Abstract.
Visceral leishmaniasis (VL) is endemic in Asia, East and North Africa, South America, and Southern Europe, and is a major public health problem in the Indian subcontinent. Miltefosine received approval in 2002 to treat VL in India, and the Indian National Vector Borne Disease Control Programme later adopted a single dose (10 mg/kg) of liposomal amphotericin B. We report results of a randomized trial comparing the efficacy of combination therapy with an Indian preparation of liposomal amphotericin B (single dose of 7.5 mg/kg) and short-course miltefosine (2.5 mg/kg/day for 14 days; n = 66) in comparison to miltefosine monotherapy (2.5 mg/kg/day for 28 days; n = 78). Nine patients in the miltefosine group and three in the combination therapy group had to discontinue therapy because of serious adverse events. At the end of the therapy, the clinical and parasitological cure rate was 100% in both groups. By per-protocol analysis, by 6 months after completion of treatment, 12 of 69 patients in the miltefosine monotherapy arm (17.4%, 95% CI: 10.24–28%) and none in the combination therapy arm had relapse. Over 5 years of follow-up, 10 patients in the miltefosine monotherapy arm (all within 0.5–2 years after completing therapy) and none in the combination therapy arm experienced post–kala-azar dermal leishmaniasis. Combination therapy offered benefits over miltefosine monotherapy for VL in India.
INTRODUCTION
Visceral leishmaniasis (VL, also known as kala-azar) is endemic in Asia, East and North Africa, South America, and Southern Europe, and is a major public health problem in the Indian subcontinent. Worldwide, 200,000–400,000 new cases of VL occur annually,1 and most of these cases occur in the Indian subcontinent, with Bihar and West Bengal being the worst affected areas. The Indian National Vector Borne Disease Control Programme recommendation for treatment of VL is liposomal amphotericin B (AmBisome, Gilead Sciences, Foster City, CA) single dose 10 mg/kg with options for other modalities like miltefosine, paromomycin, and combination therapy. Oral miltefosine has a high cure rate, up to 94% in a phase III trial,2 and was considered a game changer in the VL control strategy. Miltefosine received approval in 2002 to treat VL in India, but its successful use is problematic because of poor compliance owing to the long treatment course (28 days), possible teratogenic effect, and prolonged half-life with resultant high resistance potential.3,4 Although most VL trials show good efficacy over a short period of time (usually 6 months), long-term outcome is rarely reported. Prevention of development of post–kala-azar dermal leishmaniasis (PKDL), an important outcome measure, is often overlooked in VL clinical trials.
Combination therapy is a natural choice due to the possible additive action of two drugs in lower doses with different mechanisms of action resulting in increased efficacy, reduced toxicity, short course, and improved compliance and possible prevention of PKDL. Three short-course combination regimens including AmBisome, miltefosine, and paromomycin have been evaluated in a phase III clinical trial conducted in India (2008–2010), with excellent safety and efficacy profiles.5 Although high cure rates (> 90%) were described for each of the combinations, the follow-up duration was only 6 months. No information on PKDL was available.
A liposomal amphotericin B preparation, developed in India and commercially available as Fungisome™ (Lifecare Innovations Ltd., Gurgaon, Haryana, India), has been used for the treatment of VL. Fungisome is somewhat different chemically from AmBisome in its composition. Each milligram of amphotericin B in Fungisome is encapsulated in liposomes composed of phosphatidylcholine and cholesterol suspended in 1 mL of physiological saline. Fungisome requires sonication for 45 minutes before administration to transform multi-lamellar vesicles into small unilamellar vesicles. Like AmBisome, Fungisome is also an intravenous infusion (normal saline in contrast to 5% dextrose for AmBisome), and previous experience demonstrated a total dose of 15–21 mg/kg shows an efficacy of 90.9–100% against stibogluconate responsive and unresponsive cases of VL.6 We previously used Fungisome in varying doses and demonstrated that a total of 10 or 15 mg/kg body weight given over two consecutive days provide 90% and 100% definitive cure rates, respectively.7,8 Sundar et al.9 demonstrated the safety of a single dose of Fungisome as high as 15 mg/kg with a definitive cure rate of 93.3% at the 6-month follow-up of Indian VL.
The present study evaluated single-dose Fungisome in combination with a short course of miltefosine, with long-term follow-up, to demonstrate the safety and efficacy of the combination therapy and potential for prevention of the development of PKDL.
MATERIALS AND METHODS
This was an open-label, parallel-group, randomized, controlled trial based on a non-inferiority design. The present study was conducted at the Calcutta School of Tropical Medicine, a tertiary care referral center for VL catering to the states of West Bengal and Bihar, India. The patients were screened and recruited into the study from 2008 to 2012 and followed up regularly till 2018 (at an interval of 3 months for the first year and then biannually for 5 years).
Patients of both genders aged between 5 and 65 years with a corroborative clinical history (patients from endemic areas with a prolonged fever not responding to antimalarials or antibiotics), physical signs (anemia, splenomegaly, and hepatomegaly), and presence of parasites confirmed by examination of Giemsa-stained slides of splenic or bone marrow aspirates were enrolled into the study.
The following groups of patients were excluded: HIV-positive individuals, infants and children with body weight < 10 Kgs, patients with severe concurrent illnesses, patients who received any antileishmanial drugs or antifungal drugs in the previous 45 days, pregnant patients, and those with withdrawal of contraceptive measures. We also excluded patients with known hypersensitivity to the study drugs and those with diabetes, hypertension, tuberculosis, and heart, liver, or kidney disease.
The protocol was approved by the Institutional Clinical Research Ethics Committee of the Calcutta School of Tropical Medicine. The protocol was designed and completed in accordance with the general ethical principles outlined in the Declaration of Helsinki, 2000, and the International Conference on Harmonization guidelines for good clinical practice. Informed consent was obtained from all participants. The trial is registered with the Clinical Trials Registry—India with registration number CTRI/2016/08/007190.
Randomization and masking.
A computer-generated, randomization code was generated. Enrolled patients were randomly assigned to treatment with miltefosine or with the combination chemotherapy in a 1:1 ratio. Microscopists were masked to the treatment given.
Procedures.
Patients were screened by clinical examination and rk39 immunochromatographic strip test (InBios International, Seattle, WA). Confirmation of VL was performed by Giemsa-stained slides of spleen or bone marrow aspirates with demonstration of Leishman-Donovan (LD) bodies. These invasive tests are routinely performed in our institution under expert trained guidance. The density of parasites was graded on a log scale from 0 (no parasite per 1,000 high power fields [HPF]) to 6 (> 100 parasites per HPF). Eligible patients were then informed of the study procedures and were asked for voluntary consent. For children, consent of parents was obtained. Patients who met the entry criteria and provided voluntary informed written consent were then randomly assigned to treatment arms after completion of baseline evaluations (weight, liver and spleen size, routine hematology, and blood biochemistry), which did not take more than a week for any patient. Electrocardiograms and chest radiography were performed whenever deemed necessary.
Treatments and follow-up.
The following drugs were used: an Indian preparation of liposomal amphotericin B (FUNGISOME™, Lifecare Innovations Ltd., Gurgaon, Haryana, India) single dose of 7.5 mg/kg body weight infused in normal saline at a rate of 100 mg/100 mL/hour and miltefosine at 2.5 mg/kg/day (50 mg twice daily (body weight ≥ 25 kg) or 50 mg once daily (body weight ≤ 25 kg)) after food. The two treatment arms were miltefosine monotherapy for 28 days (group A) and liposomal amphotericin B single dose plus miltefosine for 14 days (group B). Adherence was ensured daily by staff nurses and physicians. All the subjects were periodically assessed by clinical examinations (fever, hepatosplenomegaly, new symptoms, and possible adverse drug reactions). Laboratory investigations and parasitological examination were performed on completion of treatment in each treatment arm (14 days for group A and 28 days for group B).
After completion of therapy, patients were discharged and followed up periodically at an interval of 3 months for the first year and then biannually for 5 years.
Cure was defined as clinical cure (absence of fever, clinical improvement, and reduction in spleen size) and absence of parasites at the end of therapy plus no relapse during the first 6 months of follow-up. Relapse was defined by signs or symptoms suggestive of leishmaniasis, appearing after initial cure, followed by identification of LD bodies in a splenic aspirate, within 6 months. Treatment failure was defined as either the lack of initial cure or relapse.
The primary objective was to compare the efficacy (cure rate) of the combination treatment versus oral miltefosine monotherapy. The secondary objectives were to compare the safety and tolerability of the combination treatment against miltefosine monotherapy, and rate of relapse or reinfection, rate of development of PKDL during follow-up of two regimens. To assess safety of the treatments, the Common Terminology Criteria for Adverse Events version 3.0 was used. Patients with adverse events of grade 3 or higher were withdrawn from the study. Those withdrawn because of adverse events or relapses after initial cure were given rescue treatment with either conventional amphotericin B deoxycholate in doses of 1 mg/kg daily or on alternate days for a total of 20 doses or liposomal amphotericin B (AmBisome, Gilead Sciences) 3 mg/kg per day for 5 days.
Statistical analysis.
We assumed a definitive cure rate of 90.3% with the reference drug (miltefosine monotherapy) and a non-inferiority margin of 7% for the test groups. With a power of 90% and equal allocation ratio, the sample size per group was 53, with a total sample size of 106. A non-inferiority margin of 7% was chosen because 83% was thought to be the minimum acceptable rate of definitive cure as it was the rate attained by a phase IV trial of miltefosine for the treatment of Indian patients with VL.
The primary end point of definitive cure rate was analyzed for all randomly assigned patients (intention to treat [ITT]) and for the per-protocol patients. The per-protocol group consisted of all patients who were enrolled, had no major protocol deviation or serious adverse effect mandating complete withdrawal of drug therapy, received the full treatment, and were assessed both at baseline and at the end of treatment. The cure rate comparisons are presented as the difference in proportion cured and the two-sided 95% CI for the difference in proportions using normal approximation to the binomial law. The decision rule stated that if the lower limit of the CI was more than −7%, then non-inferiority could be concluded.
The Common Terminology Criteria for Adverse Events v3.0 was used to code adverse events and concomitant treatment.10 The frequency of patients with at least one adverse event or serious adverse events was calculated along with the number and frequency of adverse events (including serious adverse events) per body system. The frequencies of adverse effects per organ system were compared between the two treatment arms.
Baseline and end of treatment clinical and laboratory characteristics of patients randomized to the treatment arms were compared using the analysis of variance. Pre- and posttreatment clinical and laboratory values were compared using paired sample Student’s t-test. All analyses were two tailed. All statistical tests were performed with SPSS version 20 (IBM Corp., Armonk, NY).
RESULTS
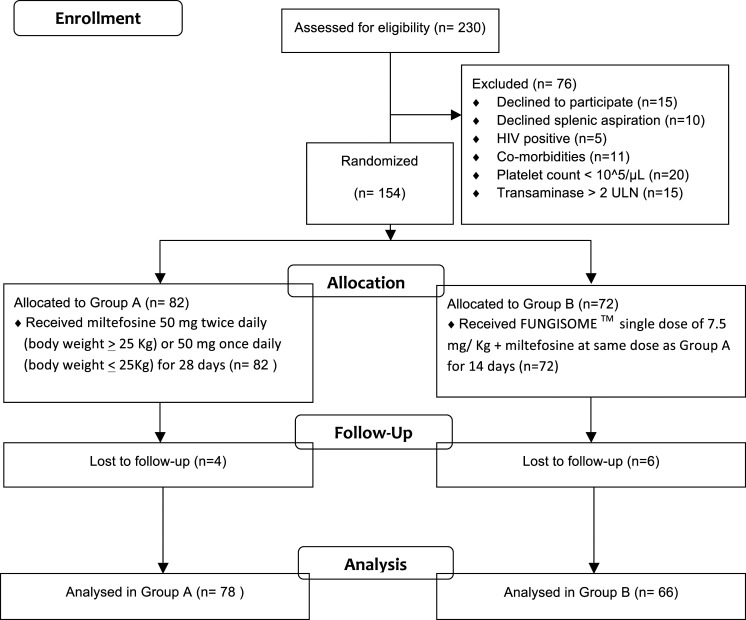
During the study period (from 2008 to 2012), a total of 230 patients were seen by the investigators, of which 76 were excluded because of various reasons (Consolidated Standards of Reporting Trials [CONSORT] flowchart in Figure 1). The remaining 154 patients were included in the study and were randomized between the two groups. Ten patients were lost to follow-up before 5 years after the end of treatment (EOT). So, a total of 144 patients were analyzed for the study—78 patients were in group A (miltefosine group) and 66 in group B (combination therapy). All of these patients completed treatment and follow-up visits for 5 years.
Figure 1.
CONSORT flow diagram. Comorbidities: uncontrolled diabetes (n = 3), untreated hypertension (n = 1), ischemic heart diseases (n = 3), and chronic kidney disease (n = 4). ULN = upper limit of normal.
Baseline characteristics.
Baseline characteristics were similar in the two groups (Table 1). The mean age of the population was 30.69 years (SD 14.59 years), and the range was 7–75 years. Overall, there were 35 males (44.9%) in the miltefosine group and 39 males (59.1%) in the combination chemotherapy group (P-value = 0.09).
Table 1.
Baseline characteristics of all patients (intention-to-treat analysis sample)
| Parameters | Miltefosine (n = 78) | Combination chemotherapy (n = 66) | P-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Mean age (years) | 28.87 | 13.81 | 32.83 | 15.29 | 0.1 |
| Mean body weight (kg) | 44.65 | 14.39 | 47.24 | 11.26 | 0.2 |
| Mean duration of fever before initiation of therapy (months) | 3.80 | 2.36 | 3.11 | 2.11 | 0.07 |
| Liver span (cm) | 3.96 | 1.45 | 3.55 | 1.69 | 0.1 |
| Centimeters of spleen palpable below the left costal margin | 9.04 | 3.03 | 8.29 | 2.58 | 0.1 |
| Mean hemoglobin (gm/dL) | 7.38 | 1.52 | 7.75 | 1.29 | 0.1 |
| Mean total leukocyte count | 2,469.48 | 790.47 | 2,775.75 | 1,334.75 | 0.09 |
| Mean erythrocyte sedimentation rate | 86.21 | 45.21 | 96.29 | 31.68 | 0.1 |
| Mean platelet count (/μL) | 119,705.13 | 47,522.82 | 136,727.27 | 65,571.69 | 0.07 |
| Mean total bilirubin (mg/dL) | 0.89 | 0.19 | 0.82 | 0.48 | 0.3 |
| Mean conjugated bilirubin (mg/dL) | 0.41 | 0.25 | 0.39 | 0.39 | 0.7 |
| Mean total protein (gm/dL) | 9.17 | 1.18 | 9.49 | 1.56 | 0.1 |
| Mean albumin (gm/dL) | 2.48 | 0.67 | 2.68 | 0.71 | 0.1 |
| Mean serum glutamic oxaloacetic transaminase (IU/L) | 55.31 | 42.47 | 65.11 | 55.59 | 0.2 |
| Mean serum glutamic pyruvic transaminase (IU/L) | 32.83 | 33.79 | 36.27 | 31.71 | 0.5 |
| Mean urea (mg/dL) | 25.52 | 6.41 | 26.23 | 7.15 | 0.5 |
| Mean creatinine (mg/dL) | 0.82 | 0.34 | 0.91 | 0.25 | 0.1 |
| Mean serum sodium (meq/L) | 134.83 | 4.77 | 134.91 | 3.85 | 0.9 |
| Mean serum potassium (meq/L) | 3.83 | 0.38 | 3.95 | 0.33 | 0.07 |
Treatment outcomes.
Table 2 shows the initial and definitive cure rates in both ITT and per-protocol analyses. In the ITT analysis, the patients who were withdrawn because of adverse events are regarded as failures. Nine patients in the miltefosine group and three patients in the combination therapy group had to discontinue their therapy because of serious adverse effects.
Table 2.
Intention-to-treat and per-protocol cure rates of both treatment groups
| Treatment arms | Intention-to-treat analysis | Per-protocol analyses | ||
|---|---|---|---|---|
| Initial cure rate | Definitive cure rate | Initial cure rate | Definitive cure rate | |
| Miltefosine | 75/78, (96.6%; 95% CI: 88.4–99%) | 63/78 (80.8%; 95% CI: 69.95–88.5%) | 75/75 (100%; 95% CI: 93.93–100%) | 63/75 (84%; 95% CI: 73.32–91.1%) |
| Combination therapy | 63/66 (95.5%; 95% CI: 86.43–98.8%) | 63/66 (95.5%; 95% CI: 86.43–98.8%) | 63/63 (100%; 95% CI: 92.84–100%) | 63/63 (100%; 95% CI: 92.84–100%) |
At the EOT, all patients were clinically asymptomatic and afebrile and had gained weight. Splenic aspiration was obtained from patients who had spleen size > 4 cm. No parasites were identified from splenic aspiration. So, at the end of therapy, the clinical and parasitological cure rate was 100%.
By the per-protocol analysis, by 6 months after completion of treatment, 12 of 69 patients in the miltefosine monotherapy (17.4%, 95% CI: 10.2–28%) relapsed. Therefore, the definitive cure rate in the miltefosine monotherapy arm for 57 of 69 was 82.6% (95% CI: 72.02–89.8%). None in the combination therapy group relapsed in the first 6 months, giving a definitive cure rate of 100% (95% CI: 92.84–100%).
The difference in definitive cure rates of the two groups is 17.4% (95% CI: 5.91–26.7%). The lower limit of the CI of the difference did not reach −7%, indicating non-inferiority of the combination therapy group.
Toxic effects.
The distribution and severity of the toxic effects are detailed in Table 3. Overall, constitutional symptoms were commoner with combination therapy, and gastrointestinal symptoms were more frequent with miltefosine. In the combination therapy group, all of the patients developed minor infusion reaction in the form of rigor and fever, both well-described complications associated with amphotericin B infusion, all of which were self-limited and needed not more than a single dose of antipyretic and antiallergic. All 66 patients had grade I fever (100.4–102.2°F) and grade I rigor (mild, not needing narcotics). Although the total number of episodes of vomiting and diarrhea was equally common among both modalities of therapy, severe vomiting was significantly more common in the miltefosine group and severe diarrhea occurred only in the miltefosine group.
Table 3.
Adverse effects (intention-to-treat sample)
| Adverse effects | Miltefosine group (n = 78) | Combination therapy group (n = 66) | P-value |
|---|---|---|---|
| Constitutional | |||
| Fever, grade 1 | 6 (7.7%) | 63 (95.5%) | < 0.001 |
| Rigors, grade 1 | 0 | 63 (95.5%) | < 0.001 |
| Insomnia | 12 (15.9%) | 5 (7.6%) | 0.197 |
| Metabolic (raised creatinine) | |||
| Grade 2 | 0 | 2 (3.0%) | 0.21 |
| Grade 3 | 3 (3.9%) | 1 (1.5%) | 0.625 |
| Total | 3 (3.9%) | 3 (4.6%) | 1.0 |
| Seizure | |||
| Grade 1 | 1 (1.9%) | 0 | 1.0 |
| Gastrointestinal | |||
| Vomiting | |||
| Grade 1 | 30 (38.5%) | 54 (81.8%) | < 0.001 |
| Grade 2 | 21 (26.9%) | 0 | < 0.001 |
| Grade 3 | 9 (11.5%) | 0 | 0.004 |
| Total | 60 (76.9%) | 54 (81.8%) | 0.24 |
| Diarrhea | |||
| Grades 1 and 2 | 0 | 0 | |
| Grade 3 | 1 (1.9%) | 0 | 1.0 |
| Total | 1 (1.9%) | 0 | 1.0 |
| Epistaxis, grade 1 | 9 (11.5%) | 0 | 0.004 |
| Pain in the abdomen, not otherwise specified | |||
| Grade 1 | 17 (21.8%) | 0 | < 0.001 |
| Grade 3 | 1 (1.9%) | 0 | 1.0 |
| Total | 18 (23.1%) | 0 | < 0.001 |
Discontinuations.
Three patients in the miltefosine group discontinued treatment because of nephrotoxicity. All three of them developed rise in creatinine within the first two weeks of starting miltefosine and developed grade 3 toxicity (0.3 mg/dL baseline for all three of them and at 2 weeks, 2.4 mg/dL, 3.1 mg/dL, and 4 mg/dL, respectively). Treatment was stopped, and creatinine gradually normalized. None of the three patients required any form of renal replacement therapy. In the miltefosine group, 6 other patients had to temporarily discontinue therapy for 1 week because of gastrointestinal intolerance. One patient developed grade 3 diarrhea requiring intravenous crystalloid replacement for 2 days followed by improvement in clinical condition that led to successful reinstitution of therapy. One of the patients with nephrotoxicity also developed convulsions and bleeding diathesis. Another patient had nonspecific colicky abdominal pain during the second week of miltefosine therapy, severe enough to hamper activities of daily living that mandated temporary discontinuation. Ultrasound abdomen did not show any stones or obstructive pathology. After resolution of pain, resumption of therapy was uneventful. Four other patients had to stop therapy for 1 week because of grade 3 vomiting not responding to oral antiemetics. All of them were successfully resumed on miltefosine after abetment of symptoms.
Three patients in the combination therapy group also had to discontinue because of rising creatinine. All three of them had normal baseline creatinine (1.1 mg/dL, 1.2 mg/dL, and 0.4 mg/dL, respectively). The rise of creatinine started to occur on an average of 10 days after the start of miltefosine (8 days, 11 days, and 10 days, respectively). At the time of discontinuation, the creatinine levels were 4 mg/dL, 3.1 mg/dL, and 2.8 mg/dL, respectively. All of them had a favorable outcome after 3–7 days of discontinuation of therapy with no need for renal replacement therapy.
End of treatment clinical and laboratory parameters.
Table 4 compares the end of treatment clinical and laboratory parameters of the two treatment arms. End of treatment hemoglobin, leukocyte count, platelet count, total protein, and albumin are significantly higher in the combination therapy arm. There is a trend toward higher mean urea and creatinine in the miltefosine arm, which does not attain statistical significance. Serum potassium is significantly higher in the miltefosine treatment arm.
Table 4.
End of treatment comparison of clinical and laboratory parameters across treatment groups
| Parameter | Therapeutic groups | Mean ± SD | 95% CI | P-value |
|---|---|---|---|---|
| Mean liver span (cm) | Miltefosine | 1.29 ± 1.57 | 0.94–1.64 | 0.3 |
| Combination therapy | 1.01 ± 1.73 | 0.58–1.44 | ||
| Centimeters of spleen palpable below the left costal margin | Miltefosine | 3.92 ± 2.43 | 3.37–4.47 | 0.1 |
| Combination therapy | 3.27 ± 2.7 | 2.6–3.93 | ||
| Mean hemoglobin (gm/dL) | Miltefosine | 9.31 ± 1.24 | 9.03–9.59 | < 0.001 |
| Combination therapy | 10.15 ± 1.55 | 9.77–10.54 | ||
| Mean total leukocyte count | Miltefosine | 3,663.97 ± 923.41 | 3,455.77–3,872.17 | < 0.001 |
| Combination therapy | 4,983.33 ± 1,379.69 | 4,644.16–5,322.51 | ||
| Mean erythrocyte sedimentation rate | Miltefosine | 61.96 ± 43.77 | 52.09–71.83 | 0.8 |
| Combination therapy | 60.8 ± 40.77 | 50.77–70.82 | ||
| Mean platelet count | Miltefosine | 170,333.33 ± 46,158.86 | 159,926.11–180,740.54 | < 0.001 |
| Combination therapy | 220,500 ± 71,676.24 | 202,879.77–238,120.22 | ||
| Mean total bilirubin (mg/dL) | Miltefosine | 0.81 ± 0.18 | 0.77–0.85 | 0.3 |
| Combination therapy | 0.76 ± 0.4 | 0.67–0.86 | ||
| Mean conjugated bilirubin (mg/dL) | Miltefosine | 0.38 ± 0.15 | 0.35–0.41 | 0.3 |
| Combination therapy | 0.33 ± 0.36 | 0.24–0.42 | ||
| Mean total protein (gm/dL) | Miltefosine | 8.66 ± 1.24 | 8.38–8.94 | < 0.001 |
| Combination therapy | 9.73 ± 1.68 | 9.32–10.15 | ||
| Mean albumin (gm/dL) | Miltefosine | 3.79 ± 0.92 | 3.59–4.01 | 0.01 |
| Combination therapy | 4.19 ± 1.03 | 3.93–4.44 | ||
| Mean serum glutamic oxaloacetic transaminase (IU/L) | Miltefosine | 53.43 ± 26.23 | 47.52–59.35 | 0.5 |
| Combination therapy | 57.19 ± 53.38 | 44.07–70.32 | ||
| Mean serum glutamic pyruvic transaminase (IU/L) | Miltefosine | 47.73 ± 37.42 | 39.29–56.16 | 0.7 |
| Combination therapy | 45.12 ± 38.37 | 35.68–54.55 | ||
| Mean urea (mg/dL) | Miltefosine | 36.19 ± 30.82 | 29.24–43.14 | 0.2 |
| Combination therapy | 30.51 ± 20.85 | 25.38–35.64 | ||
| Mean creatinine (mg/dL) | Miltefosine | 1.06 ± 0.47 | 0.95–1.16 | 0.3 |
| Combination therapy | 0.97 ± 0.51 | 0.84–1.1 | ||
| Mean serum sodium (meq/L) | Miltefosine | 137.83 ± 4.4 | 136.84–138.82 | 0.8 |
| Combination therapy | 137.96 ± 4.39 | 136.88–139.05 | ||
| Mean serum potassium (meq/L) | Miltefosine | 4.14 ± 0.38 | 4.05–4.22 | 0.008 |
| Combination therapy | 3.98 ± 0.309 | 3.91–4.05 |
Long-term follow-up.
Patients were recruited consecutively from 2008 to 2012. The last patient was recruited in 2012. After completion of therapy, patients were discharged and followed up periodically at an interval of 3 months for the first year and then biannually for 5 years (Table 5).
Table 5.
Outcome of long-term follow-up over 5 years
| Parameter | Miltefosine group (n = 78) | Combination therapy group (n = 66) | P-value |
|---|---|---|---|
| Reinfection | 0 | 0 | Not applicable |
| Post–kala-azar dermal leishmaniasis | 10 | 0 | 0.0027 |
Ten patients were lost to follow-up 1–5 years after the end of treatment. They were not included in the analysis. Only those patients (78 in miltefosine monotherapy and 66 in combination therapy) who completed at least 5 years of follow-up after the end of treatment were analyzed for the study.
Only one patient from group B (combination therapy) returned with a fever for 2 weeks and splenomegaly. Splenic aspiration did not reveal any LD body. Fever subsided with injection ceftriaxone for 7 days with presumptive diagnosis of enteric fever. No other patient of both groups returned with clinical, laboratory, or parasitological feature of VL.
No patient in combination therapy developed PKDL over 5 years of follow-up. Ten patients who received miltefosine monotherapy developed PKDL, hypopigmented macular and popular types, within 6 months to 2 years of the EOT.
All patients remained positive for rk39 immunochromatographic strip tests after 5 years of the EOT.
DISCUSSION
Combination therapy has the potentiality of increased efficacy and reduced toxicity. It has a relatively shorter course, with consequent improved compliance. Previous studies on combination therapies were of short duration and provided no information on the development of PKDL. The present study was undertaken to demonstrate efficacy in treatment of VL and prevention of PKDL.
In the present study, combination therapy was non-inferior to miltefosine monotherapy. Combination therapy was well tolerated and generated no new safety signals. The internationally accepted parameter for efficacy of VL treatment (≥ 95%) was also met with combination therapy.11 Only a minority (4.5%) developed reversible rise in creatinine, leading to treatment discontinuation. Long-term follow-up data showed prevention of development of PKDL in combination group up to 5 years.
Previously, two major studies were performed on combination therapies, one from India and another from Bangladesh.5,12 Sundar et al.5 compared 1 mg/kg amphotericin B infusion on alternate days for 30 days with three drug combinations (single injection of 5 mg/kg AmBisome and 7-day 50 mg oral miltefosine; or 10-day 11 mg/kg single intramuscular paromomycin; or 10 days each of miltefosine and paromomycin) from Bihar, India, with a follow-up of up to 6 months. Definitive cure rates for the ITT population were 93.0% (95% CI: 87.5–96.3) for amphotericin B, 97.5% (95% CI: 93.3–99.2) for AmBisome + miltefosine, 9.5% (95% CI: 93.24–99.2) for AmBisome + paromomycin, and 98.7% (95% CI: 95.1–99.8) for miltefosine + paromomycin. Patients in the combination groups had fewer adverse events than those assigned to standard treatment. No information on development of PKDL was available from this trial. In the study from Bangladesh, authors compared the safety and efficacy of three combination regimens: 5 mg/kg single dose of AmBisome + 7 days of miltefosine (2.5 mg/kg/day), 5 mg/kg single dose of AmBisome + 10 days of paromomycin (15 mg/kg/day), and 10 days of paromomycin + miltefosine, with AmBisome 15 mg/kg given in 5 mg/kg doses on days 1, 3, and 5. Six-month final cure rates for the ITT population were 98.1% (95% CI: 96.0–100) for AmBisome monotherapy, 99.4% (95% CI: 98.2–100) for AmBisome + paromomycin, 94.4% (95% CI: 90.6–98.2) for AmBisome + miltefosine, and 97.9% (95% CI: 95.5–100) for paromomycin + miltefosine. There were no relapses or PKDL up to 6 months of follow-up. All treatment regimens were well tolerated without any unexpected side effects.
The main difference with our study is inclusion of single-arm miltefosine. However, at the time of inception of the trial, miltefosine monotherapy was one of the accepted first-line therapies in Indian VL. Another important point of consideration is the use of an Indian product of liposomal amphotericin B (FungisomeTM) in contrast to the international product, AmBisome, used in the previous trials. We have previously established the safety and efficacy of this indigenous product in Indian patients with VL mono-infection and VL/HIV coinfection.8,13 AmBisome costs $240 per 50 mg vial in the commercial market, whereas the equivalent cost of Fungisome is $133. AmBisome was available through Gilead Sciences AmBisome Access Program at $18 per 50 mg vial for kala-azar elimination program of government of India.8 After 2012, Gileads has been donating AmBisome to the WHO, which distributes and delivers to VL-endemic regions at no cost. Donation is not a sustainable solution for the Indian program, and as such, more cost-effective alternatives, such as Fungisome, should be considered for program.
The absence of development of PKDL in the combination therapy group is especially interesting. It has been reported from India that rates of development of PKDL decreased after the introduction of amphotericin B as first-line treatment for VL.14,15 Post–kala-azar dermal leishmaniasis has been reported after treatment of VL with stibogluconate, AmBisome, miltefosine, and paromomycin, but the exact PKDL rate after each drug is unknown as there are no studies with long-term follow-up.16–19 In Bangladesh, a total dose of 15 mg/kg AmBisome for VL resulted in a PKDL rate of 10% in one prospective study.20
Previously, it was not known whether a single dose of AmBisome 10 mg/kg could lead to diminished incidence of PKDL or not. The Bangladesh study cited above showed combination therapy was effective in preventing development of PKDL for up to 12 months. Ours is the first study to show prevention of development of PKDL for up to 5 years with combination therapy of single-dose Fungisome and short-course miltefosine.
Control of PKDL is currently not included in the main intervention programs and guidelines, and the treatment options are limited. Straightforward but operationally difficult strategies could entail active PKDL case-finding and treatment, long-term follow-up of VL patients, patient education on the need to report to the clinic in case of a rash, and educating community workers on recognizing PKDL, all requiring the availability of safe and effective treatment. For the future, better VL treatment, including a vaccine or immunomodulator to avert any PKDL development is essentially needed.21
Our study has several limitations, such as small sample size, not including liposomal amphotericin B monotherapy as a separate arm, and single-center institutional nature of study. Single-dose AmBisome is currently the primary preferred therapy for Indian VL. Its inclusion followed the publication of high efficacy and safety of this regimen in 2010.22 Our study was initiated in 2008, and recruitment began in 2008 and ended in 2012. Therefore, single-dose Fungisome was not conceived as one of the arms for comparison. There is a need to explore the use of amphotericin liposomal formulations other than AmBisome for the treatment of Indian VL. We have previously published on the treatment of VL cases using a multi-lamellar Indian liposomal formulation, which is marketed in India, called Fungisome.8 The lack of significant attrition and availability of long-term follow-up are two important strengths of this study. Importantly, long-term follow-up (up to 5 years) after the use of combination therapy using miltefosine and liposomal amphotericin B has not been published before. In this study, we have observed excellent efficacy of combination therapy in the treatment of VL and prevention of PKDL (almost 100%) through long-term follow-up. We do concede that, given the establishment of the safety and efficacy of liposomal amphotericin B and miltefosine combination therapy from the present study, comparison of combination therapy of Fungisome and miltefosine versus Fungisome monotherapy over long-term follow-up in the prevention of relapse and development of PKDL should be the next step.
In conclusion, combination therapy of Fungisome and miltefosine is non-inferior to miltefosine monotherapy. Combination therapy is found to be highly efficient (∼100%) and tolerable, and can be applied in remote peripheral areas where VL is endemic, and, above all, it effectively prevents the development of PKDL for up to 5 years in our study. All these features make this combination therapy an important part of armamentarium in kala-azar elimination programs, particularly in India.
Acknowledgments
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M; WHO Leishmaniasis Control Team , 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, Junge K, Bryceson A, Berman J, 2002. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med 347: 1739–1746. [DOI] [PubMed] [Google Scholar]
- 3.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, Boelaert M, Dujardin JC, Chakravarty J, 2012. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis 55: 543–550. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, Sundar S, Schonian G, Dujardin JC, Salotra P, 2012. Drug susceptibility in leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis 6: e1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar S, et al. 2011. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 377: 477–486. [DOI] [PubMed] [Google Scholar]
- 6.Bodhe PV, Kotwani RN, Kirodian BG, Pathare AV, Pandey AK, Thakur CP, Kshirsagar NA, 1999. Dose-ranging studies on liposomal amphotericin B (L-AMP-LRC-1) in the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg 93: 314–318. [DOI] [PubMed] [Google Scholar]
- 7.Mondal S, Bhattacharya P, Rahaman M, Ali N, Goswami RP, 2010. A curative immune profile one week after treatment of Indian kala-azar patients predicts success with a shortcourse liposomal amphotericin B therapy. PLoS Negl Trop Dis 27: e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goswami RP, Goswami RP, Das S, Satpati A, Rahman M, 2016. Short-course treatment regimen of Indian visceral leishmaniasis with an Indian liposomal amphotericin B preparation (Fungisome™). Am J Trop Med Hyg 94: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundar S, Singh A, Rai M, Chakravarty J, 2015. Single-dose indigenous liposomal amphotericin B in the treatment of Indian visceral leishmaniasis: a phase 2 study. Am J Trop Med Hyg 92: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Therapy Evaluation Program , 2006. Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. Available at: http://ctep.cancer.gov. Accessed August 5, 2010. [Google Scholar]
- 11.World Health Organization , 2010. Control of the Leishmaniases. Report of a WHO Expert Committee. World Health Organization Technical Report Series 949. Geneva, Switzerland: WHO, 1–186. [Google Scholar]
- 12.Rahman R, et al. 2017. Safety and efficacy of short course combination regimens with AmBisome, miltefosine and paromomycin for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Negl Trop Dis 30: e0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami RP, Goswami RP, Basu A, Ray Y, Rahman M, Tripathi SK, 2017. Protective efficacy of secondary prophylaxis against visceral leishmaniasis in human immunodeficiency virus coinfected patients over the past 10 years in eastern India. Am J Trop Med Hyg 96: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha S, et al. 2007. IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of leishmania donovani infection in India. J Immunol 179: 5592–5603. [DOI] [PubMed] [Google Scholar]
- 15.Thakur CP, Kumar A, Mitra G, Thakur S, Sinha PK, Das P, Bhattacharya SK, Sinha A, 2008. Impact of amphotericin-B in the treatment of kala-azar on the incidence of PKDL in Bihar, India. Indian J Med Res 128: 38–44. [PubMed] [Google Scholar]
- 16.Pandey K, et al. 2012. Post-kala-azar dermal leishmaniasis in a patient treated with injectable paromomycin for visceral leishmaniasis in India. J Clin Microbiol 50: 1478–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koley S, Mandal RK, Choudhary S, Bandyopadhyay A, 2013. Post-kala-azar dermal leishmaniasis developing in miltefosine-treated visceral leishmaniasis. Indian J Dermatol 58: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burza S, Sinha PK, Mahajan R, Sanz MG, Lima MA, Mitra G, Verma N, Das P, 2014. Post kala-azar dermal leishmaniasis following treatment with 20 mg/kg liposomal amphotericin B (Ambisome) for primary visceral leishmaniasis in Bihar, India. PLoS Negl Trop Dis 8: e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das VN, Pandey K, Singh D, Forwood C, Lal CS, Das P, 2016. Development of post-kala-azar dermal leishmaniasis in AmBisome treated visceral leishmaniasis: a possible challenge to elimination program in India. J Postgrad Med 59: 226–228. [DOI] [PubMed] [Google Scholar]
- 20.Zijlstra EE, 2016. The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasit Vectors 9: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Rutte EA, Ziilstra EE, de Vlas SJ, 2019. Post-kala-azar dermal leishmaniasis as a reservoir for visceral leishmaniasis transmission. Trends Parasitol 35: 590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW, 2010. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med 362: 504–512. [DOI] [PubMed] [Google Scholar]