Abstract
Essential oils (EOs) have been described as promising eco-friendly secondary products of aromatic plants with several biological activities. The present study aimed to characterize the chemical composition and explore phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus EOs extracted using hydrodistillation (HD) and microwave-assisted extraction (MAE) methods. Twenty-seven and twenty-eight compounds were identified from HD and MAE extracted EOs of T. polium, respectively. The oxygenated sesquiterpenes (57.68%) were characterized as the main components of the hydrodistilled EO with a prominence of 6-epi-shyobunol (33.00%), while sesquiterpene hydrocarbons (54.48%) were the main components of the MAE method, with a prominence of delta-cadinene (25.13%). Eighteen and nineteen compounds, were characterized in T. decussatus EOs extracted using HD and MAE methods, respectively, and oxygenated monoterpenes represented the main components of both EOs with carvacrol (94.40% and 75.91%, respectively) as the main compound. The EOs extracted using the MAE method were slightly more phytotoxic than those extracted using the HD method. The T. decussatus EO extracted using the MAE method showed a higher inhibitory effect than T. polium by 16-, 32-, and 24-fold, regarding seed germination, shoot, and root growth of lettuce, respectively. Moreover, EOs extracted by HD method showed a similar pattern with 16-, 28-, and 14-fold effects. Both T. decussatus EOs exhibited potent inhibitory effect against all tested bacteria with an inhibition zone of 34–39 mm and the lowest minimum inhibitory concentration (MIC) of 0.49, 0.98, and 1.95 μg/mL against Aspergillus niger, Escherichia coli, and Staphylococcus aureus, respectively. However, the EOs of T. polium showed weak antibacterial activity and no antifungal effect. Further studies are needed for the characterization of bioactive major compounds, either singular or synergistic, at field scale and to determine their modes of action and safety.
Keywords: Lamiaceae, Teucrium polium, Thymus decussatus, essential oils, phytotoxicity, antimicrobial
1. Introduction
From the first days of ancient civilizations, especially the Egyptian one, medicinal herbs have been used as the main source of medicinal agents. Egypt is one of the countries that is characterized by unique biodiversity. With ecological variations, from deserts to seas (the Mediterranean and Red Seas), the Nile River, Nile delta, oases, depressions, and mountains [1], Egypt is also characterized with verifications of medicinal plants, including 529 medicinal plants, 60 endemic plants, and 13 pharmacopeias [2,3]. The Sinai Peninsula, especially the Saint Katherine protectorate, represents the most promising source of traditional herbs in Egypt. The plant species collected from Sinai are characterized by unique metabolites and potent pharmacological effects [4,5].
Aromatic plants are considered a natural source of essential oils (EOs). EOs are considered as a promising source of complex mixtures of metabolites, especially terpenoids [6,7]. Many biological activities have been investigated for EOs, such as phytotoxic [8,9,10], antimicrobial [11], anti-inflammatory, antipyretic [12], antiulcer [13], and hepatoprotective activities [14]. The Lamiaceae (Labiatae) family is considered an important family, having many aromatic plants. Lamiaceae contains 236 genera that embrace 6900–7200 species [15,16]. The most famous genera of the Lamiaceae family are Salvia, Scutellaria, Stachys, Plectranthus, Hyptis, Teucrium, Vitex, Thymus, and Nepeta [16].
T. polium L., one of the most important aromatic plants, is described as an important medicinal plant with several traditional uses, such as treatment of gastrointestinal disorders, inflammations, diabetes, and rheumatologic diseases [17]. In addition, many biological activities were deduced for different extracts of this plant, such as antioxidant, hepatoprotective [17,18], anti-cancer, antimicrobial, antinociceptive, and analgesic activities [19]. The reported chemical characterization confirmed that this plant is auspicious, and has high terpenoid content, especially sesquiterpenes [20,21], iridoid glycoside [21], triterpenes [20], phenyl-ethanoid glycosides, and flavonoids [20,21]. Some chemical profiles and biological investigations of the EOs of T. polium were investigated around the world, as in Iran [22], Tunisia [23], and Saudi Arabia [24].
On the other hand, plants belonging to Thymus genus are described to have numerous traditional applications for several diseases, such as headache, ulcers, eczema, renal diseases, asthenia, wounds, verrucae, and diabetes [25]. T. decussatus Benth. is a very rare plant growing in Saini, Egypt [3]. This plant is very famous for the treatment of nausea [25]. Few reports described T. decussatus EO chemical profiles and biological activities, including antimicrobial and cytotoxicity activities [26,27].
Continuing our target for finding eco-friendly natural bioactive compounds [6,7,12,21,28,29,30,31], our study aimed to (1) characterize the chemical profiles of the EOs of two medicinal plants belonging to Family Lamiaceae, T. polium and T. decussatus, collected from the Saint Katherine Protectorate, Sinai Peninsula, Egypt, (2) evaluate the phytotoxic effects of the extracted EOs on Lactuca sativa (lettuce), (2) determine the antimicrobial activities of EOs on different microorganisms, and (4) evaluate the significance of the extraction method on the chemical composition via chemometrics analysis.
2. Results and Discussion
2.1. Chemical Profiles of EOs
The EOs of T. polium were extracted using hydrodistillation (HD) and microwave-assisted extraction (MAE) methods and produced 0.045% and 0.061% (v/w) of yellow oils, respectively. The variation of the oil yield might be attributed to the extraction method used [32]. The extracted EOs were analyzed by Gas chromatography–mass spectrometry (GC–MS) and the chemical constituents were identified and described in Table 1. Twenty-seven compounds were identified from the hydro-distilled EO, representing 100% of the total mass, while 28 compounds were identified from MAE extracted EO.
Table 1.
Chemical profiles of Essential oils (EOs)of Teucrium polium and Thymus decussatus extracted using hydrodistillation (HD) and microwave-assisted extraction (MAE) methods.
| No. | Compound | Rt | KIExp | KILit | T. polium | T. decussatus | Identification b | ||
|---|---|---|---|---|---|---|---|---|---|
| HD | MAE | HD | MAE | ||||||
| Monoterpene hydrocarbons | |||||||||
| 1 | α-Thujene | 4.57 | 925 | 925 | 0.52 ± 0.01 a | 1.26 ± 0.03 | 0.04 ± 0.01 | ---- | MS, KI |
| 2 | α-Pinene | 4.77 | 935 | 936 | 8.94 ± 0.06 | 11.58 ± 0.09 | ---- | ---- | MS, KI |
| 3 | Camphene | 5.80 | 954 | 954 | ---- | 0.44 ± 0.02 | ---- | 0.30 ± 0.02 | MS, KI |
| 4 | 2,4(10)-Thujadiene | 5.96 | 961 | 960 | ---- | 0.32 ± 0.01 | ---- | ---- | MS, KI |
| 5 | Sabinene | 7.44 | 975 | 975 | 2.94 ± 0.04 | 1.14 ± 0.03 | ---- | ---- | MS, KI |
| 6 | β-Pinene | 7.54 | 978 | 979 | 7.38 ± 0.05 | 8.20 ± 0.09 | 0.09 ± 0.01 | 0.54 ± 0.03 | MS, KI |
| 7 | α-Myrcene | 10.19 | 986 | 986 | 2.90 ± 0.04 | ---- | 0.16 ± 0.01 | 1.04 ± 0.03 | MS, KI |
| 8 | p-Cymene | 10.20 | 1024 | 1026 | ---- | ---- | 3.61 ± 0.04 | 16.98 ± 0.10 | MS, KI |
| 9 | Limonene | 10.94 | 1034 | 1033 | 1.10 ± 0.02 | 0.58 ± 0.03 | ---- | ---- | MS, KI |
| 10 | α-Terpinene | 12.18 | 1018 | 1018 | ---- | ---- | ---- | 1.01 ± 0.03 | MS, KI |
| 11 | β-Terpinene | 12.23 | 1031 | 1032 | ---- | ---- | 0.23 ± 0.01 | ---- | MS, KI |
| 12 | β-Phellandrene | 12.64 | 1045 | 1044 | ---- | ---- | ---- | 0.21 ± 0.01 | MS, KI |
| 13 | γ-Terpinene | 14.43 | 1061 | 1061 | 0.34 ± 0.02 | ---- | 0.49 ± 0.01 | 2.51 ± 0.06 | MS, KI |
| 14 | β-Terpinolene | 15.15 | 1084 | 1086 | ---- | ---- | 0.12 ± 0.01 | 0.34 ± 0.01 | MS, KI |
| Oxygenated monoterpenes | |||||||||
| 15 | α-Fenchyl alcohol | 12.70 | 1106 | 1107 | 0.39 ± 0.02 | ---- | ---- | ---- | MS, KI |
| 16 | cis-Verbenol | 13.65 | 1142 | 1144 | 0.34 ± 0.03 | ---- | ---- | ---- | MS, KI |
| 17 | Menthol | 14.17 | 1181 | 1182 | ---- | ---- | ---- | 0.54 ± 0.01 | MS, KI |
| 18 | α-Terpineol | 20.28 | 1189 | 1189 | 0.44 ± 0.03 | ---- | ---- | ---- | MS, KI |
| 19 | Thymol methyl ether | 20.41 | 1236 | 1237 | ---- | ---- | ---- | 0.03 ± 0.01 | MS, KI |
| 20 | Dihydrocarvone | 21.31 | 1243 | 1242 | ---- | ---- | ---- | 0.06 ± 0.01 | MS, KI |
| 21 | Carvacrol | 22.79 | 1304 | 1304 | ---- | ---- | 94.40 ± 0.52 | 75.91 ± 0.61 | MS, KI |
| 22 | α-Terpinenyl acetate | 25.21 | 1349 | 1350 | 0.51 ± 0.02 | ---- | ---- | ---- | MS, KI |
| 23 | Thymyl acetate | 28.55 | 1357 | 1357 | ---- | ---- | 0.05 ± 0.01 | 0.06 ± 0.01 | MS, KI |
| Sesquiterpene hydrocarbons | |||||||||
| 24 | α-Cubebene | 21.37 | 1351 | 1351 | ---- | 1.10 ± 0.03 | ---- | ---- | MS, KI |
| 25 | α-Bourbonene | 25.43 | 1374 | 1374 | ---- | 0.48 ± 0.04 | ---- | ---- | MS, KI |
| 26 | α-Copaene | 25.77 | 1377 | 1376 | 0.47 ± 0.02 | 1.34 ± 0.04 | ---- | ---- | MS, KI |
| 27 | β-Elemene | 26.52 | 1385 | 1384 | ---- | 0.42 ± 0.03 | ---- | ---- | MS, KI |
| 28 | Longifolene | 27.61 | 1401 | 1402 | ---- | ---- | 0.07 ± 0.01 | ---- | MS, KI |
| 29 | cis-Caryophyllene | 27.89 | 1410 | 1409 | 0.97 ± 0.03 | 1.72 ± 0.05 | 0.33 ± 0.02 | 0.17 ± 0.01 | MS, KI |
| 30 | Clovene | 29.37 | 1426 | 1425 | ---- | ---- | 0.13 ± 0.01 | ---- | MS, KI |
| 31 | Aromadendrene | 29.55 | 1440 | 1439 | 3.86 ± 0.04 | 0.33 ± 0.03 | 0.10 ± 0.01 | 0.04 ± 0.01 | MS, KI |
| 32 | β-Neoclovene | 30.56 | 1455 | 1454 | ---- | ---- | 0.04 ± 0.01 | ---- | MS, KI |
| 33 | α-Gurjunene | 30.74 | 1475 | 1475 | 0.68 ± 0.03 | ---- | ---- | ---- | MS, KI |
| 34 | Germacrene-D | 31.99 | 1480 | 1480 | 2.21 ± 0.06 | 7.25 ± 0.46 | ---- | ---- | MS, KI |
| 35 | β-Selinene | 33.44 | 1485 | 1485 | ---- | ---- | 0.04 ± 0.01 | ---- | MS, KI |
| 36 | Guaia-1(10),11-diene | 33.72 | 1488 | 1488 | ---- | 1.69 ± 0.07 | ---- | ---- | MS, KI |
| 37 | β-Muurolene | 34.08 | 1493 | 1493 | 0.57 ± 0.04 | 5.55 ± 0.06 | ---- | 0.05 ± 0.01 | MS, KI |
| 38 | α-Muurolene | 34.54 | 1498 | 1499 | 0.72 ± 0.01 | 7.64 ± 0.58 | ---- | ---- | MS, KI |
| 39 | δ-Cadinene | 35.03 | 1525 | 1524 | 7.04 ± 0.31 | 25.13 ± 0.43 | 0.06 ± 0.01 | 0.08 ± 0.01 | MS, KI |
| 40 | Cadina-1(10),4-diene | 35.89 | 1530 | 1530 | ---- | 1.83 ± 0.05 | ---- | ---- | MS, KI |
| Oxygenated sesquiterpenes | |||||||||
| 41 | Spathulenol | 28.61 | 1515 | 1516 | ---- | 0.51 ± 0.02 | ---- | ---- | MS, KI |
| 42 | 1,5-Epoxy-salvial-4(14)-ene | 29.64 | 1548 | 1548 | 0.86 ± 0.02 | 0.78 ± 0.03 | ---- | ---- | MS, KI |
| 43 | Ledol | 29.67 | 1564 | 1565 | 0.69 ± 0.04 | 0.58 ± 0.03 | ---- | ---- | MS, KI |
| 44 | 4-epi-Cubebol | 29.29 | 1572 | 1574 | ---- | 0.30 ± 0.01 | ---- | ---- | MS, KI |
| 45 | Germacrene D-4-ol | 30.71 | 1578 | 1577 | 5.65 ± 0.31 | ---- | ---- | ---- | MS, KI |
| 46 | Τ-Cadinol | 32.23 | 1638 | 1640 | 2.06 ± 0.05 | 7.61 ± 0.12 | ---- | ---- | MS, KI |
| 47 | Cubenol | 32.31 | 1641 | 1642 | ---- | 0.35 ± 0.01 | ---- | ---- | MS, KI |
| 48 | Τ-Muurolol | 32.74 | 1646 | 1646 | 12.88 ± 0.15 | ---- | ---- | ---- | MS, KI |
| 49 | α-Cadinol | 34.16 | 1654 | 1653 | 2.01 ± 0.03 | 5.49 ± 0.10 | ---- | ---- | MS, KI |
| 50 | Alloaromadendrene oxide | 39.89 | 1672 | 1672 | 0.53 ± 0.01 | 6.09 ± 0.23 | ---- | ---- | MS, KI |
| 51 | 6-Epi-shyobunol | 41.92 | 1682 | 1680 | 33.00 ± 0.72 | 0.29 ± 0.01 | ---- | ---- | MS, KI |
| Non-oxygenated hydrocarbons | |||||||||
| 52 | Nonadecane | 45.04 | 1900 | 1900 | ---- | ---- | 0.03 ± 0.01 | 0.03 ± 0.01 | MS, KI |
| 53 | Heptacosane | 51.54 | 2700 | 2700 | ---- | ---- | 0.01 ± 0.00 | 0.10 ± 0.02 | MS, KI |
| Monoterpene hydrocarbons | 24.12 | 23.52 | 4.74 | 22.93 | |||||
| Oxygenated monoterpenes | 1.68 | 0 | 94.45 | 76.6 | |||||
| Sesquiterpene hydrocarbons | 16.52 | 54.48 | 0.73 | 0.34 | |||||
| Oxygenated sesquiterpenes | 57.68 | 22 | 0 | 0 | |||||
| Non-oxygenated hydrocarbons | 0 | 0 | 0.04 | 0.13 | |||||
| Total | 100% | 100% | 100% | 100% | |||||
Rt: retention time, KILit: published Kovats retention indices; KIexp: Kovats index determined experimentally relative to C8–C28 n-alkanes; a Values are mean ± standard deviation; b the identification of EO constituents based on the comparison of the mass spectral data and Kovats indices (KI) with those of NIST Mass Spectral Library (2011) and Wiley Registry of Mass Spectral Data 8th edition and literature. HD: hydrodistillation, MAE: microwave-assisted extraction.
In the case of the hydro-distilled EO sample, oxygenated sesquiterpenes were characterized as the main components, with a concentration of 57.68%, followed by monoterpene hydrocarbons (24.12%) and sesquiterpene hydrocarbons (16.52%), with the absence of non-terpenoid compounds. Oxygenated sesquiterpene, 6-epi-shyobunol (33.00%), was found to be the main compound, followed by t-muurolol (12.88%) and germacrene D-4-ol (5.65%). Delta-cadinene (7.04%) and aromadendrene (3.86%) were identified as the major compounds of the sesquiterpene hydrocarbons.
The results showed that sesquiterpene hydrocarbons (54.48%) were found as the main identified compounds of the T. polium EO extracted using the MAE method, followed by monoterpene hydrocarbons (23.52%) and oxygenated sesquiterpenes (22.0%). In the case of the MAE method, delta-cadinene (25.13%) was found to be the main compound of the identified sesquiterpene hydrocarbons, alongside α-muurolene (7.64%), germacrene-D (7.25%), and β-muurolene (5.55%). τ-cadinol (7.61%), alloaromadendrene oxide (6.09%), and α-cadinol (5.49%) were identified as the main oxygenated sesquiterpene compounds, while α-pinene (11.58%) and β-pinene (8.20%) were found as the main constituents of the identified monoterpene hydrocarbons, with a complete absence of oxygenated monoterpenes.
The preponderance of sesquiterpenes in the present study is consistent with other studies reporting the chemical characterization of EOs derived from other ecotypes of T. polium growing in Jordan [33], France [34], Algeria [35,36,37], Serbia, the Balkans [38], and Iran [39].
However, other studies showed a different composition of T. polium EOs [40,41,42,43,44], and this discrepancy in the composition could be ascribed to the variation in the environmental and climatic conditions [7,9]. This observation revealed the presence of various chemotypes of T. polium. Therefore, further study is needed for the discrimination of this species at a molecular level.
It is worth mentioning here that in the Egyptian flora, T. polium is mentioned without reference to any subspecies [3,45]. Therefore, from a taxonomic point of view, our results recommend further identification of Egyptian ecospecies of T. polium, which could help for more detailed identification at the sublevel.
On the other hand, the EOs extracted from T. decussatus by HD and MAE were colorless or faint yellow with a yield of 0.023% and 0.031% (v/w), respectively. This variation could also be ascribed to the method of extraction used [32]. Significant variations of the chemical compositions and antimicrobial activities were described among the extracted EOs of Artemisia argyi by HD, simultaneous distillation, and subcritical extraction techniques [46]. Moreover, the yield and composition of the EOs from Carum carvi, Anethum graveolens, and Pimpinella anisum were varied according to the method of extraction used [47].
In the case of T. decussatus, GC–MS analysis of the extracted oil revealed a lower number of identified compounds compared to T. polium (Table 1). The results also showed that the type of extraction method used affected the chemical profile of the extracted EO. Extraction using HD showed the presence of eighteen compounds, while the extracted EO with MAE method showed the presence of nineteen compounds. Monoterpenes were the main identified components of both extracted EOs, using HD and MAE methods, with concentrations of 99.19% and 98.53%, respectively. Overall, the identified monoterpenes were categorized into (1) oxygenated monoterpenes (94.53% and 76.93%, respectively) and (2) monoterpene hydrocarbons (4.66% and 21.59%, respectively). Carvacrol (94.40% and 75.91%, respectively) was the main oxygenated monoterpene identified, while p-cymene (3.61% and 16.98%, respectively) was the main identified monoterpene hydrocarbon. In the present study, the superiority of monoterpenes in the EOs of T. decussatus is in complete agreement with the previously documented studies of the EOs of Egyptian T. decussatus [26], as well as Italian T. pulegioides [48]. In contrast to our results, De Martino, et al. [48] described that the EO of T. longicaulis collected from Italy is rich with sesquiterpenes. These chemical diversities could be attributed to different environmental and genetic variations, for example plant age, seasonality, temperature, moisture, altitude, nutrient availability, and salinity variations [6].
Numerous reports described that the diterpenoids are rare and/or completely absent constituents in EOs derived from plants, with some exceptions [8]. The results of GC–MS of all extracted EOs from the two plants, T. polium and T. decussatus, deduced this to be true by the complete absence of diterpenoids.
From all the discussed data, the variation of the chemical constituents of the EOs is deduced to the correlation with the variations in plant organ, collection place, and environmental conditions including salinity, temperature, etc., as described in several reports. Additionally, our study supported the previously documented results that deduced the variations of chemical compositions of EOs with the extraction method used.
2.2. Principal Component Analysis (PCA) of EO Chemical Compounds
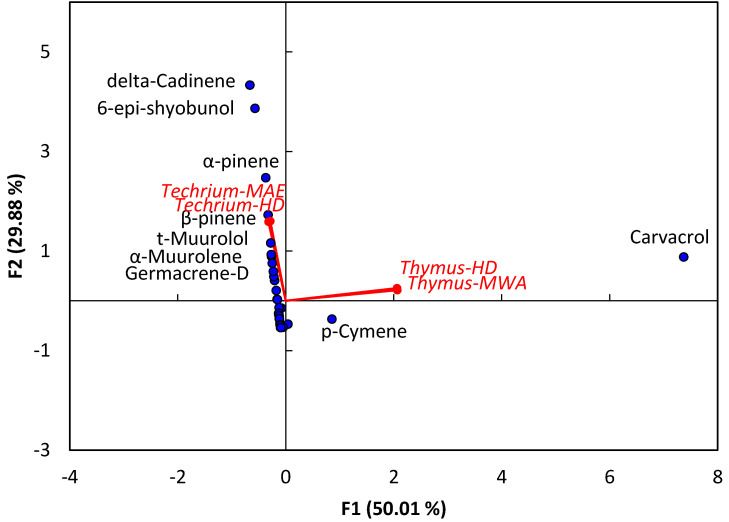
The PCA analysis of the EO chemical compositions showed the difference between the EOs of T. polium and T. decussatus (Figure 1). Moreover, both samples of the extracted EOs (HD and MAE) are closely correlated for both plant species. Regarding the variables (compounds), T. polium EOs showed a correlation with α-pinene, β-pinene, t-muurolol, α-muurolene, germacrene-D, delta-cadinene, and 6-epi-shyobunol. However, the EOs of T. decussatus revealed a correlation with carvacrol and p-cymene.
Figure 1.
Principal component analysis (PCA) of EO compounds extracted from Teucrium polium and Thymus decussatus using microwave-assisted extraction (MAE) and hydrodistillation (HD) methods.
2.3. Phytotoxicity Activity
The EOs of both T. polium and T. decussatus, extracted using the HD and MAE methods, exhibited a significant phytotoxic effect on the tested plant; L. sativa, and the inhibition was dose-dependent (Table 2). Compared to T. polium EOs, T. decussatus EOs showed more a phytotoxic effect at the highest concentration (100 μL L−1), as T. decussatus EO extracted using HD method inhibited the seed germination, shoot growth, and root growth of lettuce by 86.6%, 87.4%, and 89.9%, respectively, while the EO extracted using the MAE method inhibited lettuce by 77.7%, 85.8%, and 84.6%, respectively.
Table 2.
Phytotoxic inhibitory activity of EOs extracted from Teucrium polium and Thymus decussatus using microwave-assisted extraction (MAE) and hydrodistillation (HD) methods.
| Plant Species | Extraction Method | Conc. (µL L−1) | Inhibition (%) * | ||
|---|---|---|---|---|---|
| Germination | Shoot Growth | Root Growth | |||
| Teucrium polium | Hydrodistillation | 250 | 19.6 ± 0.95 | 8.2 ± 0.74 | 14.9 ± 2.76 |
| 500 | 33.0 ± 1.50 | 10.8 ± 0.35 | 23.2 ± 2.03 | ||
| 750 | 43.0 ± 1.98 | 21.5 ± 1.47 | 35.8 ± 1.52 | ||
| 1000 | 51.9 ± 2.09 | 31.4 ± 1.63 | 54.4 ± 1.90 | ||
| Microwave-assisted extraction | 250 | 24.1 ± 1.01 | 0.4 ± 0.35 | 15.3 ± 3.41 | |
| 500 | 37.5 ± 1.13 | 6.0 ± 2.03 | 18.7 ± 2.45 | ||
| 750 | 40.0 ± 2.01 | 17.7 ± 1.44 | 26.0 ± 2.94 | ||
| 1000 | 50.9 ± 2.25 | 39.8 ± 1.30 | 42.3 ± 2.75 | ||
| p-value (0.05) Extraction method (E) | 0.438 ns | 0.0150 * | <0.001 *** | ||
| p-value (0.05) Concentration (C) | <0.001 *** | <0.001 *** | <0.001 *** | ||
| p-value (0.05) Interaction (E × C) | 0.878 ns | <0.001 *** | 0.031 * | ||
| Thymus decussatus | Hydrodistillation | 25 | 50.9 ± 1.59 | 33.0 ± 2.37 | 11.6 ± 0.98 |
| 50 | 55.4 ± 1.55 | 45.7 ± 1.42 | 25.2 ± 3.06 | ||
| 75 | 68.8 ± 2.11 | 52.1 ± 1.48 | 49.8 ± 1.22 | ||
| 100 | 77.7 ± 2.36 | 85.8 ± 1.18 | 84.6 ± 1.02 | ||
| Microwave-assisted extraction | 25 | 42.0 ± 1.11 | 45.6 ± 1.18 | 42.1 ± 2.54 | |
| 50 | 50.9 ± 1.99 | 58.5 ± 1.78 | 56.1 ± 1.47 | ||
| 75 | 68.8 ± 2.25 | 65.7 ± 1.18 | 70.6 ± 1.22 | ||
| 100 | 86.6 ± 2.98 | 87.4 ± 3.55 | 89.9 ± 2.04 | ||
| p-value (0.05) Extraction method (E) | 0.715 ns | <0.001 *** | <0.001 *** | ||
| p-value (0.05) Concentration (C) | <0.001 *** | <0.001 *** | <0.001 *** | ||
| p-value (0.05) Interaction (E × C) | 0.250 ns | 0.007 ** | <0.001 *** | ||
* values are means of triplicates ± SE. Different superscript letters within the column of each treatment mean values significant variation at p ≤ 0.05 where ns: not significant. The statistical significance was indicated by * p ≤ 0.05, ** p ≤ 0.01 or *** p ≤ 0.001
On the other hand, the EO extracted using HD from the above-ground parts of T. polium inhibited the seed germination, shoot growth, and root growth of lettuce by 51.9%, 31.4%, and 54.4%, respectively, at a concentration of 1000 μL L−1, while the EO extracted using MAE method reduced the seed germination, shoot growth, and root growth of lettuce by 50.9%, 39.8%, and 42.3%, respectively (Table 2).
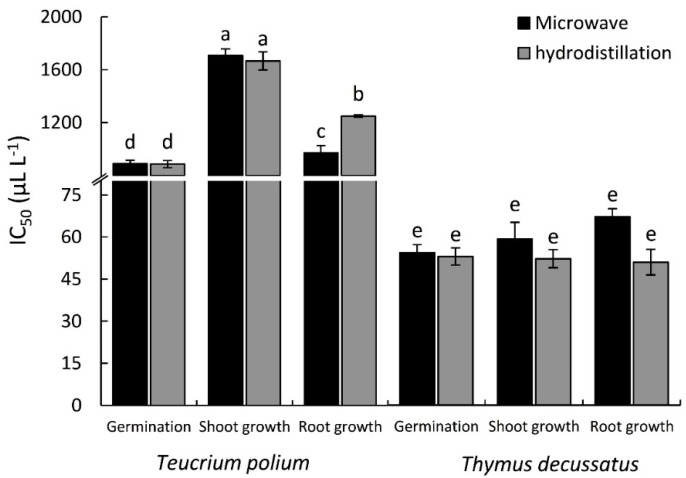
Based on the IC50 values, the EOs extracted by MAE method showed a slightly higher phytotoxic activity than those extracted by HD method (Figure 2). However, the EO extracted using the MAE method from T. decussatus showed a higher inhibitory effect than T. polium by 16-, 32-, and 24-fold, regarding the seed germination, shoot growth, and root growth of lettuce, respectively. Moreover, the EO extracted by HD showed a similar pattern with a 16-, 28-, and 14-fold change, respectively (Figure 2).
Figure 2.
The IC50 values of the essential oils extracted from Teucrium polium and Thymus decussatus using the microwave-assisted extraction (MAE) and hydrodistillation (HD) methods, based on the inhibition of the seed germination and seedling growth of lettuce. Different letters of columns mean values are significantly different at p ≤ 0.05.
Summing up, the data reflect the high potency of T. decussatus EOs, which could be attributed to its content of terpenoid compounds, particularly the monoterpenes, such as the major compounds carvacrol and p-cymene. It is worth mentioning that p-cymene is represented as the major phytotoxic compound of many plants, such as Origanum acutidens [49], Zataria multiflora [50], Eucalyptus citriodora, Eucalyptus grandis [51], Thymbra spicata [52], Thymus eigii [53], and Thymus daenensis [54].
It is important here to mention that oxygenated terpenoid compounds showed more phytotoxic activity than non-oxygenated compounds [6,7,9]. Carvacrol represents 94.4% and 75.9% of the total mass of the EOs extracted by HD and MAE methods from T. decussatus, respectively. Thereby, it could play the main role of the phytotoxic activity of both the extracted EOs. Additionally, carvacrol was previously reported to exhibit phytotoxic activity in many plants, such as Plectranthus amboinicus [55], Melissa officinalis, Origanum vulgare [56], Zataria multiflora [50], Eriocephalus africanus [57], Origanum acutidens [49], Thymbra spicata [52], Thymus eigii [53], Satureja [58], and Thymus daenensis [54].
The mode of action of carvacrol is attributed to the presence of the free hydroxyl group and proton exchange delocalized system [59]. This mode of action is ascribed to the antimicrobial activity of the carvacrol [60]. By simulation, it could also be responsible for the phytotoxic activity in the present study.
2.4. Antimicrobial Activities
The extracted EOs using HD and MAE methods showed variable antimicrobial activities (Table 3 and Table 4). The EOs of T. polium, extracted by HD and MAE methods, showed only antibacterial activity against the tested strains (Gram-positive and Gram-negative bacteria). However, the EOs of T. decussatus revealed a higher activity, as they showed both antibacterial and antifungal activities. T. decussatus EOs extracted using two different extraction methods exhibited a potent inhibitory effect against all bacterial strains, with an inhibition zone of 34–39 mm, and very strong antifungal activity, especially against the filamentous fungi, Aspergillus niger and Fusarium solani, with an inhibition zone of 40–48 mm.
Table 3.
Antimicrobial activities of EOs with a concentration of 10 μL/disc against pathogenic bacteria and fungi.
| Plant | Extraction Method | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | ||||||
| B. subtilis ATCC6633 | S. aureus ATCC29213 | E. coli ATCC25922 | C. albicans ATCC10321 | A. niger NRC53 | F. solani NRC15 | ||
| Teucrium polium | HD | 10.00 ± 0.79 * | 10.00 ± 2.12 | 10.00 ± 0.71 | NA | NA | NA |
| MAE | 11.00 ± 2.12 | 11.00 ± 0.71 | 10.00 ± 1.41 | NA | NA | NA | |
| Thymus decussatus | HD | 39.00 ± 0.71 | 34.00 ± 1.40 | 35.00 ± 2.12 | 34.00 ± 2.12 | 47.00 ± 2.12 | 40.00 ± 2.83 |
| MAE | 38.00 ± 2.90 | 35.00 ± 0.70 | 36.00 ± 1.34 | 35.00 ± 0.00 | 48.00 ± 3.14 | 40.00 ± 1.78 | |
| Thiophenicol (100 μg/disc) | 30.0 ± 0.71 | 30.00 ± 0.71 | 27.00 ± 0.77 | 25.00 ± 1.55 | ---- | ---- | |
| Treflucan (100 μg/disc) | ---- | ---- | ---- | 29.00 ± 0.70 | 13.00 ± 0.14 | 11.00 ± 0.71 | |
* values are the average (n = 3) of the inhibition zone diameter (mm) ± standard deviation, HD: Hydrodistillation, MAE: Microwave-assisted extraction. NA: No activity.
Table 4.
Minimum inhibitory concentrations (MICs) in μg/mL of the EOs extracted using hydrodistillation (HD) and microwave-assisted extraction (MAE) methods against different bacterial and fungal strains.
| Plant | Extraction Method | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | ||||||
| B. subtilis ATCC6633 | S. aureus ATCC29213 |
E. coli ATCC25922 |
C. albicans ATCC10321 | A. niger NRC53 | F. solani NRC15 | ||
| Teucrium polium | HD | 62.50 | 125.00 | 250.00 | ---- | ---- | ---- |
| MAE | 62.50 | 125.00 | 250.00 | ---- | ---- | ---- | |
| Thymus decussatus | HD | 3.91 | 1.95 | 0.98 | 3.91 | 0.49 | 7.81 |
| MAE | 3.91 | 1.95 | 0.98 | 3.91 | 0.49 | 7.81 | |
| Thiophenicol | 3.13 | 3.13 | 6.25 | ---- | ---- | ---- | |
| Treflucan | ---- | ---- | ---- | 12.5 | 50 | 50 | |
Based on the data of the MICs, both samples of Teucrium polium EOs (HD and MAE) showed MIC values of 62.50 and 125.00 μg/mL against B. subtilis and S. aureus, respectively, while they showed an MIC of 250.00 μg/mL against Gram-negative bacteria (E. coli). On the other hand, the T. decussatus EOs revealed MIC values of 3.91, 1.95, and 0.98 μg/mL against B. subtilis, S. aureus, and E. coli, respectively. Additionally, T. decussatus EOs showed strong antifungal activity on the tested fungi, with MIC values of 0.49, 3.91, and 7.81 μg/mL against A. niger, C. albicans, and F. solani, respectively (Table 4).
Belmekki, et al. [61] reported moderate antimicrobial activity of the EO from Algerian T. polium with MIC value of 3 to 5 µL/mL. Meanwhile, another study by Kerbouche, et al. [37], reported a two-fold increase in the antimicrobial activity of the T. polium ecospecies growing in Algeria. The reported antimicrobial activity of the EOs of T. polium could be attributed to the sesquiterpenes, particularly the oxygenated compounds such as 6-epi-shyobunol, t-muurolol, and germacrene D-4-ol.
The strong antimicrobial activity of T. decussatus could be attributed to the major compounds of the EO, such as carvacrol and p-cymene. Several studies reported the antimicrobial activity of carvacrol and p-cymene [59,62,63,64].
3. Materials and Methods
3.1. Plant Materials
The above-ground parts of T. polium and T. decussatus were collected from Wadi Jibaal in St Katherine Protectorate (28°32′32″ N 33°57′30.2″ E), south Sinai, Egypt, during the flowering stage. Voucher specimens (SK-105 (T. polium) and SK122 (T. decussatus)) have been deposited in the herbarium of the National Research Centre, Cairo, Egypt. The collection took place under the permission of the St Katherine Protectorate for scientific purposes. The specimens were authenticated by Dr. Ahmed Abd-ElGawad, Associate Professor of Plant Ecology, Faculty of Science, Mansoura University, Egypt.
3.2. EO Extraction, Analysis, and Constituent Identification
The EOs of the aerial parts (300 gm, each) of T. polium and T. decussatus were extracted separately via the HD and MAE methods, analyzed by GC–MS, and the constituents were identified as described previously by our team [8,32]. In brief, the HD-EOs were extracted via a Clevenger-type apparatus for 3 h. Meanwhile, the extractions of the MAE-EOs were performed using a focused microwave apparatus (CEM Corporation, Matthews, NC, USA), model (MARS 240/50, No. 907511, frequency 2450 MHz) operating at 2450 MHz with a maximum power of 1600 W. A sample of each plant was separately placed in a 5000 mL round-bottomed flask connected to a Clevenger-type apparatus outside of a microwave oven. The extraction was operated using 800 W power for 60 min. The temperature was adjusted to 100 °C. The essential oil was recovered and its volume was determined using a micropipette. The oily layer was separated using diethyl ether and dried with anhydrous sodium sulfate (0.5 g). This extraction was repeated two times and afforded two samples of EO. The extracted two samples of EO were stored in sealed air-tight glass vials at 4 °C until further analysis. The extracted EO constituents were analyzed and recognized by GC–MS analysis. The GC–MS analysis was performed by gas chromatography–mass spectrometry at the department of Medicinal and Aromatic Plants Research, National Research Center, Dokki, Giza, Egypt. This instrument consists of TRACE GC Ultra Gas Chromatographs (THERMO Scientific™ Corporate, Waltham, MA, USA), coupled with a Thermo Scientific ISQ™ EC single quadrupole mass spectrometer. The GC–MS system is equipped with a TR-5 MS column (30 m × 0.32 mm i.d., 0.25 μm film thickness). Helium was used as carrier gas at a flow rate of 1.0 mL min−1 with a split ratio of 1:10 following a temperature program of 60 °C for 1 min, rising by 4.0 °C min−1 to 240 °C and held for 1 min. Both injector and detector were held at 210 °C. One μL of the diluted samples in hexane at a ratio of 1:10 (v/v) was injected. Mass spectra were recorded by electron ionization (EI) at 70 eV, using a spectral range of m/z 40–450.
The identification of the chemical constituents of the EOs was achieved using Automated Mass spectral Deconvolution and Identification (AMDIS) software, Wiley spectral library collection, NIST library database, retention indices relative to n-alkanes (C8-C22) or appraisal of the mass spectrum with authentic standards.
3.3. Phytotoxic Activity of the Extracted EOs
The phytotoxic activity of the extracted EOs from T. polium and T.decussatus using HD and MAE methods was assessed on the germination and seedling growth of the test plant Lactuca sativa (lettuce). The seeds were purchased from the Agriculture Research Center, Mansoura, Egypt. Seeds with a uniform color and size were selected and sterilized by immersion in a solution of sodium hypochlorite (0.3%) for three min and immediately rinsed three times with distilled and sterilized water [65].
To perform the phytotoxic activity, various concentrations (250, 500, 750, and 1000 µL L−1) of T. polium and T. decussatus EOs were prepared using 1% (v/v) Tween®80 (Sigma-Aldrich, Darmstadt, Germany) as an emulsifying agent. In sterilized Petri plates (90 mm) lined with filter papers (Whatman No. 1), 20 sterilized seeds were added and 4 mL of each concentration of the EO was poured. The seeds were arranged away from each other, allowing spread within all the plates, and the plate was sealed with Parafilm® tape (Sigma, St. Louis, MO, USA) and kept at 25 °C in a growth chamber with controlled conditions of light (16 h/8 h light/dark cycle). Control plates were prepared as previously mentioned but using Tween 1% alone instead of EO. The plates were checked daily and after six days of incubation, the germinated seeds were counted and the lengths of the root and shoot were measured [65]. The inhibition of seed germination or seedling growth was calculated as follows:
| (1) |
According to the data obtained, all concentrations of the EOs of T. decussatus showed complete inhibition, i.e., no germination at all. Therefore, lower concentrations (25, 50, 75, and 100 µL L−1) were prepared and the experiment was repeated again.
3.4. Antimicrobial Properties of EOs
The extracted EOs of T. polium and T. decussatus were tested against a panel of pathogenic bacterial and fungal strains using agar diffusion technique, according to Prabuseenivasan, et al. [66] and Rota, et al. [67]. In detail, the EOs were extracted using the HD and MAE methods from the plants under study, T. polium and T. decussatus, and were tested in vitro against Gram-positive bacteria (Bacillus subtilis ATCC6633, Staphylococcus aureus ATCC29213), Gram-negative bacteria (Escherichia coli ATCC25922), unicellular yeast fungi (Candida albicans ATCC10321), and filamentous fungi (Aspergillus niger NRC53, Fusarium solani NRC15). Bacteria and yeast strains are from the American Type Culture Collection, and fungal isolates were obtained from the culture collection of the Department of Chemistry of Natural and Microbial Products, National Research Center, Cairo, Egypt.
The EOs were individually mounted on sterile paper discs (6 mm in diameter) at a concentration of 10 μL/disc. Thiophenicol and treflucan were used as positive controls for antibacterial and antifungal activities at a concentration of 100 μg/disc. All discs were placed on plates inoculated with 1 × 106 spores−mL of fungi (potato dextrose agar medium) and 1 × 108 spores−mL of bacteria (nutrient agar medium). Inoculated agar plates were left for 30 min at 4 °C for oil diffusion; after that, the plates were incubated for 24 h at 30 °C for bacteria and 72 h at 28 °C for fungi. The diameter of the inhibition zones was measured in millimeters, at three different points, and the average values were reported.
For the determination of the minimum inhibitory concentration (MIC), the plant EOs were tested at the final concentrations, ranging from 1000 to 0.49 µg/mL. The lowest concentration showing an inhibition zone around the disc was taken as the MIC.
3.5. Statistical Analysis
The phytotoxic bioassay experiments were performed in a completely randomized design, and the experiment was repeated three times with five replicates per treatment. Based on two factors, the extraction methods (two types) and the concentration (four concentrations), the data was subjected to two-way ANOVA, followed by Duncan’s test at the probability level of 0.05 using the CoStat software program (CoHort Software, Monterey, CA, USA). According to the approach proposed by Legendre and Legendre [68], the data of EO chemical composition extracted by HD and MAE methods were submitted to principal component analysis (PCA), to reproduce a synthetic representation of chemical compounds. PCA was performed using XLSTAT software (version 2018, Addinsoft, NY, USA).
4. Conclusions
The present study revealed that MAE method of T. polium and T. decussatus yielded more EOs than those obtained using HD method, while the number of identified compounds were comparable. The EOs of T. polium are characterized by the preponderance of sesquiterpenes, especially 6-epi-shyobunol, delta-cadinene, α-pinene, β-pinene, germacrene-D, and t-muurolol. However, the EOs of T. decussatus are dominated by monoterpenes, with carvacrol and p-cymene as major compounds. The EOs of T. decussatus showed strong phytotoxic, antibacterial, and antifungal activities. However, the T. polium EOs revealed moderate phytotoxic and antibacterial activities and no antifungal activity. Therefore, the T. decussatus EOs could be integrated as ecofriendly bio-herbicides or as antimicrobial agents. However, further studies are needed for more characterization of the reported major compounds, either singular or in synergism, and to explore their activity at the field scale. On the other hand, from a taxonomic point of view, our results revealed either close similarity or differences of the EO composition of T. polium with other ecospecies, therefore we recommend further identification of Egyptian ecospecies of T. polium, which could help for more detailed identification at the sublevel.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1440-016). Furthermore, the authors would like to sincerely thank the National Research Centre and Department of Botany, Faculty of Science, Mansoura University, Egypt. Mohamed Hegazy gratefully acknowledges the financial support from the Alexander von Humboldt Foundation “Georg Foster Research Fellowship for Experienced Researchers”.
Author Contributions
Conceptualization, T.M., A.A.-E., A.E. and M.-E.F.H.; Investigation, A.E. and M.-E.F.H.; Methodology, T.M., A.A.-E., A.E.-N.E.G., A.A.E.A., I.S., A.E. and M.-E.F.H.; Software, A.A.-E. and A.E.; Validation, A.A.-E., A.E. and M.-E.F.H.; Writing—original draft, A.A-E., A.E. and M.-E.F.H.; Writing—review & editing, T.M., A.A.-E., A.E.-N.E.G., A.A.E.A., I.S., H.K., F.A., A.E. and M.-E.F.H. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research at King Saud University funded this work through research group No (RG-1440-016) and the APC was also funded by Deanship of Scientific Research at King Saud University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zahran M.A., Willis A.J. The Vegetation of Egypt. Volume 2 Springer Science & Business Media; Berlin, Germany: 2008. [Google Scholar]
- 2.Eissa T., Palomino O., Carretero M., Gómez-Serranillos M. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014;151:317–332. doi: 10.1016/j.jep.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 3.Boulos L. Flora of Egypt Checklist. Al-Hadara Publishing; Cairo, Egypt: 2009. pp. 198–201. [Google Scholar]
- 4.Batanouny K.H., Aboutabl E., Shabana M.F.S. Wild Medicinal Plants in Egypt. An Inventory to Support Conservation and Sustainable Use. The Palm Press; Cairo, Egypt: 1999. [Google Scholar]
- 5.Elshamy A.I., Abd El-Gawad A.M., El Gendy A.E.-N.G., Assaeed A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodivers. 2019;16:e1900051. doi: 10.1002/cbdv.201900051. [DOI] [PubMed] [Google Scholar]
- 6.Abd El-Gawad A.M., Elshamy A.I., El Gendy A.E.-N., Gaara A., Assaeed A.M. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules. 2019;24:584. doi: 10.3390/molecules24030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaeed A., Elshamy A., El Gendy A.E.-N., Dar B., Al-Rowaily S., Abd-ElGawad A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy. 2020;10:399. doi: 10.3390/agronomy10030399. [DOI] [Google Scholar]
- 8.Abd-ElGawad A.M., Elshamy A., El Gendy A.E.-N., Al-Rowaily S.L., Assaeed A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019;16:e1900278. doi: 10.1002/cbdv.201900278. [DOI] [PubMed] [Google Scholar]
- 9.Elshamy A., Abd-ElGawad A.M., El-Amier Y.A., El Gendy A., Al-Rowaily S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019;34:316–328. doi: 10.1002/ffj.3512. [DOI] [Google Scholar]
- 10.Abd-ElGawad A.M., Elshamy A., Al-Rowaily S., El-Amier Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium Curassavicum. Plants. 2019;8:482. doi: 10.3390/plants8110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng W., Liu K., Cao S., Sun J., Zhong B., Chun J. Chemical composition, antimicrobial, antioxidant, and antiproliferative properties of grapefruit essential oil prepared by molecular distillation. Molecules. 2020;25:217. doi: 10.3390/molecules25010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elshamy A.I., Ammar N.M., Hassan H.A., Al-Rowaily S.L., Raga T.R., El Gendy A., Abd-ElGawad A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crop. Prod. 2020;148:112272. doi: 10.1016/j.indcrop.2020.112272. [DOI] [Google Scholar]
- 13.Arunachalam K., Balogun S.O., Pavan E., de Almeida G.V.B., de Oliveira R.G., Wagner T., Cechinel Filho V., de Oliveira Martins D.T. Chemical characterization, toxicology and mechanism of gastric antiulcer action of essential oil from Gallesia integrifolia (Spreng.) Harms in the in vitro and in vivo experimental models. Biomed. Pharmacother. 2017;94:292–306. doi: 10.1016/j.biopha.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 14.Damtie D., Braunberger C., Conrad J., Mekonnen Y., Beifuss U. Composition and hepatoprotective activity of essential oils from Ethiopian thyme species (Thymus serrulatus and Thymus schimperi) J. Essent. Oil Res. 2019;31:120–128. doi: 10.1080/10412905.2018.1512907. [DOI] [Google Scholar]
- 15.Heywood V.H., Brummitt R., Culham A., Seberg O. Flowering Plant Families of the World. Volume 88 Firefly Books; Ontario, ON, Canada: 2007. [Google Scholar]
- 16.Harley R.M., Atkins S., Budantsev A.L., Cantino P.D., Conn B.J., Grayer R.J., Harley M.M., de Kok R.P., Morales R., Paton A.J., et al. Labiatae. In: Kubitzki K., editor. The Families and Genera of Vascular Plants. Volume 7. Springer; Berlin, Germany: 2004. pp. 2275–2283. [Google Scholar]
- 17.Rafieian-Kopaei M., Nasri H., Baradaran A. Teucrium polium: Liver and kidney effects. J. Res. Med Sci. 2014;19:478–479. [PMC free article] [PubMed] [Google Scholar]
- 18.Forouzandeh H., Azemi M.E., Rashidi I., Goudarzi M., Kalantari H. Study of the protective effect of Teucrium polium L. extract on acetaminophen-induced hepatotoxicity in mice. Iran. J. Pharm. Res. 2013;12:123–129. [PMC free article] [PubMed] [Google Scholar]
- 19.Khazaei M., Nematollahi-Mahani S.N., Mokhtari T., Sheikhbahaei F. Review on Teucrium polium biological activities and medical characteristics against different pathologic situations. J. Contemp. Med Sci. 2018;4:1–6. [Google Scholar]
- 20.Venditti A., Frezza C., Trancanella E., Zadeh S.M.M., Foddai S., Sciubba F., Delfini M., Serafini M., Bianco A. A new natural neo-clerodane from Teucrium polium L. collected in Northern Iran. Ind. Crop. Prod. 2017;97:632–638. doi: 10.1016/j.indcrop.2017.01.010. [DOI] [Google Scholar]
- 21.Elmasri W.A., Yang T., Tran P., Hegazy M.-E.F., Hamood A.N., Mechref Y., Paré P.W. Teucrium polium phenylethanol and iridoid glycoside characterization and flavonoid inhibition of biofilm-forming Staphylococcus aureus. J. Nat. Prod. 2015;78:2–9. doi: 10.1021/np5004092. [DOI] [PubMed] [Google Scholar]
- 22.Purnavab S., Ketabchi S., Rowshan V. Chemical composition and antibacterial activity of methanolic extract and essential oil of Iranian Teucrium polium against some of phytobacteria. Nat. Prod. Res. 2015;29:1376–1379. doi: 10.1080/14786419.2014.1000320. [DOI] [PubMed] [Google Scholar]
- 23.Ben Othman M., Salah-Fatnassi K.B.H., Ncibi S., Elaissi A., Zourgui L. Antimicrobial activity of essential oil and aqueous and ethanol extracts of Teucrium polium L. subsp. gabesianum (LH) from Tunisia. Physiol. Mol. Biol. Plants. 2017;23:723–729. doi: 10.1007/s12298-017-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim S.R., Abdallah H.M., Mohamed G.A., Farag M.A., Alshali K.Z., Alsherif E.A., Ross S.A. Volatile oil profile of some lamiaceous plants growing in Saudi Arabia and their biological activities. Z. Für Nat. C. 2017;72:35–41. doi: 10.1515/znc-2015-0234. [DOI] [PubMed] [Google Scholar]
- 25.Li X., He T., Wang X., Shen M., Yan X., Fan S., Wang L., Wang X., Xu X., Sui H. Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem. Biodivers. 2019;16:e1900254. doi: 10.1002/cbdv.201900254. [DOI] [PubMed] [Google Scholar]
- 26.El-Hela A. Chemical composition and biological studies of the essential oil of Thymus decussatus Benth growing in Egypt. Egypt. J. Biomed. Sci. 2007;23:146–153. doi: 10.4314/ejbs2.v23i1.40300. [DOI] [Google Scholar]
- 27.Abd El-Mohsen M.M., Abd El-Mohsen M.M., Ehsan N.A., Hussein A.A., Hammouda F.M., Ismail S.I., Hiffnawy M.S. Hepatoprotective and antioxidative activities of the essential oils of Origanum syriacum (L.), Thymus decussatus (Benth) and Salvia multicaulis (Vahl Enum.) J. Arab Soc. Med Res. 2009;4:19–24. [Google Scholar]
- 28.Amin S.M., Hassan H.M., El Gendy A.E.N.G., El-Beih A.A., Mohamed T.A., Elshamy A.I., Bader A., Shams K.A., Mohammed R., Hegazy M.E.F. Comparative chemical study and antimicrobial activity of essential oils of three Artemisia species from Egypt and Saudi Arabia. Flavour Fragr. J. 2019;34:450–459. doi: 10.1002/ffj.3525. [DOI] [Google Scholar]
- 29.Elmasri W.A., Hegazy M.E.F., Aziz M., Koksal E., Amor W., Mechref Y., Hamood A.N., Cordes D.B., Paré P.W. Biofilm blocking sesquiterpenes from Teucrium polium. Phytochemistry. 2014;103:107–113. doi: 10.1016/j.phytochem.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Gamal-Eldeen A.M., Hegazy M.-E. A crystal lapiferin derived from Ferula vesceritensis induces apoptosis pathway in MCF-7 breast cancer cells. Nat. Prod. Res. 2010;24:246–257. doi: 10.1080/14786410802685398. [DOI] [PubMed] [Google Scholar]
- 31.Abd El-Gawad A.M., El-Amier Y.A., Bonanomi G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers. 2018;15:e1800392. doi: 10.1002/cbdv.201800392. [DOI] [PubMed] [Google Scholar]
- 32.Reda E., Saleh I., El Gendy A.N., Talaat Z., Hegazy M.-E., Haggag E. Chemical constituents of Euphorbia sanctae-catharinae Fayed essential oil: A comparative study of hydro-distillation and microwave-assisted extraction. J. Adv. Pharm. Res. 2017;1:155–159. doi: 10.21608/aprh.2017.3383. [DOI] [Google Scholar]
- 33.Aburjai T., Hudaib M., Cavrini V. Composition of the essential oil from Jordanian germander (Teucrium polium L.) J. Essent. Oil Res. 2006;18:97–99. doi: 10.1080/10412905.2006.9699398. [DOI] [Google Scholar]
- 34.Cozzani S., Muselli A., Desjobert J.M., Bernardini A.F., Tomi F., Casanova J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005;20:436–441. doi: 10.1002/ffj.1463. [DOI] [Google Scholar]
- 35.Kabouche A., Kabouche Z., Ghannadi A., Sajjadi S. Analysis of the essential oil of Teucrium polium ssp. aurasiacum from Algeria. J. Essent. Oil Res. 2007;19:44–46. doi: 10.1080/10412905.2007.9699227. [DOI] [Google Scholar]
- 36.Lograda T., Ramdani M., Chalard P., Figueredo G., Deghar A. Chemical analysis and antimicrobial activity of Teucrium polium L. essential oil from eastern Algeria. Am. J. Adv. Drug Deliv. 2014;2:697–710. [Google Scholar]
- 37.Kerbouche L., Hazzit M., Ferhat M.-A., Baaliouamer A., Miguel M.G. Biological activities of essential oils and ethanol extracts of Teucrium polium subsp. capitatum (L.) Briq. and Origanum floribundum Munby. J. Essent. Oil Bear. Plants. 2015;18:1197–1208. doi: 10.1080/0972060X.2014.935065. [DOI] [Google Scholar]
- 38.Kovacevic N.N., Lakusic B.S., Ristic M.S. Composition of the essential oils of seven Teucrium species from Serbia and Montenegro. J. Essent. Oil Res. 2001;13:163–165. doi: 10.1080/10412905.2001.9699649. [DOI] [Google Scholar]
- 39.Sabzeghabaie A., Asgarpanah J. Essential oil composition of Teucrium polium L. fruits. J. Essent. Oil Res. 2016;28:77–80. doi: 10.1080/10412905.2015.1082947. [DOI] [Google Scholar]
- 40.Kabouche Z., Boutaghane N., Laggoune S., Kabouche A., Ait-Kaki Z., Benlabed K. Comparative antibacterial activity of five Lamiaceae essential oils from Algeria. Int. J. Aromather. 2005;15:129–133. doi: 10.1016/j.ijat.2005.03.006. [DOI] [Google Scholar]
- 41.Sadeghi H., Jamalpoor S., Shirzadi M.H. Variability in essential oil of Teucrium polium L. of different latitudinal populations. Ind. Crop. Prod. 2014;54:130–134. doi: 10.1016/j.indcrop.2014.01.015. [DOI] [Google Scholar]
- 42.De Martino L., Formisano C., Mancini E., Feo V.D., Piozzi F., Rigano D., Senatore F. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat. Prod. Commun. 2010;5:1969–1976. doi: 10.1177/1934578X1000501230. [DOI] [PubMed] [Google Scholar]
- 43.Menichini F., Conforti F., Rigano D., Formisano C., Piozzi F., Senatore F. Phytochemical composition, anti-inflammatory and antitumour activities of four Teucrium essential oils from Greece. Food Chem. 2009;115:679–686. doi: 10.1016/j.foodchem.2008.12.067. [DOI] [Google Scholar]
- 44.Mitić V., Jovanović O., Stankov-Jovanović V., Zlatkovic B., Stojanovic G. Analysis of the Essential oil of Teucrium polium ssp. capitatum from the Balkan Peninsula. Nat. Prod. Commun. 2012;7:83–86. [PubMed] [Google Scholar]
- 45.Boulos L. Flora of Egypt, Vol. III (Verbenaceae—Compositae) Volume 3 Al-Hadara Publishing; Cairo, Egypt: 2002. [Google Scholar]
- 46.Guan X., Ge D., Li S., Huang K., Liu J., Li F. Chemical composition and antimicrobial activities of Artemisia argyi Lévl. et Vant essential oils extracted by simultaneous distillation-extraction, subcritical extraction and hydrodistillation. Molecules. 2019;24:483. doi: 10.3390/molecules24030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammed M.S.A., Tawfik M.S.H., Ibrahim A.E.-G. Influence of two extraction methods on essential oils of some Apiaceae family plants. Egypt. Pharm. J. 2019;18:160–166. [Google Scholar]
- 48.De Martino L., Bruno M., Formisano C., De Feo V., Napolitano F., Rosselli S., Senatore F. Chemical composition and antimicrobial activity of the essential oils from two species of Thymus growing wild in southern Italy. Molecules. 2009;14:4614–4624. doi: 10.3390/molecules14114614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kordali S., Cakir A., Ozer H., Cakmakci R., Kesdek M., Mete E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008;99:8788–8795. doi: 10.1016/j.biortech.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 50.Saharkhiz M.J., Smaeili S., Merikhi M. Essential oil analysis and phytotoxic activity of two ecotypes of Zataria multiflora Boiss. growing in Iran. Nat. Prod. Res. 2010;24:1598–1609. doi: 10.1080/14786411003754280. [DOI] [PubMed] [Google Scholar]
- 51.Aragão F., Palmieri M., Ferreira A., Costa A., Queiroz V., Pinheiro P., Andrade-Vieira L. Phytotoxic and cytotoxic effects of Eucalyptus essential oil on lettuce (Lactuca sativa L.) Allelopath. J. 2015;35:259–272. [Google Scholar]
- 52.Ulukanli Z., Bozok F., Cenet M., Ince H., Demirci S.C., Sezer G. Secondary metabolites and bioactivities of Thymbra spicata var. spicata in Amanos mountains. J. Essent. Oil Bear. Plants. 2016;19:1754–1761. doi: 10.1080/0972060X.2016.1209132. [DOI] [Google Scholar]
- 53.Ulukanli Z., Cenet M., Ince H., Yilmaztekin M. Antimicrobial and herbicidal activities of the essential oil from the Mediterranean Thymus eigii. J. Essent. Oil Bear. Plants. 2018;21:214–222. doi: 10.1080/0972060X.2017.1327824. [DOI] [Google Scholar]
- 54.Kashkooli A.B., Saharkhiz M.J. Essential oil compositions and natural herbicide activity of four Denaei Thyme (Thymus daenensis Celak.) ecotypes. J. Essent. Oil Bear. Plants. 2014;17:859–874. doi: 10.1080/0972060X.2014.884946. [DOI] [Google Scholar]
- 55.Pinheiro P.F., Costa A.V., Alves T.d.A., Galter I.N., Pinheiro C.A., Pereira A.F., Oliveira C.M.R., Fontes M.M.P. Phytotoxicity and cytotoxicity of essential oil from leaves of Plectranthus amboinicus, carvacrol, and thymol in plant bioassays. J. Agric. Food Chem. 2015;63:8981–8990. doi: 10.1021/acs.jafc.5b03049. [DOI] [PubMed] [Google Scholar]
- 56.De Almeida L.F.R., Frei F., Mancini E., De Martino L., De Feo V. Phytotoxic activities of Mediterranean essential oils. Molecules. 2010;15:4309–4323. doi: 10.3390/molecules15064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdeguer M., Blázquez M.A., Boira H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009;37:362–369. doi: 10.1016/j.bse.2009.06.003. [DOI] [Google Scholar]
- 58.Taban A., Saharkhiz M.J., Hadian J. Allelopathic potential of essential oils from four Satureja spp. Biol. Agric. Hortic. 2013;29:244–257. doi: 10.1080/01448765.2013.830275. [DOI] [Google Scholar]
- 59.Ben Arfa A., Combes S., Preziosi-Belloy L., Gontard N., Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006;43:149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 60.Roller S., Seedhar P. Carvacrol and cinnamic acid inhibit microbial growth in fresh-cut melon and kiwifruit at 4 and 8 °C. Lett. Appl. Microbiol. 2002;35:390–394. doi: 10.1046/j.1472-765X.2002.01209.x. [DOI] [PubMed] [Google Scholar]
- 61.Belmekki N., Bendimerad N., Bekhechi C., Fernandez X. Chemical analysis and antimicrobial activity of Teucrium polium L. essential oil from Western Algeria. J. Med. Plants Res. 2013;7:897–902. [Google Scholar]
- 62.Magi G., Marini E., Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015;6:165. doi: 10.3389/fmicb.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eusepi P., Marinelli L., García-Villén F., Borrego-Sánchez A., Cacciatore I., Di Stefano A., Viseras C. Carvacrol prodrugs with antimicrobial activity loaded on clay nanocomposites. Materials. 2020;13:1793. doi: 10.3390/ma13071793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aznar A., Fernández P.S., Periago P.M., Palop A. Antimicrobial activity of nisin, thymol, carvacrol and cymene against growth of Candida lusitaniae. Food Sci. Technol. Int. 2015;21:72–79. doi: 10.1177/1082013213514593. [DOI] [PubMed] [Google Scholar]
- 65.Abd El-Gawad A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crop. Prod. 2016;80:36–41. doi: 10.1016/j.indcrop.2015.10.054. [DOI] [Google Scholar]
- 66.Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rota C., Carraminana J., Burillo J., Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004;67:1252–1256. doi: 10.4315/0362-028X-67.6.1252. [DOI] [PubMed] [Google Scholar]
- 68.Legendre P., Legendre L.F. Numerical Ecology. Elsevier; Amsterdam, The Netherlands: 2012. [Google Scholar]