Abstract
Toxigenic moulds can develop on the surface of dry-cured meat products during ripening due to their ecological conditions, which constitutes a risk for consumers. A promising strategy to control this hazard is the use of antifungal microorganisms usually found in these foods. However, to date, the effectiveness of gram-positive catalase-positive cocci (GCC+) has not been explored. The aim of this work was to select GCC+ isolates with antifungal activity to study its effectiveness in a dry-cured ham model system at the environmental conditions reached during the ripening. Forty-five strains of GCC+ were evaluated and the isolate Staphylococcus xylosus Sx8 was selected to assess its efficacy at two different concentrations (106 and 104 cfu/mL) against Penicillium nordicum, Aspergillus flavus, Aspergillus parasiticus, and Penicillium griseofulvum at 15, 20, and 25 °C. The results showed that the inoculation of 106 cfu/mL of S. xylosus completely inhibited the growth of most fungi. In addition, in the presence of this strain at 104 cfu/mL, a significant reduction in fungal growth and mycotoxins production was observed at the three temperatures studied. In conclusion, S. xylosus Sx8 possesses great potential as a biological agent to control toxigenic moulds in dry-cured meat products.
Keywords: biocontrol, S. xylosus, toxigenic mould, ochratoxin A, aflatoxins, cyclopiazonic acid, dry-cured ham
1. Introduction
Moulds become the predominant population on the surface of dry-cured meat products due to their ability to adapt to the environmental conditions reached during their ripening [1,2,3]. These microorganisms significantly contribute to the development of the characteristic sensorial attributes of these foods [4,5]. However, it is well known that moulds isolated from this type of products are capable of producing mycotoxins [2,3,6].
Ochratoxin A (OTA) is the most prevalent mycotoxin in meat products [7]. It can be produced by several moulds species such as Aspergillus westerdijkiae, Penicillium verrucosum, and Penicillium nordicum [8,9,10], although P. nordicum is the most common ochratoxigenic mould isolated from dry-cured meats [8,11]. OTA is nephrotoxic, being involved in kidney diseases and urinary tract tumors [12] and it has been classified by the International Agency for Research on Cancer (IARC) as a possible human carcinogen in the 2B category [13]. Moreover, the presence of other mycotoxins in dry-cured ham such as aflatoxins (AFs) [14,15,16,17] and cyclopiazonic acid (CPA) [18] has also been reported.
AFs are mainly produced by Aspergillus flavus and Aspergillus parasiticus and they can be found in dry-cured meat products [15,16,17,19,20]. They are immunosuppressive and hepatotoxic and have been classified by the IARC as carcinogenic to humans in the 1A group [21]. On the other hand, CPA has been detected in dry-cured ham [18,22] and can be produced by species frequently isolated in this product such as Penicillium griseofulvum [22,23]. CPA provokes weight loss, nausea, muscle necrosis, convulsions and neurochemical and mutagenic toxicity due to a specific inhibitor of calcium-dependent ATPase in the sarcoplasmic reticulum [23].
Therefore, it is of utmost importance to control the growth of toxigenic moulds and their mycotoxin production. Among the different antifungal strategies, one of the most promising for dry-cured meat products is the use of microorganisms usually found in them as bioprotective agents. In this sense, several yeasts [12,24,25,26,27,28], non-toxigenic moulds [25,29,30,31], and lactic acid bacteria [32,33] have shown their capacity to reduce the development of toxigenic moulds and decrease mycotoxins production and they have been proposed as potential protective cultures in dry-cured meat products. However, the possibility of using gram-positive, catalase-positive cocci (GCC+) in dry-cured ham has not been evaluated yet.
GCC+, including coagulase-negative Staphylococcus sp., Micrococcus sp. and Kocuria sp., are one of the predominant microbial groups for most of the ripening time in dry-cured ham [34]. Among them, the most prevalent are several species of coagulase-negative staphylococci such as Staphylococcus equorum, Staphylococcus saprophyticus and Staphylococcus succinus, being the predominant species Staphylococcus xylosus [35,36,37]. These microorganisms provide beneficial effects such as colour stabilization because of its ability to reduce nitrates to nitrites and thus, to form nitrosylmyoglobin; inhibition of rancidity due to its catalase activity that breaks down hydrogen peroxide; and flavour development as a result of proteolytic and lipolytic activities [34,38,39,40]. For all these reasons, Staphylococcus sp. is usually used as a starter culture in dry-cured meat products manufacturing [38,39], being frequently added at levels around 5–6 log cfu/g and reaching counts higher than 7–8 log cfu/g during the processing [41]. Therefore, it can be of great interest in the evaluation of the antifungal potential of GCC+ as a possible protective culture to control mycotoxins production in dry-cured meat products.
However, some GCC+, including coagulase-negative staphylococci isolated from dry-cured meat products, are able to produce staphylococcal enterotoxins [34]. Then, before a staphylococcus isolate was proposed as a biocontrol agent, it must be verified that it is not an enterotoxin-producing strain.
The main objective of this work was the evaluation of the antifungal potential of non-enterotoxigenic GCC+ isolated from dry-cured ham against toxigenic moulds to select a candidate to be proposed as a protective culture. The effectiveness of the selected isolate was studied in a dry-cured ham-based agar at environmental conditions usually found during the ripening of dry-cured meat products.
2. Materials and Methods
2.1. Bacteria and Fungi
A total of 45 GCC+ and 8 mycotoxin-producing moulds were used in this study. The GCC+ strains were isolated from the surface of dry-cured Iberian hams during their processing using mannitol salt agar (MSA, Conda Pronadisa, Madrid, Spain) as described by Rodríguez et al. [37]. The mycotoxin-producing moulds were obtained from different culture collections: P. nordicum FHSCC 15 (Pn15) from the Food Hygiene and Safety Culture Collection at the University of Extremadura (Cáceres, Spain); P. nordicum CBS 323.92 (Pn92), and A. flavus CBS 573.65 (Af65) from the Centraalbureau voor Schimmelcultures (Utrecht, Netherland); P. nordicum BFE 856 (Pn856) from the Federal Research Centre for Nutrition and Food (Kulmbach, Germany); A. parasiticus CECT 2681 (Ap2681) and P. griseofulvum CECT 2919 (Pg2919) from the Spanish Type Culture Collection (Valencia, Spain); P. griseofulvum IBT 14319 (Pg14319) from the Type Culture Collection of the Department of Biotechnology from the Technical University of Denmark, and A. westerdijkiae 6B (Aw6B) was kindly supplied by Dr. Paula Rodrigues from the Mountain Research Centre, Polytechnic Institute of Bragança (Portugal).
2.2. Staphylococcal Enterotoxin Analysis
A TECRA™ Staphylococcal Enterotoxin Visual Immunoassay (TECRA Diagnostics, Roseville, Australia) for staphylococcal enterotoxins types A to E was used. All the 45 GCC+ isolates were grown in brain heart infusion (BHI, Conda Pronadisa) at 37 °C for 18 h. After centrifugation at 10,000× g for 20 min, the supernatants were collected and tested according to the manufacturer’s instructions.
2.3. 16S rDNA Sequence Analysis
The isolates with the highest antifungal activity were identified by 16S rDNA sequencing. Total genomic DNA was extracted from 1 mL overnight cultures in BHI broth (Conda Pronadisa), using a Masterpure complete DNA and RNA Purification Kit (Lucigen, Middleton, WI, USA), and processed according to the manufacturer’s instructions. The quantity and quality of DNA were spectrophotometrically determined in a biophotometer (Eppendorf AG, Hamburg, Germany). Universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′), and 1492R (5′-TACGGTTACCTTGTTACGACTT-3′) were used to amplify the 16S rDNA gene. The amplification program consisted of initial denaturation at 95 °C for 5 min; 30 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 40 s; and a final extension step at 72 °C for 1 min in a thermal cycler (Eppendorf AG 22331). Cycle sequencing reaction products were purified with a Sephadex column. Sequence analysis of PCR products was carried out by the Applied Bioscience Techniques Service of the University of Extremadura (STAB, Badajoz, Spain). The sequence results obtained were aligned with the NCBI database using the BLAST program (http://blast.ncbi.nlm.nih.gov, NCBI, Bethesda MD, USA). The identities of the isolates were determined on the basis of the highest score.
2.4. Inocula Preparation
The inocula of cocci were obtained by inoculating 100 µL of a culture preserved at −80 °C in BHI broth (Conda Pronadisa) and incubating at 30 °C for 48 h. Then, the count of microorganisms was obtained by plating them on MSA. Once the concentration of cells was known, the appropriate dilutions were made to achieve the concentrations of 108, 106 and 104 cfu/mL used as inoculum.
The moulds were grown on potato dextrose agar (PDA, Conda Pronadisa) at 25 °C for 7 days. The spores were collected using 4 mL of saline phosphate buffer (PBS) and rubbing the surface with a glass rod. The spore suspensions were quantified by using a Thoma counting chamber Blaubrand® (Brand, Germany) and adjusted to 106 spores/mL and used as inoculum.
2.5. Effect of GCC+ on Growth of Ochratoxigenic Moulds
The antifungal activity of 45 strains of GCC+ was evaluated against A. westerdijkiae 6B and P. nordicum Pn15. Suspensions of 108 cfu/mL cocci were mixed with 20 mL of melted meat extract agar (MEA; 30 g/L of meat extract (Conda Pronadisa), 20 g/L Bacto agar (Conda Pronadisa), 30 g/L of NaCl and 1000 mL of distilled water), and poured into Petri dishes, which were left for 2 h to solidify. After the solidification, drops of 10 µL at a concentration of 106 spores/mL of each mould inoculum were inoculated at a single point on the centre of each plate. Drops of mould spore suspension were also placed on GCC+-free MEA plates, which were used as control. The fungal growth was estimated after 5 days of incubation at 25 °C by measuring the diameter of the mould colonies in two perpendicular directions. The experiment was carried out in triplicates.
2.6. Effect of S. xylosus on Growth and Mycotoxins Production by Mycotoxigenic Moulds
This study was carried out in dry-cured ham-based agar in Petri dishes of 5 cm diameter against 7 toxigenic fungi (P. nordicum Pn15, Pn 92 and Pn856, A. flavus Af65, A. parasiticus Ap2681, and P. griseofulvum Pg2919 and Pg14319). It was made by mixing 30 g/L of lyophilised dry-cured ham, 20 g/L Bacto agar (Conda Pronadisa), 50 g/L of NaCl, and 1000 mL of distilled water. Finally, the water activity (aw) was 0.95. For each fungus, two batches were prepared (treated and untreated control), and three temperatures were used (15, 20, and 25 °C), like those found during the dry-cured ham ripening [37]. A control batch was inoculated only with the mould and another batch was inoculated with the mould and S. xylosus. First, 50 µL of the solution containing bacteria (106 or 104 cfu/mL) were spread on the surface of the culture medium in the corresponding plates to reach an initial count of 2.5·103 and 2.5·10 cfu/cm2, respectively. These concentrations of GCC+ are commonly found in dry-cured hams [37]. Then, 2 μL of the mould spore suspension (106 spores/mL) were inoculated superficially at a single point in the plate centre in all batches. The experiment was carried out in triplicates.
2.6.1. Growth Assessment of Toxigenic Moulds
The diameter of the growing colonies was measured each day in two perpendicular directions until the mould colonies covered the full surface of the plate in the control batches, during a maximum of 30 days.
2.6.2. Extraction and Quantification of Mycotoxins
After removing the mycelium, 1 g of agar was picked up from the centre of fungal colonies and it was stored at −20 °C until the extraction of each mycotoxin.
OTA Extraction
OTA was extracted following the QuEChERS methodology proposed by Kamala et al. [42] with modifications. One gram of agar was mixed with 2 mL of water acidified with 0.1% acetic acid, and shaken for 30 s. Then, 2 mL acetonitrile acidified with 0.1% acetic acid was added and shaken for 1 min. Thereupon, 0.4 g NaCl and 1.6 g MgSO4 were added and shaken manually for 30 s. The samples were centrifuged for 5 min at 5000 rpm, and an aliquot of 1 mL supernatant was collected. Finally, the samples were diluted to 1:1 with water and filtered through a 0.22 μm pore-size nylon membrane (RephiLe Bioscience, Philadelphia, PA, USA).
Aflatoxins Extraction
For the extraction of AFs, the methodology described by Peromingo et al. [14] was applied. One gram of sample was mixed with 5 mL of chloroform and shaken at 150 rpm during 24 h in an orbital shaker. Then, the supernatant was transferred to amber tubes and dried in the dark. Reconstitution was carried out by adding 500 µL of HPLC-grade acetonitrile. Finally, the samples were diluted 1:1 with ultrapure water and filtered through a 0.22 μm pore-size nylon membrane (RephiLe Bioscience).
CPA Extraction
CPA was extracted following the QuEChERS procedure described by Peromingo et al. [18]. One gram of sample was mixed with 1 mL of water containing 0.1% of acetic acid, and mixed with a vortex for 30 s. Thereupon, 0.2 g NaCl and 0.8 g MgSO4 were added, and shaken manually for 30 s. The samples were centrifuged for 5 min at 5000 rpm, and an aliquot of 0.5 mL supernatant was collected into amber glass roller bottles. The extracts were dried in the dark and then, were reconstituted with 500 µL of HPLC-grade acetonitrile. Finally, the samples were diluted 1:1 with ultrapure water and filtered through a 0.22 μm pore-size nylon membrane (RephiLe Bioscience).
Recovery Experiments and Linearity Range
Recovery for OTA, AFs, and CPA extraction methods had been previously calculated [18,43] showing a recovery rate >90%. Linearity was estimated in the working range for every mycotoxin by spiking the acetonitrile recovered from non-inoculated samples extracted with QuEChERS in order to subtract the matrix effect. For OTA, the calibration curve was done using 14 calibration levels from 0.05 to 100 ng/mL. For AFB1, two curves were made, the first one with 6 calibration levels from 0.01 to 1 ng/mL, and the second one included 8 calibration levels from 1 to 30 ng/mL. Similarly, two curves were prepared for AFG1, one with 6 calibration levels from 0.01 to 1 ng/mL, and the other one with 7 calibration levels from 1 to 25 ng/mL. Finally, for CPA 11, calibration levels were used from 1 to 1500 ng/mL. Each calibration curve showed an R2 ≥ 0.99 for every working range.
Mycotoxin Quantification by Orbitrap Q Exactive Plus Analysis
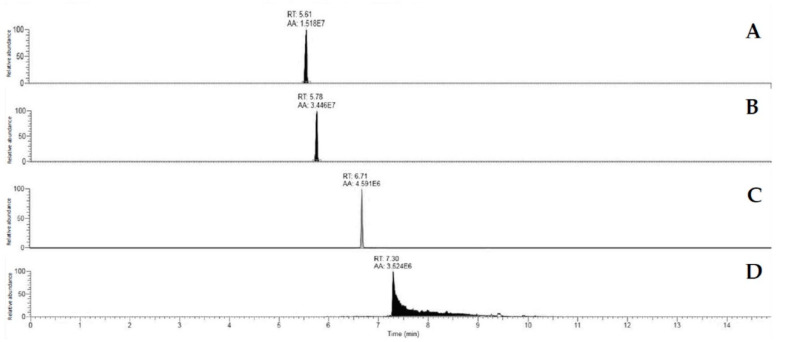
The chromatographic analysis was common for every mycotoxin determination. Ten microliters of every sample were injected in a Dionex Ultimate 3000 UHPLC apparatus (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a degassing system, a Quaternary HPLC pump, an autosampler device, and a thermostated (45 °C) Thermo Fisher Accucore Aq C18 (150 × 2.1 mm) column 2.6 μm particle size. Mobile phases were prepared with HPLC grade solvents: phase A (H2O 0.1% formic acid (FA)), phase B (acetonitrile 0.1% FA). The flow was 0.3 mL/min, and the mycotoxin separation was performed with a pre-conditioning of 10% B for 3 min, separation slope for 5 min, 25–98% B, column washing (98% B) for 6 min and 1 min of postconditioning (10% B). AFG1 eluted by 5.61 ± 0.1 min, and AFB1 by 5.78 ± 0.1 min, showing successful discrimination among them (Figure 1). OTA was eluted by 6.71 ± 0.1 min, and CPA by 7.30 ± 0.1 min.
Figure 1.
Chromatograms showing the signal obtained from Q Exactive Plus apparatus for the four mycotoxins tested (A: AFG1, B: AFB1, C: OTA and D: CPA).
The identification and quantification of mycotoxins was carried out by using a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific). For AFs determination, the Full Scan methodology was utilised. OTA and CPA were quantified by using T-SIM mode, setting an isolation window of 2 m/z.
For every analysis, an ESI source (HESI II, Thermo Fischer Scientific) operating in positive ion mode (ESI+) was used. Ion source parameters in this positive mode were spray voltage 4 kV, sheath gas (N2 > 95%) 30, auxiliary gas (N2 > 95%) 10, capillary temperature 350 °C, S-lens RF level 55, auxiliary gas heater temperature 350 °C.
For t-SIM analysis, the value for automatic gain control (AGC) target was set at 5 × 104; for Full Scan, it was 1 × 106, and a scan rate in the range between 150 and 800 m/z. Every analysis was carried out with a resolution of 70,000 full widths at half maximum (FWHM). Data analysis and processing were performed using the FreeStyle™ software, v. 1.5 (Thermo Fisher Scientific). Each mycotoxin was identified by its retention time and its exact mass at ≤5 ppm.
Mycotoxin Determination Sensitivity
Limit of detection (LOD) and limit of quantification (LOQ) were calculated by using the same acetonitrile matrix from untreated samples undergone to QuEChERS method, and spiked with OTA, AFs or CPA standards, as the lowest evaluable concentration level at which the qualifier ion signal exceeds the noise level by a factor of 3.5 and 10, respectively. Being the LOD and LOQ 0.005 ng/mL and 0.01 ng/mL for AFB1, 0.036 ng/mL and 0.05 ng/mL for AFG1, 0.014 ng/mL and 0.05 ng/mL for OTA, and 0.29 ng/mL and 1 ng/mL for CPA.
2.7. Statistical Analysis
Statistical analysis was performed using the IBM SPSS Statistics 22.0 software (IBM, Armonk, NY, USA). All data were tested for normality using the Shapiro-Wilk test, and the homogeneity of variances using the Levene test. All data sets failed the normality test, so variable transformation was performed to improve normality or homogenise the variances but without any success. The analysis of non-parametric data was performed using the Kruskal-Wallis test. After that, the Mann-Whitney U test was applied to compare the mean values obtained. The statistical significance was set at p ≤ 0.05.
3. Results
3.1. Enterotoxigenic Potential of Gram-Positive, Catalase-Positive Cocci
The enterotoxigenic potential of the 45 GCC+ isolated from dry-cured hams was evaluated by the TECRATM immunological assay. The obtained results revealed that none of these isolates produced detectable amounts of staphylococcal enterotoxins.
3.2. Selection and Identification of Gram-Positive, Catalase-Positive Cocci Isolates with Antifungal Activity
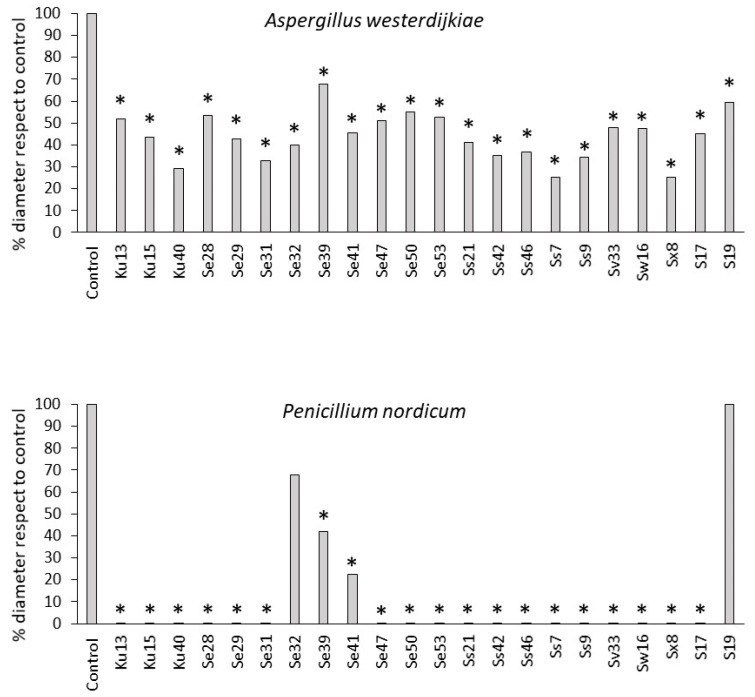
The antifungal activity of 45 GCC+ isolated from dry-cured ham at different stages of ripening was studied. The screening revealed that 22 isolates (48.9%) produced a significative decrease in the growth of at least one of the reference moulds used (Figure 2). These 22 active isolates were then identified by sequencing of 16S rDNA region, resulting in 3 Kocuria uropygioeca, 9 S. equorum, 5 S. saprophyticus, 1 Staphylococcus vitulinus, 1 Staphylococcus warneri, 1 S. xylosus, and 2 isolates of uncertain identification between Staphylococcus pasteuri and S. warneri (Table 1).
Figure 2.
Antifungal effect of Gram + catalase + cocci on A. westerdijkiae and P. nordicum growth represented as the percentage of the diameter of the mould colonies in the presence of cocci with respect to the growth of the control mould in the absence of the bacteria. * Significant differences (p ≤ 0.05) for treated samples with respect to control are indicated by an asterisk.
Table 1.
Identification by sequencing of 16S rDNA region of Gram + catalase + cocci with antifungal activity.
| Isolate | Identification | Accession Number | % Identity |
|---|---|---|---|
| Ku13 | K. uropygioeca | NR_157676.1 | 95.88 |
| Ku15 | K. uropygioeca | NR_157676.1 | 98.72 |
| Ku40 | K. uropygioeca | NR_157676.1 | 100 |
| Se28 | S. equorum | MK015791.1 | 99.86 |
| Se29 | S. equorum | MN229550.1 | 99.79 |
| Se31 | S. equorum | MN229551.1 | 99.91 |
| Se32 | S. equorum | MN758799.1 | 98.28 |
| Se39 | S. equorum | KY940339.1 | 99.85 |
| Se41 | S. equorum | MK796070.1 | 99.92 |
| Se47 | S. equorum | MN758799.1 | 99.64 |
| Se50 | S. equorum | KP224447.1 | 99.56 |
| Se53 | S. equorum | KY940339.1 | 99.86 |
| Ss21 | S. saprophyticus | KU922358.1 | 99.02 |
| Ss42 | S. saprophyticus | MF457583.1 | 99.4 |
| Ss46 | S. saprophyticus | MF457583.1 | 99.7 |
| Ss7 | S. saprophyticus | MN229561.1 | 98.52 |
| Ss9 | S. saprophyticus | KT717631.1 | 99 |
| Sv33 | S. vitulinus | KM378591.1 | 99.69 |
| Sw16 | S. warneri | MK414943.1 | 98.8 |
| Sx8 | S. xylosus | HM854231.1 | 99 |
| S17 | S. pasteuri/S. warneri | MH930440.1/MN181250.1 | 99.79 |
| S19 | S. pasteuri/S. warneri | MH930440.1/MN421516.1 | 99.79 |
In general, P. nordicum was more sensitive than A. westerdijkiae, since it did not grow in the presence of 18 tested GCC+. Although A. westerdijkiae was able to grow in all cases, all 22 active GCC+ reduced their development, recording its greatest inhibition in the presence of S. saprophyticus Ss7 and S. xylosus Sx8. Then, S. xylosus Sx8 was selected to conduct the following experiment according to its overall activity against both ochratoxigenic moulds, and considering that this species is predominant during the processing of dry-cured ham.
3.3. Antifungal Activity of S. xylosus in a Dry-Cured Ham Model System
3.3.1. Effect on Mould Growth
The effect of S. xylosus Sx8 on growth rates of P. nordicum, A. flavus, A. parasiticus, and P. griseofulvum was studied in a dry-cured ham-based medium under ecological conditions usually found during the processing of this product. All the fungal strains tested showed a satisfactory growth in this culture medium when they were individually cultured without the presence of S. xylosus Sx8 (untreated control batch, Figure 3). The pattern of development for the strains that produce the same mycotoxin was quite similar (Figure 4), showing A. flavus and A. parasiticus the fastest growth, as they covered the whole surface plate in a range of 11–14 days, depending on the incubation temperature. In addition, each mould reached almost the same growth levels regardless of the incubation temperature, except A. flavus and A. parasiticus whose growth was lower at 15 °C.
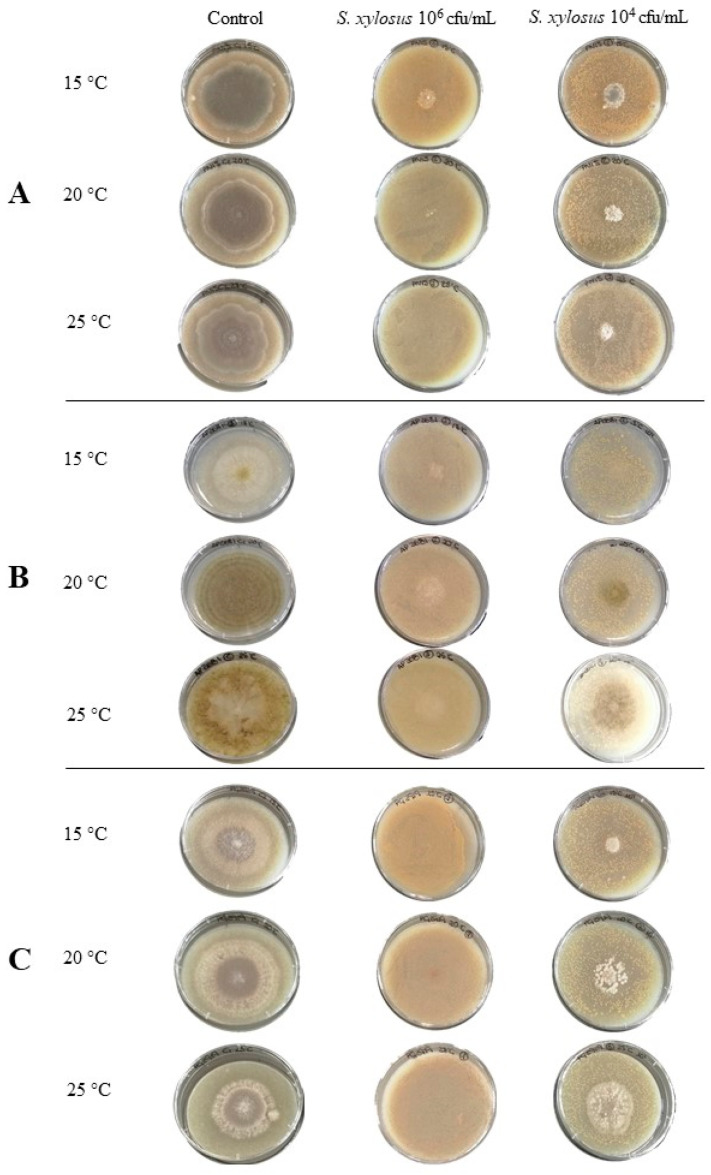
Figure 3.
Effect of S. xylosus Sx8 at different initial concentrations (106 and 104 cfu/mL) on the final growth of P. nordicum Pn15 (A), A. parasiticus Ap2681 (B), and P. griseofulvum Pg2919 (C) at 15, 20, and 25 °C.
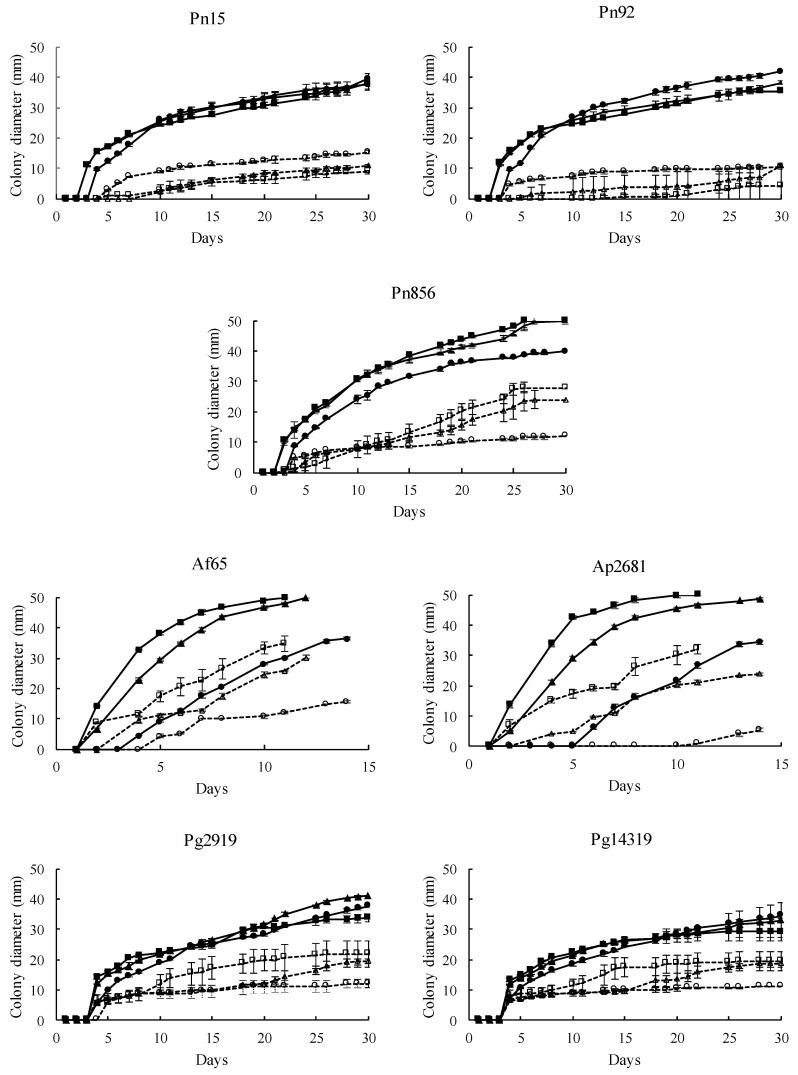
Figure 4.
Growth of OTA-producing P. nordicum (Pn15, Pn92, Pn856), AFs-producing A. flavus Af65 and A. parasiticus Ap2681, and CPA-producing P. griseofulvum (Pg2919, Pg14319) strains at 15 (●), 20 (▲), and 25 °C (■), in the presence (discontinuous line, open symbols) or absence (continuous line, closed symbols) of S. xylosus Sx8.
S. xylosus had a marked influence on the development of all toxigenic moulds. When the inoculum level of 106 cfu/mL of Sx8 was used (reaching an initial load of approximately 2.5·103 cfu/cm2), no growth was observed at 15–25 °C for most of the treated fungi, while in untreated control batches, moulds had covered almost the whole surface of the plates (Figure 3). When the assay was performed using 104 cfu/mL of Sx8 (reaching approximately 2.5·10 cfu/cm2), a significant growth diminution of all P. nordicum, A. flavus, A. parasiticus, and P. griseofulvum strains was recorded at every temperature (Figure 4).
3.3.2. Effect on Mycotoxin Production
The OTA amount produced by P. nordicum Pn15 and Pn92 in the presence of S. xylosus Sx8 was significantly reduced to below LOQ and LOD respectively at all tested temperatures (Table 2). For P. nordicum Pn856, there was a reduction in OTA production of 86.59% and 75.35% at 15 and 20 °C, respectively. However, at 25 °C, an increase of OTA production by Pn856 in the presence of the Sx8 was observed.
Table 2.
Effect of S. xylosus Sx8 on ochratoxin A (OTA) production (ng/g) by P. nordicum Pn15, Pn92, and Pn856 in dry-cured ham-based media during 30 days of incubation at 15, 20, and 25 °C.
| 15 °C | 20 °C | 25 °C | ||||
|---|---|---|---|---|---|---|
| Mould | Control | Sx8 | Control | Sx8 | Control | Sx8 |
| Pn15 | 3.93 ± 1.36 | <LOQ *,a | 18.76 ± 3.22 | <LOQ * | 66.59 ± 19.52 | <LOQ * |
| Pn92 | 2.75 ± 0.49 | <LOD *,b | 26.61 ± 4.82 | <LOD * | 89.53 ± 14.90 | <LOD * |
| Pn856 | 31.54 ± 0.07 | 4.23 ± 0.35 * | 260.64 ± 71.93 | 64.26 ± 11.72 * | 389.73 ± 57.65 | 860.13 ± 357.03 * |
* Significant differences with respect to control p ≤ 0.05; a Values under the limit of quantification; b Values under the limit of detection.
The production of AFB1 by A. flavus Af65 and A. parasiticus Ap2681 was significantly reduced in the presence of S. xylosus Sx8 (Table 3). In A. flavus, the greater reduction (99.32%) was obtained at 20 °C. The production of this mycotoxin by A. parasiticus Ap2681 was considerably lower in every condition, and Sx8 led to a reduction to non-detectable level at 25 °C. On the other hand, the production of AFG1 was also significantly reduced to non-detectable level when A. parasiticus was co-inoculated with S. xylosus Sx8 at three temperatures (Table 4).
Table 3.
Effect of S. xylosus Sx8 on aflatoxin B1 (AFB1) production (ng/g) by A. flavus Af65, and A. parasiticus Ap2681 in dry-cured ham-based media during 14 days of incubation at 15, 20, and 25 °C.
| 15 °C | 20 °C | 25 °C | ||||
|---|---|---|---|---|---|---|
| Mould | Control | Sx8 | Control | Sx8 | Control | Sx8 |
| Af65 | 2.09 ± 0.25 | 0.18 ± 0.03 * | 41.06 ± 6.23 | 0.28 ± 0.09 * | 28.49 ± 3.07 | 2.08 ± 2.21 * |
| Ap2681 | 0.08 ± 0.02 | 0.01 ± 0.01 * | 0.06 ± 0.05 | 0.01 ± 0.01 | 0.10 ± 0.02 | <LOD *,a |
* Significant differences with respect to control p ≤ 0.05; a Values under the limit of detection.
Table 4.
Effect of S. xylosus Sx8 on aflatoxin G1 (AFG1) production (ng/g) by A. parasiticus Ap2681 in dry-cured ham-based media during 14 days of incubation at 15, 20, and 25 °C.
| 15 °C | 20 °C | 25 °C | ||||
|---|---|---|---|---|---|---|
| Mould | Control | Sx8 | Control | Sx8 | Control | Sx8 |
| Ap2681 | 0.73 ± 0.34 | <LOD *,a | 4.85 ± 8.24 | <LOD * | 0.91 ± 0.30 | <LOD * |
* Significant differences with respect to control p ≤ 0.05; a Values under the limit of detection.
CPA produced by P. griseofulvum was the mycotoxin that reached the higher concentrations in the dry-cured ham-based medium (Table 5). Sx8 triggered a significant reduction of CPA production by Pg2919 at 20 and 25 °C, and by Pg14319 at 15 and 25 °C.
Table 5.
Effect of S. xylosus Sx8 on cyclopiazonic acid (CPA) production (ng/g) by P. griseofulvum Pg2919, and Pg14319 in dry-cured ham-based media during 30 days of incubation at 15, 20, and 25 °C.
| 15 °C | 20 °C | 25 °C | ||||
|---|---|---|---|---|---|---|
| Mould | Control | SX8 | Control | SX8 | Control | SX8 |
| Pg2919 | 1270.4 ± 431.9 | 888.5 ± 258.3 | 2427.8 ± 724.8 | 1793.9 ± 34.7 * | 4492.9 ± 227.0 | 2694.1 ± 881.9 * |
| Pg14319 | 2804.2 ± 802.8 | 1160.0 ± 165.0 * | 4120.4 ± 944.7 | 2932.9 ± 651.9 | 6298.7 ± 2.9 | 5358.9 ± 382.4 * |
* Significant differences with respect to control p ≤ 0.05.
4. Discussion
The use of lactic acid bacteria [32], yeasts [12,24,25,26,27,28,44], and non-toxigenic moulds [25,30,31,45] isolated from dry-cured meat products as protective cultures against toxigenic moulds in these foods has been widely proposed. However, to the best of our knowledge, the feasibility of using GCC+ for this purpose has not been explored yet, even though this group is among the prevailing ones in dry-cured meat products [35,36,37]. Therefore, the antifungal activity of cocci isolated throughout the processing of dry-cured ham deserved to be evaluated.
The selection of the suitable isolates to be used as protective cultures should be based not only on their antifungal activity but also on the safety criteria. In this sense, some GCC+ such as coagulase-negative staphylococci, coming from dry-cured meat products may produce staphylococcal enterotoxins [34]. Thus, the toxin-producing staphylococci must be excluded. Then, the enterotoxigenic potential of the isolates was checked by TECRATM immunological assay. None of the 45 tested cocci produced staphylococcal enterotoxins at detectable levels. Therefore, they should be considered as safe, and they could be eventually proposed as biocontrol agents.
A high ratio of active antifungal isolates was obtained, since 22 out of the 45 tested GCC+ (48.9%) showed inhibitory activity against at least one of the ochratoxigenic reference moulds used (Figure 2). The environmental conditions reached in the processing of dry-cured meat products are suitable for the persistence of GCC+ for long periods of time [37]. In this context, their antifungal ability could represent an ecological advantage in these foods in which moulds also develop extensively [2,3,22].
According to the overall results obtained against ochratoxigenic moulds, S. xylosus Sx8 was selected as a biocontrol agent candidate. This isolate was able to completely prevent the growth of P. nordicum, and was also showing the highest inhibition against A. westerdijkiae along with S. saprophyticus Ss7 (Figure 2). On the other hand, S. xylosus is the overwhelmingly predominant species in dry-cured meats products throughout the processing, even after 16 months of ripening [34,36]. Moreover, the S. xylosus strain has been proposed to be used as starter cultures for dry-cured meat products, because of its contribution to the typical flavour and coloration of these foods [46,47].
The antifungal activity of S. xylosus Sx8 was evaluated against a range of toxigenic moulds belonging to species commonly isolated from dry-cured meat products. Then, the screening was performed by coculturing the active Sx8 against several strains of the ochratoxigenic P. nordicum, the aflatoxigenic A. flavus and A. parasiticus, and P. griseofulvum producers of CPA in a dry-cured ham-based medium at three temperatures (15, 20, and 25 °C) usually found during the processing of this food. S. xylosus Sx8 decreased the growth of all the toxigenic moulds during the whole incubation period at every temperature tested. The efficiency in preventing fungal growth depended on the level of inoculation of Sx8. Then, when a rate of 106 cfu/mL was inoculated (reaching about 2.5·103 cfu/cm2 in plate surface of initial load), no mycelium development of most of the toxigenic strains studied was observed (Figure 3). Similar results were found regarding the antifungal activity of Debaryomyces hansenii and Saccharomycopsis fibuligera against P. nordicum and Aspergillus ochraceus in speck, recording a greater growth reduction with a yeasts concentration of 106 cfu/mL than with 104–102 cfu/mL [26]. In the same way, Andrade et al. [24] found that increasing the inoculum concentration from 104 to 106 cfu/mL of D. hansenii significantly improved the growth inhibition of P. nordicum in culture media.
The antagonistic effect of staphylococci has been linked to the generation of antifungal volatile compounds. S. saprophyticus L-38 inhibited A. flavus conidial germination and mycelial proliferation, through the production of 3,3-dimethyl-1,2-epoxybutane [48]. The inhibition of the mycelial growth and conidial germination of the plant pathogen Colletotrichum nymphaeae by Staphylococcus sciuri MarR44 has been also attributed mainly to volatile compounds, but without ruling out the role of other metabolites such as siderophore, chitinase, protease, hydrogen cyanide, indole-3-acetic acid, and gibberellin and the biofilm formation [49]. In this sense, the production of extracellular metabolites, including volatile compounds, proteins, lytic enzymes or organic acids has been reported in a range of microorganisms with antifungal activity [44,50,51,52]. In addition, competition by nutrients and space is considered one of the most common antagonistic mechanisms of antifungal agents, including bacteria, moulds and yeasts [12,44,53,54]. Considering all these possibilities, it is necessary to conduct further researches to elucidate the mechanism of action involved in the capability of S. xylosus Sx8 to reduce fungal growth.
In addition to the reduction of mould growth, S. xylosus Sx8 significantly decreased the mycotoxin concentration in most of the conditions tested. For OTA-producing moulds, the presence of S. xylosus Sx8 significantly reduced (p ≤ 0.05) the amount of this mycotoxin regardless of temperatures. In this way, the effect of this staphylococcus strain provokes that the OTA production by P. nordicum Pn15 and Pn92 drops to levels below the LOQ and LOD respectively (Table 2) at 15, 20, and 25 °C. However, even though OTA production by P. nordicum Pn856 also decreased at 15 and 20 °C, there was an increase at 25 °C. Although supposedly a reduction in mould growth would mean a lower mycotoxin production, previous studies have shown that OTA production by P. nordicum and P. verrucosum may increase under sub-optimal growth conditions [9]. Moreover, some stressful conditions seem to stimulate secondary metabolism with the consequent increase of mycotoxin production [10,55]. In fact, fungal secondary metabolism is strongly stimulated by sub-optimal conditions like nutrient depletion or metabolites produced by other organisms [56]. Nevertheless, this phenomenon only occurs in P. nordicum Pn856 at 25 °C at the lowest concentration tested of S. xylosus Sx8, but not at any other temperatures, nor for other P. nordicum strains, when an outstanding reduction in OTA amount was recorded.
S. xylosus also triggered a decrease in the production of AFB1 (Table 3) by A. flavus Af65 and A. parasiticus Ap2681, in the production of AFG1 (Table 4) by A. parasiticus, and in production of CPA by P. griseofulvum Pg2919 and Pg14319 (Table 5) at the full range of temperature evaluated (15–25 °C).
The reduction of mycotoxins amounts by S. xylosus reported in the present work could be related to the lower fungal growth reported. However, other modes of action may be involved. In this sense, the ability of S. saprophyticus to reduce the AFB1 amount in cultures of A. flavus in corn and peanuts was linked to the abolition of mycelia growth due to the volatile 3,3-dimethyl-1,2-epoxybutane [48]. In other bacteria, such as Bifidobacterium and Lactobacillus, mycotoxin reduction is due to the enzymatic degradation or to the adsorption on the cell wall [57]. The yeasts D. hansenii decreased OTA and AFs production in meat substrates by the blockage in the expression of genes related to the synthesis of these mycotoxins [25,27,28]. On the other hand, the effect of P. chrysogenum on decreasing OTA production by P. nordicum has been attributed to the repression of genes of the OTA-biosynthetic pathway [25], but also to the nutritional competition that implies a reduction in the secondary metabolism [53,54]. Nevertheless, no information about the mode of action of S. xylosus in the mycotoxin reduction in meat substrates is still available, and further investigations are required to elucidate it.
Therefore, the use of S. xylosus Sx8 to control toxigenic moulds in dry-cured meat products should be considered as a promising approach, since this species is adapted to this ecological niche. However, the inoculation size of Sx8, the environmental conditions, and even the specific mould strain seem to be key factors for its effectiveness. Thus, to assure a successful outcome, a level of 2.5·103 cfu/cm2 of this microorganism should be reached on the food surface. Its inoculation on the earlier stages of the processing will ensure its implantation in the food reaching high counts to prevent the growth of toxigenic moulds and the presence of mycotoxins. In this sense, although the counts of GCC+ decreased continuously until the end of the processing of dry-cured ham, the counts usually are higher to 104 cfu/g for a long time [37].
On the other hand, according to the obtained results, the effect of S. xylosus Sx8 is not enough for the whole suppression of mycotoxins production by all the tested moulds, but it contributed to reducing the accumulation of these mycotoxins. This effect can be enhanced by using Sx8 in mixed cultures together with other microorganisms with complementary activities against toxigenic fungi in dry-cured meat products, such as the yeast D. hansenii or the mould Penicillium chrysogenum [25,27,28,30]. In order to design these mixed protective cultures, it is crucial to understand the mode of action of each biocontrol agent and their plausible synergistic effect. Then, further studies must be carried out to establish the antifungal mechanism of S. xylosus Sx8 as well as other active GCC+ isolated from dry-cured meat products to optimise the appropriate timing and managing their application during the dry-cured ham processing, in order to successfully prevent hazard posed by mycotoxins.
In conclusion, S. xylosus Sx8 exhibits a broad antifungal activity against important toxigenic fungi commonly found on dry-cured ham. In addition, Sx8 provokes a significant decrease on mycotoxins accumulation in meat substrate. Then, the results of the present study suggest that this staphylococcus possesses great potential as a biological agent to control toxigenic moulds in dry-cured meat products.
Acknowledgments
The authors wish to thank Víctor Caballero for his technical assistance during the method development for the Orbitrap Q Exactive Plus analysis. The authors would like to thank Paula Rodrigues from the Polytechnic Institute of Bragança (Portugal) for providing the strain A. westerdijkiae 6B.
Author Contributions
The authors F.N. and M.R. conceived, proposed the idea and designed the study. E.C. F.J.G., E.B. and J.D. performed the experiments and analyzed the data. E.C., M.R. and F.N. writing—original draft preparation. E.C., E.B., J.D., M.R. and F.N. writing—review and editing. F.N. and M.R. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financed by the Spanish Ministry of Economy and Competitiveness, Government of Extremadura and FEDER (AGL2016-80209-P and GR18056).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Battilani P., Pietri A., Giorni P., Formenti S., Bertuzzi T., Toscani T., Virgili R., Kozakiewicz Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food Prot. 2007;70:975–980. doi: 10.4315/0362-028X-70.4.975. [DOI] [PubMed] [Google Scholar]
- 2.López-Díaz T.-M., Santos J.-A.A., García-López M.-L., Otero A. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium isolates. Int. J. Food Microbiol. 2001;68:69–74. doi: 10.1016/S0168-1605(01)00472-X. [DOI] [PubMed] [Google Scholar]
- 3.Núñez F., Rodríguez M.M., Bermúdez M.E., Córdoba J.J., Asensio M.A. Composition and toxigenic potential of the mould population on dry-cured Iberian ham. Int. J. Food Microbiol. 1996;32:185–197. doi: 10.1016/0168-1605(96)01126-9. [DOI] [PubMed] [Google Scholar]
- 4.Martín A., Córdoba J.J., Aranda E., Córdoba M.G., Asensio M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006;110:8–18. doi: 10.1016/j.ijfoodmicro.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 5.Martín A., Córdoba J.J., Núñez F., Benito M.J., Asensio M.A. Contribution of a selected fungal population to proteolysis on dry-cured ham. Int. J. Food Microbiol. 2004;94:55–66. doi: 10.1016/j.ijfoodmicro.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Núñez F., Westphal C.D., Bermúdez E., Asensio M.A. Production of secondary metabolites by some terverticillate penicillia on carbohydrate-rich and meat substrates. J. Food Prot. 2007;70:2829–2836. doi: 10.4315/0362-028X-70.12.2829. [DOI] [PubMed] [Google Scholar]
- 7.Bertuzzi T., Gualla A., Morlacchini M., Pietri A. Direct and indirect contamination with ochratoxin A of ripened pork products. Food Control. 2013;34:79–83. doi: 10.1016/j.foodcont.2013.04.011. [DOI] [Google Scholar]
- 8.Rodríguez A., Rodríguez M., Martín A., Delgado J., Córdoba J.J. Presence of ochratoxin A on the surface of dry-cured Iberian ham after initial fungal growth in the drying stage. Meat Sci. 2012;92:728–734. doi: 10.1016/j.meatsci.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Montero L., Córdoba J.J., Peromingo B., Álvarez M., Núñez F. Effects of environmental conditions and substrate on growth and ochratoxin A production by Penicillium verrucosum and Penicillium nordicum: Relative risk assessment of OTA in dry-cured meat products. Food Res. Int. 2019;121:604–611. doi: 10.1016/j.foodres.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Vipotnik Z., Rodríguez A., Rodrigues P. Aspergillus westerdijkiae as a major ochratoxin A risk in dry-cured ham based-media. Int. J. Food Microbiol. 2017;241:244–251. doi: 10.1016/j.ijfoodmicro.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Sonjak S., Ličen M., Frisvad J.C., Gunde-Cimerman N. Salting of dry-cured meat. A potential cause of contamination with the ochratoxin A-producing species Penicillium nordicum. Food Microbiol. 2011;28:1111–1116. doi: 10.1016/j.fm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Virgili R., Simoncini N., Toscani T., Camardo Leggieri M., Formenti S., Battilani P. Biocontrol of Penicillium nordicum growth and Ochratoxin A production by native yeasts of dry cured ham. Toxins. 2012;4:68–82. doi: 10.3390/toxins4020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostry V., Malir F., Toman J., Grosse Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017;33:65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- 14.Peromingo B., Rodríguez A., Bernáldez V., Delgado J., Rodríguez M. Effect of temperature and water activity on growth and aflatoxin production by Aspergillus flavus and Aspergillus parasiticus on cured meat model systems. Meat Sci. 2016;122:76–83. doi: 10.1016/j.meatsci.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Pleadin J., Malenica M., Vah N., Milone S., Safti L. Survey of aflatoxin B1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control. 2015;52:71–77. doi: 10.1016/j.foodcont.2014.12.027. [DOI] [Google Scholar]
- 16.Rodríguez A., Rodríguez M., Martín A., Nuñez F., Córdoba J.J. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control. 2012;27:118–126. doi: 10.1016/j.foodcont.2012.03.009. [DOI] [Google Scholar]
- 17.Rojas F.J., Jodral M., Gosalvez F., Pozo R. Mycoflora and toxigenic Aspergillus flavus in Spanish dry-cured ham. Int. J. Food Microbiol. 1991;13:249–256. doi: 10.1016/0168-1605(91)90082-Z. [DOI] [PubMed] [Google Scholar]
- 18.Peromingo B., Rodríguez M., Núñez F., Silva A., Rodríguez A. Sensitive determination of cyclopiazonic acid in dry-cured ham using a QuEChERS method and UHPLC–MS/MS. Food Chem. 2018;263:275–282. doi: 10.1016/j.foodchem.2018.04.126. [DOI] [PubMed] [Google Scholar]
- 19.Cvetnić Z., Pepeljnjak S. Aflatoxin-producing potential of Aspergillus flavus and Aspergillus parasiticus isolated from samples of smoked-dried meat. Food Nahrung. 1995;39:302–307. doi: 10.1002/food.19950390409. [DOI] [PubMed] [Google Scholar]
- 20.Markov K., Pleadin J., Bevardi M., Vahčić N., Sokolić-Mihalak D., Frece J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control. 2013;34:312–317. doi: 10.1016/j.foodcont.2013.05.002. [DOI] [Google Scholar]
- 21.IARC (International Agency for Research on Cancer) Chemical Agents and Related Occupations. A Review of Human Carcinogens. Volume 100 F. IARC; Lyon, France: 2012. Aflatoxins; pp. 225–248. [Google Scholar]
- 22.Alapont C., López-Mendoza M.C., Gil J.V., Martínez-Culebras P.V. Mycobiota and toxigenic Penicillium species on two Spanish dry-cured ham manufacturing plants. Food Addit. Contam. Part A. 2014;31:93–104. doi: 10.1080/19440049.2013.849007. [DOI] [PubMed] [Google Scholar]
- 23.Ostry V., Toman J., Grosse Y., Malir F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018;11:1–14. doi: 10.3920/WMJ2017.2243. [DOI] [Google Scholar]
- 24.Andrade M.J., Thorsen L., Rodríguez A., Córdoba J.J., Jespersen L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry-cured meat products. Int. J. Food Microbiol. 2014;170:70–77. doi: 10.1016/j.ijfoodmicro.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Cebrián E., Rodríguez M., Peromingo B., Bermúdez E., Núñez F. Efficacy of the combined protective cultures of Penicillium chrysogenum and Debaryomyces hansenii for the control of ochratoxin a hazard in dry-cured ham. Toxins. 2019;11:710. doi: 10.3390/toxins11120710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacumin L., Manzano M., Andyanto D., Comi G. Biocontrol of ochratoxigenic moulds (Aspergillus ochraceus and Penicillium nordicum) by Debaryomyces hansenii and Saccharomycopsis fibuligera during speck production. Food Microbiol. 2017;62:188–195. doi: 10.1016/j.fm.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Peromingo B., Núñez F., Rodríguez A., Alía A., Andrade M.J. Potential of yeasts isolated from dry-cured ham to control ochratoxin A production in meat models. Int. J. Food Microbiol. 2018;268:73–80. doi: 10.1016/j.ijfoodmicro.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Peromingo B., Andrade M.J., Delgado J., Sánchez-Montero L., Núñez F. Biocontrol of aflatoxigenic Aspergillus parasiticus by native Debaryomyces hansenii in dry-cured meat products. Food Microbiol. 2019;82:269–276. doi: 10.1016/j.fm.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Bernáldez V., Rodríguez A., Martín A., Lozano D., Córdoba J.J. Development of a multiplex qPCR method for simultaneous quantification in dry-cured ham of an antifungal-peptide Penicillium chrysogenum strain used as protective culture and aflatoxin-producing moulds. Food Control. 2014;36:257–265. doi: 10.1016/j.foodcont.2013.08.020. [DOI] [Google Scholar]
- 30.Delgado J., Peromingo B., Rodríguez A., Rodríguez M. Biocontrol of Penicillium griseofulvum to reduce cyclopiazonic acid contamination in dry-fermented sausages. Int. J. Food Microbiol. 2019;293:1–6. doi: 10.1016/j.ijfoodmicro.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez A., Bernáldez V., Rodríguez M., Andrade M.J., Núñez F., Córdoba J.J. Effect of selected protective cultures on ochratoxin A accumulation in dry-cured Iberian ham during its ripening process. LWT-Food Sci. Technol. 2015;60:923–928. doi: 10.1016/j.lwt.2014.09.059. [DOI] [Google Scholar]
- 32.Álvarez M., Rodríguez A., Peromingo B., Núñez F., Rodríguez M. Enterococcus faecium: A promising protective culture to control growth of ochratoxigenic moulds and mycotoxin production in dry-fermented sausages. Mycol. Res. 2020;36:137–145. doi: 10.1007/s12550-019-00376-6. [DOI] [PubMed] [Google Scholar]
- 33.Delgado J., Rodríguez A., García A., Núñez F., Asensio M. Inhibitory effect of PgAFP and protective cultures on Aspergillus parasiticus growth and aflatoxins production on dry-fermented sausage and cheese. Microorganisms. 2018;6:69. doi: 10.3390/microorganisms6030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez M., Núñez F., Córdoba J.J., Bermúdez E., Asensio M.A. Gram-positive, catalase-positive cocci from dry cured Iberian ham and their enterotoxigenic potential. Appl. Environ. Microbiol. 1996;62:1897–1902. doi: 10.1128/AEM.62.6.1897-1902.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leroy S., Vermassen A., Ras G., Talon R. Insight into the genome of Staphylococcus xylosus, a ubiquitous species well adapted to meat products. Microorganisms. 2017;5:52. doi: 10.3390/microorganisms5030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martín B., Garriga M., Hugas M., Bover-Cid S., Veciana-Nogués M.T., Aymerich T. Molecular, technological and safety characterization of Gram-positive catalase-positive cocci from slightly fermented sausages. Int. J. Food Microbiol. 2006;107:148–158. doi: 10.1016/j.ijfoodmicro.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez M., Núñez F., Córdoba J.J., Sanabria C., Bermúdez E., Asensio M.A. Characterization of Staphylococcus spp. and Micrococcus spp. isolated from Iberian ham throughout the ripening process. Int. J. Food Microbiol. 1994;24:329–335. doi: 10.1016/0168-1605(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 38.Fiorentini Â.M., Sawitzki M.C., Bertol T.M., Sant’Anna E.S. Viability of Staphylococcus xylosus isolated from artisanal sausages for application as starter cultures in meat products. Braz. J. Microbiol. 2009:129–133. doi: 10.1590/S1517-83822009000100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonová M., Strompfová V., Marciňáková M., Lauková A., Vesterlund S., Moratalla M.L., Bover-Cid S., Vidal-Carou C. Characterization of Staphylococcus xylosus and Staphylococcus carnosus isolated from Slovak meat products. Meat Sci. 2006;73:559–564. doi: 10.1016/j.meatsci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Vermassen A., de la Foye A., Loux V., Talon R., Leroy S. Transcriptomic analysis of Staphylococcus xylosus in the presence of nitrate and nitrite in meat reveals its response to nitrosative stress. Front. Microbiol. 2014;5:1–15. doi: 10.3389/fmicb.2014.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbiere Morot-Bizot S., Leroy S., Talon R. Monitoring of staphylococcal starters in two French processing plants manufacturing dry fermented sausages. J. Appl. Microbiol. 2007;102:238–244. doi: 10.1111/j.1365-2672.2006.03041.x. [DOI] [PubMed] [Google Scholar]
- 42.Kamala A., Ortiz J., Kimanya M., Haesaert G., Donoso S., Tiisekwa B., De Meulenaer B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control. 2015;54:208–215. doi: 10.1016/j.foodcont.2015.02.002. [DOI] [Google Scholar]
- 43.Sánchez-Montero L., Córdoba J.J., Alía A., Peromingo B., Núñez F. Effect of Spanish smoked paprika “Pimentón de La Vera” on control of ochratoxin A and aflatoxins production on a dry-cured meat model system. Int. J. Food Microbiol. 2019;308:108303. doi: 10.1016/j.ijfoodmicro.2019.108303. [DOI] [PubMed] [Google Scholar]
- 44.Núñez F., Lara M.S., Peromingo B., Delgado J., Sánchez-Montero L., Andrade M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015;46:114–120. doi: 10.1016/j.fm.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Bernáldez V., Córdoba J.J., Rodríguez M., Cordero M., Polo L., Rodríguez A. Effect of Penicillium nalgiovense as protective culture in processing of dry-fermented sausage “salchichón”. Food Control. 2013;32:69–76. doi: 10.1016/j.foodcont.2012.11.018. [DOI] [Google Scholar]
- 46.dos Cruxen C.E.S., Funck G.D., da Dannenberg G.S., Haubert L., de Marques J.L., Kroning I.S., Chaves F.C., da Silva W.P., Fiorentini Â.M. Characterization of Staphylococcus xylosus LQ3 and its application in dried cured sausage. LWT-Food Sci. Technol. 2017;86:538–543. doi: 10.1016/j.lwt.2017.08.045. [DOI] [Google Scholar]
- 47.Olesen P.T., Meyer A.S., Stahnke L.H. Generation of flavour compounds in fermented sausages-the influence of curing ingredients, Staphylococcus starter culture and ripening time. Meat Sci. 2004;66:675–687. doi: 10.1016/S0309-1740(03)00189-X. [DOI] [PubMed] [Google Scholar]
- 48.Gong A.D., Sun G.J., Zhao Z.Y., Liao Y.C., Zhang J.B. Staphylococcus saprophyticus L-38 produces volatile 3,3-dimethyl-1,2-epoxybutane with strong inhibitory activity against Aspergillus flavus germination and aflatoxin production. World Mycotoxin J. 2019:1–12. doi: 10.3920/WMJ2019.2495. [DOI] [Google Scholar]
- 49.Alijani Z., Amini J., Ashengroph M., Bahramnejad B. Antifungal activity of volatile compounds produced by Staphylococcus sciuri strain MarR44 and its potential for the biocontrol of Colletotrichum nymphaeae, causal agent strawberry anthracnose. Int. J. Food Microbiol. 2019;307:108276. doi: 10.1016/j.ijfoodmicro.2019.108276. [DOI] [PubMed] [Google Scholar]
- 50.Asensio M.A., Núñez F., Delgado J., Bermúdez E. Control of toxigenic molds in food processing. In: Rai V.R., Bai A.J., editors. Microbial Food Safety and Preservation Techniques. CRC Press (Taylor and Francis); Boca Raton, FL, USA: 2014. pp. 329–357. [Google Scholar]
- 51.Dalié D.K.D., Deschamps A.M., Richard-Forget F. Lactic acid bacteria-Potential for control of mould growth and mycotoxins: A review. Food Control. 2010;21:370–380. doi: 10.1016/j.foodcont.2009.07.011. [DOI] [Google Scholar]
- 52.Delgado J., Acosta R., Rodríguez-Martín A., Bermúdez E., Núñez F., Asensio M.A. Growth inhibition and stability of PgAFP from Penicillium chrysogenum against fungi common on dry-ripened meat products. Int. J. Food Microbiol. 2015;205:23–29. doi: 10.1016/j.ijfoodmicro.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 53.Álvarez M., Núñez F., Delgado J., Andrade M.J., Rodríguez M., Rodríguez A. Competitiveness of three biocontrol candidates against ochratoxigenic Penicillium nordicum under dry-cured meat environmental and nutritional conditions. Fungal Biol. 2020 doi: 10.1016/j.funbio.2020.03.006. in press. [DOI] [PubMed] [Google Scholar]
- 54.Delgado J., Núñez F., Asensio M.A., Owens R.A. Quantitative proteomics of Penicillium nordicum profiles and ochratoxin A repression by protective cultures. Int. J. Food Microbiol. 2019;305:108243. doi: 10.1016/j.ijfoodmicro.2019.108243. [DOI] [PubMed] [Google Scholar]
- 55.Rodríguez A., Medina Á., Córdoba J.J., Magan N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014;178:113–119. doi: 10.1016/j.ijfoodmicro.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Meftah S., Abid S., Dias T., Rodrigues P. Mechanisms underlying the effect of commercial starter cultures and a native yeast on ochratoxin A production in meat products. LWT. 2020;117:108611. doi: 10.1016/j.lwt.2019.108611. [DOI] [Google Scholar]
- 57.Luz C., Ferrer J., Mañes J., Meca G. Toxicity reduction of ochratoxin A by lactic acid bacteria. Food Chem. Toxicol. 2018;112:60–66. doi: 10.1016/j.fct.2017.12.030. [DOI] [PubMed] [Google Scholar]