Abstract
Age-related macular degeneration (AMD) is a complex, multifactorial, progressive disease which represents a leading cause of irreversible visual impairment and blindness in older individuals. Human cytomegalovirus (HCMV), which infects 50–80% of humans, is usually acquired during early life and persists in a latent state for the life of the individual. In view of its previously described pro-angiogenic properties, we hypothesized that cytomegalovirus might be a novel risk factor for progression to an advanced form, neovascular AMD, which is characterized by choroidal neovascularization (CNV). The purpose of this study was to investigate if latent ocular murine cytomegalovirus (MCMV) infection exacerbated the development of CNV in vascular endothelial growth factor (VEGF)-overexpressing VEGF-Ahyper mice. Here we show that neonatal infection with MCMV resulted in dissemination of virus to various organs throughout the body including the eye, where it localized principally to the choroid in both VEGF-overexpressing-VEGF-Ahyper and wild-type(WT) 129 mice. By 6 months post-infection, no replicating virus was detected in eyes and extraocular tissues, although virus DNA was still present in all eyes and extraocular tissues of both VEGF-Ahyper and WT mice. Expression of MCMV immediate early (IE) 1 mRNA was detected only in latently infected eyes of VEGF-Ahyper mice, but not in eyes of WT mice. Significantly increased CNV was observed in eyes of MCMV-infected VEGF Ahyper mice compared to eyes of uninfected VEGF-Ahyper mice, while no CNV lesions were observed in eyes of either infected or uninfected WT mice. Protein levels of several inflammatory/angiogenic factors, particularly VEGF and IL-6, were significantly higher in eyes of MCMV-infected VEGF-Ahyper mice, compared to uninfected controls. Initial studies of ocular tissue from human cadavers revealed that HCMV DNA was present in four choroid/retinal pigment epithelium samples from 24 cadavers. Taken together, our data suggest that ocular HCMV latency could be a significant risk factor for the development of AMD. © 2020 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Keywords: cytomegalovirus, age-related macular degeneration, choroidal neovascularization, inflammation, VEGF
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible visual impairment and blindness in the elderly population [1]. The advanced stage, neovascular (or ‘wet’) AMD, is characterized by the invasion of new blood vessels from the choriocapillaris into the sub-retinal pigment epithelium (RPE) and/or subretinal spaces (choroidal neovascularization, CNV), which may ultimately result in blindness and accounts for the majority of cases of vision loss in AMD [2–4]. While the precise events which contribute to the development of AMD remain uncertain, a number of studies, using various animal models, have implicated several possible immunological/inflammatory mechanisms [5–7]. In particular, the laser-induced CNV mouse model outlines the angiogenic aspect of neovascular AMD [8,9] and is one of the most widely studied. Although this model of acute injury is reproducible and cost-effective [10], its clinical relevance is limited by the somewhat artificial nature of the CNV induced via laser-stimulated photocoagulation [9].
Changes in levels of pro- and anti-angiogenic growth factors derived from the RPE, particularly vascular endothelial growth factor (VEGF) [11–13], have been considered as a primary, underlying cause of CNV pathogenesis. As a result, VEGF-A-overexpressing mice (VEGF-Ahyper), initially developed by Nagy and coworkers [14], have been used as a model of neovascular AMD. These mice exhibit an approximately two- to three-fold increase in VEGF-A protein levels compared with wild-type mice, and develop CNV later in life [15–17]. Since it has previously been reported that ocular neovascularization may occur during the development of cytomegalovirus (CMV) retinitis, a disease with acute symptoms that is found predominantly in immunosuppressed individuals [18–20], we wondered whether neovascular AMD might also have a viral component, especially since it exhibits a significantly higher association with anti-human CMV (HCMV) IgG titers compared with either dry AMD or non-AMD controls [21].
HCMV infects the majority of the human population [22] and is typically acquired in early life when the immune system is immature [23]. The incidence of congenital infection ranges from 0.5% to 2.4% of all live births [24–28], and more than 12% of 1-year-old US infants were observed to be infected with HCMV [23]. Following primary infection, HCMV persists for the life of the host through cycles of latency and reactivation [29]. Latency can occur in multiple sites/cell types including vascular endothelial and hematopoietic cells [30], while HCMV can be reactivated by a variety of factors including immunosuppression, inflammation [31–34], and cell differentiation [35–37]. Although it is usually kept in check by a healthy immune system and thought to be mostly asymptomatic, increasing evidence suggests that HCMV infection is associated with some long-term diseases including atherosclerosis [38–40], vascular restenosis [41,42], and other vascular pathologies such as hypertension [43,44].
Since CMVs are species-specific, murine cytomegalovirus (MCMV) infection of mice has been widely used to model human diseases in which HCMV is thought to be involved. Although MCMV DNA could not be detected within eye tissues several months after systemic MCMV infection of adult C57 mice, Cousins et al [45] showed that systemic MCMV infection was associated with more severe laser-induced CNV, possibly due to activation of macrophages. Our recent studies [46] have produced the novel finding that acquisition of systemic MCMV infection by BALB/c mice during early life is associated with MCMV persistence at several sites including the choroid/RPE. Therefore, the purpose of this study was to investigate whether ocular MCMV could exacerbate the development of CNV following neonatal infection of VEGF-overexpressing VEGF-Ahyper and wild-type 129 mice.
Materials and methods
Ethics statement
The human eyes used were de-identified samples purchased at the Georgia Eye Bank Inc and were not from living individuals. The IRB Augusta University (AU) Committee determined that this project did not meet the definition of human subject research under the purview of the IRB according to federal regulations. The breeding and treatment of animals in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Augusta University (Protocol #2009–0252), and also adhered to the ARVO (the Association for Research in Vision and Ophthalmology) Statement and Guidance for the Use of Animals in Ophthalmic and Vision Research.
Virus
The MCMV strain K181 was prepared and used as described in supplementary material, Supplementary materials and methods.
Mice
The VEGF-A hypermorphic mouse (VEGF-Ahyper) was purchased from the Jackson Laboratory (Bar Harbor, ME, USA; stock No 027314) and maintained on the 129S1/SvImJ background. Information about this mouse is described in supplementary material, Supplementary materials and methods.
Experimental design – animal model
A hundred plaque-forming units (pfu) of MCMV or culture medium as control was injected intraperitoneally (i.p.) into VEGF-Ahyper or wild-type 129 mice at < 3 days after birth. At several subsequent time points, spectral-domain optical coherence tomography (SDOCT) and fluorescein angiography were performed on four groups of mice to determine if pathology indicative of CNV was present, as well as to verify the overall integrity of retinal structure as described in supplementary material, Supplementary materials and methods. Virus- and mock-infected VEGF-Ahyper and wild-type 129 mice were sacrificed at 2 weeks or 6 months post-infection (p.i.) and the tissues were harvested for further examination as described in supplementary material, Supplementary materials and methods.
Experimental design – human eyes
Twenty-four pairs of fresh eyes from 24 human cadavers (death-to-preservation times < 12 h) were obtained from Georgia Eye Bank, Inc (Atlanta, GA, USA). Donor information, including age, gender, primary cause of death, and medical history, is listed in supplementary material, Table S1. Immediately following reception of eye tissues, the anterior segment (cornea, ciliary body, and iris), posterior eye cup (choroid/RPE), and retina (neural retina) were carefully dissected and stored at −80 °C. Tissues were subsequently pulverized in a mortar under liquid nitrogen and tissue powders stored at −80 °C for DNA extraction and droplet digital PCR (ddPCR) assay for the presence of HCMV DNA.
Statistical analysis
Means ± standard error of the mean (SEM) were calculated for all data. Unpaired nonparametric Mann– Whitney tests were used to calculate the statistical significance of observed differences between groups in all experiments using the GraphPad Prism software (version 7; GraphPad Software, San Diego, CA, USA). P values less than 0.05 were considered statistically significant.
Results
MCMV disseminates to the eye of both VEGF-Ahyper and wild-type 129 mice following systemic infection of neonatal mice
100 pfu of MCMV was injected i.p into VEGF-Ahyper or wild-type 129 mice at < 3 days after birth. All mice survived viral infection and appeared healthy, with no significant difference in body weight among virus-infected and uninfected VEGF-Ahyper and wild-type 129 mice at all time points analyzed (Figure 1A).
Figure 1.
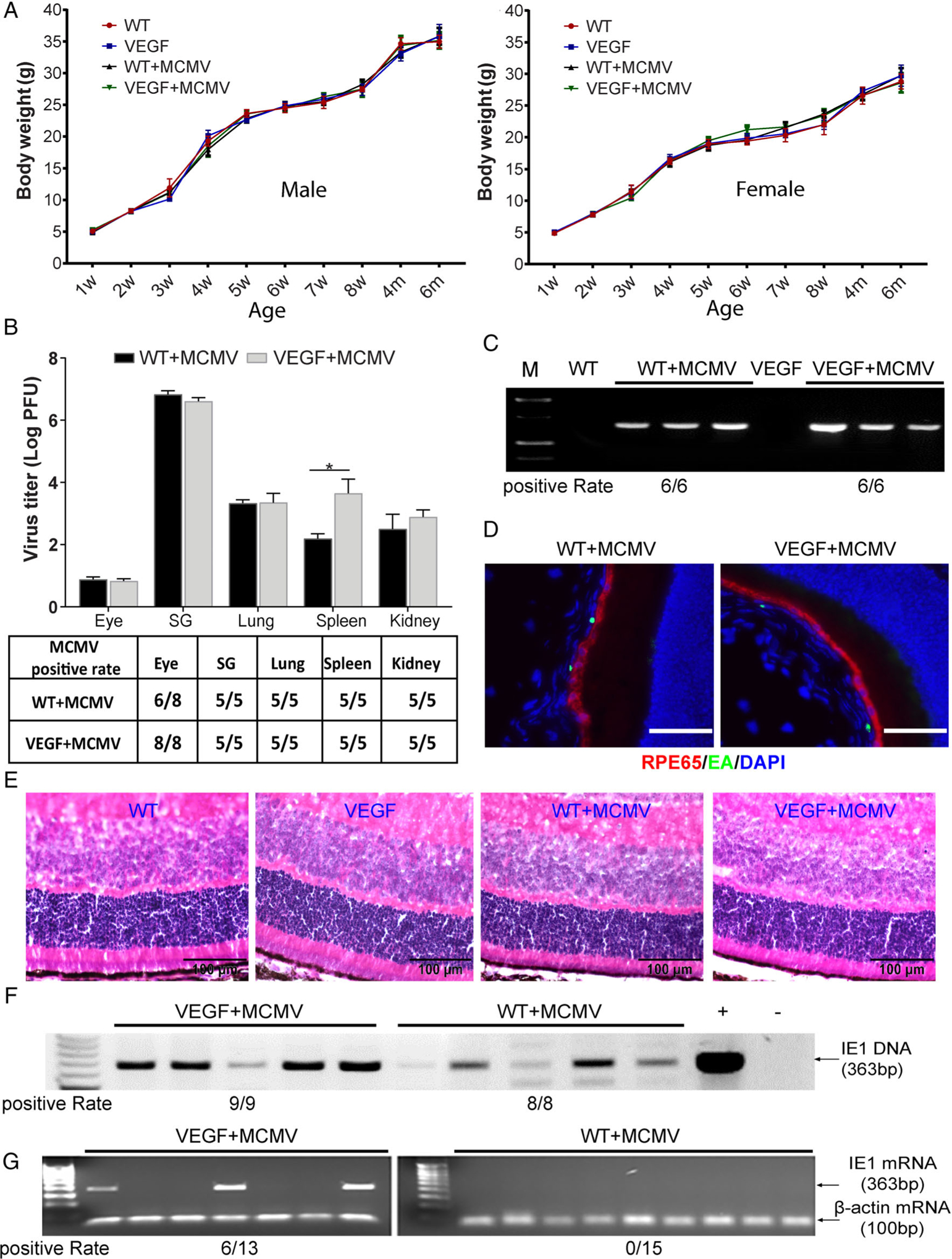
MCMV disseminates to and becomes latent in the eye following systemic infection of neonatal VEGF-Ahyper or wild-type 129 mice. (A) Postnatal changes in body weight in male (left) and female (right) neonatally-infected VEGF-Ahyper or WT mice, compared with uninfected control male and female mice of the same age (n = 20–30). (B) Titer of MCMV (upper) and MCMV positivity rate (lower) at day 14 p.i. in eyes, salivary glands, lungs, and kidneys determined by plaque assay (n = 5–8; *p < 0.05). (C) PCR assay for MCMV DNA in eyes of VEGF-Ahyper and WT mice at day 14 p.i. All mice tested positive. (D) Merged photomicrographs of ocular staining for RPE65 (red), MCMV EA (green), and DAPI (blue) from an infected WT mouse (left) and an infected VEGF-Ahyper mouse (right) at day 14 p.i. The majority of MCMV EA-positive cells are located in the choroid (scale bars: 50 μm). (E) Photomicrographs of ocular H&E staining of an infected WT mouse; an infected VEGF-Ahyper mouse at day 14 p.i.; an age-matched, uninfected control WT mouse; and an age-matched uninfected VEGF-Ahyper mouse (scale bars: 100 μm). (F) PCR assay for MCMV DNA in eyes of VEGF-Ahyper and WT mice at 6 months p.i. All eyes tested positive. (G) RT-PCR assay for MCMV IE1 mRNA in MCMV-infected VEGF-Ahyper and WT mice at 6 months p.i. IE1 mRNA was detected in 6 of 13 eyes from VEGF-Ahyper mice, while all 15 eyes from MCMV-infected WT mice tested negative.
At day 14 post-infection (p.i.), mice were sacrificed and eyes and extraocular tissues were collected for analyses. Replicating virus was recovered from the majority of eyes of both VEGF-Ahyper and wild-type 129 mice (Figure 1B). Likewise, MCMV DNA was detected in all eyes from both groups of MCMV-infected mice at day 14 p.i. (Figure 1C). In ocular tissues, MCMV infection was observed mainly in the choroid (Figure 1D) and sclera, and was present in all eyes of both VEGF-Ahyper (5/5) and WT mice (5/5). Although a few MCMV EA-positive cells were observed in the ciliary body and iris, no MCMV EA staining was located in the neural retina. The average number of EA-positive cells in the choroid (in nine sections) was 19.2 ± 1.6 per eye for VEGF-Ahyper mice and 21.2 ± 1.3 per eye for WT mice, while in the sclera, there were 45.6 ± 2.1 per eye for VEGF-Ahyper and 40.2 ± 1.8 per eye for WT mice. Not surprisingly, MCMV was also recovered from systemic tissues/organs including salivary glands, lungs, and kidneys in both groups of MCMV-infected mice (6/6 in each group, Figure 1B). H&E staining showed no remarkable pathological changes in the eyes of VEGF-Ahyper or wild-type 129 mice at 14 days p.i. (Figure 1E).
MCMV persists in ocular tissue of both VEGF-Ahyper and wild-type 129 mice, but expression of the MCMV IE1 gene occurs only in VEGF-Ahyper mice
At 6 months p.i., no replication-competent MCMV was recovered from eyes or systemic tissues/organs including lungs (0/6), kidneys (0/6), and salivary glands (0/6) of either VEGF-Ahyper or wild-type 129 mice. Nevertheless, as reported in our previous investigation of BALB/c mice [46], which are more susceptible to MCMV infection compared with other mouse strains [47,48], MCMV DNA was detected in all eyes of both VEGF-Ahyper (9/9) and WT mice (8/8) infected with virus at < 3 days after birth (Figure 1F), whereas MCMV DNA was undetectable in peripheral blood leukocytes of all latently infected mice (0/4, results not shown). Although latent ocular infection of BALB/c mice was associated with expression of MCMV immediate early (IE) genes [46], MCMV IE1 and IE3 mRNA was undetectable in all eyes of latently infected wild-type 129 mice (Figure 1G). In contrast, MCMV IE1 mRNA was detected in 6/13 eyes of VEGF-Ahyper mice at 6 months post-neonatal infection (Figure 1G), while no MCMV IE3 or late gene gB transcripts were detected in eyes of either VEGF-Ahyper mice or wild-type 129 mice at this time (results not shown).
MCMV-infected VEGF-Ahyper mice exhibit increased choroidal neovascularization compared with uninfected VEGF-Ahyper mice
Multiple techniques were used to evaluate CNV lesions in infected and uninfected VEGF-Ahyper and WT mice at 6 months p.i.
SD-OCT is a noninvasive imaging technique providing high-resolution, cross-sectional images of the retinal microstructure in vivo [49,50] and is widely used to monitor retinal abnormalities including CNV lesions [51,52]. Similar to previous observations on VEGF-Ahyper C57 mice or white mice [17], cataracts were present in eyes of most VEGF-Ahyper mice at 6 months of age. Although we did not perform SD-OCT examinations in some infected (3/18) and uninfected (1/22) VEGF-Ahyper eyes due to the presence of severe cataracts, we did perform SD-OCT examinations in the majority of mice using an Envisu R2210 system. This experiment demonstrated that CNV lesions were observed in significantly more eyes (13/15) from MCMV-latently-infectedVEGF-Ahyper mice compared with uninfected VEGF-Ahyper mice (9/21) (chi-square, p < 0.01). In addition to an increased frequency of mice exhibiting CNV, MCMV-infected VEGF-Ahyper mice (Figure 2A, lower right) also exhibited significantly more lesions per eye (Figure 2B, left) and a larger average lesion volume (Figure 2B, right), compared with uninfected VEGF-Ahyper mice (Figure 2A, upper right). CNV lesions were not observed in any eyes of infected (0/8) or uninfected (0/8) wild-type 129 mice (Figure 2A, lower left and upper left).
Figure 2.
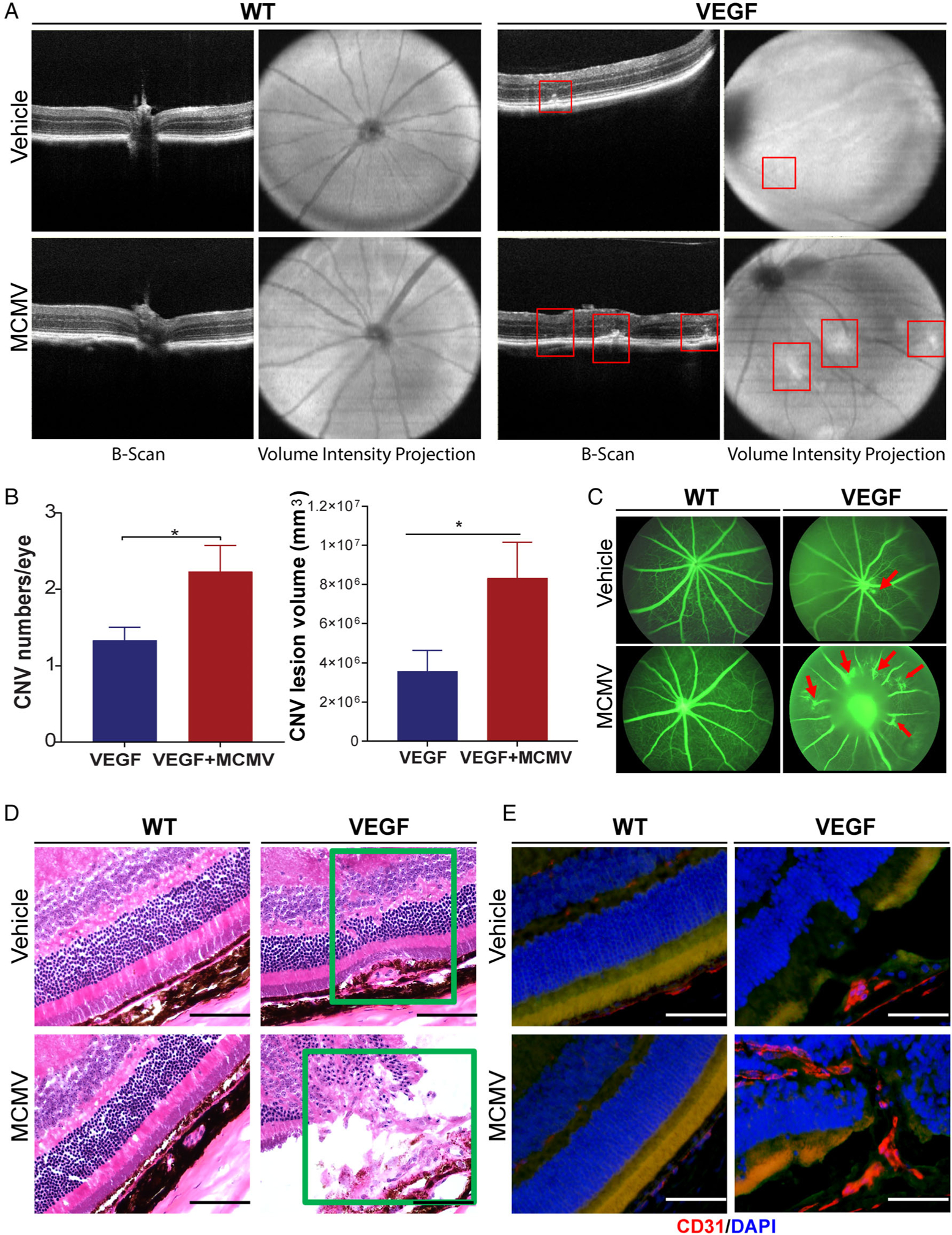
MCMV infection exacerbates CNV in VEGF-Ahyper but not in WT mice. (A) Representative images of SD-OCT from infected WT (lower left) and VEGF-Ahyper mice (lower right) at 6 months p.i.; also age-matched, uninfected WT (upper left) and VEGF-Ahyper mice (upper right). CNV lesions were found in MCMV-infected and uninfected VEGF-Ahyper mice (indicated by red squares). (B) The numbers (left) and volumes (right) of CNV lesions in MCMV-infected and uninfected VEGF-Ahyper mice (*p < 0.05). (C) Fluorescein angiography images of MCMV-latently-infected and uninfected VEGF-Ahyper and WT mice. (D) Photomicrographs of ocular H&E staining of MCMV-infected WT (lower left) and VEGF-Ahyper mice (lower right) at 6 months p.i., as well as from age-matched, uninfected WT (upper left) and VEGF-Ahyper mice (upper right). CNV lesions were observed in the subretinal space (upper right) and inside the inner retina (lower right) (scale bars: 100 μm). (E) Merged photomicrographs of ocular staining for CD31 (red) and DAPI (blue) from infected WT (lower left) and VEGF-Ahyper mice (lower right) at 6 months p.i., as well as from age-matched, uninfected WT (upper left) and VEGF-Ahyper mice (upper right). CD31-stained neovessels are present in the inner retina (lower right) (scale bars: 100 μm).
Due to the presence of cataracts, fluorescein angiography was successful in only half of infected (10/22) and uninfected (12/24) VEGF-Ahyper eyes, although the results were similar to the SD-OCT data. As shown in Figure 2C, an increased frequency of CNV was observed in MCMV-infected VEGF-Ahyper mice (6/10 positive), compared with uninfected VEGF-Ahyper mice (3/12 positive) (chi-square, p < 0.01).
To more precisely localize CNV lesions, frozen eye sections were stained with hematoxylin and eosin (H&E) (Figure 2D) as well as with anti-CD31 antibody (Figure 2E), a widely used marker of endothelial differentiation, although not entirely specific. The majority of CNV lesions in both MCMV-infected and uninfected VEGF-Ahyper mice were located above the RPE layer, either within the subretinal space as indicated in Figure 2D (upper right corner) or growing into the inner retina as indicated by Figure 2D,E (both in lower right corner). Consistent with previous studies by other investigators, which have shown that progressive, age-dependent photoreceptor degeneration occurs in VEGF-Ahyper mice [15–17], we observed a significantly thinner outer nuclear layer (ONL) in 6-month-old VEGF-Ahyper mice, compared with wild-type 129 mice in non-CNV areas (Figure 3A,B). In contrast, latent MCMV infection appeared to produce no remarkable effects on photoreceptor degeneration in VEGF-Ahyper mice as there was no significant difference in the thickness of the ONL between MCMV-infected and uninfected VEGF-Ahyper mice (Figure 3A,B).
Figure 3.
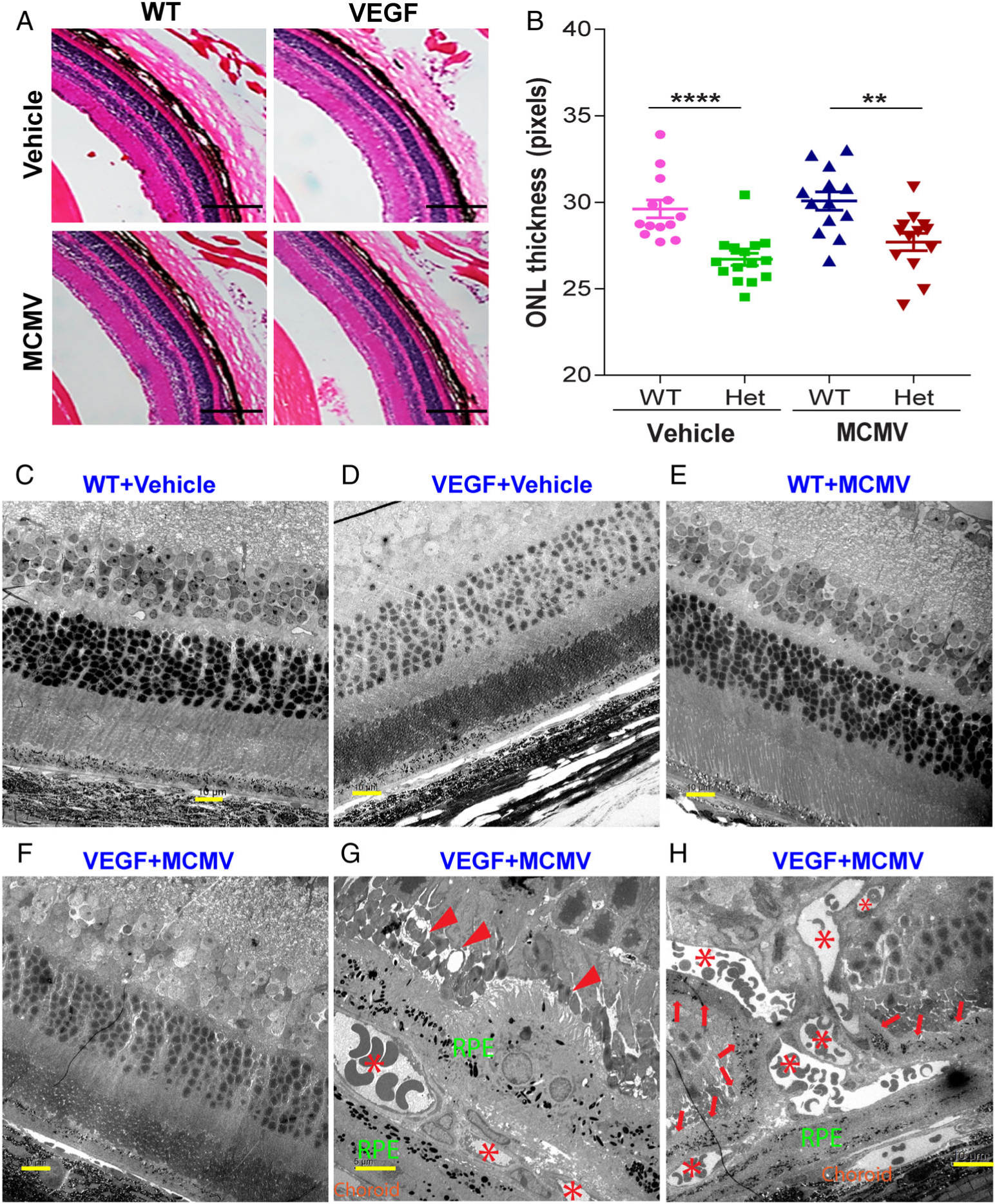
Features of photoreceptor degeneration and CNV lesions in VEGF-Ahyper mice. (A, B) Photomicrographs of ocular H&E staining (A) and analysis of ONL thickness (B) among infected and uninfected WT and VEGF-Ahyper mice 6 months p.i. A significantly thinner outer nuclear layer (ONL) was observed in MCMV-infected and uninfected VEGF-Ahyper mice, compared with infected and uninfected WT mice (scale bars: 400 μm). **p < 0.01; ****p < 0.0001. (C–H) Representative electron micrographs of ocular ultrastructure from infected WT (E) and VEGF-Ahyper mice (F, G, H) at 6 months p.i.; also age-matched, uninfected WT (C) and VEGF-Ahyper mice (D). No remarkable pathological changes were observed in the RPE layer or inner retina of non-CNV areas of both infected (F) and uninfected (D) VEGF-Ahyper mice. Some CNV lesions (indicated by aster isks) were observed in the RPE layer, surrounded by pigmented RPE-like cells (G). In addition, the morphology of nearby photoreceptors was disturbed, with shortening and loss of outer segments (G, indicated by arrowheads). Some CNV lesions (indicated by asterisks) grew into the inner retina (H) and many pigmented RPE-like cells were observed adjacent to new vessels in the inner retina (H, indicated by arrows). No remarkable pathological changes were observed in the RPE layer or inner retina of infected (E) or uninfected (C) WT mice.
Electron microscopy
To determine if other pathological changes typical of AMD, such as deposits at basal or apical aspects of the RPE, are associated with VEGF high expression and/or MCMV infection, eyes were collected from MCMV-infected VEGF-Ahyper and WT mice at 6 months p.i., as well as from age-matched, uninfected controls, and processed for examination by electron microscopy. Although a thinner ONL was observed in infected and uninfected VEGF-Ahyper mice in non-CNV areas, as shown in Figure 3D,F, no remarkable pathological changes such as deposits or cell death were observed in the RPE layer or inner retina. However, in areas where CNV developed, the morphology of nearby photoreceptors was disturbed, with shortening and loss of outer segments (Figure 3G, indicated by arrowheads). Furthermore, we observed that pigmented RPE-like cells appeared to proliferate and/or migrate along new vessels into the inner retina, as indicated by arrows in Figure 3H.
MCMV infection of VEGF-Ahyper mice increases ocular VEGF-A levels
Protein levels of VEGF-A in plasma and eyes of VEGF-Ahyper and WT mice were assayed by ELISA. Significantly higher levels of VEGF were present in plasma (Figure 4A) and eyes (Figure 4B) of VEGF-Ahyper mice, compared with WT mice, irrespective of the presence of virus. However, significantly more VEGF was detected in eyes of MCMV-infected VEGF-Ahyper mice compared with eyes of uninfected VEGF-Ahyper mice at 6 months p.i. (Figure 4B), whereas similar amounts of VEGF were detected in eyes of infected and uninfected WT mice. These results indicate that the presence of latent ocular MCMV further increased VEGF protein levels in eyes of VEGF-Ahyper mice.
Figure 4.
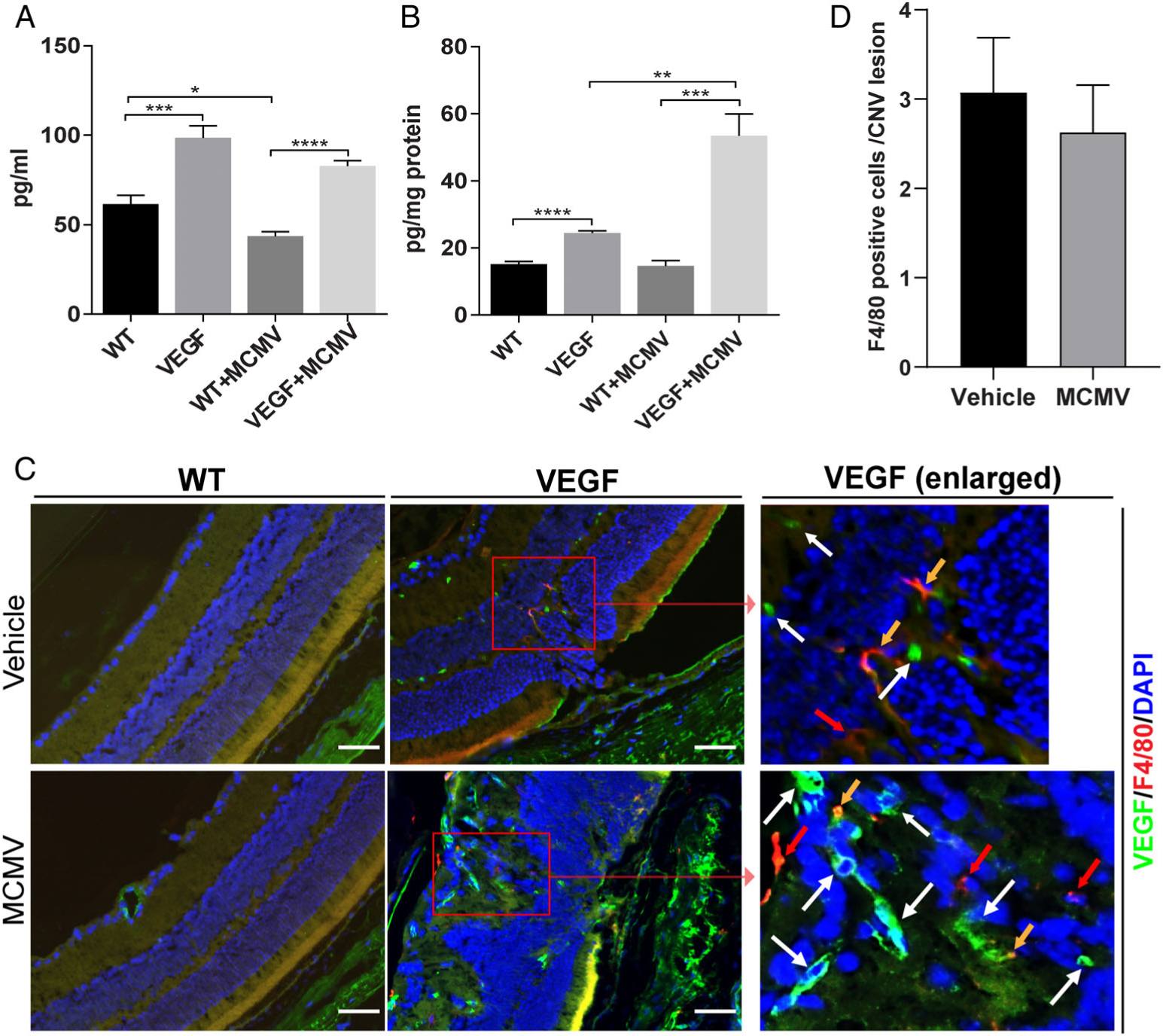
MCMV-infected VEGF-Ahyper mice contain increased ocular VEGF-A compared with uninfected VEGF-Ahyper mice at 6 months p.i. (A) Protein levels of VEGF in plasma assayed by ELISA (n = 6; *p < 0.05; ***p < 0.001; ****p < 0.0001). (B) Protein levels of ocular VEGF assayed by ELISA (n = 6; **p < 0.01; ***p < 0.001; ****p < 0.0001). (C) Merged photomicrographs of ocular staining for F4/80 (red), VEGF (green), and DAPI (blue) of MCMV-infected WT (lower left) and VEGF-Ahyper mice (lower middle and right) at 6 months p.i., as well as from age-matched, uninfected WT (upper left) and VEGF-Ahyper mice (upper middle and right). A few F4/80-stained cells were observed in CNV lesions, some of which co-stained with VEGF (yellow arrows), while some were VEGF-negative (red arrows). The majority of VEGF-stained cells were not F4/80 macrophages (white arrows) (scale bars: 100 μm). (D) The number of F4/80-stained cells per CNV lesion in MCMV-infected and uninfected VEGF-Ahyper mice at 6 months p.i.
The previous studies of Cousins et al [45] showed that systemic MCMV infection was associated with more severe laser-induced CNV in the C57 mouse strain. One possible mechanism might be related to macrophage activation [45]. Therefore, in order to localize VEGF production in the eye and also to determine if MCMV infection was related to recruitment of macrophages to CNV lesions, we performed immunofluorescence staining for both VEGF and the macrophage marker F4/80. As shown in Figure 4C, only a few VEGF-positive cells were observed in the choroid and retinal vessels from MCMV-infected and uninfected WT mice. In contrast, many VEGF-positive cells were found in the choroid, RPE layer, and inner retina of both MCMV-infected and uninfected VEGF-Ahyper mice. Not surprisingly, VEGF staining was particularly strong in areas with CNV lesions. F4/80-positive cells were rarely observed in WT eyes whether MCMV-infected or uninfected, whereas some F4/80-positive macrophages were observed in CNV lesions in both MCMV-infected(Figure 4C, lower right) and uninfected (Figure 4C, upper right) VEGF-Ahyper mice. Although some F4/80-positive macrophages were also VEGF-positive in both infected (Figure 4C, lower right) and uninfected (Figure 4C, upper right) VEGF-Ahyper mice (indicated by yellow arrows), the majority of VEGF-positive cells were not macrophages. Furthermore, as shown in Figure 4D, similar numbers of macrophages were observed in CNV lesions of infected VEGF-Ahyper, and uninfected VEGF-Ahyper mice.
Latent ocular MCMV infection is associated with upregulation of inflammatory/angiogenetic factors in VEGF-Ahyper mice
HCMV has been shown to induce angiogenesis via the production of many inflammatory/angiogenic factors [53] including VEGF [45,54,55], transforming growth factor beta (TGF-β) [56], and the cytokines/chemokines IL-6 [57,58] and C-C motif chemokine ligand 5 (CCL5) [59], while our previous studies have indicated that the receptor-interacting protein kinase 3 (RIP3) enhances innate immune responses against ocular MCMV infection [60,61]. In addition, previous studies have showed that GFAP-positive glial cells are activated following ocular MCMV infection [62], and activated glial cells might promote CNV [16]. To determine if latent ocular MCMV infection or VEGF high expression was associated with increased expression of inflammatory/angiogenic factors, the protein levels of these factors were measured by western blot and ELISA. Protein levels of ocular RIP3 (Figure 5A,B) and GFAP (Figure 5A,F) were altered by both MCMV infection and VEGF overexpression since they were significantly higher in MCMV-infected WT eyes, as well as in both infected and uninfected VEGF-Ahyper eyes, compared with uninfected WT eyes at 6 months p.i., although significantly more RIP3 protein was detected in infected VEGF-Ahyper eyes compared with MCMV-infected WT eyes or uninfected VEGF-Ahyper eyes. Protein levels of ocular TGF-β1 (Figure 5A,C) and CCL5 (Figure 5G) spiked during initial systemic MCMV infection but there was no association with VEGF overexpression since the ocular protein levels of both factors were similarly elevated in eyes of both MCMV-infected WT and MCMV-infected VEGF-Ahyper mice, compared with uninfected VEGF-Ahyper eyes. In contrast, IL-6 protein levels were significantly elevated in eyes of latently infected VEGF-Ahyper mice only, but not in eyes of mice belonging to the other three experimental groups (Figure 5H).
Figure 5.
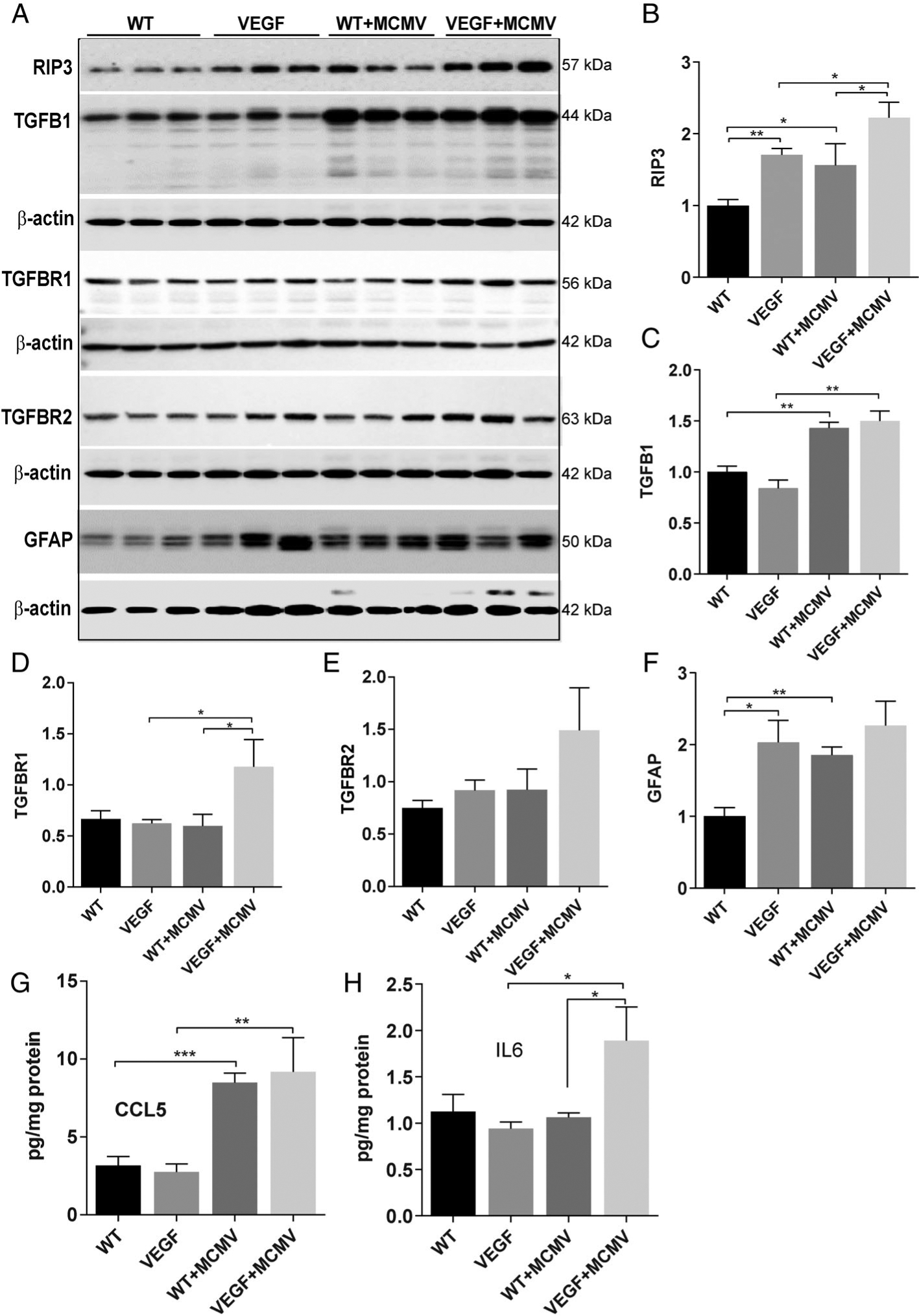
Protein levels of several inflammatory/angiogenic factors were increased in eyes of infected VEGF-Ahyper mice at 6 months p.i. (A–F) Western blots of RIP3, TGF-β1, TGF-β-R1, TGF-β-R2, and GFAP in eyes of MCMV-infected or uninfected WT and VEGF-Ahyper mice at 6 months p.i. Ratio of RIP3 to β-actin(B), TGF-β1 to β-actin(C), TGF-β-R1 to β-actin(D), TGF-β-R2 to β-actin(E), and GFAP to β-actin(F). (G, H) Protein levels of CCL5 (G) and IL-6(H) in eyes of MCMV-infected or uninfected WT and VEGF-Ahyper mice at 6 months p.i. Data are shown as mean ± SEM (for western blot, n = 3; for ELISA, n = 4) and compared by t-test. ***p < 0.001; **p < 0.01; *p < 0.05.
HCMV DNA is present in human ocular tissue
Since systemic MCMV infection of mice early in life resulted in MCMV latency in the choroid/RPE, we hypothesized that HCMV might spread to and become latent in the choroid/RPE of human eyes. To test this hypothesis, the anterior segment (cornea, ciliary body, and iris), posterior eye cup (choroid/RPE), and retina (neural retina) were dissected from 48 fresh eyes of 24 human cadavers obtained from Georgia Eye Bank, Inc. DNA was extracted and analyzed for the presence of HCMV DNA by ddPCR, a technology emulsifying an oil-based PCR reaction into thousands of droplets, each of which then acts as a PCR micro-reaction and increases the chances of rare target sequences being detected [63], as evidenced by its use in the detection of various pathogens [64–66] including latent HCMV infections [67,68]. As shown in supplementary material, Table S2, HCMV DNA was detected in four posterior eye cups (choroid/RPE) of four individual cadavers. In all four cases, infection was uniocular and no HCMV DNA was detected in contralateral eye tissue from the same cadaver. In addition, very low HCMV DNA copy numbers were detected in the retina of a fifth cadaver (1 of 48 retina samples), while no HCMV DNA could be detected in all 48 anterior segment samples. This result confirms our hypothesis that the human choroid/ RPE is a relatively common site for HCMV latency.
Discussion
The data presented in this article indicate that latent ocular MCMV infection exacerbates choroidal neovascularization in VEGF-A-overexpressing mice, suggesting that closer study of the role of cytomegaloviruses in AMD may be warranted. Systemic MCMV infection spreads to the choroid and becomes latent in both VEGF-overexpressing VEGF-Ahyper and wild-type 129 mice but MCMV IE1 gene expression is activated only in the eyes of latently infected VEGF-Ahyper mice. Since MCMV IE genes are first activated in the absence of de novo synthesis of viral proteins during virus reactivation [35], overexpression of VEGF-A could activate IE gene expression via the following pathways:
Cell proliferation and differentiation. Cytomegalovirus can be reactivated by several forms of stimulation including cell differentiation [35–37]. CNV requires proliferation/differentiation of choroidal endothelia and pericytes [69,70], in addition to differentiation of blood hematopoietic stem cells and monocytes to macrophages [69]. Our previous studies have shown that choroidal endothelia, pericytes, and RPE cells may contain latent virus following systemic neonatal MCMV-infection of BALB/c mice [46]. Macrophages originating from CD34+ progenitor cell-derived monocytes may also contain latent MCMV [30,34], although MCMV DNA was not detected in peripheral blood leukocytes in this study. Therefore, proliferation and differentiation of latently infected ocular cells or even macrophages – during the development of CNV in VEGF-Ahyper mice – could activate MCMV IE genes.
Immunosuppression. Cytomegaloviruses can be reactivated from latency by immunosuppression [31–34]. In addition to its crucial role in promoting the growth of new blood vessels, VEGF also exhibits some immunosuppressive properties. For instance, VEGF can suppress the function of T cells and modulate anti-tumor immunity via the recruitment of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and inhibition of the differentiation and activation of dendritic cells [71]. In addition, VEGF might also modulate anti-virus immunity [72]. Therefore, overexpression of VEGF-A in the choroid/RPE layer of VEGF-Ahyper mice could create an immunosuppressed environment to benefit MCMV latency and facilitating reactivation, resulting in IE gene expression. In addition, signaling events associated with inflammation are related to HCMV reactivation [30]. Recent studies suggest that the key inflammatory mediator IL-6 has multiple effects on HCMV reactivation ranging from increased IE gene expression to more efficient reactivation of infectious progeny [36,73,74]. Reactivation of latent virus from dendritic cells of healthy individuals can be significantly abrogated using neutralizing antibodies specific for IL-6 [74,75]. Therefore, increased production of IL-6 during ocular MCMV latency of VEGF-Ahyper mice might also contribute to the activation of MCMV gene expression.
Expression of MCMV IE1, but not IE3 or the gB late gene, was detected in eyes of MCMV-infected VEGF-Ahyper mice at 6 months p.i. IE1 is a protein considered to be a key activator of the virus productive cycle and expression of the IE1 gene has been used as a marker of reactivation, since it is presumed that the classic cascade of gene expression and progeny virus production will follow. However, the latent state is more dynamic than originally appreciated, and major immediate-early enhancer (MIE) expression alone does not guarantee full reactivation and production of progeny virus [35]. Ectopic expression of the IE1 proteins might not be sufficient to drive viral genome synthesis or infectious progeny production in infected cells [76], although it does influence the cellular environment [35]. Previous studies have suggested that HCMV IE1 alone can trigger a pro-inflammatory host transcriptional response via a STAT1-dependent mechanism [77,78]. In contrast, another study reported that the presence of MCMV IE1 potently inhibits the pro-inflammatory cytokine response including TNFα and IFNβ [79]. Therefore, expression of IE1 in the eye could modulate the production of inflammatory/angiogenetic factors in MCMV latently infected VEGF-Ahyper mice, thereby facilitating the development of CNV. In this study, approximately one half of the eyes (7/13) of infected mice did not express the IE1 gene at 6 months p.i. Since there is evidence which suggests that CMV latency is a highly dynamic condition, in which IE genes are expressed intermittently [80], latently infected eyes which did not express IE1 at 6 months p.i. would still express IE1 at other times during the course of the disease. Nevertheless, there is still the possibility that other viral genes or non-coding RNAs are expressed and contribute to disease. Previous results indicate that HCMV IL-10 [81] can bind to the human IL-10 receptor and induce the production of VEGF and TGF-β [82].
Several inflammatory/angiogenetic factors including CCL5, TGF-β1, RIP3, and IL-6 were significantly upregulated in eyes of both latently infected VEGF-Ahyper and wild-type 129 mice, compared with uninfected control eyes from age-matched VEGF-Ahyper or WT mice. Interestingly, levels of several cytokines, particularly IL-6, a principal cytokine in infection, cancer, inflammation, and angiogenesis [83], were significantly higher in eyes of latently infected VEGF-Ahyper mice compared with latently infected WT mice. IL-6 can be produced by almost all stromal cells and immune cells as a result of various types of stimulation [83]. It is a potent pleio-tropic cytokine and has context-dependentpro- and anti-inflammatory properties [84] while mediating a plethora of physiological functions [85–89]. IL-6 also plays a significant role in ocular angiogenesis. Previous studies from Izumi-Nagaiet al [90] have demonstrated that IL-6 plays a critical role in CNV generation associated with AMD via activation of signal transducer and activator of transcription-3(STAT3). Likewise, a study by Rojas et al [91] showed that IL-6 expression was essential for angiotensin II-induced increases in retinal VEGF expression, leukostasis, and vascular remodeling. Furthermore, IL-6 contributes to HCMV-induced angiogenesis [57,58] and was significantly induced during HCMV infection of endothelial cells. Virus-free super-natants from infected endothelial cells induced angiogenesis and neovessel formation, which was significantly reduced following neutralization of IL-6 [58]. Therefore, increased production of IL-6 during ocular MCMV infection of VEGF-Ahyper mice might exacerbate the development of CNV directly or indirectly via activation of STAT3 and subsequent production of VEGF.
In summary, the results presented herein demonstrate that acquiring systemic MCMV infection neonatally leads to ocular latency in 129 mice; prior overexpression of VEGF-A in MCMV latently infected VEGF-Ahyper mice induces activation of ocular virus gene expression and production of inflammatory/angiogenic factors including IL-6 and VEGF, and therefore enhances the development of CNV. Since a significant percentage of the human population acquires HCMV during early life and HCMV DNA was detected in the choroid/RPE of approximately 17% of the human cadavers analyzed, we would predict that ocular HCMV latency could be a significant risk factor for the development of AMD, although further studies are needed to determine which cells/tissue types within the RPE/choroid contain latent virus and virus gene expression in human AMD eyes.
Supplementary Material
Table S1. Donor information
Table S2. Copy number of HCMV DNA by ddPCR
Table S3. PCR primers
Acknowledgement
This work was supported by NIH grant EY026642.
Footnotes
No conflicts of interest were declared.
Data availability statement
The data that support the findings of this study are available from the corresponding author MZ, upon reasonable request.
References
*Cited only in supplementary material.
- 1.Friedman E Update of the vascular model of AMD. Br J Ophthalmol 2004; 88: 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowes Rickman C, Farsiu S, Toth CA, et al. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci 2013; 54: ORSF68–ORSF80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest 2010; 120: 3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorin MB. Genetic insights into age-related macular degeneration: controversies addressing risk, causality, and therapeutics. Mol Aspects Med 2012; 33: 467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Aspects Med 2012; 33: 487–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher EL, Jobling AI, Greferath U, et al. Studying age-related macular degeneration using animal models. Optom Vis Sci 2014; 91: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding JD, Kelly U, Groelle M, et al. The role of complement dysregulation in AMD mouse models. Adv Exp Med Biol 2014; 801: 213–219. [DOI] [PubMed] [Google Scholar]
- 8.Montezuma SR, Vavvas D, Miller JW. Review of the ocular angiogenesis animal models. Semin Ophthalmol 2009; 24: 52–61. [DOI] [PubMed] [Google Scholar]
- 9.Liu CH, Wang Z, Sun Y, et al. Animal models of ocular angiogenesis: from development to pathologies. FASEB J 2017; 31: 4665–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert V, Lecomte J, Hansen S, et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc 2013; 8: 2197–2211. [DOI] [PubMed] [Google Scholar]
- 11.Amin R, Puklin JE, Frank RN. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci 1994; 35: 3178–3188. [PubMed] [Google Scholar]
- 12.Kvanta A, Algvere PV, Berglin L, et al. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 1996; 37: 1929–1934. [PubMed] [Google Scholar]
- 13.Lopez PF, Sippy BD, Lambert HM, et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1996; 37: 855–868. [PubMed] [Google Scholar]
- 14.Miquerol L, Gertsenstein M, Harpal K, et al. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol 1999; 212: 307–322. [DOI] [PubMed] [Google Scholar]
- 15.Marneros AG. VEGF-A and the NLRP3 inflammasome in age-related macular degeneration. Adv Exp Med Biol 2016; 854: 79–85. [DOI] [PubMed] [Google Scholar]
- 16.Marneros AG. NLRP3 inflammasome blockade inhibits VEGF-A-inducedage-related macular degeneration. Cell Rep 2013; 4: 945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marneros AG. Increased VEGF-A promotes multiple distinct aging diseases of the eye through shared pathomechanisms. EMBO Mol Med 2016; 8: 208–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne JE, Jabs DA, Kempen JH, et al. Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology 2006; 113: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 19.Bogie GJ, Nanda SK. Neovascularization associated with cytomegalovirus retinitis. Retina 2001; 21: 85–87. [DOI] [PubMed] [Google Scholar]
- 20.Chan CK, Lin SG. Subfoveal choroidal neovascularization associated with cytomegalovirus retinitis and AIDS. Can J Ophthalmol 2008; 43: 488–489. [DOI] [PubMed] [Google Scholar]
- 21.Miller DM, Espinosa-Heidmann DG, Legra J, et al. The association of prior cytomegalovirus infection with neovascular age-related macular degeneration. Am J Ophthalmol 2004; 138: 323–328. [DOI] [PubMed] [Google Scholar]
- 22.Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis 1991; 13: 315–329. [DOI] [PubMed] [Google Scholar]
- 23.Lanzieri TM, Kruszon-Moran D, Amin MM, et al. Seroprevalence of cytomegalovirus among children 1 to 5 years of age in the United States from the National Health and Nutrition Examination Survey of 2011 to 2012. Clin Vaccine Immunol 2015; 22: 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pass RF, Stagno S, Myers GJ, et al. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 1980; 66: 758–762. [PubMed] [Google Scholar]
- 25.Hanshaw JB. Congenital cytomegalovirus infection: a fifteen year perspective. J Infect Dis 1971; 123: 555–561. [DOI] [PubMed] [Google Scholar]
- 26.Starr JG, Bart RD Jr, Gold E. Inapparent congenital cytomegalovirus infection. Clinical and epidemiologic characteristics in early infancy. N Engl J Med 1970; 282: 1075–1078. [DOI] [PubMed] [Google Scholar]
- 27.Stagno S, Reynolds DW, Huang ES, et al. Congenital cytomegalovirus infection. N Engl J Med 1977; 296: 1254–1258. [DOI] [PubMed] [Google Scholar]
- 28.Leinikki P, Granstrom ML, Santavuori P, et al. Epidemiology of cytomegalovirus infections during pregnancy and infancy. A prospective study. Scand J Infect Dis 1978; 10: 165–171. [DOI] [PubMed] [Google Scholar]
- 29.Presti RM, Pollock JL, Dal Canto AJ, et al. Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med 1998; 188: 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dupont L, Reeves MB. Cytomegalovirus latency and reactivation: recent insights into an age old problem. Rev Med Virol 2016; 26: 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins-McMillen D, Buehler J, Peppenelli M, et al. Molecular determinants and the regulation of human cytomegalovirus latency and reactivation. Viruses 2018; 10: E444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinclair J Human cytomegalovirus: latency and reactivation in the myeloid lineage. J Clin Virol 2008; 41: 180–185. [DOI] [PubMed] [Google Scholar]
- 33.Wills MR, Poole E, Lau B, et al. The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immuno-therapeutic strategies? Cell Mol Immunol 2015; 12: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu XF, Hummel M, Abecassis M. Epigenetic regulation of cellular and cytomegalovirus genes during myeloid cell development. Intern Med Rev (Wash D C) 2017; 3 10.18103/imr.v3i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodrum F Human cytomegalovirus latency: approaching the Gordian knot. Annu Rev Virol 2016; 3: 333–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hargett D, Shenk TE. Experimental human cytomegalovirus latency in CD14+ monocytes. Proc Natl Acad Sci U S A 2010; 107: 20039–20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reeves MB, MacAry PA, Lehner PJ, et al. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci U S A 2005; 102: 4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikitskaya E, Lebedeva A, Ivanova O, et al. Cytomegalovirus-productive infection is associated with acute coronary syndrome. J Am Heart Assoc 2016; 5: e003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nerheim PL, Meier JL, Vasef MA, et al. Enhanced cytomegalovirus infection in atherosclerotic human blood vessels. Am J Pathol 2004; 164: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorlie PD, Nieto FJ, Adam E, et al. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med 2000; 160: 2027–2032. [DOI] [PubMed] [Google Scholar]
- 41.Popovic M, Smiljanic K, Dobutovic B, et al. Human cytomegalovirus infection and atherothrombosis. J Thromb Thrombolysis 2012; 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 42.Skowasch D, Jabs A, Andrie R, et al. Pathogen burden, inflammation, proliferation and apoptosis in human in-stent restenosis. Tissue characteristics compared to primary atherosclerosis. J Vasc Res 2004; 41: 525–534. [DOI] [PubMed] [Google Scholar]
- 43.Firth C, Harrison R, Ritchie S, et al. Cytomegalovirus infection is associated with an increase in systolic blood pressure in older individuals. QJM 2016; 109: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haarala A, Kahonen M, Lehtimaki T, et al. Relation of high cytomegalovirus antibody titres to blood pressure and brachial artery flow-mediated dilation in young men: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol 2012; 167: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cousins SW, Espinosa-Heidmann DG, Miller DM, et al. Macrophage activation associated with chronic murine cytomegalovirus infection results in more severe experimental choroidal neovascularization. PLoS Pathog 2012; 8: e1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Xu J, Liu X, et al. Murine cytomegalovirus (MCMV) disseminates to and remains latent in the choroid following systemic infection of neonatal BALB/c mice. Invest Ophthalmol Vis Sci 2018; 59: 718 [Abstract]. [Google Scholar]
- 47.Brown MG, Dokun AO, Heusel JW, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 2001; 292: 934–937. [DOI] [PubMed] [Google Scholar]
- 48.Scalzo AA, Fitzgerald NA, Simmons A, et al. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J Exp Med 1990; 171: 1469–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991; 254: 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drexler W, Morgner U, Ghanta RK, et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med 2001; 7: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giani A, Thanos A, Roh MI, et al. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2011; 52: 3880–3887. [DOI] [PubMed] [Google Scholar]
- 52.Alnawaiseh M, Rosentreter A, Hillmann A, et al. OCT angiography in the mouse: a novel evaluation method for vascular pathologies of the mouse retina. Exp Eye Res 2016; 145: 417–423. [DOI] [PubMed] [Google Scholar]
- 53.Caposio P, Orloff SL, Streblow DN. The role of cytomegalovirus in angiogenesis. Virus Res 2011; 157: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maussang D, Langemeijer E, Fitzsimons CP, et al. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res 2009; 69: 2861–2869. [DOI] [PubMed] [Google Scholar]
- 55.Reinhardt B, Schaarschmidt P, Bossert A, et al. Upregulation of functionally active vascular endothelial growth factor by human cytomegalovirus. J Gen Virol 2005; 86: 23–30. [DOI] [PubMed] [Google Scholar]
- 56.Dumortier J, Streblow DN, Moses AV, et al. Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing. J Virol 2008; 82: 6524–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caposio P, Musso T, Luganini A, et al. Targeting the NF-κB pathway through pharmacological inhibition of IKK2 prevents human cytomegalovirus replication and virus-induced inflammatory response in infected endothelial cells. Antiviral Res 2007; 73: 175–184. [DOI] [PubMed] [Google Scholar]
- 58.Botto S, Streblow DN, DeFilippis V, et al. IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. Blood 2011; 117: 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penfold ME, Dairaghi DJ, Duke GM, et al. Cytomegalovirus encodes a potent alpha chemokine. Proc Natl Acad Sci U S A 1999; 96: 9839–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Mo J, Liu X, et al. Depletion of the receptor-interacting protein kinase 3 (RIP3) decreases photoreceptor cell death during the early stages of ocular murine cytomegalovirus infection. Invest Ophthalmol Vis Sci 2018; 59: 2445–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Liu X, Mo J, et al. Inflammation and outer blood–retina barrier (BRB) compromise following choroidal murine cytomegalovirus (MCMV) infections. Mol Vis 2018; 24: 379–394. [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Xin H, Roon P, et al. Infection of retinal neurons during murine cytomegalovirus retinitis. Invest Ophthalmol Vis Sci 2005; 46: 2047–2055. [DOI] [PubMed] [Google Scholar]
- 63.Taylor SC, Laperriere G, Germain H. Droplet digital PCR versus qPCR for gene expression analysis with low abundant targets: from variable nonsense to publication quality data. Sci Rep 2017; 7: 2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nyaruaba R, Mwaliko C, Kering KK, et al. Droplet digital PCR applications in the tuberculosis world. Tuberculosis (Edinb) 2019; 117: 85–92. [DOI] [PubMed] [Google Scholar]
- 65.Rutsaert S, Bosman K, Trypsteen W, et al. Digital PCR as a tool to measure HIV persistence. Retrovirology 2018; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibellini L, Pecorini S, De Biasi S, et al. Exploring viral reservoir: the combining approach of cell sorting and droplet digital PCR. Methods 2018; 134–135: 98–105. [DOI] [PubMed] [Google Scholar]
- 67.Lin CT, Leibovitch EC, Almira-Suarez MI, et al. Human herpesvirus multiplex ddPCR detection in brain tissue from low- and high-grade astrocytoma cases and controls. Infect Agent Cancer 2016; 11: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gianella S, Anderson CM, Var SR, et al. Replication of human herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol 2016; 90: 3944–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol 2004; 137: 496–503. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Wen F, Lim PN, et al. Nanomaterial scaffolds to regenerate musculoskeletal tissue: signals from within for neovessel formation. Drug Discov Today 2017; 22: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol 2018; 9: 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Savory LJ, Stacker SA, Fleming SB, et al. Viral vascular endothelial growth factor plays a critical role in orf virus infection. J Virol 2000; 74: 10699–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kew VG, Yuan J, Meier J, et al. Mitogen and stress activated kinases act co-operatively with CREB during the induction of human cytomegalovirus immediate-early gene expression from latency. PLoS Pathog 2014; 10: e1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reeves MB, Compton T. Inhibition of inflammatory interleukin-6 activity via extracellular signal-regulatedkinase-mitogen-activated protein kinase signaling antagonizes human cytomegalovirus reactivation from dendritic cells. J Virol 2011; 85: 12750–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang MM, Kew VG, Jestice K, et al. Efficient human cytomegalovirus reactivation is maturation dependent in the Langerhans dendritic cell lineage and can be studied using a CD14+ experimental latency model. J Virol 2012; 86: 8507–8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yee LF, Lin PL, Stinski MF. Ectopic expression of HCMV IE72 and IE86 proteins is sufficient to induce early gene expression but not production of infectious virus in undifferentiated promonocytic THP-1 cells. Virology 2007; 363: 174–188. [DOI] [PubMed] [Google Scholar]
- 77.Knoblach T, Grandel B, Seiler J, et al. Human cytomegalovirus IE1 protein elicits a type II interferon-like host cell response that depends on activated STAT1 but not interferon-gamma. PLoS Pathog 2011; 7: e1002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reitsma JM, Sato H, Nevels M, et al. Human cytomegalovirus IE1 protein disrupts interleukin-6 signaling by sequestering STAT3 in the nucleus. J Virol 2013; 87: 10763–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez-Martin S, Kropp KA, Wilhelmi V, et al. Ablation of the regulatory IE1 protein of murine cytomegalovirus alters in vivoproinflammatoryTNF-alpha production during acute infection. PLoS Pathog 2012; 8: e1002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lemmermann NA, Bohm V, Holtappels R, et al. In vivo impact of cytomegalovirus evasion of CD8 T-cell immunity: facts and thoughts based on murine models. Virus Res 2011; 157: 161–174. [DOI] [PubMed] [Google Scholar]
- 81.Kotenko SV, Saccani S, Izotova LS, et al. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc Natl Acad Sci U S A 2000; 97: 1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dziurzynski K, Wei J, Qiao W, et al. Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clin Cancer Res 2011; 17: 4642–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hunter CA, Jones SA. Corrigendum: IL-6 as a keystone cytokine in health and disease. Nat Immunol 2017; 18: 1271. [DOI] [PubMed] [Google Scholar]
- 84.Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813; 2011: 878–888. [DOI] [PubMed] [Google Scholar]
- 85.Schett G, Elewaut D, McInnes IB, et al. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat Med 2013; 19: 822–824. [DOI] [PubMed] [Google Scholar]
- 86.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 2007; 7: 429–442. [DOI] [PubMed] [Google Scholar]
- 87.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest 2011; 121: 3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasinghormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A 2000; 97: 9317–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kraakman MJ, Kammoun HL, Allen TL, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab 2015; 21: 403–416. [DOI] [PubMed] [Google Scholar]
- 90.Izumi-Nagai K, Nagai N, Ozawa Y, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol 2007; 170: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rojas M, Zhang W, Lee DL, et al. Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Invest Ophthalmol Vis Sci 2010; 51: 1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *92.Mo J, Marshall B, Covar JA, et al. Role of Bax in death of uninfected retinal cells during murine cytomegalovirus (MCMV) retinitis. Invest Ophthalmol Vis Sci 2014; 55: 7137–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *93.Sulaiman RS, Quigley J, Qi X, et al. A simple optical coherence tomography quantification method for choroidal neovascularization. J Ocul Pharmacol Res 2015; 31: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *94.Wang J, Cui X, Roon P, et al. Role of Sigma 1 receptor in retinal degeneration of the Ins2Akita/+ murine model of diabetic retinopathy. Invest Ophthalmol Vis Sci 2016; 57: 2770–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *95.Zhang M, Xin H, Duan Y, et al. Ocular reactivation of MCMV after immunosuppression of latently infected BALB/c mice. Invest Ophthalmol Vis Sci 2005; 46: 252–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Donor information
Table S2. Copy number of HCMV DNA by ddPCR
Table S3. PCR primers


