | 0.77 ± 0.10 | 0.99 ± 0.05 | 1.64 ± 0.05 | 1vs2,3,4,5,6//2vs1,3,4,5,6 3vs1,2,6,7//4vs1,2,7//5vs1,2,7 6vs1,2,3,7//7vs3,4,5,6 |
| Total ascorbic acid (AsATOT) mg AsATOT /100 g FW |
2.42 ± 0.03 | 2.34 ± 0.44 | 1.76 ± 0.07 | 1.45 ± 0.21 | 1.61 ± 0.05 | 1.14 ± 0.07 | 2.57 ± 0.31 | 1vs4,5,6//2vs4,6//3vs7 4vs1,2,7//5vs1,7 6vs1,2,7//7vs3,4,5,6 |
| Radical scavenging assay (IC50 DPPH-mg/mL) | 4.26 ± 0.20 | 2.05 ± 0.17 | 0.81 ± 0.03 | 1.08 ± 0.05 | 0.43 ± 0.05 | 1.20 ± 0.17 | 4.83 ± 0.49 | 1vs2,3,4,5,6//2vs1,3,4,5,7 3vs1,2,7//4vs1,2,7//5vs1,2,7 6vs1,7//7vs2,3,4,5,6 |
The total crude proteins were higher in the Ocimeae tribe than in the Mentheae members. The three different Ocimum spp. showed a proteins percentage in the range of 9.62–16.16%. In the Mentheae tribe only N. × faas evidenced similar proteins percentage (12.69%), while low content was observed in the sage flowers and M. did (3.19–6.29% and 6.79%, respectively).
The carotenoids and anthocyanins amounts were determined and reported in Table 2. The higher contents of carotenoids were detected in the Ocimum genotypes, 51.59 µg/g FW in the “Blue spice” (Ob-BS), 68.33 µg/g FW in the “cinnamon” (Ob-Cn) and the highest amount in Ob-Ct (Thai lemon basil) with 81.86 µg/g FW. Within the Mentheae tribe, S. disc had the highest content of carotenoids (61.34 µg/g FW), due to the dark color, while the lowest amount was detected in M. did (1.91 µg/g FW). In relation to the color of flowers, S. disc and M. did showed the highest content of anthocyanins, while the flowers with pale color had lower content, especially Ob-Ct and the Ob-Cn measured 0.03 and 0.06 mg/g FW, respectively. Anthocyanins were abundant in the following sequence: M. did = S. disc > S. micro > Ocimum species (0.98, 0.2, 0.16, 0.06 and 0.03 mg/g FW). The higher polyphenols content was detected in the Ocimum species, in the range between 7.42–8.06 mg/g FW, and the lowest amount in the S. micro (Andean sage, 2.41 mg/g FW). The ascorbic acid content (ASATOT, vitamin C), an important nutritional value, was of highest measured in S. micro (2.57 mg/g FW), M. did and N. × faas (2.42 and 2.34 mg/g FW respectively. Lower amounts of total ASA were detected in the S. disc and in the flowers of Ocimeae tribe.
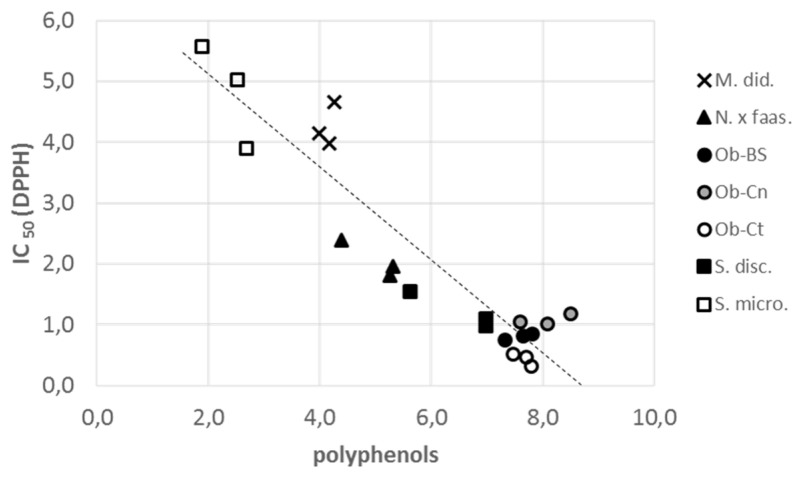
The radical scavenger activity by DPPH assay was monitored as the IC50 value: the highest activity was revealed in Ob-Ct (Thai lemon basil, 0.43 mg/mL), followed by the other two Ocimum and the Andean sage (S. disc). The lower antioxidant activity was measured in M. did and S. micro plants. Related to the higher antioxidant activity observed in the Ocimum flowers a negative correlation was observed with the highest content of total polyphenols, and is underlined in Figure 2.
Figure 2.
Correlation between polyphenols content in Lamiaceae flowers and the radical scavenger activity (DPPH). Straight line equation: y = −0.7654x + 6.6163; R2 = 0.8698.
2.2. Phytochemical Analyses
Overall, 118 chemical constituents were identified in the volatiles from Lamiaceae spp. samples (Table 3) with the number of peaks detected varying between 21 (N. × faas and S. micro) and 51 (O. basilicum ‘Cinnamon’, ’Ob-Cn). Sesquiterpene hydrocarbons represented the main class in all O. basilicum varieties as well as in S. disc (58.0% in S. disc to 77.9% in Ob-Ct), nevertheless they did not have the same characteristic compounds. β-caryophyllene, which was the only compound in common among all the studied species, represented the highest amount in both S. disc (36.2%) and Ob-Ct (23.7%). Ob-BS evidenced β-bisabolene (26.2%) as main constituent, while germacrene D (17.3%) and β-elemene (16.8%) prevailed in Ob-Cn. The presence of these latter constituents is conspicuous in all the previous species even though with different amounts, except for β-bisabolene, which was almost the exclusive compound of Ob-BS, present with lesser amount in S. disc (4.0%).
Table 3.
Volatile chemical composition (by headspace solid phase microextraction, HS-SPME) of flowers from the studied Lamiaceae species (n = 3) 1.
| Compounds | Class | RI (esp) | RI (lit) | M. did. | N. × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disco | S. micro | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Abundance % | |||||||||||
| 1 | ethyl isovalerate | NT | 854 | 856 $ | - | - | 0.5 ± 0.35 * | - | - | - | - |
| 2 | β-myrcene | MH | 991 | 988 | 0.8 ± 0.23 | - | - | 0.1 ± 0.07 | - | - | - |
| 3 | oxime, methoxy phenyl | NT-N | 926 | - | - | - | - | - | - | 1.1 ± 0.55 | - |
| 4 | α-thujene | MH | 929 | 924 | - | - | - | 0.1 ± 0.10 | - | - | - |
| 5 | α-pinene | MH | 937 | 932 | - | - | - | 0.1 ± 0.10 | - | - | 0.4 ± 0.40 |
| 6 | camphene | MH | 952 | 946 | - | - | - | 0.1 ± 0.08 | - | - | - |
| 7 | β-thujene | MH | 966 | 971 $ | - | - | - | - | - | 0.4 ± 0.37 | - |
| 8 | β-myrcene | MH | 991 | 988 | - | - | - | 0.1 ± 0.10 | - | - | - |
| 9 | α-phellandrene | MH | 1005 | 1002 | - | - | - | 0.1 ± 0.06 | - | - | - |
| 10 | (+)-4-carene | MH | 1009 | 1004 $ | 1.6 ± 0.19 | - | - | - | - | - | - |
| 11 | (E,E)-2,4-nonadiene | NT | 1014 | 1014 $ | - | - | - | 0.1 ± 0.08 | - | - | - |
| 12 | α-terpinene | MH | 1017 | 1014 | - | - | - | 0.1 ± 0.10 | - | - | - |
| 13 | o-cymene | MH | 1022 | 1022 | 13.3 ± 3.98 | - | - | - | - | 2.0 ± 0.96 | - |
| 14 | p-cymene | MH | 1025 | 1020 | - | - | - | - | - | - | 4.0 ± 0.23 |
| 15 | limonene | MH | 1030 | 1224 | - | - | - | 0.5 ± 0.09 | - | - | 25.8 ± 2.11 |
| 16 | eucaliptol | OM | 1032 | 1026 | - | 2.3 ± 0.17 | - | 0.6 ± 0.06 | - | - | 4.8 ± 0.10 |
| 17 | (Z)-β-ocimeme | MH | 1038 | 1032 | - | - | 0.2 ± 0.02 | 0.2 ± 0.05 | - | - | - |
| 18 | (E)-β-ocimeme | MH | 1049 | 1044 | - | - | 19.8 ± 0.25 | 2.4 ± 0.78 | 0.3 ± 0.28 | - | - |
| 19 | γ-terpinene | MH | 1060 | 1054 | 13.3 ± 3.08 | - | - | 1.0 ± 0.48 | - | - | 6.0 ± 0.34 |
| 20 | cis-sabinene hydrate | OM | 1070 | 1065 | 0.3 ± 0.27 | - | - | 0.2 ± 0.20 | - | - | - |
| 21 | 1-octanol | NT | 1071 | 1063 | - | - | - | - | 0.1 ± 0.08 | - | - |
| 22 | terpinolene | MH | 1088 | 1086 | - | - | - | 0.6 ± 0.06 | - | - | - |
| 23 | benzoic acid, methyl ester | NT | 1094 | 1091 $ | 0.2 ± 0.17 | - | - | - | - | - | - |
| 24 | linalool | OM | 1099 | 1095 | 17.1 ± 0.92 | - | - | 13.7 ± 0.75 | 1.6 ± 0.10 | 0.3 ± 0.07 | - |
| 25 | n-nonanal | NT | 1100 | 1100 | 0.2 ± 0.09 | - | - | - | - | - | 0.6 ± 0.10 |
| 26 | (E)-myroxide | OM | 1141 | 1140 | - | - | - | 0.7 ± 0.27 | 0.4 ± 0.05 | - | - |
| 27 | camphor | OM | 1145 | 1141 | - | - | - | 1.7 ± 0.68 | - | 0.4 ± 0.08 | 6.5 ± 0.28 |
| 28 | borneol | OM | 1167 | 1165 | - | - | - | 0.3 ± 0.14 | - | - | - |
| 29 | isoneral | OM | 1170 | 1175 $ | - | - | - | - | 0.1 ± 0.02 | - | - |
| 30 | terpinen-4-ol | OM | 1177 | 1174 | - | - | - | 2.5 ± 0.20 | - | - | - |
| 31 | isogeranial | OM | 1185 | 1184 $ | - | - | - | - | 0.3 ± 0.05 | - | - |
| 32 | α-terpineol | OM | 1189 | 1186 | - | - | - | 0.1 ± 0.06 | - | - | - |
| 33 | 3,7-octadiene-2,6-diol,2,6-dimethyl- | OM | 1190 | 1189 $ | - | - | - | 0.1 ± 0.06 | - | - | - |
| 34 | methyl salicylate | NT | 1192 | 1190 | 0.4 ± 0.03 | - | - | - | - | - | - |
| 35 | n-decanal | NT | 1206 | 1201 | 1.9 ± 0.35 | - | - | - | - | 0.5 ± 0.04 | 0.7 ± 0.11 |
| 36 | ethanol, 2-phenoxy- | NT | 1226 | 1221 $ | 0.1 ± 0.10 | - | - | - | - | - | - |
| 37 | nerol | OM | 1228 | 1227 | - | - | - | - | 1.8 ± 0.44 | - | - |
| 38 | 6-octenol, 7-methyl-3-methylene- | NT | 1229 | 1221 | - | - | - | - | 0.1 ± 0.09 | - | - |
| 39 | thymol methyl ether | OM | 1235 | 1232 | 19.9 ± 1.45 | - | - | - | - | - | - |
| 40 | β-citral | OM | 1240 | 1235 | - | - | - | - | 5.5 ± 0.53 | - | - |
| 41 | geraniol | OM | 1255 | 1249 | - | - | - | - | 1.4 ± 0.32 | - | - |
| 42 | chavicol | PP | 1256 | 1247 | - | - | 0.2 ± 0.07 | - | - | - | - |
| 43 | α-citral | OM | 1270 | 1264 | - | - | - | - | 9.2 ± 0.34 | - | - |
| 44 | bornyl acetate | OM | 1285 | 1284 | - | - | - | 0.9 ± 0.03 | - | - | - |
| 45 | isobornyl acetate | OM | 1286 | 1283 | - | - | - | - | - | - | 14.3 ± 1.66 |
| 46 | thymol | OM | 1292 | 1289 | 19.4 ± 1.59 | - | - | - | - | - | - |
| 47 | carvacrol | OM | 1299 | 1298 | 0.6 ± 0.10 | - | - | - | - | - | - |
| 48 | tridecane | NT | 1300 | 1300 | - | - | - | - | 0.1 ± 0.10 | - | - |
| 49 | elemene isomer | SH | 1344 | 1343 $ | - | - | - | 0.1 ± 0.07 | - | - | - |
| 50 | α-cubebene | SH | 1351 | 1345 | - | - | - | 0.4 ± 0.01 | 0.2 ± 0.01 | - | - |
| 51 | eugenol | PP | 1357 | 1356 | - | - | 6.9 ± 1.80 | 3.4 ± 1.14 | - | - | - |
| 52 | neryl acetate | OM | 1364 | 1359 | - | - | - | - | 0.1 ± 0.08 | - | - |
| 53 | α-copaene | SH | 1376 | 1374 | 0.1 ± 0.05 | - | 0.4 ± 0.01 | 1.8 ± 0.10 | 2.6 ± 0.13 | - | 6.3 ± 1.02 |
| 54 | cis-trans-nepetalactone | OM | 1377 | 1386 | - | 64.2 ± 0.47 | - | - | - | - | - |
| 55 | β-bourbonene | SH | 1384 | 1387 | - | - | - | - | 0.1 ± 0.06 | - | - |
| 56 | β-cubebene | SH | 1385 | 1387 | - | - | - | 0.1 ± 0.02 | 0.1 ± 0.03 | - | - |
| 57 | β-cubebene | SH | 1389 | 1387 | - | - | 0.6 ± 0.16 | 0.4 ± 0.09 | 1.2 ± 0.23 | - | - |
| 58 | β-elemene | SH | 1391 | 1389 | 0.3 ± 0.08 | 0.4 ± 0.15 | 0.1 ± 0.04 | 16.8 ± 1.69 | 0.2 ± 0.02 | 5.7 ± 0.43 | - |
| 59 | sesquithujene | SH | 1402 | 1405 | - | - | 0.2 ± 0.03 | - | 0.1 ± 0.03 | - | - |
| 60 | α-gurjunene | SH | 1409 | 1409 | - | - | - | 0.1 ± 0.06 | - | - | - |
| 61 | isodihydronepetalactone | OM | 1413 | 1414 § | - | 0.3 ± 0.13 | - | - | - | - | - |
| 62 | β-caryophyllene | SH | 1419 | 1417 | 3.1 ± 1.15 | 19.0 ± 1.17 | 4.6 ± 0.19 | 2.5 ± 1.03 | 23.7 ± 2.00 | 36.2 ± 7.93 | 2.2 ± 0.27 |
| 63 | β-copaene | SH | 1432 | 1430 | - | 0.2 ± 0.03 | 0.4 ± 0.25 | 1.1 ± 0.84 | 0.7 ± 0.41 | - | - |
| 64 | β-gurjunene | SH | 1434 | 1431 | - | - | - | 0.1 ± 0.08 | - | - | - |
| 65 | cis-geranylacetone | AC | 1435 | 1445 $ | - | - | - | - | - | - | 0.6 ± 0.08 |
| 66 | trans-α-bergamotene | SH | 1435 | 1432 | - | - | 6.4 ± 0.03 | - | 11.6 ± 0.57 | - | - |
| 67 | α-guaiene | SH | 1439 | 1437 | - | - | - | 9.0 ± 0.03 | - | - | - |
| 68 | (Z)-β-farnesene | SH | 1444 | 1440 | - | - | 1.4 ± 0.10 | - | - | - | - |
| 69 | isogermacrene D | SH | 1448 | 1446 § | - | - | - | - | 0.8 ± 0.11 | - | - |
| 70 | trans-geranylacetone | AC | 1453 | 1452 $ | 0.2 ± 0.19 | - | - | - | - | - | - |
| 71 | cis-muurola-3,5-diene | SH | 1454 | 1448 | - | - | - | 1.0 ± 0.40 | - | - | - |
| 72 | α-humulene | SH | 1455 | 1452 | - | 0.8 ± 0.11 | 1.9 ± 0.05 | 2.1 ± 0.31 | 3.2 ± 0.36 | 6.0 ± 0.93 | - |
| 73 | (E)-β-famesene | SH | 1457 | 1454 | - | 0.5 ± 0.23 | 2.5 ± 0.11 | - | 0.3 ± 0.02 | 0.7 ± 0.21 | - |
| 74 | cis-muurola-4(14),5-diene | SH | 1463 | 1465 | - | - | 0.3 ± 0.11 | 1.4 ± 0.18 | 0.5 ± 0.04 | - | - |
| 75 | γ-muurolene | SH | 1477 | 1478 | - | - | 0.1 ± 0.07 | 0.3 ± 0.05 | 0.3 ± 0.04 | - | 0.9 ± 0.39 |
| 76 | germacrene D | SH | 1481 | 1484 | 6.7 ± 0.73 | 8.0 ± 2.13 | 8.4 ± 1.25 | 17.3 ± 1.07 | 13.4 ± 1.35 | 1.7 ± 0.70 | - |
| 77 | 2-isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphtalene | SH | 1485 | 1485 $ | - | - | - | 0.4 ± 0.09 | - | - | - |
| 78 | β-selinene | SH | 1486 | 1489 | - | - | - | 0.3 ± 0.03 | - | 0.7 ± 0.09 | - |
| 79 | bicyclosesquiphellandrene | SH | 1489 | 1488 $ | - | - | - | 0.1 ± 0.04 | 0.2 ± 0.03 | - | - |
| 80 | bicyclo[7.2.0undec-4-ene,4,11,11-trimethyl-8-methylene- | NT | 1490 | 1504 $ | - | - | 0.8 ± 0.06 | - | 1.1 ± 0.06 | - | - |
| 81 | (Z,E)-α-farnesene | SH | 1491 | 1498 $ | - | - | - | - | - | 0.8 ± 0.07 | - |
| 82 | cis-muurola-4(14),5-diene | SH | 1492 | 1491 § | - | - | - | - | 0.2 ± 0.04 | - | - |
| 83 | valencene | SH | 1493 | 1496 | - | - | - | - | - | - | - |
| 84 | epi-cubebol | OS | 1493 | 1493 | - | - | - | - | - | - | 1.5 ± 0.19 |
| 85 | α-zingiberene | SH | 1495 | 1493 | - | 1.0 ± 0.25 | - | - | - | 0.9 ± 0.09 | - |
| 86 | γ-amorphene | SH | 1496 | 1495 | - | - | - | - | 0.1 ± 0.10 | - | - |
| 87 | aciphyllene | SH | 1499 | 1501 | - | - | - | 1.1 ± 0.19 | - | - | - |
| 88 | β-bulnesene | SH | 1505 | 1508 $ | - | - | - | 9.5 ± 0.68 | - | - | - |
| 89 | cis-α-bisabolene | SH | 1507 | 1506 | - | - | 0.1 ± 0.00 | - | - | - | - |
| 90 | β-bisabolene | SH | 1509 | 1505 | - | 0.5 ± 0.16 | 26.2 ± 1.56 | - | 0.9 ± 0.06 | 4.0 ± 1.60 | - |
| 91 | γ-cadinene | SH | 1513 | 1513 | - | 0.2 ± 0.01 | - | 2.9 ± 0.33 | 0.6 ± 0.09 | - | - |
| 92 | cubebol | OS | 1515 | 1514 | - | - | - | - | - | 0.5 ± 0.20 | 3.0 ± 0.50 |
| 93 | β-sesquiphellandrene | SH | 1524 | 1521 | - | - | - | - | - | 1.3 ± 0.02 | - |
| 94 | δ-cadinene | SH | 1525 | 1522 | - | 0.5 ± 0.05 | 0.6 ± 0.10 | 0.8 ± 0.01 | 1.3 ± 0.10 | - | 5.3 ± 0.55 |
| 95 | trans-γ-bisabolene | SH | 1533 | 1531 $ | 0.2 ± 0.08 | - | - | - | - | - | |
| 96 | α-cadinene | SH | 1538 | 1537 | - | - | 0.1 ± 0.06 | 0.2 ± 0.03 | 0.2 ± 0.04 | - | - |
| 97 | trans-α-bisabolene | SH | 1545 | 1545 $ | - | - | 17.3 ± 2.00 | - | 15.4 ± 0.47 | - | - |
| 98 | elemol | OS | 1549 | 1548 | - | - | - | - | - | 1.1 ± 0.28 | - |
| 99 | guaiol | OS | 1596 | 1600 | - | - | - | - | - | 0.2 ± 0.04 | 11.5 ± 0.41 |
| 100 | 10-epi-γ-eudesmol | OS | 1619 | 1622 | - | - | - | - | - | - | 0.4 ± 0.04 |
| 101 | τ-cadinol | OS | 1640 | 1638 | - | - | - | 0.2 ± 0.01 | - | - | - |
| 102 | β-eudesmol | OS | 1649 | 1649 | - | - | - | - | - | - | 1.1 ± 0.17 |
| 103 | Methyl dihydrojasmonate | NT | 1650 | 1648 § | - | - | - | - | - | 0.2 ± 0.20 | - |
| 104 | α-eudesmol | OS | 1653 | 1652 | - | - | - | - | - | - | 2.7 ± 0.27 |
| 105 | (+)-valeranone | OS | 1677 | 1674 | - | - | - | - | - | - | 1.0 ± 0.13 |
| 106 | elemol acetate | OS | 1679 | 1680 | - | - | - | - | - | 9.0 ± 1.87 | - |
| 107 | (E)-α-santalol | OS | 1680 | 1687 $ | - | - | - | 0.1 ± 0.10 | - | - | - |
| 108 | β-bisabolol | OS | 1684 | 1674 | - | - | - | - | - | 1.0 ± 0.17 | - |
| 109 | 2,2,6-trimethyl-1-(3-methylbuta-1,3-dienyl)-7-oxabicyclo[4.1.0] heptan-3-ol | NT | 1692 | 1692 $ | - | - | - | 0.2 ± 0.18 | - | - | - |
| 110 | β-sinensal | NT | 1695 | 1700 | - | 0.1 ± 0.10 | - | - | - | - | - |
| 111 | benzyl benzoate | NT | 1762 | 1759 | 0.2 ± 0.20 | - | - | - | - | - | - |
| 112 | α-sinensal | OS | 1752 | 1755 | - | - | - | - | - | 0.7 ± 0.25 | - |
| 113 | hexahydrofarnesyl acetone | AC | 1844 | 1845 $ | 0.1 ± 0.10 | - | - | - | - | - | - |
| 114 | pentylcurcumene | NT | 1950 | 1951 $ | - | 0.1 ± 0.10 | - | - | - | - | - |
| 115 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | NT | 2116 | 2116 & | - | - | - | - | - | 2.5 ± 0.12 | - |
| 116 | sandaracopimarinol | OD | 2279 | 2269 | - | - | - | - | - | 2.2 ± 0.44 | - |
| 117 | communic acid | NT | 2405 | 2365 | - | - | - | - | - | 3.6 ± 0.65 | - |
| 118 | methyl neoabietate | OD | 2435 | 2443 | - | - | - | - | - | 6.3 ± 0.89 | - |
| Number of identified peaks | 21 | 16 | 24 | 51 | 38 | 27 | 21 | ||||
| Class of Compounds | M. did. | N × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disc | S. micro | ||||
| Monoterpene hydrocarbons (MH) | 29.0 ± 4.71 | 2.3 ± 0.28 | 20.0 ± 0.50 | 5.3 ± 0.44 | - | 2.4 ± 0.34 | 36.8 ± 5.91 | ||||
| Oxygenated monoterpenes (OM) | 57.3 ± 4.32 | 66.8 ± 1.35 | - | 20.8 ± 0.21 | 20.4 ± 1.52 | 0.7 ± 0.13 | 25.6 ± 3.83 | ||||
| Sesquiterpene hydrocarbons (SH) | 10.2 ± 1.55 | 31.3 ± 2.03 | 71.6 ± 0.08 | 69.8 ± 4.06 | 77.9 ± 2.21 | 58.0 ± 8.11 | 14.7 ± 0.97 | ||||
| Oxygenated sesquiterpenes (OS) | - | - | - | 0.3 ± 0.16 | - | 12.5 ± 2.61 | 21.2 ± 0.89 | ||||
| Oxygenated diterpenes (OD) | - | - | - | - | - | 8.5 ± 0.45 | - | ||||
| Phenylpropanoids (PP) | - | - | 7.1 ± 1.87 | 3.4 ± 1.14 | - | - | - | ||||
| Apocarotenoids (AC) | 0.3 ± 0.05 | - | - | - | - | - | - | ||||
| Non-terpene derivatives (NT) | 3.0 ± 0.32 | 0.2 ± 0.03 | 1.3±0.59 | 0.3 ± 0.06 | 1.4 ± 0.21 | 7.9 ± 1.27 | 1.3 ± 0.38 | ||||
| Total Identified (%) | 99.8 ± 0.20 | 98.5 ± 0.50 | 100 ± 0.00 | 99.9 ± 0.01 | 100 ± 0.00 | 90.0 ± 4.41 | 99.6 ± 0.53 | ||||
1 value in tables are the mean of 3 triplicates; * Standard deviation; RI (exp): relative retention index determined on HP-5MS capillary column; RI (lit) relative retention index from Adams (1996); §: relative retention index found in pherobase.com; $: relative retention index found in NIST 2014; &: relative index found in pubchem (pubchem.ncbi.nlm.nih.gov).
More than the half of M. did volatile organic compounds (VOCs) was represented by oxygenated monoterpenes (57.3%), especially constituted by thymol (19.4%) and its methyl ether (19.9%) together with linalool (17.1%). This plant species showed also a good amount of monoterpene hydrocarbons (29.0%), with both o-cymene and γ-terpinene as the same highest amount (13.3%).
N. × faas aroma profile was divided into two classes of compounds: oxygenated monoterpenes (OM), which was the predominant one (66.8%), and sesquiterpene hydrocarbons (SH, 31.3%). This species showed β-caryophyllene (19.0%) as the most abundant sesquiterpene together with germacrene D (8.0%). Furthermore, cis-trans-nepetalactone, an iridoid monoterpenoid (64.2%), was the chief constituent seen that it represents more than 96% of OM class.
The second species of the Salvia genus (S. microphylla) showed a heterogeneous profile because all the classes were present. In fact, the EO composition evidenced the presence of MH (36.8%), OM (25.6%), OS (21.2%) and SH (14.7%) in this decreasing order. This species was characterized by limonene (25.8%) followed by isobornyl acetate (14.3%) and guaiol (11.5%) as principal components.
2.3. Multivariate Explorer Analyses
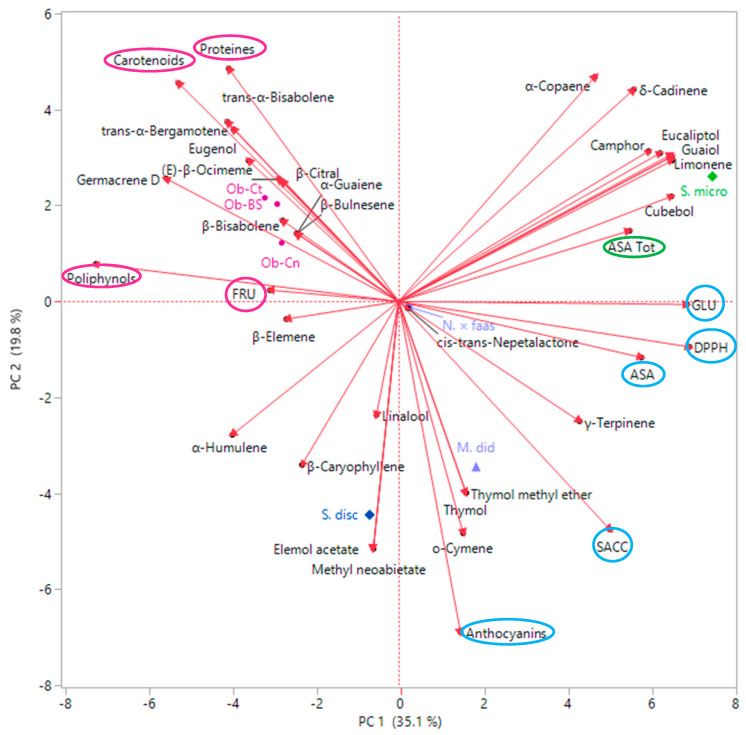
Principal component analysis (PCA) was performed with the spontaneous emission compounds present in a percentage greater than 3% in addition to the nutritional values of flowers. The result of this multivariate analysis (Figure 3) where the first two axes account for 54.9% for a correlation matrix, showed two first macro groups, one with positive loading on PC1 and the other one with a negative loading in the same axis. All Ocimum varieties, scored negatively along PC1, were located in the upper left quadrant. This loading was generated mostly by the content of both distinguished compounds such β-citral in Ob-Ct (5.5%), (E)-β-ocimene (19.8%), eugenol (6.9%) and β-bisabonene (26.2%) in Ob-BS, and common compounds such as trans-α-bergamotene (11.6% and 6.4%, respectively), trans-α-bisabolene (15.7% and 17.3%, respectively), in addition to their nutritional value as regards proteins (13.8 in Ob-Ct and 16.16 in Ob-BS, respectively) and carotenoids (81.86 in Ob-Ct and 51.59 in Ob-BS respectively). Ob-Cn, even though it had a negative loading along PC1 and plotted in the same quadrant as the other two varieties, was slightly separated from them. In fact, this basil was distinguished by the presence of α-bulnesene (9.5%) and α-guaiene (9.0%) together with the high value of polyphenols (8.06 mg/g FW) and fructose (FRU) (6.85 mg/g FW). S. disc, with its negative loading along both axes, was positioned deep down in the left quadrant by dint of characteristic compounds: elemol acetate (9.0%) and methyl neoabietate (6.3%), together with their amount in saccharose (SACC) and anthocyanins. In the opposite quadrant relative to the Y-axis two out of three remaining species were present: M. did and N. × faas. These species were scored positively along PC1 and negatively along PC2. This position is mainly due to the main compounds as for Monarda and Nepeta. S. micro was the only sample with a positive loading on both PC1 and PC2, and it was located in the upper right quadrant because both its main constituents were previously cited as well as other specific compounds such as camphor (6.5%), α-copaene (6.3%), δ-cadinene (5.3%), eucalyptol (4.8%) and cubebol (3.0%), with the addition of glucose (GLU) and ASATOT content.
Figure 3.
Principal component analysis (PCA) plot depicting phytochemical proximities among VOCs of the studied spp.
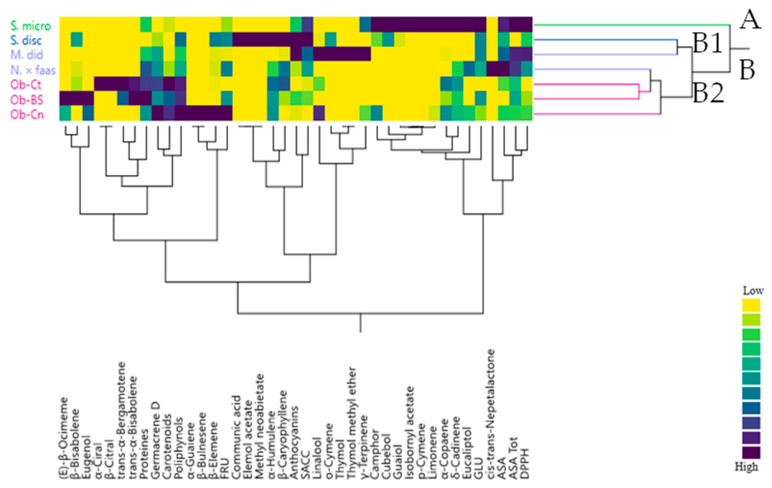
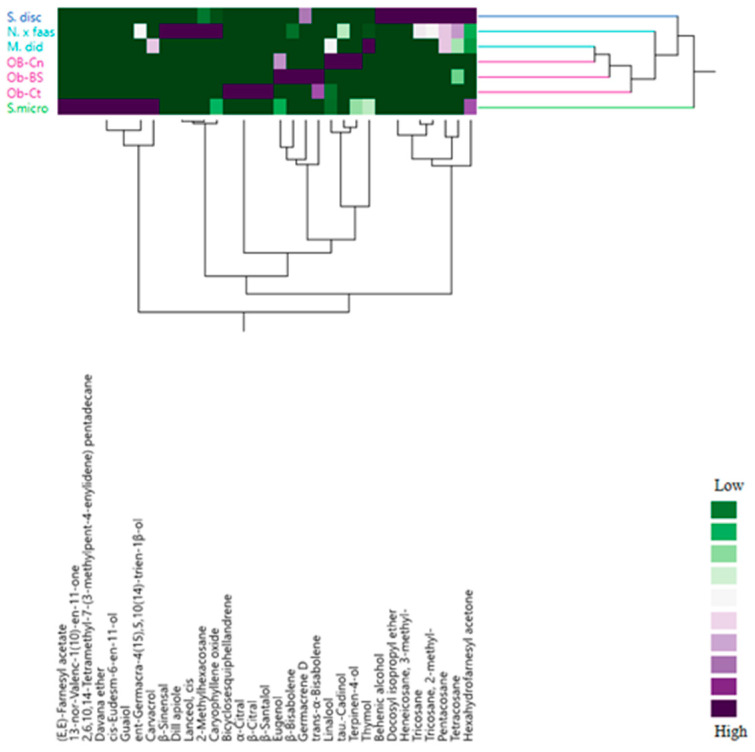
These results were confirmed by the heat map of the two-way HCA analysis (Figure 4) which differentiated S. microphylla (A) from other studied species gathered together in the group (B). This latter was further divided into two clusters. The cluster B1 was composed by S. disc and M. did, which in spite they showed a VOC with different compositions, the two species pointed out the highest amount in anthocyanins (0.98 in both spices). The cluster B2 included the remaining plant species. Including Nepeta with basil varieties was not strange, owing to fact that this species shared with basil its high percentage of germacrene D as well as proteins and carotenoids.
Figure 4.
Dendrogram of cluster hierarchical analysis performed on VOCs from the studied Lamiaceae species.
2.4. Essential Oil (EO) Analysis
The different constituents of the EOs from the seven Lamiaceae species studied herein, identified by gas chromatography-mass spectrometry (GC-MS) analysis, are reported in Table 4. Ninety-five compounds were present accounting for 92.7% in Nepeta to 100% of the total identification in the oil composition of Ob-BS. The striking thing was the drastic decrease of the number of identified peaks in all the Ocimum varieties. This decrease was about 58% in Ob-BS to more than 85% in Ob-Cn.
Table 4.
Chemical composition of the flower EOs from the studied Lamiaceae species (n = 3) 1.
| Compounds | Class | RI (exp) | RI (lit) | M. did | N. × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disco | S. micro | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative abudance (%) | |||||||||||
| 1 | 5,5-dimethyl-2(5H)-furanone | nt | 952 | 952 | - | - | - | - | - | - | 2.3 ± 0.38 |
| 2 | eucalyptol | om | 1032 | 1026 | - | 0.3 ± 0.09 * | - | - | - | - | - |
| 3 | 3,5-octadien-2-ol | nt | 1038 | 1037 | - | - | - | - | - | - | 1.2 ± 0.61 |
| 4 | cis-sabinene hydrate | om | 1070 | 1068 | 0.6 ± 0.06 | - | - | - | - | - | - |
| 5 | linalool | om | 1099 | 1095 | 10.2 ± 1.12 | - | - | 48.6 ± 1.64 | 1.4 ± 0.06 | - | 1.3 ± 0.90 |
| 6 | terpinen-4-ol | om | 1177 | 1074 | - | - | - | 23.7 ± 1.90 | - | - | 2.3 ± 0.12 |
| 7 | isocreosol | pp | 1201 | 1202 | - | 0.9 ± 0.32 | - | - | - | - | - |
| 8 | nordavanone | om | 1230 | 1234 | - | - | - | - | - | - | 2.4 ± 0.76 |
| 9 | pulegone | om | 1237 | 1237 | - | 0.1 ± 0.08 | - | - | - | - | - |
| 10 | β-citral | om | 1240 | 1245 | - | - | - | - | 18.8 ± 0.90 | - | - |
| 11 | camphor | om | 1245 | 1143 | - | - | - | - | - | - | 1.4 ± 0.41 |
| 12 | α-citral | om | 1270 | 1271 | - | - | - | - | 32.2 ± 1.68 | - | - |
| 13 | benzenepropanoic acid, methyl ester | nt | 1279 | 1280 | - | 0.3 ± 0.09 | - | - | - | - | - |
| 14 | isobornyl acetate | om | 1286 | 1290 | - | - | - | - | - | - | 0.6 ± 0.14 |
| 15 | thymol | om | 1291 | 1289 | 68.6 ± 3.43 | 0.4 ± 1.15 | - | - | - | - | 8.1 ± 0.95 |
| 16 | carvacrol | om | 1299 | 1298 | 4.5 ± 0.99 | - | - | - | - | - | 10.9 ± 1.95 |
| 17 | eugenol | pp | 1357 | 1356 | - | - | 17.6 ± 0.25 | 10.7 ± 0.43 | - | - | 1.6 ± 0.76 |
| 18 | cis-trans-nepetalactone | om | 1377 | 1393 | - | 0.8 ± 0.22 | - | - | - | - | - |
| 19 | β-bourbonene | sh | 1384 | 1385 | - | 0.3 ± 0.04 | - | - | - | - | - |
| 20 | β-caryophyllene | sh | 1419 | 1417 | - | 2.4 ± 0.26 | - | - | - | 1.2 ± 0.61 | - |
| 21 | α-bergamotene | sh | 1435 | 1438 | - | - | 0.5 ± 0.01 | - | - | - | - |
| 22 | 2,6,10-trimethyltridecane | nt | 1449 | 1461 | - | - | - | - | - | 0.2 ± 0.11 | - |
| 23 | α-humulene | sh | 1454 | 1452 | - | 0.4 ± 0.04 | - | - | - | 0.3 ± 0.16 | - |
| 24 | (E)-β-famesene | sh | 1457 | 1454 | - | 0.4 ± 0.05 | 0.4 ± 0.02 | - | - | - | - |
| 25 | germacrene D | sh | 1481 | 1484 | - | - | 3.4 ± 0.04 | - | - | 2.3 ± 0.51 | - |
| 26 | α-curcumene | sh | 1483 | 1486 | - | 0.8 ± 0.04 | - | - | - | - | - |
| 27 | 1-(3,6,6-trimethyl-1,6,7,7a-tetrahydrocyclopenta[c]pyran-1-yl) ethanone | nt | 1484 | - | - | 0.3 ± 0.07 | - | - | - | - | - |
| 28 | bicyclosesquiphellandrene | sh | 1489 | 1488 | - | - | - | - | 3.3 ± 0.21 | - | - |
| 29 | davana ether | os | 1490 | 1491 | - | - | - | - | - | - | 16.3 ± 1.51 |
| 30 | α-farnesene | sh | 1508 | 1509 | - | - | - | - | - | 0.2 ± 0.06 | - |
| 31 | β-bisabolene | sh | 1509 | 1505 | - | 0.8 ± 0.02 | 34.4 ± 2.02 | - | - | - | - |
| 32 | trans-α-bisabolene | sh | 1512 | 1545 $ | - | - | 38.7 ± 2.94 | - | 29.3 ± 0.80 | - | - |
| 33 | γ-cadinene | sh | 1513 | 1511 | - | 0.3 ± 0.00 | - | - | - | - | - |
| 34 | β-sesquiphellandrene | sh | 1524 | 1521 | - | 0.4 ± 0.01 | - | - | - | - | - |
| 35 | cyclohexanemethanol, 4-ethenyl-α,α,4-trimethyl-3-(1-methylethenyl)-, acetate, [1R-(1α,3α,4β)]- | nt | 1569 | 1562 | - | - | - | - | - | 1.5 ± 0.03 | - |
| 36 | cis-eudesm-6-en-11-ol | os | 1571 | 1575 | - | - | - | - | - | - | 4.1 ± 0.15 |
| 37 | caryophyllene oxide | os | 1581 | 1583 | - | 17.2 ± 1.19 | - | - | - | 0.1 ± 0.03 | 1.1 ± 0.51 |
| 38 | davanone | os | 1588 | 1586 | - | - | - | - | - | - | 2.8 ± 0.19 |
| 39 | guaiol | os | 1596 | 1597 | - | - | - | - | - | - | 4.0 ± 0.65 |
| 40 | humulene epoxide II | os | 1606 | 1608 | - | 1.1 ± 0.03 | - | - | - | - | - |
| 41 | zingiberenol | os | 1616 | 1620 | - | 1.1 ± 0.19 | - | - | - | - | - |
| 42 | dill apiole | os | 1622 | 1625 | - | 3.0 ± 0.31 | - | - | - | - | - |
| 43 | 13-nor-valenc-1(10)-en-11-one | os | 1629 | 1628 | - | - | - | - | - | 3.1 ± 0.75 | |
| 44 | selin-6-en-4α-ol | os | 1636 | 1636 | - | 0.2 ± 0.04 | - | - | - | - | - |
| 45 | tau.-cadinol | os | 1640 | 1640 | - | 1.6 ± 0.19 | - | 13.8 ± 2.94 | - | - | - |
| 46 | cubenol | os | 1642 | 1643 | - | 0.4 ± 0.06 | - | - | - | - | - |
| 47 | 10,10-dimethyl-2,6-dimethylenebicyclo[7.2.0] undecan-5β-ol |
os | 1644 | 1644 | - | 1.2 ± 0.11 | - | - | - | - | - |
| 48 | β-eudesmol | os | 1649 | 1651 | - | - | - | - | - | - | 2.0 ± 0.13 |
| 49 | α-eudesmol | os | 1653 | 1652 | - | - | - | - | - | - | 2.8 ± 0.41 |
| 50 | precocene II | pp | 1658 | 1659 | - | 2.3 ± 0.30 | - | - | - | - | - |
| 51 | aromadendrene oxide-(2) | os | 1678 | 1678 | - | 2.0 ± 0.08 | - | - | - | - | - |
| 52 | α-bisabolol | os | 1684 | 1683 | - | - | 1.3 ± 0.07 | - | - | - | |
| 53 | β-sinensal | os | 1695 | 1704 | - | 4.1 ± 0.91 | - | - | - | - | - |
| 54 | germacra-4(15),5,10(14)-trien-1β-ol | os | 1695 | 1686 $ | - | 0.6 ± 0.13 | - | - | - | - | 3.5 ± 0.36 |
| 55 | heptadecane | nt | 1700 | 1700 | - | 0.6 ± 0.25 | - | - | - | 0.2 ± 0.04 | - |
| 56 | Z-α-trans-bergamotol | os | 1701 | 1708 | - | 0.1 ± 0.00 | - | - | - | - | - |
| 57 | longifolenaldehyde | os | 1707 | 1708 | - | 0.5 ± 0.07 | - | - | - | - | - |
| 58 | cuprenenol | os | 1709 | 1702 | - | - | - | - | - | - | 2.0 ± 0.06 |
| 59 | β-santalol | os | 1715 | 1720 | - | - | 12.0 ± 1.29 | - | - | ||
| 60 | cis-nuciferol | pp | 1735 | 1730 | - | 2.2 ± 0.55 | - | - | - | - | - |
| 61 | (6R,7R)-bisabolone | os | 1747 | 1737 | - | 1.2 ± 0.39 | - | - | - | - | - |
| 62 | cis-lanceol | os | 1763 | 1761 | - | 4.6 ± 1.57 | - | - | - | - | - |
| 63 | costol | os | 1778 | 1774 | - | 0.4 ± 0.15 | - | - | - | - | - |
| 64 | hexadecanal | nt | 1817 | 1818 | - | - | - | - | - | 0.1 ± 0.08 | - |
| 65 | (E,E)-farnesyl acetate | os | 1843 | 1843 | - | - | - | - | - | - | 4.4 ± 1.15 |
| 66 | hexahydrofarnesyl acetone | ac | 1844 | 1845 | 1.3 ± 0.77 | 1.6 ± 0.69 | - | - | - | 15.7 ± 2.04 | 11.9 ± 1.12 |
| 67 | 2,6,10,15-tetramethyl-benzoic acid, 2-phenylethyl ester | nt | 1856 | 1860 | - | 0.1 ± 0.08 | - | - | - | - | - |
| 68 | 3-methyl-nonadecane | nt | 1970 | 1972 | - | - | - | - | - | 0.2 ± 0.04 | - |
| 69 | octadecanal | nt | 2021 | 2021 | - | - | - | - | - | 0.2 ± 0.03 | - |
| 70 | 2,6,10,14-tetramethyl-7-(3-methylpent-4-enylidene) pentadecane | nt | 2071 | 2068 | - | - | - | - | - | - | 5.3 ± 0.88 |
| 71 | heneicosane | nt | 2100 | 2100 | - | 0.5 ± 0.35 | - | - | - | 1.5 ± 0.26 | - |
| 72 | phytol | od | 2114 | 2122 | - | 0.3 ± 0.04 | - | - | - | 0.3 ± 0.06 | 2.5 ± 0.65 |
| 73 | 1 N-phenyl-naphthalenamine | nt-N | 2135 | 2135 | - | 1.2 ± 0.80 | - | - | - | - | - |
| 74 | 3-methyl-heneicosane | nt | 2171 | 2172 | - | - | - | - | - | 9.9 ± 1.75 | - |
| 75 | docosane | nt | 2200 | 2200 | - | - | - | - | - | 0.4 ± 0.11 | - |
| 76 | eicosanal | nt | 2224 | 2224 | - | - | - | - | - | 0.4 ± 0.14 | - |
| 77 | sclareol | od | 2227 | 2225 | - | 0.3 ± 0.14 | - | - | - | - | - |
| 78 | 4-methyldocosane | nt | 2257 | 2258 | - | 0.9 ± 0.07 | - | - | - | 0.6 ± 0.21 | - |
| 79 | larixol | od | 2264 | 2265 | - | 0.1 ± 0.03 | - | - | - | - | - |
| 80 | kolavenol | od | 2297 | 2297 | - | - | - | - | - | 0.3 ± 0.12 | - |
| 81 | carbonic acid, octadecyl vinyl ester | nt | 2299 | 2299 $ | - | 1.4 ± 0.47 | - | - | - | - | - |
| 82 | tricosane | nt | 2300 | 2300 | - | 1.3 ± 0.90 | - | - | - | 5.0 ± 1.24 | - |
| 93 | 2-methyl-tricosane | nt | 2363 | 2365 | - | 0.7 ± 0.48 | - | - | - | 4.3 ± 1.52 | - |
| 84 | 1-heneicosanol | nt | 2380 | 2365 | 0.5 ± 0.07 | - | - | - | - | - | - |
| 85 | tetracosane | nt | 2400 | 2400 | 4.5 ± 0.83 | 14.7 ± 3.63 | 3.7 ± 0.53 | - | - | 24.3 ± 1.87 | - |
| 86 | undec-10-ynoic acid, dodecyl ester | nt | 2409 | 2409 $ | - | 0.3 ± 0.04 | - | - | - | - | - |
| 87 | docosanal | nt | 2430 | 2430 | - | - | - | - | - | 0.4 ± 0.05 | - |
| 88 | 2-methyltetracosane | nt | 2462 | 2456 | - | 0.2 ± 0.09 | - | - | - | - | - |
| 89 | (Z)-13-docosen-1-ol | nt | 2467 | 2466 | - | - | - | - | - | 0.2 ± 0.06 | - |
| 90 | retinol | od | 2473 | 2473 $ | - | 1.9 ± 0.45 | - | - | - | - | - |
| 91 | retinal | od | 2486 | 2486 $ | - | 0.2 ± 0.03 | - | - | - | - | - |
| 92 | behenic alcohol | nt | 2493 | 2501 | - | - | - | - | - | 3.1 ± 0.95 | - |
| 93 | pentacosane | nt | 2500 | 2500 | 6.8 ± 1.42 | 6.8 ± 1.46 | - | - | - | 14.6 ± 2.76 | - |
| 94 | docosyl isopropyl ether | nt | 2524 | - | - | - | - | - | - | 10.2 ± 0.71 | - |
| 95 | 2-methylhexacosane | nt | 2661 | 2663 | - | 6.9 ± 0.87 | - | - | - | 0.2 ± 0.03 | - |
| Number of identified peaks | 8 | 52 | 8 | 4 | 6 | 28 | 24 | ||||
| Class of compounds | M. did. | N × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disco | S. micro | ||||
| Oxygenated monoterpenes (om) | 83.9 ± 2.49 | 1.6 ± 0.42 | - | 72.3 ± 3.11 | 52.4 ± 2.51 | - | 27.0 ± 1.84 | ||||
| Sesquiterpene hydrocarbons (sh) | - | 5.8 ± 0.43 | 77.4 ± 2.96 | - | 32.6 ± 1.00 | 4.0 ± 1.87 | - | ||||
| Oxygenated sesquiterpenes (os) | - | 35.2 ± 2.29 | 1.3 ± 0.07 | 13.8 ± 1.94 | 12.0 ± 1.29 | 0.1 ± 0.03 | 46.1 ± 3.98 | ||||
| Oxygenated diterpenes (od) | - | 2.8 ± 0.84 | - | - | - | 0.6 ± 0.18 | 2.5 ± 0.56 | ||||
| Apocarotenoides (ac) | 1.3 ± 0.77 | 1.6 ± 0.69 | - | - | - | 15.7 ± 2.04 | 11.9 ± 1.12 | ||||
| Non-terpenes derivatives (nt) | 11.8 ± 1.02 | 41.2 ± 4.11 | 3.7 ± 0.53 | - | - | 77.5 ± 3.87 | 8.8 ± 1.87 | ||||
| Phenylpropanoids (pp) | - | 4.5 ± 0.85 | 17.6 ± 0.25 | 10.7 ± 0.43 | - | - | 1.6 ± 0.76 | ||||
| Total Identified (%) | 97.0 ± 2.31 | 92.7 ± 3.3 | 100 ± 0.00 | 96.8 ± 0.22 | 97.0 ± 0.17 | 97.9 ± 0.01 | 97.9 ± 0.63 | ||||
1 value in tables are the mean of 3 triplicates; * Standard deviation; RI (exp): relative retention index determined on HP5MS capillary column; RI (lit) relative retention index from ADAMS (1996).
Another important thing to note was how the fragile and thermosensitive constituents decomposed into artefacts due to the heating during the hydrodistillation. All the O. basilicum volatiles were dominated by sesquiterpene compounds which were biosynthesized by the mevalonic acid (MVA) pathway, while the EO distillation originated the volatile monoterpenes (C10) [44]. This is because the two varieties of basil, Ob-Cn and Ob-Ct, showed OM as the main class of compounds in their EOs (72.3% and 52.4%, respectively) except for the Ob-BS that seemed not to be affected by heating since the EO profile evidenced SH (77.4%) as in VOCs. Linalool (48.6%) and terpinene-4-ol (23.7%) were the main monoterpenes in Ob-Cn; α- and β-citral in Ob-Ct (32.2% and 18.8%, respectively). This latter species evidenced also a good percentage of SH (32.6%), represented by trans-α-bisabolene (29.3%). This compound (38.7%), together with β-bisabolene (34.4%), were peculiar in Ob-BS.
M. didyma showed a trend not very different from its spontaneous emission because it conserved the predominance of the same class of compounds: OM (84.5%). Thymol (68.6%) became the chief compound, while thymol methyl ether completely disappears. By contrast, Nepeta evidenced aliphatic hydrocarbons as the most abundant class (NT, 41.2%) together with a good amount of OS (35.2%). In detail of composition, caryophyllene oxide (17.2%) and tetracosane (14.7%) were most abundant constituents.
S. disc had a radically different profile, and its EO was distinguished by its high rate of NTs (77.5%). More than the 63% of this fraction was represented only by three compounds: tetracosane (24.3%), pentacosane (14.6%) and docosyl-isopropyl ether (10.2%). Important was also the amount of apocarotenoids exclusively represented by hexahydrofarnesyl acetone (15.7%). This constituent was also present in the second species of the Salvia genus in a notable amount (11.9%). The membership class of this compound was one of the main class in S. micro, even though it was not the prevalent one. In fact, OS (46.1%) and OM (27.0%) were mainstream. Davana ether (16.3%) and carvacrol (10.9%) showed to be the most representative compounds.
2.5. Multivariate Explorer Analyses
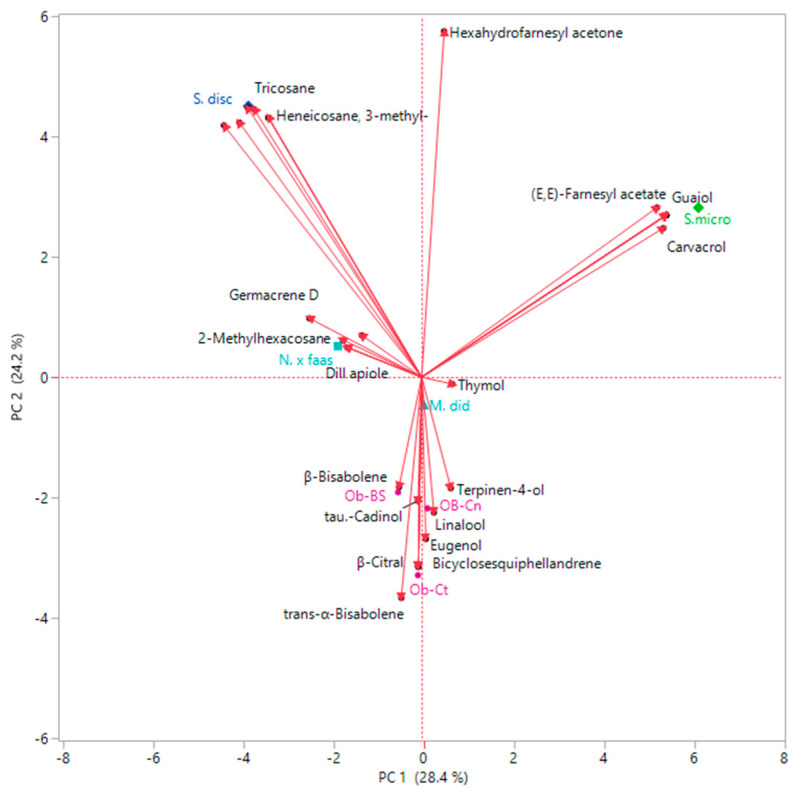
The PCA analysis performed with compounds of EOs > 3% was reported in Figure 5. The first two axes account for more than the half (52.6%) of a correlation matrix. Here PC2 plays a key role in the agglomeration of the species rather than PC1. In fact, two macro groups were present: S. micro, S. disc and N × faas were of positive loading on PC2 while the remaining ones were of negative loading. It is interesting to note that only S. micro was positioned in the upper right quadrant (load positively in both axes) and this was due to the exclusive compound (E,E)-farnesyl acetone and guaiol as well as its high amount of carvacrol. The species with the highest percentage of NTs, S. disc and N. × faas, were positioned on the opposite quadrant. All the basil species together with Monarda were located in the borderline along Y-axis, except for Ob-BS which shifted a little to the left, this was because of its content in β-bisabonene.
Figure 5.
PCA plot depicting phytochemical proximities among the essential oils (EOs) of the studied spp.
The heat map of the two-way HCA analysis (Figure 6) confirmed what observed in PCA analysis and distinguish S. micro from the others (group I). The second group II was further divided into two subgroups: II.1 homogeneous constituted only by S. disc; II.2 which gathered M. did with all the basil species.
Figure 6.
Dendrogram of cluster hierarchical analysis performed on EOs from the studied Lamiaceae species.
3. Discussion
3.1. Bioactive Compounds
Carbohydrates are the most abundant macronutrient in edible flowers, reaching even 90% of Rosa micrantha’s dry weight [13,45]. Nectar is a relevant source of soluble sugars [14], and it is composed of water, sucrose, glucose, fructose, and traces of 10 minor sugars [46]. Most Lamiaceae flowers are known to produce nectar in significant amounts and several species are cultivated as melliferous plants [47,48]. In our study, Salvia spp. and M. did flowers contained the highest quantities of glucose and sucrose, while Ob-Cn was characterised by the highest amount of fructose (Table 2). Soluble sugars were poorly represented in Ob-Ct, compared to the other six flowers under evaluation (Table 2). Sucrose amount in M. did was fully comparable with the results obtained by Stefaniak and Grzeszczuk [49] who analysed the same species. However, some discrepancy in the amount of total reducing sugars was evidenced. This could be due to other components of reducing sugars and/or to the genetic difference between M. did and plant material, the origin of the outset plant material and the cultivation methods used in other reports. Very few studies were performed on the detection of soluble sugars in O. basilicum edible flowers. Shanaida et al. [50] quantified total soluble sugars and reducing sugars in O. americanum, with similar range of contents as those presented here.
Usually, in edible flowers carbohydrates are followed by proteins, ranging between 2.0 and 52.3 g/100 g DW (reviewed in [13]). In our work, these primary metabolites were of the highest amount in Ocimum spp. and N. × faas, exceeding 10% of the flowers’ dry weight. Similar results were obtained analysing other well-known edible flowers, such as Allium schoenoprasum [51] and Cucurbita pepo [52], although these flowers belong to different families. However, previous work on flowers of M. didyma showed higher percentage of proteins, due to the cultivation systems [49].
Secondary metabolites are classified in phenolics, terpenes and steroids, and alkaloids [53]. They are usually involved in the adaptation of plants to their environment, playing a role in plant defense against biotic and abiotic stresses [53], ultraviolet radiation and oxidants [54]. Flowers assigned on these molecules the role to attract pollinators as well as the fragrance and brightness [53,54,55]. Flowers’ colours are determined by flavonoids (mostly anthocyanins), betalains and carotenoids [53,56], that often contribute in mixture to the final flowers’ hue [56]. Carotenoids are involved in yellow, orange and red flowers’ pigmentation [57], while anthocyanins are mainly responsible for the bluish to purple and reddish colors [54]. Betalains are the yellow and violet pigments that replace anthocyanins in plants belonging to the order Caryophyllales [58] and, for this reason, they were not evaluated in this work. In our study, carotenoids were higher in S. disc and Ocimum spp. flowers (Table 2) than in the other three varieties under evaluation and some species reported in literature, such as the pale colored Telosma minor (Andrews) W. G. Craib and Piper retrofractum Vahl (12.9 each μg/g DW) [59], as well as other 11 species (0.020–0.992 μg/g FW), including Lavandula angustifolia Mill. and Salvia spendens Sellow ex Roem. et Shult. [60].
On the other hand, the examined flowers contained less carotenoids than Hemerocallis × hybrida Hort., Mimulus × hybridus ‘Magic Yellow’, and black Dianthus chinensis L. ‘Chianti’ [49]. In fact, all of them, with the only exception for D. chinensis ‘Chianti’, were decribed as flowers with intense yellow, orange or red colourations. This feature makes these flowers very different from those described herein, which are characterised by softer tones. Anyway, regarding M. did, there was a strong discrepancy between our carotenoid quantification and the one obtained by Stefaniak et al. [49] (1.91 vs. 167.20 μg/g FW). This could be due to different genetic background and cultivation systems.
S. disc and M. did were rich in anthocyanins, as their colour suggested. This class of metabolites is higher in these two flowers than in Begonia semperflorens Link and Otto (0.05 mg/g FW), Fuchsia hybrida Hort. Ex Siebert and Voss (0.08 mg/g FW) and Pelargonium peltatum (L) L’Hér. (0.14 mg/g FW), which are characterised by red petals [61]. Nevertheless, D. chinensis ‘Chianti’ and M. didyma contain more than 2 mg/g FW of anthocyanins [49]. These last results were obtained with different methods, therefore the comparison may be not similar.
Between the species studied herein, S. disc and Ocimum spp. showed the highest content in total polyphenols (TPC). The same metabolites are comparable between S. micro (2.41 mg/g FW) and S. splendens (2.16 mg/g FW) [60]. To the best of our knowledge, the TPC of O. basilicum flowers was reported in only one paper [62], with the quantification of three different basil cultivars (‘Subja’, ‘Holy green’ and ‘Red rubin’) in freeze-dried samples, making difficult the comparison with the fresh flowers used in this work. No studies were published on the TPC in N. × faas flowers, but some data are available for other species belonging to the same genus, such as N. cataria L. [63] and N. nepetella L. [64]. Dried flowers of these two species are characterised by 2- and 4-fold more TPC than N. × faas fresh flowers [63,64].
Ascorbic acid (vitamin C, ASA) is known to take part in essential human biochemical and physiological processes. This molecule plays a relevant role in the development and maintenance of connective tissues, in bone formation and wound healing [65]. ASA is also involved in several metabolic pathways, in the proper functioning of the immune system and it protects the human body from free radicals’ damages [65]. However, human organisms are not able to synthetize this vitamin, since humans lack the terminal enzyme of its biosynthetic pathway [66]. For this reason, ASA must be present in a well-balanced diet, and the consumption of edible flowers can help to supply the EU daily requirements intakes (80 mg per days) [67]. In fact, Tagetes tenuifolia Cav. and Viola tricolor L. are considered good sources of vitamin C, containing 241.20 and 182.16.20 mg/100g FW respectively [60].
In this work, M. did, S. micro and N. × faas were characterised by higher levels of total (ASATOT) and the reduced form (ASA) than the other species under evaluation (Table 2). Nevertheless, the amounts of vitamin C were very low, and due to their small size, thousands of flowers would be needed to reach the EU recommended intakes. However, compared to other Lamiaceae flowers, these two varieties and N. × faas were characterized by ASATOT content similar to some Agastache spp. [17]. On the other hand, M. did and S. micro contained around −18 and −15 fold less vitamin C than S. splendens and L. angustifolia [60].
Carotenoids, TPC and ASA are known as antioxidant molecules [68,69]. The radical scavenging activity of the flowers under evaluation was highest in the Ocimeae tribe. This parameter is remarkable in Ob-Ct species, since it is higher than other edible flowers such as Agastache ‘Blue Boa’ (IC50 0.86 mg/mL) [17], Crithmum maritimum L. (IC50 0.71 mg/mL) [70], and Centaurea cyanus L. (IC50 0.79 mg/mL) [71]. A strong correlation between polyphenols and radical scavenging activity (R2 = 0.8698) (Figure 2) were observed, as already evinced in other edible flowers, such as Bellis perennis L. [72], Calendula officinalis L. [73], and 19 Chinese species [74].
3.2. Spountaneous Emissions
In this study, we evaluated the aroma profile spontaneously emitted from the seven selected Lamiaceae flowers. The analyses of the volatile organic compounds increased our knowledge concerning their ecological role. In spite of this, the species that have been investigated for their spontaneous emission were too negligible compared to the high number of plants present in nature. The spontaneous emission of Ocimum basilicum (Ob) was already widely studied [75,76,77,78,79], but only a few papers investigated its varieties and none of these works reported the varieties studied herein. In 2008, Klimánková et al., [80] evaluated five cultivars of basil green cultivar I (Prava zelena), green cultivar II (Trpaslici), red cultivar III (Cinamonette), red cultivar IV (Purple Opaal), and red cultivar V (Rot), and their volatile composition was characterized by linalool, methyl chavicol, eugenol, bergamotene, and methyl cinnamate. A more recent study reported the SPME components of two basil varieties (Violetto and Genovese) where linalool (18.94% and 22.57% respectively), eugenol (3.95% and 15.02%, respectively) and methyl eugenol (39.17% and 19.39%, respectively) were identified as the main constituents [77]. On the contrary, the whole spontaneous emission of Malaysian O. basilicum flower was represented by estragole (88.18%) [78]. In the three Ocimum varieties studied herein only linalool and eugenol were detected even though their presence was not detected in all varieties (Ob-Cn and Ob-Ct for linalool and Ob-Cn and Ob-BS for eugenol) obviously with lesser percentage. Bergamoptene was observed in Ob-BS studied here. Methyl eugenol and methyl cinnamate were not present while chavicol (= eustragol) was of lesser amount and only present in Ob-BS.
To the best of our knowledge, no works considered the aroma profile of M. didyma or any other species belonging to the same genus.
Concerning the studied Nepeta × faassenii no work was found related to its VOC composition. Moreover, this genus seemed to be not attractive seeing the scant reports in this context. The first work found dates back to 2010 when the authors used the Proton-Transfer-Reaction Mass Spectrometry (PTR-MS) method to evaluate the VOCs of three Nepata species cultivated in vitro. High concentration of nepetalactone was evidenced in N. sibirica L. and especially in N. rtanjensis Diklic and Milojevic shoot cultures, even though this constituent was detected in traces in N. nervosa Royle ex Benth. [81]. Recently Yayali and collaborators [82] investigated the Turkish Nepeta conferta Hedge and Lamond and reported that p-cymene (25.5%), eucalyptol (9.8%), limonene (5.0%), sabinene (4.8%), carvacrol (3.7%), (E)-linalool oxide (3.3%), (Z)-linalool oxide (3.0%) [82]. Moreover, Barhoumi et al. [83] studied the VOCs of two wild Nepeta curviflora Boiss originating in two Jordan regions (Salt, Northwest of Amman capital and Irbid, in the Northern of Jordan). Fully expanded flowers from Salt were characterized by a high SH content (75.94%) especially represented by trans-caryophyllene (26.50%) and OMs (18.53%) represented mainly by 4aα,7α,7aα-nepetalactone (12.74%). The main sesquiterpene hydrocarbons detected in the emission profiles of the flowers from the northern species, collected during the full blossoming stage, included β-bourbonene (19.45%), α-copaene (13.37%) and bicyclogermacrene (7.09%) [83]. All these compounds were completely absent in the VOCs of the species studied herein, except for nepetalactone and trans-caryophyllene.
No work was also found in both the studied species of Salvia genus, notwithstanding numerous published works on this subject [84,85,86,87,88,89,90,91,92,93,94]. The two studied species S. disc and S. micro, originating from South America, were grown under uniform conditions in CREA-Sanremo (Italy). As reported by Ascrizzi et al. [93], with the exception of only 3 species, all the South American studied plants were rich in SHs with a percentage ranging from 54.4% and 96.5% and showed β-caryophyllene and germacrene D as the most abundant ones. This is in a total agreement with the class of compounds in S. disc also for the presence of these two compounds because the first one showed a similar amount likewise what reported by the cited work while the second one had a very low amount. The same work underlined the presence of other sesquiterpene constituents in South American species such valencene, α-copaene, cis-muurola-3,5-diene, β-bisabolene and γ-muurolene. Except for β-bisabolene, all the other compounds were lacking. S. micro profile followed the same trend of one of the exceptions of South American species: S. dorisiana [93]. In this latter the whole volatile emission profile was mainly composed by MH (77.9%), with limonene of the most abundant compound (11.65%).
3.3. Essential Oils
Numerous reports are present in the literature on the EO composition of O. basilicum taxa which are very complex and show wide compositional variability according to the presence of several chemotypes within the species and according to the varied climatic/geographical conditions and agronomic practices [95]. Regardless of these factors, monoterpenes were commonly distributed in basil EOs and the linalool percentage was very high (ranging from 29.2% to 75.9%) as in the ‘Cinnamon’ variety, reported by many studies [96,97,98,99,100,101,102,103,104,105,106]. Only few papers reported the EO composition of O. basilicum varieties and among these we can find the study of Sajjadi [107], who investigated two Iranian basil varieties (O. basilicum L. cv. purple and O. basilicum L. cv. Green). Methyl chavicol was the characteristic compounds of both (52.4% and 40.5%, respectively). Although the oil of green basil was characterized by a high content of citral (both neral and geranial, 46.1%), citral was not detected in purple basil oil [107]. The same varieties from Yemen evidenced a completely different behaviour where linalool prevailed in both varieties (44.3% in O. basilicum var. purpurascens (purple) vs. 46.2% in O. basilicum var. basilicum (green) [108]. In the same year, another work was published by Tsasi and co-workers [109], where the effect of harvesting was studied in five O. basilicum varieties. The Ob-Cn EO were in agreement with O. basilicum var. latifolia and O. basilicum var. minimum, cultivated in the field, concerning their linalool content (49.5% and 52.0%, respectively) and with var. violetto (11.9) and var. latifolia (10.1%) cultivated in the greenhouse regarding the eugenol amount. Among the studied varieties in this work, Ob-Ct was the only one reported by the literature. The first work was done by Turkish scientists, who actually did not study directly this variety, but compared some investigated EOs with a high amount of citral compared to what is found in lemon balm basil, known as O. × citriodorum or O. basilicum var citriodorum (a hybrid of O. basilicum × O. americanum) [110]. Further on, in 2000, another Turkish research team succeeded in the cultivation of O. × citriodorum and confirmed the domination of neral (43.3%) and geranial (43.4%) in the flower EO [111]. A quite recent work confirmed a good percentage of citral (20.7%) in O. × citriodorum even though it was not the main compound which was represented on the contrary by nerol (23.0%) [112]. Asian O. × citriodorum showed the presence of two chemotypes: the first one was rich in geranial/neral, which is the same as this study, and another one with methyl chavicol [113]. The behaviour of the investigated Ob-BS followed the O. basilicum var. ‘Blue Spice’ [29].
The studied species of M. didyma showed an EO almost exclusively formed by thymol. The richness in this compound was confirmed by many scientific publications. In fact, Fraternale et al. [114], showed the prevalence of thymol (51.7%) and γ-terpinene (14.3%) in the flower EO of M. didyma. Also, a Monarda species grown in Canada underlined thymol (41.17%), γ-terpinene (15.88%), carvacrol (15.20%), and p-myrcene (12.58%) as main constituents [115]. Two other studies published in 2017 reported the EO composition from this plant species cultivated in central Italy: the EO from the flowering aerial parts pointed to thymol (59.3%) and p-cymene (10.3%) as major compounds [116], while the second work evidenced thymol 62% [43]. Other Monarda species, always cultivated in Italy, were very rich in monoterpenes, but with o-cymene (13.42), γ-terpinene (22.15), and carvacrol (13.80%) as the main constituents, and thymol with a lesser amount (5.87%) [42]. The chemical characterisation of the EO from Nepeta can be traced back to 1967 when Regnier [117] studied three species and each one showed a different main compound: nepetalactone in N. cataria L. (77%), epi nepetalactone in N. mussini Spreng. Ex Henckel (70%) and citronellol in N. citriodora Dumort. Since then several species were studied. The bulk of investigated plants were distinguished by the presence of a good amount of at least one of the nepetalactone isomers (ranging between 16% to 72%) such as N. cataria [118], N. rtanjensis [119], N. cataria var. citriodora and N. nuda L. [120]. All these works disagree with what was found in the analysed Nepeta × faassenii where these compounds were completely absent. The presence of non-terpene compounds was observed in the Lebanese Nepeta species such as N. cilicica Boiss. ex Benth [121], N. nuda ssp. Pubescens and N. curviflora Boiss [122]. These results did not agree with the data found herein since, despite this class was the main one, the constituents were completely different. Caryophyllene oxide, one of the most important compounds in our Nepeta × faassenii (17.2%), was evidenced in the higher amount in N. melissifolia Lam. and N. sibirica (22.06 and 20.35%, respectively) [120]. The only work which analysed the studied Nepeta hybrid was that of Ali and his co-workers [123], who found an EO rich in 1,8-cineole. This compound was present in very fewer amount in our study.
Leafing through the literature, the chemical composition of S. microphylla EO dates back to 1992 when Chialva et al. [124] identified compounds such as α-pinene, β-pinene, camphene, δ-3-carene, limonene, 1,8-cineole, camphor, borneol, bornyl acetate, (E)-caryophyllene, α-copaene, globulol, spatulenol, α-eudesmol and β-eudesmol. Later, Aydogmus et al. [125] observed the presence of β-eudesmol and 8-α-hydroxy-β-eudesmol. In the last decade, two works analysed the EO composition of this Salvia spp. The former found that (E)-caryophyllene (15.35%), α-eudesmol (14.06%), β-eudesmol (8.74%) and γ-eudesmol (7.64%) were the principal compounds [126], while the latest one evidenced α-eudesmol (20.5%), β-caryophyllene (13.7%) γ-eudesmol (8.2%), spathulenol (7%), and bornyl acetate (6.8%) [127]. In 2019, Wróblewska and collaborators [128], found linalool (46.91%), thymol (17.72%), its methyl ether (6.4%) and p-cymene (9.66%). In the current study, the EO composition greatly differed from the others seen before. As far as we know, the S. discolor EO profile was reported only in the paper of Sharopov et al. [129] who investigated the German species and underlined its richness in intermediol (57.37%) and (E)-caryophyllene (17.81%).
4. Materials and Methods
4.1. Plant Material and Cultivation
Monarda didyma “Fireball” and Nepeta × faassenii “Six Hills Giant” plants were bought at L’Erbaio della Gorra (Str. Gianardo, 11 Casalborgone, To, Italy,) plant nursery, and then grown in open field for two years in private garden. Cutting were used for plant propagation in greenhouse. Seeds of Ocimum basilicum ‘Blue Spice’, Ocimum basilicum ‘Cinnamon’ and Ocimum × citriodorum were provided to the Conservatoire National des Plantes à Parfum, Medicinales et Aromatiques (Milly-la-Forêt, France). Salvia discolor and S. microphylla “Hot Lips” are currently part of the plants collection at CREA—Research Centre for Vegetable and Ornamental Crops (CREA, Sanremo, IM, Italy, GPS: 43.816887, 7.758900) where they were propagated by cuttings. All the plants used in this work, both deriving from seed or cutting, were cultivated in pots kept in an unheated greenhouse covered with an anti-insect net at CREA, as reported by Najar et al. [17]. Briefly, the plants were cultured in substrate (Hochmoor—Terflor, Capriolo, BS, Italy) with slow release fertilizer (Nitrophoska, Eurochem Agro, Cesano Maderno, MB, Italy) and irrigated with nutrient solution (Ferti 3, Planta-Dȕngemittel, Regenstauf, Germany) every week. Supplemental irrigations with water were carried out according to the needs of the plants and the season in order to avoid water stress to the plants. The plants were grown applying the organic cultivation method (without pesticides), using antagonist insects (Koppert Italia Srl., Bussolengo, VR, Italy) and microorganisms [17]. Full-bloom flowers were picked during their flowering time (see Table 1).
4.2. Biochemical Analyses
Fresh flowers were picked early in the morning, divided into three homogeneous biological replica, and stored at −80 °C. Frozen samples (200 mg) were used to quantify total carotenoid [130], total polyphenolic content (TPC) (Folin-Ciocalteu method, according to [17]), and total anthocyanins content [17]. Radical scavenging activity was determined by DPPH assay [131], reporting the results as IC50 (mg/mL). Soluble sugars (D-glucose, D-fructose and sucrose), total ascorbate (ASATOT) and reduced ascorbate (ASA) were quantified as described in Najar et al. [17]. All measurements were performed with an ultraviolet (UV)-1800 spectrophotometer (Shimadzu Corp., Kyoto, Japan). Total nitrogen content determination was performed by Kjeldhal method following the protocol described in Jones et al., [132]. Data were reported as percentage of crude protein content, obtained by multiplying the percentage of nitrogen by 6.25 as conversion factor (%N × 6.25).
4.3. Phytochemical Analysis
A fresh flower of each plant was picked (an average of 0.5 to 1 g), placed separately in a glass conical flask (20 mL) and sealed with a cap provided with aluminium foil for 30 min (equilibration time). The evaluation of VOC emission was performed with the use of 100 μm polydimethylsiloxanes (PDMS) fibre manufactured by Supelco Ltd (St. Louis, MO, USA). Prior to the analyses, the fibre was conditioned according to the manufacturer’s instruction, at 250 °C for a duration of 30 min in the injector of a gas chromatograph. Exposition of the fibre in the headspace phase of the samples took place for 15 min at a temperature of 23 °C. Subsequently the fibre was reinserted back into the needle and immediately transferred to the injector of the gas chromatograph (temperature 250 °C), where the analytes were thermally desorbed for a duration of 30 min. The composition of the compounds desorbed from SPME fibre was examined using GC-MS.
Essential oil (EO) was extracted from fresh flowers even though the weight of these plant material was barely sufficient to undertake a microdistillation. Therefore, the fresh flowers were separately hydrodistilled for 2 h using a micro-Clevenger like apparatus as recommended by the European Pharmacopeia [133]. The yield of the EOs were very low and were collected directly in high-performance liquid chromatography (HPLC)-grade n-hexane and immediately analysed by GC-MS.
GC-MS analyses were performed with an Agilent 7890B gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an Agilent HP-5MS (Agilent Technologies Inc., Santa Clara, CA, USA) capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and an Agilent 5977B single quadrupole mass detector (Agilent Technologies Inc., Santa Clara, CA, USA). Analytical conditions were as follows: injector and transfer line temperatures 220 and 240 °C, respectively; oven temperature programmed to raise from 60 to 240 °C at 3 °C/min; carrier gas helium at 1 mL/min; injection of 1 μL (0.5% HPLC grade n-hexane solution); split ratio 1:25. The acquisition parameters were as follows: full scan; scan range: 30-300 m/z; scan time: 1.0 sec. Identification of the constituents was based on a comparison of the retention times with those of the authentic samples, comparing their linear retention indices relative to the series of n-hydrocarbons. Computer matching was also used against commercial [134,135] and laboratory-developed mass spectra library built up from pure substances and components of known oils and MS literature data [135,136,137,138,139,140].
4.4. Statistical Analysis
Biochemical results were statistically analysed using either Tukey’s honest significant difference (HDS) or the Games-Howell test according to the homogeneity of variance (Levene’s test) [141]. The analyses were performed using IBM SPSS software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
Linear correlation between polyphenols and radical scavenging activity were determined using Microsoft Excel ® 2013 (Microsoft Corporation, Redmond, WA, USA).
Multivariate explorer, principal component and hierarchical clustering analyses were carried out which allowed the co-evaluation of all variables [142]. For each treatment, the chemical compounds and their proportions (%) were plotted in Excel spreadsheets. Compounds present with amounts less than 5% were omitted from the analysis. The data were transformed by orthogonal rotation into latent variables named as the principal components. These are linear combinations of original variables created from the eigenvalues of the data correlation matrix. The Euclidean distance was used as a dissimilarity metric to represent the straight-line distance between the centroids of each cluster of chemical compounds identified in EO analysis. The unweighted pair group method with arithmetic averages (UPGMA) was used to cluster the compounds. The results were presented in a dendrogram that characterized the clusters. Both analyses were run in the JMP software package 13.0.0 (SAS Institute, Cary, NC, USA).
Acknowledgments
The authors thank the Conservatoire National des Plantes à Parfum, Medicinales et Aromatiques (Milly-la-Forêt, France) for providing the seeds of Ocimum basilicum ‘Blue Spice’, O. basilicum ‘Cinnamon’ and Ocimum × citriodorum; Carlo Mascarello, Sergio Ariano and Alberto Lanteri for the production and cultivation of plant material.
Author Contributions
Conceptualization, L.P. (Laura Pistelli), L.P. (Luisa Pistelli), B.R.; methodology, I.M., B.N.; software, I.M., B.N.; validation, I.M., B.N., L.P. (Laura Pistelli) and L.P. (Luisa Pistelli); formal analysis, B.N., I.M.; investigation, B.N., I.M., and A.C.; writing—original draft preparation, B.N., I.M.; writing—review and editing, I.M., B.N., B.R., A.C., L.P. (Luisa Pistelli), L.P. (Laura Pistelli); supervision, L.P. (Laura Pistelli), L.P. (Luisa Pistelli); project administration, B.R.; funding acquisition, B.R., L.P. (Laura Pistelli), L.P. (Luisa Pistelli). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the INTERREG-ALCOTRA UE 2014-2020 Project “ANTEA” - Attività innovative per lo sviluppo della filiera transfrontaliera del fiore edule (n. 1139), grant number: CUP C12F17000080003.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Azzazy M.F. Systematic Importance of Pollen Morphology of Some Plants of (Lamiaceae) Curr. Bot. 2016;7:5. doi: 10.19071/cb.2016.v7.3029. [DOI] [Google Scholar]
- 2.Karpiński T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules. 2020;10:103. doi: 10.3390/biom10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gul S., Ahmad M., Zafar M., Bahadur S., Sultana S., Begum N., Shah S.N., Zaman W., Ullah F., Ayaz A., et al. Taxonomic study of subfamily Nepetoideae (Lamiaceae) by polynomorphological approach. Microsc. Res. Tech. 2019;82:1021–1031. doi: 10.1002/jemt.23249. [DOI] [PubMed] [Google Scholar]
- 4.Echeverría J., Niemeyer H.M. Essential oil of Kurzamra pulchella (Clos) Kuntze (Lamiaceae, Nepetoideae, Mentheae, Menthinae): Relationship with chemotype groups in the subtribe Menthinae. Nat. Prod. Res. 2017;31:108–112. doi: 10.1080/14786419.2016.1214828. [DOI] [PubMed] [Google Scholar]
- 5.Wojdylo A., Oszmianski J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- 6.Bonesi M., Loizzo M.R., Acquaviva R., Malfa G.A., Aiello F., Tundis R. Anti-inflammatory and antioxidant agents from Salvia genus (Lamiaceae): An assessment of the current state of knowledge. Antiinflamm. Antiallergy Agents Med. Chem. 2017;16:70–86. doi: 10.2174/1871523016666170502121419. [DOI] [PubMed] [Google Scholar]
- 7.Süntar I., Nabavi S.M., Barreca D., Fischer N., Efferth T. Pharmacological and chemical features of Nepeta L. genus: Its importance as a therapeutic agent. Phyther. Res. 2018;32:185–198. doi: 10.1002/ptr.5946. [DOI] [PubMed] [Google Scholar]
- 8.Elansary H.O., Szopa A., Kubica P., Ekiert H., El-Ansary D.O., Al-Mana F.A., Mahmoud E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. Polyphenols and Biological Activities. Processes. 2020;8:446. doi: 10.3390/pr8040446. [DOI] [Google Scholar]
- 9.Mamadalieva N.Z., Akramov D.K., Böhmdorfer S., Azimova S.S., Rosenau T. Extractives and biological activities of Lamiaceae species growing in Uzbekistan. Holzforschung. 2020;74:96–115. doi: 10.1515/hf-2018-0296. [DOI] [Google Scholar]
- 10.Caser M., D’Angiolillo F., Chitarra W., Lovisolo C., Ruffoni B., Pistelli L., Pistelli L., Scariot V. Ecophysiological and phytochemical responses of Salvia sinaloensis Fern. to drought stress. Plant Growth Regul. 2018;84:383–394. doi: 10.1007/s10725-017-0349-1. [DOI] [Google Scholar]
- 11.Trivellini A., Lucchesini M., Maggini R., Mosadegh H., Villamarin T.S.S., Vernieri P., Mensuali-Sodi A., Pardossi A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crop. Prod. 2016;83:241–254. doi: 10.1016/j.indcrop.2015.12.039. [DOI] [Google Scholar]
- 12.Mishra L.K., Sarkar D., Shetty K. Functional Foods and Biotechnology: Sources of Functional Foods and Ingredients. CRC Press, Taylor & Francis Group, LLC; Boca Raton, FL, USA: 2019. Human health-relevant bioactives and associated functionalities of herbs in the Lamiaceae family; pp. 115–131. [DOI] [Google Scholar]
- 13.Fernandes L., Casal S., Pereira J.A., Saraiva J.A., Ramalhosa E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017;60:38–50. doi: 10.1016/j.jfca.2017.03.017. [DOI] [Google Scholar]
- 14.Mlcek J., Rop O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011;22:561–569. doi: 10.1016/j.tifs.2011.04.006. [DOI] [Google Scholar]
- 15.Husti A., Cantor M., Buta E., Horţ D. Current trends of using ornamental plants in culinary arts. ProEnvironment. 2013;6:52–58. [Google Scholar]
- 16.Lu B., Li M., Yin R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review (2000–2015) Crit. Rev. Food Sci. Nutr. 2016;56:S130–S148. doi: 10.1080/10408398.2015.1078276. [DOI] [PubMed] [Google Scholar]
- 17.Najar B., Marchioni I., Ruffoni B., Copetta A., Pistelli L., Pistelli L. Volatilomic Analysis of Four Edible Flowers from Agastache Genus. Molecules. 2019;24:4480. doi: 10.3390/molecules24244480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pires T.C.S.P., Barros L., Santos-Buelga C., Ferreira I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019;93:244–258. doi: 10.1016/j.tifs.2019.09.020. [DOI] [Google Scholar]
- 19.Loizzo M.R., Pugliese A., Bonesi M., Tenuta M.C., Menichini F., Xiao J., Tundis R. Edible flowers: A rich source of phytochemicals with antioxidant and hypoglycemic properties. J. Agric. Food Chem. 2016;64:2467–2474. doi: 10.1021/acs.jafc.5b03092. [DOI] [PubMed] [Google Scholar]
- 20.Nowicka P., Wojdyło A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants. 2019;8:308. doi: 10.3390/antiox8080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes L., Ramalhosa E., Pereira J.A., Saraiva J.A., Casal S. The unexplored potential of edible flowers lipids. Agriculture. 2018;8:146. doi: 10.3390/agriculture8100146. [DOI] [Google Scholar]
- 22.Oladeji O., Amusan T. Proximate, vitamin and mineral assays of an underutilised indigenous vegetable in West Africa Salvia elegans Vahl (Lamiales: Lamiaceae) in enhancing diet diversification. Rev. Bras. Gestão Ambient. E Sustentabilidade. 2016;3:327–336. doi: 10.21438/rbgas.030607. [DOI] [Google Scholar]
- 23.Rop O., Mlcek J., Jurikova T., Neugebauerova J., Vabkova J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules. 2012;17:6672–6683. doi: 10.3390/molecules17066672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grzeszczuk M., Stefaniak A., Meller E., Wysocka G. Mineral composition of some edible flowers. J. Elem. 2018;23:151–162. doi: 10.5601/jelem.2017.22.2.1352. [DOI] [Google Scholar]
- 25.Fernandes L., Casal S., Pereira J.A., Malheiro R., Rodrigues N., Saraiva J.A., Ramalhosa E. Borage, calendula, cosmos, Johnny Jump up, and pansy flowers: Volatiles, bioactive compounds, and sensory perception. Eur. Food Res. Technol. 2019;245:593–606. doi: 10.1007/s00217-018-3183-4. [DOI] [Google Scholar]
- 26.Chen N.-H., Wei S. Factors influencing consumers’ attitudes towards the consumption of edible flowers. Food Qual Prefer. 2017;56:93–100. doi: 10.1016/j.foodqual.2016.10.001. [DOI] [Google Scholar]
- 27.Makri O., Kintzios S. Ocimum sp.(basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants. 2008;13:123–150. doi: 10.1300/J044v13n03_10. [DOI] [Google Scholar]
- 28.Poonkodi K. Chemical composition of essential oil of Ocimum basilicum L. (Basil) and its biological activities-an overview. J. Crit. Rev. 2016;3:56–62. [Google Scholar]
- 29.Beatovic D., Krstic-Milosevic D., Trifunovic S., Siljegovic J., Glamoclija J., Ristic M., Jelacic S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015;9:62–75. [Google Scholar]
- 30.Copetta A., Lingua G., Berta G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza. 2006;16:485–494. doi: 10.1007/s00572-006-0065-6. [DOI] [PubMed] [Google Scholar]
- 31.Lim Ah Tock M.J., Kamatou G.P.P., Combrinck S., Sandasi M., Viljoen A.M. A chemometric assessment of essential oil variation of three Salvia species indigenous to South Africa. Phytochemistry. 2020;172:112249. doi: 10.1016/j.phytochem.2019.112249. [DOI] [PubMed] [Google Scholar]
- 32.Talebi S.M., Behzadpour S., Matsyura A. Morphological and essential oil variations among Iranian populations of Salvia chloroleuca (Lamiaceae) Biosyst. Divers. 2019;27:233–237. doi: 10.15421/011932. [DOI] [Google Scholar]
- 33.Giffen J.E., Lesiak A.D., Dane A.J., Cody R.B., Musah R.A. Rapid species-level identification of salvias by chemometric processing of ambient ionisation mass spectrometry-derived chemical profiles. Phytochem. Anal. 2017;28:16–26. doi: 10.1002/pca.2639. [DOI] [PubMed] [Google Scholar]
- 34.Landi M., Ruffoni B., Combournac L., Guidi L. Nutraceutical value of edible flowers upon cold storage. Ital. J. Food Sci. 2017;30:1–18. doi: 10.14674/IJFS-756. [DOI] [Google Scholar]
- 35.Jenks A.A., Kim S.C. Medicinal plant complexes of Salvia subgenus Calosphace: An ethnobotanical study of new world sages. J. Ethnopharmacol. 2013;146:214–224. doi: 10.1016/j.jep.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Sharopov F., Valiev A., Sobeh M., Arnold E., Winka M. Bioactivity of three Salvia species in relation to their total phenolic and flavonoid contents. Pharm. Chem. J. 2018;52:596–600. doi: 10.1007/s11094-018-1866-6. [DOI] [Google Scholar]
- 37.Asadollahi M., Firuzi O., Heidary Jamebozorgi F., Alizadeh M., Jassbi A.R. Ethnopharmacological studies, chemical composition, antibacterial and cytotoxic activities of essential oils of eleven Salvia in Iran. J. Herb. Med. 2019;17–18:100250. doi: 10.1016/j.hermed.2018.11.006. [DOI] [Google Scholar]
- 38.Karafakıoglu Y., Aksoy L. Evaluation of Minerals, Phenolics, Radical Scavenging Activity, Total Oxidant Status and Total Antioxidant Status of Nepeta Viscida Boiss. Int. J. Agric. Life Sci. 2019;3:98–105. [Google Scholar]
- 39.Anishchenko I.E., Zhigunov O.Y.U. On biology of some reprentatives of the genus Nepeta L. under cultivationconditions in the bashkir cis-Urals. B. Acad. Sci. 2016;1:32–37. [Google Scholar]
- 40.Salehi B., Valussi M., Jugran A.K., Martorell M., Ramírez-Alarcón K., Stojanović-Radić Z.Z., Antolak H., Kręgiel D., Mileski K.S., Sharifi-Rad M., et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018;80:104–122. doi: 10.1016/j.tifs.2018.07.030. [DOI] [Google Scholar]
- 41.Radulović N., Blagojević P.D., Rabbitt K., de Sousa Menezes F. Essential oil of Nepeta x faassenii Bergmans ex Stearn (N. mussinii Spreng. x N. nepetella L.): A comparison study. Nat. Prod. Commun. 2011;6:1015–1022. doi: 10.1177/1934578X1100600724. [DOI] [PubMed] [Google Scholar]
- 42.Laquale S., Avato P., Argentieri M.P., Bellardi M.G., D’Addabbo T. Nematotoxic activity of essential oils from Monarda species. J. Pest Sci. 2018;91:1115–1125. doi: 10.1007/s10340-018-0957-1. [DOI] [Google Scholar]
- 43.Mattarelli P., Epifano F., Minardi P., Di Vito M., Modesto M., Barbanti L., Bellardi M.G. Chemical composition and antimicrobial activity of essential oils from aerial parts of Monarda didyma and Monarda fistulosa cultivated in Italy. J. Essent. Oil-Bearing Plants. 2017;20:76–86. doi: 10.1080/0972060X.2016.1278184. [DOI] [Google Scholar]
- 44.Trettel J.R., Gazim Z.C., Gonçalves J.E., Stracieri J., Magalhães H.M. Volatile essential oil chemical composition of basil (Ocimum basilicum L. ‘Green’) cultivated in a greenhouse and micropropagated on a culture medium containing copper sulfate. In Vitro Cell. Dev. Biol. Plant. 2017;53:631–640. doi: 10.1007/s11627-017-9868-8. [DOI] [Google Scholar]
- 45.Guimarães R., Barros L., Carvalho A.M., Ferreira I.C. Studies on chemical constituents and bioactivity of Rosa micrantha: An alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J. Agric. Food Chem. 2010;58:6277–6284. doi: 10.1021/jf101394w. [DOI] [PubMed] [Google Scholar]
- 46.Petanidou T. Sugars in Mediterranean floral nectars: An ecological and evolutionary approach. J. Chem. Ecol. 2005;31:1065–1088. doi: 10.1007/s10886-005-4248-y. [DOI] [PubMed] [Google Scholar]
- 47.Cresswell J.E. How and why do nectar-foraging bumblebees initiate movements between inflorescences of wild bergamot Monarda fistulosa (Lamiaceae)? Oecologia. 1990;82:450–460. doi: 10.1007/BF00319785. [DOI] [PubMed] [Google Scholar]
- 48.Mačukanović-Jocić M., Stevanović Z.D., Mladenović M., Jocić G. Flower morphophysiology of selected Lamiaceae species in relation to pollinator attraction. J. Apic. Res. 2011;50:89–101. doi: 10.3896/IBRA.1.50.2.01. [DOI] [Google Scholar]
- 49.Stefaniak A., Grezeszczuk M.E. Nutritional and biological value of five edible flower species. Not. Bot. Horti Agrobo. 2019;47:128–134. doi: 10.15835/nbha47111136. [DOI] [Google Scholar]
- 50.Shanaida M., Kernychna I., Shanaida Y. Chromatographic analysis of organic acids, amino acids, and sugars in Ocimum americanum L. Acta Pol. Pharm. Drug Res. 2017;74:729–732. [PubMed] [Google Scholar]
- 51.Grzeszczuk M., Wesolowska A., Jadczak D., Jakubowska B. Nutritional value of chive edible flowers. Acta Sci. Pol. Hortoru. 2011;10:85–94. [Google Scholar]
- 52.Sotelo A., López-García S., Basurto-Peña F. Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant Foods Hum. Nutr. 2007;62:133–138. doi: 10.1007/s11130-007-0053-9. [DOI] [PubMed] [Google Scholar]
- 53.Bourgaud F., Gravot A., Milesi S., Gontier E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001;161:839–851. doi: 10.1016/S0168-945200490-3. [DOI] [Google Scholar]
- 54.Daayf F., Lattanzio V. Recent Advances in Polyphenol Research. John Wiley & Sons; Hoboken, NJ, USA: 2009. [Google Scholar]
- 55.Theis N., Lerdau M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003;164:S93–S102. doi: 10.1086/374190. [DOI] [Google Scholar]
- 56.Mol J., Grotewold E., Koes R. How genes paint flowers and seeds. Trends Plant Sci. 1998;3:212–217. [Google Scholar]
- 57.Zhu C., Bai C., Sanahuja G., Yuan D., Farré G., Naqvi S., Shi L., Capell T., Christou P. The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 2010;504:132–141. doi: 10.1016/j.abb.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 58.Gandía-Herrero F., García-Carmona F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013;18:334–343. doi: 10.1016/j.tplants.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Chanwitheesuk A., Teerawutgulrag A., Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005;92:491–497. doi: 10.1016/j.foodchem.2004.07.035. [DOI] [Google Scholar]
- 60.Grzeszczuk M., Stefaniak A., Pachlowska A. Biological value of various edible flower species. Acta Sci. Pol-Hortoru. 2016;15:109–119. doi: 10.15835/nbha47111136. [DOI] [Google Scholar]
- 61.Benvenuti S., Bortolotti E., Maggini R. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci. Hortic. 2016;199:170–177. doi: 10.1016/j.scienta.2015.12.052. [DOI] [Google Scholar]
- 62.Srivastava S., Adholeya A., Conlan X.A., Cahill D.M. Acidic potassium permanganate chemiluminescence for the determination of antioxidant potential in three cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2016;71:72–80. doi: 10.1007/s11130-016-0527-8. [DOI] [PubMed] [Google Scholar]
- 63.Simon J.E. Phytochemical Analysis and Anti-Inflammatory Activity of Nepeta cataria Accessions. J. Med. Active Plants. 2018;7:19–27. [Google Scholar]
- 64.Seladji M., Bekhechi C., Beddou F., Hanane D.I.B., Bendimerad N. Antioxidant activity and phytochemical screening of Nepeta nepetella aqueous and methanolic extracts from Algeria. J. Appl. Pharm. Sci. 2014;4:12. doi: 10.7324/JAPS.2014.40203. [DOI] [Google Scholar]
- 65.Iqbal K., Khan A., Khattak M.M.A.K. Biological significance of ascorbic acid (vitamin C) in human health—A review. Pak. J. Nutr. 2004;3:5–13. [Google Scholar]
- 66.Nishikimi M., Fukuyama R., Minoshima S., Shimizu N., Yagi K. Cloning and chromosomal mapping of the Human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994;269:13685–13688. [PubMed] [Google Scholar]
- 67.European Parliament . Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011, Daily Reference Intakes for vitamins and minerals (adults), Annex XII. European Parliament; Strasbourg, France: 2011. [Google Scholar]
- 68.Carović-Stanko K., PeteK M., Martina G., Pintar J., Bedeković D., Ćustić M.H., Šatović Z. Medicinal Plants of the Family Lamiaceaeas Functional Foods-a Review. Czech J. Food Sci. 2016;34:377. doi: 10.17221/504/2015-CJFS. [DOI] [Google Scholar]
- 69.Cavaiuolo M., Cocetta G., Ferrante A. The antioxidants changes in ornamental flowers during development and senescence. Antioxidants. 2013;2:132–155. doi: 10.3390/antiox2030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Houta O., Akrout A., Neffati M., Amri H. Phenolic contents, antioxidant and antimicrobial potentials of Crithmum maritimum cultivated in Tunisia arid zones. J. Biol. Active Prod. Nat. 2011;1:138–143. doi: 10.1080/22311866.2011.10719081. [DOI] [Google Scholar]
- 71.Anvari D., Jamei R. A comparative study between the leaf and flowers of some Asteraceae plants with respect to their antioxidant activity compounds. Curr. Nutr. Food Sci. 2016;12:296–303. doi: 10.2174/1573401312666160909112745. [DOI] [Google Scholar]
- 72.Siatka T., Kašparová M. Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules. 2010;15:9450–9461. doi: 10.3390/molecules15129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butnariu M., Coradini C.Z. Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem. Cent. J. 2012;6:35. doi: 10.1186/1752-153X-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng Y., Deng M., Lv Z., Peng Y. Evaluation of antioxidant activities of extracts from 19 Chinese edible flowers. SpringerPlus. 2014;3:315. doi: 10.1186/2193-1801-3-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tarchoune I., Baâtour O., Harrathi J., Cioni P.L., Lachaâl M., Flamini G., Ouerghi Z. Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to NaCl or Na2SO4 salinity. J. Plant Nutr. Soil Sci. 2013;176:748–755. doi: 10.1002/jpln.201200278. [DOI] [Google Scholar]
- 76.Ronga D., Pellati F., Brighenti V., Laudicella K., Laviano L., Fedailaine M., Benvenuti S., Pecchioni N., Francia E. Testing the influence of digestate from biogas on growth and volatile compounds of basil (Ocimum basilicum L.) and peppermint (Mentha x piperita L.) in hydroponics. J. Appl. Res. Med. Aromat. Plants. 2018;11:18–26. doi: 10.1016/j.jarmap.2018.08.001. [DOI] [Google Scholar]
- 77.Matłok N., Gorzelany J., Stępień A.E., Figiel A., Balawejder M. Effect of fertilization in selected phytometric features and contents of bioactive compounds in dry matter of two varieties of basil (Ocimum basilicum L.) Sustainability. 2019;11:6590. doi: 10.3390/su11236590. [DOI] [Google Scholar]
- 78.Khairun Fadila S., Chun Hui A., Sook Mei K., Cheng Hock C. Chemical constituents and antioxidant capacity of Ocimum basilicum and Ocimum sanctum. Iran. J. Chem. Chem. Eng. 2019;38:139–152. [Google Scholar]
- 79.Açıkgöz M. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crop. Prod. 2020;148:112278. doi: 10.1016/j.indcrop.2020.112278. [DOI] [Google Scholar]
- 80.Klimánková E., Holadová K., Hajšlová J., Čajka T., Poustka J., Koudela M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008;107:464–472. doi: 10.1016/j.foodchem.2007.07.062. [DOI] [Google Scholar]
- 81.Nestorović J., Mišić D., Šiler B., Soković M., Glamočlija J., Ćirić A., Maksimović V., Grubišić D. Nepetalactone content in shoot cultures of three endemic Nepeta species and the evaluation of their antimicrobial activity. Fitoterapia. 2010;81:621–626. doi: 10.1016/j.fitote.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Yayli B., Tosun G., Karaköse M., Renda G., Yayli N. SPME/GC-MS analysis of volatile organic compounds from three lamiaceae species (Nepeta conferta Hedge & Lamond, Origanum onites L. and Satureja cuneifolia Ten.) growing in Turkey. Asian J. Chem. 2014;26:2541–2544. doi: 10.14233/ajchem.2014.15719. [DOI] [Google Scholar]
- 83.Barhoumi L. Volatile organic compounds and essential oil composition of selected organs of nepeta curviflora collected from two regions in Jordan. Jordan J. Chem. 2017;12:101–112. [Google Scholar]
- 84.Lušić D., Koprivnjak O., Ćurić D., Sabatini A.G.S., Conte L.S. Volatile profile of croatian lime tree (Tilia sp.), fir honeydew (Abies alba) and sage (Salvia officinalis) honey. Food Technol. Biotechnol. 2007;45:156–165. [Google Scholar]
- 85.Znini M., Majidi L., Desjobert J.M., Paolini J., Costa J. GC-MS analysis and comparison of volatile compounds of Salvia aucheri Boiss. var. mesatlantica Maire. obtained by hydrodistillation and headspace solid phase microextraction (HS-SPME) Acta Chromatogr. 2014;26:495–505. doi: 10.1556/AChrom.26.2014.3.8. [DOI] [Google Scholar]
- 86.D’Auria M., Racioppi R. The Effect of Drying of the Composition of Volatile Organic Compounds in Rosmarinus officinalis, Laurus nobilis, Salvia officinalis and Thymus serpyllum. A HS-SPME-GC-MS Study. J. Essent. Oil-Bearing Plants. 2015;18:1209–1223. doi: 10.1080/0972060X.2014.895213. [DOI] [Google Scholar]
- 87.Mohammadhosseini M. Chemical composition of the volatile fractions from flowers, leaves and stems of Salvia mirzayanii by HS-SPME-GC-MS. J. Essent. Oil Bear. Plants. 2015;18:464–476. doi: 10.1080/0972060X.2014.1001185. [DOI] [Google Scholar]
- 88.Mohammadhosseini M. Chemical composition of the essential oils and volatile fractions from flowers, stems and roots of Salvia multicaulis Vahl. by Using MAHD, SFME and HS-SPME Methods. J. Essent. Oil Bear. Plants. 2015;18:1360–1371. doi: 10.1080/0972060X.2015.1024447. [DOI] [Google Scholar]
- 89.Al Jaber H. Salvia ceratophylla from Jordan: Volatile Organic Compounds, Essential oil composition and antioxidant activity. Jordan J. Chem. 2016;11:108–119. [Google Scholar]
- 90.Cozzolino R., Ramezani S., Martignetti A., Mari A., Piacente S., De Giulio B. Determination of volatile organic compounds in the dried leaves of Salvia species by solid-phase microextraction coupled to gas chromatography mass spectrometry. Nat. Prod. Res. 2016;30:841–848. doi: 10.1080/14786419.2015.1076817. [DOI] [PubMed] [Google Scholar]
- 91.Hatipoglu S.D., Zorlu N., Dirmenci T., Goren A.C., Ozturk T., Topcu G. Determination of volatile organic compounds in fourty five Salvia species by thermal desorption-GC-MS technique. Rec. Nat. Prod. 2016;10:659–700. [Google Scholar]
- 92.Koutsaviti A., Tzini D.I., Tzakou O. Greek Salvia sclarea L. essential oils: Effect of hydrodistillation time, comparison of the aroma chemicals using hydrodistillation and HS-SPME techniques. Rec. Nat. Prod. 2016;10:800–805. [Google Scholar]
- 93.Ascrizzi R., Cioni P.L., Amadei L., Maccioni S., Flamini G. Geographical patterns of in vivo spontaneously emitted volatile organic compounds in Salvia species. Microchem. J. 2017;133:13–21. doi: 10.1016/j.microc.2017.03.002. [DOI] [Google Scholar]
- 94.Nekoei M., Mohammadhosseini M. Chemical Composition of the Essential Oils and Volatiles of Salvia leriifolia by Three Different Extraction Methods Prior to Gas Chromatographic-Mass Spectrometric Determination: Comparison of HD with SFME and HS-SPME. J. Essent. Oil Bear. Plants. 2017;20:410–425. doi: 10.1080/0972060X.2017.1305918. [DOI] [Google Scholar]
- 95.Padalia R.C., Verma R.S., Chauhan A., Chanotiya C.S. Changes in aroma profiles of 11 Indian Ocimum taxa during plant ontogeny. Acta Physiol. Plant. 2013;35:2567–2587. doi: 10.1007/s11738-013-1293-y. [DOI] [Google Scholar]
- 96.Opalchenova G., Obreshkova D. Comparative studies on the activity of basil - An essential oil from Ocimum basilicum L. - Against multidrug resistant clinical isolates of the genera Staphylococcus, Enterococcus and Pseudomonas by using different test methods. J. Microbiol. Methods. 2003;54:105–110. doi: 10.1016/S0167-7012(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 97.Hussain A.I., Anwar F., Hussain Sherazi S.T., Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 98.Pozzatti P., Scheid L.A., Spader T.B., Atayde M.L., Santurio J.M., Alves S.H. in vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008;54:950–956. doi: 10.1139/W08-097. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J.-W., Li S.-K., Wu W.-J. The main chemical composition and in vitro antifungal activity of the essential oils of Ocimum basilicum Linn. var. pilosum (Willd.) Benth. Molecules. 2009;14:273–278. doi: 10.3390/molecules14010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soković M., Glamočlija J., Marin P.D., Brkić D., Griensven L.J.L.D. antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orhan I.E., Ozcelik B., Kan Y., Kartal M. Inhibitory effects of various essential oils and individual components against extended-spectrum Beta-Lactamase (ESBL) produced by Klebsiella pneumoniae and their chemical compositions. J. Food Sci. 2011;76 doi: 10.1111/j.1750-3841.2011.02363.x. [DOI] [PubMed] [Google Scholar]
- 102.Rajeswara Rao B.R., Kothari S.K., Rajput D.K., Patel R.P., Darokar M.P. Chemical and biological diversity in fourteen selections of four Ocimum species. Nat. Prod. Commun. 2011;6:1705–1710. doi: 10.1177/1934578x1100601134. [DOI] [PubMed] [Google Scholar]
- 103.Govindarajan M., Sivakumar R., Rajeswary M., Yogalakshmi K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae) Exp. Parasitol. 2013;134:7–11. doi: 10.1016/j.exppara.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 104.Nardoni S., Giovanelli S., Pistelli L., Mugnaini L., Profili G., Pisseri F., Mancianti F. in vitro activity of twenty commercially available, plant-derived essential oils against selected dermatophyte species. Nat. Prod. Commun. 2015;10:1473–1478. doi: 10.1177/1934578X1501000840. [DOI] [PubMed] [Google Scholar]
- 105.Cardoso N.N.R., Alviano C.S., Blank A.F., Romanos M.T.V., Fonseca B.B., Rozental S., Rodrigues I.A., Alviano D.S. Synergism Effect of the Essential Oil from Ocimum basilicum var. Maria Bonita and its major components with fluconazole and its influence on ergosterol biosynthesis. evidence-based complement. Altern. Med. 2016:1–12. doi: 10.1155/2016/5647182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Snoussi M., Dehmani A., Noumi E., Flamini G., Papetti A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016;90:13–21. doi: 10.1016/j.micpath.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 107.Sajjadi S.E. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. Daru J. 2006;3:128–130. [Google Scholar]
- 108.Ahmed A., Hussein K., Alsyari A. Chemotaxonomy and Spectral Analysis (GC/MS and FT-IR) of Essential Oil Composition of Two Ocimum basilicum L. Varietiesv and their Morphological Characterization. Jordan J. Chem. 2017;12:147–160. [Google Scholar]
- 109.Tsasi G., Mailis T., Daskalaki A., Sakadani E., Razis P., Samaras Y., Skaltsa H. The effect of harvesting on the composition of essential oils from five varieties of Ocimum basilicum L. cultivated in the Island of Kefalonia, Greece. Plants. 2017;6:41. doi: 10.3390/plants6030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Telci I., Bayram E., Yilmaz G., Avci B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.) Biochem. Syst. Ecol. 2006;34:489–497. doi: 10.1016/j.bse.2006.01.009. [DOI] [Google Scholar]
- 111.Tansi S., Nacar S. First cultivation trials of lemon basil (Ocimum basilicum var. citriodorum) in Turkey. Pak. J. Biol. Sci. 2000;3:395–397. doi: 10.3923/pjbs.2000.395.397. [DOI] [Google Scholar]
- 112.Avetisyan A., Markosian A., Petrosyan M., Sahakyan N., Babayan A., Aloyan S., Trchounian A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017;17 doi: 10.1186/s12906-017-1587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raina A.P., Gupta V. Chemotypic characterization of diversity in essential oil composition of Ocimum species and varieties from India. J. Essent. Oil Res. 2018;30:444–456. doi: 10.1080/10412905.2018.1495109. [DOI] [Google Scholar]
- 114.Fraternale D., Giamperi L., Bucchini A., Ricci D., Epifano F., Burini G., Curini M. Chemical composition, antifungal and in vitro antioxidant properties of Monarda didyma L. Essent. Oil. J. Essent. Oil Res. 2006;18:581–585. doi: 10.1080/10412905.2006.9699174. [DOI] [Google Scholar]
- 115.Adebayo O., Bélanger A., Khanizadeh S. Variable inhibitory activities of essential oils of three Monarda species on the growth of Botrytis cinerea. Can. J. Plant Sci. 2013;93:987–995. doi: 10.4141/cjps2013-044. [DOI] [Google Scholar]
- 116.Ricci D., Epifano F., Fraternale D. The essential oil of Monarda didyma L. (Lamiaceae) exerts phytotoxic activity in vitro against various weed seeds. Molecules. 2017;22:222. doi: 10.3390/molecules22020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Regnier F.E., Eeisenbraun E.J., Waller G.R. Nepetalactone and epinepetalactone from Nepeta cataria L. Phytochemistry. 1967;6:1271–1280. doi: 10.1016/S0031-9422(00)86089-6. [DOI] [Google Scholar]
- 118.Saharkhiz M.J., Zadnour P., Kakouei F. Essential oil analysis and phytotoxic activity of catnip (Nepeta cataria L.) Am. J. Essent. Oils Nat. Prod. 2016;4:40–45. [Google Scholar]
- 119.Dmitrović S., Perišić M., Stojić A., Živković S., Boljević J., Nestorović Živković J., Aničić N., Ristić M., Mišić D. Essential oils of two Nepeta species inhibit growth and induce oxidative stress in ragweed (Ambrosia artemisiifolia L.) shoots in vitro. Acta Physiol. Plant. 2015;37:1–15. doi: 10.1007/s11738-015-1810-2. [DOI] [Google Scholar]
- 120.Baranauskienė R., Bendžiuvienė V., Ragažinskienė O., Venskutonis P.R. Essential oil composition of five Nepeta species cultivated in Lithuania and evaluation of their bioactivities, toxicity and antioxidant potential of hydrodistillation residues. Food Chem. Toxicol. 2019;129:269–280. doi: 10.1016/j.fct.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 121.Formisano C., Rigano D., Arnold N.A., Piozzi F., Senatore F. GC and GC-MS analysis of the essential oil of Nepeta cilicica Boiss. ex Benth. From Lebanon. Nat. Prod. Res. 2013;27:1975–1981. doi: 10.1080/14786419.2013.805332. [DOI] [PubMed] [Google Scholar]
- 122.Musso L., Scaglia B., Al Haj G., Arnold N.A., Adani F., Scarì G., Dallavalle S., Iriti M. Chemical characterization and nematicidal activity of the essential oil of Nepeta nuda L. ssp. pubescens and Nepeta curviflora boiss. From Lebanon. J. Essent. Oil-Bear. Plants. 2017;20:1424–1433. doi: 10.1080/0972060X.2017.1407678. [DOI] [Google Scholar]
- 123.Ali A., Tabanca N., Demirci B., Blythe E.K., Baser K.H.C., Khan I.A. Chemical composition and biological activity of essential oils from four Nepeta species and hybrids against Aedes aegypti (L.) (Diptera: Culicidae) Rec. Nat. Prod. 2015;10:137–147. [Google Scholar]
- 124.Chialva F., Monguzzi F., Manitto P. Composition of the Essential Oils of Five Salvia Species. J. Ess. Oil Res. 1992;4:447–455. doi: 10.1080/10412905.1992.9698108. [DOI] [Google Scholar]
- 125.Aydoǧmuş Z., Yeşilyurt V., Topcu G. Constituents of Salvia microphylla. Nat. Prod. Res. 2006;20:775–781. doi: 10.1080/14786410500462843. [DOI] [PubMed] [Google Scholar]
- 126.Lima R.K., Cardoso M.D.G., Andrade M.A., Guimarães P.L., Batista L.R., Nelson D.L. Bactericidal and Antioxidant Activity of Essential Oils from Myristica fragrans Houtt and Salvia microphylla H.B.K. J. Am. Oil Chem. Soc. 2012;89:523–528. doi: 10.1007/s11746-011-1938-1. [DOI] [Google Scholar]
- 127.Koutsaviti A., Antonopoulou V., Vlassi A., Antonatos S., Michaelakis A., Papachristos D.P., Tzakou O. Chemical composition and fumigant activity of essential oils from six plant families against Sitophilus oryzae (Col: Curculionidae) J. Pest Sci. 2018;91:873–886. doi: 10.1007/s10340-017-0934-0. [DOI] [Google Scholar]
- 128.Wróblewska K., Szumny A., Żarowska B., Kromer K., Dębicz R., Fabian S. Impact of mulching on growth essential oil composition and its biological activity in Monarda didyma L. Ind. Crop. Prod. 2019;129:299–308. doi: 10.1016/j.indcrop.2018.11.076. [DOI] [Google Scholar]
- 129.Sharopov F.S., Satyal P., Setzer W.N., Wink M. Chemical compositions of the essential oils of three Salvia species cultivated in Germany. Am. J. Essent. Oils Nat. Prod. 2015;3:26–29. [Google Scholar]
- 130.Lichtenthaler H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzym. 1987;148:350–382. doi: 10.1016/0076-687948036-1. [DOI] [Google Scholar]
- 131.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. [Google Scholar]
- 132.Jones J.B., Jr., Wolf B., Mills H.A. Plant Analysis Handbook: A Practical SAMPLING, preparation, Analysis, and Interpretation Guide. Micro-Macro Publishing, Inc.; Athens, Greece: 1991. [Google Scholar]
- 133.European Pharmacopoeia . European Pharmacopoeia. 9th ed. EDQM, Council of Europe; Strasbourg, France: 2017. [Google Scholar]
- 134.NIST 14/EPA/NIH . Mass Spectra Library. I. Willy and Sons, Inc.; Hoboken, NJ, USA: 2014. [Google Scholar]
- 135.Adams R.P. Identification of essential oil components by gas chromatography/mass spectrometry. Biochem. Syst. Ecol. 1996;24:594. [Google Scholar]
- 136.Davies N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. 1990;A 503:1–24. doi: 10.1016/S0021-9673(01)81487-4. [DOI] [Google Scholar]
- 137.Jennings W., Shibamoto T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography, Food/Nahrung. Academic Press; Cambridge, MA, USA: 1982. [Google Scholar]
- 138.Masada Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry. John Wiley & Sons, Inc.; New York, NY, USA: 1976. [Google Scholar]
- 139.Stenhagen E., Abrahamsson S., McLafferty F.W. Registry of Mass Spectral Data. Wiley & Sons; New York, NY, USA: 1974. [Google Scholar]
- 140.Swigar A.A., Silverstein R.M. Monoterpenes. Aldrich Chemical Company; Milwaukee, WI, USA: 1981. [Google Scholar]
- 141.Lee S., Lee D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018;71:353. doi: 10.4097/kja.d.18.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moita Neto J.M., Moita G.C. An introduction analysis exploratory multivariate date. Quimica Nova. 1998;21:467–469. doi: 10.1590/S0100-40421998000400016. [DOI] [Google Scholar]