Abstract.
Hookworm infections are classified as the most impactful of the human soil-transmitted helminth (STH) infections, causing a disease burden of ∼4 million disability-adjusted life years, with a global prevalence of 406–480 million infections. Until a decade ago, epidemiological surveys largely assumed Necator americanus and Ancylostoma duodenale as the relevant human hookworm species implicated as contributing to iron-deficiency anemia. This assumption was based on the indistinguishable morphology of the Ancylostoma spp. eggs in stool and the absence of awareness of a third zoonotic hookworm species, Ancylostoma ceylanicum. The expanded use of molecular diagnostic assays for differentiating hookworm species infections during STH surveys has now implicated A. ceylanicum, a predominant hookworm of dogs in Asia, as the second most common hookworm species infecting humans in Southeast Asia and the Pacific. Despite this, with the exception of sporadic case reports, there is a paucity of data available on the impact of this emerging zoonosis on human health at a population level. This situation also challenges the current paradigm, necessitating a One Health approach to hookworm control in populations in which this zoonosis is endemic. Here, we have summarized the available research studies and case reports on human A. ceylanicum infections in Southeast Asia and the Pacific after 2013 using a systematic review approach. We summarized eight research articles and five clinical case studies, highlighting the importance of future in-depth investigation of zoonotic A. ceylanicum infections using sensitive and cost-effective diagnostic tools.
Hookworm infections in humans occur predominantly in countries with low socioeconomic status located in tropical and subtropical areas of the world.1 Symptomology is measured in disability-adjusted life years (DALYs), which include the years a human host has lived with the infection (years lost due to disability (YLD)) and the years that are lost because of early death (years of life lost (YLL)).2 The global infection prevalence for any hookworm in 2016 was around 450.68 million (1,297 million in 1994),3 with a disease burden of around 1.8 million DALYs in 2015.4,5 Primary symptoms associated with infection include iron-deficiency anemia caused by intestinal blood loss within the small intestine,6,7 which particularly impacts pregnant women8 and children.9 For hookworms, as well as other soil-transmitted helminths (STHs), disease burden has decreased significantly over the last two decades.3 This decrease can be attributed principally to targeted mass drug administration (preventive chemotherapy) in populations at risk of infection10–12 and integrated intervention programs targeting improved access to safe water, sanitation, and hygiene13,14 and development of molecular high-sensitivity diagnostic methods (improved infection prevalence estimation)15 and socioeconomic development.16 The WHO-recommended and -approved diagnostic tool for STH detection is the Kato–Katz thick smear.17 However, this microscopy-based diagnostic method has important limitations: innately low diagnostic sensitivity in regard to hookworm detection,18 the requirement for rapid sample screening to avoid over-clearance of hookworm eggs following slide preparation,19 and the inability of microscopy-based diagnostics to identify human hookworms to a species level.20 These factors together suggest an underestimation of the true species-specific prevalence and intensity of human hookworms.
Hookworm infections in humans have largely been attributed to Necator americanus and Ancylostoma duodenale.6 However, contrary to this belief, recent molecular studies have unequivocally demonstrated that Ancylostoma ceylanicum is highly endemic, comprising the second most common species of hookworm, after N. americanus, in many parts of Southeast Asia and the Pacific and estimated to infect ∼100 million people.21 This situation challenges the current hookworm paradigm. Despite the global efforts to meet the WHOs 2020 road map for eliminating morbidity associated with STH infections, A. ceylanicum is not included as a causal agent of human hookworm infection and therefore not included within this WHO framework.11,22 This is despite a growing body of evidence to demonstrate that its prevalence in certain restricted areas or countries outcompetes that of A. duodenale.23
Importantly, A. ceylanicum is a zoonosis and transmissible to humans from animals. This species comprises the predominant hookworm of dogs and cats throughout Southeast Asia and the Pacific, which act as reservoirs for human infections.21,24,25 This situation contrasts that of N. americanus and A. duodenale, which are both specific to humans. In 2013, a review by Traub highlighted the history of the reported distribution and overlooked public health significance and the impact of A. ceylanicum infections in humans in Southeast Asia and the Pacific.21 The review hypothesizes that mass anthelmintic programs may contribute to the re-emergence of A. ceylanicum infections in humans unless a One Health approach toward its control is undertaken.21 In this review, we aim at summarizing and updating information spanning the 6 years since the last major review by Traub, on the prevalence, distribution, and impacts of A. ceylanicum on human health.
Here, we conducted a systematic review, searching the publicly available database Google Scholar on June 14, 2019 with no restrictions in terms of scientific journal or author for publications in English only. We limited the output to novel original research articles published from 2013 to 2019 using the following search term in the title: “Ancylostoma ceylanicum.” Publication titles and abstracts of 70 peer-reviewed articles were screened and relevant articles included in this systematic review, as well as two other known case reports. 26,35 Review articles were excluded, with only original research articles included within the context of this mini-review. A list of all included publications is found in Tables 1 and 2.
Table 1.
Summary of publicly available data on the proportion of hookworm infections attributed to A. ceylanicum in humans worldwide published between 2013 and 2019
| Author and year | Reference number | Study country | Diagnostic tools used | Proportion of hookworm-positive samples | Type of infection |
|---|---|---|---|---|---|
| Koehler et al., 2013 | 26 | Australia | PCR-coupled SSCP | 18.2% (2/11) | Mono-infection |
| Phosuk et al., 2013 | 27 | Thailand | Agar plate culture + PCR | 10% (3/30) | Mono-infection |
| Inpankaew et al., 2014 | 25 | Cambodia | PCR + Sanger sequencing for Ancylostoma spp. | 46.0% (57/124) | Mono-infection |
| 3.2% (4/124) | N. americanus/A. ceylanicum | ||||
| 1.6% (2/124) | A. duodenale/A. ceylanicum | ||||
| 0.8% (1/124) | N. americanus/A. duodenale/A. ceylanicum | ||||
| George et al., 2015 | 28 | India | Semi-nested PCR-RFLP | 4.9% (2/41) | Mono-infection |
| Aung et al., 2017 | 29 | Myanmar | PCR | 27.3% (3/11) | Mono-infection |
| Bradbury et al., 2017 | 30 | Solomon Islands | Kato–Katz + PCR | 16.7% (11/66) | Mono-infection |
| 1.5% (1/66) | N. americanus/A. ceylanicum | ||||
| Papaiakovou et al., 2017 | 31 | Timor-Leste | Semi-nested PCR-RFLP | 95.5% (21/22) | Ancylostoma spp. |
| Argentina | PCR | 0% (0/8) | Ancylostoma spp. | ||
| O’ Connell et al., 2018 | 32 | Thailand (Myanmar refugees) | Semi-nested PCR-RFLP, qPCR | 17.4% (83/476 baseline) 4.7% (6/128, follow-up) 0% (0/29, follow-up) | – |
A. ceylanicum = Ancylostoma ceylanicum; N. americanus = Necator americanus. Systematic review findings are summarized chronologically.
Table 2.
Summary of A. ceylanicum–positive clinical cases from 2013 to 2018
| Yoshikawa et al.34 | Brunet et al.35 | Speare et al.36 | Kaya et al.37 | Ngui et al.38 | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years), gender | 25, male | 47, male | 26, male | 26, male | 33, male | 26, male | 47, male | 58, female |
| Country | Malaysia | Laos | India | Papua New Guinea | Myanmar | Solomon Islands | Thailand/Laos | Malaysia |
| Tourist | Yes | Yes | No | Yes | No | |||
| Symptoms | Abdominal pain, watery diarrhea | Asymptomatic | Fever, weight loss, dyspnea, abdominal pain, bloody diarrhea | Abdominal pain, peripheral eosinophilia | Intermittent diarrhea, extreme eosinophilia, fever, abdominal pain, watery diarrhea, vomiting | Melena, discolored stool, dizziness, tightness of chest, cold sweats | ||
| Eosinophil count/μL (range, 30–500 or < 6%) | 3,000 | 20,470 | 7,050 | 1,570 | 59% | 6.20 × 109/L | 43%, 74% | 1% |
| Anemia | No | No | No | No | No | No | No | Yes |
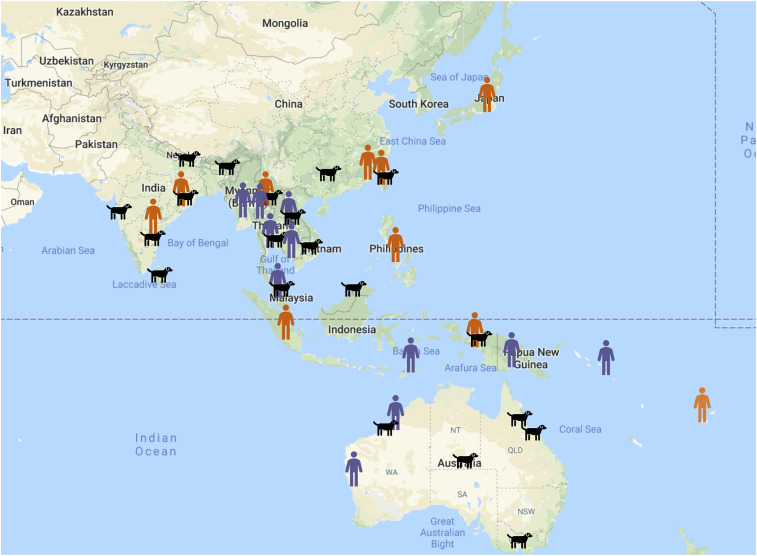
Since 2013, A. ceylanicum infections in humans continue to be identified across Southeast Asia and the Pacific, including Thailand, Myanmar, India, Cambodia, and the Solomon Islands (Table 1, Figure 1). The proportion of A. ceylanicum infections among all hookworm-positive samples was relatively high in some regions (16.7–46.0%). Five of 13 publications describe individual cases of returning travelers or personnel from Southeast Asia and the Pacific, highlighting the relevance of this emerging zoonosis to the field of travel medicine (Table 2). Most of the aforementioned patients had a severely increased eosinophil count, which is a known immune response to hookworm infections.33 Symptoms ranged from abdominal pain to weight loss, fever, diarrhea, vomiting, and other generalized symptoms (dizziness, tightness of chest, and sweats). Interestingly, only one of these clinical cases presented with anemia (Table 2), which is likely due to the general health status of travelers from developed countries compared with that of the local community. Although these were sporadic case reports from developed countries, there is no attention to ascertain morbidity caused on an endemic population scale. Moreover, these “rare” singular occurrence findings highlight the neglect of other symptomatic or subclinical cases in the country of origin.
Figure 1.
Known distribution of Ancylostoma ceylanicum infections in Southeast Asia and the Pacific. Human infections from 2013 or earlier are depicted in orange, human infections after 2013 to date are depicted in purple, and all canine infections are highlighted in black. Not depicted here are studies of canine A. ceylanicum infections from Madagascar, Kenya, Tanzania, and South Africa.
The inconsistency in diagnostic tools used to estimate infection prevalence may additionally contribute to this underestimation.39 In the context of some studies that used a traditional microscopy-based gold standard diagnosis tool, only hookworm-positive samples were further investigated for species-specific infections using molecular tools.25,30 Not only does this lead to an underestimation of overall hookworm prevalence,40 but it also underestimates the proportion of A. ceylanicum infections even further,25 owing to the relatively lower egg-shedding intensities of A. ceylanicum–infected individuals than those infected with N. americanus.41
Despite the increased availability and application of molecular diagnostic assays,40 there remains a lack of large-scale mapping of A. ceylanicum in areas other than Southeast Asia and the Pacific, with a clear lack of available data in regard to infection prevalence and intensity, particularly in human populations. This lack of knowledge is partly due to the application of microscopy-based diagnostic tools that are not able to differentiate between hookworm species.42 Apart from Southeast Asia and the Pacific, A. ceylanicum has also been reported in 3% of canines in Tanzania.43 To date, the global distribution of cases in humans reflects that of its distribution in dogs. During the previous 6 years after publication of Traub’s 2013 review, the focus of STH research has been on molecular biological areas such as genomics, transcriptomics, and diagnostics.44 At the same time, diagnostic tool application is shifting from invasive endoscopy38 to molecular laboratory–based techniques such as semi-nested polymerase chain reaction (PCR)-RFLP, quantitative PCR (qPCR), and multiplexed-tandem qPCR.28,45 Moving toward these advanced molecular biology–based diagnostic tools using PCR systems can prove beneficial in increasing general knowledge of hookworm infection prevalence, the species-specific burden they cause and examining effects of coinfections within hosts.46 Currently, however, novel diagnostic techniques are limited to research settings and still need to be completely transferable to the endemic field in regard to resources, expertise, and simple feasibility in remote settings.47
As evidently shown within the literature, A. ceylanicum continues to present itself as an increasingly emergent zoonosis, with a potentially wider than previously assumed geographical distribution. For the majority of countries, there is a paucity of epidemiological information available, which would serve as the basis for monitoring and diagnosing infections with this zoonosis for both clinical and population settings. Of the 13 epidemiological studies on Ancylostoma ceylanicum retrieved from Google Scholar (since 2013) (70 total hits), nearly all used molecular-based screening to further identify hookworms to a species level. Traub hypothesized the emergence of A. ceylanicum owing to multiple factors, including wide-scale mass deworming programs, resulting in the disproportional increase in infective larvae in the environment compared with that of N. americanus larvae, exacerbated by the lack of an “allergy-based” elimination of any new incoming larvae of “unnatural” hookworms. If this is due to differential benzimidazole drug efficacy for different hookworm species remains unclear.
There is a clear need for further in-depth investigation of A. ceylanicum infection prevalence, intensity, and morbidity data in human hosts worldwide. These data will aid in mapping a more detailed picture of STH infections, inform governmental agencies about targeted treatment programs (including One Health approaches), and ultimately contribute to an eradication of not only hookworm infections but also helminth infections more broadly.
REFERENCES
- 1.Jourdan PM, Lamberton PH, Fenwick A, Addiss DG, 2017. Soil-transmitted helminth infections. Lancet 391: 252–265. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990—2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 3.Chan M, Medley G, Jamison D, Bundy D, 1994. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology 109: 373–387. [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ, Fenwick A, Ray SE, Hay SI, Molyneux DH, 2018. “Rapid impact” 10 years after: the first “decade”(2006–2016) of integrated neglected tropical disease control. PLoS Negl Trop Dis 12: e0006137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, Casey DC, Charlson FJ, Coates MM, Coggeshall M, 2016. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S, 2004. Hookworm infection. N Engl J Med 351: 799–807. [DOI] [PubMed] [Google Scholar]
- 7.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ, 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 8.Brooker S, Hotez PJ, Bundy DA, 2008. Hookworm-related anaemia among pregnant women: a systematic review. PLoS Negl Trop Dis 2: e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crompton DWT, 2000. The public health importance of hookworm disease. Parasitology 121: S39–S50. [DOI] [PubMed] [Google Scholar]
- 10.Albonico M, Allen H, Chitsulo L, Engels D, Gabrielli A-F, Savioli L, 2008. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl Trop Dis 2: e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization , 2012. Soil-Transmitted Helminthiasis: Eliminating Soil-Transmitted Helminthiasis as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020. Geneva, Switzerland: WHO. [Google Scholar]
- 12.World Health Organization , 2017. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 13.Clarke NE, Clements AC, Amaral S, Richardson A, McCarthy JS, McGown J, Bryan S, Gray DJ, Nery SV, 2018. (S) WASH-D for Worms: a pilot study investigating the differential impact of school-versus community-based integrated control programs for soil-transmitted helminths. PLoS Negl Trop Dis 12: e0006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC, 2014. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med 11: e1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connell EM, Nutman TB, 2016. Molecular diagnostics for soil-transmitted helminths. Am J Trop Med Hyg 95: 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L, 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization , 2011. Helminth Control in School-Age Children: a Guide for Managers of Control Programmes. Geneva, Switzerland: WHO. [Google Scholar]
- 18.Tarafder MR, Carabin H, Joseph L, Balolong E, Jr., Olveda R, McGarvey ST, 2010. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘gold standard’. Int J Parasitol 40: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin LK, Beaver PC, 1968. Evaluation of Kato thick-smear technique for quantitative diagnosis of helminth infections. Am J Trop Med Hyg 17: 382–391. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Sanguankiat S, Yoonuan T, Pongvongsa T, Keomoungkhoun M, Phimmayoi I, Boupa B, Moji K, Waikagul J, 2010. Copro-molecular identification of infections with hookworm eggs in rural Lao PDR. Trans R Soc Trop Med Hyg 104: 617–622. [DOI] [PubMed] [Google Scholar]
- 21.Traub RJ, 2013. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol 43: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 22.Crompton DWT, 2006. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: WHO. [Google Scholar]
- 23.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, Piyaraj P, Naaglor T, Taamasri P, Leelayoova S, 2011. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg 84: 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng-Nguyen D, Stevenson MA, Dorny P, Gabriël S, Van Vo T, Nguyen V-AT, Van Phan T, Hii SF, Traub RJ, 2017. Comparison of a new multiplex real-time PCR with the Kato Katz thick smear and copro-antigen ELISA for the detection and differentiation of Taenia spp. in human stools. PLoS Negl Trop Dis 11: e0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S, Odermatt P, 2014. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis 20: 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koehler AV, Bradbury RS, Stevens MA, Haydon SR, Jex AR, Gasser RB, 2013. Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning‐coupled approach. Electrophoresis 34: 1720–1728. [DOI] [PubMed] [Google Scholar]
- 27.Phosuk I, Intapan PM, Thanchomnang T, Sanpool O, Janwan P, Laummaunwai P, Aamnart W, Morakote N, Maleewong W, 2013. Molecular detection of Ancylostoma duodenale, Ancylostoma ceylanicum, and Necator americanus in humans in northeastern and southern Thailand. Korean J Parasitol 51: 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George S, Kaliappan SP, Kattula D, Roy S, Geldhof P, Kang G, Vercruysse J, Levecke B, 2015. Identification of Ancylostoma ceylanicum in children from a tribal community in Tamil Nadu, India using a semi-nested PCR-RFLP tool. Trans R Soc Trop Med Hyg 109: 283–285. [DOI] [PubMed] [Google Scholar]
- 29.Aung WPP, Htoon TT, Tin HH, Sanpool O, Jongthawin J, Sadaow L, Phosuk I, Ropai R, Intapan PM, Maleewong W, 2017. First molecular identifications of Necator americanus and Ancylostoma ceylanicum infecting rural communities in lower Myanmar. Am J Trop Med Hyg 96: 214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradbury RS, Hii SF, Harrington H, Speare R, Traub R, 2017. Ancylostoma ceylanicum hookworm in the Solomon Islands. Emerg Infect Dis 23: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papaiakovou M, Pilotte N, Grant JR, Traub RJ, Llewellyn S, McCarthy JS, Krolewiecki AJ, Cimino R, Mejia R, Williams SA, 2017. A novel, species-specific, real-time PCR assay for the detection of the emerging zoonotic parasite Ancylostoma ceylanicum in human stool. PLoS Negl Trop Dis 11: e0005734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell EM, Mitchell T, Papaiakovou M, Pilotte N, Lee D, Weinberg M, Sakulrak P, Tongsukh D, Oduro-Boateng G, Harrison S, 2018. Ancylostoma ceylanicum hookworm in Myanmar refugees, Thailand, 2012–2015. Emerg Infect Dis 24: 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loukas A, Prociv P, 2001. Immune responses in hookworm infections. Clin Microbiol Rev 14: 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshikawa M, Ouji Y, Hirai N, Nakamura-Uchiyama F, Yamada M, Arizono N, Akamatsu N, Yoh T, Kaya D, Nakatani T, 2018. Ancylostoma ceylanicum, novel etiological agent for traveler’s diarrhea–report of four Japanese patients who returned from southeast Asia and Papua New Guinea. Trop Medicine Health 46: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunet J, Lemoine J-P, Lefebvre N, Denis J, Pfaff AW, Abou-Bacar A, Traub RJ, Pesson B, Candolfi E, 2015. Bloody diarrhea associated with hookworm infection in traveler returning to France from Myanmar. Emerg Infect Dis 21: 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speare R, Bradbury RS, Croese J, 2016. A case of Ancylostoma ceylanicum infection occurring in an Australian soldier returned from Solomon Islands. Korean J Parasitol 54: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaya D, Yoshikawa M, Nakatani T, Tomo-Oka F, Fujimoto Y, Ishida K, Fujinaga Y, Aihara Y, Nagamatsu S, Matsuo E, 2016. Ancylostoma ceylanicum hookworm infection in Japanese traveler who presented chronic diarrhea after return from Lao People’s Democratic Republic. Parasitol Int 65: 737–740. [DOI] [PubMed] [Google Scholar]
- 38.Ngui R, Lim YA, Ismail WHW, Lim KN, Mahmud R, 2014. Zoonotic Ancylostoma ceylanicum infection detected by endoscopy. Am J Trop Med Hyg 91: 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergquist R, Johansen MV, Utzinger J, 2009. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol 25: 151–156. [DOI] [PubMed] [Google Scholar]
- 40.Hii SF, Senevirathna D, Llewellyn S, Inpankaew T, Odermatt P, Khieu V, Muth S, McCarthy J, Traub RJ, 2018. Development and evaluation of a multiplex quantitative real-time PCR for hookworm species in human stool. Am J Trop Med Hyg 99: 1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M, 2003. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ 81: 343–352. [PMC free article] [PubMed] [Google Scholar]
- 42.Chidambaram M, Parija SC, Toi PC, Mandal J, Sankaramoorthy D, George S, Natarajan M, Padukone S, 2017. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol 7: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merino-Tejedor A, Nejsum P, Mkupasi E, Johansen M, Olsen A, 2019. Molecular identification of zoonotic hookworm species in dog faeces from Tanzania. J Helminthol 93: 313–318. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz EM, Hu Y, Antoshechkin I, Miller MM, Sternberg PW, Aroian RV, 2015. The genome and transcriptome of the zoonotic hookworm Ancylostoma ceylanicum identify infection-specific gene families. Nat Genet 47: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stracke K, Clarke N, Awburn CV, Nery SV, Khieu V, Traub RJ, Jex AR, 2019. Development and validation of a multiplexed-tandem qPCR tool for diagnostics of human soil-transmitted helminth infections. PLoS Negl Trop Dis 13: e0007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements AC, Gomes SJ, Traub R, McCarthy JS, 2016. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis 10: e0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon CA, Gray DJ, Gobert GN, McManus DP, 2011. DNA amplification approaches for the diagnosis of key parasitic helminth infections of humans. Mol Cell Probes 25: 143–152. [DOI] [PubMed] [Google Scholar]