Abstract
Background
Standard treatment for International Federation of Gynecology and Obstetrics (FIGO) 2018 stage 1B2 cervical cancer (i.e., tumor size between 2 and 4 cm) is a radical hysterectomy (RH) with pelvic lymph node dissection (PLND). We evaluated the oncological and fertility outcomes treatment in patients receiving a fertility‐sparing alternative consisting of neoadjuvant chemotherapy (NACT) followed by vaginal radical trachelectomy (VRT).
Methods
Patients with stage 1B2 cervical cancer who wished to preserve fertility were included from September 2009 to September 2018. NACT consisted of 6‐week cycles of cisplatin or carboplatin with paclitaxel. If tumor size decreased to 2 cm or smaller, NACT was followed by a robot‐assisted PLND and VRT.
Results
Eighteen patients were included. Median follow‐up time was 49.7 months (range 11.4–110.8). Median tumor size was 32 mm (range 22–40 mm). Complete remission after NACT occurred in seven women. Four women had a poor response on NACT. Three underwent RH with PLND; one received chemoradiation after PLND instead of VRT because of positive lymph nodes. The remaining 14 patients received VRT 3–4 weeks after NACT. Four recurrences occurred: three after NACT and VRT and one after NACT and RH. Median time to recurrence was 20.8 months (range 17.0–105.7). Three recurrences occurred in women with adenocarcinoma with lymph vascular space invasion (LVSI). In four women fertility could not be preserved. To date, four women had six pregnancies, including three live births born at term, two first trimester miscarriages, and one currently ongoing pregnancy.
Conclusion
NACT and VRT in women with stage 1B2 cervical cancer showed promising results. In 78% fertility was preserved. However, patients with poor response on NACT and with adenocarcinoma and/or LVSI were possibly at risk for recurrence. Long‐term results in relation to fertility and oncological outcome are needed to corroborate these findings.
Implications for Practice
Standard treatment for women with International Federation of Gynecology and Obstetrics (FIGO) 2018 stage 1B2 cervical cancer (tumor size 2–4 cm) is a radical hysterectomy and pelvic lymph node dissection (PLND). However, many of these women are young and wish to preserve fertility. Data on fertility‐sparing treatment options are sparse, but neoadjuvant chemotherapy followed by a vaginal radical trachelectomy and PLND could be an alternative. Since 2009 we performed an observational cohort study in which 18 women opted for this treatment in our center. In 14 women fertility could be preserved. In four patients the tumor recurred. In four women six pregnancies occurred. After careful selection this treatment could be a good fertility‐sparing treatment option.
Keywords: Cervical cancer, Vaginal trachelectomy, Neoadjuvant chemotherapy, Recurrence, Fertility preservation
Short abstract
Many women with early stage cervical cancer want to preserve fertility. This article assesses oncological, fertility, and obstetrical outcome in patients with FIGO 2018 stage 1B2 cervical cancer treated with neoadjuvant chemotherapy followed by a vaginal radical trachelectomy and pelvic lymph node dissection.
Introduction
Cervical cancer is the fourth most common malignancy in women worldwide with an estimated 570,000 new cases in 2018 representing 6.6% of all female malignancies 1. Many women diagnosed with cervical cancer are young. With a peak incidence of cervical cancer between ages 35 and 45 years 2, women are often nulliparous, as the mean age for women having their first child has increased from 24 to 30 years over the past decades 3. The number of patients diagnosed with early stage cervical cancer (i.e., stage 1A–1B) has increased in the past years, partially because of the use of screening programs. Consequently, many patients with early stage cervical cancer wish to preserve fertility.
Radical hysterectomy with pelvic lymph node dissection (PLND) is the recommended treatment for women with International Federation of Gynecology and Obstetrics (FIGO) stage 1B1 and 1B2 cervical cancer (FIGO staging 2018 4). Vaginal radical trachelectomy (VRT) with PLND is a safe alternative for women with stage 1B1 (<2 cm) cervical cancer who wish to preserve their fertility. This treatment has shown to have both good oncological and obstetrical outcomes 5, 6, 7. However, data on fertility‐sparing treatment options for women with larger tumors (between 2 and 4 cm; i.e., FIGO 2018 stage 1B2) are sparse 8. VRT does not seem to be a safe option for these women because of the high risk of recurrence (20% 9). Abdominal radical trachelectomy (ART) seems safe from an oncological perspective but has poorer fertility and obstetrical outcomes (live birth rate 42% 10).
Another option for this group of women could be the combination of neoadjuvant chemotherapy (NACT) followed by VRT and PLND. A recent systematic review comparing NACT with VRT and ART showed similar recurrence rates (10% and 6.9%, respectively) but a better live birth rate in the first treatment group (63% vs. 42% 10). Pregnancy rates were 70% for the NACT group and 21% for the ART group. It should, however, be noted that sample sizes of the studies included in this review were small.
Our center has over 10 years of experience with VRT with good results 5. In this observational cohort study, we assessed the oncological, fertility, and obstetrical outcomes in patients with FIGO 2018 stage 1B2 cervical cancer treated with neoadjuvant chemotherapy followed by a VRT and PLND.
Materials and Methods
This is an observational cohort study in which patients treated between September 2009 and September 2018 were included, with a follow‐up until September 2019.
Ethical approval was obtained to collect data from patients undergoing this treatment from the Radboudumc Committee for Ethics in Research in the region Arnhem and Nijmegen.
Setting
Gynecologic oncological care in The Netherlands is centralized in eight gynecologic oncological centers. Women diagnosed with cervical cancer are referred to one of these centers, usually in the same region, for further diagnostic workup and treatment. Vaginal radical trachelectomy is only performed in two of these centers.
Patients
Patients with FIGO 2018 stage 1B2 cervical cancer (i.e., tumor size 2–4 cm) who wished to preserve fertility were discussed in the multidisciplinary tumor board to assess eligibility for fertility‐sparing treatment. Patients gave informed consent, after thorough counseling, to receive NACT followed by VRT with bilateral pelvic lymph node dissection. Diagnosis was confirmed by either biopsy or large loop excision of transitional zone. All patients received a gynecological examination with or without anesthesia and magnetic resonance imaging (MRI) for staging and evaluation of pelvic lymph nodes. All patients were examined whether they were fit for receiving chemotherapy by checking criteria such as adequate renal function, no preexisting deafness, and no severe neuropathy.
Treatment: NACT with VRT and Pelvic Lymph Node Dissection
Patients received cisplatin 70 mg/m2 and paclitaxel 70 mg/m2 weekly for six cycles, with 1 week's rest between cycles 3 and 4. This chemotherapy schedule was based on the Cochrane analysis by Lissoni et al. 11. After three cycles of NACT, clinical response was evaluated by MRI and gynecological examination with or without anesthesia. Patients were then again discussed in the tumor board. If disease had progressed, patients were no longer eligible for VRT and would receive a radical hysterectomy with pelvic lymph node dissection. If there was stable disease or regression of disease, patients would continue with another 3‐week cycle. In case of stable disease or partial response with a tumor size still larger than 2 cm after 3 weeks of chemotherapy, the response on chemotherapy was again evaluated with an MRI and gynecological examination after the six cycles of chemotherapy were completed. The surgical procedure was performed 3 to 4 weeks after the last chemotherapy course.
The operational procedure started with robot‐assisted PLND. In case of a suspicious lymph node, frozen section analysis (FSA) was performed during surgery. The procedure continued with VRT if no nodes were suspicious or when FSA showed no lymph node metastases. The surgical technique of VRT has been described thoroughly before 5, 12. In short, a 2‐cm vaginal cuff was dissected. A limited parametrial resection up to or lateral from the ureter was performed, meaning that less “length” of the parametrium was dissected compared with abdominal trachelectomy or radical hysterectomy 13. At least 1 cm of the cervix was preserved. The removed vaginal specimen was always sent for FSA to check the surgical margins for tumor or dysplasia at 3 and 6 mm from the cutting surface (side of the uterus). If margins were positive, additional cervical tissue was removed if possible. Then, a permanent isthmic cerclage (Mersilene, Ethicon 6, v40 needle) was placed followed by the vagino‐isthmic anastomosis using modified Sturmdorf sutures. An intrauterine balloon catheter (Charrière 8) which remained in place for 2 weeks, was inserted to prevent cervical stenosis.
If the final pathological report showed residual tumor >2 cm, positive lymph nodes, positive parametrial margins, or positive vaginal margins, we would recommend patients for adjuvant treatment, that is, (chemo)radiation or additional surgery.
Follow‐Up
Patients received follow‐up either in our hospital or in the initial referring Dutch gynecologic oncological center. Follow‐up consisted of gynecological examination, including cervical cytology every 3 months for 2 years, followed by every 6 months for a total of at least 5 years. Standard imaging is not part of follow‐up protocol in The Netherlands. In case of pregnancy, patients were recommended to seek specialized antenatal obstetrical care and to have a cesarean section as their mode of delivery.
Data Collection
We collected the following data: histological type and grade, tumor size, lymph vascular space invasion (LVSI), date and localization of recurrence, number of chemotherapy cycles, ideal dose of paclitaxel and cisplatin, actual doses received and delays of these drugs, and fertility and obstetrical outcomes (e.g., need of fertility treatment, number of pregnancies, number of live births). Data were collected from our patient records or were collected by contacting the lead gynecologist from other gynecologic oncological centers where follow‐up was performed.
Data Analysis
Descriptive statistics were used. We used SPSS 23.0 software (IBM, Armonk, NY) for statistical analyses.
Results
Between September 2009 and September 2018, 19 patients diagnosed with stage 1B2 cervical cancer (FIGO 2018) were scheduled to receive fertility‐preserving treatment by neoadjuvant chemotherapy and VRT with PLND (intention to treat). One patient was excluded from this study because she was pregnant (gestational age 24 weeks) and received a different treatment regimen. Median time of follow‐up for the remaining 18 patients was 49.7 months (range 11.4–110.8 months).
Patient Characteristics
Median age at time of diagnosis was 29 years (range 23–36 years). Median body mass index was 23.1 (range 20.0–31.2). Squamous cell carcinoma was diagnosed in 12 patients (67%) and adenocarcinoma in 6 patients (33%). Median tumor size was 32 mm (range 22–40 mm), with a median invasion depth of 6 mm (range 5–15 mm). LVSI was found in tumors in 56% of patients (n = 10) of whom five also had a poorly differentiated tumor (grade 3). An overview of patient, tumor, and treatment characteristics of all patients is provided in Tables 1 and 2.
Table 1.
Patients with stage 1B2 cervical cancer who completed neoadjuvant chemotherapy followed by vaginal radical trachelectomy and pelvic lymph node dissection
| Patient | Age, yr | Histology | Grade | Invasion depth, mm | Tumor size, mm | Lymph nodes | LVSI | Cycles of NACT | Path response to NACT | Recurrence | Treatment recurrence | DFS, months | OS, months | Current status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | SCC | 3 | 5 | 37 | 0/19 | No | 6/6 | Partial | No | n/a | 109.8 | 109.8 | NED |
| 2 | 30 | SCC | 1 | 6 | 25 | 0/12 | Yes | 5/6 | Partial | Yes | Surgery + CRT | 105.7 | 110.8 | NED |
| 3 | 24 | SCC | 3 | 5 | 35 | 0/19 | Yes | 4/6 | Complete | No | n/a | 103.5 | 103.5 | NED |
| 4 | 30 | SCC | 2 | 5 | 20 | 0/14 | No | 2/6 | Partial | No | n/a | 100.3 | 100.3 | NED |
| 5 | 29 | SCC | 3 | 5 | 35 | 0/23 | No | 6/6 | Complete | No | n/a | 51.4 | 51.4 | NED |
| 6 | 25 | SCC | 2 | 8 | 30 | 0/20 | Yes | 6/6 | Complete | No | n/a | 52.0 | 52.0 | NED |
| 7 | 36 | AC | 3 | 6 | 38 | 0/19 | Yes | 6/6 | Partial | Yes | Surgery + CT + HPV16 vaccine | 23.6 | 67.5 | Palliative care |
| 8 | 30 | ASC | 2 | 6 | 35 | 0/31 | Yes | 6/6 | Partial | yes | CRT | 18.1 | 32.7 | NED |
| 9 | 29 | AC | 2 | 15 | 28 | 0/43 | No | 6/6 | Partial | No | n/a | 48.1 | 48.1 | NED |
| 10 | 31 | SCC | 3 | 5 | 20 | 0/16 | Yes | 6/6 | Complete | No | n/a | 12.4 | 12.4 | NED |
| 11 | 23 | SCC | 3 | 4 | 20 | 0/18 | Yes | 6/6 | Complete | No | n/a | 37.1 | 37.1 | NED |
| 12 | 25 | SCC | 2 | 5 | 36 | 0/28 | No | 6/6 | Complete | No | n/a | 29.2 | 29.2 | NED |
| 13 | 31 | AC | 1 | 7 | 35 | 0/19 | No | 6/6 | Partial | No | n/a | 19.3 | 19.3 | NED |
| 14 | 30 | SCC | 3 | 6 | 40 | 0/11 | Yes | 6/6 | Partial | No | n/a | 11.4 | 11.4 | NED |
All patients had stage IB2 and received cisplatin/carboplatin and paclitaxel (70 mg/m2) as NACT.
Abbreviations: AC, adenocarcinoma; ASC, adenosquamous carcinoma; CRT, chemoradiation; CT, chemotherapy; DFS, disease‐free survival; HPV16, human papillomavirus 16; LVSI, lymph vascular space invasion; n/a, not applicable; NACT, neoadjuvant chemotherapy; NED, no evidence of disease; OS, overall survival; Path response to NACT, pathological response after surgery; SCC, squamous cell carcinoma.
Table 2.
Patients with stage 1B2 cervical cancer who failed to undergo VRT and pelvic lymph node dissection after neoadjuvant chemotherapy
| Patient | Age, yr | Histology | Grade | Invasion depth, mm | Tumor size, mm | Lymph nodes | LVSI | Cycles of NACT | Path response to NACT | Treatment instead of VRT | Reason | Recurrence | Treatment recurrence | DFS, months | OS, months | Current status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 25 | SCC | 2 | 11 | 30 | 3/23 | Yes | 3/6 | Partial | Chemo‐radiation | Mets lymph nodes | No | n/a | 87.9 | 87.9 | NED |
| 2 | 27 | AC | 2 | 9 | 25 | 0/15 | Yes | 4/6 | Partial | Rad hys | Poor response to CT | Yes | Debulking + RT | 17.0 | 85.9 | NED |
| 3b | 30 | AC | 2 | 4 | 25 | 0/20 | No | 4/6 | Complete | Rad hys | Poor response to CT | No | n/a | 13.1 | 13.1 | NED |
| 4 | 31 | SCC | 3 | 10 | 35 | 0/21 | No | 6/6 | Partial | Rad hys | Poor response to CT | No | n/a | 14.7 | 14.7 | NED |
All patients had stage IB2 and received cisplatin/carboplatin and paclitaxel (70 mg/m2) as NACT.
Patient received chemoradiation because of macrometastases in right obturator pelvic lymph nodes.
Response evaluated as poor response after examination under anesthesia; magnetic resonance imaging showed no residual disease. On final histology no residual disease after neoadjuvant chemotherapy. Abbreviations: AC, adenocarcinoma; CT, chemotherapy; DFS, disease‐free survival; LVSI, lymph vascular space invasion; Mets, metastases; n/a, not applicable; NACT, neoadjuvant chemotherapy; NED, no evidence of disease; OS, overall survival; Path response to NACT, pathological response after surgery; Rad hys, radical hysterectomy; RT, radiotherapy; SCC, squamous cell carcinoma; VRT, vaginal radical trachelectomy.
Neoadjuvant Chemotherapy
All 18 patients received cisplatin and paclitaxel as NACT. During chemotherapy, three patients switched from cisplatin to carboplatin (area under the curve 4) after four, two, and three cycles, respectively, because of tinnitus symptoms and renal impairment. After three cycles, clinical response on chemotherapy was evaluated by MRI and gynecological examination with or without anesthesia. Eventually, 12 patients (67%) completed all six cycles of chemotherapy. Three patients (patients 2, 3, and 4; Table 1) stopped early because of renal impairment (also after dose reduction) after five, four, and two cycles, respectively. One patient completed only four cycles because of severe bone marrow toxicity (patient 3, Table 2). Another patient (patient 2, Table 2) showed no response to chemotherapy, and it was decided to perform a radical hysterectomy with PLND instead. Finally, one patient (patient 1, Table 2) stopped early after three cycles because of psychological problems.
Surgery
Seven patients had a pathologically proven complete response to chemotherapy, of whom two only completed four cycles. Fourteen patients underwent a VRT and PLND. One patient had a suspicious lymph node during surgery. FSA showed metastatic disease. The VRT was cancelled, and chemoradiation was given instead. During evaluation of clinical response on NACT by gynecological examination and MRI, it was found that two patients had a poor response on chemotherapy and had a remaining tumor size larger than 2 cm after chemotherapy (Table 2, patients 2 and 4). They both underwent a radical hysterectomy with PLND (Fig. 1). One patient (Table 2, patient 3) showed good response on MRI, but on EUA it was suspected that tumor size was still >2 cm. Therefore, this patient also underwent a radical hysterectomy instead of VRT. After pathological examination it appeared, however, that the response to chemotherapy was complete.
Figure 1.
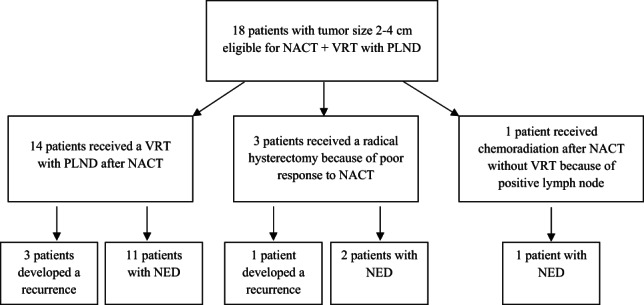
Flow chart. Abbreviations: NACT, neoadjuvant chemotherapy; NED, no evidence of disease; PLND, pelvic lymph node dissection; VRT, vaginal radical trachelectomy.
Of the patients who underwent surgery (n = 17), seven (41%) had a complete pathological response, and ten (59%) had residual disease, with depth of invasion ranging from 3 to 9 mm and linear extension ranging from 5 to 21 mm (partial response). All surgical margins were ≥ 6 mm free of tumor or dysplasia. Median number of pelvic lymph nodes removed was 19 (range 11–43).
No major complications occurred during the surgical procedures.
Oncological Outcome
Table 1 shows data for all 14 patients who underwent the intended treatment. Table 2 shows the data for those four patients who received a different treatment. Median follow‐up for the total group was 49.7 months (range 11.4–110.8 months). During follow‐up two patients developed cervical stenosis, and both required dilatation. Four patients developed a recurrence: three after VRT (Table 1; recurrence rate 21%) and one after radical hysterectomy (Table 2). For the VRT group, median time to recurrence was 23.6 months (range 18.1–105.7). One recurrence occurred in a patient with squamous cell carcinoma (LVSI positive), three recurrences occurred in patients with adenocarcinoma, and in all tumors of these patients LVSI was present. All patients with recurrent disease had residual disease after NACT. From the VRT group (Table 1) one patient (patient 8) developed recurrent disease locoregionally and was treated with chemoradiation that resulted in complete remission (overall survival [OS], 32.7 months). Another patient (patient 7) was treated by a radical hysterectomy for a recurrence. Preoperatively she appeared to have both lymphogenic and hematogenic (liver and diaphragm) recurrence of disease. She was therefore additionally treated with chemotherapy and a human papillomavirus 16 cancer vaccine (ISA101/ISA101b) 14. She never achieved complete remission. Currently, she has stable disease with palliative therapy (OS, 67.5 months). The third patient (patient 2) had a locoregional recurrence after 105.7 months (metastasis in iliac external lymph node) and has recently been treated by surgery followed by chemoradiation. The fourth recurrence (patient 2, Table 2) occurred in one of the patients who underwent a radical hysterectomy with PLND after NACT because of poor response on chemotherapy. She relapsed locoregionally and was treated with a debulking procedure and adjuvant radiotherapy. Up until now she shows no evidence of disease (OS, 85.9 months).
Fertility and Obstetrical Outcomes
Table 3 describes fertility and obstetrical outcomes. Fourteen out of 18 patients (78%) underwent the intended treatment, and fertility was thus preserved. The remaining four had either a radical hysterectomy or chemoradiation as described above. In addition, three women were diagnosed with premature ovarian insufficiency (POI), most likely as a consequence of the chemotherapy.
Table 3.
Pregnancy and obstetrical outcomes after NACT and VRT
| Outcome | Measure |
|---|---|
| NACT + VRT, n | 14/18 |
| Unknown fertility data, n | 1 |
| Fertility preservation, n (%) | 12/14 (86) |
| Desire to conceive, n (%) | 7/14 (50) |
| Referral to fertility unit, n (%) | 5/14 (36) |
| Pregnancies, n | 6 |
| Conception (n = 4), n | |
| Spontaneous | 2 |
| IUI | 3 |
| IVF / IVF‐ICSI | 0 |
| Donor oocytes/IVF | 1 |
| Outcome pregnancies (n = 4), n (%) | |
| Current ongoing pregnancy | 1 (16.7) |
| First trimester abortion | 2 (33.3) |
| >37 weeks’ gestation | 3 (50) |
Abbreviations: IUI, intrauterine insemination; IVF, in vitro fertilization; IVF‐ICSI, in vitro fertilization–intra cytoplasmatic sperm injection; NACT, neoadjuvant chemotherapy; VRT, vaginal radical trachelectomy.
All women who were treated with NACT followed by VRT and PLND were nulliparous. One of them had a previous tubectomy because of an ectopic pregnancy. Another woman had a previous termination of pregnancy in the first trimester. Five patients underwent in vitro fertilization (IVF) treatment immediately before NACT was started. Two of them succeeded in obtaining oocytes for vitrification. In one patient nine oocytes were obtained, of which six were frozen, and in the other woman five oocytes were obtained, of which four were frozen. In the third patient IVF was unsuccessful. Unfortunately, she lost fertility potential after treatment because she underwent a radical hysterectomy after NACT because of poor response on chemotherapy. A fourth patient was referred to the fertility clinic after a recurrence was found. The response to ovarian hyperstimulation was very poor, and as a result the procedure was waived. The fifth patient did not show up at the referral appointment. To date, none of these patients has tried to conceive. Of the remaining patients who did not receive any fertility treatment prior to chemotherapy, only four tried to conceive after treatment (NACT and VRT).
To date, four women had six pregnancies, including three live births born at term, two first trimester miscarriages, and one currently ongoing pregnancy. One spontaneous pregnancy is currently ongoing in one of the three patients who was diagnosed with POI. One other pregnancy in another patient was also spontaneous; the other four were achieved through either intrauterine insemination or IVF treatment.
Discussion
Our study showed that neoadjuvant chemotherapy followed by vaginal radical trachelectomy and PLND could be a safe and fertility‐sparing option in a selected group of women with stage 1B2 cervical cancer. In 14 (78%) of the women who were eligible fertility preservation was achieved.
Comparison with Other Literature
ART is an alternative therapy, which is currently most frequently performed worldwide. ART is meant to be more radical in terms of parametrial and paracervical resection 13, however, in most cases at the expense of the uterine artery. The main advantages are that it requires no special instrumentation, no skills in vaginal surgery, and minimal additional training 10, 11, 13. Nevertheless, literature shows that women who undergo ART have a higher risk of losing fertility potential and have a poorer obstetrical outcome because of the more radical procedure. Furthermore, a significant proportion of patients receiving ART will require adjuvant treatment because of unfavorable factors such as larger tumor size and deep stromal invasion. Plante et al. described that 26% received adjuvant chemotherapy, radiotherapy or both after ART 15, 16, 17, 18, 19. VRT is less radical by preserving the uterine artery but leads to lesser parametrial resection. We showed previously that fertility and obstetrical results are good with fertility preserved in 85%–100% 20, 21, 22, 23.
Literature is scarce on neoadjuvant chemotherapy followed by VRT and PLND. So far, eight studies have been published, including three overlapping studies, describing oncological and obstetrical outcomes of, in total, 35 patients (Table 3) 6, 21, 22, 23, 24, 25, 26, 27, 28. Literature review shows that the rate of fertility preservation after ART varies, ranging from 31% to 100%, with a median of 79% 20, 21, 22. NACT followed by VRT showed similar recurrence rates (VRT recurrence rate 10% vs. ART 6.9%) with better fertility and obstetrical outcome (VRT: pregnancy rate 70% and 63% live births vs. ART: pregnancy rate 21% and 42% live births) 10. Mean fertility preservation rate was 94%, and 11 out of 35 patients attempted to conceive, resulting in 12 pregnancies in 10 women (overall pregnancy rate of 29%; Table 4). Patients with cervical cancer stage 1B2 have similar oncological outcomes when treated with NACT with VRT as compared with ART. However, pregnancy rates are better after NACT and VRT as compared to ART.
Table 4.
Literature overview: Oncological and obstetrical outcomes after NACT and VRT
| Study | Planned NACT+VRT | Underwent NACT+VRT | Adjuvant treatment | Median/mean FU (months) | Fertility preservation n (%) | Recurrences n (%) | Deaths | Attempted pregnancies | Pregnancies | Live births |
|---|---|---|---|---|---|---|---|---|---|---|
| Maneo 2008a 28 | 21 | 16 | 2 (RT) | 69 | 100% | 0 | 0 | 9/16 |
10 in 6 patients |
9 |
| Plante 2011 6, 22 | 4 | 4 | 0 | 95 | 4 (100) | 0 | 0 | 3/4 |
4 |
1 preterm 2 term |
| Singh 2011 23 | 1 | 1 | 1 (CT) | 14 | 1 (100) | 0 | 0 | — | — | — |
| Marchiolè 2018 20 | 19 | 19 |
1 (RT) 2 women refused adjuvant treatment |
63 | 17 (89) | 2/19 (10) | 0 | 6/19 | 8 | 3 term |
| Wang 2013 24 | 2 | 2 | 0 | 84 | 2 (100) | 0 | 0 | 0 | n/a | n/a |
| Lanowska 2014 21 | 20 | 18 | 1 (RT) | 23 | 17 (85) | 1 (5.5) | 0 | 7/7 |
6 1 ongoing |
2 preterm 2 term |
| Robova 2014 9 | 28 | 20 | 0 | 42 | 20 (71) | 4/20 (20) | 2/20 (10) | 12/20 |
10 |
In 8 women: 6 term 4 preterm |
| Hauerberg 2015 25 | 1 | 1 | 0 | 68 | 1 (100) | 0 | 0 | — | — | — |
| Our report 2019 | 18 | 14 | 0 | 49.7 | 14 (18) | 3/14 (21) | 0 | 6/4 | 6 | 3 live births |
Abbreviations: —, unknown; CT, chemotherapy; FU, follow‐up; n/a, not applicable; NACT, neoadjuvant chemotherapy; RT, radiotherapy; VRT, vaginal radical trachelectomy.
Cold knife conization, max 3 cm diameter of tumor before treatment.
We found a fertility preservation rate of 78% in patients who underwent VRT and PLND after NACT, which is comparable to rates in the review by Plante et al. 22 and van de Kol et al. 10. In our study's follow‐up period four women (33%) tried to conceive, resulting in six pregnancies in four women.
Because three women developed POI after NACT, we started referring women to the fertility unit to discuss fertility‐preserving options, such as oocyte vitrification, prior treatment.
The most commonly used NACT is a triplet drug regimen containing paclitaxel (T), ifosfamide (I) or epirubicin (E), and cisplatin (P) (TIP/TEP), and patients receive an average of three 3‐week cycles. A triplet drug regimen results in good responses; it reduces tumor volume, thus making fertility‐preserving surgery possible. On the other hand, these regimens are more toxic, and it is not possible to use them in dose‐dense schemes 11. Little is known about the effects of ifosfamide on fertility. Plante et al., Marchiolè et al., and Lanowska et al. all used the TIP/TEP regimen every 3 weeks, and they gave three cycles of chemotherapy. They found an optimal or complete response rate of 57%–100%, and fertility was preserved in 85%–100% of the patients 20, 21, 22. Our patients received a dose‐dense two‐drug chemotherapeutic regimen consisting of cisplatin and paclitaxel. Although we gave lower doses that are less toxic, patients received cycles more often, and similar complete response rates and fertility outcome were found. According to a Cochrane analysis, a dose‐dense weekly schedule seems more effective in neoadjuvant chemotherapy for cervical cancer than 3‐week schedules 11, 29, 30. In addition, the 6‐week cycles have been proven effective in ovarian cancer as well and resulted in at least equivalent doses of cisplatin and paclitaxel as the three 3‐week schedules 31. Still, some patients had to switch to carboplatin because of ototoxicity, which is a known side effect of cisplatin.
One could argue that one needs to perform a lymphadenectomy first to exclude metastastic lymph nodes (LN) before administering NACT. Positive lymph nodes in FIGO 2018 stage 1B2 cervical cancer are found in 10%–15%. Lanowska et al. 21 performed upfront laparoscopic lymphadenectomy in all 20 patients; none had positive lymph nodes. However, another article from the same group showed that 67% (12/18) patients were diagnosed with metastasis in one or more pelvic and/or paraaortic lymph nodes. This high number of node metastases was unexpected and could (partially) be explained by a high incidence (42%) of stage 1B3 tumors (>4 cm) and adeno(squamous) carcinoma (42%), combined with a high incidence of LVSI and grade 3 tumors. These are all known risk factors for lymph node involvement 27. It is also known that lymph node metastases also respond to NACT 32. This means that it is possible that NACT can convert LN‐positive patients into LN‐negative patients, which may lead to a higher number of patients eligible for fertility‐preserving treatment. By performing a PLND before NACT, some patients may wrongly be excluded from fertility‐preserving surgery. However, treatment should be individualized. For some patients who opt for fertility‐preserving treatment and have several risk factors for lymph node metastases, lymphadenectomy before NACT should be considered, such as the presence of adenocarcinoma grade 3, positive lymph vascular space invasion, or some suspicion of the pelvic nodes on MRI and positron emission tomography–computed tomography.
In our hospital three (21%) patients were diagnosed with a recurrence after NACT and VRT. Median disease‐free survival was 23.6 months. Previous studies report recurrence rates of 0 to 20% (Table 4), but all have small numbers. Plante et al. published an overview of patients with cervical cancer size 2–4 cm who received NACT followed by a variety of fertility‐preserving surgical procedures. A total of 77 patients were described, of whom 56 underwent a vaginal trachelectomy and 21 underwent a simple conization after NACT. Five patients (7%) had recurrent disease, three of them locally in the residual cervix and one regionally in the pelvis after a simple vaginal trachelectomy (SVT). An SVT is a less radical procedure that consists of vaginal resection of the cervix, with resection of the upper 1–2 cm of the vaginal cuff and the medial portion of the cardinal and uterosacral ligaments. The cervix is cut at the lower uterine segment, and a prophylactic cerclage is placed during surgery. The fifth patient had a distant recurrence (ovary) after VRT 21, 22, 23. Recurrence rates were more often found in LVSI‐positive patients, adenocarcinomas (46%), and grade 2 and 3 tumors (82%). In our study all patients with recurrent disease had a partial response to chemotherapy with >10 mm residual disease. Three had adenocarcinoma with LVSI present. This is in agreement with the results of our cohort of patients who received only VRT in (FIGO 2018) stage 1B1 cervical cancer. This study showed that both adeno(squamous) carcinoma and LVSI are important prognostic factors 33 and that patient selection for this treatment is therefore important.
There is an ongoing debate on whether fertility‐preserving surgery could be less radical, presumably leading to better fertility outcome. So far, no prospective data are available yet, but data from three randomized trails concerning this issue are expected 34, 35, 36. However, none of these studies focuses on primary surgery without NACT. A new trail, CONTESSA‐NEOCON, recently opened, focusing on the feasibility of preserving fertility in women with LN‐negative, FIGO 2018 stage IB2 cervical cancer with lesions measuring >2 cm to <4 cm by administering NACT followed by fertility‐sparing surgery using either cone biopsy or simple trachelectomy with pelvic lymph node dissection and no adjuvant therapy. The study will lead to interesting data. However, patients are not randomized between radical vaginal trachelectomy and cone biopsy/simple trachelectomy 37.
Strengths and Limitations
This study has some strengths. First of all, compared with existing literature this study has a fair sample of 18 patients receiving this experimental treatment. Second, we were able to follow up on these patients and collect both oncological and obstetrical outcomes.
A limitation is that it is an observational cohort study, with risk of selection bias. Also, more extensive statistics, such as analyzing variables that would influence recurrence rate, was not possible because of the sample size.
Conclusion
Treatment of patients with stage 1B2 cervical cancer (FIGO 2018) with NACT and VRT showed promising results with 78% of patients in whom fertility could be preserved. However, preoperative selection of patients for fertility‐sparing surgery, in particular LVSI status, histology type, and response to NACT, is very important. Patients with adenocarcinoma and presence of LVSI seem to have a higher risk of recurrence and should be counseled as such. Furthermore, we recommend referring every patient who is eligible for NACT and VRT to a fertility unit because of the risk of POI. Prospective trials are needed in order to investigate various fertility‐sparing strategies and to determine which patients with cervical cancer are suitable for these different treatments.
Author Contributions
Conception/design: Petra L.M. Zusterzeel, Johanna W.M. Aarts, Fraukje J.M. Pol, Petronella B. Ottevanger, Maaike A.P.C. van Ham
Provision of study material or patients: Petra L.M. Zusterzeel, Johanna W.M. Aarts, Fraukje J.M. Pol, Petronella B. Ottevanger, Maaike A.P.C. van Ham
Collection and/or assembly of data: Petra L.M. Zusterzeel, Johanna W.M. Aarts, Fraukje J.M. Pol, Petronella B. Ottevanger, Maaike A.P.C. van Ham
Data analysis and interpretation: Petra L.M. Zusterzeel, Johanna W.M. Aarts, Fraukje J.M. Pol, Petronella B. Ottevanger, Maaike A.P.C. van Ham
Manuscript writing: Petra L.M. Zusterzeel, Johanna W.M. Aarts, Fraukje J.M. Pol, Petronella B. Ottevanger, Maaike A.P.C. van Ham
Final approval of manuscript: Petra L.M. Zusterzeel, Johanna W.M. Aarts, Fraukje J.M. Pol, Petronella B. Ottevanger, Maaike A.P.C. van Ham
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Cervical cancer . World Health Organization Web site. Available from https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed September 20, 2019.
- 2. Vaccarella S, Lortet‐Tieulent J, Plummer M et al. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur J Cancer 2013;49:3262–3273. [DOI] [PubMed] [Google Scholar]
- 3. Fertility statistics . Eurostat Web site. Available from http://ec.europa.eu/eurostat/statistics‐explained/index.php/Fertility_statistics. Accessed April 1, 2017.
- 4. Bhatla N, Berek J, Cuello Fredes M et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019;145:129–135. [DOI] [PubMed] [Google Scholar]
- 5. Zusterzeel PL, Pol FJ, van Ham M et al. Vaginal radical trachelectomy for early‐stage cervical cancer: Increased recurrence risk for adenocarcinoma. Int J Gynecol Cancer 2016;26:1293–1299. [DOI] [PubMed] [Google Scholar]
- 6. Plante M, Gregoire J, Renaud MC et al. The vaginal radical trachelectomy: An update of a series of 125 cases and 106 pregnancies. Gynecol Oncol 2011;121:290–297. [DOI] [PubMed] [Google Scholar]
- 7. Plante M. Evolution in fertility‐preserving options for early‐stage cervical cancer: Radical trachelectomy, simple trachelectomy, neoadjuvant chemotherapy. Int J Gynecol Cancer 2013;23:982–989. [DOI] [PubMed] [Google Scholar]
- 8. Bentivegna E, Gouy S, Maulard A et al. Oncological outcomes after fertility‐sparing surgery for cervical cancer: A systematic review. Lancet Oncol 2016;17:e240–e253. [DOI] [PubMed] [Google Scholar]
- 9. Robova H, Halaska MJ, Pluta M et al. Oncological and pregnancy outcomes after high‐dose density neoadjuvant chemotherapy and fertility‐sparing surgery in cervical cancer. Gynecol Oncol 2014;135:213–216. [DOI] [PubMed] [Google Scholar]
- 10. van de Kol KGC, Vergeldt TFM, Bekkers RLM. Abdominal radical trachelectomy versus chemotherapy followed by vaginal radical trachelectomy in stage 1B2 (FIGO 2018) cervical cancer. A systematic review on fertility and recurrence rates. Gynecol Oncol 2019;155:515–521. [DOI] [PubMed] [Google Scholar]
- 11. Lissoni AA, Colombo N, Pellegrino A et al. A phase II, randomized trial of neo‐adjuvant chemotherapy comparing a three‐drug combination of paclitaxel, ifosfamide, and cisplatin (TIP) versus paclitaxel and cisplatin (TP) followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: The Snap‐02 Italian Collaborative Study. Ann Oncol 2009;20:660–665. [DOI] [PubMed] [Google Scholar]
- 12. Dargent D, Brun JL, Roy M, et al. Pregnancies following radical trachelectomy for invasive cervical cancer. Gynecol Oncol 1994;13:683–690. [Google Scholar]
- 13. Einstein MH, Park KJ, Sonoda Y et al. Radical vaginal versus abdominal trachelectomy for stage IB1 cervical cancer: A comparison of surgical and pathologic outcomes. Gynecol Oncol 2009;112:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Study of the therapeutic vaccine (ISA101) to treat advanced or recurrent cervical cancer (CervISA) . ClinicalTrials.gov identifier NCT02128126. Available from https://clinicaltrials.gov/ct2/show/NCT02128126. Accessed December 2, 2019.
- 15. Pareja R, Rendon GJ, Vasquez M et al. Immediate radical trachelectomy versus neoadjuvant chemotherapy followed by conservative surgery for patients with stage IB1 cervical cancer with tumors 2cm or larger: A literature review and analysis of oncological and obstetrical outcomes. Gynecol Oncol 2015;137:574–580. [DOI] [PubMed] [Google Scholar]
- 16. Wethington SL, Sonoda Y, Park KJ et al. Expanding the indications for radical trachelectomy: A report on 29 patients with stage IB1 tumors measuring 2 to 4 centimeters. Int J Gynecol Cancer 2013;23:1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lintner B, Saso S, Tarnai L et al. Use of abdominal radical trachelectomy to treat cervical cancer greater than 2 cm in diameter. Int J Gynecol Cancer 2013;23:1065–1070. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Wu X, Li X et al. Abdominal radical trachelectomy: Is it safe for IB1 cervical cancer with tumors ≥2 cm? Gynecol Oncol 2013;131:87–92. [DOI] [PubMed] [Google Scholar]
- 19. Plante M. Bulky early‐stage cervical cancer (2‐4 cm lesions): Upfront radical trachelectomy or neoadjuvant chemotherapy followed by fertility‐preserving surgery: Which is the best option? Int J Gynecol Cancer 2015;25:722–728. [DOI] [PubMed] [Google Scholar]
- 20. Marchiolè P, Ferraioli D, Moran E et al. NACT and laparoscopic‐assisted radical vaginal trachelectomy in young patients with large (2‐5 cm) high risk cervical cancers: Safety and obstetrical outcome. Surg Oncol 2018;27:236–244. [DOI] [PubMed] [Google Scholar]
- 21. Lanowska M, Mangler M, Speiser D et al. Radical vaginal trachelectomy after laparoscopic staging and neoadjuvant chemotherapy in women with early‐stage cervical cancer over 2 cm: Oncologic, fertility, and neonatal outcome in a series of 20 patients. Int J Gynecol Cancer 2014;24:586–593. [DOI] [PubMed] [Google Scholar]
- 22. Plante M, Lau S, Brydon L et al. Neoadjuvant chemotherapy followed by vaginal radical trachelectomy in bulky stage IB1 cervical cancer: Case report. Gynecol Oncol 2006;101:367–370. [DOI] [PubMed] [Google Scholar]
- 23. Singh P, Nicklin J, Hassall T. Neoadjuvant chemotherapy followed by radical vaginal trachelectomy and adjuvant chemotherapy for clear cell cancer of the cervix: A feasible approach and review. Int J Gynecol Cancer 2011;21:137–140. [DOI] [PubMed] [Google Scholar]
- 24. Wang D, Yang J, Shen K et al. Neoadjuvant chemotherapy followed by fertility‐sparing surgery for women with stage IB1 cervical cancer. J Gynecol Oncol 2013;24:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hauerberg L, Hogdall C, Loft A et al. Vaginal radical trachelectomy for early stage cervical cancer. Results of the Danish National Single Center Strategy. Gynecol Oncol 2015;138:304–310. [DOI] [PubMed] [Google Scholar]
- 26. Gottschalk E, Mangler M, Schneider A et al. Pregnancy after lymphadenectomy and neoadjuvant chemotherapy followed by radical vaginal trachelectomy in FIGO stage IB1 cervical cancer. Fertil Steril 2011;95:2431.e5–e7. [DOI] [PubMed] [Google Scholar]
- 27. Vercellino GF, Piek JM, Schneider A et al. Laparoscopic lymph node dissection should be performed before fertility preserving treatment of patients with cervical cancer. Gynecol Oncol 2012;126:325–329. [DOI] [PubMed] [Google Scholar]
- 28. Maneo A, Chiari S, Bonazzi C et al. Neoadjuvant chemotherapy and conservative surgery for stage IB1 cervical cancer. Gynecol Oncol 2008;111:438–443. [DOI] [PubMed] [Google Scholar]
- 29. Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta‐Analysis Collaboration . Neoadjuvant chemotherapy for locally advanced cervical cancer: A systematic review and meta‐analysis of individual patient data from 21 randomised trials. Eur J Cancer 2003;39:2470–2486. [DOI] [PubMed] [Google Scholar]
- 30. Neoadjuvant Chemotherapy for Cervical Cancer Meta‐Analysis Collaboration (NACCCMA) Collaboration . Neoadjuvant chemotherapy for locally advanced cervix cancer. Cochrane Database Syst Rev 2004;(2):CD001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Burg ME, Onstenk W, Boere IA et al. Long‐term results of a randomized phase III trial of weekly versus three‐weekly paclitaxel/platinum induction therapy followed by standard or extended three‐weekly paclitaxel/platinum in European patients with advanced epithelial ovarian cancer. Eur J Cancer 2014;50:2592–2601. [DOI] [PubMed] [Google Scholar]
- 32. Bader AA, Winter R, Moinfar F et al. Is intraoperative frozen section analysis of pelvic lymph nodes accurate after neoadjuvant chemotherapy in patients with cervical cancer? Gynecol Oncol 2006;103:106–112. [DOI] [PubMed] [Google Scholar]
- 33. Pol FJ, Zusterzeel PL, van Ham MA et al. Satellite lymphovascular space invasion: An independent risk factor in early stage cervical cancer. Gynecol Oncol 2015;138:579–584. [DOI] [PubMed] [Google Scholar]
- 34. Schmeler KM, Frumovitz M, Ramirez PT. ConServ. Conservative management of early stage cervical cancer: Is there a role for less radical surgery? Gynecol Oncol 2011;120:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Plante M. The SHAPE trial; a randomized phase III trial comparing radical hysterectomy and pelvic node dissection vs simple hysterectomy and pelvic node dissection in patients with low‐risk early stage cervical cancer. Available at https://clinicaltrials.gov/ct2/show/NCT01658930. Accessed March 3, 2020. [Google Scholar]
- 36. Covens A. GOG Protocol 278. Available at https://gcigtrials.org/system/files/GOG-F%20Monk.pdf. Accessed March 3, 2020. [Google Scholar]
- 37. Plante M, van Trommel N, Lheureux S et al. FIGO 2018 stage IB2 (2‐4 cm) cervical cancer treated with neo‐adjuvant chemotherapy followed by fertility sparing surgery (CONTESSA); neo‐adjuvant chemotherapy and conservative surgery in cervical cancer to preserve fertility (NEOCON‐F). A PMHC, DGOG, GCIG/CCRN and multicenter study. Int J Gynecol Cancer 2019;29:969–975. [DOI] [PubMed] [Google Scholar]


