Abstract
Background:
The process of aortic injury, repair, and remodeling during aortic aneurysm and dissection is poorly understood. We examined the activation of bone marrow (BM)-derived and resident aortic cells in response to aortic injury in a mouse model of sporadic aortic aneurysm and dissection.
Materials and methods:
Wild-type C57BL/6 mice were transplanted with green fluorescent protein (GFP)+ BM cells. For 4 wk, these mice were either unchallenged with chow diet and saline infusion or challenged with high-fat diet and angiotensin II infusion. We then examined the aortic recruitment of GFP+ BM-derived cells, growth factor production, and the differentiation potential of GFP+ BM-derived and GFP− resident aortic cells.
Results:
Aortic challenge induced recruitment of GFP+ BM cells and activation of GFP− resident aortic cells, both of which produced growth factors. Although BM cells and resident aortic cells equally contributed to the fibroblast populations, we did not detect the differentiation of BM cells into smooth muscle cells. Interestingly, aortic macrophages were both of BM-derived (45%) and of non−BM-derived (55%) origin. We also observed a significant increase in stem cell antigen-1 (Sca-1)+ stem/progenitor cells and neural/glial antigen 2 (NG2+) cells in the aortic wall of challenged mice. Although some of the Sca-1+ cells and NG2+ cells were BM derived, most of these cells were resident aortic cells. Sca-1+ cells produced growth factors and differentiated into fibroblasts and NG2+ cells.
Conclusions:
BM-derived and resident aortic cells are activated in response to aortic injury and contribute to aortic inflammation, repair, and remodeling by producing growth factors and differentiating into fibroblasts and inflammatory cells.
Keywords: Aortic aneurysm, Bone marrow cells
Introduction
Aortic aneurysm and dissection (AAD) are major diseases of the aorta, accounting for more than 10,000 deaths in the United States each year.1 Despite improvements in diagnostic and therapeutic techniques, patients with AAD have a high mortality rate. Understanding the mechanisms of AAD formation is important for developing new treatment options.
The aortic wall is constantly subjected to biologic insults and hemodynamic stress, which can cause aortic inflammation, smooth muscle cell (SMC) damage,2 and extracellular matrix destruction.3 When the aorta is injured, complex interconnected programs may be triggered to quickly restore tissue homeostasis,4 such as the recruitment of inflammatory cells to the injured area to clear damaged tissue,5,6 the activation and differentiation of stem cells to replace the damaged cells, the proliferation of SMCs to replace the lost cells, and the rapid proliferation of fibroblasts that produce collagen to strengthen the aortic wall and prevent rupture.7–9 However, the reparative process that occurs in response to aortic injury is poorly understood.
Bone marrow (BM)-derived cells have been shown to directly participate in vascular repair and regeneration10–12 by producing growth factors.13 To understand the reparative process after AAD and to determine the relative contributions of BM-derived versus resident aortic cells in aortic repair and remodeling, we systematically examined the recruitment, activation, differentiation potential, and growth factor production of BM-derived and resident aortic cells in response to aortic injury in a mouse model of sporadic AAD.
Materials and methods
Experimental design and model of sporadic AAD
All animal procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine in accordance with the guidelines of the National Institutes of Health. Eight-week-old male wild-type C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) (n = 28) were lethally irradiated and then subjected to BM transplantation as described in the following section. Four weeks after transplantation, the mice were either challenged with a high-fat diet and continuous angiotensin II infusion (2000 ng/kg/min; SigmaeAldrich Corporation, St. Louis, MO) for 4 wk (n = 19) or unchallenged with a chow diet and continuous saline infusion for 4 wk (n = 9). At the end of the study, the mice were euthanized, and their aortas were harvested, fixed in 4% paraformaldehyde, dehydrated in 20% sucrose, and embedded in an optimal cutting temperature compound for immunofluorescence studies (see Immunofluorescence studies section). Aortic dilatation was defined as an aortic diameter 1.25× greater than that of unchallenged mice, and aortic aneurysm was defined as an aortic diameter 1.5× greater than that of unchallenged mice.
Bone marrow transplantation
BM cells from green fluorescent protein (GFP) transgenic mice (Jackson Laboratory, Bar Harbor, ME) were used as donor cells. BM cells were harvested from 8-wk-old male GFP transgenic mice. The cellular content of the BM was analyzed by means of flow cytometry analysis (BD fluorescence-activated cell sorting [FACS] LSR; BD Biosciences, Heidelberg, Germany) by using antibodies against fibroblast-specific protein-1 (FSP-1) (Abcam, Cambridge, MA), CD68 (Santa Cruz Biotechnology, Santa Cruz, CA), stem cell antigen-1 (Sca-1) (Abcam, Cambridge, MA), and neural/glial antigen 2 (NG2) (Abcam, Cambridge, MA). The recipient mice were lethally irradiated with a total dose of 10 Gy (1000 rad), which was administered in 2 doses 3 h apart. The mice then received 5 × 106 BM donor cells via tail-vein injection. To confirm the success of BM transplantation, FACS analysis was used to compare peripheral blood from recipient mice 4 wk after transplantation to that from control mice that did not receive BM transplantation.
Blood pressure measurement
Systolic blood pressure was measured on the day of pump implantation and once a week thereafter by means of a computerized tail-cuff system (Visitech Systems, Inc, Apex, NC).
Immunofluorescence studies
Frozen sections (5 μm) of the aorta were stained with primary antibodies, including anti–FSP-1, anti-CD68, anti–α-smooth muscle actin (SMA), anti-SM22α, anti–vascular endothelial growth factor (VEGF) (Abcam), anti–insulin-like growth factor-1 (IGF-1), and anti–platelet-derived growth factor beta (PDGF-B) (Santa Cruz Biotechnology). Sections were incubated with secondary antibodies conjugated to an Alexa Fluor dye (Invitrogen, Carlsbad, CA). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The sections were examined with an Olympus DP70 fluorescence microscope (Olympus, Tokyo, Japan) or a Leica SP5 confocal microscope (Leica Microsystems Inc, Buffalo Grove, IL). GFP+ cells and immunostained cells were counted from four randomly selected fields (at a magnification of × 600) per slide by using Image-Pro Plus 6.0 (Media Cybernetics, Inc, Bethesda, MD). Cells within the thrombus in the false lumen were not counted.
Statistical analysis
All statistical analyses were performed with SPSS (Version 13, SPSS Inc, Chicago, IL). The normality of the data was examined by using the Shapiro–Wilk test. For normally distributed data, the equality of variances was examined by using Levene’s test. Two-group comparisons were performed by using the two-sample t-test. Multigroup comparisons were performed by using one-way analysis of variance. Data are reported as the mean ± standard deviation. For all statistical analyses, two-tailed probability values were used. A probability value of P < 0.05 was considered significant.
Results
Reconstitution of BM in irradiated wild-type recipient mice
To facilitate the tracking of BM-derived cells, recipient mice were transplanted with BM cells from GFP+ mice. FACS analysis of the donor BM cells showed the absence of FSP-1+ fibroblasts and CD68+ macrophages (data not shown). Four weeks after transplantation, FACS analysis of the peripheral blood from the 28 mice showed that 97.1%−99.0% of all nucleated cells expressed GFP, indicating near complete reconstitution of BM in the recipient mice (online-only Supplemental Fig). Furthermore, the leukocyte subpopulations in the recipient mice were similar to those in mice that did not receive BM transplants (data not shown), confirming that the composition of the leukocyte population was within the physiologic range before the mice were infused with angiotensin II.
Development of AAD in challenged mice
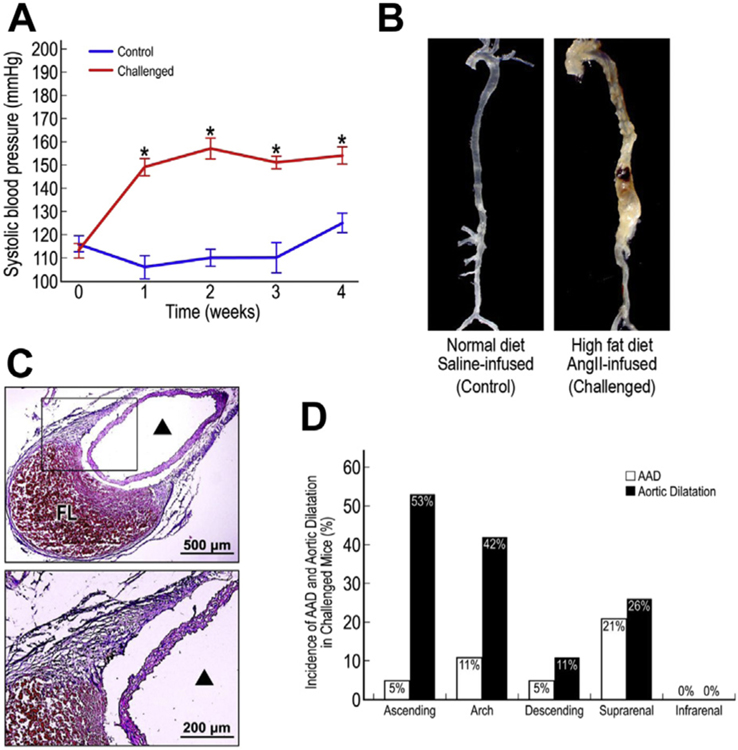
Compared with unchallenged mice, challenged mice showed a significant increase in systolic blood pressure (Fig. 1A). Whereas no obvious aortic changes were observed in the unchallenged mice, 68% (13/19) of the challenged mice developed aortic dilatation, and 37% (7/19) developed aortic dissection (Fig. 1B and C) with false lumen and thrombus formation between the medial and adventitial layers (Fig. 1C). Of the seven mice that developed dissection, six had an enlarged aorta and met the criteria for an aneurysm (aortic diameter 1.5× greater than normal), and one showed aortic dilation (aortic diameter 1.25× greater than that normal) but did not meet the criteria for an aneurysm. AAD and aortic dilatation occurred in the ascending aorta, aortic arch, descending thoracic aorta, and suprarenal abdominal aorta (Fig. 1D). The infrarenal abdominal aorta was relatively normal in all of the mice.
Fig. 1 –
Development of aortic aneurysm and dissection (AAD) in challenged mice. Mean systolic blood pressure in control and challenged mice during the 4-wk challenge period (A). *P < 0.001. Representative images of an aorta from a control mouse and an aorta from a challenged mouse with suprarenal AAD (B). Cross-section of the aneurysmal suprarenal aorta in a challenged mouse showing dissection between the medial and adventitial layers and thrombus formation in the false lumen (C). ▲ indicates the true aortic lumen. Incidence of AAD and aortic dilatation in different aortic segments of challenged mice (D). FL, false lumen.
Recruitment of BM cells to the injured aortic wall
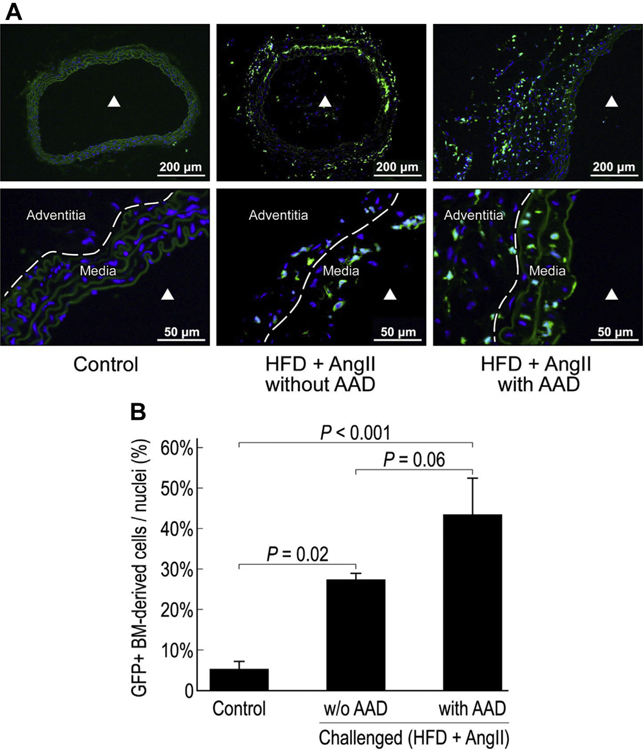
We next examined the presence of BM-derived cells. In unchallenged mice, a few GFP+ BM-derived cells were detected in the adventitia but not in the media of the aortic wall (Fig. 2A). In contrast, significantly more GFP+ BM-derived cells were observed in the aortic media and adventitia of challenged mice, particularly those with AAD. In the aortas of challenged mice with AAD, BM-derived cells contributed to 43% of the cell population (Fig. 2B). These findings suggest that aortic stress and injury induce the recruitment of BM-derived cells to the aortic wall.
Fig. 2 –
Recruitment of bone marrow (BM) cells to the injured aortic wall. Representative images show GFP + BM-derived cells in the suprarenal aorta of challenged mice (with or without AAD) and control mice (A). ▲ indicates the aortic lumen. The percentage of GFP + BM-derived cells in the total cell population in the suprarenal aorta of challenged mice (with or without AAD) and control mice (n = 4 per group) (B). HFD, high-fat diet.
Production of growth factors by BM-derived cells and resident aortic cells
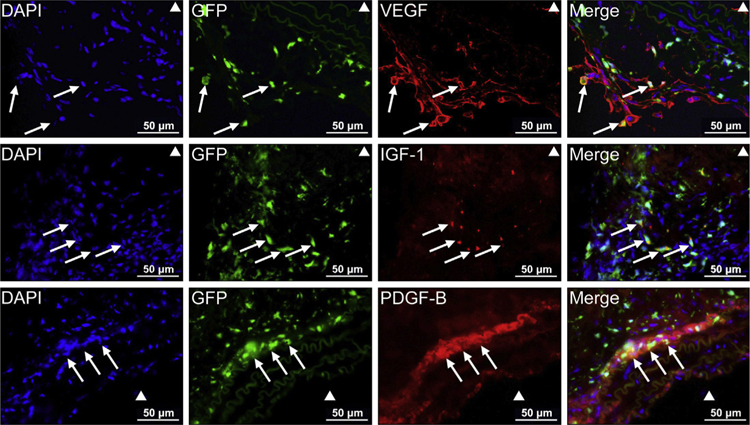
VEGF,14 IGF-1,15 and PDGF-B16 are multifunctional factors that have been shown to promote cardiovascular repair. Thus, we examined the presence of these growth factors in the injured aortic wall. Immunofluorescence staining showed that VEGF, IGF-1, and PDGF-B were highly expressed in the adventitia and in the media-adventitia boundary area in AAD tissue (Fig. 3). Interestingly, BM-derived cells and resident aortic cells showed different patterns of growth factor production. Whereas VEGF was expressed in both GFP+ and GFP− cells, IGF-1 and PDGF-B were expressed mostly in GFP+ cells.
Fig. 3 –
Production of growth factors by bone marrow (BM)-derived cells and resident aortic cells. Immunofluorescence staining showing the production of growth factors by GFP + BM-derived cells and GFP − resident cells in the injured aorta. White arrows indicate BM-derived cells producing the indicated growth factor (scale bar = 5 μm).
Absence of BM-derived SMCs in the injured aortic wall
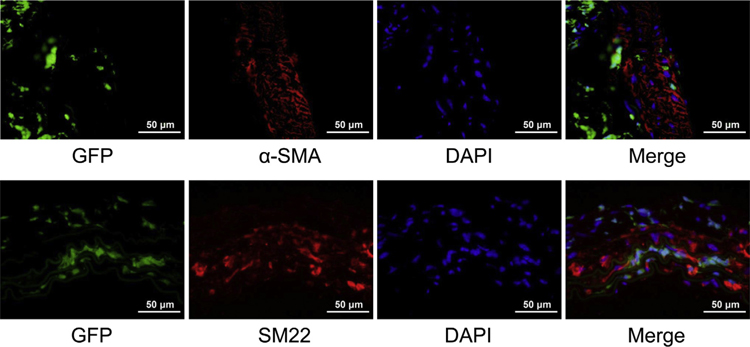
To explore whether BM-derived cells contribute to regeneration of the injured aorta in AAD by differentiating into vascular SMCs, we examined the expression of the early SMC markers α-SMA and SM22α in GFP+ BM-derived cells. In the aortas of all 19 challenged mice, no GFP+ α-SMA+ cells or GFP+ SM22α+ cells were detected by using confocal microscopy analysis (Fig. 4), suggesting that BM-derived cells in the aortic wall may not differentiate into vascular SMCs in this experimental model of AAD.
Fig. 4 –
Vascular smooth muscle cells (SMCs) not derived from differentiated bone marrow (BM)-derived cells. Representative confocal microscopy images showing that no GFP + α-SMA + cells or GFP + SM22α+ cells were detected in the aortas of challenged mice, suggesting that BM-derived cells in the aortic wall may not differentiate into vascular SMCs in this experimental model of AAD.
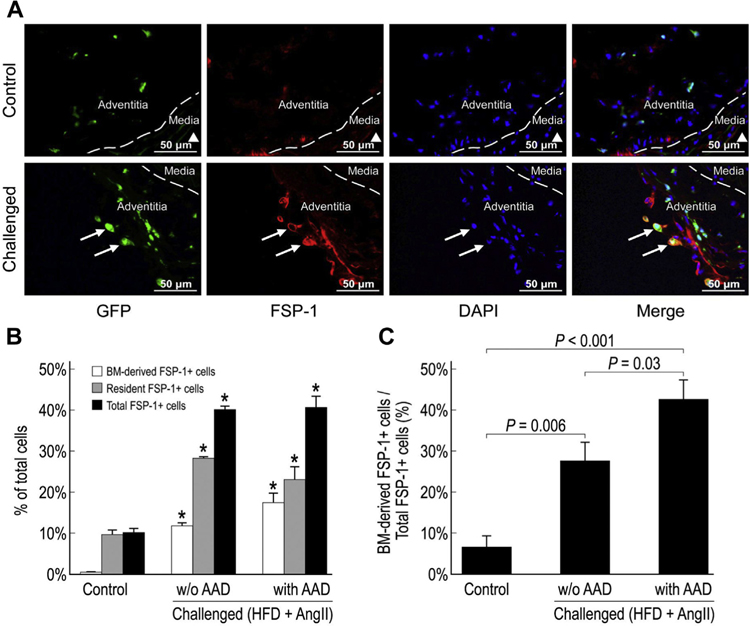
Equal distribution of BM-derived and resident fibroblasts in the injured aortic wall
Fibroblasts are important components of the aortic wall and play diverse roles in tissue repair, remodeling, and inflammation.7,9 Therefore, we examined the presence of fibroblasts in the injured aortic wall by detecting FSP-1, a marker of fibroblasts in organs undergoing tissue remodeling.17 Immunofluorescence staining showed that FSP-1+ cells were significantly increased in the aortas of challenged mice and composed up to 40% of the aortic cell population (Fig. 5A). Both GFP+ BM-derived FSP-1+ cells and GFP− resident FSP-1+ cells were increased, suggesting that BM-derived cells and resident aortic cells equally contributed to the increased number of FSP-1+ cells in the aortas of the challenged mice (Fig. 5B). These findings indicate the important contribution of BM-derived cells and resident aortic cells in aortic fibrotic remodeling.
Fig. 5 –
Equal distribution of bone marrow (BM)-derived fibroblasts and resident fibroblasts in the injured aortic wall. Representative images showing FSP-1+ cells in the suprarenal aorta of control and challenged mice (A). ▲ indicates the aortic lumen. White arrows indicate FSP-1+ BM-derived cells. The percentage of FSP-1+ BM-derived cells, FSP-1+ resident cells, and FSP-1+ cells in the total cell population of the suprarenal aorta (*P < 0.05 versus control group) (B) and the percentage of FSP-1+ BM-derived cells in the total FSP-1+ cell population of the suprarenal aorta in challenged mice (with or without AAD) and control mice (n = 4 per group) (C). HFD, high-fat diet.
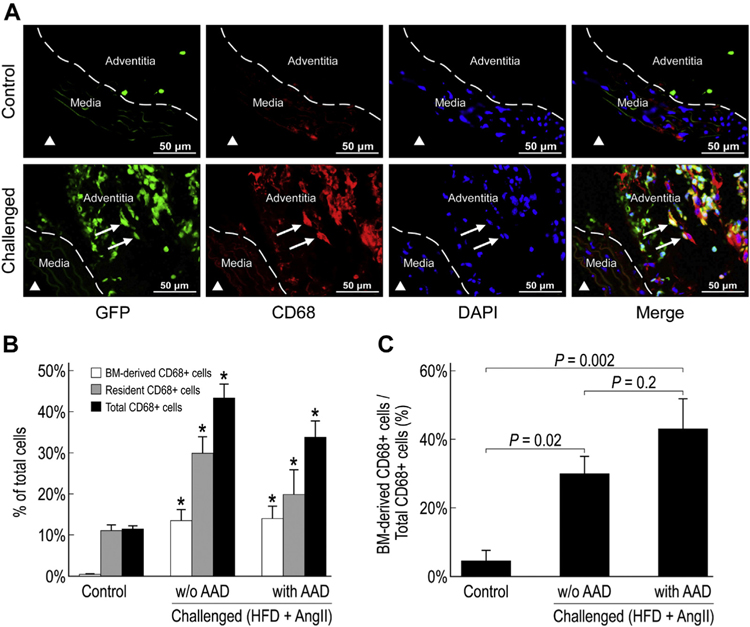
Equal distribution of BM-derived and nonBM-derived inflammatory cells in the injured aortic wall
Inflammatory cells, especially macrophages, play an important role in aortic destruction in AAD.5,6 The number of CD68+ macrophages was significantly increased in the aortas of challenged mice when compared with the aortas of control mice, particularly in the aortic adventitia (Fig. 6A and B). Interestingly, in challenged mice, only 30%−45% of the aortic macrophages were GFP+ BM-derived (Fig. 6C), whereas the remaining cells were GFP− cells. These findings suggest that the macrophages from a non-BM source also contribute to aortic inflammation.
Fig. 6 –
Equal distribution of bone marrow (BM)-derived and non − BM-derived inflammatory cells in the injured aortic wall. Representative images showing CD68+ inflammatory cells in the suprarenal aorta of control and challenged mice (A). ▲ indicates the aortic lumen. White arrows indicate CD68+ BM-derived cells. The percentage of CD68+ BM-derived cells, CD68+ resident cells, and CD68+ cells in the total cell population of the suprarenal aorta (*P < 0.05 compared to control group) (B) and the percentage of CD68+ BM-derived cells in the total CD68+ population of the suprarenal aorta, in challenged (with or without AAD) and control mice (n = 4 in each group) (C). HFD, high-fat diet.
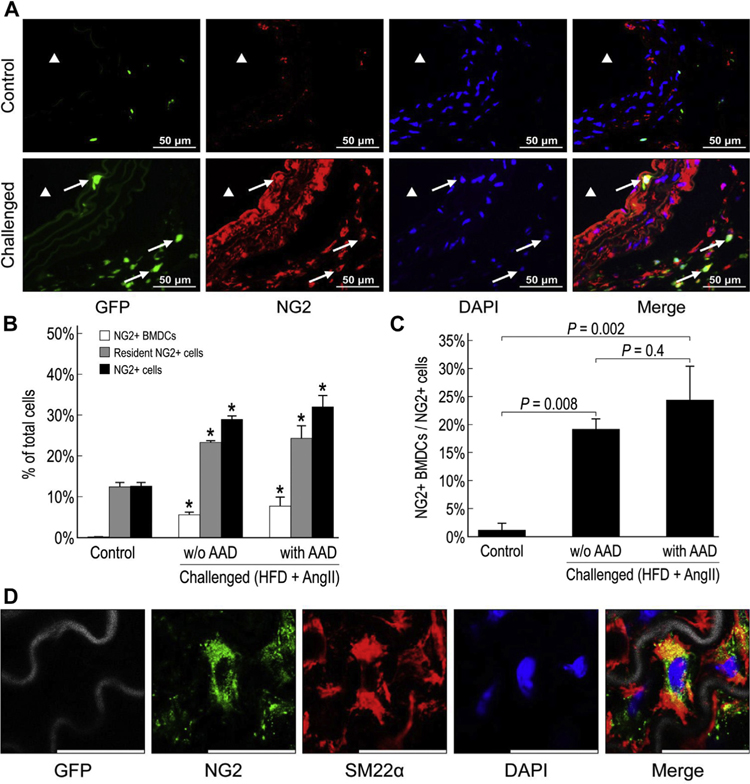
BM-derived and aortic resident NG2+ cells are activated in the injured aortic wall
NG2+ cells are multipotent progenitor cells that are critical for the development and maturation of neural and vascular tissues.18 We therefore explored the presence of NG2+ cells and their potential differentiation into SMCs and contribution to aortic regeneration in AAD. Whereas only a few NG2+ cells were detected in the aortic adventitia of unchallenged mice, significantly more GFP+ BM-derived NG2+ cells and GFP− resident NG2+ cells were observed in the aortic adventitia of challenged mice (Fig. 7A and B), indicating the recruitment of BM-derived cells and the activation of resident aortic cells in response to aortic injury. Although the percentage of BM-derived NG2+ cells in the total NG2+ cell population was increased in the aortas of challenged mice, resident NG2+ cells accounted for 75%−80% of the NG2+ cells in those aortas (Fig. 7C). Interestingly, we also observed significant NG2 expression in the aortic media of challenged mice (Fig. 7A). Although a significant amount of GFP− NG2+ SM22α+ cells were observed (Fig. 7D), GFP+ NG2+ SM22α+ cells were not detected, indicating that resident aortic NG2+ cells, but not BM-derived NG2+ cells, can transform into SMCs.
Fig. 7 –
Activation of bone marrow (BM)-derived and resident aortic NG2+ cells in the injured aortic wall. Representative images of NG2+ cells in the suprarenal aorta of control and challenged mice (A). ▲ indicates the aortic lumen. White arrows indicate NG2+ BM-derived cells. The percentage of NG2+ BM-derived cells, NG2+ resident cells, and NG2+ cells in the total cell population of the suprarenal aorta (*P < 0.05 compared to control group) (B) and the percentage of NG2+ BM-derived cells in the total NG2+ cell population of the suprarenal aorta in challenged and control mice (n = 4 per group) (C). Resident NG2+ pericytes (GFP−) differentiated into vascular smooth muscle cells (scale bar = 25 μm) (D). BMDC, bone marrow–derived cells; HFD, high-fat diet.
Activation of BM-derived and resident Sca-1 + stem/progenitor cells in the injured aortic wall
Because Sca-1+ stem/progenitor cells are important in cardiovascular repair,19 we examined their presence in the injured aortic wall. In aortas from unchallenged mice, we detected Sca-1+ stem/progenitor cells, most of which were GFP− resident cells (Fig. 8A and B). However, in aortas from challenged mice, we observed significantly more Sca-1+ cells, particularly in the adventitia. Some Sca-1+ cells were also seen in the endothelial layer, and very few were detected in the aortic media (Fig. 8A). These Sca-1+ cells were composed of both BM-derived Sca-1+ and resident Sca-1+ cells (Fig. 8C). Interestingly, although the proportion of BM-derived Sca-1+ cells was significantly higher in challenged mice (20%−25%) than in control mice (5%), most of the Sca-1+ cells were GFP− resident stem/progenitor cells, indicating a predominant role of resident Sca-1+ stem/progenitor cells in aortic repair and remodeling.
Fig. 8 –
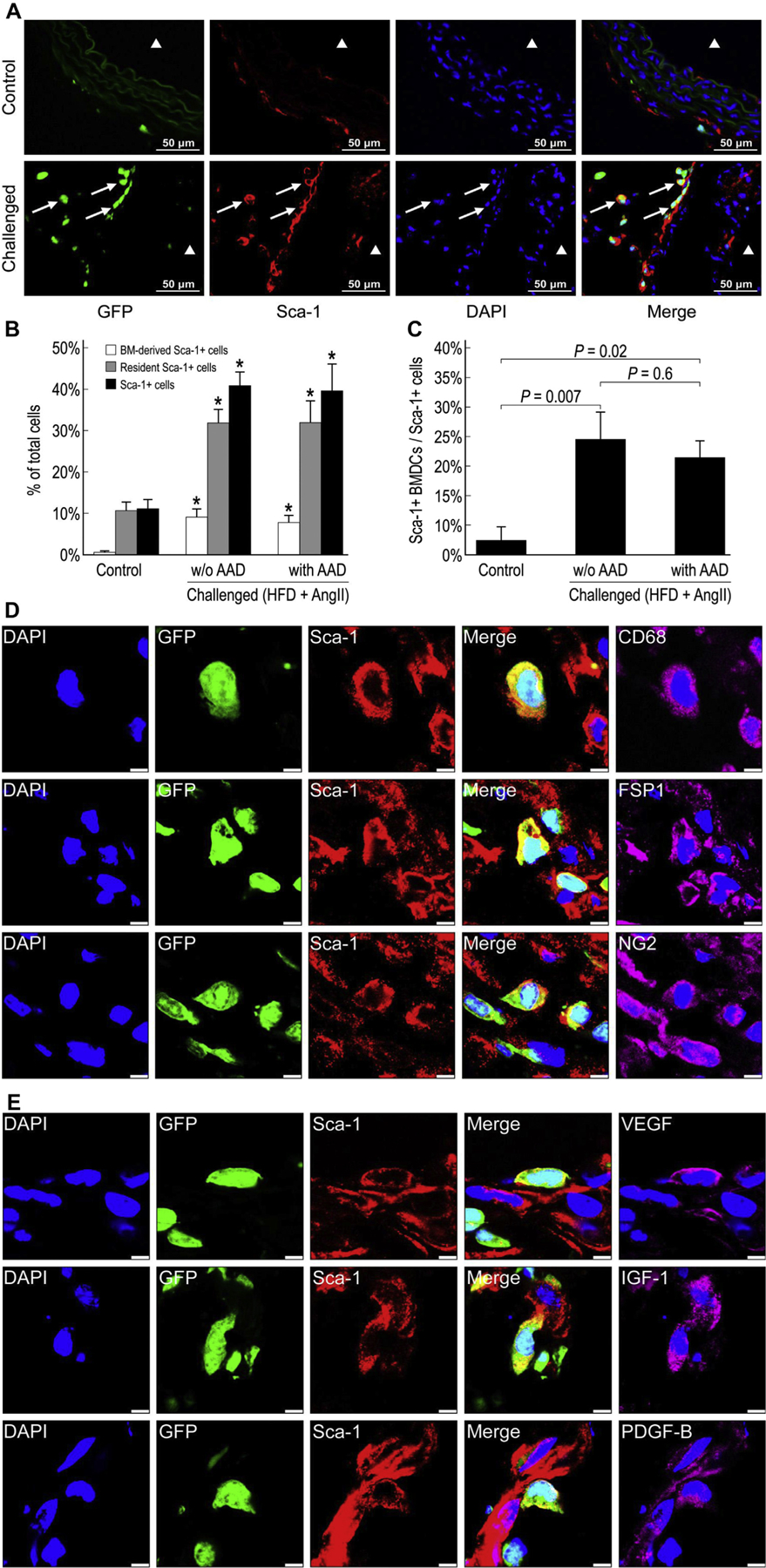
Activation of bone marrow (BM)-derived and resident Sca-1+ stem/progenitor cells in the injured aortic wall. Representative images showing BM-derived Sca-1+ cells in the suprarenal aorta of control and challenged mice (A). ▲ indicates the aortic lumen. White arrows indicate BM-derived Sca-1+ cells. The percentage of BM-derived Sca-1+ cells, resident Sca-1+ cells, and Sca-1+ cells in the total cell population of the suprarenal aorta (*P <0.05 compared with control group) (B) and the percentage of BM-derived Sca-1+ cells in the total Sca-1+ cell population of the suprarenal aorta in challenged and control mice (n = 4 per group) (C). Immunofluorescence staining showing both BM-derived (GFP+) and resident (GFP−) Sca-1+ cells expressing CD68, FSP-1, or NG2 in the aortic adventitia of challenged mice (scale bar = 5 μm) (D). Both BM-derived (GFP+) and resident (GFP−) Sca-1+ cells expressing VEGF, IGF-1, and PDGF-B in the aortic adventitia of challenged mice (scale bar = 5 μm) (E). HFD, high-fat diet.
We next explored whether Sca-1+ stem/progenitor cells can differentiate into vascular SMCs. Using immunofluorescence staining, we did not detect either BM-derived (GFP+) Sca-1+ SM22α+ cells or resident (GFP−) Sca-1+ SM22α+ cells, suggesting that Sca-1+ cells may not differentiate into SMCs. In contrast, we detected a significant amount of Sca-1+ FSP-1+ cells, Sca-1+ CD68+ cells, and Sca-1+ NG2+ cells in the aortas of challenged mice (Fig. 8D), which were either BM-derived or resident cells. These findings suggest the potential differentiation of both BM-derived and resident Sca-1+ cells into fibroblasts, inflammatory cells, and NG2+ cells. Finally, triple staining showed that the growth factors VEGF, IGF-1, and PDGF-B were expressed mostly in BM-derived Sca-1+ cells (Fig. 8E).
Discussion
In this study, we showed that both BM-derived and resident aortic cells were activated in response to aortic stress, produced growth factors, and formed fibroblasts and inflammatory cells. Resident stem/progenitor cells were more prominent than BM-derived stem/progenitor cells in the aortas of challenged mice. Our findings suggest that BM-derived and resident stem/progenitor cells are activated in response to aortic stress and may contribute to aortic repair and remodeling by developing into functionally relevant phenotypes and/or by producing growth factors.
VEGF,14,20–22 IGF-1,23–25 and PDGF-B16,26,27 play potent roles in vascular protection and regeneration by promoting vascular cell survival and proliferation. We observed a profound increase in the production of VEGF, IGF-1, and PDGF-B from BM-derived cells and resident aortic cells in the adventitia of AAD tissue, suggesting that these cells may contribute to aortic repair and regeneration by producing growth factors that promote vascular cell survival and proliferation. Interestingly, VEGF was expressed in both BM-derived cells and in resident aortic cells, whereas IGF-1 and PDGF-B were expressed mostly in BM-derived cells. These findings suggest that BM-derived cells and resident aortic cells differ in growth factor production.
SMCs are the main cell type in the aortic media and are crucial for maintaining aortic structure and function. SMC apoptosis and depletion are common features of aortic aneurysms28 and dissections.29 Effective repair and replacement of damaged SMCs are essential in aortic repair and in the prevention of AAD formation. We previously observed the expression of an SMC marker in stem/progenitor cells in human thoracic AAD tissue, suggesting the differentiation of stem/progenitor cells into SMCs.30 However, it is unclear whether those SMC progenitors were resident cells or BM-derived cells. In the present study, we observed very few BM-derived cells in the aortic media and did not detect the expression of vascular SMC markers in BM-derived cells. Although the differentiation of BM-derived progenitor cells into SMC lineages has been demonstrated in vitro,31 this event has been reported to be extremely rare in vivo.32 Thus, it is most likely that SMC progenitor cells observed in AAD tissue are resident cells rather than BM-derived cells.
Fibroblasts participate in tissue repair by migrating, proliferating rapidly, and synthesizing connective tissue components.8 They can also produce cytokines, such as interleukin-6, induce monocyte chemotactic protein-1 secretion, and promote monocyte recruitment and activation,9 thus mediating vascular inflammation and promoting aortic destabilization. In the aortas of challenged mice, we observed a significant increase in fibroblasts derived from both BM cells and aortic cells, near the dissection area between the aortic media and adventitia and in the adventitia. The quick and profound increase in fibroblasts in response to aortic injury suggests that fibroblasts may play a pivotal role in increasing aortic strength and preventing aortic rupture. However, they may also drive the inflammatory response or maladaptive remodeling and aggravate the injury.
Macrophages have been shown to play a destructive role in the formation and progression of AAD. Previous studies have shown an association between aortic macrophage accumulation and abdominal aortic aneurysm expansion.5 Macrophage-mediated vascular inflammation leads to aortic dissection9 and contributes to aortic aneurysm formation.6 In addition, macrophages are the main source of elastase activity in aneurysmal tissues.33 In our study, CD68+ macrophages were significantly increased in the aortic adventitia of challenged mice, indicating an increased level of inflammation. Interestingly, we found that only 45% of the aortic macrophages in challenged mice were derived from BM cells, whereas the remaining macrophages were derived from a non-BM source. It has recently been shown that the proliferation of BM-derived inflammatory and tissue-resident macrophage lineage branches is a key feature of the inflammatory process.34 Thus, aortic resident macrophages may exist and contribute to aortic inflammation.
NG2+ cells are vascular mural cells with progenitor-like properties and have been shown to function as vascular smooth muscle cell progenitor cells.35,36 In addition, NG2+ cells secrete growth factors such as VEGF and angiopoietin-135,36 and promote endothelial survival,25 thus playing a critical role in cardiovascular development and repair.35,37 In our study, aortic challenge significantly increased BM-derived NG2+ cells and resident NG2+ cells, either by proliferation and/or transformation. Importantly, although BM-derived NG2+ cells were significantly increased in response to injury, resident NG2+ cells were the predominant type observed in the injured aorta, indicating that resident NG2+ cells may play a major role in aortic repair. Furthermore, we observed the transformation of resident aortic NG2+ cells but not BM-derived NG2+ cells into SMCs, supporting the notion that resident aortic cells may play a major role in aortic SMC repair and/or regeneration.
When cardiovascular tissue is injured, stem cells/progenitors are activated to participate in cardiac38–40 and vascular10,41 repair by producing growth factors and differentiating into endothelial cells,42–44 SMCs,19 and inflammatory cells.44 We previously observed stem/progenitor cells in human thoracic AAD tissue.30 However, it is unclear whether those stem/progenitor cells were resident aortic cells or BM-derived cells. In the present study, we showed that both BM-derived and resident Sca-1+ stem/progenitor cells were significantly increased in the aortas of challenged mice, suggesting that aortic injury not only induced the recruitment of BM-derived stem cells but also stimulated the proliferation of resident stem cells. Interestingly, most of the Sca-1+ cells (>80%) that were increased in response to aortic challenge were of resident aortic origin. These Sca-1+ cells can produce growth factors and differentiate into SMCs, fibroblasts, and inflammatory cells. A growing body of evidence has suggested that resident stem/ progenitor cells contribute to angiogenesis and vascular repair.42–44 Thus, our study indicates a predominant role of resident aortic cells in aortic repair and remodeling.
Conclusions
In summary, we found using an experimental model of AAD that BM-derived and resident aortic cells are activated in response to aortic stress. BM-derived cells and aortic resident cells contribute equally to aortic inflammatory cells and fibroblasts. Resident aortic cells contribute to the majority of progenitors. Our findings suggest that BM-derived cells and aortic resident cells play differential and dynamic roles in aortic inflammation, repair, and remodeling.
Supplementary Material
Acknowledgment
This study was supported by the National Institutes of Health [grant R01 HL085341 (to S.A.L.). S.A.L.’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine. S.Z. was supported by a program from SHMEC (15ZZ042) and a program from NSFC (81700408). The authors thank Darren Woodside, PhD, and Deenadayalan Bakthavatsalam, PhD (Texas Heart Institute), for their expert advice regarding use of the confocal microscope. The authors gratefully acknowledge Nicole Stancel, PhD, ELS(D) (Texas Heart Institute), for providing editorial support, and Scott Weldon, MA, CMI, FAMI (Baylor College of Medicine), for assistance with figures.
Footnotes
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article. Dr LeMaire serves as an advisory panel member for Biom’up and Acer Therapeutics, serves as a consultant and as a principal investigator for clinical studies sponsored by Terumo Aortic and CytoSorbents, and serves as a coinvestigator for clinical studies sponsored by W.L. Gore & Associates. Dr LeMaire also serves as Editor in Chief for the Journal of Surgical Research; as such, he recused himself from the entire peer-review and editorial process for this manuscript.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jss.2019.07.013.
REFERENCES
- 1.Minino AM, Murphy SL, Xu J, et al. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 2.Collins MJ, Dev V, Strauss BH, et al. Variation in the histopathological features of patients with ascending aortic aneurysms: a study of 111 surgically excised cases. J Clin Pathol. 2008;61:519–523. [DOI] [PubMed] [Google Scholar]
- 3.Michel JB, Martin-Ventura JL, Egido J, et al. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321. [DOI] [PubMed] [Google Scholar]
- 5.Rateri DL, Howatt DA, Moorleghen JJ, et al. Prolonged infusion of angiotensin II in apoE(−/−) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol. 2011;179:1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tazume H, Miyata K, Tian Z, et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32:1400–1409. [DOI] [PubMed] [Google Scholar]
- 7.Duan J, Gherghe C, Liu D, et al. Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 2012;31:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javelaud D, Laboureau J, Gabison E, et al. Disruption of basal JNK activity differentially affects key fibroblast functions important for wound healing. J Biol Chem. 2003;278:24624–24628. [DOI] [PubMed] [Google Scholar]
- 9.Tieu BC, Lee C, Sun H, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napoli C, Williams-Ignarro S, de Nigris F, et al. Beneficial effects of concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proc Natl Acad Sci U S A. 2005;102:17202–17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Liu X, Jiang Y, et al. MAPK/ERK signalling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med. 2008;12:2395–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. [DOI] [PubMed] [Google Scholar]
- 14.Zachary I, Mathur A, Yla-Herttuala S, et al. Vascular protection: a novel nonangiogenic cardiovascular role for vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2000;20:1512–1520. [DOI] [PubMed] [Google Scholar]
- 15.Thum T, Hoeber S, Froese S, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. [DOI] [PubMed] [Google Scholar]
- 16.Zymek P, Bujak M, Chatila K, et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–2323. [DOI] [PubMed] [Google Scholar]
- 17.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A. 2011;108:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Zhang Z, Torsney E, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco M, Roswall P, Cortez E, et al. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinugasa M, Amano H, Satomi-Kobayashi S, et al. Necl-5/ poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Circ Res. 2012;110:716–726. [DOI] [PubMed] [Google Scholar]
- 22.Nosaka M, Ishida Y, Kimura A, et al. Absence of IFN-gamma accelerates thrombus resolution through enhanced MMP-9 and VEGF expression in mice. J Clin Invest. 2011;121:2911–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellison GM, Torella D, Dellegrottaglie S, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/ hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–986. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Mossman BT, Kolpa E, et al. Age-related differences in MAP kinase activity in VSMC in response to glucose or TNF-alpha. J Cell Physiol. 2003;197:418–425. [DOI] [PubMed] [Google Scholar]
- 25.Suleiman MS, Singh RJ, Stewart CE. Apoptosis and the cardiac action of insulin-like growth factor I. Pharmacol Ther. 2007;114:278–294. [DOI] [PubMed] [Google Scholar]
- 26.Caglayan E, Vantler M, Leppanen O, et al. Disruption of platelet-derived growth factor-dependent phosphatidylinositol 3-kinase and phospholipase Cgamma 1 activity abolishes vascular smooth muscle cell proliferation and migration and attenuates neointima formation in vivo. J Am Coll Cardiol. 2011;57:2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S, Hansson EM, Tikka S, et al. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–1491. [DOI] [PubMed] [Google Scholar]
- 28.Nataatmadja M, West M, West J, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl 1):II329–II334. [DOI] [PubMed] [Google Scholar]
- 29.He R, Guo DC, Estrera AL, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–678. [DOI] [PubMed] [Google Scholar]
- 30.Shen YH, Hu X, Zou S, et al. Stem cells in thoracic aortic aneurysms and dissections: potential contributors to aortic repair. Ann Thorac Surg. 2012;93:1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurpinski K, Lam H, Chu J, et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28:734–742. [DOI] [PubMed] [Google Scholar]
- 32.Daniel JM, Bielenberg W, Stieger P, et al. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. [DOI] [PubMed] [Google Scholar]
- 33.Yamanouchi D, Morgan S, Kato K, et al. Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:702–707. [DOI] [PubMed] [Google Scholar]
- 34.Davies LC, Rosas M, Jenkins SJ, et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majesky MW, Dong XR, Hoglund V, et al. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majesky MW, Dong XR, Regan JN, et al. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katare R, Riu F, Mitchell K, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. [DOI] [PubMed] [Google Scholar]
- 39.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. [DOI] [PubMed] [Google Scholar]
- 40.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au P, Tam J, Fukumura D, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naito H, Kidoya H, Sakimoto S, et al. Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J. 2011;31:842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasquinelli G, Tazzari PL, Vaselli C, et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. [DOI] [PubMed] [Google Scholar]
- 44.Zengin E, Chalajour F, Gehling UM, et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.