Abstract
Background
Fundamental Research in Oncology and Thrombosis (FRONTLINE) is a global survey of physicians' perceptions and practice in the management of venous thromboembolism (VTE) in patients with cancer.
Materials and Methods
The present survey, FRONTLINE 2, follows the original FRONTLINE survey (published in The Oncologist in 2003) and provides insights into how physicians perceive risk of VTE in cancer and approach its prophylaxis and treatment.
Results
Between November 2015 and February 2016, 5,233 respondents participated, representing cancer physicians and surgeons. Most believed that less than one in five patients with any cancer might be at risk of VTE, with a slightly higher risk in patients with brain, pancreatic, and lung tumors. The most frequently reported reasons for giving prophylaxis were prior history of VTE (74.6%), abnormal platelet count (62.0%), and obesity (59.5%). In surgical and medical cancer patients, low‐molecular‐weight heparin (LMWH) was the most popular prophylactic measure, used by 74.2% and 80.6%, respectively. Oral anticoagulants (OACs) were given in less than one fifth of cases. In surgical patients, prophylaxis was usually provided for 1 month postoperatively. Following a diagnosis of VTE, patients initially received treatment with LMWH and were maintained long term on OACs, primarily warfarin, dabigatran, and rivaroxaban. Most surgical and medical cancer patients underwent treatment of VTE for 3–6 months.
Conclusion
Compared with the original FRONTLINE survey, FRONTLINE 2 reveals some differences in the management of VTE in patients with cancer. Newer anticoagulants such as fondaparinux, dabigatran, and rivaroxaban are being incorporated into the contemporary management of VTE in patients with cancer.
Implications for Practice
This globally conducted survey of more than 5,000 cancer clinicians revealed a number of insights into the perceived risk for venous thromboembolism as well as contemporary approaches to its prevention and treatment. Although guidelines have consistently recommended anticoagulant medications for prevention and treatment of cancer‐associated thrombosis, clinicians report substantial variation in their practice.
Keywords: Venous thromboembolism • Cancer‐associated thrombosis • Deep vein thrombosis • Pulmonary embolism • Central venous catheter
Short abstract
Improvements in molecular analysis hold promise for improved identification and treatment of cancers of unknown primary. This article presents the case of a patient with a cancer of unknown primary and metastases in the brain and lung, detailing the genomic profiling performed to establish targeted therapy options.
Introduction
Thrombosis is a common complication in patients with cancer and is associated with high mortality 1, 2, 3, 4, 5, 6. Its pathogenesis is multifactorial, with patient‐, tumor‐, and treatment‐related factors (e.g., antineoplastic agents and central lines) influencing the frequency of venous and arterial thromboembolic events 7, 8, 9, 10, 11. Identifying patients at risk for the development of cancer‐associated thrombosis is challenging, especially because the absolute risk of these complications also varies with the natural history of malignant disease 11, 12. In surgical patients, the type of cancer and invasiveness of procedures have been the basis for initiating prophylaxis 13; however, derived risk scores have been advocated. In medical oncology patients receiving systemic therapies, several risk scores based on patient characteristics have been developed 14, 15, 16.
Pharmacologic thromboprophylaxis has demonstrated good efficacy and safety in surgical cancer patients 17, 18 and may be usefully extended up to 1 month postoperatively 13, 19, 20. In medical oncology patients, the use of low‐molecular‐ and ultra‐low‐molecular‐weight heparin, although effective and safe 21, 22, 23, is not routinely recommended because absolute reductions in thromboembolic complications in unselected patient populations have not been considered sufficiently clinically meaningful 13. More recently, direct oral anticoagulants (DOACs) have been assessed for the prevention of cancer‐associated thrombosis in medical oncology patients 24, 25, 26, 27, 28, 29, 30. Although these drugs improve prognosis, they have failed to demonstrate any impact on enhancing survival 13, 31.
The first Fundamental Research in Oncology and Thrombosis (FRONTLINE) Survey was conducted in 2001 and published in The Oncologist shortly thereafter 32; the present survey, FRONTLINE 2, will provide an opportunity to understand the recent evolution in perceptions and patterns of practice against cancer‐associated thrombosis. The survey was designed to capture a large, highly representative sample of respondents so as to generate new insights into VTE of cancer and potentially stimulate further research into this often‐serious paraneoplastic complication.
Materials and Methods
Study Design
As with FRONTLINE 32, FRONTLINE 2 was developed by the Thrombosis Research Institute (TRI; London, U.K.) in collaboration with a dedicated steering committee of clinicians with expertise in VTE. Between November 2015 and February 2016, news of the survey was distributed by a series of mailings, advertisements, and congress activities. It was estimated that if 50,000 medical professionals were invited to participate, approximately 5,000 might complete the survey. The survey was accessible across all platforms and browsers, on any computer, laptop, iPad, or mobile device (Apple, Android). Respondents were asked to enter their e‐mail address and generate an identifier before entering the survey website.
The survey questionnaire was divided into five parts (sections A–E). In section A, respondents were asked to provide data on their patients' demographics. Section B was devised specifically to investigate the incidence and management of VTE in surgical cancer patients. Section C sought similar information in nonsurgical cancer patients (medically ill patients with cancer), defined as those with active cancer receiving outpatient treatment and in whom surgery was not planned. Section D contained questions on thrombosis associated with vascular access devices, and section E on incidental thrombosis in patients with cancer. Each section contained 10–20 multiple‐choice questions and the entire survey was supposed to take each respondent no longer than 20–30 minutes to complete.
The survey was designed to assess perceptions and patterns of practice; therefore, the findings are presented simply as percentages exclusive of missing values. No formal statistical analysis of the study data was performed.
Results
Demographics
In all, 5,233 respondents completed the survey. The largest group, accounting for approximately one third overall, were from Europe; 18.4% were from North America, 5.9% from South America, 15.3% from Asia, and 31.1% “rest of the world”—mostly Middle Eastern nations. Roughly half of physicians (47.0%) were affiliated with university hospitals, with one third (33.2%) at community/district hospitals and the remainder (19.8%) private practitioners. Among them, they treated a wide variety of different cancer types, most prominently those of the breast, lung, and colon, with lymphoma, prostate and hematologic malignancies also highly represented.
Perception of Risk by Cancer Type
For the most part, respondents believed that less than one in five patients with each type of cancer might be at risk of VTE, with a slightly higher perception of risk in patients with brain, pancreatic, and lung tumors.
Patterns of Prophylaxis
For all types of cancer except those affecting children and adults with leukemia, physicians were almost evenly divided between those who routinely administered VTE prophylaxis to most of their patients (>50%) and those who did not. Physicians who treated pediatric cancers and adult leukemia were least likely to administer any prophylaxis. The most frequently selected reasons for giving prophylaxis indicated by more than half of respondents treating surgical patients were prior history of VTE (74.6%), thrombophilia/thrombocytosis (62.0%), and obesity (59.5%).
Among physicians treating surgical cancer patients, 63.1% reported that they routinely provided prophylaxis against VTE, whereas the remainder did so on a case‐by‐case basis. Most (73.5%) themselves initiated prophylaxis, with a minority referring their patients to a specialist thrombosis service or hematologist to provide prophylaxis. Physicians' general approach to giving prophylaxis against VTE in surgical cancer patients is displayed in Figure 1. LMWH and unfractionated heparin (UFH) were overwhelmingly the most common pharmacologic methods, used by 74.2% and 21.9% of respondents, respectively, and most respondents (69.3%) used physical methods such as compression hosiery. Aspirin was more commonly used as prophylaxis than oral anticoagulants (warfarin and DOACs)—in 20.6% and 18.8% of cases, respectively. Vena cava filters were rarely or never placed. In the highest proportion of cases, prophylaxis was provided for 1 month postoperatively (32.0%); few respondents administered prophylaxis for longer periods of 3 months (12.3%) or indefinitely (7.3%). Most respondents (71.7%) reported mobilizing their patients within 48 hours or within 2–5 days following surgery (19.3%).
Figure 1.

Respondents' general approach to prophylaxis against venous thromboembolism. Note: Respondents could give more than one answer and totals may exceed 100%.Abbreviations: LMWH, low‐molecular‐weight heparin; UFH, unfractionated heparin.
In medically ill patients with cancer, the major reasons underlying any decision to administer prophylaxis against VTE were prior episode of VTE (reported by 26.3% respondents) and high‐risk individuals (14.4%). The main barriers to providing VTE prophylaxis were presence or perceived high risk of bleeding (both >80%), whereas history of bleed was less commonly a reason for withholding VTE prophylaxis (reported by 43.0%). The respondents' general approach to prophylaxis is shown in Figure 1. Likewise as with surgical cancer patients, most respondents indicated that they used LMWH (80.6%) or UFH (21.7%) in the setting of prophylaxis, with a slightly higher proportion using fondaparinux and oral anticoagulants compared with respondents who dealt with surgical cancer patients (20.0% vs. 12.1% and 30.9% vs. 18.8%, respectively).
Diagnosis and Treatment of VTE
For the diagnosis of VTE (deep vein thrombosis [DVT] and pulmonary embolism [PE]), most respondents managing surgical patients used clinical judgment plus standard objective imaging—86.5% reported using ultrasound for the diagnosis of DVT and 78.4% computed tomography/magnetic resonance imaging scan for PE.
Respondents' initial treatment approaches for VTE (DVT or PE) and long‐term strategies for DVT and PE in surgical and medical cancer patients are displayed in Figures 2 and 3. No obvious difference was noted between the approach to treatment of these complications in surgical and medical cancer patients. Nearly all patients (99%) with a diagnosis of DVT or PE were treated. Treatment was initiated for the most part using heparins—LMWH or UFH—or oral anticoagulants (Vitamin K antagonist (VKA) or DOAC). Thereafter, over the long term, LMWH was largely discontinued and patients were maintained on oral anticoagulant therapy (primarily warfarin, dabigatran, or rivaroxaban). Aspirin was used in just over 20% of surgical cancer patients but less frequently in medical patients. Responses to optimal duration of long‐term anticoagulant therapy against DVT are depicted in Figure 4. The most frequently selected response in surgical and medical cancer patients was 3–6 months, as indicated by roughly half (>40%–50%) of respondents. Longer duration of therapy (6–12 months or indefinitely) was less commonly selected following an episode of DVT than after PE.
Figure 2.

Initial treatment approach for venous thromboembolism (deep vein thrombosis or pulmonary embolism). Note: Respondents could give more than one answer and totals may exceed 100%.Abbreviations: LMWH, low‐molecular‐weight heparin; UFH, unfractionated heparin.
Figure 3.
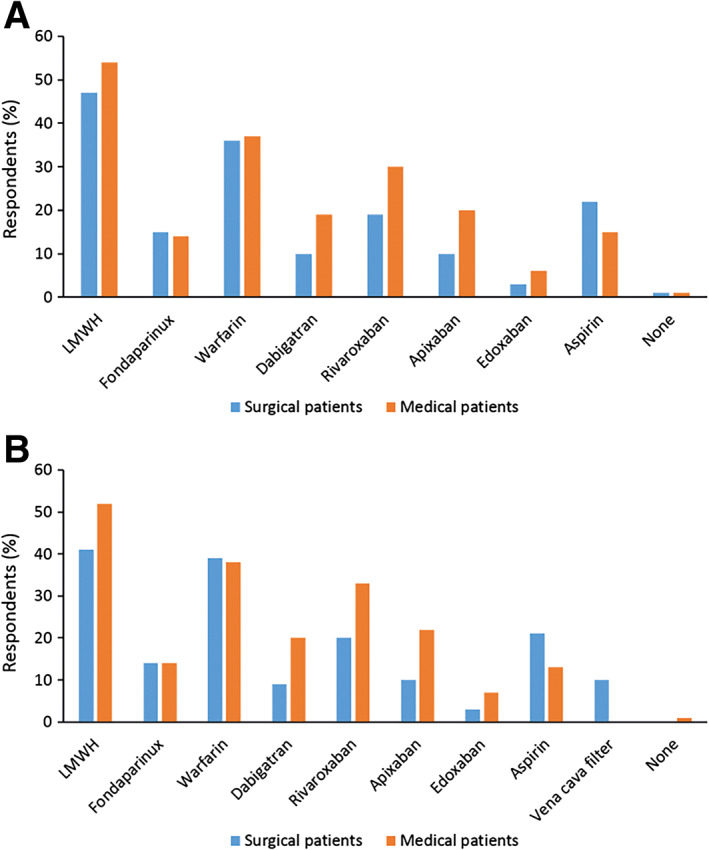
Long‐term treatment approach for deep vein thrombosis (A) and pulmonary embolism (B). Note: Respondents could give more than one answer and totals may exceed 100%.Abbreviations: LMWH, low‐molecular‐weight heparin.
Figure 4.
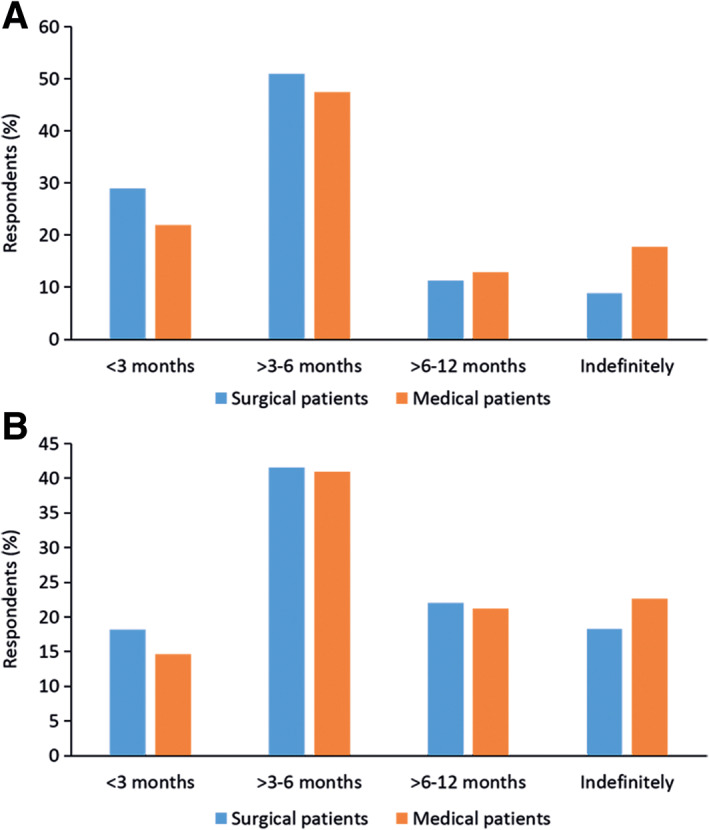
Duration of long‐term anticoagulant therapy in surgical and medical cancer patients with deep vein thrombosis (A) and pulmonary embolism (B). Note: Respondents could give more than one answer and totals may exceed 100%.
Central Venous Access
The majority of respondents (76.2%) indicated that insertion of a central venous catheter (CVC) increases the risk of thrombosis. However, half (51.6%) reported that they rarely or never gave prophylaxis against DVT in patients with CVC placement. Among those who choose to anticoagulate, most (55.5%) reported that they use LMWH, with UFH, fondaparinux, and oral anticoagulants (mainly warfarin) also frequently selected. Treatment would typically last 3–6 months, and most respondents (61.3%) would discontinue anticoagulant therapy once the CVC was removed.
Comparison of FRONTLINE 2 Versus FRONTLINE
The original FRONTLINE survey was conducted in 2001 32. Because the FRONTLINE program was designed to probe current real‐world practices, survey questions were identical in the original survey and the present survey. For comparison, the FRONTLINE and FRONTLINE 2 respondents' geographical location, practice setting, and approach to prophylaxis against VTE in surgical and medical cancer patients are depicted in Figure 5.
Figure 5.

Comparison between FRONTLINE and FRONTLINE 2. Respondents' geographical region (A) and practice setting (B). Methods for VTE prophylaxis in patients with cancer managed surgically (C) and medically (D). Note: Respondents could give more than one answer and totals may exceed 100%.Abbreviations: LMWH, low‐molecular‐weight heparin; OAC, oral anticoagulant; ROW, rest of world; UFH, unfractionated heparin.
Discussion
FRONTLINE 2 is a global survey of perceptions and clinical practices of oncologists (surgical, medical, radiation, hematologic, and pediatric oncologists and specialist care nurses) who treat cancer‐associated thrombosis. More than 5,000 respondents dealing with a broad range of cancers from around the world completed the survey. They confirmed that VTE is a fairly commonplace manifestation in patients with cancer. Although respondents indicated that they were aware of the risk of VTE in their patients, they did not always administer prophylaxis. Treatment of VTE events, however, was universally provided (in 99% of cases). Respondents reported using mainly LMWH, UFH, and fondaparinux over the short term and maintaining treatment using a range of oral anticoagulant medications in the longer term: warfarin was most widely used, followed by DOACs such as dabigatran and rivaroxaban. Prophylaxis was administered usually for 1 month, which may reflect guideline‐recommended practice to administer anticoagulation to patients with cancer during periods of hospitalization or after undergoing anticancer surgery 13, 20. Treatment of VTE events typically lasted 3–6 months, with a somewhat higher proportion of patients with PE than those with DVT remaining on anticoagulants for longer periods (6–12 months or beyond). This observation is supported by the EINSTEIN program of studies 33, 34, in which intended duration of treatment (with either rivaroxaban or standard therapy) was determined by treating physicians and tended to be longer for PE than VTE.
Compared with the original FRONTLINE survey conducted in 2001, the present FRONTLINE 2 is overall consistent and shows some small differences in the routine management of VTE in patients with cancer. Newer anticoagulant agents that entered the market after FRONTLINE have expanded the therapeutic options against VTE. Fondaparinux (approved by the European Medicines Agency in 2002) is currently widely used, as are the DOACs dabigatran (approved 2008) and rivaroxaban (approved 2008). These DOACs have become the mainstays of treatment against DVT and PE. Use of aspirin has increased especially in surgical cancer patients. Whether these changes are entirely due to the more recent introduction of newer agents or differences between the FRONTLINE and FRONTLINE 2 survey respondents' geographical location and/or clinical practice setting is unknown; FRONTLINE 2 included a much higher proportion of “rest of the world” and fewer western European participants than original FRONTLINE as well as input from private practitioners, who were not petitioned in the earlier survey.
Guidelines for the prophylaxis and treatment of VTE in patients with cancer such as those disseminated by the American Society of Clinical Oncology 13 and others 35, 36 recommend that VTE be managed similarly to that arising in individuals without cancer: anticoagulation using LMWH (especially in medical oncology)/UFH, VKA, or DOAC underlying the basis of therapy. Although guidelines are highly useful education materials backed by evidence mainly from clinical trials, their actual implementation is uncertain; in reality, patients with cancer are more likely treated individually. Moreover, “cancer” is a very broad term used to describe a great variety of solid tumors and malignant blood disorders of early and more advanced stages in elderly, not‐so‐elderly, and children treated with or without surgery (an independent risk factor for VTE), hospitalized to receive chemotherapy or at end of life, or outpatients managed in the community. Hence, real‐world data are important because they tell us what clinicians are indeed doing based on their perceptions and patients' preferences.
Combined analysis of data from the EINSTEIN‐DVT and EINSTEIN‐PE trials demonstrated similar efficacy between rivaroxaban and LMWH/VKA for secondary prophylaxis against VTE in patients with active cancer 24. The SELECT‐D randomized trial compared 6‐month treatment with rivaroxaban versus LMWH in patients with cancer and observed low rates of recurrence in either arm 37. Recent evidence from North America suggests that warfarin and rivaroxaban are at least as commonly used as prophylactic agent as LMWH, and for longer treatment periods 38. Moreover, a meta‐analysis of randomized controlled phase III trials suggested a trend, albeit nonsignificant, toward better efficacy and safety of DOACs versus VKA for the treatment of VTE in patients with cancer 39. Additionally, in a large‐scale study, antithrombotic prophylaxis significantly reduced systemic VTE and mortality in patients with cancer with a CVC implant 9. The HOKUSAI‐VTE trial showed that edoxaban was noninferior to conventional anticoagulation using warfarin in patients with cancer and VTE and led to less clinically relevant bleeding 25. Subsequently, the same investigators demonstrated noninferiority of edoxaban versus dalteparin at preventing VTE recurrence and major bleeding in a large cohort of patients with cancer 28. The present survey reveals that although warfarin is more commonly used prophylactically or therapeutically than any individual DOACs, use of these latter agents taken together as a class (that is, any DOAC) exceeds that of warfarin in contemporary practice.
FRONTLINE 2 respondents reported that less than one in five patients with cancer overall experience VTE events, and of those, individuals with brain, pancreatic, and lung tumors are at highest risk. These data are in line with those provided in an extensive literature review by Timp et al. 40, wherein the cumulative incidence rate of VTE in newly diagnosed patients with cancer either enrolled in observational cohort registries or admitted as inpatients to oncology departments varied at 1%–8% over approximately 2 years of follow‐up. These researchers also noted a pattern of higher risk for VTE in more aggressive versus classically indolent tumor types 40. It is possible that both surgical and medical oncologists who tend to deal with the same tumors on a day‐to‐day basis may be alert that their own patients are at either higher or lower risk for VTE and thereby administer prophylaxis accordingly. Because clinical practice guidelines such as those issued by the International Initiative on Thrombosis and Cancer (endorsed by the International Society on Thrombosis and Haemostasis 41) grade risk assessment based on primary site (Khorana score), it seems likely that the perception of certain cancers as conferring higher risk of VTE, rather than cancer per se, exerts primary influence on prevention strategy.
The present study has limitations. Although a large sample of participants (more than 5,000) responded, whether the data collected truly reflect actual clinical practice worldwide is unknown. Moreover, comparisons between the latest FRONTLINE 2 findings versus original FRONTLINE are hampered by the large switch in geographical location of respondents to the two surveys, from mostly Europe and North America at first to rest of world in the latter survey. On the other hand, to the authors' knowledge, the present survey is the largest of its kind to date and provides a wealth of insights.
Conclusion
This study shows that across the globe, practice in the setting of cancer‐related VTE varies somewhat. However, many notable innovations in anticoagulation therapy are being adopted. As our knowledge of VTE in cancer increases, so will the promise of better outcomes for those affected. Indeed, the recently published AVERT 29 and CASSINI 30 trials provide compelling evidence for the use of the DOACs apixaban and rivaroxaban for the prevention of VTE in high‐risk patients with cancer in a range of clinical scenarios. It is hoped that the present FRONTLINE 2 survey elevates clinicians' awareness of the risk and optimal management choices for VTE in cancer.
Author Contributions
Conception/design: Ajay K. Kakkar, Rupert Bauersachs, Anna Falanga, John Wong, Gloria Kayani, Alex Kahney, Rodney Hughes, Mark Levine
Collection and/or assembly of data: Ajay K. Kakkar, Rupert Bauersachs, Anna Falanga, John Wong, Gloria Kayani, Alex Kahney, Rodney Hughes, Mark Levine
Data analysis and interpretation: Ajay K. Kakkar, Rupert Bauersachs, Anna Falanga, John Wong, Gloria Kayani, Alex Kahney, Rodney Hughes, Mark Levine
Manuscript writing: Ajay K. Kakkar, Rupert Bauersachs, Anna Falanga, John Wong, Gloria Kayani, Alex Kahney, Rodney Hughes, Mark Levine
Final approval of manuscript: Ajay K. Kakkar, Rupert Bauersachs, Anna Falanga, John Wong, Gloria Kayani, Alex Kahney, Rodney Hughes, Mark Levine
Disclosures
Ajay K. Kakkar: Bayer AG (RF); Bayer AG, Janssen Pharma, Pfizer, Verseon (C/A, H), Bayer AG, Sanofi S.A., Verseon (SAB); Rupert Bauersachs: Bayer AG, Bristol‐Myers Squibb, Pfizer, Daiichi‐Sankyo (H); Rodney Hughes: Bayer (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Madhusudana Rao of Thrombosis Research Institute (London, U.K.) provided support with programming and statistical analysis. FRONTLINE 2 is an independent academic research initiative sponsored by the Thrombosis Research Institute (London, U.K.) and supported by an unrestricted research grant from Bayer AG (Berlin, Germany).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Levitan N, Dowlati A, Remick SC et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285–291. [DOI] [PubMed] [Google Scholar]
- 2. Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb Haemost 2002;87:575–579. [PubMed] [Google Scholar]
- 3. Huerta C, Johansson S, Wallander MA et al. Risk factors and short‐term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007;167:935–943. [DOI] [PubMed] [Google Scholar]
- 4. Castelli R, Ferrari B, Cortelezzi A et al. Thromboembolic complications in malignant haematological disorders. Curr Vasc Pharmacol 2010;8:482–494. [DOI] [PubMed] [Google Scholar]
- 5. Streiff MB, Holmstrom B, Angelini D et al. NCCN Guidelines Insights: Cancer‐Associated Venous Thromboembolic Disease, version 2.2018. J Natl Compr Canc Netw 2018;16:1289–1303. [DOI] [PubMed] [Google Scholar]
- 6. Puurunen MK, Gona PN, Larson MG et al. Epidemiology of venous thromboembolism in the Framingham Heart Study. Thromb Res 2016;145:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee AY, Levine MN. Venous thromboembolism and cancer: Risks and outcomes. Circulation 2003;107(suppl 2):I17–I21. [DOI] [PubMed] [Google Scholar]
- 8. Monreal M, Falgá C, Valdés M et al.; RIETE Investigators. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: Findings from the RIETE registry. J Thromb Haemost 2006;4:1950–1956. [DOI] [PubMed] [Google Scholar]
- 9. Fagnani D, Franchi R, Porta C et al.; POLONORD Group. Thrombosis‐related complications and mortality in cancer patients with central venous devices: An observational study on the effect of antithrombotic prophylaxis. Ann Oncol 2007;18:551–555. [DOI] [PubMed] [Google Scholar]
- 10. Akl EA, Vasireddi SR, Gunukula S et al. Anticoagulation for patients with cancer and central venous catheters. Cochrane Database Syst Rev 2011;2:CD006468. [DOI] [PubMed] [Google Scholar]
- 11. Falanga A, Russo L, Milesi V et al. Mechanisms and risk factors of thrombosis in cancer. Crit Rev Oncol Hematol 2017;118:79–83. [DOI] [PubMed] [Google Scholar]
- 12. Falanga A, Schieppati F, Russo D. Cancer tissue procoagulant mechanisms and the hypercoagulable state of patients with cancer. Semin Thromb Hemost 2015;41:756–764. [DOI] [PubMed] [Google Scholar]
- 13. Lyman GH, Khorana AA, Kuderer NM et al.; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2189–2204. [DOI] [PubMed] [Google Scholar]
- 14. Khorana AA, Kuderer NM, Culakova E et al. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood 2008;111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ay C, Dunkler D, Marosi C et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377–5382. [DOI] [PubMed] [Google Scholar]
- 16. Verso M, Agnelli G, Barni S et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern Emerg Med 2012;7:291–292. [DOI] [PubMed] [Google Scholar]
- 17. Akl EA, Terrenato I, Barba M et al. Low‐molecular‐weight heparin vs unfractionated heparin for perioperative thromboprophylaxis in patients with cancer: A systematic review and meta‐analysis. Arch Intern Med 2008;168:1261–1269. [DOI] [PubMed] [Google Scholar]
- 18. Kakkar VV, Balibrea JL, Martínez‐González J et al.; CANBESURE Study Group. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: The CANBESURE randomized study. J Thromb Haemost 2010;8:1223–1229. [DOI] [PubMed] [Google Scholar]
- 19. Bergqvist D, Agnelli G, Cohen AT et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975–980. [DOI] [PubMed] [Google Scholar]
- 20. Mandalà M, Falanga A, Roila F; on behalf of the ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(suppl 6):vi85–vi92. [DOI] [PubMed] [Google Scholar]
- 21. Lee AY, Levine MN, Baker RI et al.; Randomized Comparison of Low‐Molecular‐Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 22. Cohen AT, Gurwith MM, Dobromirski M. Thromboprophylaxis in non‐surgical cancer patients. Thromb Res 2012;129(suppl 1):S137–S145. [DOI] [PubMed] [Google Scholar]
- 23. Francis CW, Kessler CM, Goldhaber SZ et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: The DALTECAN Study. J Thromb Haemost 2015;13:1028–1035. [DOI] [PubMed] [Google Scholar]
- 24. Prins MH, Lensing AW, Brighton TA et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN‐DVT and EINSTEIN‐PE): A pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 2014;1:e37–e46. [DOI] [PubMed] [Google Scholar]
- 25. Raskob GE, van Es N, Segers A et al.; Hokusai‐VTE investigators. Edoxaban for venous thromboembolism in patients with cancer: Results from a non‐inferiority subgroup analysis of the Hokusai‐VTE randomised, double‐blind, double‐dummy trial. Lancet Haematol 2016;3:e379–e387. [DOI] [PubMed] [Google Scholar]
- 26. Bott‐Kitslaar DM, Saadiq RA, McBane RD et al. Efficacy and safety of rivaroxaban in patients with venous thromboembolism and active malignancy: A single‐center registry. Am J Med 2016;129:615–619. [DOI] [PubMed] [Google Scholar]
- 27. Pignataro BS, Nishinari K, Cavalcante RN et al. Oral rivaroxaban for the treatment of symptomatic venous thromboembolism in 400 patients with active cancer: A single‐center experience. Clin Appl Thromb Hemost 2017;23:883–887. [DOI] [PubMed] [Google Scholar]
- 28. Raskob GE, van Es N, Verhamme P et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med 2018;378:615–624. [DOI] [PubMed] [Google Scholar]
- 29. Carrier M, Abou‐Nassar K, Mallick R. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380:711–719. [DOI] [PubMed] [Google Scholar]
- 30. Khorana AA, Soff GA, Kakkar AK et al. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med 2019;380:720–728. [DOI] [PubMed] [Google Scholar]
- 31. Akl EA, Kamath G, Kim SY et al. Oral anticoagulation for prolonging survival in patients with cancer. Cochrane Database Syst Rev 2007;CD006466. [DOI] [PubMed] [Google Scholar]
- 32. Kakkar AK, Levine M, Pinedo HM et al. Venous thrombosis in cancer patients: Insights from the FRONTLINE survey. The Oncologist 2003;8:381–388. [DOI] [PubMed] [Google Scholar]
- 33. Investigators EINSTEIN, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–2510. [DOI] [PubMed] [Google Scholar]
- 34. Investigators EINSTEIN‐PE, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–1297. [DOI] [PubMed] [Google Scholar]
- 35. Khorana AA, Noble S, Lee AYY et al. Role of direct oral anticoagulants in the treatment of cancer‐associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost 2018;16:1891–1894. [DOI] [PubMed] [Google Scholar]
- 36. Connolly GC, Francis CW. Cancer‐associated thrombosis. Hematol Am Soc Hematol Educ Program 2013;2013:684–691. [DOI] [PubMed] [Google Scholar]
- 37. Young AM, Marshall A, Thirlwall J et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT‐D). J Clin Oncol 2018;36:2017–2023. [DOI] [PubMed] [Google Scholar]
- 38. Khorana AA, McCrae KR, Milentijevic D et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost 2017;1:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larsen TB, Nielsen PB, Skiøth F et al. Non‐vitamin K antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: A semi systematic review and meta‐analysis of safety and efficacy outcomes. PLoS One 2014;9:e114445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Timp JF, Braekkan SK, Versteeg HH et al. Epidemiology of cancer‐associated venous thrombosis. Blood 2013;122:1712–1723. [DOI] [PubMed] [Google Scholar]
- 41. Farge D, Frere C, Connors JM et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566–e581. [DOI] [PubMed] [Google Scholar]


