Abstract
Background
One of the most common psychological morbidities of cancer is depression. Routine depression symptoms screening (DSS) is recommended, but its ability to lead to psychosocial interventions in clinical practice is limited. We examined the use of and factors associated with psychosocial interventions for positive DSS following cancer diagnosis.
Materials and Methods
We conducted a population‐based cohort study of patients with diagnoses from 2010 to 2017 who reported ≥1 patient‐reported Edmonton Symptom Assessment System (ESAS) score. Positive DSS was defined as ESAS ≥2 out of 10 for the depression item within 6 months of diagnosis. Outcomes were psychosocial interventions around the time of positive DSS: palliative care assessment, psychiatry/psychology assessment, social work referral, and antidepressant therapy (in patients ≥65 years of age with universal drug coverage). We examined reduction in depression symptom score (≥1 point) following intervention. Modified Poisson regression examined factors associated with interventions.
Results
Of 142,270 patients, 65,424 (46.0%) reported positive DSS at a median of 66 days (interquartile range: 34–105) after diagnosis. Of those with depression symptoms, 17.1% received palliative assessment, 1.7% psychiatry/psychology assessment, 8.4% social work referral, and 4.3% antidepressant therapy. Depression symptom score decreased in 67.2% who received palliative assessment, 63.7% with psychiatry/psychology assessment, 67.3% with social work referral, and 71.4% with antidepressant therapy. On multivariable analysis, patients with older age, rural residence, lowest income quintile, and genitourinary or oropharyngeal cancer were more likely to not receive intervention other than palliative care.
Conclusion
The proportion of patients reporting positive DSS after cancer diagnosis receiving psychosocial intervention is low. We identified patients vulnerable to not receiving interventions, who may benefit from additional support. These data represent a call to action to modify practice and optimize the usefulness of systematic symptom screening.
Implications for Practice
Patient‐reported depression symptoms screening should be followed by targeted interventions to improve symptoms and patient‐centered management.
Keywords: Patient‐reported outcomes, Depression, Supportive care, Distress
Short abstract
This population‐based study examined psycho‐social interventions for patient‐reported depression symptoms after a new diagnosis of cancer and assessed changes in depression scores with intervention. The results reported here are a call to action to modify practice for symptom screening and intervention in supportive care for cancer patients.
Introduction
A cancer diagnosis is a life‐altering event associated with psychological morbidity. One of the most common psychological diagnoses in patients with cancer is depression, affecting 20%–25% of patients depending on the timing in the cancer journey 1, 2. Management of psychological distress in patients with cancer, of which depression is one of the main constructs, has become a growing focus in oncology, with distress proposed as the sixth vital sign in cancer care by national and international societies 3, 4. Cancer‐related depressive symptoms and associated distress lead to significant repercussions for patients, providers, and health care systems, including reduced understanding of the disease and adherence to therapy, decreased quality of life, lower satisfaction with care, increased suicide rates, and suggested inferior long‐term survival 5, 6, 7, 8.
There is a large body of literature demonstrating the limitations of clinical interviews and physician judgment in identifying depressive symptoms, suggesting the need for, and use of, effective screening tools to improve detection 9, 10. Routine and systematic psychosocial screening is now recommended by cancer societies, with hopes that it will improve detection and management, and is even integrated as an accreditation criterion by the American College of Surgeons Commission on Cancer 3, 4, 11, 12, 13. Screening programs for psychosocial wellbeing, including depression, have thus been implemented in a number of cancer centers across North America 4, 14, 15. However, few data are available as to whether screening for depressive symptoms facilitates access to psychosocial interventions or if identified symptoms are actually addressed in clinical practice.
Screening programs may include the use of patient‐reported outcomes (PROMs), which have been shown to improve patient engagement, outcomes, and satisfaction in oncology practice 16, 17, 18. In 2007, the province of Ontario initiated population‐level, routine prospective screening with the Edmonton Symptom Assessment System (ESAS) during outpatient oncology visits 19, 20, 21. However, information regarding the usefulness and actionability of such programs to support patients in clinical practice outside of controlled trials settings is limited 22, 23. The availability of prospective ESAS scores at the population level offers a unique opportunity to assess practice patterns for patients reporting higher symptom burden, in particular symptoms of depression.
We conducted a population‐based study to examine the use of and factors associated with psychosocial interventions for patient‐reported depression symptoms (DS) following a new cancer diagnosis as well as to assess change in depression scores with intervention.
Materials and Methods
Study Design
Using data linked from prospectively maintained administrative databases stored at ICES in Ontario, Canada, we conducted a population‐level cohort study. Under the Canada Health Act, the 14 million residents of Ontario benefit from universally accessible and publicly funded health care though the Ontario Health Insurance Plan (OHIP). All residents of Ontario are eligible for OHIP after they have resided in the province for 3 months.
The study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board and meets the data confidentiality and privacy guidelines of ICES. It was conducted and reported following the Reporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement 24.
Study Population and Cohort
This study was conducted in all patients with a valid OHIP number diagnosed with any cancer from 2010 to 2017. Patients with a new cancer diagnosis were identified in the Ontario Cancer Registry (OCR) using International Classification of Diseases for Oncology codes (ICD‐O.3), survived at least 6 months following the date of diagnosis, and reported at least one ESAS score during this time (supplemental online Table 1). Patients were excluded if they had an invalid or missing unique identification number, if date of death was missing, if they died before or on the date of diagnosis (indicating administrative coding error or diagnosis on autopsy), if date of last contact was missing, if they had two cancer diagnoses on the same date as the most responsible cancer type could not be attributed, or if they were <18 years of age at the time of diagnosis. We further excluded patients who had received psychiatry or psychology services, or antidepressant therapy, in the 5 years to 90 days prior to date of cancer diagnosis, in order to avoid capturing recurring interventions aimed at treatment of a chronic condition.
Patients were followed from time of diagnosis to the end of the follow‐up period, defined as the date of death, last clinical encounter, or end of study as of September 30, 2017, whichever came first.
Data Sources
This study linked administrative data sets at ICES. The OCR includes all patients with a cancer diagnosis (excluding nonmelanoma skin cancer) in Ontario since 1964 25. The reliability of its data has previously been reported 21, 22, 23. The Registered Persons Database (RPDB) contains vital status and demographic data on all individuals covered under OHIP 26. Information regarding health services is included in the Canadian Institute of Health Information Discharge Abstract Database for acute inpatient hospitalizations, the National Ambulatory Care Reporting System for same‐day surgery admissions, emergency room visits, and oncology clinic visits, and the OHIP Claims Database for billing from health care providers, including physicians, groups, laboratories, and out‐of‐province providers 25. The Cancer Activity Level Reporting (ALR) database is maintained by the OCR and includes chemotherapeutics and medications administered to patients with cancer. The Symptom Management Database contains data on all ESAS assessments collected as part of the provincial screening program, including date and location. The Ontario Drug Database (ODB) contains prescription drugs received under public insurance for those ≥65 years of age, of low income, or with disabilities. These databases have been validated for a variety of diagnoses and services 25.
The data sets were linked using unique encoded identifiers and analyzed at ICES. The research team's analyst (H.Z.) had complete access to all the data sets used in this study in order to create the study cohorts, proceed to linkage, and perform the analyses.
Exposure
The main exposure was DS, defined as an ESAS score ≥2 for depression. ESAS is a validated and reliable PROM assessing the severity of nine common cancer‐associated symptoms, including depression [35, 37, 39]. Patients are asked to rate each symptom on a 11‐point numeric scale, from 0 (absence of symptom) to 10 (worst possible symptom). We captured the first ESAS score for depression ≥2 within the first 6 months following diagnosis. ESAS DS ≥2 has been determined as the optimal cutoff for screening of clinical depression in patients with cancer, with 73% sensitivity and 74% specificity 27.
ESAS is routinely captured during outpatient oncology visits in Ontario, as part of a province‐wide screening program 19, 20, 21. Provincial management algorithms for patient‐reported symptoms have been developed and distributed; however, there is no standardized protocol regarding use of ESAS. Variability in ESAS screening exists by cancer site, cancer center, and patient characteristics, with patients diagnosed with melanoma or endocrine cancers, visiting regional cancer centers, living in urban areas, of older age, and who are immigrants, are less likely to undergo symptom screening 40.
Outcomes Measures
The primary outcomes of interest were receipt of psychosocial intervention, defined as palliative care assessment, psychiatry or psychology assessment, social work referral, and antidepressant therapy (supplemental online Table 1). Palliative care assessments were identified via physicians’ claims, as previously described 28. Psychiatry and psychology assessments were captured using physician claims and the ALR. Social work referral was captured in the ALR. Antidepressant therapy was defined as filling of a prescription for antidepressant as per the ODB, using drug identification numbers. Because the ODB covers Ontario patients ≥65 years of age, we restricted the cohort to patients ≥65 years of age for assessment of antidepressant therapy. Considering the opportunistic nature of ESAS collection, it is possible that patients may be experiencing depression symptoms prior to their index assessment. To address this, we measured receipt of intervention during time windows around the date of DS, consistent with other studies using routinely recorded ESAS scores 29. Psychosocial interventions were captured from 30 days prior to 30 days following the date of DS (supplemental online Fig. 1A).
The secondary outcome was change in ESAS score following receipt of intervention, categorized as increased, stable, or decreased. This was examined in a subgroup analysis of patients with DS receiving an intervention. A clinically significant increase or decrease was defined as a change of ≥1/10 compared with the preintervention score 30. The postintervention ESAS DS was captured during a 30‐day time window starting from 42 days after the intervention, to allow time for treatment effect (supplemental online Fig. 1B). If more than one score was recorded during this time window, the first one was retained. The denominator for this analysis was the number of patients receiving the intervention and recording an ESAS score within the time window for measurement of the postintervention score.
Covariates
Age and sex were abstracted from the RPDB. Rural living was determined with postal code of residence 31. Income quintile was assessed with an ecologic measure based on the median income of a patient's postal code of residence using national census data 32, 33. The comorbidity burden was measured using the Johns Hopkins Adjusted Clinical Groups system score. The 32 aggregated diagnosis groups were summed to create a total score, then dichotomized with a cutoff of 10 for high comorbidity burden, consistent with previous reports 34, 35. The time period of diagnosis was defined as the year of diagnosis in the OCR and categorized as 2010–2014 and 2015–2017.
Statistical Analysis
Descriptive analyses were used to define baseline characteristics and outcomes. Categorical variables were reported as absolute number (n) and proportion (%), and continuous variables as means with SD or median with interquartile range (IQR). Comparisons were undertaken with chi‐square test for categorical variables and the Kruskal‐Wallis or t test for continuous variables.
We examined factors associated with receipt of intervention (palliative care assessment, psychiatry/psychology assessment, social work referral, and receipt of antidepressant therapy) for DS using multivariable regression models. Relevant demographic and clinical characteristics were identified a priori for inclusion in the model based on clinical relevance (markers of complexity of cancer care) and existing literature (known relationship with symptom burden). The following covariates were included: age (categorical), comorbidity burden, income, rural living, cancer type, subsequent cancer diagnosis, and time period of diagnosis (categorical). We constructed one model for each intervention of interest using modified Poisson regression with robust variance and an offset for length of follow‐up. In order to examine the association between sex and receipt of intervention, we constructed a second set of models excluding patients with sex‐related cancers (breast, gynecologic, prostate, and male genital organs). The model for the outcome of antidepressant therapy was restricted to patients ≥65 years of age. Results are reported as relative risks (RR) with 95% confidence interval (95% CI).
Finally, we conducted two sensitivity analyses to assess the robustness of the results to our analytic choices. First, we defined DS as ESAS depression score ≥4 to explore whether the established score of ≥2 overestimates the potential number of patients needing intervention and reduces the proportion of patients with DS receiving an intervention. Second, we extended the time period to capture an intervention in 90 days following the date of DS, in order to examine whether delays in referrals and care may have lowered the observed proportion of patients receiving an intervention.
All analyses were two‐sided and statistical significance was set at p ≤ .05. Analyses were conducted using SAS Enterprise Guide 6.1 (SAS Institute, Cary, NC).
Results
We identified 142,270 patients with a new cancer diagnosis who survived at least 6 months following diagnosis and reported at least one ESAS score during those first 6 months (Fig. 1). Patients reported a median of 5 (IQR: 2–7) ESAS scores during this period. Of those, 65,626 were aged ≥65 years and included in the antidepressant analysis. Median follow‐up from the time of diagnosis was 32 months (IQR: 16–56) for the entire cohort, during which 47.3% of patients died. DS was reported by 65,424 (46.0%) patients overall, of whom 26,125 were aged ≥65 years. The median time from diagnosis to DS was 66 days (IQR: 34–105), and median time from DS to date of death in nonsurvivors was 431 days (IQR: 244–809). The characteristics of patients with and without DS are presented in Table 1. Younger patients, those with a higher comorbidity burden, and those receiving systemic therapy as part of their treatment were more likely to report DS. Among type of cancer diagnoses, patients with oropharyngeal and respiratory cancers were more likely to report DS.
Figure 1.
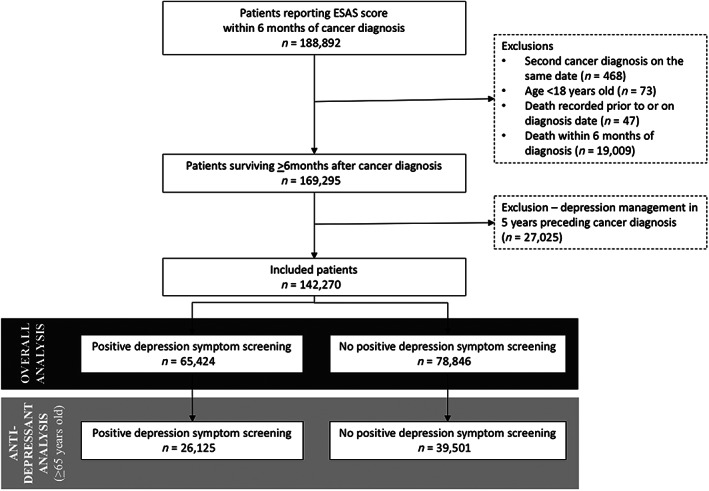
Flow diagram of cohort creation. Abbreviation: ESAS, Edmonton Symptom Assessment System.
Table 1.
Demographic and clinical characteristics of included patients, stratified by patient‐reported depression
| Variable | All patients (n = 142,270) | Patient‐reported depression (n = 65,424) | No patient‐reported depression (n = 76,846) | p value | |
|---|---|---|---|---|---|
| Age, years | ≤60 | 57,979 (40.7) | 30,460 (52.5) | 27,519 (47.5) | <.001 |
| 61–70 | 43,734 (30.3) | 19,124 (43.7) | 24,610 (56.3) | ||
| 71–80 | 29,309 (20.6) | 11,453 (39.1) | 17,856 (60.9) | ||
| ≥81 | 11,248 (7.9) | 4,387 (39.0) | 6,861 (61.0) | ||
| Sex | Female | 83,084 (58.4) | 40,236 (48.4) | 42,848 (51.6) | <.001 |
| Male | 59,186 (41.6) | 25,188 (42.6) | 33,998 (57.4) | ||
| Rural residence | Yes | 14,942 (10.5) | 6,127 (41.0) | 8,815 (59.0) | <.001 |
| No | 127,328 (89.5) | 59,297 (46.6) | 68,031 (53.4) | ||
| High comorbidity burden (ACG ≥10) | Yes | 28,028 (19.7) | 14,499 (51.7) | 13,529 (48.3) | <.001 |
| No | 114,242 (80.3) | 50,925 (44.6) | 63,317 (55.4) | ||
| Income quintile | 1st (lowest) | 24,103 (16.9) | 11,585 (36.9) | 15,218 (63.1) | <.001 |
| 2nd | 27,250 (19.1) | 12,713 (46.7) | 14,537 (53.3) | ||
| 3rd | 28,325 (19.9) | 12,979 (45.8) | 15,346 (54.2) | ||
| 4th | 31,063 (21.8) | 14,163 (45.6) | 16,900 (54.4) | ||
| 5th (highest) | 31,529 (22.2) | 13,984 (44.4) | 17,545 (55.6) | ||
| Cancer diagnosis type | Breast | 41,088 (28.9) | 18,720 (45.6) | 22,368 (54.4) | <.001 |
| Gastrointestinal | 33,872 (23.8) | 16,021 (47.3) | 17,851 (52.7) | ||
| Genitourinary | 23,524 (16.4) | 7,530 (32.0) | 15,994 (68.0) | ||
| Gynecologic | 15,762 (11.1) | 7,403 (47.0) | 8,359 (53.0) | ||
| Oropharyngeal | 7,034 (4.9) | 4,075 (57.9) | 2,959 (42.1) | ||
| Respiratory | 20,990 (14.7) | 11,675 (55.6) | 9,315 (44.4) | ||
| Subsequent second cancer diagnosis | Yes | 4,772 (3.4) | 2,184 (45.8) | 2,588 (54.2) | .76 |
| No | 137,498 (96.6) | 63,240 (46.0) | 74,258 (54.0) | ||
| Time period of diagnosis | 2010–2014 | 92,626 (65.1) | 42,285 (45.7) | 50,341 (54.3) | <.001 |
| 2015–2017 | 49,644 (34.9) | 23,139 (46.6) | 26,505 (53.4) | ||
| Receipt of systemic therapy | Yes | 72,546 (51.0) | 38,685 (55.5) | 31,039 (44.5) | <.001 |
| No | 69,724 (49.0) | 26,739 (36.9) | 45,807 (63.1) |
Data are presented as n (%).
Abbreviation: ACG, aggregated clinical groups.
Receipt of psychosocial interventions is detailed in Figure 2. Of all patients with DS, 17.1% received a palliative care assessment, 1.7% psychiatry or psychology assessment, and 8.4% social work referral. In patients ≥65 years of age, 5.8% filled a prescription for antidepressants. Interventions were more likely in patients with DS than without DS. The receipt of psychosocial interventions varied depending on the type of cancer diagnosis as depicted in Figure 3. Overall, a higher proportion of patients with oropharyngeal and respiratory cancers received interventions. Changes in ESAS score following psychosocial intervention for DS were assessed in patients who received an intervention and had ESAS scores in the pre‐ and postintervention time windows (6,645 for palliative assessment, 523 for psychiatry/psychology assessment, 3,723 for social work referral, and 716 for antidepressant therapy). The results are presented in Figure 4. Reduction in ESAS depression score was identified in a majority of patients, between 63.7% and 72.5% of patients depending on the type of intervention.
Figure 2.

Receipt of psychosocial intervention stratified by patient‐reported depression.
Figure 3.

Receipt of psychosocial intervention with patient‐reported depression. Proportions represent the proportion of patients receiving the intervention within each cancer type (row percentages).
Figure 4.

Change in ESAS depression score following psychosocial intervention with patient‐reported depression. Abbreviation: ESAS, Edmonton Symptom Assessment System.
We examined factors associated with the receipt of each psychosocial intervention in multivariable analyses (Table 2). With regard to patient‐level characteristics, older age was associated with less receipt of psychiatry or psychology assessment and social work referral, but higher receipt of palliative care assessment, compared with age ≤ 65 years. Patients with a higher comorbidity burden were more likely to receive any of the interventions. Rural residence was associated with a lower likelihood of receiving psychiatry or psychology assessment (RR: 0.39, 95% CI: 0.28–0.53), but it was not associated with other interventions. Finally, the association between income quintile and receipt of intervention varied; patients in the lowest quintile had fewer chances of psychiatry or psychology assessment (RR 0.79, 95%CI: 0.66‐0.95), but higher receipt of social work referral (RR: 1.15, 95% CI: 1.06–1.24). Cancer type was also associated with receipt of certain interventions. Patients with genitourinary cancers presented lower chances of receipt for all the interventions, compared with those with respiratory cancers. Breast and gynecologic cancers were associated with lower likelihood for palliative care assessment, social work referral, and antidepressant therapy but not psychiatry or psychology assessment. Patients with oropharyngeal cancers were less likely to receive palliative care assessment and antidepressant therapy. Finally, a more recent diagnosis from 2015 to 2017 was associated with a higher likelihood of palliative care assessment (RR: 1.10, 95% CI: 1.06–1.13) and social work referral (RR: 2.83, 95% CI: 2.69–2.98). The models examining the impact of sex on receipt of intervention excluded sex‐related cancers and showed that female patients were more likely to get psychiatry or psychology assessment (RR: 1.19, 95% CI: 1.01–1.41) but less likely to receive palliative care assessment (RR: 0.95, 95% CI: 0.92–0.99). Sex was not associated with social work referral (RR: 1.00, 95% CI: 0.93–1.07) or antidepressant therapy (RR: 1.09, 95% CI: 0.96–1.24).
Table 2.
Factors associated with receipt of psychosocial intervention for patient‐reported depression (multivariable analysis)
| Palliative care assessment (n = 11,185) | Psychiatry/psychology assessment (n = 1,080) | Social work referral (n = 5,520) | Antidepressant therapya(n = 1,111) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Relative risk | 95% CI | Relative risk | 95% CI | Relative risk | 95% CI | Relative risk | 95% CI | |||||
| Age, years | <65 | Reference | Reference | Reference | — | ||||||||
| 65–70 | 1.08 | 1.03 | 1.13 | 0.59 | 0.48 | 0.73 | 0.58 | 0.53 | 0.64 | Reference | |||
| 71–80 | 1.09 | 1.05 | 1.14 | 0.36 | 0.29 | 0.45 | 0.46 | 0.42 | 0.50 | 0.88 | 0.78 | 0.99 | |
| ≥81 | 1.26 | 1.19 | 1.33 | 0.29 | 0.20 | 0.42 | 0.33 | 0.28 | 0.38 | 0.95 | 0.82 | 1.11 | |
|
Rural residence (ref: urban) |
0.96 | 0.91 | 1.02 | 0.39 | 0.28 | 0.53 | 0.95 | 0.87 | 1.04 | 1.16 | 0.98 | 1.38 | |
| High comorbidity burden (ACG ≥10) | 1.07 | 1.03 | 1.11 | 1.78 | 1.56 | 2.03 | 1.03 | 0.96 | 1.09 | 1.34 | 1.20 | 1.50 | |
|
Income quintile (ref: 5th – highest) |
1st (lowest) | 1.06 | 1.01 | 1.12 | 0.79 | 0.66 | 0.95 | 1.15 | 1.06 | 1.24 | 0.97 | 0.82 | 1.15 |
| 2nd | 0.97 | 0.93 | 1.03 | 0.76 | 0.64 | 0.91 | 1.08 | 0.99 | 1.17 | 0.86 | 0.73 | 1.01 | |
| 3rd | 1.01 | 0.96 | 1.07 | 0.68 | 0.56 | 0.81 | 1.07 | 0.99 | 1.16 | 0.87 | 0.74 | 1.03 | |
| 4th | 1.00 | 0.95 | 1.05 | 0.81 | 0.68 | 0.96 | 1.13 | 1.04 | 1.22 | 1.00 | 0.85 | 1.17 | |
|
Cancer diagnosis type (ref: respiratory) |
Breast | 0.18 | 0.17 | 0.19 | 1.03 | 0.86 | 1.23 | 0.93 | 0.86 | 1.00 | 0.53 | 0.45 | 0.63 |
| Gastrointestinal | 0.69 | 0.67 | 0.72 | 0.98 | 0.82 | 1.19 | 0.94 | 0.87 | 1.01 | 0.83 | 0.73 | 0.94 | |
| Genitourinary | 0.35 | 0.33 | 0.38 | 0.66 | 0.51 | 0.86 | 0.43 | 0.38 | 0.48 | 0.63 | 0.53 | 0.75 | |
| Gynecologic | 0.36 | 0.34 | 0.39 | 0.80 | 0.63 | 1.01 | 0.77 | 0.69 | 0.84 | 0.55 | 0.44 | 0.69 | |
| Oropharyngeal | 0.36 | 0.33 | 0.39 | 1.03 | 0.86 | 1.23 | 1.05 | 0.95 | 1.17 | 0.60 | 0.45 | 0.80 | |
|
Subsequent second cancer diagnosis (ref: none) |
0.95 | 0.86 | 1.05 | 0.83 | 0.57 | 1.22 | 0.86 | 0.72 | 1.03 | 1.07 | 0.83 | 1.38 | |
|
Time period of diagnosis 2015–2017 (ref: 2010–2014) |
1.10 | 1.06 | 1.13 | 1.00 | 0.89 | 1.13 | 2.83 | 2.69 | 2.98 | 0.91 | 0.81 | 1.02 | |
Patients ≥65 years of age.
Abbreviations: —, not relevant; ACG, aggregated clinical groups; ref, reference.
The sensitivity analyses for DS cutoff of ≥4 on the ESAS score and time window for psychosocial intervention prolonged to 90 days following DS did not substantially alter the results (supplemental online Tables 2, 3), except for a higher proportion of patients receiving antidepressants therapy to 9.4% when extending the measuring window to 90 days following DS.
Discussion
In this large cohort of patients with new cancer diagnoses, we identified a high prevalence of patient‐reported DS (46%) using the ESAS score at the population level, with higher prevalence in patients with oropharyngeal and respiratory cancers. However, a disappointingly low proportion of patients received psychosocial interventions around the time of recording DS, outlining unmet needs in patients with cancer. The most common intervention was palliative care assessment in 17.1% of patients with DS, followed by social work referral in 8.4%, psychiatry or psychology assessment in 1.7%, and antidepressant therapy in 4.3% aged ≥65 years. The majority of patients receiving an intervention reported a lower ESAS depression score following the intervention. Finally, we described patient and cancer factors associated with receipt of psychosocial intervention that can be used to identify patients more vulnerable to not receiving intervention. Although it is encouraging that the risk of palliative care assessment and social work referral was higher in the most recent time period from 2015 to 2017, our results highlight a need to modify practice in order to better support patients reporting DS.
The existing oncology literature has well‐established risk factors for depression and the need for screening in the cancer population 3, 4, 9, 10, 13, 36, 37, 38, 39, 41. Distress and depression have been recognized as a significant burden in patients with cancer, negatively impacting engagement, compliance, satisfaction with care, quality of life, and health care services use 5, 6, 7, 8. Routine screening using validated instruments, rather than inconsistent clinicians’ judgment, has been recommended and implemented by cancer programs 3, 4, 13, 14, 15. Routine screening with tools such as the ESAS is meant to identify patients at risk and trigger tailored intervention. Thus, implemented screening programs are ineffective if not followed by tailored care 42. It has been recommended that future work addresses how tools such as the ESAS score trigger interventions 12, 43. To answer this, the current study examined practice patterns for psychosocial interventions in patients reporting DS following cancer diagnosis in a large population‐level cohort of patients that allowed for a detailed analysis of trends and predictors.
The usefulness of screening for DS is contingent on following up with psychosocial interventions 8, 44, 45. Interventions are available to effectively address cancer‐related DS with level‐1 evidence for efficacy, including psychotherapy, social support, and pharmacotherapy 46, 47, 48, 49. The proportion of patients receiving psychosocial interventions is disappointingly, and somewhat alarmingly, low. Prior studies have identified that only 20%–25% of patients identified with DS receive psychosocial interventions, but they were limited to single‐center analyses and smaller sample size or relied on voluntary survey data 50, 51. Furthermore, although some patients may adjust to a new cancer diagnosis and report lower levels of DS over time, this is not the case for all. Indeed, patients who initially report DS tend to experience sustained symptoms, and support for these patients should be in place from the time of diagnosis 41, 51, 52. Our results highlight an important gap in the management of DS for patients with cancer. This study is an important contribution to the literature as it provides an understanding of practice patterns for patient‐reported DS necessary to raise awareness and support change in practice for better patient support and minimizing the negative impacts of cancer‐related depression on outcomes and the health care system. It is a call to action for the global community, clinicians, policy makers, and mental health stakeholders to develop more effective strategies and pathways to bridge the gap between screening of DS and actual psychosocial interventions for patients with cancer.
Our definition of depression may have influenced results. The use of a screening tool to assess DS rather than a clinical diagnosis of depression may result in an overestimation of depression. However, it is consistent with recent trends in assessing cancer‐related depression whereby assessment of symptoms is more easily accessible and pragmatic in the oncology clinical setting 3, 4, 53, 54. If the definition is too sensitive, the use of ESAS depression ≥2 could inflate the number of patients at risk and reduce the overall proportion of patients receiving interventions; the sensitivity analysis using a cutoff at ≥4 did not alter the results. It is also not known whether interventions were targeted at DS or other symptoms, especially knowing that there is often overlap and cross‐management of symptoms with cancer‐related depression 54. This would have overestimated interventions for DS; thus, the high proportion of lack of intervention remains worrisome. The time window to measure psychosocial intervention can also influence the results. Extending the post‐DS time window to 90 days, the only difference was a higher proportion of patients receiving antidepressants. However, this number remained below 10%, and worrisomely low, thus not altering the conclusions. It is estimated that 10%–13% of the general population is treated with antidepressants 55, 56; it seems reasonable to expect that a higher proportion of patients with cancer with high depression scores may require antidepressants. It is also possible that the low proportions observed are due to patients declining intervention, which can happen 20% of the time 57.
Integration of psychosocial care in contemporary cancer care is a challenge; it requires symptom screening, in‐depth assessment of at‐risk patients, triage for intervention, and referral for intervention. From a provider perspective, use of screening results, perceptions of need and effectiveness of interventions, and clinical experience can impact practice. Seven years after the implementation of the province‐wide ESAS screening in Ontario, a survey of cancer centers’ physicians revealed a lack of use of scores, with one out of three respondents indicating rarely or never looking at the scores, and one out of two not incorporating the scores into care plans 23. Investigations of cancer physicians’ attitudes regarding treatment of DS revealed that lack of knowledge about psychosocial services, lack of confidence about the help provided by those services, and negative perceptions impacted their integration in practice 44. In particular, a majority of physicians have negative views regarding antidepressants for cancer‐related depression, owing to “potential for over‐reliance” 58. With regard to patients, there is documented reluctance to use psychosocial interventions, including pharmacotherapy 59. The stigma of mental health diseases still impacts perceptions, and up to 50% of patients reporting significant distress do not intend to use any therapy 60, 61, 62. Finally, from a system perspective, organization and availability of resources appear to play a role in low use, and mental health services can be difficult to access 63, 64. Insufficient psycho‐oncology coverage can impede referral in up to 70% of patients referred 64. Furthermore, psychosocial services are not equally available to all patients; access to psychosocial services is more difficult in rural regions 64. This is confirmed in our results, with lower risk of psychiatry or psychology assessment for patients in rural areas, whereas other interventions were not impacted. Therefore, the reasons underlying the low rate of psychosocial interventions observed herein are likely multifactorial, and solutions should address provider‐, patient‐, and system‐level factors 44, 59.
Standardized protocols for integrated psychosocial interventions are needed to overcome cultural and organization barriers 12, 44. Recommendations exist for the implementation of such programs 12. These efforts should focus on populations most vulnerable for not receiving interventions, as described in this study. Special attention should be paid to older patients and those not previously having regular contact with the health care system (lower comorbidity burden), and any program should ensure equal access to care in urban and rural communities and across socioeconomic statuses. Cancers to focus initial efforts on include genitourinary, oropharyngeal, breast, and gynecologic cancers; those patients experienced lower likelihood of interventions potentially due to higher vulnerability due to risk factors associated with the cancer, more demanding therapy, or higher prevalence of DS.
Provision of integrated counselling and treatment closer to the point of oncology care could avoid delays. This includes formal support and referral pathways involving oncologists, palliative care physicians, social workers, psychiatrists, psychologists, and nursing. Education for health care providers regarding the current gap in practice as well as the rationale and benefit for interventions should be established 65. Patient information is also key to address the perceptions of psychosocial care, including the stigma of mental health 62. New models of assessment and triage may be looked at, such as telemedicine or phone assessments as previously described 65, 66, 67. Prescription of antidepressants by oncologists or primary care providers could be another option. A survey of patients and oncologists has revealed that patients would welcome prescription by non–mental health providers and that oncologists would consider increasing this practice providing they receive education to improve their knowledge and competence in that area 57, 58. Our results indicate a higher proportion of antidepressant use than psychiatry assessments, indicating that antidepressants are already prescribed by non–mental health providers and expanding this approach could be feasible. Such a strategy has been successfully used by specialties managing other physical illnesses associated with DS, such as gastroenterology and rheumatology 68, 69.
There are some study limitations. This is a retrospective cohort study using health care administrative data sets that were not collected specifically to address the research question. As such, we lacked some patient and disease details, including stage, to decipher the decision‐making process or indications for interventions or lack thereof. We also could not capture the use of private psychosocial services or patient support groups; however, prior evidence suggests that only 8% of patients use such services 50. The antidepressant therapy analysis could only be conducted for patients ≥65 years of age who benefit from drug coverage under the ODB. Older patients have a different risk profile for DS such that this may have underestimated the use of antidepressants. However, with such a low rate of use, even if the use of medication was doubled in patients <65 years of age, underuse would remain problematic. Finally, the report of ESAS scores is opportunistic; although DS is captured on a specific date, manifestations of pain and report to health care providers might have happened prior to capture of the score. To address this issue, we assessed interventions during time windows encompassing the period before and after ESAS score acquisition, as previously suggested for other intervention in other symptoms 29, 70.
Conclusion
In this large population‐level analysis, we described practice patterns for patient‐reported DS following a new cancer diagnosis. We identified a worrisomely low proportion of patients reporting DS who received psychosocial interventions. We identified patients at a higher risk for not receiving psychosocial interventions, who may benefit from more support services. These data are important as they represent a call to action to modify current practice to facilitate interventions and optimize the usefulness of systematic symptom screening. They can support the development of new strategies for initiating psychosocial interventions for DS, inform decisions about how to implement such strategies, and provide a benchmark to monitor their results.
Author Contributions
Conception/design: Julie Hallet, Laura E. Davis, Elie Isenberg‐Grzeda, Natalie G. Coburn
Provision of study material or patients: Julie Hallet, Laura E. Davis, Haoyu Zhao, Victoria Zuk, Natalie G. Coburn
Collection and/or assembly of data: Julie Hallet, Laura E. Davis, Haoyu Zhao, Victoria Zuk, Natalie G. Coburn
Data analysis and interpretation: Julie Hallet, Laura E. Davis, Elie Isenberg‐Grzeda, Alyson L. Mahar, Haoyu Zhao, Victoria Zuk, Lesley Moody, Natalie G. Coburn
Manuscript writing: Julie Hallet, Laura E. Davis, Alyson L. Mahar, Natalie G. Coburn
Final approval of manuscript: Julie Hallet, Laura E. Davis, Elie Isenberg‐Grzeda, Alyson L. Mahar, Haoyu Zhao, Victoria Zuk, Lesley Moody, Natalie G. Coburn
Disclosures
Julie Hallet: Ipsen Biopharmaceuticals Canada, Novartis Oncology (H); Elie Isenberg‐Grzeda: Celgene USA (C/A); Natalie G. Coburn: Cancer Care Ontario's Patient‐Reported Outcomes and Symptom Management Program (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures and Tables
Acknowledgments
This work was supported by the Sherif and Mary‐Lou Hanna Chair in Surgical Oncology. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute of Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Mitchell AJ, Chan M, Bhatti H et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: A meta‐analysis of 94 interview‐based studies. Lancet Oncol 2011;12:160–174. [DOI] [PubMed] [Google Scholar]
- 2. Mojtabai R, Jorm AF. Trends in psychological distress, depressive episodes and mental health treatment‐seeking in the United States: 2001‐2012. J Affect Disord 2015;174:556–561. [DOI] [PubMed] [Google Scholar]
- 3. Holland JC, Bultz BD, National Comprehensive Cancer Network (NCCN) . The NCCN guideline for distress management: A case for making distress the sixth vital sign. J Natl Compr Canc Netw 2007;5:3–7. [PubMed] [Google Scholar]
- 4. Bultz BD, Groff SL, Fitch M et al. Implementing screening for distress, the 6th vital sign: A Canadian strategy for changing practice. Psychooncology 2011;20:463–469. [DOI] [PubMed] [Google Scholar]
- 5. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta‐analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 6. Deshields T, Tibbs T, Fan M‐Y et al. Differences in patterns of depression after treatment for breast cancer. Psychooncology 2006;15:398–406. [DOI] [PubMed] [Google Scholar]
- 7. Walker J, Waters RA, Murray G et al. Better off dead: Suicidal thoughts in cancer patients. J Clin Oncol 2008;26:4725–4730. [DOI] [PubMed] [Google Scholar]
- 8. Bidstrup PE, Johansen C, Mitchell AJ. Screening for cancer‐related distress: Summary of evidence from tools to programmes. Acta Oncol 2011;50:194–204. [DOI] [PubMed] [Google Scholar]
- 9. Söllner W, DeVries A, Steixner E et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? Br J Cancer 2001;84:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Passik SD, Dugan W, McDonald MV et al. Oncologists' recognition of depression in their patients with cancer. J Clin Oncol 1998;16:1594–1600. [DOI] [PubMed] [Google Scholar]
- 11. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons commission on cancer distress screening standard. J Natl Compr Canc Netw 2013;11:214–221. [DOI] [PubMed] [Google Scholar]
- 12. Pirl WF, Fann JR, Greer JA et al. Recommendations for the implementation of distress screening programs in cancer centers: Report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer 2014;120:2946–2954. [DOI] [PubMed] [Google Scholar]
- 13. Andersen BL, Rowland JH, Somerfield MR. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. J Oncol Pract 2015;11:133–134. [DOI] [PubMed] [Google Scholar]
- 14. Loscalzo M, Clark KL, Holland J. Successful strategies for implementing biopsychosocial screening. Psychooncology 2011;20:455–462. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell AJ, Lord K, Slattery J et al. How feasible is implementation of distress screening by cancer clinicians in routine clinical care? Cancer 2012;118:6260–6269. [DOI] [PubMed] [Google Scholar]
- 16. Kotronoulas G, Kearney N, Maguire R et al. What is the value of the routine use of patient‐reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32:1480–1501. [DOI] [PubMed] [Google Scholar]
- 17. Basch E. Patient‐reported outcomes ‐ Harnessing patients' voices to improve clinical care. N Engl J Med 2017;376:105–108. [DOI] [PubMed] [Google Scholar]
- 18. Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruera E, Kuehn N, Miller MJ et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 20. Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;88:2164–2171. [DOI] [PubMed] [Google Scholar]
- 21. Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: A 15‐year retrospective review of validation studies (1991–2006). Palliat Med 2008;22:111–122. [DOI] [PubMed] [Google Scholar]
- 22. Carli Buttenschoen D, Stephan J, Watanabe S et al. Health care providers' use and knowledge of the Edmonton Symptom Assessment System (ESAS): Is there a need to improve information and training? Support Care Cancer 2014;22:201–208. [DOI] [PubMed] [Google Scholar]
- 23. Pereira JL, Chasen MR, Molloy S et al. Cancer care professionals' attitudes toward systematic standardized symptom assessment and the Edmonton Symptom Assessment System after large‐scale population‐based implementation in Ontario, Canada. J Pain Symptom Manage 2016;51:662–672.e8. [DOI] [PubMed] [Google Scholar]
- 24. Benchimol EI, Smeeth L, Guttmann A et al. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) Statement. PLoS Med 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robles SC, Marrett LD, Clarke EA et al. An application of capture‐recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 1988;41:495–501. [DOI] [PubMed] [Google Scholar]
- 26. Iron K, Zagorski BM, Sykora K et al. Living and dying in Ontario: An opportunity for improved health information. ICES Investigative Report; Toronto, 2008. [Google Scholar]
- 27. Rhondali W, Perceau E, Berthiller J et al. Frequency of depression among oncology outpatients and association with other symptoms. Support Care Cancer 2012;20:2795–2802. [DOI] [PubMed] [Google Scholar]
- 28. Merchant SJ, Brogly SB, Goldie C et al. Palliative care is associated with reduced aggressive end‐of‐life care in patients with gastrointestinal cancer. Ann Surg Oncol 2018;25:1478–1487. [DOI] [PubMed] [Google Scholar]
- 29. Barbera L, Seow H, Husain A et al. Opioid prescription after pain assessment: A population‐based cohort of elderly patients with cancer. J Clin Oncol 2012;30:1095–1099. [DOI] [PubMed] [Google Scholar]
- 30. Hui D, Shamieh O, Paiva CE et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer 2015;121:3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kralj B. Measuring “rurality” for purposes of health‐care planning: An empirical measure for Ontario. Ont Med Rev 2000;67:33–52. [Google Scholar]
- 32. Wilkins R. Use of postal codes and addresses in the analysis of health data. Health Rep 1993;5:157–177. [PubMed] [Google Scholar]
- 33. Alter DA, Naylor CD, Austin P et al. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med 1999;341:1359–1367. [DOI] [PubMed] [Google Scholar]
- 34. Reid RJ, Roos NP, MacWilliam L et al. Assessing population health care need using a claims‐based ACG morbidity measure: A validation analysis in the province of Manitoba. Health Serv Res 2002;37:1345–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiner JP, Starfield BH, Steinwachs DM et al. Development and application of a population‐oriented measure of ambulatory care case‐mix. Med Care 1991;29:452–472. [DOI] [PubMed] [Google Scholar]
- 36. Bardwell WA, Natarajan L, Dimsdale JE et al. Objective cancer‐related variables are not associated with depressive symptoms in women treated for early‐stage breast cancer. J Clin Oncol 2006;24:2420–2427. [DOI] [PubMed] [Google Scholar]
- 37. Shimizu K, Nakaya N, Saito‐Nakaya K et al. Clinical biopsychosocial risk factors for depression in lung cancer patients: A comprehensive analysis using data from the Lung Cancer Database Project. Ann Oncol 2012;23:1973–1979. [DOI] [PubMed] [Google Scholar]
- 38. Rodin G, Walsh A, Zimmermann C et al. The contribution of attachment security and social support to depressive symptoms in patients with metastatic cancer. Psychooncology 2007;16:1080–1091. [DOI] [PubMed] [Google Scholar]
- 39. Trask PC. Assessment of depression in cancer patients. J Natl Cancer Inst Monographs 2004;2004:80–92. [DOI] [PubMed] [Google Scholar]
- 40. Mahar AL, Davis LE, Bubis LD, Li Q, Sutradhar R, Coburn NG, Barbera L. Factors associated with receipt of symptom screening in the year after cancer diagnosis in a universal health care system: A retrospective cohort study. Curr Oncol 2019. Feb;26(1):e8‐e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bubis LD, Davis L, Mahar A et al. Symptom burden in the first year after cancer diagnosis: An analysis of patient‐reported outcomes. J Clin Oncol 2018;36:1103–1111. [DOI] [PubMed] [Google Scholar]
- 42. Mitchell AJ, Vahabzadeh A, Magruder K. Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary‐care research. Psychooncology 2011;20:572–584. [DOI] [PubMed] [Google Scholar]
- 43. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: Past, present, and future developments. J Pain Symptom Manage 2017;53:630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dilworth S, Higgins I, Parker V et al. Patient and health professional's perceived barriers to the delivery of psychosocial care to adults with cancer: A systematic review. Psychooncology 2014;23:601–612. [DOI] [PubMed] [Google Scholar]
- 45. Carlson LE, Groff SL, Maciejewski O et al. Screening for distress in lung and breast cancer outpatients: A randomized controlled trial. J Clin Oncol 2010;28:4884–4891. [DOI] [PubMed] [Google Scholar]
- 46. Newell SA, Sanson‐Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J Natl Cancer Inst 2002;94:558–584. [DOI] [PubMed] [Google Scholar]
- 47. Hart SL, Hoyt MA, Diefenbach M et al. Meta‐analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst 2012;104:990–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker AE, Grimshaw JM, Armstrong EM. Salient beliefs and intentions to prescribe antibiotics for patients with a sore throat. Br J Health Psychol 2001;6:347–360. [DOI] [PubMed] [Google Scholar]
- 49. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: A systematic review and meta‐analysis. BMC Psychiatry 2013;13:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pascoe S, Edelman S, Kidman A. Prevalence of psychological distress and use of support services by cancer patients at Sydney hospitals. Aust N Z J Psychiatry 2000;34:785–791. [DOI] [PubMed] [Google Scholar]
- 51. Carlson LE, Waller A, Groff SL et al. What goes up does not always come down: Patterns of distress, physical and psychosocial morbidity in people with cancer over a one year period. Psychooncology 2013;22:168–176. [DOI] [PubMed] [Google Scholar]
- 52. Couper JW, Love AW, Duchesne GM et al. Predictors of psychosocial distress 12 months after diagnosis with early and advanced prostate cancer. Med J Aust 2010;193(suppl 5):S58–S61. [DOI] [PubMed] [Google Scholar]
- 53. Rayner L, Loge JH, Wasteson E et al. The detection of depression in palliative care. Curr Opin Support Palliat Care 2009;3:55–60. [DOI] [PubMed] [Google Scholar]
- 54. Trask PC, Griffith KA. The identification of empirically derived cancer patient subgroups using psychosocial variables. J Psychosom Res 2004;57:287–295. [DOI] [PubMed] [Google Scholar]
- 55. Davey CG, Chanen AM. The unfulfilled promise of the antidepressant medications. Med J Aust 2016;204:348–350. [DOI] [PubMed] [Google Scholar]
- 56. Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United States, 2011–2014. NCHS Data Brief 2017;283:1–8. [PubMed]
- 57. Mandeli J, Holland J, McFarland DC et al. ReCAP: Would women with breast cancer prefer to receive an antidepressant for anxiety or depression from their oncologist? J Oncol Pract 2016;12:172–174; e197–e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Holch P, Absolom KL, Pini S et al. Oncology professionals' views on the use of antidepressants in cancer patients: A qualitative interview study. BMJ Support Palliat Care 2011;1:301–305. [DOI] [PubMed] [Google Scholar]
- 59. Rankin NM, Butow PN, Thein T et al. Everybody wants it done but nobody wants to do it: An exploration of the barrier and enablers of critical components towards creating a clinical pathway for anxiety and depression in cancer. BMC Health Serv Res 2015;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carlson LE, Angen M, Cullum J et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer 2004;90:2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Steele R, Fitch MI. Why patients with lung cancer do not want help with some needs. Support Care Cancer 2008;16:251–259. [DOI] [PubMed] [Google Scholar]
- 62. Holland JC, Kelly BJ, Weinberger MI. Why psychosocial care is difficult to integrate into routine cancer care: Stigma is the elephant in the room. J Natl Compr Canc Netw 2010;8:362–366. [DOI] [PubMed] [Google Scholar]
- 63. Malowney M, Keltz S, Fischer D et al. Availability of outpatient care from psychiatrists: A simulated‐patient study in three U.S. cities. Psychiatr Serv 2015;66:94–96. [DOI] [PubMed] [Google Scholar]
- 64. Zimmermann‐Schlegel V, Hartmann M, Sklenarova H et al. Accessibility, availability, and potential benefits of psycho‐oncology services: The perspective of community‐based physicians providing cancer survivorship care. The Oncologist 2017;22:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Turner J, Kelly B, Clarke D et al. A randomised trial of a psychosocial intervention for cancer patients integrated into routine care: The PROMPT study (promoting optimal outcomes in mood through tailored psychosocial therapies). BMC Cancer 2011;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marcus AC, Garrett KM, Cella D et al. Can telephone counseling post‐treatment improve psychosocial outcomes among early stage breast cancer survivors? Psychooncology 2010;19:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Helmes AW, Culver JO, Bowen DJ. Results of a randomized study of telephone versus in‐person breast cancer risk counseling. Patient Educ Couns 2006;64:96–103. [DOI] [PubMed] [Google Scholar]
- 68. Spiller R, Aziz Q, Creed F et al. Guidelines on the irritable bowel syndrome: Mechanisms and practical management. Gut 2007;56:1770–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Häuser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome ‐ A systematic review. Eur J Pain 2010;14:5–10. [DOI] [PubMed] [Google Scholar]
- 70. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: A population‐based matched cohort study among individuals with and without a history of cancer. Cancer 2017;123:4286–4293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures and Tables


