Abstract
Early‐phase clinical trials using oral inhibitors of MEK, the mitogen‐activated protein kinase kinase, have demonstrated benefit for patients with neurofibromatosis type 1 (NF1)‐associated tumors, particularly progressive low‐grade gliomas and plexiform neurofibromas. Given this potential of MEK inhibition as an effective medical therapy, the use of targeted agents in the NF1 population is likely to increase substantially. For clinicians with limited experience prescribing MEK inhibitors, concern about managing these treatments may be a barrier to use. In this manuscript, the Clinical Care Advisory Board of the Children's Tumor Foundation reviews the published experience with MEK inhibitors in NF1 and outlines recommendations for side‐effect management, as well as monitoring guidelines. These recommendations can serve as a beginning framework for NF providers seeking to provide the most effective treatments for their patients.
Implications for Practice
Neurofibromatosis type 1 (NF1) clinical care is on the cusp of a transformative shift. With the success of recent clinical trials using MEK inhibitors, an increasing number of NF1 patients are being treated with MEK inhibitors for both plexiform neurofibromas and low‐grade gliomas. The use of MEK inhibitors is likely to increase substantially in NF1. Given these changes, the Clinical Care Advisory Board of the Children's Tumor Foundation has identified a need within the NF1 clinical community for guidance for the safe and effective use of MEK inhibitors for NF1‐related tumors. This article provides a review of the published experience of MEK inhibitors in NF1 and provides recommendations for monitoring and management of side effects.
Keywords: Neurofibromatosis type 1, MEK inhibitor, Plexiform neurofibroma
Short abstract
As treatment for neurofibromatosis type 1 (NF1)‐associated complex and inoperable tumors, MEK inhibitor therapy is likely to become more widely used. To aid clinicians who may not have experience with the use of this class of agents, the Clinical Care Advisory Board of the Children's Tumor Foundation presents an overview of the use of MEK inhibitors in the NF1 population, covering relevant clinical trial results, common side effects, basic symptom management, recommended screening guidelines, and patient counseling approaches.
Introduction
Neurofibromatosis type 1 (NF1) is a common autosomal dominant tumor predisposition syndrome affecting approximately 1 in 3,000 individuals at birth 1, 2. NF1 is associated with a number of clinical manifestations including café‐au‐lait macules, axillary/inguinal freckling, Lisch nodules, skeletal abnormalities, and learning disabilities. Individuals with NF1 are at risk for the development of both benign and malignant tumors throughout their lifetime, with the most common neoplasms being cutaneous neurofibromas, plexiform neurofibromas (PNs), and optic pathway or other low‐grade gliomas. Although some tumors in NF1 are associated with few to no symptoms, others can have a significant impact on an individual's appearance and/or function. NF1 is caused by the loss of neurofibromin, the protein product of the NF1 gene. Neurofibromin is a ras‐GAP protein, a negative regulator of RAS signaling. Loss of functional neurofibromin results in activation of the classic RAS‐MAPK signaling cascade, cell proliferation, and subsequent tumor formation. NF1 is a classic tumor suppressor disorder, with tumor cells demonstrating biallelic loss of the functional NF1 gene. Mitogen‐activated protein kinase kinase (MEK1/MEK2) is a kinase in the RAS‐MAPK pathway, which phosphorylates and activates MAPK (mitogen‐activated protein kinase). Overactivation of the RAS‐MAPK signaling cascade has been implicated in the development of a number of malignancies; perhaps the most well known is melanoma. In melanoma, inhibition of the signaling pathway components RAF and MEK results in improved response rates and overall survival in comparison with conventional chemotherapy or BRAF inhibitors alone 3, 4, 5, 6. Clinical trials evaluating MEK inhibitors in NF1 patients with two different tumor types, low‐grade gliomas and plexiform neurofibromas, have shown encouraging results. Table 1 outlines the NF1‐specific recently published or ongoing clinical trials. As an overall well‐tolerated treatment for NF1‐associated complex and inoperable tumors, MEK inhibitor therapy is likely to become more widely used in the NF1 population. To aid clinicians who may not have experience with the use of this class of agents, the Clinical Care Advisory Board of the Children's Tumor Foundation has developed this review to present an overview of the use of MEK inhibitors in the NF1 population, covering relevant clinical trial results, common side effects, basic symptom management, recommended screening guidelines, and patient counseling approaches.
Table 1.
Current state of MEK inhibitor trials in neurofibromatosis type 1
| MEK inhibitor | FDA approved indication | NF‐focused trial | Status |
|---|---|---|---|
| Binimetinib | Melanoma | Phase II, LGG and PN | Ongoing |
| Cobimetinib | Melanoma | None | None |
| Mirdametinib | None/Orphan Drug Status | Phase II, PN | Completed, Weiss (2018) |
| Selumetinib | None/Orphan Drug Status | Phase I/II, LGG and PN | Dombi (2016); Gross (2018); Fangusaro (2019) |
| Trametinib | Melanoma | Phase II, PNs | McCowage (2018) |
Abbreviations: FDA, Food and Drug Administration; LGG, low‐grade glioma; NF, neurofibromatosis; PN, plexiform neurofibroma.
Low‐Grade Gliomas
Low‐grade gliomas (LGGs) are benign tumors of the glia in the brain and are the most common brain tumor in the general pediatric population. Activating mutations in the Ras‐MAPK signaling pathway are found in a majority of pediatric patients, and individuals with NF1 are at increased risk for the development of LGGs 7, 8. LGGs in pediatric NF1 patients most commonly develop along the optic apparatus or in the brainstem 9. Compared with LGGs in the general population, LGGs associated with NF1 tend to be more indolent and usually require therapy only in the setting of symptomatic disease 10, 11, 12, 13. First‐line therapy for these patients is typically conventional chemotherapy including a carboplatin‐based regimen as surgical resection is often not feasible owing to tumor location. Radiation therapy is usually avoided when treating NF1‐associated LGG given the increased risk of vision loss, potential vascular changes such as moyamoya, and secondary malignancies 14, 15. Carboplatin‐containing regimens are effective at halting tumor progression for the majority of individuals with NF1 and a clinically progressive LGG, but in patients whose tumors continue to progress, morbidity associated with the tumor can be quite high and novel therapy is essential 16, 17. Several individual or small series patient studies have demonstrated an encouraging response to MEK inhibition, including a recent case report of the MEK inhibitor trametinib in a child with NF1 and a refractory LGG 18. A recently published phase II study of selumetinib in recurrent LGGs in NF1 patients evaluated 25 patients, 40% of whom demonstrated a partial response to the medication. In addition, 96% of patients had 2 years of progression free survival and only one subject had progression of their tumor while on therapy 19, 20. Of the NF1 patients treated, 68% had no tumor progression, even while off selumetinib for 25–40 months. Given these initial results, MEK inhibition may represent a novel and promising therapy approach for NF1‐associated clinically significant LGGs. Although these data are very exciting, several caveats remain. First, although well tolerated in current trials for 1–2 years, the safety and potential long‐term toxicity of MEK inhibition is not yet known. Second, MEK inhibition has not yet been demonstrated to be equivalent to conventional chemotherapy in newly diagnosed NF1 patients requiring therapy. In order to explore this question, the Children's Oncology Group currently has recently opened a phase III trial randomizing between carboplatin/vincristine and selumetinib specifically in NF1 patients.
Plexiform Neurofibromas
One of the most common and clinically problematic NF1‐associated tumors, PNs, arise in approximately 50% of individuals with NF1 21, 22. PNs are benign, but often invasive, tumors of the peripheral nervous system made up of Schwann cells, blood vessels, fibroblasts, and mast cells. These tumors can arise along any group of peripheral nerves and can result in significant morbidity such as pain, disfigurement, neurological deficits, and local organ compromise. PNs are thought to be congenital, often becoming clinically apparent in young childhood, with the most rapid growth occurring prior to the adult years 23, 24. PNs are also associated with a risk of malignant transformation to malignant peripheral nerve sheath tumors, the leading cause of mortality in NF1 25, 26, 27.
Until recently, therapy for PNs was limited to surgical debulking, which is often associated with subtotal resection, plexiform regrowth, and significant surgical risks 28, 29. Hence, there is a clear need for effective medical therapy for these tumors. As with LGGs, early‐phase plexiform clinical trials have shown promising response with the use of MEK inhibitors. In 2016, Dombi et al. demonstrated that inhibition of MEK with the oral inhibitor selumetinib in pediatric NF1‐associated PNs resulted in >20% volumetric tumor shrinkage in 70% of patients 30. Response to selumetinib therapy in NF1‐associated PNs was further demonstrated in a phase II study (SPRINT) that showed improvement in motor skills as well as plexiform‐related quality of life measures such as pain 31. In response to the results of these early studies, selumetinib has been granted orphan drug status by the Food and Drug Administration (FDA) and is currently undergoing the process of FDA approval.
Mirdametinib, formerly known as PD‐0325901, has also received orphan drug status by the FDA for treatment of NF1 associated plexiform neurofibromas. In a phase II study in adolescents and young adults, 19 patients were treated and 42% demonstrated an objective response of >20% shrinkage in tumor volume 32. Finally, in a phase II study of the MEK inhibitor trametinib, at least 50% of patients met the partial response target of >20% plexiform volume reduction 33. Other trials using MEK inhibitors in NF1 are currently underway, including binimetinib for PNs in both pediatric and adult NF1 patients.
Of note, when assessing plexiform growth as well as treatment response in clinical trials, volumetric analysis is more sensitive in assessment of tumor response to therapy than standard magnetic resonance imaging two‐dimensional measurements 34. For institutions where volumetric assessment is not readily available, significant changes in tumor size may not be readily apparent on two‐dimensional measurements and functional/symptom improvement may provide a better marker of response.
Given the unprecedented response of PNs to this class of medications, there is significant excitement about their ongoing use and anticipated FDA approval. The selumetinib trials have also demonstrated that continued use of the medication is important, as breaks in therapy for side effects, procedures, or significant dose reductions can be associated with tumor regrowth 30. These results indicate that individuals with NF1‐associated PNs on MEK inhibitor therapy may need to remain on the medications for a significant length of time without frequent or prolonged time off for maximal benefit. As such, monitoring for and managing side effects becomes especially important, particularly as there are limited data about potential side effects and toxicities associated with long‐term use.
MEK Inhibitor Toxicity and Management
Overall, MEK inhibitors, particularly in pediatric patients, appear to be well tolerated but may have both mild and severe side effects that can affect quality of life and medication compliance. Table 2 outlines the most commonly reported MEK inhibitor side effects in the trials of NF1 patients. Gastrointestinal toxicities, elevation of creatinine kinase, and skin toxicity (grading as per the National Cancer Institute Common Terminology Criteria for Adverse Events) are seen most frequently 20, 30. Abdominal pain, nausea, weight gain, and diarrhea are usually grade 1–2 for each of the MEK inhibitors and usually do not require dose adjustments. In the two pediatric selumetinib trials, creatinine kinase elevations and rash were the most common grade 3 toxicities. Fatigue was also commonly reported but did not generally require dosing adjustments. The most severe dose‐limiting side effects of MEK inhibitors involve dermatologic, cardiac, and ophthalmic toxicities.
Table 2.
Summary of the most common side effects of individual MEK inhibitors
| Side effect | Mirdametinib | Selumetinib | Trametinib |
|---|---|---|---|
| Cardiac (decreased EF/SF) | NRD | 38% (Gr 1–2); 2% (Gr 3) | NRD |
| Diarrhea | NRD | 54% (Gr 1–2); 4% (Gr 3) | NRD |
| Fatigue | 26% (Gr ≥2) | 56% (Gr 1–2) | NRD |
| Nausea/vomiting | 21% (Gr ≥2) | 44% (Gr 1–2) | NRD |
| Ophthalmologic | No DL toxicity | No DL toxicity | NRD |
| Paronychia | NRD | 38% (Gr 1–2); 6% (Gr 3) | 50% |
| Rash/skin toxicity | 53% (Gr ≥2) | 52%–58% (Gr 1–2); 4%–10% (Gr 3) | 40% |
Percentages represent percentages of patients for each grade.
Abbreviations: DL, dose limiting; EF, ejection fraction; Gr, grade; NRD, not reported to date; SF, shortening fraction.
Dermatologic Toxicity
Dermatologic toxicity is the primary toxicity of MEK inhibitor use in children and adults and is seen in patients both with and without NF1. These toxicities include folliculitis, eczematous dermatitis, acneiform eruptions to the face (Fig. 1A), palmar‐plantar erythrodysesthesia syndrome (hand foot syndrome), photosensitivity, xerosis, pruritus, alopecia, lightening of hair color, and chronic paronychia (Fig. 1B). The majority of these adverse effects can be ameliorated with proper recognition treatment. Each MEK inhibitor has a slightly different reported frequency of skin toxicity reported in trials. Most dermatologic toxicities begin within the first 2 weeks of initiation of therapy 35. Most skin rashes are grade 1, but vemurafenib, trametinib, and selumetinib have the highest reported rates of grade 3 and 4 skin toxicity 36. In the pediatric selumetinib trials, 25%–40% of patients required dose reductions or therapy cessations as a result of dermatologic toxicity. Lower doses, however, did not affect response rates, indicating that lower doses may be effective and better tolerated 20.
Figure 1.
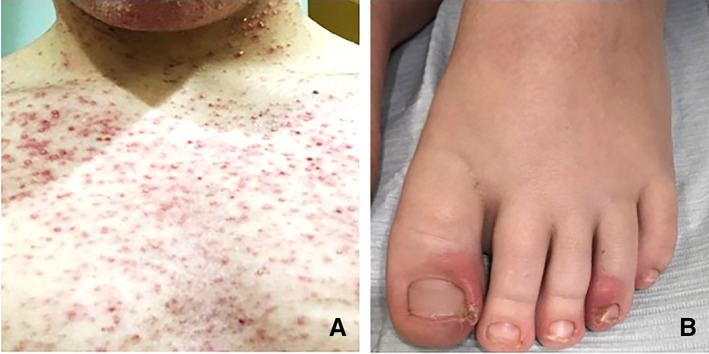
Skin toxicity associated with MEK inhibitors. (A): Acneiform rash in a postpubertal adolescent. (B): Paronychia in a child. Photo B credit to Dr. V. Oza, New York University School of Medicine.
Evidence‐based approaches for treating dermatologic toxicities specifically in individuals treated with MEK inhibitors are lacking. The following recommendations are based on expert opinion for the treatment of comparable skin conditions unless otherwise specified and are outlined in Figure 2.
Figure 2.

Schema for management of skin toxicity associated with MEK inhibitors.
Management of dermatologic toxicities should include both preventive measures and symptomatic treatments 37. Education regarding preventive management should commence with the initiation of MEK therapy for all patients in order to maintain skin moisture and minimize the risk of infection. Daily baths (not showers) using mild cleansers and a high‐quality skin moisturizer applied over the entire body twice a day and within 5 minutes after taking a bath is recommended. Bleach baths 3–4 times per week may reduce the risk of infection and ameliorate inflammation and pruritus 38. For a bleach bath, one‐quarter to one‐half cup of bleach is added to a full bathtub, or 1 tsp per gallon, and the patient soaks for 5–10 minutes, followed by a rinse with water and application of an emollient diffusely on the skin. Hair should be washed daily, and a selenium‐based shampoo can be used for a dry or itchy scalp. Products with added fragrance such as soaps, laundry detergent, and scented creams should be avoided. Ultraviolet light can trigger acneiform rash and skin inflammation in patients on MEK inhibitors. All patients should be advised to practice good sun safety including the use of cover‐ups, such as a wide‐brimmed hat and lightweight clothing that covers the skin. Sunscreen that contains zinc oxide or titanium dioxide should also be applied every 2–3 hours when outdoors and applied to the face daily even when not planning to be outdoors.
For prevention of facial acneiform rash, pimecrolimus cream and clindamycin lotion can be applied to acne‐prone areas (often the “T” zone) morning and night in postpubertal patients at initiation of MEK therapy. For patients who develop a mild acneiform rash despite preventive treatment, topical application of clindamycin 2% lotion and a low‐potency topical steroid such as hydrocortisone 1% lotion can be used twice daily. For moderate to severe acneiform rashes, oral antibiotic treatment is recommended. Doxycycline is typically prescribed for children 8 years of age or older, whereas cephalexin is used for children under 8. Antibiotics should be used for 4 weeks at a minimum and may be continued as long as the patient remains symptomatic.
Eczematous dermatitis can be treated initially with the topical application of pimecrolimus or low‐potency topical steroid such as hydrocortisone 2.5%. For more eczematous rashes that progress, we recommend a mid‐potency topical steroid such as triamcinolone 0.1% ointment applied twice per day until the rash resolves (typically 5–7 days).
Folliculitis can initially be treated with the application of clindamycin lotion to affected areas and the use of germicidal skin cleanser, such as chlorohexidine, in the bath daily. For moderate to severe folliculitis, oral antibiotic treatment is recommended as described above for acneiform rashes.
For patients who develop a mild paronychia, gentle nail care with moisturizers and antiseptic soaks with chlorhexidine for 10–15 minutes used 3–4 times per day are recommended. For moderate paronychia mupirocin, high‐potency topical steroids such as fluocinonide 0.05% ointment can be applied around the inflamed nails twice a day. In these cases, patients should be examined to rule out the presence of a superinfection, which might necessitate oral antibiotics, or an abscess or felon, which may necessitate incision and drainage 39.
Overall, early and aggressive management dermatologic toxicity associated with MEK therapy can minimize the need for breaks in treatment. In a phase II clinical trial of panitumumab (an epidermal growth factor receptor inhibitor) in adults with metastatic colorectal cancer, pre‐emptive management of skin toxicity using a regimen that included skin moisturizers, sunscreen, topical steroid, and doxycycline reduced the incidence of grade 2 or greater skin toxicities to 29%, compared with 62% of patients treated reactively 37. Providers should work closely with a dermatologist for management of moderate and severe skin toxicity. Despite symptomatic management, dose reductions and treatment holds may still become necessary. Dose reductions are commonly used and may not affect overall efficacy of MEK inhibitor therapy. If a dose reduction does not lead to resolution or improvement of symptoms, a drug hold should be considered.
Cardiac Toxicity
Cardiac toxicity due to MEK inhibitor use is a rare, but significant, side effect. In early trials of trametinib in adults in melanoma, 7% of patients experienced a decrease in left ventricular ejection fractions of >10% 40. In the majority of reported trials, the decline was asymptomatic, but grade 3 (or symptomatic) cardiac toxicity has been reported 41. Patients with a history of cardiac disease, such as QTc prolongation, arrhythmias, or uncontrolled hypertension, appear to be more at risk for serious cardiac toxicities. Melanoma clinical trials also demonstrated increased rates of cardiac toxicity including decreased ejection fractions for patients receiving trametinib. However, cardiac function was reported to normalize after discontinuation of therapy 3, 42, 43, 44. The NF1 pediatric published literature on this topic is limited, but cardiac toxicity has also been reported in a case report of a child on trametinib 45. In NF1 pediatric patients with PNs who were treated with selumetinib, an asymptomatic decrease in the ejection fraction was noted in one child (grade 3) in the highest dose range (above the current recommended dose) 30. The patient recovered during drug halt and the cardiac change did not recur when restarting therapy at a lower dose 30. In the pediatric LGG selumetinib trial, asymptomatic ejection fraction decreases were noted in 38% of patients, and one patient had grade 3 dysfunction 20.
Given the potential long‐term toxicity and reversibility of cardiac toxicity, most providers recommend routine cardiac monitoring while on MEK inhibitor therapy. As outlined in Table 3, an echocardiogram is often performed as a baseline assessment, after 1 month on therapy, and then every 3–6 months. Providers should also monitor patients for common cardiac symptoms such as peripheral edema, murmurs, and dyspnea. In the reported trials, left ventricular dysfunction developed as early as 2 weeks on therapy and as late as 2 years. For changes in left ventricular ejection fraction of >10% from baseline, MEK inhibitor therapy should be held for up to 4 weeks (Novartis guidelines). If heart function returns to baseline within 4 weeks, a lower dose of the MEK inhibitor can be restarted. If left ventricular systolic function does not recover within 4 weeks, or the patient has ongoing cardiac symptoms, MEK inhibitor therapy should be permanently discontinued.
Table 3.
Proposed surveillance of patients on MEK inhibitors
| Examination | Frequency |
|---|---|
| Physical examination with vitals | At therapy start and then monthly |
| Skin examination | At therapy start and then monthly |
| Laboratory evaluation | At therapy start and then monthly |
| Echocardiogram | At start, after 1 month, and then every 3–6 months |
| MRI evaluation of the affected area | At start and then every 3–6 months |
| Ophthalmology evaluation | At start, after 1 month, and then every 3–6 months |
Laboratory evaluation to include complete blood count, creatinine kinase; metabolic panel to include electrolytes, creatinine, glucose, and aspartate aminotransferase/alanine aminotransferase.
Abbreviation: MRI, magnetic resonance imaging.
Ophthalmic Toxicity
Ocular toxicities, particularly those involving the retina, have been reported with the use of MEK inhibitors and are thought to be a class effect of these medications. Ocular toxicities occur in a significant proportion of patients (10%–20%) and are usually associated with mild symptoms. The most common toxicities reported are dry eye, periorbital edema, and retinopathy 46. MEK inhibitor–associated retinopathy may present as blurred vision, photophobia, transient visual disturbances, and subretinal fluid 47, 48. It is usually associated with minimal symptoms and often does not require adjustments in therapy. However, in patients with significant retinopathy symptoms, cessation of therapy has been reported to improve the majority of symptoms. Retinal vein occlusion and bilateral retinal detachment with the potential for development of intraretinal cysts are the most significant ocular toxicities associated with MEK inhibitor use and may lead to permanent blindness. Fortunately, in adult MEK inhibitor trials, these serious retinal toxicities are reported in fewer than 1% of patients 46, 47.
Ocular toxicity has been reported less commonly in NF1 than in non‐NF1 clinical trials. In the pediatric PN selumetinib trial, serial ophthalmologic evaluation revealed only a grade 1 cataract, not clearly associated with MEK inhibitor therapy 30. No ocular toxicity was reported in the low‐grade glioma phase II selumetinib trial 20. Other MEK inhibitors in NF1 patients, such as binimetinib, are currently undergoing evaluation for efficacy as well as toxicity.
As ocular toxicities are a clearly documented side effect of MEK inhibitors, ongoing monitoring by an ophthalmologist is strongly recommended. Given that the majority of the retinopathy occurs early in therapy with MEK inhibitors in adults (as early as 2 weeks) 49, more frequent monitoring early in the treatment course is warranted. Most clinical protocols have recommended ophthalmologic evaluation at baseline prior to starting therapy, at 1 month of treatment, and then every 3–6 months thereafter. The ophthalmologic examination should be particularly focused on visual acuity (Table 3). Optical coherence tomography (OCT) can detect small retinal detachments and retinal fluid as well as evaluate the retina and choroid in real time, even when no symptoms are present (Fig. 3) 47, 50. The utility of OCT in regular screening of NF1 patients on MEK inhibitor therapy, however, has not yet been established. Where available, it may provide an important adjuvant method of evaluating the retina in a patient with a new change in vision or other concern on clinical examination.
Figure 3.
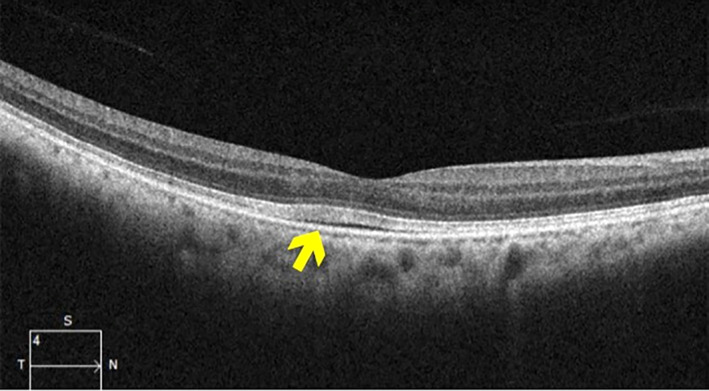
Ocular coherence tomography demonstrating a small retinal detachment and underlying fluid (arrow). Photo credit to Dr. Silas Wang, Massachusetts Eye and Ear Infirmary.
Rare Toxicities
Providers should be aware that several rare complications have been reported in a variety of clinical trials. These include liver toxicity, thrombosis, intestinal obstruction, pneumonia, and pneumonitis. Ongoing studies will help elucidate the risk of such rare side effects in individuals with NF1.
Future Directions
Several key aspects of treatment remain to be studied in MEK inhibition in the NF1 population. First, the studies presented to date have primarily been undertaken in the pediatric NF1 population. The efficacy of MEK inhibitors in PNs in adult patients has not yet been verified, but this class of medications may be less useful in adults, especially given that research has shown that PNs progress more rapidly in childhood. The efficacy of each of the individual MEK inhibitors in NF1 patients, as well as the frequency of side effects, is also not yet known. Preliminary evidence indicates that selumetinib, mirdametinib, and trametinib have efficacy in NF1‐associated PNs, but no comparison trials to identify relative benefit have been performed. Next, optimal dosing and length of therapy needs to be more carefully studied for the treatment of these unique, slow‐growing NF1‐associated tumors, as it is recognized that recommendations may be different than for other tumors/malignancies. Preliminary trials indicate that consistent dosing of MEK inhibitors, without long breaks, may be associated with improved tumor response in NF1 patients. Prolonged use is also likely to be necessary and may require dose adjustments to balance efficacy and toxicity. Important to note is the very limited information on the risks and side effects of long‐term use of MEK inhibitors in patients. The ideal length of therapy, risks and benefits of drug holidays, and risks of prolonged exposure have not been established. NF providers should use care in balancing side effects with potential benefits, as response is often not quickly apparent but may require many months of sustained therapy.
Counseling Patients About the Use of MEK Inhibitors
As with any therapy, potential medication effectiveness, side effects, and treatment monitoring should be thoroughly discussed in detail with the patient. Families should be aware of the lack of a response in some patients and the toxicities that may require immediate attention with possible cessation of therapy. Prior to initiation of treatment, a review of the recommended MEK inhibitor guidelines in Table 2 is suggested. A patient information sheet may be useful to cover general topics of MEK inhibitor therapies and reinforce the most important aspects of their use (supplemental online Appendix 1).
Conclusion
MEK inhibitor therapy appears to be beneficial for the treatment of neurofibromatosis type 1–associated tumors, including progressive LGGs and inoperable PNs. Therefore, MEK inhibition may represent a novel medical therapy in a disease population with previously limited therapy options. Although generally well tolerated, MEK inhibitors have potential toxicities that can affect treatment plans, limit patient compliance, and impact overall effectiveness. In order to maximize the potential benefit to patients, appropriate counseling of therapy risks/benefits, screening recommendations, and symptom control of side effects is paramount. Care should be taken to limit the time off medication, as interruptions in treatment have been associated with tumor regrowth. Long‐term use of these medications in individual patients may be warranted; therefore, appropriate monitoring for toxicity is critical. The early recognition of potential toxicities with appropriate dose adjustments is likely to result in lower risks of long‐term consequences. As further trials are undertaken in this patient population, specific recommendations for each individual MEK inhibitor are likely to emerge.
Author Contributions
Conception/design: Laura J. Klesse, Justin T. Jordan, Heather B. Radtke, Tena Rosser, Elizabeth Schorry, Nicole Ullrich, David Viskochil, Pamela Knight, Scott R. Plotkin, Kaleb Yohay
Manuscript writing: Laura J. Klesse, Justin T. Jordan, Heather B. Radtke, Tena Rosser, Elizabeth Schorry, Nicole Ullrich, David Viskochil, Pamela Knight, Scott R. Plotkin, Kaleb Yohay
Final approval of manuscript: Laura J. Klesse, Justin T. Jordan, Heather B. Radtke, Tena Rosser, Elizabeth Schorry, Nicole Ullrich, David Viskochil, Pamela Knight, Scott R. Plotkin, Kaleb Yohay
Disclosures
Laura J. Klesse: AstraZeneca (SAB); Justin T. Jordan: Recursion Pharmaceuticals, Neurofibromatosis Network (C/A), American Academy of Neurology (H), Burke Foundation (RF), Elsevier Board of Directors of Neurofibromatosis Northeast (royalties); Nicole Ullrich: AstraZeneca (C/A); David Viskochil: Springworks Therapeutics (RF), AstraZeneca (H); Scott Plotkin: NFlection Therapeutics, AstraZeneca (C/A), NFlection Therapeutics (OI). Kaleb Yohay: AstraZeneca (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Miller DT, Freedenberg D, Schorry E et al. Health supervision for children with neurofibromatosis type 1. Pediatrics 2019;143. [DOI] [PubMed] [Google Scholar]
- 2. Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. J Med Genet 1989;26:712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flaherty KT, Infante JR, Daud A et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flaherty KT, Robert C, Hersey P et al. Improved survival with MEK inhibition in BRAF‐mutated melanoma. N Engl J Med 2012;367:107–114. [DOI] [PubMed] [Google Scholar]
- 5. Davies MA, Saiag P, Robert C et al. Dabrafenib plus trametinib in patients with BRAF(V600)‐mutant melanoma brain metastases (COMBI‐MB): A multicentre, multicohort, open‐label, phase 2 trial. Lancet Oncol 2017;18:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schadendorf D, Hauschild A, Santinami M et al. Patient‐reported outcomes in patients with resected, high‐risk melanoma with BRAF(V600E) or BRAF(V600K) mutations treated with adjuvant dabrafenib plus trametinib (COMBI‐AD): A randomised, placebo‐controlled, phase 3 trial. Lancet Oncol 2019;20:701–710. [DOI] [PubMed] [Google Scholar]
- 7. Jones DTW, Kieran MW, Bouffet E et al. Pediatric low‐grade gliomas: Next biologically driven steps. Neuro Oncol 2018;20:160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Packer RJ, Pfister S, Bouffet E et al. Pediatric low‐grade gliomas: Implications of the biologic era. Neuro Oncol 2017;19:750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillamo JS, Creange A, Kalifa C et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): A retrospective study of 104 patients. Brain 2003;126:152–160. [DOI] [PubMed] [Google Scholar]
- 10. Helfferich J, Nijmeijer R, Brouwer OF et al. Neurofibromatosis type 1 associated low grade gliomas: A comparison with sporadic low grade gliomas. Crit Rev Oncol Hematol 2016;104:30–41. [DOI] [PubMed] [Google Scholar]
- 11. Laithier V, Grill J, Le Deley MC et al. Progression‐free survival in children with optic pathway tumors: Dependence on age and the quality of the response to chemotherapy‐‐Results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol 2003;21:4572–4578. [DOI] [PubMed] [Google Scholar]
- 12. Stokland T, Liu JF, Ironside JW et al. A multivariate analysis of factors determining tumor progression in childhood low‐grade glioma: A population‐based cohort study (CCLG CNS9702). Neuro Oncol 2010;12:1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ater JL, Xia C, Mazewski CM et al. Nonrandomized comparison of neurofibromatosis type 1 and non‐neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low‐grade glioma: A report from the Children's Oncology Group. Cancer 2016;122:1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grill J, Couanet D, Cappelli C et al. Radiation‐induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol 1999;45:393–396. [DOI] [PubMed] [Google Scholar]
- 15. Sharif S, Upadhyaya M, Ferner R et al. A molecular analysis of individuals with neurofibromatosis type 1 (NF1) and optic pathway gliomas (OPGs), and an assessment of genotype‐phenotype correlations. J Med Genet 2011;48:256–260. [DOI] [PubMed] [Google Scholar]
- 16. Packer RJ, Lange B, Ater J et al. Carboplatin and vincristine for recurrent and newly diagnosed low‐grade gliomas of childhood. J Clin Oncol 1993;11:850–856. [DOI] [PubMed] [Google Scholar]
- 17. Packer RJ, Ater J, Allen J et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low‐grade gliomas. J Neurosurg 1997;86:747–754. [DOI] [PubMed] [Google Scholar]
- 18. Knight T, Shatara M, Carvalho L et al. Dramatic response to trametinib in a male child with neurofibromatosis type 1 and refractory astrocytoma. Pediatr Blood Cancer 2019;66:e27474. [DOI] [PubMed] [Google Scholar]
- 19. Banerjee A, Jakacki RI, Onar‐Thomas A et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low‐grade glioma: A Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol 2017;19:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fangusaro J, Onar‐Thomas A, Young Poussaint T et al. Selumetinib in paediatric patients with BRAF‐aberrant or neurofibromatosis type 1‐associated recurrent, refractory, or progressive low‐grade glioma: A multicentre, phase 2 trial. Lancet Oncol 2019;20:1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mautner VF, Asuagbor FA, Dombi E et al. Assessment of benign tumor burden by whole‐body MRI in patients with neurofibromatosis 1. Neuro Oncol 2008;10:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plotkin SR, Bredella MA, Cai W et al. Quantitative assessment of whole‐body tumor burden in adult patients with neurofibromatosis. PLoS One 2012;7:e35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dombi E, Solomon J, Gillespie AJ et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: Relationship to age and body weight. Neurology 2007;68:643–647. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen R, Dombi E, Widemann BC et al. Growth dynamics of plexiform neurofibromas: A retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis 2012;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res 2002;62:1573–1577. [PubMed] [Google Scholar]
- 26. Ferner RE, Lucas JD, O'Doherty MJ et al. Evaluation of (18)fluorodeoxyglucose positron emission tomography ((18)FDG PET) in the detection of malignant peripheral nerve sheath tumours arising from within plexiform neurofibromas in neurofibromatosis 1. J Neurol Neurosurg Psychiatry 2000;68:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsai LL, Drubach L, Fahey F et al. [18F]‐Fluorodeoxyglucose positron emission tomography in children with neurofibromatosis type 1 and plexiform neurofibromas: Correlation with malignant transformation. J Neurooncol 2012;108:469–475. [DOI] [PubMed] [Google Scholar]
- 28. Needle MN, Cnaan A, Dattilo J et al. Prognostic signs in the surgical management of plexiform neurofibroma: The Children's Hospital of Philadelphia experience, 1974‐1994. J Pediatr 1997;131:678–682. [DOI] [PubMed] [Google Scholar]
- 29. Canavese F, Krajbich JI. Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J Pediatr Orthop 2011;31:303–311. [DOI] [PubMed] [Google Scholar]
- 30. Dombi E, Baldwin A, Marcus LJ et al. Activity of selumetinib in neurofibromatosis type 1‐related plexiform neurofibromas. N Engl J Med 2016;375:2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gross AM, Wolters P, Baldwin A et al. SPRINT: Phase II study of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY‐142886) in children with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PN). J Clin Oncol 2018;35(suppl 15):10503a. [Google Scholar]
- 32. Weiss B, Plotkin S, Widemann B et al. NFM‐06. NF106: Phase 2 trial of the MEK inhibitor PD‐0325901 in adolescents and adults with NF1‐related plexiform neurofibromas: An NF Clinical Trials Consortium Study. Neuro Oncol 2018;20(suppl 2):i143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCowage GB, Mueller S, Pratilas CA et al. Trametinib in pediatric patients with neurofibromatosis type 1 (NF‐1)–associated plexiform neurofibroma: A phase I/IIa study. J Clin Oncol 2018;36(suppl 15):10504a. [Google Scholar]
- 34. Dombi E, Ardern‐Holmes SL, Babovic‐Vuksanovic D et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology 2013;81(21 Suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rizzo D, Ruggiero A, Amato M et al. BRAF and MEK inhibitors in pediatric glioma: New therapeutic strategies, new toxicities. Expert Opin Drug Metab Toxicol 2016;12:1397–1405. [DOI] [PubMed] [Google Scholar]
- 36. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol 2015;7:122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lacouture ME, Mitchell EP, Piperdi B et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open‐label, randomized trial evaluating the impact of a pre‐Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351–1357. [DOI] [PubMed] [Google Scholar]
- 38. Maarouf M, Shi VY. Bleach for atopic dermatitis. Dermatitis 2018;29:120–126. [DOI] [PubMed] [Google Scholar]
- 39. Piraccini BM, Bellavista S, Misciali C et al. Periungual and subungual pyogenic granuloma. Br J Dermatol 2010;163:941–953. [DOI] [PubMed] [Google Scholar]
- 40. Falchook GS, Lewis KD, Infante JR et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: A phase 1 dose‐escalation trial. Lancet Oncol 2012;13:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banks M, Crowell K, Proctor A et al. Cardiovascular effects of the MEK inhibitor, trametinib: A case report, literature review, and consideration of mechanism. Cardiovasc Toxicol 2017;17:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Long GV, Stroyakovskiy D, Gogas H et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–1888. [DOI] [PubMed] [Google Scholar]
- 43. Robert C, Karaszewska B, Schachter J et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30–39. [DOI] [PubMed] [Google Scholar]
- 44. Robert C, Grob JJ, Stroyakovskiy D et al. Five‐year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;281:626–636. [DOI] [PubMed] [Google Scholar]
- 45. Modak S, Asante‐Korang A, Steinherz LJ et al. Trametinib‐induced left ventricular dysfunction in a child with relapsed neuroblastoma. J Pediatr Hematol Oncol 2015;37:e381–e383. [DOI] [PubMed] [Google Scholar]
- 46. Mendez‐Martinez S, Calvo P, Ruiz‐Moreno O et al. Ocular adverse events associated with MEK inhibitors. Retina 2019;39:1435–1450. [DOI] [PubMed] [Google Scholar]
- 47. Urner‐Bloch U, Urner M, Jaberg‐Bentele N et al. MEK inhibitor‐associated retinopathy (MEKAR) in metastatic melanoma: Long‐term ophthalmic effects. Eur J Cancer 2016;65:130–138. [DOI] [PubMed] [Google Scholar]
- 48. Montana CL, Apte RS. MEKanisms of a serous complication. JAMA Ophthalmol 2017;135:413–414. [DOI] [PubMed] [Google Scholar]
- 49. Duncan KE, Chang LY, Patronas M. MEK inhibitors: A new class of chemotherapeutic agents with ocular toxicity. Eye (Lond) 2015;29:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stjepanovic N, Velazquez‐Martin JP, Bedard PL. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol 2016;27:998–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information


