Abstract.
Nucleic acid amplification tests are increasingly used to detect ocular chlamydia infection in trachoma research and programs. To evaluate the reliability of Chlamydia trachomatis detection by the Abbott RealTime CT/NG assay (Abbott Molecular, Inc., Des Plaines, IL) on the m2000 platform, three conjunctival samples were collected from each of 200 children aged 0–9 years in Ethiopia: two from the right eye and one from the left eye. Four aliquots were processed for each child: two from the first right eye sample, one from the second right eye sample, and one from the left eye sample. Sixty-nine swabs were processed in a U.S. laboratory and 131 in an Ethiopian laboratory. Intra-class correlation coefficients (ICCs) were high when comparing two aliquots from the same swab (ICC ranged from 0.96 to 0.99), two separate swabs from the right eye (0.89–0.91), and one right and one left eye swab (0.87–0.89), indicating reliable chlamydial load assessment across different samples and laboratory settings.
Trachoma researchers and program managers are increasingly using commercial nucleic acid amplification tests (NAATs) to assess the community burden of ocular chlamydia when monitoring trachoma.1–4 The Abbott RealTime polymerase chain reaction (PCR) assay provides both qualitative information about the presence of Chlamydia trachomatis and quantitative information about the load of infection. Previous studies have compared the diagnostic performance of this assay with that of other NAATs for chlamydia detection,5–12 although few have evaluated the precision of this assay or compared results between eyes.
In this cross-sectional study, 200 children aged 0–9 years were randomly sampled in October 2012 from six communities in the Goncha Siso Enese woreda of the Amhara region in Ethiopia. These communities were participating in the same arm of a cluster-randomized trial in which they had received four rounds of annual mass azithromycin distributions, with the last round taking place 2 years before sampling.3 The trial included an annual door-to-door census, from which a simple random sample of 40 children aged 0–9 years was recruited per community until the desired sample size was reached. The trial received ethical approval from the University of California, San Francisco (UCSF); Emory University; and the Ethiopian Ministry of Science and Technology. Informed consent was obtained from all participants and caregivers of participants.
Trained examiners collected three conjunctival samples per child, including two from the right eye and one from the left eye. Examiners passed a Dacron polyester-tipped swab (Fisher Scientific, Waltham MA) three times against the everted superior tarsal conjunctiva, rotating 120° with each pass. Swabs were placed dry into 2.0-mL Nunc tubes, stored on ice for less than 8 hours in the field, then at −20°C for several weeks until transportation to the laboratory, then at 4°C for less than 48 hours while being transported to the laboratory, and finally at −20°C (Ethiopia laboratory) or −80°C (U.S. laboratory) until processing.
To assess the precision of C. trachomatis detection, swabs from a simple random sample of 69 children (group 1) were processed at UCSF, and swabs from the remaining 131 children (group 2) were processed by the Amhara Public Health Institute in Bahir Dar, Ethiopia. During processing, 1,000 µL of molecular grade water was added to each of the thawed samples, samples were then vortexed, and then 100 µL aliquots were created. At each site, swabs were handled such that laboratory personnel were masked to child identifiers and clinical information. For each child, four aliquots were processed with the RealTime CT/NG PCR assay on the Abbott m2000 platform: two from the first swab of the right eye (runs 1 and 2), one from the second swab of the right eye (run 3), and one from the swab of the left eye (run 4). All assays were performed according to the manufacturer’s package insert. The output of interest was the delta cycle (DC), which provides an estimate of chlamydial DNA quantity. Qualitative results were generated by instrument software that calculated the DC cutoff from the mean target cycle number of the controls and the addition of a predetermined number of cycles.
Bland–Altman plots were constructed to visualize each comparison of DC values by group. A linear mixed-effects model with child, eye, and swab included as crossed random effects was used to calculate intra-class correlation coefficients (ICCs) for the following comparisons of DC values for each group: two aliquots from the same swab (runs 1 versus 2), two separate swabs from the right eye (runs 1 versus 3), and swabs from the right and left eye (runs 1 versus 4) of the same child. Analyses were conducted in Stata version 14 (StataCorp LLC, College Station, TX) and R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Group 1 samples were processed in February 2013 in San Francisco, USA, and group two samples were processed in October 2017 in Bahir Dar, Ethiopia. Table 1 displays characteristics of children and samples from each processing group. Of the total sample, 95 (48%) were female, and the median age was 5 years (interquartile range: 3–7). Overall, 42 children (21%) were PCR positive for ocular chlamydia from any swab, including 17 (25%) in group 1 and 25 (19%) in group 2. Median DC values for each group are displayed in Table 1. Delta cycle values were higher in all samples from group 1.
Table 1.
Characteristics of study population by processing group
| Characteristic | Group 1, United States (N = 69) | Group 2, Ethiopia (N = 131) | P-value* |
|---|---|---|---|
| Age (years), median (IQR) | 5 (3–7) | 5 (3–7) | 0.62 |
| Gender, female, n (%) | 29 (42.0%) | 66 (50.4%) | 0.29 |
| Polymerase chain reaction positive, n (%) | |||
| Run 1: right eye swab 1 (no. 1) | 18 (26.1%) | 24 (18.3%) | 0.21 |
| Run 2: right eye swab 1 (no. 2) | 19 (27.5%) | 24 (18.3%) | 0.15 |
| Run 3: right eye swab 2 | 15 (21.7%) | 24 (18.3%) | 0.58 |
| Run 4: left eye swab | 17 (24.6%) | 25 (19.1%) | 0.38 |
| DC value,† median (IQR) | |||
| Run 1: right eye swab 1 (no. 1) | 10.2 (7.0–13.3) | 4.5 (3.4–7.1) | 0.001 |
| Run 2: right eye swab 1 (no. 2) | 9.7 (6.3–13.3) | 4.7 (3.4–7.5) | 0.003 |
| Run 3: right eye swab 2 | 7.9 (6.3–11.8) | 5.6 (3.6–8.4) | 0.03 |
| Run 4: left eye swab | 10.9 (7.5–12.7) | 7.2 (5.5–8.3) | 0.004 |
| Log-transformed EB count,† median (IQR) | |||
| Run 1: right eye swab 1 (no. 1) | 4.8 (2.5–7.0) | NA | NA |
| Run 2: right eye swab 1 (no. 2) | 4.4 (2.0–7.0) | NA | NA |
| Run 3: right eye swab 2 | 5.3 (3.3–6.6) | NA | NA |
| Run 4: left eye swab | 5.3 (2.8–6.5) | NA | NA |
DC = delta cycle; EB = elementary body; IQR = interquartile range; NA = not applicable.
Wilcoxon rank sum used to compare continuous variables by group, and the Fisher exact test used to compare proportions by group.
Among positives. The assay was standardized against quantified EB suspensions provided by the University of California, San Francisco laboratory. Using these DC outputs, calibration curves were produced to determine the known concentrations of EBs in a sample. The Ethiopian laboratory had not yet established a chlamydia calibration protocol and thus reported DC values only.
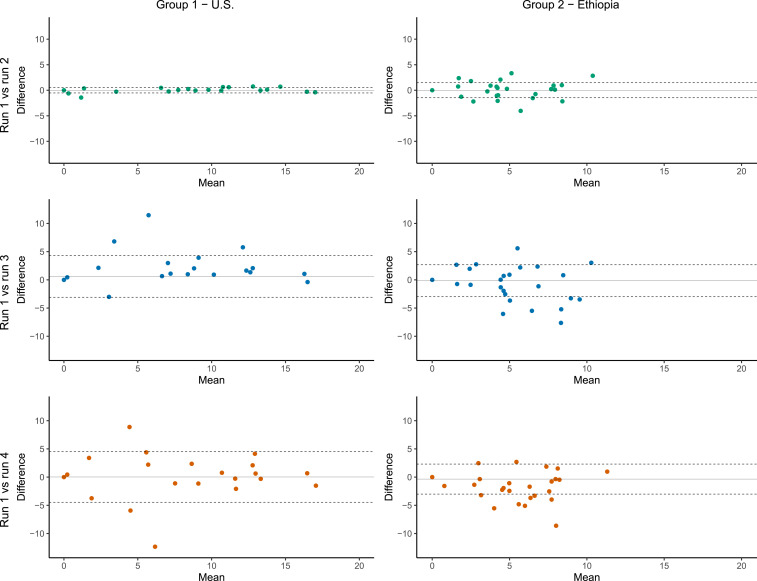
Figure 1 and Table 2 summarize each comparison by group. Intra-class correlation coefficient estimates were close to 1.0 when comparing two aliquots from the same swab in both groups, suggesting high repeatability of the assay. The ICC was approximately 0.9 when comparing two separate swabs of the right eye in both groups, indicating some minor variation in sample collection between serial swabs. Overall, we did not find evidence that the quantitative results were consistently higher in the swab collected first in either group (Table 2). However, in group 1, the first swab of the right eye was on average 0.61 (95% CI: 0.16–1.06) DCs higher than the second swab. The qualitative results of the first and second swabs from the right eye disagreed in three children from group 1 (but no children from group 2); in all three of these children, the swab collected first was positive and the swab collected second was negative. The positive swab’s DC value was 0.45 for one of these discrepant pairs, raising the possibility that insufficient chlamydial DNA remained after the initial swabbing. But the DC values for the other two discrepant pairs were 6.81 and 11.46, suggesting that the swab technique was most likely to blame (i.e., fewer epithelial cells removed during the second swabbing). The ICC for the comparison of swabs of the right and left eye was similar to the ICC comparing two swabs from the right eye in both groups. Across groups, statistically significant differences in the ICC were found in the comparison of runs 1 and 2 (P < 0.001) and in mean differences in the comparison of runs 1 and 3 (P = 0.002), although the practical differences in the ICC and mean differences across groups for all comparisons were minor.
Figure 1.
Comparison of delta cycle (DC) values from different runs and swabs by processing group. Bland–Altman plots for each processing group comparing runs 2, 3, or 4 against run 1. The y axis indicates the difference in DC value for run 1 (the first aliquot from the first right eye swab) minus the comparison run; positive values indicate higher load in run 1. Solid lines display mean difference, and dashed lines display 95% CIs for the mean difference. This figure appears in color at www.ajtmh.org.
Table 2.
Intra-class correlation coefficients (ICCs) for comparisons of DC values by processing group
| Group 1, U.S. (N = 69) | Group 2, Ethiopia (N = 131) | |||
|---|---|---|---|---|
| ICC (95% CI) | Mean difference† (95% CI) | ICC* (95% CI) | Mean difference† (95% CI) | |
| Intra-eye, intra-swab: runs 1 and 2 | 0.998 (0.997 to 0.999) | 0.01 (−0.06 to 0.07) | 0.96 (0.95 to 0.97) | 0.01 (−0.12 to 0.14) |
| Intra-eye, inter-swab: runs 1 and 3 | 0.91 (0.87 to 0.95) | 0.61 (0.16 to 1.06) | 0.89 (0.86 to 0.92) | −0.16 (−0.40 to 0.09) |
| Inter-eye, inter-swab: runs 1 and 4 | 0.87 (0.81 to 0.92) | 0.02 (−0.53 to 0.57) | 0.89 (0.86 to 0.92) | −0.35 (−0.58 to −0.11) |
As calculated from linear mixed-effects regression models with crossed random effects for child, eye, and swab.
Difference in DC value for run 1 minus the other run; positive number indicates higher load in run 1.
Limitations of this study include the lack of chlamydia calibration in group 2, which did not allow estimation of elementary bodies and the delay in processing the swabs in Ethiopia. However, within each group, swabs were processed within a short time on a machine with the same optical calibration, so this should not change the conclusions of the study.
Previous studies comparing chlamydia detection by the Abbott RealTime assay with other assays have demonstrated excellent agreement.5–12 The present study demonstrates that in addition to being a reliable test for the presence and amount of C. trachomatis in a conjunctival swab, the RealTime assay is also highly reproducible in different laboratory settings, even when not standardizing to a host gene that might provide an indication of the amount of material on the swab. Moreover, chlamydial load was similar when comparing right and left eyes, suggesting that swabbing a single eye is likely sufficient for monitoring trachoma elimination. Assessment of chlamydial load may provide more information for trachoma researchers and program managers in the effort to eliminate trachoma.
Acknowledgment:
We would like to thank Abbott for its donation of the m2000 RealTime molecular diagnostics system and consumables.
REFERENCE
- 1.Taylor HR, Burton MJ, Haddad D, West S, Wright H, 2014. Trachoma. Lancet 384: 2142–2152. [DOI] [PubMed] [Google Scholar]
- 2.Gebre T, et al. 2012. Comparison of annual versus twice-yearly mass azithromycin treatment for hyperendemic trachoma in Ethiopia: a cluster-randomised trial. Lancet 379: 143–151. [DOI] [PubMed] [Google Scholar]
- 3.Keenan JD, et al. 2018. Mass azithromycin distribution for hyperendemic trachoma following a cluster-randomized trial: a continuation study of randomly reassigned subclusters (TANA II). PLoS Med 15: e1002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash SD, et al. 2018. Ocular Chlamydia trachomatis infection under the surgery, antibiotics, facial cleanliness, and environmental improvement strategy in Amhara, Ethiopia, 2011–2015. Clin Infect Dis 67: 1840–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaydos CA, et al. 2010. Performance of the Abbott RealTime CT/NG for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 48: 3236–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Møller JK, Pedersen LN, Persson K, 2010. Comparison of the Abbott RealTime CT new formulation assay with two other commercial assays for detection of wild-type and new variant strains of Chlamydia trachomatis. J Clin Microbiol 48: 440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng A, Qian Q, Kirby JE, 2011. Evaluation of the Abbott RealTime CT/NG assay in comparison to the Roche cobas amplicor CT/NG assay. J Clin Microbiol 49: 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dize L, West S, Williams JA, Van Der Pol B, Quinn TC, Gaydos CA, 2013. Comparison of the Abbott m2000 RealTime CT assay and the cepheid geneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. J Clin Microbiol 51: 1611–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geelen TH, et al. 2013. Performance of cobas(R) 4800 and m2000 real-time assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in rectal and self-collected vaginal specimen. Diagn Microbiol Infect Dis 77: 101–105. [DOI] [PubMed] [Google Scholar]
- 10.Chernesky M, Jang D, Gilchrist J, Hatchette T, Poirier A, Flandin JF, Smieja M, Ratnam S, 2014. Head-to-head comparison of second-generation nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae on urine samples from female subjects and self-collected vaginal swabs. J Clin Microbiol 52: 2305–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y, Yin YP, Shi MQ, Zheng BJ, Zhong MY, Jiang N, Chen SC, Chen XC, 2014. Evaluation of Abbott RealTime CT/NG assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in cervical swabs from female sex workers in China. PLoS One 9: e89658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncada J, Shayevich C, Philip SS, Lucic D, Schachter J, 2015. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in rectal and oropharyngeal swabs and urine specimens from men who have sex with men with abbott's M2000 RealTime. Sex Transm Dis 42: 650–651. [DOI] [PubMed] [Google Scholar]