Abstract
Lessons Learned
Modified vaccinia Ankara‐Bavarian Nordic (MVA‐BN)‐Brachyury followed by fowlpox virus‐BN‐Brachyury was well tolerated upon administration to patients with advanced cancer.
Sixty‐three percent of patients developed CD4+ and/or CD8+ T‐cell responses to brachyury after vaccination.
BN‐Brachyury vaccine also induced T‐cell responses against CEA and MUC1, which are cascade antigens, that is, antigens not encoded in the vaccines.
Background
Brachyury, a transcription factor, plays an integral role in the epithelial–mesenchymal transition, metastasis, and tumor resistance to chemotherapy. It is expressed in many tumor types, and rarely in normal tissues, making it an ideal immunologic target. Bavarian Nordic (BN)‐Brachyury consists of vaccination with modified vaccinia Ankara (MVA) priming followed by fowlpox virus (FPV) boosting, each encoding transgenes for brachyury and costimulatory molecules.
Methods
Patients with metastatic solid tumors were treated with two monthly doses of MVA‐brachyury s.c., 8 × 108 infectious units (IU), followed by FPV‐brachyury s.c., 1 × 109 IU, for six monthly doses and then every 3 months for up to 2 years. The primary objective was to determine safety and tolerability.
Results
Eleven patients were enrolled from March 2018 to July 2018 (one patient was nonevaluable). No dose‐limiting toxicities were observed. The most common treatment‐related adverse event was grade 1/2 injection‐site reaction observed in all patients. Best overall response was stable disease in six patients, and the 6‐month progression‐free survival rate was 50%. T cells against brachyury and cascade antigens CEA and MUC1 were detected in the majority of patients.
Conclusion
BN‐Brachyury vaccine is well tolerated and induces immune responses to brachyury and cascade antigens and demonstrates some evidence of clinical benefit.
Discussion
BN‐Brachyury is a novel prime‐boost therapeutic cancer immunotherapy strategy composed of an MVA‐vector vaccine followed by an FPV‐vector booster vaccine targeting the transcription factor brachyury. The prime‐boost approach used in this trial was intended to optimize immunogenicity and improve clinical benefit.
The primary objectives of this trial were to assess the safety and to determine the recommended phase II dose. All patients were monitored for dose‐limiting toxicities (DLTs) 28 days after the first FPV‐brachyury booster vaccine since MVA‐Brachyury was already evaluated in a separate phase I trial 1. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Radiologic imaging was performed at baseline, weeks 12, 24, and 40, and then every 12 weeks thereafter. This study enrolled 11 patients from March 2018 to July 2018 (one patient was nonevaluable owing to the discovery of brain metastases within 1 week after enrollment). The data cutoff for this analysis was October 1, 2019. Six patients were male. Three patients had chordoma, six had gastrointestinal cancers, and one had papillary thyroid cancer. BN‐Brachyury vaccine was well tolerated with no DLTs observed; no patients stopped treatment as a result of side effects. There was one serious grade 3 treatment‐related adverse event (TRAE), depressed level of consciousness associated with fever, which resolved spontaneously and did not recur with subsequent cycles. All other TRAEs were grade 1 or 2; the most common were injection‐site reactions, which occurred in all evaluable patients (n = 10), fatigue (n = 6), and chills (n = 5). The recommended phase II dose is two doses of priming MVA‐BN‐Brachyury vaccine (8 × 108 IU) followed by monthly booster of FPV‐Brachyury vaccine (1 × 109 IU). The best overall response (per RECIST version 1.1) was stable disease in six patients (60%). Time on treatment ranged from 12 to 65 weeks. One patient with metastatic chordoma is still on treatment (week 65) with stable disease per RECIST as of week 52. The majority of patients (5/8, 63%) developed brachyury‐specific T cells after vaccination (Fig. 1). T‐cell responses against the cascade antigens CEA and MUC1 (not encoded in the vaccine), were developed in 4/8 (50%) and 6/8 (75%) of patients, respectively. Development of T‐cell responses to brachyury and CEA, but not MUC1, were associated with disease control. Multifunctional T cells (i.e., a more stringent analysis of T cells positive for two or more of the following: interferon gamma [IFNg], interleukin 2 [IL2], tumor necrosis factor alpha [TNFa], or CD107a) against at least one of the tumor‐associated antigens tested were generated after vaccination in 38% of patients.
Figure 1.
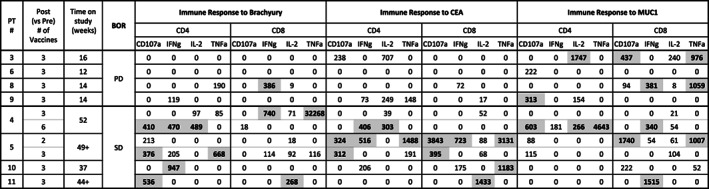
Tumor‐associated antigen‐specific T‐cell responses developed after vaccination with BN‐Brachyury. Immune responses are calculated by comparing the absolute number of CD4+ or CD8+ T cells producing cytokine (IFNg, IL‐2, TNFa) or positive for the degranulation marker CD107a per 1 × 106 PBMCs plated at the start of the in vitro stimulation at the specified time points post (vs. pre) vaccine. Background (obtained with the negative control peptide pool, HLA) and any response prior to vaccine are subtracted: [TAA post vaccine − HLA post vaccine] − [TAA pre vaccine − HLA pre vaccine]. Positive immune responses are defined as >250 (bold and highlighted in gray).Abbreviations: BOR, best overall response; HLA, human leukocyte antigen; PBMC, peripheral blood mononuclear cell; PD, progressive disease; SD, stable disease; TAA, tumor‐associated antigen.
This study demonstrated that BN‐Brachyury is well tolerated and induces immune responses to brachyury and cascade antigens. Future trials with this vaccine are planned.
Trial Information
| Disease | Advanced cancer/solid tumor only |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study – 1 | Phase I |
| Type of Study – 2 | Dose evaluation |
| Primary Endpoint | Safety |
| Primary Endpoint | Tolerability |
| Secondary Endpoint | Clinical benefit per objective response or progression‐free survival (RECIST version 1.1) |
| Additional Details of Endpoints or Study Design | Exploratory endpoint: immune analysis |
| Investigator's Analysis | Drug tolerable, some efficacy observed in chordoma |
Drug Information
| Drug 1 | |
| Generic/Working Name | MVA‐BN‐Brachyury |
| Company Name | Bavarian Nordic |
| Drug Type | Poxviral‐vector vaccine |
| Dose | 8 × 108 IU given as four injections (1 injection = 2 × 108 IU) |
| Route | Subcutaneous |
| Schedule of Administration | Two doses were administered at monthly (28 ± 4 days) intervals for 2 months. Each dose consisted of four injections, one in each extremity. |
| Drug 2 | |
| Generic/Working Name | FPV‐BN‐Brachyury |
| Company Name | Bavarian Nordic |
| Drug Type | Poxviral‐vector vaccine |
| Dose | Single injection of 1 × 109 IU |
| Route | Subcutaneous |
| Schedule of Administration | The first dose of FPV‐BN‐Brachyury was administered beginning 1 month (28 days ± 4 days) after the second dose of MVA‐BN‐Brachyury. It was administered at monthly (28 days ± 4 days) intervals for six doses then every 12 weeks (−4 days up to +14 days) for a total treatment duration of 2 years. |
Patient Characteristics
| Number of Patients, Male | 6 |
| Number of Patients, Female | 4 |
| Stage | Advanced or metastatic |
| Age | Median (range): 55 (40–73) |
| Number of Prior Systemic Therapies | Median (range): 4 (1–8) |
| Performance Status: ECOG |
0 — 5 1 — 5 2 — 3 — Unknown — |
| Other |
White = 9 Native American = 1 |
| Cancer Types or Histologic Subtypes |
Microsatellite stable colorectal cancer 4 Chordoma 3 Pancreatic cancer 1 Appendiceal carcinoma 1 Papillary thyroid cancer 1 |
Primary Assessment Method
| Title | New assessment |
| Number of Patients Screened | 11 |
| Number of Patients Enrolled | 11 |
| Number of Patients Evaluable for Toxicity | 10 |
| Number of Patients Evaluated for Efficacy | 10 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 6 (60%) |
| Response Assessment PD | n = 4 (40%) |
| Outcome Notes | Best overall response was stable disease in six patients (60%), and the 6‐month progression‐free survival rate was 50%. |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Injection site reaction | 0% | 40% | 60% | 0% | 0% | 0% | 100% |
| Fatigue | 40% | 50% | 10% | 0% | 0% | 0% | 60% |
| Chills | 50% | 50% | 0% | 0% | 0% | 0% | 50% |
| Fever | 60% | 20% | 20% | 0% | 0% | 0% | 40% |
| Headache | 70% | 20% | 10% | 0% | 0% | 0% | 30% |
| Abdominal soft tissue necrosis | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
| Rash maculo‐papular | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
| Depressed level of consciousness | 90% | 0% | 0% | 10% | 0% | 0% | 10% |
| Testicular pain | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
| Pleural effusion | 90% | 0% | 10% | 0% | 0% | 0% | 10% |
| Weight gain | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
| Localized edema | 90% | 0% | 10% | 0% | 0% | 0% | 10% |
| Myalgia | 80% | 10% | 10% | 0% | 0% | 0% | 20% |
| Edema limbs | 80% | 10% | 10% | 0% | 0% | 0% | 20% |
| Edema trunk | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
| Cough | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
| Respiratory, thoracic, and mediastinal disorders—nasal congestion | 80% | 20% | 0% | 0% | 0% | 0% | 20% |
Highest adverse event grade is noted for each patient. Adverse events at least possibly related to research.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Depressed level of consciousness | 3 | Possible |
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Drug tolerable, some efficacy observed in chordoma
This phase I study evaluated safety and systemic immunogenicity of Bavarian Nordic (BN)‐Brachyury, a novel prime‐boost therapeutic cancer vaccine platform composed of a modified vaccinia Ankara (MVA)‐vector vaccine that acts as the prime followed by a fowlpox virus (FPV)‐vector booster vaccine. Both are novel poxviral vector‐based therapeutic cancer vaccines that encode the transgenes for the brachyury gene as well as a triad of human T‐cell costimulatory molecules (TRICOM: B7.1, LFA‐3, and ICAM‐1) 2. The MVA‐prime and FPV‐boost strategy has been shown to stimulate higher levels of antigen‐specific T cells compared with other heterologous vaccine combinations 3, 4 but has never been tested with the brachyury transgene. Repeated boosting with FPV has been shown to limit neutralizing antiviral antibody formation 5 that can occur with repeated vaccinia dosing 6.
The transcription factor brachyury has been shown to play an integral role in the acquisition of mesenchymal features by epithelial cancer cells (i.e., epithelial–mesenchymal transition), metastasis 7, and resistance to chemotherapy, radiation, and possibly immunotherapy by reducing the susceptibility of cancer cells to lysis by antigen‐specific T cells and natural killer cells 8, 9, 10. The Laboratory of Tumor Immunology and Biology at the National Cancer Institute has identified expression of brachyury in many tumor types, including lung, colorectal, breast, and thyroid. Brachyury is universally expressed in chordoma 11, 12, and in May 2018, BN‐Brachyury was granted orphan drug designation by the U.S. Food and Drug Administration for the treatment of chordoma, a disease lacking effective therapies. Brachyury is rarely expressed in normal tissue 13, 14, making it an ideal target. Polymerase chain reaction analysis has shown that brachyury mRNA expression increases with tumor stage and histologic tumor grade 7. High levels of brachyury in primary tumors correlate with poor patient prognosis in carcinomas of the lung 15, colon 16, breast 17, and chordomas 18. In vitro silencing of brachyury expression in human lung carcinoma cells results in downregulation of mesenchymal markers and upregulation of epithelial markers with concomitant loss of cell migration and invasion 7. Taken together, these findings indicate that brachyury expression is detrimental and is an excellent target for the treatment of advanced malignancies.
The MVA‐BN‐Brachyury vaccine was previously tested in patients with advanced cancer in a phase I dose‐escalation study that demonstrated an acceptable safety profile and ability to generate brachyury‐specific T cells; however, immunological impact was limited owing to the lack of booster dosing 1. The safety and immunologic data in the current study are consistent with findings from the prior clinical trial 1.
Based on peripheral blood mononuclear cell (PBMC) availability, immune analyses were performed in 8 of 10 patients (Table 1). PBMCs were assayed from all patients prior to receiving BN‐Brachyury, as well as after the third vaccination (two primes, one boost). PBMCs were also evaluated in one patient after only two primes, and in another patient after receiving multiple boosts. Considering all time points evaluated, the majority of patients (5/8, 63%) developed brachyury‐specific T cells after BN‐Brachyury vaccination. BN‐Brachyury also induced T‐cell responses against cascade antigens not encoded in the vaccine; CEA‐ and MUC1‐specific T cells were developed in 4/8 (50%) and 6/8 (75%) patients, respectively, after vaccination. Tumor‐associated antigen responses were evaluated for potential association with clinical response by comparing immune responses in four patients with a best overall response of stable disease versus four patients who had progression or unconfirmed progression at the first restaging. It is interesting to note that brachyury‐specific T cells were developed in four of four patients with stable disease compared with only one of four patients who had progressive disease. CEA‐specific T cells were also developed at a higher frequency in patients with stable disease (four of four) compared with those with progressive disease (zero of four). In contrast, MUC1‐specific T cells were developed at the same frequency in patients with stable disease (three of four) and progressive disease (three of four). Multifunctional tumor‐associated antigen responses, a more stringent criteria, defined as CD4+ or CD8+ T cells that express two or more of the markers IFNg, IL2, TNFa, or CD107a, were also measured before and after vaccination. The generation of long‐lasting multifunctional T cells after vaccination has previously been associated with improved overall survival in patients with melanoma 19. In the present study, using the criteria of a > 10‐fold increase post‐ versus prevaccination, or the presence of >1,000 polyfunctional cells at post per 1 × 106 PBMCs (if negative at pre), polyfunctional T cells specific for brachyury, CEA, or MUC1 were generated after BN‐Brachyury vaccine in 3/8 (38%) patients (Table 2).
Based on PBMC availability, the effect of repeated fowlpox boosting could be evaluated in only one patient (patient 4). In this patient, brachyury‐specific T cells were observed after both a single boost and multiple FPV boosts; however, T cells against the cascade antigens CEA and MUC1 were detectable only after the patient received multiple FPV boosts. This is the first study to show the ability of the BN‐Brachyury vaccine to induce cascade antigen responses. Repeated fowlpox boosting increased T‐cell responses against cascade antigens. These findings illustrate the immunogenicity of the heterologous brachyury vaccine and the importance of the prime‐boost strategy.
This study also showed some evidence of clinical benefit. Best overall response was stable disease in six patients (60%), and the 6‐month progression‐free survival rate was 50%. Patients with metastatic chordoma seemed to benefit most in terms of progression‐free survival. One patient (patient 11) with metastatic chordoma achieved a maximum 14% decrease in target lesion burden at week 40 and remains on study treatment at week 65 with stable disease (as of week 52). Interestingly, volumetric measurement (segmentation) of the patient's scans showed an 83% decrease in the volume of their target lesions, which may be equivalent to partial response per RECIST version 1.1 as a 30% decrease in diameter is equivalent to 65% reduction in volume 20. This suggests that restricting response evaluations to single diameter measurements may be inappropriate for tumors with a nonspherical growth pattern (such as chordoma) and that volumetric tumor assessment, although time consuming, may better determine the potential treatment benefit 21. Another patient with chordoma (patient 1) has had stable disease for at least 40 weeks and came off treatment because of patient choice (unconfirmed disease progression at week 51). This patient has not received subsequent therapies and still has stable disease per follow‐up imaging.
This study demonstrated that heterologous MVA‐ and FPV‐brachyury vaccination is well tolerated and induces immune responses to brachyury and cascade antigens, suggesting induction of immunologically relevant tumor cell destruction. These data have informed combining BN‐Brachyury with checkpoint inhibition in patients with advanced cancer (NCT03493945) and radiation in patients with advanced chordoma (NCT03595228) to evaluate potential for synergistic activity in selected populations. In conclusion, this trial suggests that BN‐Brachyury vaccine is well tolerated and can induce antitumor immune responses. Evaluating BN‐Brachyury vaccine in a more homogenous group of less heavily pretreated patients (such as chordoma) may improve objective and immunologic responses.
Disclosures
Christopher R. Heery: Bavarian Nordic (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Table
Table 1.
Scoring criteria of multifunctional TAA response developed post‐ versus prevaccination
| Factor | Lowa | Highb |
|---|---|---|
| Brachyury | 2/8 (25%) | 1/8 (13%) |
| CEA | 1/8 (13%) | 1/8 (13%) |
| MUC1 | 4/8 (50%) | 1/8 (13%) |
| Any TAA | 6/8 (75%) | 3/8 (38%) |
3× pre or, if no pre, >100/1 × 106 cells post.
10× pre or, if no pre, >1,000/1 × 106 cells post.
Abbreviation: TAA, tumor‐associated antigen.
Acknowledgments
We thank Debra Weingarten for editorial assistance in the preparation of this manuscript. We also thank the patients and their families, as well as the clinical and research staff who participated in the study. The National Cancer Institute has a Collaborative Research and Development Agreement (CRADA) with Bavarian Nordic, as well as patents involving brachyury, MUC1, and CEA. C.R.H. is currently affiliated with Precision BioSciences, Durham, North Carolina, USA.
Footnotes
- ClinicalTrials.gov Identifier: NCT03349983
- Sponsor: Bavarian Nordic
- Principal Investigator: Marijo Bilusic
- IRB Approved: Yes
References
- 1. Heery CR, Palena C, McMahon S et al. Phase I study of a poxviral TRICOM‐based vaccine directed against the transcription factor brachyury. Clin Cancer Res 2017;23:6833–6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madan RA, Bilusic M, Heery C et al. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol 2012;39:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodge JW, Higgins J, Schlom J. Harnessing the unique local immunostimulatory properties of modified vaccinia Ankara (MVA) virus to generate superior tumor‐specific immune responses and antitumor activity in a diversified prime and boost vaccine regimen. Vaccine 2009;27:4475–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodge JW, Poole DJ, Aarts WM et al. Modified vaccinia virus Ankara recombinants are as potent as vaccinia recombinants in diversified prime and boost vaccine regimens to elicit therapeutic antitumor responses. Cancer Res 2003;63:7942–7949. [PubMed] [Google Scholar]
- 5. Robinson HL, Montefiori DC, Johnson RP et al. Neutralizing antibody‐independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med 1999;5:526–534. [DOI] [PubMed] [Google Scholar]
- 6. Smith CL, Mirza F, Pasquetto V et al. Immunodominance of poxviral‐specific CTL in a human trial of recombinant‐modified vaccinia Ankara. J Immunol 2005;175:8431–8437. [DOI] [PubMed] [Google Scholar]
- 7. Fernando RI, Litzinger M, Trono P et al. The T‐box transcription factor Brachyury promotes epithelial‐mesenchymal transition in human tumor cells. J Clin Invest 2010;120:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang B, Cohen JR, Fernando RI et al. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis 2013;4:e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton DH, Huang B, Fernando RI et al. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial‐mesenchymal transition. Cancer Res 2014;74:2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamilton DH, McCampbell KK, Palena C. Loss of the cyclin‐dependent kinase inhibitor 1 in the context of brachyury‐mediated phenotypic plasticity drives tumor resistance to immune attack. Front Oncol 2018;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vujovic S, Henderson S, Presneau N et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol 2006;209:157–165. [DOI] [PubMed] [Google Scholar]
- 12. Miettinen M, Wang Z, Lasota J et al. Nuclear brachyury expression is consistent in chordoma, common in germ cell tumors and small cell carcinomas and rare in other carcinomas and sarcomas. An immunohistochemical study of 5229 cases. Am J Surg Pathol 2015;39:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilton DH, Litzinger MT, Fernando RI et al. Cancer vaccines targeting the epithelial‐mesenchymal transition: Tissue distribution of brachyury and other drivers of the mesenchymal‐like phenotype of carcinomas. Semin Oncol 2012;39:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamilton DH, Fernando RI, Schlom J et al. Aberrant expression of the embryonic transcription factor brachyury in human tumors detected with a novel rabbit monoclonal antibody. Oncotarget 2015;6:4853–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haro A, Yano T, Kohno M et al. Expression of Brachyury gene is a significant prognostic factor for primary lung carcinoma. Ann Surg Oncol 2013;20(suppl 3):S509–S516. [DOI] [PubMed] [Google Scholar]
- 16. Kilic N, Feldhaus S, Kilic E et al. Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer 2011;47:1080–1085. [DOI] [PubMed] [Google Scholar]
- 17. Palena C, Roselli M, Litzinger MT et al. Overexpression of the EMT driver brachyury in breast carcinomas: Association with poor prognosis. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitamura Y, Sasaki H, Kimura T et al. Molecular and clinical risk factors for recurrence of skull base chordomas: Gain on chromosome 2p, expression of brachyury, and lack of irradiation negatively correlate with patient prognosis. J Neuropathol Exp Neurol 2013;72:816–823. [DOI] [PubMed] [Google Scholar]
- 19. Wimmers F, Aarntzen EH, Duiveman‐deBoer T et al. Long‐lasting multifunctional CD8(+) T cell responses in end‐stage melanoma patients can be induced by dendritic cell vaccination. Oncoimmunology 2016;5:e1067745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 21. Fenerty KE, Folio LR, Patronas NJ et al. Predicting clinical outcomes in chordoma patients receiving immunotherapy: A comparison between volumetric segmentation and RECIST. BMC Cancer 2016;16:672. [DOI] [PMC free article] [PubMed] [Google Scholar]


