Abstract
Purpose
The use of microsatellite instability (MSI) and mismatch repair (MMR) as predictive biomarkers for fluorouracil‐based adjuvant chemotherapy in colorectal cancer has been a paradigm shift. However, whether this applies to gastric cancer is questionable. Furthermore, we herein investigated whether and how autophagy plays a role in MSI‐relevant chemoresistance.
Materials and Methods
A total of 929 patients with deficient MMR (dMMR) and proficient MMR (pMMR) gastric cancers who underwent curative‐intent gastrectomy were enrolled. We compared clinicopathological variables and survival among dMMR and pMMR cohorts and tested the responses of MSI‐high and microsatellite stable (MSS) gastric cancer cell lines to 5‐fluorouracil (5‐FU) with or without chloroquine, an autophagy inhibitor.
Results
We identified an 8.9% prevalence of dMMR cases (83 out of 929) in our cohort. This was associated with old age, tumor site at the distal stomach, an intestinal phenotype, fewer nodal metastasis, and early pathological stages. MMR was an independent prognostic factor after multivariate adjustment. Overall survival (OS) of dMMR patients was better than that of the pMMR patients but was only applicable to stage III patients. There was no difference in OS between dMMR patients treated with or without adjuvant chemotherapy, although the latter showed more medical morbidities. The MSI‐high gastric cancer cell lines, versus the MSS counterparts, displayed increased resistance to 5‐FU and increased autophagy. Interestingly, autophagy inhibition abrogated the chemoresistance.
Conclusion
Our data show that fluorouracil‐based adjuvant chemotherapy does not work for dMMR cases, if not worse. Autophagy inhibition and/or immune checkpoint inhibition might be promising alternative strategies for gastric cancer treatment.
Implications for Practice
The use of microsatellite instability (MSI) and mismatch repair (MMR) as predictive biomarkers for adjuvant chemotherapy in colorectal cancer has caused a paradigm shift in cancer therapy, although its implications in gastric cancer are still questionable. The data obtained in the current study indicate that MSI‐MMR is an independent prognostic factor for gastric cancer. Standard fluorouracil‐based adjuvant chemotherapy did not work for deficient MMR cases, and was likely worse. Instead, strategies like autophagy inhibition and/or immune checkpoint inhibition should be taken into consideration in the future.
Keywords: Microsatellite instability, Mismatch repair, Gastric cancer, Autophagy
Short abstract
The use of microsatellite instability (MSI)/ mismatch repair (MMR) as predictive biomarkers for fluorouracil‐based adjuvant chemotherapy in colorectal cancer was a paradigm shift; however, it is still unclear whether this has any clinical implications for gastric cancer. This retrospective study focused on whether fluorouracil‐based adjuvant chemotherapy is beneficial for patients with deficient MMR gastric cancer and whether autophagy plays a role in MSI‐relevant chemoresistance using in vitro assays.
Introduction
Gastric cancer is the fifth most common type of cancer and the third most common cause of cancer‐related death globally 1. Radical D2 gastrectomy combined with adjuvant fluorouracil‐based chemotherapy for managing locally advanced gastric cancer emerged as a gold standard for gastric cancer therapy at the beginning of the 21st century. Two separate genomic classifications of gastric cancer have been proposed in tandem by The Cancer Genome Atlas (TCGA) 2 and the Asian Cancer Research Group (ACRG) 3. Both studies unanimously identified microsatellite instability (MSI) as a unique subgroup of heterogenous gastric cancers. A high level of MSI (MSI‐H) indicates a hypermutator phenotype secondary to frequent polymorphism in short, repetitive DNA sequences and single nucleotide substitution, as a consequence of a deficient DNA mismatch repair (dMMR) machinery 4. Defects in this process ensue from inactivation of one of the mismatch repair (MMR) proteins, usually MLH1, MSH2, MSH6, or PMS2 4. Two recent meta‐analyses demonstrated that MSI‐H/dMMR gastric cancers are associated with better survival compared with their microsatellite stable (MSS) or proficient MMR (pMMR) counterparts 5, 6. Many retrospective, institute‐level series and even meta‐analysis have tried to elucidate whether adjuvant chemotherapy is harmful to dMMR gastric cancers, as observed in colorectal cancer 7, 8. However the results were mixed, mostly because of the small number of cases analyzed, and did not reach statistical significance 9, 10, 11, 12, 13. It had not been clear whether the MSI status is an actionable biomarker for stratifying patients and predicting benefits from adjuvant chemotherapy for stage II–III gastric cancer, until two post hoc analyses derived from the MAGIC trial and the CLASSIC trials indicated that MSI status is an actionable biomarker 14, 15. Several hypotheses have been proposed to explain why fluorouracil‐based adjuvant chemotherapy does not benefit patients with MSI‐H/dMMR cancer 16. Nevertheless, an evidence of explanation for MSI‐relevant chemoresistance has not yet surfaced.
Autophagy is an evolutionary conserved eukaryotic process in which organelles and bulk proteins are turned over by lysosomal activity. In mammalian cells, microtubule‐associated protein 1 light chain 3 (LC3) is conjugated to phosphatidyl ethanolamine. LC3‐II, a lipidated form of LC3, is considered a marker of autophagy for monitoring autophagosome formation, whereas p62 protein is reported as an autophagy substrate whose level decreases upon autophagy induction; as such, p62 is used to monitor autophagy flux. Autophagy has been proposed to provide cancer cells extraordinary energy and synthesis materials to meet their increased metabolic demands, to allow for disease progression during hypoxia, and to cope with the stress induced when antineoplastic drugs are administered 17. Therefore, autophagy is increasingly conceived to be responsible for carcinogenesis and chemoresistance 18. Importantly, targeting autophagy inhibition, such as using chloroquine (CQ) or 3‐MA, has been demonstrated as a promising strategy against chemoresistance in colon cancers 18, 19, 20.
The use of MSI‐MMR as predictive biomarkers for fluorouracil‐based adjuvant chemotherapy in colorectal cancer was a paradigm shift. However, it is still unclear whether this has any clinical implications for gastric cancer. Accordingly, we investigated retrospectively whether fluorouracil‐based adjuvant chemotherapy is beneficial for patients with dMMR gastric cancer based on a considerably large surgical series, as well as tested if and how autophagy plays a role in MSI‐relevant chemoresistance using in vitro assays.
Materials and Methods
Study Cohort
This retrospective study was approved by the institutional review board of Chang Gung Memorial Hospital (CGMH). Initially, we retrospectively analyzed 1,248 patients of gastric cancer following curative‐intent gastrectomy between 1999 and 2007 at CGMH, and from which the corresponding and representative surgical sections had been constructed as tissue microarray panels 21. After exclusion of the cases of hospital mortality (n = 33, 2.6%) and the cases of pathology stage 1 (n = 319, 25.6%) which were not subjected to adjuvant chemotherapy, a total of 929 eligible cases were enrolled for outcome analysis. The clinicopathological data on demographics, tumor location, pathological findings, and pathological stage were obtained from the prospectively collected electronic medical records. Pathological stages had been systematically converted to the AJCC Staging Manual, edition 8 22. The prescribed regimens of adjuvant chemotherapy were fluorouracil‐based, including intravenous 5‐fluorouracil (5‐FU), titanium silicate‐1, uracil‐tegafur, or oxaliplatin plus capecitabine 23. To assess the severity of medical morbidities, which affected the decision of whether or not to implement adjuvant chemotherapy made by the clinicians and/or patients and their families, American Society of Anesthesiologist score (ASA), Charlson comorbidity index (CCI), and Eastern Cooperative Oncology Group (ECOG) performance status were routinely checked preoperatively 24. Survival time was evaluated from the date of surgery to the date of death or date of last follow‐up (July 31, 2017), whichever came first.
Tissue Microarray and MMR Status Detected by Immunohistochemistry
The formalin‐fixed and paraffin‐embedded tissue samples were arrayed using an automated tissue‐arraying machine (BEECHER ATA‐27, Beecher Instruments, Sun Prairie, WI) 14. After the representative tumor areas were confirmed by H&E staining, three to five 1.0‐mm tissue cores were taken from the tissue blocks and transferred to recipient blocks. The tissue microarray slides were then sectioned for the DNA MMR protein assay (MLH1, GM011, 1:50 [Genemed, Torrance, CA]; MSH2, G219‐1129, 1:100 [Zeta, Arcadia, CA]; MSH6, ERP3945, 1: 150 [Abcam, Cambridge, U.K.]; PMS2, A16‐4, 1:100, [BD Pharmingen, San Jose, CA]). The procedures were performed in an automated immunostaining machine (BOND‐MAX, Leica Microsystems; Wetzlar, Germany) with optimal negative and positive controls according to the manufacturer's instructions. Those with and without absence of any MMR proteins were defined as dMMR and pMMR, respectively.
Gastric Cancer Cell Lines
Four human gastric cancer cell lines, SNU1, SNU638, SNU719, and SNU601, were obtained from the Korean Cell Line Bank (Seoul, Korea). Of these, SNU1 and SNU638 were characterized as MSI‐H, whereas SNU719 and SNU601 were MSS 25. All cell lines were grown in PRMI1640 supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin) at 37°C in a humidified incubator with 5% CO2.
Cell Proliferation Assay
Cells were seeded at a density of 2 × 103 cells per well in 100 μL, in a 96‐well plate 1 day before treatment with various concentrations of 5‐FU, with or without chloroquine (CQ). The cell proliferation assay was performed using the reagent WST‐1 (Roche, Indianapolis, IN) at the chosen time points of 1, 2, and 3 days after treatment. Sample absorbance was analyzed at 450 nm with background subtraction at 630 nm. All experiments were performed in triplicate.
Western Blotting
Cancer cells were lysed in lysis buffer, and 10 μg of protein was loaded per lane and resolved by SDS‐polyacrylamide electrophoresis. Protein was transferred onto nitrocellulose membranes, blocked, and probed with the following primary antibodies: rabbit anti‐LC3B (1:4000 dilution, Sigma‐Aldrich, St. Louis, MO), rabbit anti‐SQSTM1/p62 (1:4000 dilution, Sigma‐Aldrich), and rabbit anti‐GAPDH (1:4000 dilution).
Statistical Analysis
All data were presented as percentage of patients or mean with SD. Numerical data were compared by independent two‐sample t tests. Nominal data were compared by Pearson chi‐squared test, Fisher's exact test, or multiple forward stepwise logistic regression test when appropriate. Survival was calculated and plots constructed according to the Kaplan‐Meier method. Furthermore, the log‐rank test was performed for a statistical univariate analysis of prognostic variables. All aforementioned factors were input for Cox proportional hazard model once statistical significance proved by univariate analysis. An “enter‐selection” procedure was used to select the most relevant prognostic factors, and only factors that remained significant (p < .05) were included in the final model. We performed all statistical analyses using IBM SPSS Statistics for Windows (ver. 20.0; IBM Corporation, Armonk, NY). Furthermore, p < .05 was considered statistically significant.
Results
Demographics and Survival
The prevalence of dMMR gastric cancers in our series was 8.9% (83 out of 929). The vast majority of dMMR cases (n = 80, 96.4%) showed MLH1/ PMS2 coabsence, whereas the remaining three cases (3.6%) showed MSH2 and/or MSH‐6 absence. The dMMR cohort was characterized by older age, tumor site at the distal stomach, an intestinal phenotype, less nodal metastasis, and thus earlier pathologic stage (Table 1). Multivariate analysis demonstrated that age (hazard ratio [HR], 1.2), lymph node ratio (HR, 4.9 for the highest tier), pathological stage (HR, 7.2 for the highest tier), and MMR status (HR, 1.6) were prognostic factors, whereas adjuvant chemotherapy was not (Table 2). Overall survival (OS) of the dMMR cohort was better than that of the pMMR cohort irrespective of chemotherapy (p < .0001) but was only applicable to stage III patients (p < .0001; Fig. 1A–D). This trend was exactly same regarding to disease‐free survival (DFS; Fig. 2A–C). OS of stage II–IV pMMR with adjuvant chemotherapy was superior to pMMR without chemotherapy (p = .002), and OS of stage II–IV dMMR with or without adjuvant chemotherapy did not differ (p = .619; Fig. 3A). Subanalysis showed that this unique discrimination of OS stratified by stage and adjuvant chemotherapy was only applicable to stage III patients (p < .0001; Fig. 3B). This unique discrimination of DFS stratified by stage and adjuvant chemotherapy was also only applicable to stage III patients (p < .0001; Fig. 3C). To take into account the comorbidities upon the decision for adjuvant chemotherapy, we summarized the severity of the medical morbidities from patients treated with (n = 724) and without (n = 205) adjuvant chemotherapy (Table 3). The results show that patients treated with adjuvant chemotherapy were associated with younger age (p < .001) and less CCI (p < .001), ECOG (p < .001), and ASA (p < .001) scores compared with those without adjuvant chemotherapy treatment.
Table 1.
Clinicopathological findings of stage II–IV gastric cancer patients classified by DNA MMR status
| Parameters | Total no. of patients (n = 929) | p value | |
|---|---|---|---|
| Defective MMR (n = 83), n (%) | Proficient MMR (n = 846), n (%) | ||
| Age, median (IQR), yr | 73.0 (12.0) | 64.0 (20.0) | <.0001 |
| Gender | .624 | ||
| Male | 53 (63.9) | 517 (61.1) | |
| Female | 30 (36.1) | 329 (38.9) | |
| Location | .007 | ||
| Upper | 7 (8.4) | 185 (21.9) | |
| Middle | 11 (13.3) | 131 (15.5) | |
| Lower | 62 (74.7) | 476 (56.3) | |
| Diffuse | 3 (3.6) | 54 (6.4) | |
| Differentiation | .212 | ||
| Well or moderately | 33 (39.8) | 279 (33.0) | |
| Poorly | 50 (60.2) | 567 (67.0) | |
| Lauren's classificationa | <.001 | ||
| Intestinal | 52 (64.2) | 351 (41.8) | |
| Diffuse | 19 (23.5) | 364 (43.4) | |
| Mix | 10 (12.3) | 124 (14.8) | |
| Depth of invasion | .386 | ||
| T1 | 0 | 18 (2.1) | |
| T2 | 8 (9.3) | 60 (7.1) | |
| T3 | 1 (1.2) | 22 (2.6) | |
| T4 | 74 (89.2) | 746 (88.2) | |
| Nodal status | <.001 | ||
| N0 | 23 (27.7) | 138 (16.3) | |
| N1 | 20 (24.1) | 108 (12.8) | |
| N2 | 18 (21.7) | 188 (22.2) | |
| N3 | 22 (26.5) | 412 (48.7) | |
| LN ratio, median (IQR) | 0.10 (0.24) | 0.26 (0.46) | <.0001 |
| Stage | .044 | ||
| II | 25 (30.1) | 181 (21.4) | |
| III | 53 (63.9) | 546 (64.5) | |
| IV | 5 (6.0) | 119 (14.1) | |
| Lymphatic invasiona | 51 (62.2) | 609 (72.2) | .055 |
| Vascular invasiona | 9 (11.1) | 175 (20.9) | .035 |
| Perineural invasiona | 40 (48.2) | 561 (67.1) | .001 |
Not all data were available in cases.
Abbreviations: IQR, interquartile range; LN ratio, ratio of metastatic to retrieved lymph nodes; MMR, mismatch repair.
Table 2.
Univariate and multivariate analysis of prognostic factors in patients with stage II–IV gastric cancer
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Median (months) | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| Age | .049 | |||||
| ≤65 (n = 473) | 33.0 | 26.9–39.2 | 1 | |||
| >65 (n = 456) | 25.6 | 21.3–30.0 | 1.293 | 1.082–1.545 | .005 | |
| Gender | .161 | |||||
| Male (n = 570) | 28.6 | 24.4–32.9 | ||||
| Female (n = 359) | 30.8 | 23.2–38.5 | ||||
| Location | <.0001 | |||||
| Upper (n = 192) | 28.7 | 16.1–41.2 | 1.059 | 0.788–1.422 | .704 | |
| Middle (n = 142) | 41.6 | 23.6–59.5 | 1 | |||
| Lower (n = 538) | 30.5 | 26.0–34.9 | 1.036 | 0.805–1.332 | .784 | |
| Diffuse (n = 57) | 12.2 | 8.1–16.3 | 1.022 | 0.691–1.513 | .912 | |
| Differentiation | .001 | |||||
| Yes (n = 312) | 39.1 | 27.4–50.8 | 1 | |||
| No (n = 617) | 25.5 | 21.6–29.4 | 1.195 | 0.934–1.531 | .157 | |
| Lauren's classification | <.001 | |||||
| Intestinal (n = 403) | 38.7 | 29.4–48.1 | 1 | |||
| Diffuse (n = 383) | 22.6 | 18.4–26.7 | 1.147 | 0.888–1.480 | .294 | |
| Mixed (n = 134) | 23.3 | 14.2–32.5 | 1.015 | 0.759–1.358 | .918 | |
| LN ratio | <.0001 | |||||
| ≤0.050 (n = 218) | N/A | 1 | ||||
| 0.051–0.240 (n = 258) | 46.0 | 24.1–67.9 | 1.412 | 1.010–1.974 | .044 | |
| 0.241–0.450 (n = 185) | 25.9 | 20.7–31.1 | 2.222 | 1.555–3.176 | <.0001 | |
| 0.451–0.760 (n = 158) | 13.2 | 11.0–15.4 | 3.210 | 2.215–4.651 | <.0001 | |
| >0.760 (n = 110) | 8.8 | 7.4–10.1 | 4.996 | 3.324–7.510 | <.0001 | |
| Stage | <.0001 | |||||
| II (n = 206) | N/A | 1 | ||||
| III (n = 599) | 27.8 | 24.6–31.1 | 2.050 | 1.483–2.834 | <.0001 | |
| IV (n = 124) | 8.3 | 6.5–10.1 | 7.247 | 4.894–10.733 | <.0001 | |
| Lymphatic invasion | <.0001 | |||||
| Yes (n = 660) | 20.7 | 18.1–23.3 | 1.020 | 0.789–1.318 | .879 | |
| No (n = 265) | 142.0 | N/A | 1 | |||
| Vascular invasion | <.0001 | |||||
| Yes (n = 184) | 16.0 | 12.2–19.8 | 0.935 | 0.759–1.152 | .529 | |
| No (n = 733) | 34.7 | 28.4–41.0 | 1 | |||
| Perineural invasion | <.0001 | |||||
| Yes (n = 601) | 23.3 | 19.9–26.8 | 1.120 | 0.919–1.365 | .263 | |
| No (n = 318) | 52.0 | 26.7–77.4 | 1 | |||
| MMR status | <.0001 | |||||
| dMMR (n = 83) | N/A | 1 | ||||
| pMMR (n = 846) | 27.6 | 24.6–30.7 | 1.673 | 1.118–2.505 | .012 | |
| Chemotherapy | <.001 | |||||
| Yes (n = 724) | 31.5 | 26.6–36.4 | 0.838 | 0.678–1.036 | .103 | |
| No (n = 205) | 16.4 | 12.1–20.7 | 1 | |||
Abbreviations: CI, confidence interval; dMMR, defective mismatch repair; LN ratio, ratio of metastatic to retrieved lymph nodes; MMR, mismatch repair; N/A, not available; pMMR, proficient mismatch repair.
Figure 1.

OS of gastric cancers stratified by MMR status. OS at stage II–IV (A), stage II (B), stage III (C), and stage IV (D).Abbreviations: CI, confidence interval; dMMR, deficient mismatch repair; HR, hazard ratio; OS, overall survival; pMMR, proficient mismatch repair.
Figure 2.

DFS of gastric cancers stratified by mismatch repair (MMR) status. MMR status at stage II–III (A), stage II (B), and stage III (C).Abbreviations: CI, confidence interval; DFS, disease‐free survival; dMMR, deficient mismatch repair; HR, hazard ratio; pMMR, proficient mismatch repair.
Figure 3.
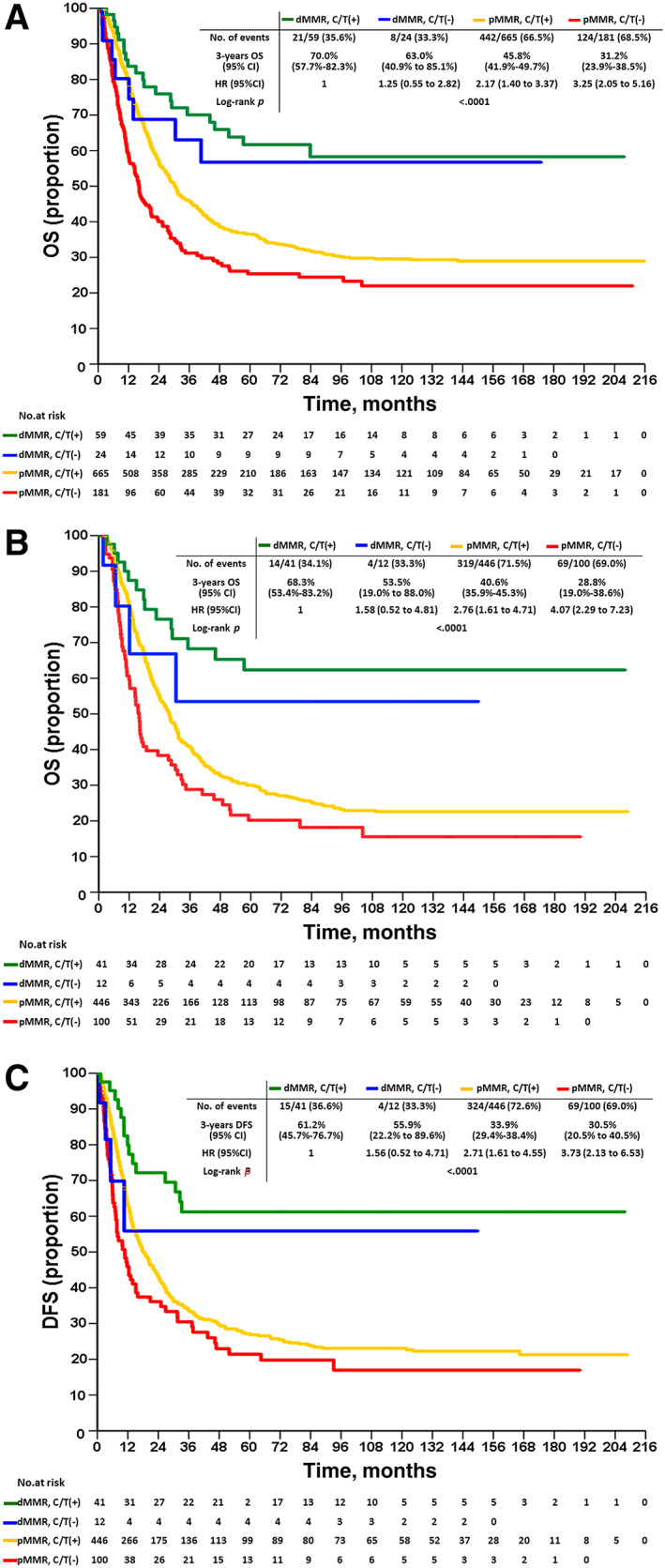
OS and DFS of gastric cancers stratified by mismatch repair (MMR) status and adjuvant chemotherapy. OS of stage II–IV gastric cancers stratified by MMR status and adjuvant chemotherapy (A). OS of stage III gastric cancers stratified by MMR status and adjuvant chemotherapy (B). DFS of stage III gastric cancers stratified by MMR status and adjuvant chemotherapy (C). C/T (+) and C/T (−) indicate with and without adjuvant chemotherapy, respectively.Abbreviations: CI, confidence interval; DFS, disease‐free survival; dMMR, deficient mismatch repair; HR, hazard ratio; OS, overall survival; pMMR, proficient mismatch repair.
Table 3.
Medical morbidity of gastric cancer patients with and without adjuvant chemotherapy
| Variables | With ACTa (n = 724), n (%) | Without ACTb (n = 205), n (%) | p value |
|---|---|---|---|
| Age, yr | |||
| ≤70 | 470 (65) | 63 (31) | <.001 |
| >70 | 254 (34) | 142 (69) | |
| BMI | |||
| <18 | 86 (12) | 26 (13) | .72 |
| 18–25 | 470 (65) | 131 (64) | |
| >25 | 168 (23) | 48 (21) | |
| CCI | |||
| 0 | 383 (53) | 67 (33) | <.001 |
| 1 | 231 (32) | 67 (33) | |
| >1 | 108 (15) | 71 (34) | |
| ECOG | |||
| 0–1 | 586 (81) | 114 (56) | <.001 |
| 2–4 | 138 (19) | 91 (44) | |
| ASA | |||
| 2 | 405 (56) | 59 (29) | <.001 |
| >2 | 319 (44) | 146 (71) |
Including 59 defective mismatch repair and 665 proficient mismatch repair cases, respectively.
Including 24 defective mismatch repair and 181 proficient mismatch repair cases, respectively.
Abbreviations: ACT, adjuvant chemotherapy; ASA, American society of Anesthesiologists; BMI, body mass index; CCI, Charlson comorbidity index; ECOG, Eastern Cooperative Oncology group performance;
In Vitro Study
The proliferation assays of two MSI‐H (SNU1 and SNU638) and two MSS (SNU601 and SNU719) gastric cancer cell lines challenged by escalating 5‐FU concentrations are shown in Figure 4A. Neither SNU1 nor SNU638 responded to 5‐FU until the concentration was titrated up to 0.5 μg/mL or higher; in contrast, both SNU601 and SNU719 responded to the minimal concentration of 0.1 μg/mL 5‐FU. LC3B and p62 expression of the four gastric cancer cell lines challenged by escalating 5‐FU concentrations were detected by immunoblot. It was found that expression of LC3B‐II in SNU1 and SNU638 was consistently increased along the 5‐FU dose escalation, whereas expression of LC3B‐II in SNU601 and SNU719 was not increased and was even lost along the 5‐FU dose escalation, suggesting failure of autophagy initiation (Fig. 4B). Meanwhile, expression of p62 in all four gastric cancer cell lines remained otherwise similar to their reference controls along the 5‐FU challenge, suggesting absence of autophagy blockade. To investigate whether the chemoresistance is derived from the augmented autophagy of the cancer cells elicited by 5‐FU, we added CQ, an autophagy inhibitor, in combination with 5‐FU, to test cell viability. When CQ was titrated to 5 μg/mL, SNU1 cells, the most chemoresistant cells we had tested, began responding to 5‐FU treatment, even at the minimal concentration of 0.1 μg/mL (Fig. 4C). Collectively, these results suggest that MSI‐H gastric cancer cell lines show a stronger resistance to 5‐FU through robust autophagy, as compared with the MSS counterparts. Such MSI‐relevant chemoresistance to 5‐FU could be effectively abrogated by autophagy inhibition.
Figure 4.
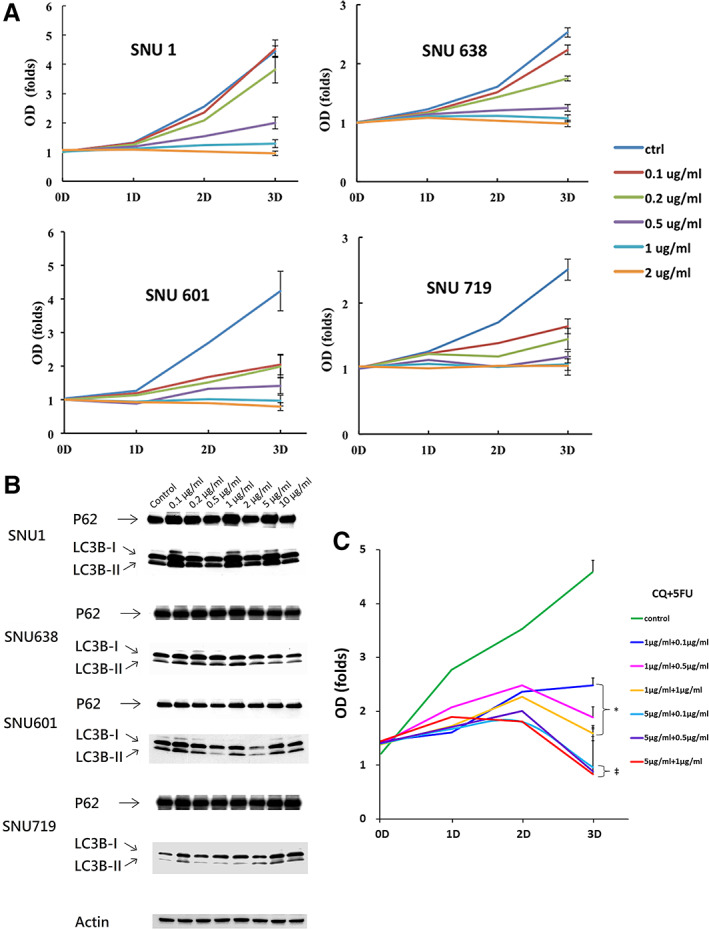
In vitro test of gastric cancer cell lines. (A): Proliferation assay of MSI‐H (SNU1 and SNU638) and MSS (SNU601 and SNU719) gastric cancer cell lines treated by 5‐FU with various concentrations. All experiments were in triplicate. (B): Representative Western blotting of LC3B and p62 of MSI‐H (SNU1 and SNU638) and MSS (SNU601 and SNU719) gastric cancer cell lines treated by 5‐FU with various concentrations, where LC3B‐II indicated lipidization of LC3B‐I, surrogate of autophagy activation, whereas p62 represented autophagy flux. (C): Proliferation assay of MSI‐H SNU1 gastric cancer cell line treated by 5‐FU and CQ with various concentrations. All experiments were in triplicate. *, p < .05 versus control; ‡, p < .01 versus control.Abbreviations: CQ, chloroquine; ctrl, control; 5‐FU, 5‐fluorouracil; MSI‐H, microsatellite instability high; MSS, microsatellite stable.
Discussion
Gastric cancer is a de facto heterogenous disease entity composed of various histological and molecular subtypes, which are currently treated by a one‐for‐all strategy; consequently, the survival rates of patients with gastric cancer is far from promising. However, this is beginning to change. In 2014, the TCGA research network divided gastric cancer into the following four molecular subtypes: Epstein‐Barr virus‐positive (9%); MSI‐H (22%), genomically stable (20%), and chromosomally instable (50%) 2. One year later, ACRG identified two major categories, MSI (23%) and MSS 3. Growing evidence indicates that the MSI‐H subtype is actionable and potentially druggable, which has triggered extensive research. The incidence of MSI cases is noticeably different geographically (i.e., more MSI cases were found to occur in South Korea and Russia) 2. In the present study, we identified that the prevalence of the dMMR cohort was 8.9%, occurring in 83 out of 929 stage II–IV gastric cancers, and it increases up to 9.5% if stage I cases are taken into account, with 114 out of 1,206 stage I–IV cases in our initial screening (data not shown). Considering that gastric cancer is the fifth most common type of cancer globally, a prevalence of MSI‐H gastric cancer around 10% is highly significant. Importantly, the methods for the detection of MSI have raised concerns. Mathiak et al. showed that MMR detected by immunohistochemistry (IHC) yielded 33 of 34 MSI cases that were identified by five microsatellite markers (BAT25, BAT26, NR21, NR24, and NR27) 26. Beghelli et al. reported a concordant rate of 95% between MMR detected by IHC and MSI detected by DNA sequencing 27. Additionally, a TCGA study found that the majority (92%, 45 out of 49) of MSI‐H gastric cancers were caused by MLH1 promoter methylation 2, which was consistent with our data that the majority of dMMR cases (96.4%) showed an MLH1 defect. Collectively, the accumulated evidence supports that the MMR phenotype detected by IHC is a surrogate of the MSI genotype. IHC possesses several advantages such as having a low cost, being easy to process, and allowing for mass screening. Therefore, our data, constructed by studying one of the largest surgical cohorts worldwide, reaffirm that the dMMR cohort is associated with older age, tumor site at the distal stomach, an intestinal phenotype, less nodal metastasis, and early pathological stage, which corresponds with the main findings from previous large series and meta‐analyses 5, 6, 10.
Our noteworthy discovery includes the finding that the previously claimed survival niche of dMMR cases is valid only at stage III irrespective of the use of adjuvant chemotherapy. This result is reasonable given that the survival of stage I, II, and IV gastric cancer are either too good or too dismal to be discriminated in terms of MMR status. Our data differ from those of Beghelli et al., as in their study, only stage II MSI‐H gastric cancer was found to be associated with a better prognosis 27. Our study also differs from the one conducted by Kim et al. in which the prognosis of MSI cases was found to be better in stage II and III if adjuvant chemotherapy is not administered 11. According to our prognostic analysis, adjuvant chemotherapy was significant in univariate analysis, whereas it failed to remain significant after multivariate adjustment. Instead, the MMR status following pathological stage and lymph node ratio ranked as the third most important prognostic factor. Because we had already excluded the stage I cases, these unexpected data should be interpreted carefully, as adjuvant chemotherapy following radical gastrectomy has been a consensus of standard care for the modern surgical society 28, 29, 30. We believed that confounding factors were diluting the power of adjuvant chemotherapy. Therefore, we examined the outcomes of our cases stratified by both MMR status and adjuvant chemotherapy. As expected, OS of pMMR with adjuvant chemotherapy was better than OS without chemotherapy; contrastingly, OS of dMMR with or without adjuvant chemotherapy did not differ. The statistical analysis proved that the use of adjuvant chemotherapy in dMMR cases was not beneficial in terms of survival and explained why the adjuvant chemotherapy per se was not an independent prognostic factor. In retrospect, the dMMR patients subjected to adjuvant chemotherapy had better physiological and cognitive conditions, as reflected by their younger age, and lower scores of CCI, ECOG, and ASA. Their outcomes were only comparable to those without adjuvant chemotherapy. It is very likely that dMMR cases would have a better chance of survival had adjuvant chemotherapy not been given routinely. Nevertheless, it is too early to recommend withdrawal of adjuvant chemotherapy from dMMR cases, particularly in the stage III ones. Even with prospective and randomized design, altogether only 59 MSI‐H patients of the MAGIC (n = 19) and CLASSIC (n = 40) trials were included in the post hoc analysis 14, 15, and it is probable that a few events of recurrence could have led to an opposite conclusion.
A mechanism to explain why the fluorouracil‐based adjuvant chemotherapy does not benefit MSI‐H cases has not yet been elucidated. One possibility is related to the high tumor mutational burden (TMB) of MSI‐H tumors, which present neoantigens and recruit TILs to counter themselves 31, 32, 33, 34. By introducing chemotherapy, the MSI‐H cases lose immune‐surveillance ability and chemoresistance ensues 15. 5‐FU, the most frequently used antimetabolite agent, exerts its cytotoxic effect through inhibition of thymidylate synthetase and incorporation of macromolecules into DNA 35. Cancer cells with functioning MMR machinery attempt to amend the modified base pairs as though they were replication errors but are unable to complete their repair. These futile efforts result in cell cycle arrest and apoptosis 14. Contrarily, cancer cells lacking MMR machinery keep idling and survive while accumulating stepwise mutations and building a phenotype of chemoresistance 16. Additionally, our in vitro experiment raised a third possibility. Autophagy plays a role in the adaptation of cancer cells to detrimental microenvironments, such as nutrient deprivation and hypoxia, and to the challenge induced by chemo‐ and/or radiation therapy 17, 18. The present data show that MSI‐H gastric cancer cells have increased chemoresistance to 5‐FU due to increased autophagy activation. Importantly, this MSI‐relevant chemoresistance could be effectively abrogated by autophagy inhibition. This preclinical study provides an initial glimpse into the potential of autophagy inhibition as a suitable treatment against MSI‐H gastric cancer in the future. Although the pursuit of more specific autophagy inhibitors and trials of the combination of autophagy inhibitors with cytotoxic drugs are undergoing, these are not yet in use in current practice 17, 18. Separately, checkpoint inhibitor immunotherapy has revolutionarily transformed treatment guidelines of several kinds of solid tumors, where the role of mismatch repair is especially relevant 36. Theoretically, patients whose tumors have high TMB attain the highest response rate to checkpoint inhibitors. As such, MSI‐H gastric cancers whose genomes are most chaotic and previously believed to be least amenable to manipulation are now the best candidates for checkpoint inhibitor immunotherapy 37. In fact, immune checkpoint blockade of programmed cell death 1 and cytotoxic T‐lymphocyte‐associated protein 4 have led to U.S. Food and Drug Administration‐approved therapies for MSI‐H/dMMR tumors 36, 38, 39.
A couple of drawbacks in this study should be addressed. First, our study was retrospective; thus, the outcome analysis was unavoidably biased. For example, because the decision to use adjuvant chemotherapy had been largely dependent on the patients’ general conditions and/or the clinicians’ preference, it might be incorrect to analyze the impact of adjuvant chemotherapy upon survival. Second, our in vitro assay was lacking in engagement of immune cells, which were deemed to display cross‐talk with MMR machinery during chemotherapy. However, an in vivo xenograft model with inherent immunity for this purpose is not available.
Conclusion
The prevalence of dMMR cases was 8.9% (83 out of 929) in our series, which was characterized by older age, tumor site at the distal stomach, an intestinal phenotype, less nodal metastasis, and early pathological stage. The claimed survival niche of dMMR cases was valid only at stage III, and the survival of dMMR cases with or without adjuvant chemotherapy did not differ. In vitro, MSI‐H gastric cancer cells exhibited chemoresistance to 5‐FU, which could be effectively abrogated by autophagy inhibition. From a clinical perspective, fluorouracil‐based adjuvant chemotherapy does not improve survival of dMMR cases; however, autophagy inhibition and/or checkpoint inhibitor immunotherapy, in combination with cytotoxic drugs, might be alternatives.
Author Contributions
Conception/design: Ta‐Sen Yeh
Provision of study material or patients: Jun‐Te Hsu, Chun‐Nan Yeh, Ta‐Sen Yeh
Collection and/or assembly of data: Chun‐Yi Tsai, Tien‐An Lin, Shih‐Chiang Huang, Jun‐Te Hsu, Chun‐Nan Yeh, Tse‐Ching Chen, Cheng‐Tang Chiu, Jen‐Shi Chen, Ta‐Sen Yeh
Data analysis and interpretation: Chun‐Yi Tsai, Ta‐Sen Yeh
Manuscript writing: Chun‐Yi Tsai, Tien‐An Lin, Shih‐Chiang Huang, Jun‐Te Hsu, Chun‐Nan Yeh, Tse‐Ching Chen, Cheng‐Tang Chiu, Jen‐Shi Chen, Ta‐Sen Yeh
Final approval of manuscript: Chun‐Yi Tsai, Tien‐An Lin, Shih‐Chiang Huang, Jun‐Te Hsu, Chun‐Nan Yeh, Tse‐Ching Chen, Cheng‐Tang Chiu, Jen‐Shi Chen, Ta‐Sen Yeh
Disclosures
The authors indicated no financial relationships.
Acknowledgments
This work was supported by the Chang Gung Medical Research Program, Taiwan (CMRPG3D1121‐3 and CMRPG3D1131‐3).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cristescu R, Lee J, Nebozhyn M et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–456. [DOI] [PubMed] [Google Scholar]
- 4. Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther 2018;189:45–62. [DOI] [PubMed] [Google Scholar]
- 5. Choi YY, Bae JM, An JY et al. Is microsatellite instability a prognostic marker in gastric cancer?: A systemic review with meta‐analysis. J Surg Oncol 2014;110:129–135. [DOI] [PubMed] [Google Scholar]
- 6. Polom K, Marano L, Marrelli D et al. Meta‐analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018;105:159–167. [DOI] [PubMed] [Google Scholar]
- 7. Ribic CM, Sargent DJ, Moore MJ et al. Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sargent DJ, Marsoni S, Monges G et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oki E, Kakeji Y, Zhao Y et al. Chemosensitivity and survival in gastric cancer patients with microsatellite instability. Ann Surg Oncol 2009;16:2510–2515. [DOI] [PubMed] [Google Scholar]
- 10. An JY, Kim H, Cheong JH et al. Microsatellite instability in sporadic gastric cancer: Its prognostic role and guidance for 5‐FU based chemotherapy after R0 resection. Int J Cancer 2012;131:505–511. [DOI] [PubMed] [Google Scholar]
- 11. Kim SY, Choi YY, An JY et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: Results from a large cohort with subgroup analyses. Int J Cancer 2015;137:819–825. [DOI] [PubMed] [Google Scholar]
- 12. Haag GM, Czinik E, Ahadova A et al. Prognostic significance of microsatellite‐instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int J Cancer 2019;144:1697–1703. [DOI] [PubMed] [Google Scholar]
- 13. Zhao F, Yuan X, Ren D et al. Predicting the efficacy of 5‐fluorouracil‐based adjuvant chemotherapy in gastric cancer by microsatellite instability: A meta‐analysis. J Environ Pathol Toxicol Oncol 2019;38:21–28. [DOI] [PubMed] [Google Scholar]
- 14. Smyth EC, Wotherspoon A, Peckitt C et al. Mismatch repair deficiency, microsatellite instability, and survival. An exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi YY, Kim H, Shin SJ et al. Microsatellite instability and programmed cell death‐ligand 1 expression in stage II/III gastric cancer: Post hoc analysis of the CLASSIC randomized controlled study. Ann Surg 2019;270:309–316. [DOI] [PubMed] [Google Scholar]
- 16. Meyers M, Wagner MW, Hwang HS et al. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine‐mediated cell death and cell cycle responses. Cancer Res 2001;61:5193–5201. [PubMed] [Google Scholar]
- 17. Galluzzi L, Bravo‐San Pedro JM, Demaria S et al. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol 2017:14:247–258. [DOI] [PubMed] [Google Scholar]
- 18. Huang Z, Zhou L, Chen Z et al. Stress management by autophagy: Implications for chemoresistance. Int J Cancer 2016;139:23–32. [DOI] [PubMed] [Google Scholar]
- 19. Li J, Hou N, Faried A et al. Inhibition of autophagy augments 5‐fluorouuracil chemotherapy in human colon cancer in vivo and in vitro model. Eur J Cancer 2010;46:1900–1909. [DOI] [PubMed] [Google Scholar]
- 20. Sasaki K, Tsuno NH, Sunami E et al. Chloroquine potentiate the anti‐cancer effect of 5‐fluorouracil on colon cancer cells. BMC Cancer 2010;10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang SC, Ng KF, Yeh TS et al. Subtraction of Epstein‐Barr virus and microsatellite instability genotype from the Lauren histotypes: Combined molecular and histologic subtyping with clinicopathological and prognostic significance validation in a cohort of 1,248 cases. Int J Cancer 2019;145:3218–3230. [DOI] [PubMed] [Google Scholar]
- 22. Hung SF, Chien TC, Fang WL et al. The 8th edition American Joint Committee on gastric cancer pathological staging classification performs well in a population with high proportion of locally advanced disease. Eur J Surg Oncol 2018;44:1634–1639. [DOI] [PubMed] [Google Scholar]
- 23. Liu YY, Fang WL, Wang F, et al. Does a Higher cutoff value of lymph node retrieval substantially improve survival in patients with advanced gastric cancer?—Time to embrace a new digit. The Oncologist 2017;22:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang SC, Liu KH, Hung CY et al. Adjuvant chemotherapy improves survival in stage III gastric cancer after D2 surgery. J Cancer 2018;9:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HS, Choi SI, Min HL et al. Mutation at intron repeats of the ataxia‐telangiectasia mutated (ATM) gene and ATM protein loss in primary gastric cancer with microsatellite instability. PLOS One 2013;8:e82769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mathiak M, Warneke VS, Behrens HM et al. Clinicopathologic characteristics of microsatellite instable gastric carcinomas revisited: Urgent need for standardization. Immunohistochem Mol Morphol 2017;25:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beghelli S, de Manozi G, Barbi S et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery 2006;139:347–356. [DOI] [PubMed] [Google Scholar]
- 28. Sakuramoto S, Sasako M, Yamaguchi T et al; ACTS‐GC Group . Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. New Eng J Med 2007;357:1810–1820. [DOI] [PubMed] [Google Scholar]
- 29. Sasako M, Sakuramoto S, Katai H et al. Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387–4393. [DOI] [PubMed] [Google Scholar]
- 30. Bang YJ, Kim YW, Yang HK et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open‐label, randomised controlled trial. Lancet 2012;379:315–321. [DOI] [PubMed] [Google Scholar]
- 31. Michel S, Benner A, Tariverdian M et al. High density of FOXP3‐positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer 2008;99:1867–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee HE, Chae SW, Lee YJ et al. Prognostic implications of type and density of tumor‐infiltrating lymphocytes in gastric cancer. Br J Cancer 2008;99:1704–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim KJ, Lee KS, Cho HJ et al. Prognostic implications of tumor‐infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite‐unstable gastric cancers. Human Pathol 2014;45:285–293. [DOI] [PubMed] [Google Scholar]
- 34. Philips SM, Banerjea A, Feakins R et al. Tumor‐infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg 2004;91:469–475. [DOI] [PubMed] [Google Scholar]
- 35. Longley DB, Harkin DP, Johnson PG. 5‐fluorouracil: Mechanisms of action and clinical strategies. Nature Rev Cancer 2003;3:330–338. [DOI] [PubMed] [Google Scholar]
- 36. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Subbiah V, Kurzrock R. The marriage between genomics and immunotherapy: Mismatch meets its match. The Oncologist 2019;24:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weinberg BA, Xiu J, Hwang JJ et al. Immuno‐oncology biomarkers for gastric and gastroesophageal junction adenocarcinoma: Why PD‐L1 testing may not be enough. The Oncologist 2018;23:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hashimoto T, Kurokawa Y, Takahashi T et al. Predictive value of MLH1 and PD‐L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer 2019;22:785–792. [DOI] [PubMed] [Google Scholar]


