Abstract
Background
Peptide receptor radionuclide therapy (PRRT) is effective for treating midgut neuroendocrine tumors (NETs); however, incorporation of PRRT into routine practice in the U.S. is not well studied. Herein we analyze the first year of PRRT implementation to determine tolerance of PRRT and factors that increase risk of PRRT discontinuation.
Materials and Methods
Medical records were reviewed and data were abstracted on all patients with NETs scheduled for PRRT during the first year of PRRT implementation at a U.S. NET referral center (August 2018 through July 2019). Logistic regression was used to identify factors associated with PRRT discontinuation.
Results
Fifty‐five patients (56% male) were scheduled for PRRT over the study period. The most common primary NET location was small bowel (47%), followed by pancreas (26%), and 84% of the NETs were World Health Organization grade 1 or 2. The cohort was heavily pretreated with somatostatin analog (SSA) therapy (98%), non‐SSA systemic therapy (64%), primary tumor resection (73%), and liver‐directed therapy (55%). At the time of analysis, 52 patients completed at least one PRRT treatment. Toxicities including bone marrow suppression and liver function test (LFT) abnormalities were comparable to prior publications. Eleven patients (21%) prematurely discontinued PRRT because of toxicity or an adverse event. Pretreatment LFT abnormality was associated with increased risk of PRRT cancellation (odds ratio: 12; 95% confidence interval: 2.59–55.54; p < .001).
Conclusion
PRRT can be administered to a diverse NET population at a U.S. NET referral center. Baseline liver function test abnormality increases the likelihood of PRRT discontinuation.
Implications for Practice
Peptide receptor radionuclide therapy (PRRT) can be successfully implemented at a U.S. neuroendocrine tumor (NET) referral center in a NET population that is diverse in tumor location, grade, and prior treatment history. Toxicity and adverse effects of PRRT are comparable to prior reports; however, 21% of individuals prematurely discontinued PRRT. Patients with baseline liver function test abnormalities were more likely to discontinue PRRT than patients with normal liver function tests, which should be taken into consideration when selecting treatment options for NETs.
Keywords: Peptide receptor radionuclide therapy, Neuroendocrine tumor, DOTATATE, Lu‐177
Short abstract
Understanding the efficacy of different treatments for patients with neuroendocrine tumors is of growing importance. This article analyzes the first‐year implementation of peptide receptor radionuclide therapy into routine practice and the factors affecting discontinuation of therapy.
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of tumors that arise from neuroendocrine cells located throughout the body, most commonly in the gastrointestinal tract, pancreas, and lung 1. Although once thought to be rare, the incidence of NETs has increased fivefold in the last 3 decades potentially owing to increased sensitivity of diagnostic techniques 1, 2, 3, 4. Some NETs are functional through secretion of peptides and neuroamines that cause clinical syndromes such as carcinoid syndrome; however, many other NETs remain nonfunctional 2. It has been estimated that more than 120,000 individuals are living in the U.S. with metastatic NETs 1. Potential therapies for NETs include surgery, somatostatin analogs (SSAs), systemic chemotherapy, liver‐directed therapy, and peptide receptor radionuclide therapy (PRRT) 5. Understanding the efficacy of these treatments as well as the ideal sequencing of therapy is of growing importance to the multidisciplinary team that manages patients with NETs including oncologists, surgeons, gastroenterologists, and radiologists specializing in nuclear medicine or interventional radiology 6.
The majority of NETs express somatostatin receptors, which is the characteristic of NETs that is specifically targeted by PRRT. PRRT uses a radionuclide, often 177Lutetium (177Lu) or 90Yttrium (90Y), linked to a somatostatin analog, which allows for selective delivery of radiation to somatostatin receptor–expressing NETs 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18. Although the use of PRRT has been studied for years in Europe, the only randomized controlled trial looking at the efficacy of PRRT is the recent NETTER‐1 trial, conducted in the U.S. and Europe. In the NETTER‐1 trial, patients with metastatic well‐differentiated (World Health Organization [WHO] grade 1/2) midgut NETs who had progressed on prior somatostatin analog therapy were randomized to receive either 177Lu‐DOTATATE plus octreotide long‐acting repeatable (LAR) or high‐dose octreotide LAR alone. Treatment with 177Lu‐DOTATATE resulted in markedly longer progression‐free survival and a significantly higher tumor response rate than the octreotide LAR–alone group, supporting the effectiveness of PRRT for the treatment of well‐differentiated midgut NETs, with data on overall survival forthcoming 19. These results, along with much of the European data, subsequently led to a Food and Drug Administration (FDA) approval for the use of PRRT for gastroenteropancreatic NETs demonstrating somatostatin avidity on imaging, which encompasses a larger group than the patients with midgut NETs included in NETTER‐1. Although PRRT is a well‐tolerated treatment, there are risks associated with it, including myelosuppression, nephrotoxicity, and hepatotoxicity 18, 19, 20. In the NETTER‐1 trial, myelosuppression was significantly greater in patients receiving PRRT compared with octreotide alone. No significant renal toxicity was seen; however, some toxicity has been variably noted in other studies 18, 20.
Since its FDA approval in 2018, PRRT has been incorporated into many NET treatment programs across the U.S. However, the efficacy, tolerability, and toxicity of PRRT in U.S.‐based patients has only been studied in limited cohorts prior to its FDA approval 6, 20, 21. We previously published our data in U.S. patients treated in Europe, and in comparison with NETTER‐1 and European data, we noticed that our heavily pretreated population appeared to be at increased risk for complications including liver toxicity 20. With PRRT now being increasingly used in routine clinical practice for the treatment of NETs in the U.S., understanding how PRRT can be incorporated effectively into the practice of NET management is critical.
In this study, we examine the first year of PRRT implementation in a tertiary U.S. NET referral center. We describe the patient cohort selected for PRRT, highlight the toxicities and intolerances that resulted during PRRT treatment, and define factors that may help predict which patients are most likely to prematurely discontinue PRRT treatment.
Materials and Methods
We prospectively collected clinical data from a cohort of consecutive patients with NETs scheduled to receive PRRT through the University of Pennsylvania NET Program during the first year after PRRT implementation, spanning August 2018 through July 2019 (n = 55). The study was approved by the University of Pennsylvania Institutional Review Board. As per current guidelines, a standard course of PRRT involves infusion of four doses of 177Lu‐DOTATATE, targeted to 7.4 GBq (200 mCi) per dose, with 8‐week intervals in between doses. PRRT was given at least 28 days after the most recent administration of a long‐acting SSA. Subsequent administration of a long‐acting SSA was performed at least 6 hours after PRRT administration. All patients received at least a portion of their PRRT therapy at the University of Pennsylvania, with two patients receiving a portion of their PRRT at a different site. Per guidelines, infusion of an amino acid solution is recommended with PRRT to reduce nephrotoxicity. Although only lysine and arginine are required in the amino acid infusion, no such infusion is currently FDA approved. FDA‐approved amino acid solutions require that more than 2 liters of fluid be administered to achieve the required lysine and arginine amounts within osmolality constraints, and these solutions contain additional unneeded amino acids, which make them highly emetogenic. Although our center initially used this FDA‐approved amino acid solution, we subsequently changed to a pharmacy‐compounded 1‐liter infusion of lysine and arginine given over 4 hours with a much improved side‐effect profile.
The electronic medical records of all patients in the cohort were manually reviewed to extract study‐related data. The data collected included sex, date of birth, date of death (if applicable), date of NET diagnosis, primary tumor location, grade, and whether liver metastases were present. Information regarding PRRT included dates of treatment and doses administered. Additionally, information about the use of other therapies before PRRT was collected, including nonhepatic surgery, liver‐directed therapies including transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiofrequency ablation or microwave ablation (RFA/MWA), bland embolization, and hepatic resection, and systemic therapy, which was defined as having systemic treatment for a malignancy with any non‐SSA agent. Laboratory data including white blood cell (WBC) count, hemoglobin (Hgb), platelets (Plt), creatinine (Cr), total bilirubin (Bili), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were also retrieved from the medical records, both prior to treatment and in between subsequent PRRT sessions.
Toxicities were determined based on Common Terminology Criteria for Adverse Events version 5.0 criteria from the National Institutes of Health/National Cancer Institute and were defined as the development of a new grade 2 or higher toxicity during or after treatment within the study period 22. More specifically, for hematologic toxicities, leukopenia, anemia, and thrombocytopenia were defined as the new development of a WBC count less than 3,000/mm3, Hgb less than 10g/dL, and Plt count less than 75,000/mm3, respectively. Nephrotoxicity was defined as the new development of a Cr more than twofold higher than baseline. Biochemical liver injury was defined as the new development of a Bili more than 1.5× the upper limit of normal, AST more than 3× the upper limit of normal, or ALT more than 3× the upper limit of normal. A baseline composite liver function test (LFT) abnormality was defined by having a total bilirubin >1.2 mg/dL, AST >41 U/L, or ALT >54 U/L, which are the upper limits of normal for our institutional assays.
Adverse effects of PRRT were also obtained during review of medical records. Any intolerance or side effect documented during the infusion visit or on subsequent office visits after the initiation of PRRT was included as an adverse effect. Finally, the medical record was reviewed for patients that delayed subsequent PRRT sessions or terminated therapy early because of toxicity, adverse effect, or death.
Statistical Analysis
Statistical analysis was performed using Stata/IC 15.1 (College Station, TX) to do Kruskal‐Wallis and Pearson's chi‐squared testing. Primary outcome was binary, whether or not a patient discontinued PRRT, and logistic regression was performed, using forward selection and inclusion of all clinically significant odds ratios (ORs), where p < .10 and/or if variables confound another exposure by 10% in either direction. Significance was defined as p < .05.
Results
Cohort Characteristics
Fifty‐five patients were scheduled to receive PRRT during the study period. The mean age at NET diagnosis for the entire cohort was 54.8 ± 10.6 years and 56% of the patients were male (Table 1). Primary NET location was predominantly in the small bowel (47%), followed by pancreas (26%), colon (9%), unknown primary location (9%), and other (9%, with two gastric, two lung, and one retroperitoneal paraganglioma). WHO tumor grade was 35%, 49%, and 11% in grades 1, 2, and 3, respectively. The cohort was heavily pretreated, with 54 patients (98%) having received prior SSA therapy and 40 patients (73%) having undergone primary tumor resection. Thirty‐five patients (64%) received non‐SSA systemic therapy, including capecitabine/temozolomide (19 patients, 35%) and everolimus (19 patients, 35%). Liver metastases were present in 49 (89%) patients, and 33 (60%) had received prior liver‐directed therapy (TACE, TARE, bland embolization, RFA/MWA, and/or hepatic resection).
Table 1.
PRRT cohort characteristics
| Characteristics | n (%) |
|---|---|
| Individuals scheduled for PRRT, n | 55 |
| Age at NET diagnosis, mean (SD), years | 54.8 (10.6) |
| Sex | |
| Male | 30 (56) |
| Female | 25 (44) |
| NET primary location | |
| Small bowel | 26 (47) |
| Pancreatic | 14 (26) |
| Colon | 5 (9) |
| Other | 5 (9) |
| Unknown | 5 (9) |
| NET grade | |
| 1 | 19 (35) |
| 2 | 27 (49) |
| 3 | 6 (11) |
| Unknown | 3 (5) |
| Prior somatostatin use | 54 (98) |
| Prior systemic chemotherapy | 35 (64) |
| Cap/Tem | 19 (35) |
| Everolimus | 19 (35) |
| Prior surgical resection | 40 (73) |
| Liver metastases present | 49 (89) |
| Prior liver‐directed therapy | 33 (60) |
| TACE | 11 (20) |
| TARE | 8 (15) |
| Bland embolization | 9 (16) |
| RFA/MWA | 4 (7) |
| Hepatic resection | 15 (27) |
| Individuals completing ≥1 PRRT session | 52 (95) |
| Age at first PRRT dose, mean (SD), years | 60.4 (9.9) |
| Time from diagnosis to PRRT treatment, mean (SD), years | 6.4 (5.2) |
Abbreviations: Cap/Tem, capecitabine‐temozolomide; NET, neuroendocrine tumor; PRRT, peptide receptor radionuclide therapy; RFA/MWA, radiofrequency ablation or microwave ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Of the initial 55 patients scheduled for PRRT, 52 had received at least one dose of PRRT by the end of the study period, including 7 who received one dose, 14 who received two doses, 10 who received three doses, and 21 who received a full course of four doses (Fig. 1). The mean age at the first dose of PRRT was 60.4 ± 9.9 years, with an average time from NET diagnosis to PRRT of 6.4 ± 5.2 years. Of the patients who had not yet completed a full course of four doses, 18 remained in treatment with a plan for additional PRRT without delay. Eleven patients stopped PRRT, and two patients experienced treatment delays but ultimately continued PRRT.
Figure 1.
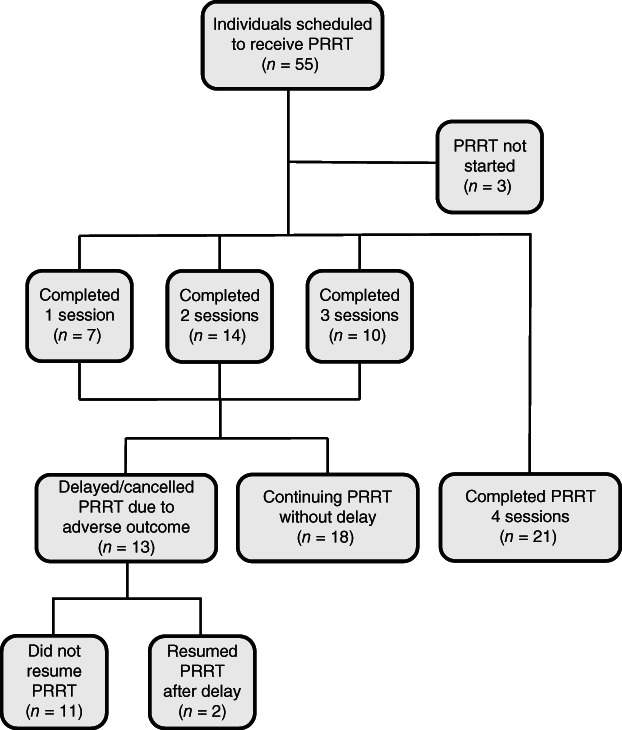
Individuals scheduled for PRRT over the first year after PRRT implementation at a U.S. neuroendocrine tumor referral center.Abbreviation: PRRT, peptide receptor radionuclide therapy
Toxicity
The most common toxicity noted in the cohort of 52 patients who received at least one dose of PRRT was bone marrow suppression, with 16 occurrences (31%) of new leukopenia, 9 occurrences (17%) of new anemia, and 6 occurrences (12%) of new thrombocytopenia (Table 2). New hyperbilirubinemia occurred in six patients (12%), and new elevations of serum transaminases occurred in four patients (8%). Kidney injury, as measured by creatinine change from baseline, occurred in only two patients (4%). There were no documented cases of myelodysplastic syndrome, severe leukopenia requiring granulocyte colony stimulating factor or antimicrobial prophylaxis, or need for initiation of hemodialysis. However, two patients received 50% dose reductions owing to myelosuppression.
Table 2.
Toxicities observed after peptide receptor radionuclide therapy (n = 52)
| Toxicity | Criteria (CTCAE grade 2 or higher) | Occurrences, n (%) |
|---|---|---|
| Leukopenia | WBC count < 3,000/μL | 16 (31) |
| Anemia | Hgb < 10 g/dL | 9 (17) |
| Thrombocytopenia | Platelets < 75,000/μL | 6 (12) |
| Acute kidney injury | Creatinine >2× baseline | 2 (4) |
| Transaminitis | AST or ALT > 3× ULN | 4 (8) |
| Hyperbilirubinemia | Bilirubin (total) > 1.5× ULN | 6 (12) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; Hgb, hemoglobin; ULN, upper limit of normal; WBC, white blood cell.
Intolerance and Adverse Effects
Over 149 administered doses of PRRT, the most common adverse effect noted during an infusion was nausea, which occurred in 21 cases (14%; supplemental online Table 1). The vast majority of these cases occurred early in the study period when a less concentrated amino acid solution was used. After changing to a specialty compounded arginine/lysine amino acid solution, the rates of nausea decreased considerably, and indeed resolved in most cases. Furthermore, a much simpler pretreatment antiemetic regimen was used for the compounded amino acid formulation. Other adverse effects during the PRRT infusion were rare (supplemental online Table 1). In the intervening periods after a PRRT dose was administered, fatigue was the most commonly reported adverse effect, observed after 43% of doses. Nausea and worsening carcinoid syndrome–related symptoms occurred after 19% and 13% of doses, respectively. Vomiting, abdominal pain, body pain, loss of appetite, mood disorders, dizziness, increased edema/ascites, and hair loss were reported, but with occurrence rates less than 10%.
Factors Associated with Discontinuation of Treatment
Thirteen patients delayed or discontinued PRRT because of toxicity, adverse outcome, or death, with two of these individuals eventually resuming treatment. The most common reasons for PRRT delay or discontinuation included myelosuppression, disease progression, and death (n = 3 for each; Table 3). Two of the patients who delayed treatment for myelosuppression were ultimately able to resume treatment. Comparisons of cohort characteristics were made between the subgroup that is continuing or completed PRRT and the subgroup that discontinued treatment (Table 4). Patients who discontinued PRRT were more likely to have a baseline elevation in total bilirubin, AST, or ALT or to have received prior systemic therapy. Additionally, patients who discontinued PRRT were more likely to have a baseline LFT abnormality, defined as a total bilirubin >1.2 mg/dL, AST >41 U/L, or ALT >54 U/L. Prior nonhepatic resection, prior liver‐directed therapy, and baseline WBC count, hemoglobin, platelets, and serum creatinine were not seen at significantly different rates between these two groups.
Table 3.
Reasons for peptide receptor radionuclide therapy delay/cancellation (n = 13)
| Cause of delay/cancellation | No. of patients |
|---|---|
| Myelosuppression | 3a |
| Disease progression | 3 |
| Death | 3 |
| Worsening liver function | 2 |
| Failure to thrive/fatigue | 2 |
Two patients resumed treatment.
Table 4.
Comparison between individuals who continued/completed peptide receptor radionuclide therapy (PRRT) and individuals who discontinued PRRT
| Variable | Continuing/completed (n = 41) | Discontinuation (n = 11) | p value |
|---|---|---|---|
| Age, median (IQR), years | 61 (54.0, 70.0) | 59.0 (50.0, 71.0) | .50 |
| Type of NET, n (%) | .26 | ||
| Pancreatic | 12 (27) | 2 (18) | |
| Small bowel | 21 (48) | 5 (45) | |
| Colonic | 5 (11) | 0 (0) | |
| Unknown primary | 6 (14) | 4 (36) | |
| Grade of NET, n (%) | .93 | ||
| 1 | 15 (36) | 4 (40) | |
| 2 | 22 (52) | 5 (50) | |
| 3 | 5 (12) | 1 (10) | |
| Unknown | 2 (5) | 1 (9) | |
| Prior SSA use, n (%) | 43 (98) | 11 (100) | .61 |
| Liver metastases, n (%) | 39 (95) | 10 (91) | .58 |
| Prior liver‐directed therapy, n (%) | 26 (59) | 7 (64) | .78 |
| Prior systemic therapy, n (%) | 24 (55) | 10 (91) | .03 |
| Prior nonhepatic surgical resection, n (%) | 32 (73) | 8 (73) | >.99 |
| Baseline labs, median (IQR) | |||
| Hemoglobin | 13.1 (11.9, 14.1) | 12.3 (11.4, 13.8) | .23 |
| White blood cells | 5.9 (4.4, 7.9) | 5.5 (3.5, 6.7) | .17 |
| Platelets | 207.0 (159.0, 243.5) | 236.0 (117.0, 302.0) | .35 |
| Creatinine | 1.0 (0.7, 1.2) | 0.8 (0.7, 1.2) | .56 |
| Total bilirubin | 0.6 (0.5, 0.8) | 1.0 (0.6, 1.4) | .033 |
| AST | 23.0 (20.0, 31.0) | 53.0 (35.0, 65.0) | <.001 |
| ALT | 19.0 (15.0, 30.0) | 44.0 (25.0, 60.0) | .015 |
| Baseline LFT abnormality, n (%)a | 8 (18) | 8 (73) | <.001 |
Defined as at least one of the following: total bilirubin > 1.2, AST > 41, ALT > 54.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; IQR, interquartile range; LFT, liver function test; NET, neuroendocrine tumor; SSA, somatostatin analog.
In logistic regression models, abnormal LFTs were evaluated both individually and as a composite. In univariable analysis, abnormal LFTs taken individually or as a composite were significantly associated with increased likelihood of discontinuation of PRRT (Table 5). Abnormal total bilirubin was associated with future discontinuation (OR: 5.65; 95% confidence interval [CI]: 1.06–30.03; p = .04); however, a baseline composite LFT abnormality was most strongly associated with future discontinuation (OR: 12; 95% CI: 2.59–55.54; p < .001). Prior systemic therapy was suggestive of an increased probability of PRRT discontinuation but did not meet statistical significance (OR: 8.33; 95% CI: 0.98–70.80; p = .05). To determine if prior history of both liver‐directed and systemic therapy would further increase the probability of PRRT discontinuation, the 21 (38%) patients who had received both therapies prior to PRRT were analyzed. Receiving both liver‐directed and systemic therapy was suggestive of an increased probability of PRRT discontinuation but, similar to systemic therapy alone, did not meet statistical significance (OR: 3.75; 95% CI: 0.94–14.9; p = .06). No other variables were statistically significant.
Table 5.
Univariable logistic regression to identify factors associated with peptide receptor radionuclide therapy discontinuation
| Factors | OR (95% CI) | p value |
|---|---|---|
| Total bilirubin >1.2 mg/dL | 5.65 (1.06–30.03) | .042 |
| AST >41 U/L | 1.09 (1.03–1.15) | .004 |
| ALT >54 U/L | 1.07 (1.01–1.12) | .011 |
| Baseline LFT abnormalitya | 12 (2.59–55.5) | <.001 |
| Prior systemic therapy | 8.33 (0.98–70.80) | .05 |
| Prior systemic and liver‐directed therapy | 3.75 (0.94–14.9) | .06 |
Defined as at least one of the following: total bilirubin >1.2 mg/dL, AST >41 U/L, or ALT >54 U/L.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; LFT, liver function test; OR, odds ratio.
Discussion
The recent FDA approval of PRRT for the treatment of somatostatin receptor–positive NETs has led to the widespread adoption of PRRT in NET centers throughout the U.S. Demonstrating that PRRT administration can be effectively incorporated into the multidisciplinary treatment of NETs in the U.S. as well as characterization of toxicities and tolerability experienced in a real‐world population are of paramount importance. Herein, we characterize our center's 1‐year experience with implementation of a PRRT program, showing that PRRT can be successfully implemented, that adverse events associated with PRRT are encountered, and that baseline LFT abnormalities may increase the likelihood of PRRT discontinuation.
The patient population selected for PRRT at our tertiary center was diverse and differed from the NETTER‐1 trial, which had strict inclusion and exclusion criteria 19. The patients treated in our center had a wider range of primary NET locations as well as some with advanced NET grades compared with NETTER‐1. Eleven percent of our cohort had grade 3 NETs. Whereas this group was excluded from the NETTER‐1 trial, in our cohort, grade 3 NETs with DOTATATE avidity were considered for PRRT if they had an indolent disease course or if they had exhausted other therapeutic options. Additionally, our patients also had higher rates of prior liver‐directed therapy and prior systemic therapy compared with the NETTER‐1 patients 19. Given the heterogeneity of patients with NETs, it is possible that diverse cohorts such as ours may be more consistent with U.S. patients with NETs who are or will be considering PRRT as part of their NET care.
Toxicities are known to be associated with PRRT 15, 16, 18, 19, 20, 23, and as expected, they were observed in the patients treated at our institution. Bone marrow suppression was the most common toxicity observed in our cohort, with rates comparable to prior studies 15, 16, 18, 19. Kidney injury was rare, similar to prior literature as well 15, 23. Hepatotoxicity was also noted in our study; however, it was not found to be as common as a previous report by our center, which saw rates as high as 59% 20, possibly as a result of increased use of 90Y PRRT in this prior study's patients, whereas all patients in our examined cohort received 177Lu. Similarly, adverse effects mirrored prior studies during and after infusions 15, 19. As many patients with NETs across the U.S. were waiting for the rollout of PRRT, it is possible that patients treated in this initial cohort may have more advanced disease that is in need of a salvage therapy and may be at higher risk for adverse events than individuals who will use PRRT in subsequent years. Therefore, it will be important to track toxicities and intolerances over time as PRRT becomes more firmly cemented in NET management algorithms in the U.S.
Discontinuation of PRRT owing to intolerance or an adverse event occurred in 11 patients (21%), which was more frequent than the 6% reported in NETTER‐1 19. Identifying factors that are associated with an increased rate of PRRT discontinuation is important as this may allow for other therapies to be considered in patients less likely to tolerate PRRT. Comparing patients who completed or were continuing PRRT with patients who discontinued PRRT revealed that prior systemic therapy and LFT elevations were seen more frequently in those who discontinued. Univariable logistic regression modeling demonstrated that the presence of any pre‐PRRT abnormal LFT increased the likelihood of PRRT discontinuation, and using a composite LFT abnormality variable was even more significant. Additionally, prior systemic therapy and the combination of prior systemic therapy and liver‐directed therapy trended toward significantly increasing risk of PRRT discontinuation; however, given our limited sample size, statistical significance was not achieved.
As PRRT‐treated cohorts in the U.S. are followed longitudinally, it will also be important to assess the efficacy of PRRT on NET growth and survival over time. Although beyond the scope of this current study, determining characteristics of PRRT‐treated patients who are most likely to respond to therapy would have significant clinical utility. Additionally, understanding where PRRT is best used in the treatment algorithm for NETs is equally important, especially in U.S.‐based populations, many of whom have had significant pretreatment of their NETs. The higher rate of discontinuation seen in our trial of heavily pretreated patients versus the NETTER‐1 cohort may indicate a possible benefit of using PRRT earlier in the treatment course of NETs prior to systemic therapy, similar to prior data from our center 6; however, larger sample sizes are needed to make any firm conclusions.
A limitation of our study is the small sample size, which limits power and model building. In a larger cohort, it is possible that other variables may be significantly associated with PRRT discontinuation on both univariable and multivariable modeling. Another limitation of our study is that all individuals had not completed their entire course of PRRT. Therefore, it is possible that some individuals who were early in their PRRT treatment and were tolerating therapy well may in fact discontinue treatment at a later time point. Finally, although toxicities were noted during PRRT, it is not always possible to determine if these toxicities were related to PRRT or to another process independent of PRRT.
Conclusion
The review of our center's early experience after U.S. approval of PRRT shows that PRRT can be successfully initiated at a NET referral center in the U.S. and that toxicity is comparable to prior reports. We found that patients with baseline elevations in their LFTs have increased likelihood of discontinuing therapy. If confirmed in a larger cohort, this finding may help identify patients more likely to be intolerant of PRRT and direct these individuals to earlier PRRT or alternative treatment modalities.
Author Contributions
Conception/design: Jason M. Heckert, Jennifer R. Eads, Michael C. Soulen, Daniel A. Pryma, David A. Mankoff, David C. Metz, Bryson W. Katona
Provision of study material or patients: Bonita Bennett, Caroline Creamer, Jennifer R. Eads, Michael C. Soulen, Daniel A. Pryma, David A. Mankoff, David C. Metz, Bryson W. Katona
Collection and/or assembly of data: Jason M. Heckert, Sarit T. Kipnis, Shria Kumar, Samuel Botterbusch, Alice Alderson
Data analysis and interpretation: Jason M. Heckert, Sarit T. Kipnis, Shria Kumar, Jennifer R. Eads, Michael C. Soulen, Daniel A. Pryma, David A. Mankoff, David C. Metz, Bryson W. Katona
Manuscript writing: Jason M. Heckert, Sarit T. Kipnis, Shria Kumar, Bonita Bennett, Caroline Creamer, Jennifer R. Eads, Michael C. Soulen, Daniel A. Pryma, David A. Mankoff, David C. Metz, Bryson W. Katona
Final approval of manuscript: Jason M. Heckert, Sarit T. Kipnis, Shria Kumar, Samuel Botterbusch, Alice Alderson, Bonita Bennett, Caroline Creamer, Jennifer R. Eads, Michael C. Soulen, Daniel A. Pryma, David A. Mankoff, David C. Metz, Bryson W. Katona
Disclosures
Jennifer R. Eads: Bristol‐Myers Squibb (E [spouse]), Novartis, Exelixis, Lexicon (C/A), Calithera Biosciences, Leap Therapeutics, Merck, Bristol‐Myers Squibb, EMD Serano, Symphogen, Medimmune, Bayer, Placon Therapeutics (RF); Michael C. Soulen: Guerbet LLC, BTG International, Sirtex Medical (RF), Guerbet LLC, Genentech (C/A); Daniel A. Pryma: 511 Pharma, Progenics, Siemens (RF), 511 Pharma, Progenics, Siemens, Nordic Nanovector, Actinium (C/A); David C. Metz: Ipsen, Advanced Accelerator Applications, Wren (RF), Advanced Accelerator Applications, Lexicon (C/A); Bryson W. Katona: Exact Sciences (C/A), Janssen (other–travel). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Yao JC, Hassan M, Phan A et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2. Modlin I, Oberg K, Chung D et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72. [DOI] [PubMed] [Google Scholar]
- 3. Huguet I, Grossman AB, O'Toole D. Changes in epidemiology of NETs. Neuroendocrinology 2017;104:105–111. [DOI] [PubMed] [Google Scholar]
- 4. Hallet J, Law CH, Cukier M et al. Exploring the rising incidence of neuroendocrine tumors: A population‐based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589–597. [DOI] [PubMed] [Google Scholar]
- 5. Tsoli M, Chatzellis E, Koumarianou A et al. Current best practice in the management of neuroendocrine tumors. Ther Adv Endocrinol Metab 2018;10:2042018818804698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katona BW, Roccaro GA, Soulen MC et al. Efficacy of peptide receptor radionuclide therapy in a United States‐based cohort of metastatic neuroendocrine tumor patients: Single‐institution retrospective analysis. Pancreas 2017;46:1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cives M, Strosberg J. Radionuclide therapy for neuroendocrine tumors. Curr Oncol Rep 2017;19:9. [DOI] [PubMed] [Google Scholar]
- 8. Kunikowska J, Pawlak D, Bąk MI et al. Long‐term results and tolerability of tandem peptide receptor radionuclide therapy with 90Y/177 Lu‐DOTATATE in neuroendocrine tumors with respect to the primary location: A 10‐year study. Ann Nucl Med 2017;31:347–356. [DOI] [PubMed] [Google Scholar]
- 9. Hamiditabar M, Ali M, Roys J et al. Peptide receptor radionuclide therapy with 177Lu‐octreotate in patients with somatostatin receptor expressing neuroendocrine tumors: Six years' assessment. Clin Nucl Med 2017;42:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim SJ, Pak K, Koo PJ et al. The efficacy of (177)Lu‐labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: A meta‐analysis. Eur J Nucl Med Mol Imaging 2015;42:1964–1970. [DOI] [PubMed] [Google Scholar]
- 11. Sabet A, Dautzenberg K, Haslerud T et al. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu‐octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging 2015;42:1238–1246. [DOI] [PubMed] [Google Scholar]
- 12. Paganelli G, Sansovini M, Ambrosetti A et al. 177 Lu‐DOTA‐octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: Results from a phase II study. Eur J Nucl Med Mol Imaging 2014;41:1845–1851. [DOI] [PubMed] [Google Scholar]
- 13. Ezziddin S, Khalaf F, Vanezi M et al. Outcome of peptide receptor radionuclide therapy with 177Lu‐octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014;41:925–933. [DOI] [PubMed] [Google Scholar]
- 14. Ezziddin S, Attassi M, Yong‐Hing CJ et al. Predictors of long‐term outcome in patients with well‐differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu‐octreotate. J Nucl Med 2014;55:183–190. [DOI] [PubMed] [Google Scholar]
- 15. Kwekkeboom DJ, de Herder WW, Kam BL et al. Treatment with the radiolabeled somatostatin analog [177Lu‐DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124–2130. [DOI] [PubMed] [Google Scholar]
- 16. Imhof A, Brunner P, Marincek N et al. Response, survival, and long‐term toxicity after therapy with the radiolabeled somatostatin analogue [90YDOTA]‐TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416–2423. [DOI] [PubMed] [Google Scholar]
- 17. Van der Zwan WA, Brabander T, Kam BLR et al. Salvage peptide receptor radionuclide therapy with [177Lu‐DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2019;46:704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garske‐Román U, Sandström M, Fröss Baron K et al. Prospective observational study of 177Lu‐DOTA‐octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry‐guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging 2018;45:970–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strosberg J, El‐Haddad G, Wolin E et al. Phase 3 trial of 177Lu‐Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riff BP, Yang YX, Soulen MC et al. Peptide receptor radionuclide therapy–induced hepatotoxicity in patients with metastatic neuroendocrine tumors. Clin Nucl Med 2015;40:845–850. [DOI] [PubMed] [Google Scholar]
- 21. Sharma N, Naraev BG, Engelman EG et al. Peptide receptor radionuclide therapy outcomes in a North American cohort with metastatic well‐differentiated neuroendocrine tumors. Pancreas 2017;46:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 . 2010. Jun 14. Available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed May 1, 2019.
- 23. Carlsen EA, Fazio N, Granberg D et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr Relat Cancer 2019;26:227–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1


