Summary
Antiproliferative factor (APF) is a potent frizzled protein 8-related sialoglycopeptide inhibitor of bladder epithelial cell proliferation that mediates its activity by binding to cytoskeletal associated protein 4 in the cell membrane. Synthetic asialylated APF (as-APF) (Galβ1–3GalNAcα-O-TVPAAVVVA) was previously shown to inhibit both normal bladder epithelial as well as T24 bladder carcinoma cell proliferation and heparin-binding epidermal growth factor-like growth factor (HB-EGF) production at low nanomolar concentrations, and an l-pipecolic acid derivative (Galβ1–3GalNAcα-O-TV-pipecolic acid-AAVVVA) was also shown to inhibit normal bladder epithelial cell proliferation. To better determine their spectrum of activity, we measured the effects of these APF derivatives on the proliferation of cells derived from additional urologic carcinomas (bladder and kidney), non-urologic carcinomas (ovary, lung, colon, pancreas, and breast), and melanomas using a 3H-thymidine incorporation assay. We also measured the effects of as-APF on cell HB-EGF and matrix metalloproteinase (MMP2) secretion plus cell invasion, using qRT-PCR, Western blot and an in vitro invasion assay. l-pipecolic acid as-APF and/or as-APF significantly inhibited proliferation of each cell line in a dose-dependent manner with IC50’s in the nanomolar range, regardless of tissue origin, cell type (carcinoma vs. melanoma), or p53 or ras mutation status. as-APF also inhibited HB-EGF and MMP2 production plus in vitro invasion of tested bladder, kidney, breast, lung, and melanoma tumor cell lines, in a dose-dependent manner (IC50=1–100 nM). Synthetic APF derivatives are potent inhibitors of urologic and non-urologic carcinoma plus melanoma cell proliferation, MMP2 production, and invasion, and may be useful for development as adjunctive antitumor therapy(ies).
Keywords: Antiproliferative factor, MMP2, HB-EGF, Invasion, Carcinoma, Melanoma
Background
Although progress has been made in the prevention and management of certain human malignancies, many cancers remain prevalent and difficult to treat. For example, lung and bronchial cancers, breast cancer, bladder cancer, and melanoma remain prevalent and leading causes of cancer deaths worldwide [1–7]. In addition, while ovarian and pancreatic cancers are less common than these other malignancies, they remain among the most fatal cancers in the U.S. [1, 6]. All of these malignancies readily metastasize, prompting the search for new or adjunctive treatments that control tumor cell growth or invasion to improve outcomes.
Activation of Wnt/β-catenin signaling has been shown to be critical for the development of several of these cancers [8–11], and an epithelial to mesenchymal transition (EMT) which is generally associated with decreased E-cadherin expression and resulting increased β-catenin signaling has also been implicated in tumor progression and/or survival for these and other epithelial malignancies [11–13]. In addition, several of these carcinomas and melanoma commonly have increased activity of various signaling pathways (including Akt and Erk/MAPK), as well as increased production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) and matrix metalloproteinase 2 (MMP2) [14–20]. Increased MMP2 production can result from increased Akt activation or canonical Wnt signaling [21], and MMP2 is one of several enzymes that can regulate cellular production of active HB-EGF by cleaving proHB-EGF in the cell membrane [22]. MMP2 also functions in tumor cell invasion and metastasis [19, 23, 24]. Therefore, identification of a factor that stimulates E-cadherin expression and decreases cellular β-catenin, while inhibiting HB-EGF and MMP2 production, plus Akt and Erk/MAPK signaling, has the potential to improve outcomes by preventing recurrence and/or progression of these malignancies to invasive or metastatic disease.
A natural growth inhibitor (antiproliferative factor, or APF) that is made by bladder epithelial cells from patients with interstitial cystitis/painful bladder syndrome (IC/PBS) was previously discovered to be a small sialoglycopeptide (Neu5Acα2–3Galβ1–3GalNAcα-O-TVPAAVVVA) [25] whose peptide backbone bears 100% homology to a segment from the 6th transmembrane segment of Frizzled 8, a receptor that functions in Wnt signaling [26]. APF profoundly inhibits cell proliferation via G2/M blockade [27, 28] and alters specific protein production in vitro, including the downstream effectors of Wnt signaling cyclin D1, JNK, and E-cadherin [29]. In addition, APF stimulates p53 expression [28] while inhibiting Akt [30] and Erk/MAPK signaling [31], as well as HB-EGF and MMP2 production by T24 bladder carcinoma cells and/or normal bladder epithelial cells in vitro [25, 30, 32]. Previous studies indicated that structural requirements for APF antiproliferative activity in normal bladder epithelial cells include the proximal 2 sugar moieties, at least 8 of the 9 N-terminal amino acids, a negative charge in the C-terminal amino acid, a free amino group at the N-terminus, maintenance of a specific amino acid sequence in the C-terminal tail, and trans conformation for the peptide bonds [33]. Therefore, subsequent studies have been performed with the active asialylated synthetic APF derivative (“as-APF”=Galβ1–3GalNAcα-O-TVPAAVVVA). Furthermore, a derivative of as-APF in which Pro3 was replaced by pipecolic acid (l-pipecolic acid as-APF) was also found to be a potent inhibitor of normal bladder cell proliferation [33], while a nonglycosylated APF peptide derivative was inactive and therefore useful as a negative control [25, 33].
Synthetic as-APF was also determined to have potent antiproliferative activity in T24 transitional carcinoma cells and HeLa cervical carcinoma cells at low nanomolar concentrations [25, 34]. We therefore sought to determine whether as-APF and/or l-pipecolic acid APF could also inhibit the proliferation of additional cancer cell lines that have abnormal expression or activity of the cell proteins involved in Akt/Wnt signalling, p53 and/or E-cadherin expression, including other bladder carcinoma lines, carcinoma cells derived from other tissues (kidney, ovary, lung, colon, pancreas, and breast), and melanoma cells. As many of these malignancies also share increased MMP2 and HB-EGF secretion as prognostic factors for tumor progression, we further determined the effect of these APF derivatives on expression of these proteins and their downstream effects on in vitro invasion by several carcinoma and melanoma cell lines.
Methods
Cell culture
T24 (HTB-4), 5637 (HTB-9), and RT4 (HTB-2) bladder transitional carcinoma cells; SCaBER (HTB-3) bladder squamous carcinoma cells; ACHN (CRL-1611) kidney carcinoma cells; Caov-3 ovarian carcinoma cells (HTB-75); A-427 (HTB-53), A549 (CCL-185) and H522 (CRL-5810) lung carcinoma cells; WiDr (CCL-218) colon carcinoma cells; PANC-1 (CRL-1469) pancreatic carcinoma cells; BT-474 (HTB-20), HCC1428 (CRL-2327), and Hs 578T (HTB-126) breast carcinoma cells; and Hs 839.T (CRL-7572) and A375 (CRL-1619) melanoma cells were obtained from ATCC.
Caov-3, PANC-1, BT-474, Hs 578T, A375, and Hs 839. T cells were grown in high glucose (4.5 g/L) DMEM (Invitrogen) containing 10% heat inactivated fetal bovine serum, 1% antibiotic/antimycotic solution, 1% l-glutamine (all from Sigma), and 1.5 g/L sodium bicarbonate (Invitrogen). A549 cells were grown in F-12K medium (Invitrogen) containing 10% heat inactivated fetal bovine serum, 1% antibiotic/antimycotic solution, and 1% l-glutamine. T24 and RT4 cells were grown in McCoy’s 5A medium (Invitrogen) containing 10% heat inactivated fetal bovine serum, 1% antibiotic/antimycotic solution, 1% l-glutamine, and 2.2 g/L sodium bicarbonate. 5637, H522, and HCC1428 cells were grown in RPMI 1640 medium containing 10% heat inactivated fetal bovine serum, 1% antibiotic/antimycotic solution, 1% l-glutamine (all from Sigma), 10 mM HEPES buffer, 1 mM sodium pyruvate, 4.5 g/L glucose, and 1.5 g/L sodium bicarbonate (Invitrogen). SCaBER, A-427, ACHN, and WiDr cells were grown in Eagle’s minimal essential medium (MEM) (Gibco-BRL, Life Technologies, Grand Island, New York) containing 10% heat inactivated fetal bovine serum, 1% antibiotic/antimycotic solution, 1% l-glutamine, 1.5 g/L sodium bicarbonate and 1 mM sodium pyruvate. All cells were incubated at 37°C in a 5% CO2 atmosphere.
Synthesis of APF and its derivatives
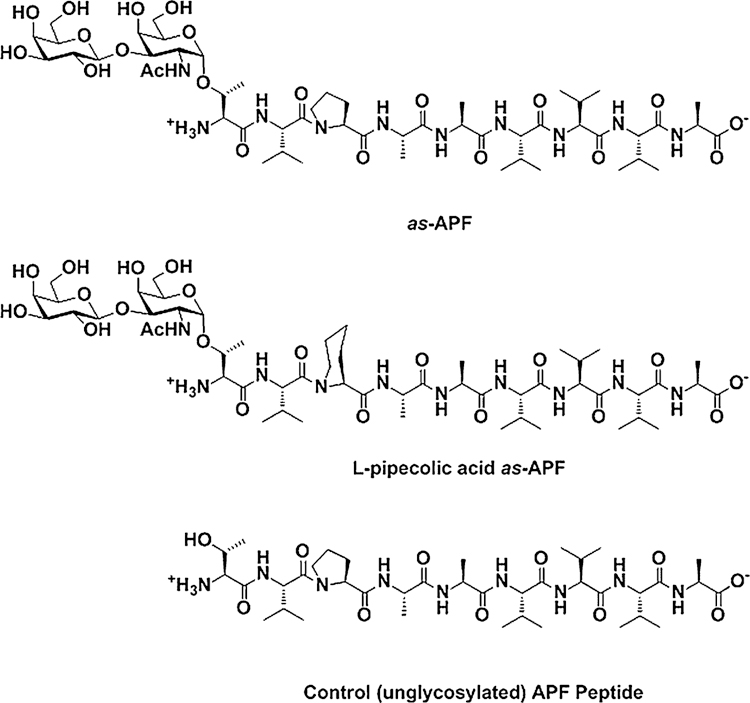
as-APF, l-pipecolic acid as-APF, and inactive control nonglycosylated peptide (structures shown in Fig. 1) were synthesized using standard Fmoc chemistry and purified using previously described methods [25, 33].
Fig. 1.
The chemical structure of as-APF, l-pipecolic acid as-APF, and unglycosylated APF control peptide
3H-thymidine cell proliferation assay
Cell proliferation was measured by 3H-thymidine incorporation into each cell type, plating cells in 150 μL of their respective medium (see above) onto a 96-well cell culture plate (Corning, NY) at a predetermined optimal cell density for APF inhibition of cell proliferation: all cell lines were plated at a density of 1.5×103 cells/well, with the exception of A549 and HCC1428 cells which were plated at a density of 3×103 cells/well. On the next day, cell growth medium was removed and replaced with serum-free medium appropriate for each cell type. On the third day, each APF derivative was resuspended in acetonitrile/distilled water (1:1) and applied to the cells in the respective serum-free medium in varying concentrations; cell controls received acetonitrile/distilled water diluted in serum-free medium alone (at the same final dilution). Cells were then incubated at 37°C in a 5% CO2 atmosphere for 48 h, after which cell contents were harvested, methanol-fixed onto glass fiber filter paper, and the amount of radioactivity incorporated determined. Each experiment was performed in triplicate at least twice.
Cell invasion assay
Cells were grown in T75 or T150 tissue culture flasks (Corning, Inc., Corning, NY) with the appropriate medium (as listed above) containing 10% heat-inactivated fetal bovine serum (FBS) in a 37°C/5% CO2 atmosphere. When cells were 80% confluent, the medium was changed to serum-free medium. Following overnight serum starvation, cells were treated with varying concentrations of as-APF or inactive control peptide for 72 h, after which they were trypsinized (using trypsin-EDTA, Invitrogen) and plated in serum-free medium in the upper chambers of ECMatrix™ -coated polycarbonate membranes (24-well insert, 8 μm pore size – ECM550) (Millipore, Billerica, MA) at a density of 1 × 106 live cells/mL; medium supplemented with 10% FBS was added to the lower chambers. Cells were then incubated for an additional 24 h, after which the invasive cells on the lower surface of the membrane were stained, dissolved with 10% acetic acid, transferred to a 96 well plate and OD determined at 560 nm.
Western blot
Cells were plated in T-75 or T-150 cell culture flasks (Corning) and cultured with growth media as described above. When each cell line reached 80–90% confluency, they were further incubated overnight in serum-free medium prior to treatment with 500 nM as-APF or negative control peptide for an additional 72 h. Medium was then collected, cells were pelleted by centrifugation at 4°C, and supernatant was aliquoted and frozen at −80°C until Western blot assay was performed.
Protein concentration was measured for Wetern blots using a Folin reagent-based protein assay kit (Bio-Rad, Hercules, CA). Cell supernatants were then incubated for 10 min at 70°C in sample reducing buffer, each lane was loaded with 20 μg of protein, and proteins were separated by electrophoresis using 4–12% NuPAGE NOVEX BisTris polyacrylamide gels (Invitrogen, Carlsbad, CA) in MOPS/SDS running buffer (Invitrogen), according to the manufacturer’s instructions. Proteins were then transferred to nitrocellulose membranes (Invitrogen) according to the NuPAGE gel manufacturer’s protocol for Western transfer (30 V constant voltage for 1 h). Following protein transfer, the nitrocellulose membranes were blocked with 5% nonfat dry milk in TBS-T buffer (Tris-buffered saline, pH 7.4, with 0.1% Tween 20) for 1 h at room temperature, then further incubated overnight at 4°C in TBS-T buffer containing rabbit polyclonal anti-MMP2 antibodies (Cell Signaling Technologies, Danvers, MA) or anti-HB-EGF antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were subsequently washed with TBS-T buffer, incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies (Santa Cruz Biotechnology) for 1 h at room temperature, and developed with ECL Chemiluminescence Reagent (Amersham Biosciences, Piscataway, NJ). GAPDH expression served as a standard control for the Western blot procedure (rabbit polyclonal primary antibodies from Cell Signaling Technologies).
RNA extraction
Following cell incubation with as-APF or its inactive control peptide, the culture medium was removed, cells were washed with 1X PBS, and RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA concentration was measured at 260 nm in a UV/VIS spectrophotometer from Perkin Elmer. Extracted RNA was stored at −80°C.
qRT-PCR
Gene expression was determined using SYBR®Green based real-time RT-PCR, QuantiTect® primers and reagents (Qiagen) and a Roche 480 LightCycler. Samples were tested in triplicate runs, and specific mRNA levels quantified and compared to mRNA levels for β-actin using Roche LC480 real-time PCR analysis software (version 1.5.0). Predetermined optimal concentrations of RNA were used for each set of primers. p53 (QT00060235), MMP2 (QT00088396), and β-actin (QT1680476) primer sets were obtained from Qiagen. (p53 served as a standard control for APF activity, while β-actin served as a standard control for the qRT-PCR procedure).
Statistical analysis
Comparisons of 3H-thymidine incorporation of cells treated with equimolar concentrations of as-APF vs. negative control peptide were performed using a two-tailed Student’s t test; statistical values are reported as the mean +/− standard error of the mean. Similar comparisons of the number of matrigel-invading cells treated with equimolar concentrations of as-APF vs. inactive control peptide were also performed, with statistical values reported as the mean +/− standard deviation of the mean. Crossover point analysis was performed for qRT-PCR data, and mRNA copy number for each gene was quantified relative to β-actin; this value is expressed as mean +/− standard error of the mean (SEM) for duplicate runs performed on three separate occasions; the significance of the difference between mean values was determined by an analysis of variance. Statistical significance was defined as p <.05 for all analyses.
Results
Inhibition of cell proliferation by synthetic APF derivatives
The cell lines tested for this study include a broad range of epithelial cancers plus two melanoma cell lines with varying status regarding known p53, H-ras, or K-ras mutations (Table 1). As APF was discovered to be made by bladder epithelial cells and was previously shown to inhibit the proliferation of T24 bladder carcinoma cells [25], we first determined the sensitivity of several additional bladder carcinoma cell lines to the growth inhibitory activity of as-APF as compared to T24 cells.
Table 1.
Human tumor cell line characteristics
| NCI designation (ATCC number) | Origin | Histologic type | ras/p53 Mutation |
|---|---|---|---|
| T24 (HTB-4) | urinary bladder | transitional cell carcinoma | H-ras, p53 |
| 5637 (HTB-9) | urinary bladder | transitional cell carcinoma | p53 |
| RT4 (HTB-2) | urinary bladder | transitional cell carcinoma | neither |
| SCaBER (HTB-3) | urinary bladder | squamous cell carcinoma | p53 |
| ACHN (CRL-1611) | kidney | renal cell carcinoma | neither |
| Caov-3 (HTB-75) | ovary | adenocarcinoma | p53 |
| A-427 (HTB-53) | lung | NSC adenocarcinoma | K-ras |
| A549 (CCL-185) | lung | NSC adenocarcinoma | K-ras |
| H522 (CRL-5810) | lung | NSC adenocarcinoma | K-ras, p53 |
| WiDr (CCL-218) | colon | adenocarcinoma | K-ras, p53 |
| PANC-1 (CRL-1469) | pancreas | epithelioid adenocarcinoma | K-ras, p53 |
| BT-474 (HTB-20) | breast | ductal adenocarcinoma | p53 |
| HCC1428 (CRL-2327) | breast | adenocarcinoma/pleural fluid | p53 |
| Hs 578T (HTB-126) | breast | ductal adenocarcinoma | H-ras, p53 |
| Hs 839.T (CRL-7572) | skin | melanoma | neither |
| A375 (CRL-1619) | skin | melanoma | neither |
As shown in Fig. 2, as-APF inhibited the proliferation of the two additional cell lines derived from bladder transitional carcinomas (5637 and RT4) as well as a bladder squamous carcinoma cell line (SCaBER) with IC50’s in the high picomolar to mid nanomolar range (p <.05 at concentrations ≥2.5 nM for all four cell lines). In addition, the l-pipecolic acid derivative of as-APF also significantly inhibited the proliferation of each of these lines plus T24 transitional carcinoma cells with comparable IC50’s (i.e., the same or within one tube dilution). Interestingly, neither the epithelial cell type of origin (transitional or squamous) nor the presence of a ras and/or p53 mutation appeared to influence sensitivity of the bladder carcinoma cells to these antiproliferative agents.
Fig. 2.
Inhibition of bladder carcinoma cell proliferation by synthetic APF derivatives. T24, 5637, RT4, and SCaBER cells were treated with varying concentrations of as-APF (■), l-pipecolic acid as-APF (○), or inactive control peptide (▼) for 48 h prior to determination of 3H-thymidine incorporation. Assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/− standard error of the mean
Therefore, we next determined whether cells derived from another urinary tract tumor (renal cell carcinoma) might also be sensitive to the antiproliferative effects of the APF derivatives. As shown in Fig. 3a, the in vitro proliferation of the ACHN renal cell carcinoma cell line was also inhibited by synthetic as-APF and l-pipecolic acid as-APF, with IC50’s in the low to mid nanomolar range (p <.05 at concentrations ≥250 pM). In addition, cells derived from a urogenital ovarian tumor (Caov-3 ovarian carcinoma cells) were also found to be sensitive to both as-APF derivatives with an IC50 in the same concentration range (Fig. 3b) (p<.05 at concentrations ≥2.5 nM).
Fig. 3.
Inhibition of kidney and ovarian carcinoma cell proliferation by synthetic APF derivatives. ACHN kidney carcinoma and Caov-3 ovarian carcinoma cells were treated with varying concentrations of as-APF (■), l-pipecolic acid as-APF (○), or inactive control peptide (▼) for 48 h prior to determination of 3H-thymidine incorporation. Assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/− standard error of the mean
We then examined whether carcinoma cells derived from tumors outside of the urogenital tract were also sensitive to these antiproliferative agents. Therefore, we tested the sensitivity of three K-ras mutant non-small cell (NSC) adenocarcinoma cell lines, one of which also carries a p53 mutation, to the active APF derivatives. As shown in Fig. 4, tumor cells derived from three NSC lung cancers with K-ras mutation were also inhibited by as-APF with IC50’s in the low nanomolar range, regardless of p53 mutation status (p <.05 at concentrations ≥250 pM for H522 cells; p <.05 at concentrations ≥2.5 nM for A549 and A-427 cells). Sensitivity of A549 cells to the l-pipecolic acid as-APF derivative was also determined to be similar to their sensitivity to as-APF.
Fig. 4.
Inhibition of lung carcinoma cell proliferation by synthetic APF derivatives. A549, H522, and A-427 cells were treated with varying concentrations of as-APF (■), l-pipecolic acid as-APF (○), or inactive control peptide (▼) for 48 h prior to determination of 3H-thymidine incorporation. Assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/− standard error of the mean
We next tested K-ras and p53 mutant cells derived from a colon adenocarcinoma (WiDr) and pancreatic epithelioid adenocarcinoma (PANC-1) for their sensitivity to l-pipecolic acid as-APF and as-APF. Both of these cell lines similarly proved to be sensitive to both APF derivatives with an IC50 in the high picomolar range (Fig. 5) (p <.05 at concentrations ≥250 pM). Sensitivity of both cell lines to the l-pipecolic acid as-APF derivative were again similar to their sensitivity to as-APF, close to within one dilution and within the low nanomolar range.
Fig. 5.
Inhibition of pancreatic and colon carcinoma cell proliferation by synthetic APF derivatives. WiDr and PANC-1 cells were treated with varying concentrations of as-APF (■), l-pipecolic acid as-APF (○), or inactive control peptide (▼) for 48 h prior to determination of 3H-thymidine incorporation. Assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/− standard error of the mean
The final carcinoma cells lines tested for sensitivity to both synthetic APF derivatives were three breast carcinoma cell lines (all with p53 mutations and one with both H-ras and p53 mutations). As shown in Fig. 6, each of these cell lines also proved to be very sensitive to as-APF and its l-pipecolic acid derivative, with IC50’s in the low to mid nanomolar range (p<.05 at concentrations ≥250 pM for Hs 578T cells; p <.05 at concentrations ≥2.5 nM for HCC1428 cells; p <.05 at concentrations ≥25 nM for BT474 cells).
Fig. 6.
Inhibition of breast carcinoma cell proliferation by synthetic APF derivatives. BT474, HCC1428, and Hs 578T cells were treated with varying concentrations of as-APF (■), l-pipecolic acid as-APF (○), or inactive control peptide (▼) for 48 h prior to determination of 3H-thymidine incorporation. Assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/− standard error of the mean
Because all of these carcinoma cell lines appeared to be fairly sensitive to as-APF, and because melanoma cells are derived from neuroepithelial cells in the neural crest, we also tested two melanoma cell lines for their sensitivity to these agents. As shown in Fig. 7, both melanoma cell lines tested were also sensitive to synthetic as-APF with IC50’s in the low nanomolar range (p <.05 at concentrations ≥250 pM for Hs 839.T cells; p <.05 at concentrations ≥2.5 nM for A375cells), and Hs 839.T was also sensitive to l-pipecolic acid as-APF with IC50 in the low nanomolar range. However, the dose response curve for both APF derivatives in Hs 839.T cells reproducibly had a markedly lower slope than that seen with the A375 melanoma cells or any of the other carcinoma cell lines tested.
Fig. 7.
Inhibition of melanoma cell proliferation by synthetic APF derivatives. Hs 839.T and A375 cells were treated with varying concentrations of as-APF (■), l-pipecolic acid as-APF (○), or inactive control peptide (▼) for 48 h prior to determination of 3H-thymidine incorporation. Assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/− standard error of the mean
Inhibition of MMP2 gene (mRNA) expression and MMP2 protein secretion by synthetic APF derivatives
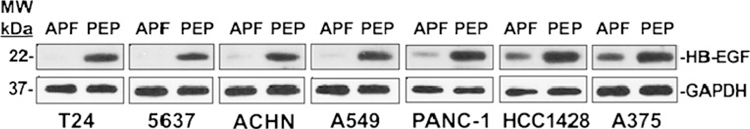
To better determine the spectrum of activity of APF derivatives, we also determined the effect of as-APF on MMP2 gene expression and secretion in seven cancer cell lines randomly selected from six tissues of origin [two from bladder, plus one each from kidney, lung, pancreas, breast, and skin (melanoma)]. Western blot was first performed on as-APF-treated (vs. negative control peptide-treated) cell supernatants from the same cancer cell lines to determine the effect of the synthetic APF derivatives on MMP2 secretion. As shown in Fig. 8a, treatment with as-APF (but not control peptide) for 72 h resulted in apparently decreased amounts of a protein at the appropriate molecular weight for MMP2 as compared to GAPDH for all of the seven cell lines, with the bladder carcinoma lines showing the greatest difference and melanoma cells showing the least difference between as-APF-treated and peptide-treated cells. We therefore next quantified mRNA by qRT-PCR in these same cells to determine whether the apparent differences in amount of MMP2 protein were from transcriptional or post-transcriptional effects of as-APF on MMP2 expression. Five of these cell lines proved to have a significant decrease in MMP2 mRNA following 72 h of as-APF (but not control peptide) treatment (p <.05 for comparison of all treated cells) (Fig. 8b). However, using the same commercially-available MMP2 primer set we were unable to detect valid amplification of MMP2 mRNA on several repeated attempts for two of the carcinoma cell lines (ACHN and HCC1428), even though they clearly secreted MMP2 protein per Western blot (Fig. 8a).
Fig. 8.
Inhibition of MMP2 mRNA expression and protein secretion by synthetic APF derivatives. T24, 5637, ACHN, A549, PANC-1, HCC1428, and A375 cells were serum-starved, then treated with 500 nM as-APF (“APF”) or negative control peptide (“PEP”) for 72 h prior to collection of supernatant and mRNA extraction. a Western blot of supernatant proteins; GAPDH was used as a control for secreted cell proteins. b qRT-PCR of cell mRNA. qRT-PCR was performed in duplicate on three separate occasions for each sample; data are expressed as the mean change in normalized mRNA copy number following treatment with as-APF (+/− standard deviation) as compared to negative control peptide (the latter set to “1”). (p=.001 for T24 cells; p=.002 for A549 cells; p=.003 for PANC-1 cells; p=.03 for 5637 cells; p=.04 for A375 cells)
Inhibition of cancer cell invasion by synthetic APF derivatives
As the collagenase MMP2 is also known to be important for tumor cell invasion [19, 23, 24], we next determined whether inhibition of MMP2 gene expression and secretion by as-APF treatment also influenced the ability of the same cancer cell lines to invade using an in vitro assay with ECMatrix™-coated polycarbonate membranes. As shown in Fig. 9, as-APF significantly inhibited invasion by all seven of these cancer cell lines in vitro at the highest concentration tested (p <.05 at concentrations of ≥250 nM). However, the IC50 differed between the cell lines, with HCC1428 and T24 cells being the most sensitive to inhibition of invasion (IC50 approximately 1 nM in both cases), and A375 and PANC-1 cells being the least sensitive (IC50 approximately 35 and 100 nM, respectively). It is interesting to note that, with the exception of PANC-1 cells, the relative sensitivity of each cancer cell line to inhibition of invasion (Fig. 9) mirrored the relative decrease in MMP2 protein secreted by each cell type (Fig. 8a) in response to as-APF treatment.
Fig. 9.
Inhibition of carcinoma and melanoma cell invasion by synthetic APF derivatives in vitro. T24, 5637, ACHN, A549, PANC-1, HCC1428, and A375 cells were serum-starved, then treated with 500 nM as-APF or negative control peptide for 72 h prior to plating in serum-free medium in the upper chambers of ECMatrix™ - coated polycarbonate membranes, with invasion determined following another 24 h of incubation. Assay was performed in duplicate; data are expressed as mean +/− standard deviation
Inhibition of HB-EGF secretion by synthetic APF derivatives
In addition to its role in collagen degradation and tumor cell invasion, MMP2 is also one of several enzymes known to cleave proHB-EGF in the cell membrane, resulting in the release of active HB-EGF [22]. As APF was previously shown to decrease the production of active HB-EGF (but not decrease proHB-EGF mRNA expression) by normal bladder epithelial cells [29, 32], we also determined whether as-APF inhibited HB-EGF secretion into the culture medium by these same cancer cell lines. As shown in Fig. 10, as-APF treatment indeed decreased the amount of HB-EGF in the cell medium of all seven cancer cell lines as compared to treatment with the control peptide. As was true for MMP2 secretion, as-APF appeared to have the greatest effect on HB-EGF secretion by the bladder carcinoma lines.
Fig. 10.
Inhibition of HB-EGF secretion by synthetic APF derivatives. T24, 5637, ACHN, A549, PANC-1, HCC1428, and A375 cells were serum-starved, then treated with 500 nM as-APF (“APF”) or negative control peptide (“PEP”) for 72 h prior to collection of supernatant. Western blot was performed using GAPDH control for secreted cell proteins
Discussion
In this manuscript we present evidence that two synthetic derivatives of APF (synthetic as-APF and its l-pipecolic acid derivative) are potent inhibitors of non-urologic carcinoma as well as bladder carcinoma, renal carcinoma and melanoma cell proliferation, with IC50’s in the high picomolar to mid nanomolar range for each cell type in vitro. In addition, we show evidence for significant inhibition of MMP2 and HB-EGF secretion, as well as in vitro invasion, of cancer cell lines from several different tissues of origin in response to as-APF. These data indicate that active APF derivatives have a potential role for development as adjunctive anti-tumor agents that inhibit the growth and invasion of certain cancers.
The bladder carcinoma cell lines chosen for this study were derived from both transitional and squamous carcinomas, which account for approximately 90% and 5% of bladder cancers, respectively [5] and which appear to be equally sensitive to growth inhibition by both APF derivatives. Certain genes known to be regulated by APF derivatives in normal bladder epithelial cells and/or T24 bladder carcinoma cells in prior studies [28, 30] are commonly found to have aberrant expression in bladder cancer cells (e.g., decreased E-cadherin [35, 36] and tumor suppressors p53 and p21 [37, 38]; increased expression of HB-EGF [39] and MMP2 [24, 40]; and increased Akt phosphorylation/activation [41]), all of which may contribute to increased tumor progression, cell proliferation, and/or metastasis. T24 bladder carcinoma cells are derived from a transitional cell carcinoma (TCC) and are known to have a p53 mutation, high levels of pRb expression, H-ras mutation, and no E-cadherin expression [42, 43]. T24 cells were found to have similar APF sensitivity to three bladder carcinoma cell lines that do not have any ras mutation but do have varying p53, Rb, and/or E-cadherin expression status [5637 cells (also a transitional cell carcinoma line, positive for p53 mutations but no pRb expression), SCaBER cells (a squamous bladder carcinoma cell line with a p53 mutation and high pRb expression), and RT4 cells (a low grade TCC cell line known to have wild type p53 and normal E-cadherin expression) [42, 43]. The similar APF sensitivity of bladder carcinoma cells with mutant p53 and/or Ras genes vs. those with wild type genes to these active APF derivatives indicates that aberrant activation of cell growth pathways involving these cell proteins is not able to abrogate the inhibition of cell proliferation by APF derivatives. APF derivatives therefore may be particularly useful for development as adjunctive therapies to control the proliferation of bladder epithelial tumors with abnormal Ras signaling and/or p53 mutation.
Patients diagnosed with transitional cell bladder cancer also have an approximately 5% risk of developing upper urinary tract carcinomas during their lifetime, and patients with kidney carcinoma have an increased risk of developing transitional bladder carcinoma as compared to the general population [5, 44] suggesting a possible etiologic association between upper and lower urinary tract malignancies. The kidney cell line chosen for this study (ACHN) was derived from renal cell carcinoma, the most common (>80%) type of kidney cancer, which is also known generally to have increased Akt signalling [45, 46], and is generally highly resistant to both radiation and chemotherapy [47]. This cell line was as sensitive to the antiproliferative effects of the APF derivatives as bladder carcinoma cells; therefore, further development of APF derivatives that can control Akt signalling and proliferation of urothelial carcinoma cells derived from various parts of the urinary tract may be useful for the adjunctive treatment of urologic epithelial tumors in general. However, whether inhibition of Wnt/Akt signalling plays a role in APF inhibition of proliferation for any of the urogenital carcinoma cell lines discovered to be sensitive to APF in the current study (as previously determined for T24 cells [30]) remains to be determined. Also unknown is whether the use of an adjunctive agent such as a synthetic APF derivative against either bladder or kidney carcinoma might be able to prevent subsequent development of related tumors in other parts of the urinary tract.
Additional carcinomas were also tested in the current study to determine whether tumors derived from non-urologic tissues that also commonly display abnormal Akt signaling and/or p53/ras mutations might be sensitive to active APF derivatives, as well. We first tested ovarian carcinoma cells, as they are not urothelial in origin but derived from another part of the urogenital tract, and ovarian carcinoma remains among the most lethal cancers [1, 2, 6]. Although these cells are also known to have a p53 mutation, they were similarly exquisitely sensitive to both synthetic APF derivatives.
The three lung carcinomas chosen for study were NSC adenocarcinoma cell lines, because up to 85% of lung carcinomas are classified as NSC carcinomas for which the overall five-year survival rate is only 15% and the five-year survival rate for Stage IV disease is approximately 1% [1, 2, 48, 49]; adenocarcinoma, which is the most common type of lung cancer in non-smokers, accounts for up to 35% of NSC carcinomas overall [49]. The prognosis for NSC lung carcinoma is also negatively associated with increased Akt phosphorylation as well as MMP2 production, K-ras mutation (which is present in 10–30%) and p53 mutation (which is present in 50–70% of NSC carcinomas) [14, 50–52]. Similarly, increased Akt signalling and MMP2 production, H-ras mutation (which occurs in approximately 5–13% of breast cancer lesions), and p53 mutation (which occurs in approximately 20–50% of breast cancer lesions) also correlate with an unfavorable prognosis for that malignancy [14, 50, 53–60]; K-ras and p53 mutations are also common in carcinomas from the GI tract where they are similarly associated with a decreased response to therapy [61]; and increased Akt signalling correlates with poor prognosis for pancreatic cancer [14, 62] while MMP2 secretion may also contribute to its invasion and metastasis [63, 64]. The discovery that the two active APF derivatives could inhibit the proliferation of, and MMP2 and HB-EGF secretion by, NSC lung, breast, and gastrointestinal carcinoma cells with ras and/or p53 mutations suggests they may be useful for development as antitumor agents for these carcinomas, as well.
Because each of the epithelial–derived tumor cells proved to be sensitive to these APF derivatives, we finally determined whether melanoma cells might also be sensitive to the effects of APF derivatives on cell proliferation, MMP2 and HB-EGF production, and invasion. Melanoma cells are also derived from neuroepithelial cells in the neural crest [65] and therefore share certain characteristics with carcinoma cells in that they can express carcinoembryonic antigen and/or keratin [66, 67]. Melanoma is also a prevalent malignancy, melanoma cells readily metastasize, the disease remains difficult to treat and it is also associated with aberrant Akt activation and MMP2 expression. In addition, both Akt and Erk/MAPK signalling pathways appear to be involved in the development and progression of melanoma [7, 68], and APF was previously shown to block HB-EGF-induced Akt [30] Erk/MAPK signalling in T24 cells [31]. Although MMP2 also appears to be important for the metastasis of melanoma [7, 19, 23, 68], the melanoma cells tested showed less sensitivity to the effect of the APF derivatives on MMP2 secretion and Matrigel invasion than the selected carcinoma lines. However, the proliferation of both melanoma lines tested was inhibited by these derivatives at concentrations comparable to those required for inhibition of the carcinoma cells, suggesting these derivatives may also be useful for development as adjunctive agents for treatment of melanoma. Whether either of the two APF derivatives used in the current study inhibits Akt and/or Erk/MAPK signalling in melanoma or any of the other non-bladder tumor cells remains to be investigated.
The bladder carcinoma cells tested in this study appeared to be the most sensitive overall to APF’s inhibition of cell proliferation, MMP2 and HB-EGF production, and invasion, while the other carcinoma and melanoma cells tested had differing levels of sensitivity to these various effects. For example, although treatment of PANC-1 cells with as-APF resulted in a significant decrease in MMP2 mRNA along with an apparent decrease in MMP2 protein secretion, these cells were the least sensitive to inhibition of cell invasion by as-APF (with IC50 around 100 nM). It is possible that the production of enzymes other than MMP2 which are also involved in pancreatic cancer cell invasion (such as matrilysin [69] or the src family kinase Fyn [70]) may not be affected by as-APF. However, because PANC-1 cells were among the most sensitive cells in terms of inhibition of HB-EGF production and cell proliferation by as-APF, it is nonetheless possible that the development of APF derivatives may be useful for adjunctive pancreatic cancer therapy.
A variety of cancers are particularly lethal because of their relative resistance to chemotherapy and radiation therapy, the latter of which can be increased by ras mutation and/or downstream Akt activation [71, 72]. Conversely, inhibition of PI3K/Akt signalling has been shown to synergistically increase the radiosensitivity of various tumor cells including urogenital cell lines (such as T24 bladder carcinoma cells [73], CaKi-1 renal carcinoma cells [74], LNCaP prostate cancer cells [75] and HeLa cells [76]), A549 NSC lung carcinoma cells [77, 78], several melanoma cell lines [79], and glioma cells [72]. One of the ways by which inhibition of PI3K/Akt signalling enhances radiosensitivity is thought to involve induction of a G2/M cell cycle block, which by itself is known to enhance cell sensitivity to radiation [80]. In addition to its ability to inhibit Akt phosphorylation (via its CKAP4 cell membrane receptor in T24 bladder carcinoma cells) [30], APF has been shown to induce a G2/M block in both normal bladder epithelial cells and T24 bladder carcinoma cells [27, 28]. Therefore, it will be important in future studies to determine whether APF derivatives are also able to enhance the radiosensitivity of resistant carcinoma and melanoma cell lines.
CKAP4/p63 has been shown to mediate as-APF inhibition of cell proliferation and Akt signalling in normal primary bladder epithelial cells and/or T24 bladder carcinoma cells (as shown by anti-CKAP4/p63 antibodies and/or CKAP4/p63 siRNA knockdown) [30, 81]. Whether this cell membrane protein is also necessary and sufficient for mediating APF activity in other carcinoma or melanoma lines remains to be determined. Each of the malignant cell lines in this report was sensitive to the antiproliferative effects of both APF derivatives in the high picomolar to mid nanomolar range, and there was no significant difference (defined as a difference in IC50 greater than one tube dilution) between the two agents for 10 of the 13 cell lines in which they were compared. However, the consistently lower slope of the dose–response curves for inhibition of Hs 893.T melanoma cell proliferation (as compared to A375 melanoma cells as well as all normal epithelial or carcinoma cells studied to date in this and other studies [25, 33, 34]), suggests the possibility of a different or additional APF receptor, or the inhibition of different or additional signaling pathway(s), in these cells as compared to the other cell lines in this study.
Conclusions
Many carcinomas and melanomas that remain prevalent and difficult to treat have in common aberrations in cell signaling (including Akt and/or Erk/MAPK) and expression of certain genes (including MMP2 and HB-EGF) that contribute to unregulated proliferation and/or invasion and metastasis. Synthetic APF derivatives that were previously shown to inhibit proliferation, Akt activation, and gene expression for a variety of cell growth-related proteins (including HB-EGF and MMP2) in normal bladder epithelial and/or T24 bladder carcinoma cells, are also potent inhibitors of cell proliferation, HB-EGF and MMP2 secretion, plus invasion, for several non-urologic carcinoma as well as urologic carcinoma and melanoma cell lines. These and other active APF derivatives may therefore be useful for development as adjunctive antitumor therapy(ies).
Acknowledgements
The authors thank Stewart Martin, Greenebaum Cancer Center and the University of Maryland School of Medicine, for providing some of the breast carcinoma cell lines, and Eunice Katz for assistance with the preparation of this manuscript. This material is based upon work supported by the Office of Research and Development (Medical Research Service), Department of Veterans Affairs, as well as funding from the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We also gratefully acknowledge the assistance of the Biophysics Resource in the Structural Biophysics Laboratory, at the Center for Cancer Research, National Cancer Institute.
Footnotes
Disclosures Susan Keay and Piotr Kaczmarek are named as inventors on patents involving APF.
Contributor Information
Kristopher R. Koch, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD 21201, USA
Chen-Ou Zhang, Department of Pathology, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Susan Keay, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD 21201, USA; Veterans Administration Maryland Health Care System, Baltimore, MD 21201, USA.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300 [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg JE, Carroll PR, Small EJ (2005) Update on chemotherapy for advanced bladder cancer. J Urol 174:14–20 [DOI] [PubMed] [Google Scholar]
- 4.Sengupta N, Siddiqui E, Mumtaz FH (2004) Cancers of the bladder. J R Soc Promot Health 124:228–229 [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DS, Shipley WU, Feldman AS (2009) Bladder cancer. Lancet 374:239–249. 10.1016/So140-6736(09)60491-60498 [DOI] [PubMed] [Google Scholar]
- 6.Ward EM, Thun MJ, Hannan LM, Jemal A (2006) Interpreting cancer trends. Ann NY Acad Sci 1076:29–53 [DOI] [PubMed] [Google Scholar]
- 7.Miller AJ, Mihm MC Jr (2006) Mechanisms of disease: Melanoma. New Engl J Med 355:51–65 [DOI] [PubMed] [Google Scholar]
- 8.Thievessen I, Seifert HH, Swiatkowski S, Flori AR, Schulz WA (2003) E-cadherin involved in inactivation of WNT/beta-catenin signalling in urothelial carcinoma and normal urothelial cells. Br J Cancer 88:1932–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates RC, Mercurio AM (2005) The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther 4:365–370 [DOI] [PubMed] [Google Scholar]
- 10.Berx G, Van Roy F (2001) The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 3:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakatsuki S, Watanabe R, Saito K, Saito T, Katagiri A, Sato S, Tomita Y (1996) Loss of human E-cadherin (ECD) correlated with invasiveness of transitional cell cancer in the renal pelvis, ureter and urinary bladder. Cancer Lett 103:11–17 [DOI] [PubMed] [Google Scholar]
- 12.Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558 [DOI] [PubMed] [Google Scholar]
- 13.Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39:305–318 [DOI] [PubMed] [Google Scholar]
- 14.Steelman LS, Stadelman KM, Chappell WH, Horn S, Bäsecke J, Cervello M, Nicoletti F, Libra M, Stivala F, Martelli AM, McCubrey JA (2008) Akt as a therapeutic target in cancer. Expert Opin Ther Targets 12:1139–1165 [DOI] [PubMed] [Google Scholar]
- 15.Grant S (2008) Co-targeting survival signalling pathways in cancer. J Clin Invest 118:3003–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo AE, Torrisi E, Bevelacqua Y, Perrotta R, Libra M, McCubrey JA, Spandidos DA, Stivala F, Malaponte G (2009) Melanoma: molecular pathogenesis and emerging target therapies. Int J Oncol 34:1481–1489 [DOI] [PubMed] [Google Scholar]
- 17.Dreesen O, Brivanlou AH (2007) Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev 3:7–17 [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto S, Yagi H, Yotsumoto F, Horiuchi S, Yoshizato T, Kawarabayashi T, Kuroki M, Mekada E (2007) New approach to cancer therapy: heparin binding-epidermal growth factor-like growth factor as a novel targeting molecule. Anticancer Res 27:3713–3721 [PubMed] [Google Scholar]
- 19.Kleiner DE, Stetler-Stevenson WG (1999) Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 43(Suppl): S42–S51 [DOI] [PubMed] [Google Scholar]
- 20.Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67. 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, Knöfler M (2010) Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology 151:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razandi M, Pedram A, Park ST, Levin ER (2003) Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278:2701–2712 [DOI] [PubMed] [Google Scholar]
- 23.Gould Rothberg BE, Bracken MB, Rimm DL (2009) Tissue biomarkers for prognosis in cutaneous melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 101:452–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanayama H, Yokota K, Kurokawa Y, Murakami Y, Nishitani M, Kagawa S (1998) Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer 82:1359–1366 [PubMed] [Google Scholar]
- 25.Keay SK, Szekely Z, Conrads TP, Veenstra TD, Barchi JJ Jr, Zhang C-O, Koch KR, Michejda CJ (2004) An antiproliferative factor from interstitial cystitis patients is a Frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA 101:11803–11808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitoh T, Hirai M, Katoh M (2001) Molecular cloning and characterization of human Frizzled-8 gene on chromosome 10p11.2. Int J Oncol 18:991–996 [DOI] [PubMed] [Google Scholar]
- 27.Rashid HH, Reeder JE, O’Connell MJ, Zhang CO, Messing EM, Keay SK (2004) Interstitial cystitis antiproliferative factor (APF) as a cell-cycle modulator. BMC Urol 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Keay SK, Dimitrakov JD, Freeman MR (2007) p53 mediates interstitial cystitis antiproliferative factor (APF)-induced growth inhibition of human urothelial cells. FEBS Lett 581:3795–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keay S, Seillier-Moiseiwitsch F, Zhang C-O, Chai TC, Zhang J (2003) Changes in human bladder cell gene expression associated with interstitial cystitis or antiproliferative factor treatment. Physiol Genomics 14:107–115 [DOI] [PubMed] [Google Scholar]
- 30.Shahjee H, Koch K, Guo L, Zhang C-O, Keay S (2010) Antiproliferative factor decreases Akt phosphorylation and alters gene expression via CKAP4 in T24 bladder carcinoma cells. J Exp Clin Cancer Res 29:160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Keay SK, Freeman MR (2009) Heparin-binding epidermal growth factor-like growth factor functionally antagonizes interstitial cystitis antiproliferative factor via mitogen-activated protein kinase pathway activation. BJU Int 103:541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keay S, Kleinberg M, Zhang C-O, Hise MK, Warren JW (2000) Bladder epithelial cells from interstitial cystitis patients produce an inhibitor of HB-EGF production. J Urol 164:2112–2118 [PubMed] [Google Scholar]
- 33.Kaczmarek P, Keay SK, Tocci GM, Koch KR, Zhang C-O, Barchi JJ Jr, Grkovic D, Guo L, Michejda CJ (2008) Structure-activity relationship studies for the peptide portion of the bladder epithelial cell antiproliferative factor from interstitial cystitis patients. J Med Chem 51:5974–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Planey SL, Keay SK, Zhang CO, Zacharias DA (2009) Palmitoylation of cytoskeleton associated protein 4 by DHHC2 regulates antiproliferative factor-mediated signaling. Mol Biol Cell 20:1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahnken A, Kausch I, Feller AC, Kruger S (2005) E-cadherin immunoreactivity correlates with recurrence and progression of minimally invasive transitional cell carcinomas of the urinary bladder. Oncol Rep 14:1065–1070 [PubMed] [Google Scholar]
- 36.Kashibuchi K, Tomita K, Schalken JA, Kume H, Yamaguchi T, Muto S, Horie S, Kitamura T (2006) The prognostic value of E-cadherin, alpha-, beta-, and gamma-catenin in urothelial cancer of the upper urinary tract. Eur Urol 49:839–845 [DOI] [PubMed] [Google Scholar]
- 37.Stein JP, Ginsberg DA, Grossfeld GD, Chatterjee SJ, Esrig D, Dickinson MG, Groshen S, Taylor CR, Jones PA, Skinner DG, Cote RJ (1998) Effect of p21WAF1/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Instit 90:1072–1079 [DOI] [PubMed] [Google Scholar]
- 38.Slaton JW, Benedict WF, Dinney CP (2001) p53 in bladder cancer: mechanism of action, prognostic value, and target for therapy. Urology 57:852–859 [DOI] [PubMed] [Google Scholar]
- 39.Thøgersen VB, Sørensen BS, Poulsen SS, Orntoft TF, Wolf H, Nexo E (2001) A subclass of HER1 ligands are prognostic markers for survival in bladder cancer patients. Cancer Res 61:6227–6233 [PubMed] [Google Scholar]
- 40.Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F (1993) Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res 53:5365–5369 [PubMed] [Google Scholar]
- 41.Ching CB, Hansel DE (2010) Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Lab Invest 90:1406–1414 [DOI] [PubMed] [Google Scholar]
- 42.Cooper MJ, Haluschak JJ, Johnson D, Schwartz S, Morrison LJ, Lippa M, Hatzivassiliou G, Tan J (1994) p53 mutations in bladder carcinoma cell lines. Oncol Res 6:569–579 [PubMed] [Google Scholar]
- 43.Sanchez-Carbayo M, Socci ND, Charytonowicz E, Lu M, Prystowsky M, Childs G, Cordon-Cardo C (2002) Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res 62:6973–6980 [PubMed] [Google Scholar]
- 44.Smith H, Weaver D, Barjenbruch O, Weinstein S, Ross G Jr (1989) Routine excretory urography in follow-up of superficial transitional cell carcinoma of bladder. Urology 34:193–196 [DOI] [PubMed] [Google Scholar]
- 45.Porta C, Figlin RA (2009) Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol 182:2569–2577 [DOI] [PubMed] [Google Scholar]
- 46.Park JY, Lin PY, Weiss RH (2007) Targeting the PI3K-Akt pathway in kidney cancer. Expert Rev Anticancer Ther 7:863–870 [DOI] [PubMed] [Google Scholar]
- 47.Bjelogrlic SK, Radulovic S, Babovic N (2008) Molecular targeting agents in renal cell carcinoma: present strategies and future perspectives. Curr Pharm Des 14:1058–1077 [DOI] [PubMed] [Google Scholar]
- 48.Herbst RS, Hermach JV, Lippman RM (2008) Molecular origins of cancer – lung cancer. N Engl J Med 359:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.“Lung Carcinoma: Tumors of the Lungs”. Merck manual professional edition, online edition. http://www.merck.com/mmpe/sec05/ch062/ch062b.html#sec05-ch062-ch062b-1405
- 50.Oren M (2003) Decision making by p53: life, death, and cancer. Cell Death Differ 10:431–442 [DOI] [PubMed] [Google Scholar]
- 51.Campling BG, El-Deiry WS (2003) Clinical implication of p53 mutation in lung cancer. Mol Biotech 24:141–156 [DOI] [PubMed] [Google Scholar]
- 52.Leinonen T, Pirinen R, Böhm J, Johansson R, Kosma VM (2008) Increased expression of matrix metalloproteinase-2 (MMP-2) predicts tumour recurrence and unfavourable outcome in non-small cell lung cancer. Histol Histopathol 23:693–700 [DOI] [PubMed] [Google Scholar]
- 53.Soussi T (2000) The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann NY Acad Sci 910:121–137 [DOI] [PubMed] [Google Scholar]
- 54.Milyavsky M, Tabach Y, Shats I, Erez N, Cohen Y, Tang X, Kalis M, Kogan I, Buganim Y, Goldfinger N, Ginsberg D, Harris CC, Domany E, Rotter V (2005) Transcriptional programs following genetic alterations in p53, INK4A, and H-Ras genes along defined stages of malignant transformation. Cancer Res 65:4530–4543. 10.1158/0008-5472.CAN-04-3880 [DOI] [PubMed] [Google Scholar]
- 55.Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR (2000) Ras activation in human breast cancer. Breast Cancer Res Treat 62:51–62 [DOI] [PubMed] [Google Scholar]
- 57.Krontiris TG, Devlin B, Karp DD, Robert NJ, Risch N (1993) An association between the risk of cancer and mutations in the HRAS1 minisatellite locus. N Engl J Med 329:517–523 [DOI] [PubMed] [Google Scholar]
- 58.Oliveira AM, Ross JS, Fletcher JA (2005) Tumor suppressor genes in breast cancer: the gatekeepers and the caretakers. Am J Clin Pathol 124(Suppl):S16–S28 [DOI] [PubMed] [Google Scholar]
- 59.Jezierska A, Motyl T (2009) Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit 15:RA32–40 [PubMed] [Google Scholar]
- 60.Hernandez-Aya LF, Gonzalez-Angulo AM (2011) Targeting the phosphatidylinositol 3-kinase signaling pathway in breast cancer. Oncologist 16:404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O (2007) Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer 43:1348–1360 [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, Nakamori S, Monden M, Aozasa K (2004) Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res 10:2846–2850 [DOI] [PubMed] [Google Scholar]
- 63.Juuti A, Lundin J, Nordling S, Louhimo J, Haglund C (2006) Epithelial MMP-2 expression correlates with worse prognosis in pancreatic cancer. Oncology 71:61–68 [DOI] [PubMed] [Google Scholar]
- 64.Mihaljevic AL, Michalski CW, Friess H, Kleeff J (2010) Molecular mechanism of pancreatic cancer–understanding proliferation, invasion, and metastasis. Langenbecks Arch Surg 395:295–308 [DOI] [PubMed] [Google Scholar]
- 65.Baxter LL, Loftus SK, Pavan WJ (2009) Networks and pathways in pigmentation, health, and disease. Wiley Interdiscip Rev Syst Biol Med 1:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Om A, Ghose T, Rowden G (1991) Keratin and carcinoembryonic antigen (CEA) in human melanoma cells. Virchows Arch B Cell Pathol 61:81–87 [DOI] [PubMed] [Google Scholar]
- 67.Selby WL, Nance KV, Park HK (1992) CEA immunoreactivity in metastatic malignant melanoma. Mod Pathol 5:415–419 [PubMed] [Google Scholar]
- 68.Palmieri G, Capone M, Ascierto ML, Gentilcore G, Stroncek DF, Casula M, Sini MC, Palla M, Mozzillo N, Ascierto PA (2009) Main roads to melanoma. J Translat Med 7:86–102. 10.1186/1479-5876-7-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto H, Itoh F, Iku S, Adachi Y, Fukushima H, Sasaki S, Mukaiya M, Hirata K, Imai K (2001) Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol 19:1118–1127 [DOI] [PubMed] [Google Scholar]
- 70.Yadav V, Denning MF (2011) Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration. Mol Carcinog 50:346–352. 10.1002/mc.20716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKenna WG, Muschel RJ, Gupta AK, Hahn SM, Bernhard EJ (2003) The RAS signal transduction pathway and its role in radiation sensitivity. Oncogene 22:5866–5875. 10.1038/sj.onc.1206699 [DOI] [PubMed] [Google Scholar]
- 72.Chautard E, Loubeau G, Tchirkov A, Chassagne J, Vermot-Desroches C, Morel L, Verrelle P (2010) Akt signaling pathway: a target for radiosensitizing human malignant glioma. Neuro Oncol 12:434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta AK, Cerniglia GJ, Mick R, Ahmed MS, Bakanauskas VJ, Muschel RJ, McKenna WG (2003) Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys 56:846–853 [DOI] [PubMed] [Google Scholar]
- 74.Krasny L, Shimony N, Tzukert K, Gorodetsky R, Lecht S, Nettelbeck DM, Haviv YS (2010) An in-vitro tumour microenvironment model using adhesion to type I collagen reveals Akt-dependent radiation resistance in renal cancer cells. Nephrol Dial Transplant 25:373–380 [DOI] [PubMed] [Google Scholar]
- 75.Gottschalk AR, Doan A, Nakamura JL, Stokoe D, Haas-Kogan DA (2005) Inhibition of phosphatidylinositol-3-kinase causes increased sensitivity to radiation through a PKB-dependent mechanism. Int J Radiat Oncol Biol Phys 63:1221–1227 [DOI] [PubMed] [Google Scholar]
- 76.Xia S, Yu S, Fu Q, Liu F, Zheng W, Fu X, Zhao Y (2010) Inhibiting PI3K/Akt pathway increases DNA damage of cervical carcinoma HeLa cells by drug radiosensitization. J Huazhong Univ Sci Technolog Med Sci 30:360–364 [DOI] [PubMed] [Google Scholar]
- 77.Schuurbiers OC, Kaanders JH, van der Heijden HF, Dekhuijzen RP, Oyen WJ, Bussink J (2009) The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J Thorac Oncol 4:761–767 [DOI] [PubMed] [Google Scholar]
- 78.Toulany M, Kehlbach R, Florczak U, Sak A, Wang S, Chen J, Lobrich M, Rodemann HP (2008) Targeting of AKT1 enhances radiation toxicity of human tumor cells by inhibiting DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Ther 7:1771–1781 [DOI] [PubMed] [Google Scholar]
- 79.Johnson GE, Ivanov VN, Hei TK (2008) Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis 13:790–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liebmann J, Cook JA, Fisher J, Teague D, Mitchell JB (1994) In vitro studies of Taxol as a radiation sensitizer in human tumor cells. J Natl Cancer Inst 86:441–446 [DOI] [PubMed] [Google Scholar]
- 81.Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK (2006) CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem 281:37836–37843 [DOI] [PubMed] [Google Scholar]