Abstract
Condensed tannins (CTs) are plant anti-herbivore compounds with antimicrobial activity that can be used in ruminant diets as ruminal microbiome manipulators. However, not all CTs from fodder legumes are bioactive due to their wide structural diversity. The aim of our study was to investigate the effect of 10 CT-containing plants (Flemingia macrophylla, Leucaena leucocephala, Stylosanthes guianensis, Gliricidia sepium, Cratylia argentea, Cajanus cajan, Desmodium ovalifolium, Macrotiloma axilare, D. paniculatum, and Lespedeza procumbens) on in vitro fermentation kinetics of Nelore beef cattle. Polyethylene glycol (PEG), a specific CT-binding agent, was added to neutralize condensed tannin. Tifton and alfalfa hay were used as controls lacking CT. The experimental layout included a randomized complete block with factorial design and four blocks. The data were subjected to analysis of variance followed by Duncan’s test to determine differences (p < 0.05) among treatment means. The addition of PEG in browse incubations resulted in increased gas production, fermentation rate, short-chain fatty acid (SCFA) and N-NH3 release. Within our study, Lespedeza procumbens, Desmodium paniculatum, Leucaena leucocephala, Desmodium ovalifolium, and Flemingia macrophylla showed superior bioactivity compared to other species evaluated, suggesting a natural alternative for replacing ionophores to modify ruminal fermentation. Condensed tannins from L. pocumbens, D. paniculatum, L. leucocephala, D. ovalifolium, and F. macrophylla have the potential to modify rumen fermentation in beef cattle.
Keywords: bioactive compounds, cattle, legumes, sustainable
1. Introduction
In tropical and subtropical environments, such as Brazil, grasses of African origin are the most pervasive pasture species for cattle grazing [1]. Because of poor digestibility and high C:N ratios, this led to low livestock productivity and increased rumen methane emissions in these climates [2,3]. In addition, Nelore, a Zebu breed that has less feed efficiency and emits more methane [2], is the predominant beef cattle in Brazilian livestock. This combination led to the search for legumes and their byproducts that can be added to diets of cattle grazing cultivated grass pastures to mitigate greenhouse gas emissions without compromising livestock productivity [4,5].
Many tropical and temperate plant species have chemical defenses, such as condensed tannins (CTs), which can complex with proteins and other macromolecules, providing bactericidal effects [3,6]. Warm-climate arboreal, brush, and herbaceous legumes were long proposed as tropical and subtropical pasture forages that provide protein to livestock and their rumen microbiome; many are also rich in CTs [7]. Legumes containing CTs possess antimicrobial properties capable of manipulating rumen microbiomes. This biodiversity could become a significant alternative for improving sustainable productivity in ruminants grazing tropical and subtropical pastures [3,8]. In primarily tropical and subtropical countries with an expanding agricultural sector, such as Brazil, the use of legume forages is a vital tool for sustainable management in livestock production because they increase dietary protein, reduce greenhouse gas emissions, and improve livestock health in myriad ways.
However, the biological properties of tropical and subtropical forage legume phenolic compounds depend on the level and type in the plant [5,9]. Knowledge of not only phenolic composition but also their effect on ruminal fermentation kinetics is needed for each individual species if we are to fully harness their potential to improve livestock production [3]. The objective of our study was to investigate the effects of 10 CT-rich legumes on in vitro fermentation kinetics in Nelore beef cattle.
2. Results
In vitro degradability differed among plants and PEG treatments (Table 1). Both control Tifton and alfalfa showed greater IVDMD and IVOMD compared to species containing CT. Among tannin-rich legumes, S. guianensis, G. sepium, and M. axilare had higher degradability, followed by L. leucocephala and C. argentea with intermediate values. Lespedeza procumbens, followed by D. paniculatum and F. macrophylla, had low in vitro degradability compared to other entries; however, when PEG was added, the degradability of these plants increased.
Table 1.
Effects of tannins from plant species on ruminal degradability, microbial efficiency and N-NH3 at 96 h of incubation in vitro.
| Variables/Plant | |||
|---|---|---|---|
| IVDMD (g/kg DM) | Without PEG | With PEG | Plant × PEG (2) |
| Cajanus cajan | 403 §D | 435 DE | ns |
| Cynodon spp (Tifton 85) | 751 A | 739 A | ns |
| Cratylia argentea | 553 aC | 458 bD | *** |
| Desmodium ovalifolium | 432 D | 474 D | ns |
| Desmodium paniculatum | 278 bF | 336 aF | * |
| Flemingia macrophylla | 300 bF | 362 aF | * |
| Gliricidia sepium | 676 B | 633 C | ns |
| Lespedeza procumbens | 352 bE | 406 aE | * |
| Leucaena leucocephala | 567 bC | 633 aC | * |
| Medicago sativa | 697 B | 703 BC | ns |
| Macrotiloma axilare | 670 B | 671 BC | ns |
| Stylosanthes guianensis | 694 B | 695 AB | ns |
| SEM (1) | 2.42 | 2.10 | 1.81 |
| p-values (2) | *** | *** | |
| IVOMD (g/kg DM) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 406 §D | 438 CDE | ns |
| Cynodon spp (Tifton 85) | 753 A | 748 A | ns |
| Cratylia argentea | 554 C | 502 C | ns |
| Desmodium ovalifolium | 429 D | 471CD | ns |
| Desmodium paniculatum | 273 bF | 409 aDE | *** |
| Flemingia macrophylla | 298 F | 357 E | ns |
| Gliricidia sepium | 685 B | 637 B | ns |
| Lespedeza procumbens | 357 E | 409 DE | ns |
| Leucaena leucocephala | 557 bC | 628 aB | * |
| Medicago sativa | 689 B | 697 AB | ns |
| Macrotiloma axilare | 672 B | 673 AB | ns |
| Stylosanthes guianensis | 700 B | 702 AB | ns |
| SEM (1) | 2.44 | 2.05 | 2.35 |
| p-values (2) | *** | *** | |
| Partitioning factor (mg DMD/mL) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 3.09 §BC | 2.84 C | ns |
| Cynodon spp (Tifton 85) | 2.73 C | 2.64 C | ns |
| Cratylia argentea | 3.82 aB | 2.87 bC | ** |
| Desmodium ovalifolium | 3.11 BC | 2.81 C | ns |
| Desmodium paniculatum | 5.50 aA | 2.14 bD | *** |
| Flemingia macrophylla | 3.86 aB | 2.73 bC | *** |
| Gliricidia sepium | 3.61 BC | 3.39 AB | ns |
| Lespedeza procumbens | 4.74 aA | 2.28 bD | *** |
| Leucaena leucocephala | 3.73 B | 3.49 A | ns |
| Medicago sativa | 3.27 BC | 3.32 AB | ns |
| Macrotiloma axilare | 3.46 BC | 3.36 AB | ns |
| Stylosanthes guianensis | 3.22 BC | 3.18 B | ns |
| SEM (1) | 0.13 | 0.07 | 0.24 |
| p-values (2) | *** | *** | |
| NNH3 (mg/100 mL) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 22.70 §CD | 27.87 AB | ns |
| Cynodon spp (Tifton 85) | 22.12 CD | 19.37 D | ns |
| Cratylia argentea | 25.87 BCD | 29.87 AB | ns |
| Desmodium ovalifolium | 12.75 bF | 20.50 aCD | * |
| Desmodium paniculatum | 15.50 bF | 30.25 aAB | *** |
| Flemingia macrophylla | 17.25 bEF | 27.87 aAB | *** |
| Gliricidia sepium | 30.50 AB | 34.00 A | ns |
| Lespedeza procumbens | 12.37 bF | 24.75 aBCD | *** |
| Leucaena leucocephala | 21.25 bDE | 29.12 aAB | * |
| Medicago sativa | 32.12 A | 26.62 BC | ns |
| Macrotiloma axilare | 25.62 BCD | 23.37 BCD | ns |
| Stylosanthes guianensis | 26.87 BC | 27.75 AB | ns |
| SEM (1) | 0.82 | 1.07 | 2.49 |
| p-values (2) | *** | *** |
IVDM = in vitro dry matter degradability; IVOMD = in vitro organic matter degradability; DMD = dry matter degradability; PEG = polyethylene glycol; (1) SEM = standard error of the mean; (2) ns = not significant (p > 0.05); * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001. § Means not followed by the same lowercase letter within rows and means not followed by the same uppercase letter within columns differ (Duncan test at 5%).
The partitioning factors in C. argentea, D. paniculatum, F. macrophylla, and L. procumbens obtained without PEG were greater than those obtained after adding PEG. The addition of PEG had no significant effect on the partitioning factor in other species.
Tannins influenced the concentrations of N-NH3 in the rumen, which were lower without PEG. Desmodium ovalifolium, D. paniculatum, F. macrophylla, L. procumbens, and L. leucocephala had lower concentrations of N-NH3 with tannin incubations (non-treated PEG) compared to other entries.
Only D. paniculatum and L. procumbens had lower levels of acetate and propionate when PEG was not used. Total tannins in most plants reduced butyrate concentrations (Table 2). Tannin-containing plant such as D. ovalifolium, D. paniculatum, F. macrophylla, and L. procumbens had higher levels of butyrate with PEG incubations. However, the effect of tannin was more pronounced on isobutyric, isovaleric, and valeric acid levels where, with the exception of the controls, G. sepium, M. axilare, and S. guianensis had lower levels of isoacids than those with PEG addition.
Table 2.
Effects of tannins from plant species on short-chain fatty acids (concentrations at 96 h of incubation in vitro.
| Short-Chain Fatty Acid/Plant | |||
|---|---|---|---|
| Acetic Acid (mmol/g OMD) | Without PEG | With PEG | Plant × PEG (2) |
| Cajanus cajan | 8.42 §AB | 8.43 BC | ns |
| Cynodon spp (Tifton 85) | 6.71 B | 6.96 C | ns |
| Cratylia argentea | 6.74 B | 7.55 C | ns |
| Desmodium ovalifolium | 8.44 AB | 8.03 BC | ns |
| Desmodium paniculatum | 8.56 bAB | 10.50 aA | ** |
| Flemingia macrophylla | 9.45 A | 9.70 AB | ns |
| Gliricidia sepium | 7.20 B | 7.52 C | ns |
| Lespedeza procumbens | 7.48 bAB | 10.48 aA | *** |
| Leucaena leucocephala | 7.61 AB | 6.90 C | ns |
| Medicago sativa | 7.52 AB | 7.15 C | ns |
| Macrotiloma axilare | 6.59 B | 6.77 C | ns |
| Stylosanthes guianensis | 7.45 AB | 7.41 C | ns |
| SEM (1) | 0.25 | 0.17 | 0.56 |
| p-values (2) | *** | *** | |
| Propionic Acid (mmol/g OMD) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 2.35 §ABC | 2.33 BC | ns |
| Cynodon spp (Tifton 85) | 2.29 ABC | 2.54 AB | ns |
| Cratylia argentea | 2.23 ABC | 2.78 AB | ns |
| Desmodium ovalifolium | 1.75 BC | 2.29 BC | ns |
| Desmodium paniculatum | 1.98 BC | 2.63 AB | ns |
| Flemingia macrophylla | 2.55 AB | 2.85 A | ns |
| Gliricidia sepium | 2.95 A | 3.01 A | ns |
| Lespedeza procumbens | 1.56 bC | 3.02 aA | *** |
| Leucaena leucocephala | 2.62 AB | 2.30 BC | ns |
| Medicago sativa | 1.99 BC | 1.93 C | ns |
| Macrotiloma axilare | 2.33 ABC | 2.31 BC | ns |
| Stylosanthes guianensis | 1.96 BC | 1.95 C | ns |
| SEM (1) | 0.09 | 0.07 | 0.24 |
| p-values (2) | * | *** | |
| Butyric acid (mmol/g OMD) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 0.92 §ABC | 1.09 AB | ns |
| Cynodon spp (Tifton 85) | 1.05 A | 1.11 AB | ns |
| Cratylia argentea | 0.77 DE | 0.89 CDE | ns |
| Desmodium ovalifolium | 0.73 bDEF | 0.94 aBCD | * |
| Desmodium paniculatum | 0.57 bFG | 1.14 aA | *** |
| Flemingia macrophylla | 0.70 bDEF | 1.08 aAB | *** |
| Gliricidia sepium | 0.73 DEF | 0.77 DE | ns |
| Lespedeza procumbens | 0.45 bG | 1.20 aA | *** |
| Leucaena leucocephala | 0.64 EF | 0.74 E | ns |
| Medicago sativa | 1.00 AB | 0.96 BC | ns |
| Macrotiloma axilare | 0.86 BCD | 0.87 CDE | ns |
| Stylosanthes guianensis | 0.82 DC | 0.79 CDE | ns |
| SEM (1) | 0.03 | 0.03 | 0.06 |
| p-values (2) | *** | *** | |
| Isobutyric acid (mmol/g OMD) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 0.19 §bABC | 0.26 aCDE | * |
| Cynodon spp (Tifton 85) | 0.14 BCD | 0.14 F | ns |
| Cratylia argentea | 0.21 ABC | 0.25 CDE | ns |
| Desmodium ovalifolium | 0.17 ABCD | 0.19 EF | ns |
| Desmodium paniculatum | n.d b | 0.37 aA | *** |
| Flemingia macrophylla | 0.12 bCD | 0.35 aAB | *** |
| Gliricidia sepium | 0.26 A | 0.26 CDE | ns |
| Lespedeza procumbens | 0.06 bD | 0.33 aABC | *** |
| Leucaena leucocephala | 0.16 bABCD | 0.29 aBCD | * |
| Medicago sativa | 0.25 AB | 0.24 DE | ns |
| Macrotiloma axilare | 0.19 ABC | 0.21 DEF | ns |
| Stylosanthes guianensis | 0.19 ABC | 0.19 EF | ns |
| SEM (1) | 0.01 | 0.01 | 0.04 |
| p-values (2) | * | *** | |
| Isovaleric acid (mmol/g OMD) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 0.22§bCD | 0.36 aBCDE | *** |
| Cynodon spp (Tifton 85) | 0.17 DE | 0.18 H | ns |
| Cratylia argentea | 0.31 B | 0.34 CDEF | ns |
| Desmodium ovalifolium | 0.14 bDEF | 0.24 bGH | *** |
| Desmodium paniculatum | 0.08 bF | 0.52 aA | *** |
| Flemingia macrophylla | 0.12 bEF | 0.44 aB | *** |
| Gliricidia sepium | 0.29 BC | 0.30 DEFG | ns |
| Lespedeza procumbens | 0.08 bF | 0.41 aBC | *** |
| Leucaena leucocephala | 0.16 bDE | 0.30 aDEFG | *** |
| Medicago sativa | 0.39 A | 0.37 BCD | ns |
| Macrotiloma axilare | 0.26 BC | 0.28 EFG | ns |
| Stylosanthes guianensis | 0.28 BC | 0.27 FG | ns |
| SEM (1) | 0.02 | 0.01 | 0.03 |
| p-values (2) | *** | *** | |
| Valeric acid (mmol/g OMD) | Without PEG | With PEG | Plant × PEG |
| Cajanus cajan | 0.22 §bAB | 0.28 aB | * |
| Cynodon spp (Tifton 85) | 0.18 BC | 0.19 DE | ns |
| Cratylia argentea | 0.17 CD | 0.20 DE | ns |
| Desmodium ovalifolium | 0.14 bD | 0.19 aE | * |
| Desmodium paniculatum | 0.08 bE | 0.23 aBCD | *** |
| Flemingia macrophylla | 0.19 bBC | 0.31 aA | *** |
| Gliricidia sepium | 0.20 BC | 0.21 CDE | ns |
| Lespedeza procumbens | 0.08 bE | 0.23 aBCDE | *** |
| Leucaena leucocephala | 0.13 bD | 0.21 aCDE | *** |
| Medicago sativa | 0.21 ABC | 0.21 CDE | ns |
| Macrotiloma axilare | 0.22 AB | 0.22 BCDE | ns |
| Stylosanthes guianensis | 0.24 A | 0.25 BC | ns |
| SEM (1) | 0.01 | 0.01 | 0.01 |
| p-values (2) | *** | *** |
(1) SEM = standard error of the mean; (2) ns = not significant (p > 0.05); * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001. § Means not followed by the same lowercase letter within rows and means not followed by the same uppercase letter within columns differ (Duncan test at 5%). PEG = polyethylene glycol; nd = not detected.
When assessing the parameters of France et al. (1993) [10] (Table 3), the maximum increase in potential of gas production (A) after 96 h of fermentation was recorded in Tifton grass (control) with and without PEG addition, followed by S. guianensis, alfalfa, and M. axilare. Reactive TC from D. paniculatum, L. procumbens, and F. macrophylla resulted in low values of A in incubations without PEG. When tannin was bound with PEG, with the exception of G. sepium, the levels of A increased in all tannin-containing plants. However, only L. procumbens had increased lag time (L) as a result of an interaction between the plant species and PEG. In D. ovalifolium, F. macrophylla, and L. procumbens, the time required to reach half of the asymptotic value (T/2) was greater than when PEG neutralized CT.
Table 3.
Kinetic parameters of gas production modeled according to France et al. (1993) [10] of legumes forages incubated with a rumen fluid inoculum.
| Model Parameters | A (mL) | L (h/min) | T/2 (h) | |||
|---|---|---|---|---|---|---|
| NON | PEG (1) | NON | PEG | NON | PEG | |
| Cajanus cajan | 122.87 §bF | 138.50 aF | 3.66 B | 3.30 A | 29.20 CD | 23.10 D |
| Cynodon spp | 254.52 bA | 258.82 aA | 4.10 AB | 4.00 A | 29.56 CD | 29.81 A |
| Cratylia argentea | 139.45 bE | 146.15 aF | 3.55 B | 4.74 A | 27.17 DE | 26.30 BC |
| Desmodium ovalifolium | 143.12 bE | 155.60 aE | 4.84 AB | 4.18 A | 41.45 aAB | 27.16 bB |
| Desmodium paniculatum | 46.43 bH | 145.40 aF | 4.47 AB | 4.53 A | 24.53 DEF | 26.37 BC |
| Flemingia macrophylla | 81.99 bG | 123.27 aG | 3.87 B | 4.25 A | 44.92 aA | 29.42 bA |
| Gliricidia sepium | 165.92 D | 166.42 D | 3.92 B | 4.47 A | 22.25 EF | 22.90 D |
| Lespedeza procumbens | 75.60 bG | 163.85 aD | 6.21 bA | 3.86 aA | 39.42 aB | 26.62 bBC |
| Leucaena leucocephala | 147.32 bE | 165.47 aD | 4.72 AB | 4.25 A | 33.49 C | 25.11 C |
| Medicago sativa | 188.01 aBC | 187.15 bC | 3.04 B | 3.41 A | 20.85 F | 21.34 E |
| Macrotiloma axilare | 175.25 bCD | 180.95 aC | 3.53 aB | 3.86 A | 23.39 EF | 23.61 D |
| Stylosanthes guianensis | 192.92 bB | 197.45 aB | 3.38 aB | 3.61 A | 20.21 F | 20.72 E |
| p-values (2) | *** | *** | *** | *** | *** | *** |
(1) PEG = polyethylene glycol; NON = without PEG; (2) ns = not significant; (p > 0.05); * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001. §Means not followed by the same lowercase letter within rows and means not followed by the same uppercase letter within columns differ (Duncan test at 5%). France (1993): p = a + b (1 – exp−ct), where p = gas production (mL), in time t, a and b = constants of model, c = production gas rate (h−1), a + b = potential gas production (mL), L = lag time, A = potential of gas production and T/2 = asymptotic value.
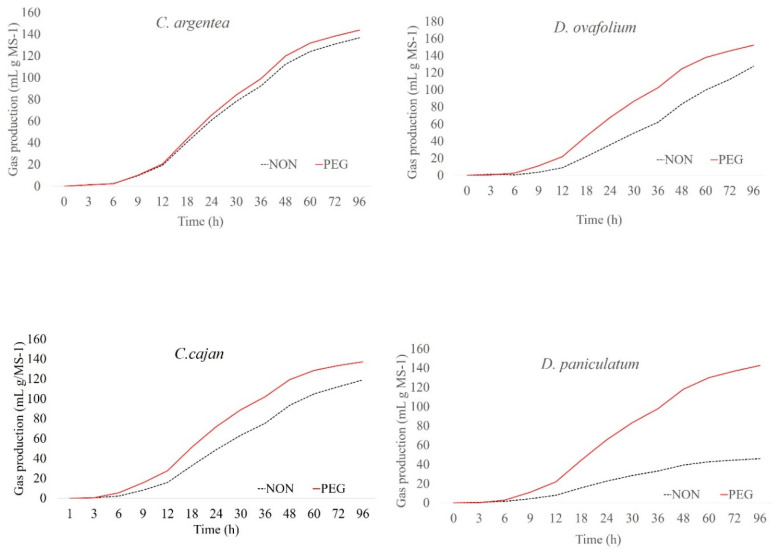
The cumulative gas production curves from plant samples after 96 h of fermentation are shown in Figure 1, Figure 2 and Figure 3. Among the plants, G. sepium and S. guianensis had similar fermentation with and without PEG. For C. argentea and M. axilare, the fermentation process was slightly increased with PEG addition. An increase in gas production obtained after incubation with PEG treatment was recorded for C. cajan, D. ovalifolium, and L. leucocephala. However, the maximum effect of tannins on gas suppression was found in D. paniculatum, F. macrophylla, and L. procumbens, whereas, in the presence of PEG, these values were greater. The inclusion of PEG for L. procumbens, F. macrophylla, D. ovalifolium, D. paniculatum, and L. leucocephala resulted in improved gas production rates (μ) (Figure 4). However, adding PEG did not improve μ values in G. sepium and S. guianensis.
Figure 1.
Cumulative gas production curves of Cratylia argentea, Cajanus cajan, Desmodium ovalifolium, and Desmodium paniculatum incubated at the end of 96 h.
Figure 2.
Cumulative gas production curves of Flemingia macrophylla, Gliricidia sepium, Lespedeza procumbens, and Leucaena leucocephala incubated at the end of 96 h.
Figure 3.
Cumulative gas production curves of Macrotiloma axilare and Stylosanthes guianensis incubated at the end of 96 h.
Figure 4.
Gas production curves of legumes forages incubated at the end of 96 h.
3. Discussion
The main goal of our study was to identify, using an in vitro gas technique, tannin-rich plants as a natural alternative to manipulate rumen microbial fermentation for methane reduction in Nelore beef cattle. Thus, by assessing data from browse fermentation kinetics, we clearly observed that the CT content in L. procumbens, D. paniculatum, L. leucocephala, D. ovalifolium, and F. macrophylla had high reactivity among the tested legumes. Their different kinetic behavior in the presence of PEG confirmed the influence of CT on the fermentation process. However, fermentation characteristics in G. sepium, S. guianensis, and M. axilare were similar with or without PEG added during incubation, reflecting no biological effect of CT in these samples. The greatest effect on the cumulative gas production was recorded in D. paniculatum, although it did not have the highest CT content. The fact that some CTs are more reactive than others is related to their chemical structure as much as to their CT concentration [9,11]; thus, our work highlights the importance of studies that predict CT reactivity in ruminant nutrition rather than simply its quantity.
Rumen degradability and kinetic characteristics were affected by browse samples and PEG treatment. As expected, the least reactive plant species (G. sepium, S. guianensis, and M. axilare) showed maximum IVDMD and IVOMD, and the most reactive browse samples (D. paniculatum, L. procumbens, and F. macrophylla) had minimal IVDMD and IVOMD. An improvement in IVDMD and DIVMO after PEG incubation was observed in our study, reflecting the inhibitory effect of CT on rumen microbial degradability. This finding is consistent with previous studies reported in the literature [12,13].
We observed that the most reactive plant samples, when incubated without PEG, resulted in high PF values (DMD/GP), whereas, in the presence of PEG, the same did not occur. This suggests greater gas production and lower herbage fiber degradation after CT neutralization. Similar results were reported by Bueno et al. (2015) [2], who also recorded high levels of PF during incubation of herbage containing CT; those authors reported that, unlike true degradability, if apparent degradability was measured, the losses of washable fractions may result in inaccurate FP values. Therefore, we hypothesize that the high PF values in our assay are a consequence of soluble fraction losses from substrates due to the washing process; the degradation of these substrates was inhibited by CT in the rumen medium. Such effects, therefore, lead us to affirm that PF is not an adequate parameter to measure microbial efficiency in CT-containing herbage trials, leading to incorrect estimates.
Because of CT–protein complexation, incubation without PEG resulted in lower concentrations of ruminal ammonia. The relatively low values of N-NH3 in D. ovalifolium, D. paniculatum, L. procumbens, F. macrophylla, and L. leucocephala incubations are probably due to inhibition of amino-acid deamination by ruminal microorganisms. The decrease in N-NH3 concentrations obtained in incubations with tannin-containing plants is a phenomenon of CT previously reported in in vitro assays [14,15]. This was also observed in our in vivo studies (Fagundes et al., unpublished data), in which goats fed Flemingia had decreased ammonia values compared to those fed a diet with PEG inclusion.
Although the most pronounced reduction in the presence of tannins constituted isoacid concentrations, in the absence of PEG, fermentation of D. ovalifolium, D. paniculatum, F. macrophylla, and L. procumbens resulted in a decrease in butyrate levels. In two samples, D. paniculatum and L. procumbens, a decrease in acetate concentration was also observed. Condensed tannins can alter ruminal microbiota profile and, depending on their structure, molecular weight, and type of binding to macromolecules, they can selectively affect specific rumen bacteria and consequently alter the metabolic processes of short-chain fatty acids in the rumen [16]. Several authors [15,17] found similar results.
As expected, compared with legume samples, the control Tifton, due to its higher fiber content, had the greatest potential gas production (A). Similar to the alfalfa (Medicago sativa) control, the least reactive species (S. guianensis, M. axilare, and G. sepium) had greater values of A. When samples were incubated with PEG, the increase in potential gas production (A) was greater in almost all plants, indicating inhibition of substrate degradation by CT. The relative decrease in potential gas production (A) in D. paniculatum, L. procumbens, and F. macrophylla in the absence of PEG reflects their superior reactivity compared to other plants. Likewise, in relation to the France (1993) [10] model, tannins from D. procumbens, L. procumbens, and F. macrophylla had decreased asymptotic time (T/2). Furthermore, CT resulted in a greater time of colonization (L) only in L. procumbens; this was probably because highly bioactive CT affected the microbial fermentation of soluble and readily available fractions in the rumen. This agrees with the reports of Baba et al. (2002) [12], which concluded that the increase in gas production not accompanied by a proportional increase in substrate degradability in PEG incubations reflects an improvement in degradation of both soluble and insoluble fractions.
Tannins also influenced fermentation rate and gas production after 96 h of incubation. In the absence of PEG, the greatest reductions in the gas production curve were recorded in D. paniculatum, F. macrophylla, and L. procumbens. Compared with non-PEG treatment, PEG addition during incubation of D. ovalifolium and F. macrophylla resulted in greater fermentation rates (μ). Studies by Kamalac et al. (2005) [18] and Calabrò et al. (2012) [19] also reported an increase in GP content with the addition of PEG.
4. Materials and Methods
4.1. Tannin-Rich Plants
Herbage material was collected from tropical (Flemingia macrophylla, Leucaena leucocephala, Stylosanthes guianensis, Gliricidia sepium, Cratylia argentea, Cajanus cajan, Desmodium ovalifolium, and Macrotiloma axilare) and temperate (Desmodium paniculatum and Lespedeza procumbens) CT-rich species. Legumes from tropical environments were obtained at the Embrapa Agrobiologia and Universidade Federal Rural of Rio de Janeiro in southeastern Brazil, in the municipality of Seropédica, Rio de Janeiro Brazil (22°44′38″ south (S); 43°42′28″ west (W); altitude 33 m). The temperate plants were obtained at the Texas AgriLife Research Center, Stephenville TX, United States of America (USA) (32°13′13″ north (N); 98°12′49″ W; 388 m altitude). Hay of Tifton 85 grass (Cynodon sp.) and alfalfa (Medicago sativa) harvested at Stephenville TX USA were used as controls because they contain little CT.
The herbaceous legumes were harvested close to the ground and assayed as whole plants. Leaves and tender shoots from trees and shrubs were hand-clipped to a height of 1.00 m, from 0.5 × 0.5 m quadrants at five different points. The samples obtained were mixed by plot, subsampled, dried at 40 °C (to avoid inactivating CT) in forced ventilation, and ground to pass a 1 mm sieve for all assays except phenols which were assayed on samples that passed through a 0.25 mm sieve.
4.2. Chemical Analysis
All dried samples were analyzed for dry matter (DM) (ID 930.15), mineral matter (MM) (ID 942.05) by combustion at 450 °C, crude protein (CP) (ID 954.01) by micro-Kjeldhal method using a conversion factor of 6.25, and ether extract (EE) (ID 920.39) by exhaustive extraction with ether [20], while neutral-detergent fiber (NDF-NDF; ID 973.18) and acid-detergent fiber (ADF) were determined according to Mertens (2002) [21], sequentially with the addition of α-amylase and inclusion of residual ash. Acid detergent lignin (ADL) was determined by solubilization of cellulose with sulfuric acid as described by Robertson and Van Soest (1981) [22]. Total phenol (TP) concentrations were quantified by the Folin–Ciocalteu reagent method [23], while total tannins (TTs) were estimated as the difference in TP concentration before and after treatment with insoluble polyvinylpolypirrolidone [24], using tannic acid as the standard. Concentrations of extractable, protein-bound, fiber-bound, and total CT were determined using the HCl–butanol method based on photospectrometric measurements as described by Terrill et al. (1992) [25]. Species-specific standards were created for each plant species analyzed [26] using CT extracts purified on Sephadex LH-20 and lyophilized to recover purified CT. All compositional data were determined in duplicate and are reported on a DM basis. The chemical compositions of the plants are presented in Table 4.
Table 4.
Chemical composition of substrates used in the in vitro bioassay (g/kg dry matter (DM)).
| Plant | CP | EE | NDF | ADF | ADL | TP (1) | TT (1) | CT (2) |
|---|---|---|---|---|---|---|---|---|
| Cajanus cajan | 200 | 38 | 552 | 438 | 191 | 50 | 39 | 34 |
| Cynodon spp (Tifton 85) | 138 | 13 | 743 | 306 | 133 | n.d §. | n.d | n.d |
| Cratylia argentea | 195 | 18 | 577 | 418 | 164 | 36 | 21 | 1.0 |
| Desmodium ovalifolium | 112 | 12 | 614 | 490 | 176 | 237 | 223 | 164 |
| Desmodium paniculatum | 162 | 20 | 515 | 414 | 216 | 171 | 154 | 70 |
| Flemingia macrophylla | 189 | 16 | 577 | 465 | 229 | 181 | 168 | 109 |
| Gliricidia sepium | 281 | 23 | 441 | 277 | 130 | 45 | 28 | 0.3 |
| Lespedeza procumbens | 141 | 20 | 475 | 349 | 161 | 108 | 87 | 198 |
| Leucaena leucocephala | 263 | 22 | 444 | 271 | 136 | 258 | 229 | 48 |
| Medicago sativa | 271 | 84 | 448 | 245 | 127 | 14 | 8 | 0.2 |
| Macrotiloma axilare | 208 | 22 | 523 | 377 | 105 | 30 | 24 | 1.2 |
| Stylosanthes guianensis | 212 | 31 | 509 | 350 | 95 | 63 | 41 | 6.4 |
DM = dry matter; CP = crude protein; EE = ether extract; NDF = neutral-detergent fiber; ADF = acid-detergent fiber; ADL = acid-detergent lignin; TP = total phenols; TT = total tannins; CT = condensed tannins. (1) Expressed as equivalent (eq.) g tannic acid/kg DM; (2) expressed as eq. g leucocyanidin/kg DM; § n.d.: not detected.
4.3. Animals and Inoculum Preparation
All methods and animal care were performed in accordance with the relevant guidelines and regulations of the Ethic Committee on Animal Use of the School of Animal Science and Food Engineering, (São Paulo University) (protocol number CEUA 2416120916).
Ruminal contents from four adult rumen-cannulated Nellore cattle grazing a tropical grass pasture were collected individually before the morning feeding. The solid phase was collected manually and stored in heated boxes at 39 °C, while the liquid phase was collected by using a vacuum pump and stored in pre-warmed thermal bottles, previously flushed with CO2. One inoculum was prepared for each donor animal (blocks). For each inoculum, the phases were homogenized in a 1:1 ratio in a blender for 10 s and filtered through three layers of cotton cloth, according to Bueno et al. (2005) [27]. The inocula were constantly saturated with CO2 and held in a water bath (39 °C) until use.
4.4. In Vitro Gas Production Assay
The effect of tannin-rich plants on fermentation kinetics was obtained using a pressure transducer according to the semi-automatic in vitro gas production technique proposed by Theodorou et al. (1994) [28] and modified by Mauricio et al. (1999) [29]. Two sub-samples of each plant (0.5 g) were placed into 160mL glass bottles in duplicate, combined or not with a CT-binding agent (polyethylene glycol; PEG 6000, Synth, Diadema, Brazil) to neutralize CT. Then, 0.5 g of PEG was added to the sample directly into the fermentation flasks. Four flasks were prepared for each sample: two with no PEG and two with PEG. The inoculation was performed by injecting 25 mL of inoculum in 50 mL of buffered mineral solution (Menke’s buffered medium) [30] into each fermentation flask. Blanks were used for each inoculum to measure the fraction of total gas production due to substrate in inocula, and these values were subtracted from the total to obtain net GP. The flasks were sealed with butyl rubber septum stoppers, manually shaken, and kept in a forced-ventilation oven at 39 °C for 96 h. All legume samples were incubated simultaneously in the run. After 3, 6, 9, 12, 18, 24, 30, 34, 48, 60, 72, and 96 h, the pressure inside each bottle was measured using a transducer (Pressure Press Data 800; Piracicaba, SP, Brazil), and those values were used to estimate the gas volume produced. The values obtained were transformed into volume by the following equation defined for the test laboratory conditions: V = p × 4.6788; where V = gas volume (mL) and p = pressure (psi). At the end of the incubation (96 h), the bottle was opened and samples of the ruminal liquid contained in each glass bottle were collected for determination of short-chain fatty acids and ammoniacal N (N-NH3). The remaining material was filtered in crucibles to determine in vitro apparent DM degradability (IVDMD) and in vitro apparent organic matter degradability (IVOMD). The partition factor (PF) was determined by the ratio between the dry matter degradation (DMD; mg) and gas production (GP; mL), according to Blümmel et al. (1997) [31].
Gas production data were used to determine the fermentation kinetics based on the model of France et al. (1993) [10].
| (1) |
| (2) |
where y = cumulate gas production (mL/g DM) in incubation time t (h), A = potential gas production (mL/g DM), L = lag time (h), b and c are constants of the model, T/2 = time to half-asymptote (h), and μ = fractional rate of gas production (h−1). The kinetic parameters (A, L, μ, and T/2) were compared in the statistical analysis.
4.5. Short-Chain Fatty Acid Determination
The short-chain fatty acid profiles in ruminal liquid were determined by gas chromatography (GC-2014, Shimadzu®, Tokyo, Japan) as described by Bueno et al. (2020) [32]. Ruminal samples were centrifuged at 14,500× g for 10 min, and the supernatant (800 µL) was transferred to a flask with 200 µL of formic acid (98–100%) and 100 µL of the internal standard (100 mM 2-ethyl butyric acid, Chem service, USA). Acetic, propionic, isobutyric, butyric, isovaleric, and valeric acids (99.5% purity, Chem service, USA) were used as quantitative external standards. The following chromatographic conditions were employed throughout this study: injector and detector temperatures, 250 °C; hydrogen flow to the flame jet at 60 kPa; helium carrier gas at 8.01 mL/min; synthetic air at 40 kPa.
4.6. N-NH3 Determination
N-NH3 concentrations in the ruminal liquid were obtained by distillation (2 mL sample) with 50% potassium hydroxide (KOH) and titration with 0.05 N sulfuric acid (micro-Kjeldahl method), according to the methodology described by Preston (1995) [33].
4.7. Experimental Design and Statistical Analysis
The experimental design included a randomized complete block with factorial design (12 forages with and without PEG) and four repetitions (blocks/inoculum donors), according to the following model:
| Yijk = μ + αi +βj + (ab)ij + bk + eijk, | (3) |
where Yijk is the dependent variable, μ is the overall mean, αi = effect of legumes (substrates) (i = 1 to 12), βj = effect of PEG addition or not (j = 1 to 2), (ab)ij = interaction of substrates and PEG addition, bk = effect of ruminal inoculum (k = 1 to 4), and eijk = random variation.
Gas production differences were compared using the non-linear procedure of SAS 9.2 Program (Institute Inc., Cary, NC, USA) [34]. Data were subjected to analysis of variance followed by a Duncan’s test to determine the significance of the difference between treatment means. Unless otherwise noted, probabilities were considered significant at p ≤ 0.05.
5. Conclusions
The neutralization of CT by adding PEG to incubations of herbage containing biologically active CT resulted in increased gas production, fermentation rate, AGCC, and N-NH3, showing that CT clearly affected the in vitro fermentative kinetics of these species in the rumen. Condensed tannins from L. procumbens, D. paniculatum, L. leucocephala, D. ovalifolium, and F. macrophylla were effective in modifying ruminal fermentation, which indicates a promising alternative to ionophores for methane reduction in beef cattle. We suggest, therefore, further research to map the main monomer components of these plant secondary compounds and to document their effects as ruminal manipulators.
Acknowledgments
The authors would like to thank the São Paulo Research Foundation FAPESP (São Paulo, Brazil) for scholarships (FAPESP Process 2014/34656-1) to G.M.F. and Seta® (Estância Velha, Brazil) for supplying the tannin extract.
Author Contributions
Designed the study, G.M.F.; performed the experiment and collected the samples, G.M.F., G.B., K.C.W., and F.A.M.; performed the laboratory analyses, G.M.F., G.B., K.C.S., and J.P.M.; analyzed and interpreted the data, G.M.F. and I.C.S.B.; wrote the manuscript, G.M.F. All authors read and approved the final manuscript.
Funding
This research was funded by National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico -CNPq). Universal Notice MCTI/CNPq grant number 405776/2016-0.
Conflicts of Interest
The authors (G.M. Fagundes, G. Benetel, K.C. Welter, F.A. Melo, J.P. Muir, and I.C.S. Bueno) have no financial or personal relationships with other people or organizations that could inappropriately influence or bias the research.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Valle C.B., Jank L., Resende R.M.S. O melhoramento de forrageiras tropicais no Brasil. Rev Ceres. 2009;56:460–472. [Google Scholar]
- 2.Bueno I.C.S., Brandi R.A., Franzolin R., Fagundes G.M., Benetel G., Abdalla A.L., Louvandini H., Muir J.P. Comparative in vitro methane production and antimethanogenic effect of tannins in five ruminant species. Anim. Feed Sci. Tech. 2015;205:1–9. doi: 10.1016/j.anifeedsci.2015.03.008. [DOI] [Google Scholar]
- 3.Fagundes G.M., Benetel G., Welter K.C., Melo F.A., Muir J.P., Carriero M.M., Souza R.L.M., Meo-Filho P., Frighetto R.T.S., Berndt A., et al. Tannin as a natural rumen modifier to control methanogenesis in beef cattle in tropical systems: Friend or foe to biogas energy production? Res. Vet. Sci. 2020;132:82–93. doi: 10.1016/j.rvsc.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Tedeschi L.O.C.A., Ramírez-Restrepo C.A., Muir J.P. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Anim. 2014;8:1095–1105. doi: 10.1017/S1751731114000974. [DOI] [PubMed] [Google Scholar]
- 5.Norris A.B., Crossland W.L., Tedeschi L.O., Foster J.L., Muir J.P., Pinchak W.E., Fonseca M.A. Inclusion of quebracho tannin extract in a high-roughage cattle diet alters digestibility, nitrogen balance, and energy partitioning. J. Anim. Sci. 2020 doi: 10.1093/jas/skaa047. (at press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagundes G.M., Modesto E.C., Fonseca C.E.M., Lima H.R.P., Muir J.P. Intake, digestibility and milk yield in goats fed Flemingia macrophylla with or without polyethylene glycol. Small Rumin. Res. 2014;116:88–93. doi: 10.1016/j.smallrumres.2013.10.018. [DOI] [Google Scholar]
- 7.Muir J.P., Tedeschi L.O., Dubeux J.C.B., Jr., Peters M., Burkart S. Enhancing food security in Latin America with forage legumes. Archivos Latinoamericanos Producción Anim. 2017;25:113–131. [Google Scholar]
- 8.Santos K.C., Magalhães A.L.R., Silva D.K.A., Araújo G.G.L., Fagundes G.M., Ybarra N.G., Abdalla A.L. Nutritional potential of forage species found in Brazilian Semiarid region. Livest. Sci. 2017;195:118–124. doi: 10.1016/j.livsci.2016.12.002. [DOI] [Google Scholar]
- 9.Naumann H.D., Armstrong S.A., Lambert B.D., Muir J.P., Tedeschi L.O., Kothmann M.M. Effect of molecular weight and concentration of legume condensed tannins on in vitro larval migration inhibition of Haemonchus contortus. J. Vet. Parasitol. 2014;199:93–98. doi: 10.1016/j.vetpar.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 10.France J., Dhanoa M.S., Theodorou M.K., Lister S.J., Davies D.R., Isac D. A model to interpret gas accumulation profiles associated with in vitro degradation of ruminant feeds. J. Theor. Biol. 1993;163:99–111. doi: 10.1006/jtbi.1993.1109. [DOI] [Google Scholar]
- 11.Bueno I.C.S., Vitti D.M.S.S., Louvandini H., Abdalla A.L. A new approach for in vitro bioassay to measure tannin biological effects based on a gas production technique. Anim. Feed Sci. Tech. 2008;141:153–170. doi: 10.1016/j.anifeedsci.2007.04.011. [DOI] [Google Scholar]
- 12.Baba A.S.H., Castro F.B., Ørskov E.R. Partitioning of energy and degradability of browse plants in vitro and the implications of blocking the effects of tannin by the addition of polyethylene glycol. Anim. Feed Sci. Technol. 2002;95:93–104. doi: 10.1016/S0377-8401(01)00283-8. [DOI] [Google Scholar]
- 13.Gemeda B.S., Hassen A. Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical browse plants. Asian Australas. J. Anim. Sci. 2015;28:188–199. doi: 10.5713/ajas.14.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatta R., Uyeno Y., Tajima K., Takenaka A., Yabumoto Y., Nonaka I., Kurihara M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009;92:5512–5522. doi: 10.3168/jds.2008-1441. [DOI] [PubMed] [Google Scholar]
- 15.Bhatta R., Baruah L., Saravanan M., Suresh K.P., Sampath K.T. Effect of medicinal and aromatic plants on rumen fermentation, protozoa population and methanogenesis in vitro. J. Anim. Physiol. Anim. Nutr. 2013;97:446–456. doi: 10.1111/j.1439-0396.2012.01285.x. [DOI] [PubMed] [Google Scholar]
- 16.Patra A.K., Saxena J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011;91:24–37. doi: 10.1002/jsfa.4152. [DOI] [PubMed] [Google Scholar]
- 17.Frutos P., Hervas G., Giraldez F.J., Mantecon A.R. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2004;2:191–202. doi: 10.5424/sjar/2004022-73. [DOI] [Google Scholar]
- 18.Kamalak A.D.E.M., Canbolat O., Sahin M., Gurbuz Y., Ozkose E., Ozkan C.O. The effect of polyethylene glycol (PEG 8000) supplementation on in vitro gas production kinetics of leaves from tannin containing trees. S. Afr. J. Anim. Sci. 2005;35:229. doi: 10.4314/sajas.v35i4.3964. [DOI] [Google Scholar]
- 19.Calabrò S., Guglielmelli A., Iannaccone F., Danieli P.P., Tudisco R., Ruggiero C., Piccolo G., Cutrignell M.I., Infascelli F. Fermentation kinetics of sainfoin hay with and without PEG. J. Anim. Physiol. Anim. Nutr. 2012;96:842–848. doi: 10.1111/j.1439-0396.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 20.Association of Official Analytical Chemists (AOAC) Official Methods of Analysis. 16th ed. AOAC; Arlington, VA, USA: 1995. [Google Scholar]
- 21.Mertens D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beaker or crucibles: Collaborative study. J. AOAC Int. 2002;85:1217–1240. [PubMed] [Google Scholar]
- 22.Robertson J.B., Van Soest P.J. The detergent system of analysis. In: James W.P.T., Theander O., editors. The Analysis of Dietary Fibre in Food. Marcel Dekker; New York, NY, USA: 1981. pp. 123–158. [Google Scholar]
- 23.Makkar H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003;49:241–256. doi: 10.1016/S0921-4488(03)00142-1. [DOI] [Google Scholar]
- 24.Makkar H.P.S., Blümmel M., Borowy N.K., Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993;61:161–165. doi: 10.1002/jsfa.2740610205. [DOI] [Google Scholar]
- 25.Terrill T.H., Rowan A.M., Douglas G.B., Barry T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992;58:321–329. doi: 10.1002/jsfa.2740580306. [DOI] [Google Scholar]
- 26.Wolfe R.M., Terrill T.H., Muir J.P. Drying method and origin of standard affect condensed tannin (CT) concentrations in perennial herbaceous legumes using simplified butanol-HCl CT analysis. J. Sci. Food Agric. 2008;88:1060–1067. doi: 10.1002/jsfa.3188. [DOI] [Google Scholar]
- 27.Bueno I.C.S., Cabral Filho S.L.S., Gobbo S.P., Louvandini H., Vitti D.M.S.S., Aballa A.L. Influence of inoculum source in a gas production method. Anim. Feed Sci. Technol. 2005;123–124:96–105. doi: 10.1016/j.anifeedsci.2005.05.003. [DOI] [Google Scholar]
- 28.Theodorou M.K., Williams B.A., Dhanoa M.S., Mcallan A.B., France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Tech. 1994;48:185–197. doi: 10.1016/0377-8401(94)90171-6. [DOI] [Google Scholar]
- 29.Mauricio R.M., Mould F.L., Dhanoa M.S., Owen E., Channa K.S., Theodorou M.K. A semi-automated in vitro gas production technnique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 1999;79:321–330. doi: 10.1016/S0377-8401(99)00033-4. [DOI] [Google Scholar]
- 30.Onodera R., Henderson C. Growth factors of bacterial origin for the culture of the rumen oligotrich protozoon, Entodinium caudatum. J. Appl. Bacteriol. 1980;48:125–134. doi: 10.1111/j.1365-2672.1980.tb05214.x. [DOI] [Google Scholar]
- 31.Blümmel M., Makkar H.P.S., Becker K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997;77:24–34. doi: 10.1111/j.1439-0396.1997.tb00734.x. [DOI] [Google Scholar]
- 32.Bueno I.C.S., Brandi R.A., Fagundes G.M., Benetel G., Muir J.P. The role of condensed tannins in the in vitro rumen fermentation kinetics in ruminant species: Feeding type involved? Animals. 2020;10:635. doi: 10.3390/ani10040635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston T.R. Tropical Animal Feeding: A Manual for Research Workers. FAO; Rome, Italy: 1995. Biological and chemical analytical methods; pp. 191–264. [Google Scholar]
- 34.Sas Institute . The SAS System for Windows. Release 8.01. SAS Institute; Cary, NC, USA: 2000. [Google Scholar]