Abstract
Hypericum L. (Hypericaceae) extracts have been used for their therapeutic effects; however, not much is known about the immunomodulatory activity of essential oils extracted from this plant. We isolated essential oils from the flowers and leaves of H. perforatum and analyzed their chemical composition and innate immunomodulatory activity. Analysis of flower (HEOFl) versus leaf (HEOLv) essential oils using gas chromatography–mass spectrometry revealed that HEOFl was comprised mainly of monoterpenes (52.8%), with an abundance of oxygenated monoterpenes, including cis-p-menth-3-en-1,2-diol (9.1%), α-terpineol (6.1%), terpinen-4-ol (7.4%), and limonen-4-ol (3.2%), whereas the sesquiterpenes were found in trace amounts. In contrast, HEOLv was primarily composed of sesquiterpenes (63.2%), including germacrene D (25.7%) and β-caryophyllene (9.5%). HEOLv also contained oxygenated monoterpenes, including terpinen-4-ol (2.6%), while monoterpene hydrocarbons were found in trace amounts. Both HEOFl and HEOLv inhibited neutrophil Ca2+ mobilization, chemotaxis, and reactive oxygen species (ROS) production, with HEOLv being much more active than HEOFl. Furthermore, the pure sesquiterpenes germacrene D, β-caryophyllene, and α-humulene also inhibited these neutrophil responses, suggesting that these compounds represented the active components of HEOLv. Although reverse pharmacophore mapping suggested that potential protein targets of germacrene D, β-caryophyllene, bicyclogermacrene, and α-humulene could be PIM1 and mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MAPKAK2), a kinase binding affinity assay did not support this finding, implying that other biological targets are involved. Our results provide a cellular and molecular basis to explain at least part of the beneficial immunotherapeutic properties of the H. perforatum essential oils.
Keywords: Hypericum perforatum, essential oil, calcium flux, neutrophil, chemotaxis, reactive oxygen species, sesquiterpene, anti-inflammatory
1. Introduction
Hypericum L. (Hypericaceae) is comprised of approximately 500 species that can be found around the world. Various products from Hypericum species have been used as antidepressant, sedative, diuretic, antiphlogistic, analgesic, astringent, and antipyretic remedies in Europe, America, Africa, and Asia [1,2,3,4,5,6,7,8]. One of the most intensively studied medicinal plants from this genus is H. perforatum L. or St. John’s wort, which is a perennial herb that his known for its beneficial pharmacological properties [2,3]. For example, H. perforatum L. has been widely used in many countries in antibacterial, antiviral, anti-inflammatory, antinociceptive, or analgesic remedies [4]. Extracts from this herb have also been reported as a therapeutic remedy for burns, skin wounds, cuts, stomach aches, and ulcers [5]. In addition, H. perforatum extracts have also been reported to have anti-angiogenic, anti-fibroblastic, and antioxidant properties [6,7,8]. The phytochemical profile of H. perforatum includes naphthodianthrones (specifically hypericin and pseudohypericin), hyperforin, proanthocyanins, flavonoids, biflavonoids, xanthones, phenylpropanes, phenolic acids, and volatile constituents [9,10,11]. Hypericum essential oils are rich sources of monoterpenes, sesquiterpenes, and their oxygenated derivatives (reviewed in [9] and Table 1, which has a listing of the more recent H. perforatum essential oil data published after this review).
Essential oils are natural mixtures of terpenes, which have a wide range of pharmacological activities [12]. The chemical composition and biological activity of essential oils can be affected by many factors, including harvesting time and which part of the plant is used for essential oil isolation [13]. Essential oils prepared from various plant species have become increasingly popular in recent decades as complementary and alternative medicines. Thus, analysis of the chemical composition of essential oils from different plant species and subsequent evaluation their biological properties, including immunomodulatory activity, can lead to the discovery of novel immunomodulatory agents that may be useful for therapeutic purposes. Although previous studies have demonstrated that Hypericum essential oils have antimicrobial, anti-proliferative, and antioxidant activities [14,15,16,17,18], the innate immunomodulatory effects of Hypericum essential oils have not been investigated.
The innate immune system is essential for host defense and provides immediate defense against infection. Among the earliest cell types responding to invasion by pathogens are innate immune cells, such as neutrophils and monocyte/macrophages [19]. Neutrophils perform a variety of microbicidal functions, including phagocytosis, chemotaxis, and biochemical destruction of pathogens [20]. Thus, neutrophils represent an ideal pharmacological target for therapeutic development, and a number of small molecules that modulate neutrophil function have been identified [21,22,23]. In addition, numerous natural products, including essential oils, have been evaluated for immunomodulatory activity. For example, we recently analyzed the chemical composition of essential oils from Artemisia kotuchovii Kupr, Ferula akitschkensis B.Fedtsch. ex Koso-Pol., and Ferula iliensis Krasn. ex Korovin and characterized their neutrophil modulatory activity [24,25,26].
Based on the reported therapeutic effects of H. perforatum extracts, we hypothesized that H. perforatum essential oils might have immunomodulatory activity. Thus, we evaluated the chemical composition and neutrophil immunomodulatory activity of essential oils obtained from flowers and leaves of H. perforatum.
2. Materials and Methods
2.1. Plant Material
Hypericum perforatum was collected in 2019 during the flowering and fruiting stages on the south side of Baldy Mountain, Gallatin Valley, Montana, USA (45.7674° N, 110.9438° W) at an elevation of ~1800 m above sea level. Flowers and leaves were air-dried for 7–10 days at room temperature in the dark before hydrodistillation. Botanical identification of the plant material was performed by botanist Robyn A. Klein from Montana State University (Bozeman, MT, USA).
2.2. Materials
Dimethyl sulfoxide (DMSO), N-formyl-Met-Leu-Phe (fMLF), phorbol 12-myristate 13-acetate (PMA), Histopaque 1077, α-terpineol, myrtenol, and γ-terpinene were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Geraniol, germacrene D, α-humulene, and β-caryophyllene were from Cayman Chemicals (Ann Arbor, MI, USA), and (-)-terpinen-4-ol was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). n-Hexane was purchased from Merck (Darmstadt, Germany). Fluo-4AM was purchased from Invitrogen (Carlsbad, CA, USA). L-012 was purchased from Tocris Bioscience (San Diego, CA, USA). Hanks’ balanced salt solution (HBSS; 0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 5.56 mM glucose, and 10 mM HEPES, pH 7.4) was purchased from Life Technologies (Grand Island, NY, USA). HBSS without Ca2+ and Mg2+ is designated as HBSS−; HBSS containing 1.3 mM CaCl2 and 1.0 mM MgSO4 is designated as HBSS+.
2.3. Essential Oil Extraction
Essential oils were obtained by hydrodistillation of dried plant material using a Clevenger apparatus, as previously described [26]. We used conditions accepted by the European Pharmacopoeia (European Directorate for the Quality of Medicines, Council of Europe, Strasbourg, France, 2014) to avoid artifacts. The yield of the essential oil was calculated based on the amount of air-dried plant material used. Stock solutions of the essential oils were prepared in DMSO (10 mg/mL) for biological evaluation and in n-hexane (10% w/v) for gas-chromatographic analysis.
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was performed using an Agilent 5975 GC-MSD system (Agilent Technologies, Santa Clara, CA, USA), as reported previously [27]. An Agilent Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness) was used with He as the carrier gas (0.8 mL/min). The GC oven temperature was kept at 60 °C for 10 min, increased to 220 °C at a rate of 4 °C/min, kept constant at 220 °C for 10 min, and then increased to 240 °C at a rate of 1 °C/min. The split ratio was adjusted to 40:1, and the injector temperature was 250 °C. MS spectra were monitored at 70 eV with a mass range of 35 to 450 m/z.
GC analysis was performed on an Agilent 6890N GC system. To obtain the same elution order as with GC-MS, the line was split for FID and MS detectors, and a single injection was performed using the same column and appropriate operational conditions. The ionization detector (FID) temperature was 300 °C. Essential oil components were identified by co-injection with standards (whenever possible), which were purchased commercially or isolated from natural sources. In addition, compound identities were confirmed by comparison of their mass spectra with those in the Wiley GC/MS Library (Wiley, NY, USA), MassFinder software 4.0 (Dr. Hochmuth Scientific Consulting, Hamburg, Germany), Adams Library, and NIST Library. Confirmation was also achieved using the in-house “Başer Library of Essential Oil Constituents” database, obtained from chromatographic runs of pure compounds performed with the same equipment and conditions. A C8–C40 n-alkane standard solution (Fluka, Buchs, Switzerland) was used to spike the samples for the determination of relative retention indices (RRI). Relative percentage amounts of the separated compounds were calculated from FID chromatograms.
2.5. Isolation of Human Neutrophils
Neutrophils were isolated from blood that was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montana State University (Protocol #MQ041017). Neutrophils were purified from the blood using dextran sedimentation, followed by Histopaque 1077 gradient separation and hypotonic lysis of red blood cells, as described previously [22]. Isolated neutrophils were washed twice and resuspended in HBSS−. Neutrophil preparations were routinely >95% pure, as determined by light microscopy, and >98% viable, as determined by trypan blue exclusion. Neutrophils were obtained from multiple different donors (n = 8); however, the cells from different donors were never pooled during experiments.
2.6. Ca2+ Mobilization Assay
Changes in neutrophil intracellular Ca2+ concentrations ([Ca2+]i) were measured using a FlexStation 3 scanning fluorometer (Molecular Devices, Sunnyvale, CA, USA). Briefly, human neutrophils suspended in HBSS− were loaded with Fluo-4AM at a final concentration of 1.25 μg/mL and incubated for 30 min in the dark at 37 °C. The cells were then washed with HBSS−, resuspended in HBSS+, and aliquoted into the wells of flat-bottom, half-area 96-well black microtiter plates (2 × 105 cells/well). Essential oils or pure compounds diluted in DMSO were added to the wells (final concentration of DMSO was 1%). The samples were preincubated for 10 min, followed by addition of 5 nM fMLF. Changes in fluorescence were monitored (λex = 485 nm, λem = 538 nm) every 5 s for 240 s at room temperature after addition of the test compound/oil. The maximum change in fluorescence, expressed in arbitrary units over baseline, was used to determine the response. Responses were normalized to the response induced by 5 nM fMLF, which was assigned a value of 100%. Curve fitting (at least five or six points) and calculation of median effective concentration values (EC50 or IC50) were performed by nonlinear regression analysis of the dose–response curves generated using Prism 7 (GraphPad Software, Inc., San Diego, CA, USA).
2.7. Chemotaxis Assay
Human neutrophils were resuspended in HBSS+ containing 2% (v/v) heat-inactivated fetal bovine serum (2 × 106 cells/mL), and chemotaxis was analyzed in 96-well ChemoTx chemotaxis chambers (Neuroprobe, Gaithersburg, MD). After preincubation with the indicated concentrations of the test sample (essential oil or pure compound) or DMSO (1% final concentration) for 30 min at room temperature, the cells were added to the upper wells of the ChemoTx chemotaxis chambers. The lower wells were loaded with 30 µL of HBSS+ containing 2% (v/v) fetal bovine serum and the indicated concentrations of test sample, DMSO (negative control), or 1 nM fMLF as a positive control. Neutrophils were allowed to migrate through the 5.0-µm pore polycarbonate membrane filter for 60 min at 37 °C and 5% CO2. The number of migrated cells was determined by measuring ATP in lysates of transmigrated cells using a luminescence-based assay (CellTiter-Glo; Promega, Madison, WI, USA), and luminescence measurements were converted to absolute cell numbers by comparison of the values with standard curves obtained with known numbers of neutrophils. Curve fitting (at least eight to nine points) and calculation of median effective concentration values (IC50) were performed by nonlinear regression analysis of the dose–response curves generated using GraphPad Prism 8.
2.8. ROS Production Assay
ROS production was determined by monitoring L-012-enhanced chemiluminescence, which is a reliable method for detecting superoxide anion (O2−) production [22]. Human neutrophils were resuspended at 2 × 105 cells/mL in HBSS+ supplemented with 40 µM L-012. Cells (100 µL) were aliquoted into wells of 96-well flat-bottomed microtiter plates containing essential oil or compounds at different concentrations (final DMSO concentration of 1%). Cells were preincubated for 10 min, and 200 nM PMA was added to each well to stimulate ROS production. Luminescence was monitored for 120 min (2-min intervals) at 37 °C using a Fluoroskan Ascent FL microtiter plate reader (Thermo Electron, Waltham, MA, USA). The curve of light intensity (in relative luminescence units) was plotted against time, and the area under the curve was calculated as total luminescence. Compound concentrations that inhibited ROS production by 50% of the PMA-induced response (positive control) were determined by graphing the percentage inhibition of ROS production versus the logarithm of concentration of test sample (IC50). Each curve was determined using five to seven concentrations.
2.9. Kinase Kd Determination
Selected sesquiterpenes were submitted for dissociation constant (Kd) determination toward PIM1 and MAPKAPK2 using KINOMEscan [28] (Eurofins Pharma Discovery, San Diego, CA, USA). For dissociation constant Kd determination, a 12-point half-log dilution series (a maximum concentration of 33 µM) was used. Assays were performed in duplicate, and their average mean value is displayed.
2.10. Human Neutrophil Elastase (HNE) Inhibition Assay
Essential oils and individual compounds were dissolved in 100% DMSO at 5 mM stock concentrations. The final concentration of DMSO in the reactions was 1%, and this level of DMSO had no effect on enzyme activity. Sivelestat, a known HNE inhibitor, was used as a positive control. The inhibition assay was performed, as described previously [29]. Briefly, a solution containing 200 mM Tris-HCl (pH 7.5), 0.01% bovine serum albumin, 0.05% Tween-20, and 20 mU/mL of human neutrophil elastase was added to black, flat-bottom 96-well microtiter plates containing different concentrations of test compounds. Reactions were initiated by addition of 25 µM elastase substrate N-methylsuccinyl-Ala-Ala-Pro-Val-7-amino-4-methylcoumarin in a final reaction volume of 100 µL/well. Kinetic measurements were obtained every 30 s for 10 min at 25 °C using a Fluoroskan Ascent FL fluorescence microplate reader (Thermo Electron, MA, USA) with excitation and emission wavelengths at 355 and 460 nm, respectively. The concentration of compound that caused 50% inhibition of the enzymatic reaction (IC50) was calculated by plotting % inhibition versus logarithm of inhibitor concentration.
2.11. Cytotoxicity Assay
Human promyelocytic leukemia HL-60 cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 10 mM HEPES, 100 µg/mL streptomycin, and 100 U/mL penicillin. Cytotoxicity was analyzed with a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA), according to the manufacturer’s protocol. Briefly, HL-60 cells were cultured at a density of 1 × 105 cells/well with different concentrations of essential oil or compound (final concentration of DMSO was 1%) for 30 min or 2 h at 37 °C and 5% CO2. Following treatment, substrate was added to the cells, and the samples were analyzed with a Fluoroskan Ascent FL microplate reader.
2.12. Molecular Modeling
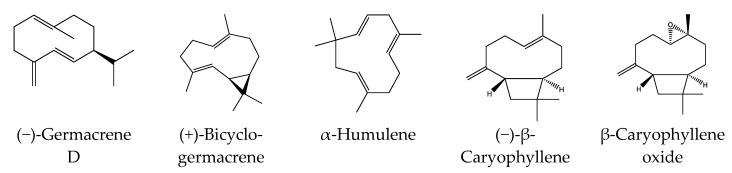
Structures of the main sesquiterpenes found in HEOLv and used for molecular modeling are shown in Figure 1. The protein targets for β-caryophyllene, (−)-germacrene D, (+)-bicyclogermacrene, and α-humulene were analyzed using the PharmMapper Server [30]. This online tool is intended to recognize potential target possibilities for a given small molecule through an “invert” pharmacophore mapping approach. The software uses several built-in reference databases of protein drug targets encoded by sets of pharmacophore points for faster mapping. Initial 3D structures of the investigated compounds were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov) and saved in Tripos MOL2 format. The MOL2 files of (−)-β-caryophyllene, (−)-germacrene D, (+)-bicyclogermacrene, and α-humulene (PubChem compound CIDs: 5281515, 5317570, 5315347, and 5281520, respectively; see Figure 1) were uploaded into the PharmMapper web server. Automatic generation of up to 300 conformers for each compound was switched on. The “Human Protein Targets Only” database containing 2241 targets was selected for pharmacophore mapping. The top 250 potential targets were retrieved and sorted by normalized fit score value. The physicochemical properties of selected compounds were computed using SwissADME (http://www.swissadme.ch) [31].
Figure 1.
Chemical structures of sesquiterpenes (−)-germacrene D, (+)-bicyclogermacrene, α-humulene, (−)-β-caryophyllene, and β-caryophyllene oxide.
3. Results and Discussion
3.1. Essential Oil Composition
Although the chemical composition of H. perforatum essential oils has been reported previously in several publications [9,11,32,33,34,35,36,37,38,39], there is a wide variation in the reported levels of secondary metabolites from different H. perforatum plant samples (see Table 1 for a summary of results from recent studies since 2010). This variability can impact the specific pharmacological activity of essential oils/extracts [40,41]. In addition, few studies have investigated flower and leaf essential oils separately, and there are no publications on the chemical composition of essential oils from H. perforatum collected in the Rocky Mountain region of the United States. Thus, we analyzed the essential oil composition of flowers and leaves from H. perforatum samples collected in this region.
Table 1.
Review of the major volatile constituents of H. perforatum essential oils (2010–2020).
| Location | Major Compound (%) | Ref. |
|---|---|---|
| Greece | α-pinene (7.5); (E)-β-caryophyllene (10.3); germacrene D (5.5); β-selinene (14.7); α-selinene (14.6) | [39] |
| Iran | decane (27.7); menthol (8.9); methyl decanoate (4.6); β-elemene (4.6) | [37] |
| eudesma-4(15),7-dien-1β-ol (8.1–7.5); thymol (7.0–7.2); 1,4-trans-1,7-trans-acorenone (5.2–5.5) | [37] | |
| decane (59.6); dodecane (12.9); ethyl cyclohexane (6.8); 5-methyl nonane (4.7); 3-methyl nonane (4.3) | [36] | |
| 2,6-dimethyl-heptane (6.2–36.1); α-pinene (5.6–26.0); δ-cadinene (0.0–22.6); γ-cadinene (0.0–16.9) | [42] | |
| α-pinene (12.5); β-pinene (8.3); (E)-β-ocimene (4.4); 2-methyl decane (4.0); undecane (7.0); germacrene D (6.9); α-selinene (4.2) | [33] | |
| α-pinene (21.9); nonane (9.8); n-octane (9.1); dodecanol (6.8) | [43] | |
| Serbia | germacrene D (18.6); β-caryophyllene (11.2); 2-methyl octane (9.5); α-pinene (6.5); bicyclogermacrene (5.0); (E)-β-ocimene (4.6) | [34] |
| Syria | β-selinenol (18.1); elemol (12.8); β-elemene (10.7) | [38] |
| Turkey | β-selinene (19.4); bicyclogermacrene (15.3); tetradecene (8.2); α-amorphene (8.1) | [35] |
The extraction yields (v/w) of essential oils obtained from H. perforatum flowers (designated as HEOFl) and leaves (HEOLv) were 0.3% (HEOFl) and 0.3% (HEOLv). The chemical composition of the oils was evaluated using GC-FID and GC/MS simultaneously, and Table 2 and Table 3 summarize the identified compounds, their percentage composition, and their relative retention indices (RRI) (compounds are listed in order of their elution). A total of 94 constituent compounds were identified in the H. perforatum essential oils. Thirty compounds were identified in HEOFl, representing around 71.3% of the total essential oil composition. The main components of HEOFl were 3-methoxy-2,3-dimethylcyclobutene (9.8%), cis-p-menth-3-en-1,2-diol (9.1%), terpinen-4-ol (7.4%), α-terpineol (6.1%), trans-ascaridol glycol (4.6%), 4-hydroxy-4-methyl-cyclohex-2-enone (3.4%), limonen-4-ol (3.2%), p-cymen-8-ol (2.9%), myrtenol (2.7%), and α-pinene (2.2%). Twenty other compounds were present at concentrations <2.0%. Seventy-five compounds were identified in HEOLv, representing around 97.2% of the total essential oil composition. The main components of HEOLv were germacrene D (25.7%), β-caryophyllene (9.5%), terpinen-4-ol (7.4%), sabinene (5.6%), α-pinene (4.9%), β-pinene (4.6%), (E)-β-ocimene (4.2%), bicyclogermacrene (2.5%), δ-cadinene (2.5%), and myrcene (2.1%). Fifty-five other compounds were present at concentrations from 0.1% to <2%. The remaining 10 volatile compounds were identified in trace amounts (<0.1%). Overall, there were significant differences in essential oil composition between H. perforatum flowers and leaves, with the major components of flowers being oxygenated monoterpenes (49.2%) and the main components of the leaves being sesquiterpene hydrocarbons (52.9%), including very high levels of germacrene D (25.7%).
Table 2.
Chemical composition of essential oils obtained from H. perforatum flowers (HEOFl) and leaves (HEOLv) a.
| No | RRI | Compound | Fl | Lv | No | RRI | Compound | Fl | Lv |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 965 | 3-methyl nonane | 0.6 | 48 | 1687 | α-humulene | 1.2 | ||
| 2 | 1032 | α-pinene | 2.2 | 4.9 | 49 | 1690 | cryptone | 0.9 | |
| 3 | 1035 | α-thujene | 1.4 | 50 | 1693 | β-acoradiene | 0.4 | ||
| 4 | 1048 | MBOL | 1.5 | 51 | 1700 | limonen-4-ol | 3.2 | ||
| 5 | 1065 | 2-methyl decane | 0.2 | 52 | 1704 | γ-muurolene | 1.4 | ||
| 6 | 1100 | undecane | 0.2 | 53 | 1706 | α-terpineol | 6.1 | ||
| 7 | 1118 | β-pinene | 0.9 | 4.6 | 54 | 1708 | ledene | 0.3 | |
| 8 | 1132 | sabinene | 5.6 | 55 | 1726 | germacrene D | 25.7 | ||
| 9 | 1174 | myrcene | 2.1 | 56 | 1740 | α-muurolene | 0.8 | ||
| 10 | 1176 | α-phellandrene | t | 57 | 1743 | α-cadinene | 0.3 | ||
| 11 | 1188 | α-terpinene | 0.5 | 58 | 1743 | eremophilene | 1.7 | ||
| 12 | 1203 | limonene | 0.5 | 59 | 1755 | bicyclogermacrene | 2.5 | ||
| 13 | 1218 | β-phellandrene | 1 | 60 | 1758 | (E,E)-α-farnesene | 0.7 | ||
| 14 | 1225 | (Z)-3-hexenal | 0.2 | 61 | 1773 | δ-cadinene | 2.5 | ||
| 15 | 1246 | (Z)-β-ocimene | 0.5 | 62 | 1776 | γ-cadinene | 1.0 | ||
| 16 | 1255 | γ-terpinene | 1.1 | 63 | 1799 | cubenene | 0.1 | ||
| 17 | 1266 | (E)-β-ocimene | 4.2 | 64 | 1804 | myrtenol | 2.7 | ||
| 18 | 1280 | p-cymene | 0.5 | 1.2 | 65 | 1815 | 2,6-dimethyl-3(E),5(Z),7-octatriene-2-ol | 0.1 | |
| 19 | 1290 | terpinolene | 0.3 | 66 | 1830 | 1.5 | |||
| 20 | 1466 | α-cubebene | 0.1 | 67 | 1853 | cis-calamenene | 0.2 | ||
| 21 | 1475 | acetic acid | 1.3 | 68 | 1857 | geraniol | 1.4 | t | |
| 22 | 1493 | α-ylangene | 0.1 | 69 | 1864 | p-cymen-8-ol | 2.9 | 0.1 | |
| 23 | 1495 | bicycloelemene | 0.1 | 70 | 1900 | epi-cubebol | t | ||
| 24 | 1497 | α-copaene | 0.2 | 71 | 1945 | 1,5-epoxy-salvial(4)14-ene | t | ||
| 25 | 1506 | decanal | t | 72 | 1953 | palustrol | 0.4 | ||
| 26 | 1525 | α-funebrene | t | 73 | 1973 | dodecanol | 1.2 | ||
| 27 | 1535 | β-bourbonene | 0.2 | 74 | 2006 | MDCB * | 9.8 | ||
| 28 | 1536 | italicene | t | 75 | 2008 | caryophyllene oxide | 1.2 | ||
| 29 | 1544 | α-gurjunene | 0.5 | 76 | 2050 | (E)-nerolidol | 0.8 | ||
| 30 | 1549 | β-cubebene | t | 77 | 2057 | ledol | 1.2 | ||
| 31 | 1553 | linalool | 1.3 | 0.2 | 78 | 2069 | germacrene D-4β-ol | t | |
| 32 | 1562 | octanol | 1.1 | 79 | 2080 | cubenol | 0.2 | ||
| 33 | 1571 | TMEOL | 0.9 | t | 80 | 2088 | 1-epi-cubenol | 0.2 | |
| 34 | 1586 | pinocarvone | 0.9 | 81 | 2093 | junenol | 0.3 | ||
| 35 | 1587 | β-funebrene | 1.1 | 82 | 2098 | globulol | 0.3 | ||
| 36 | 1589 | β-ylangene | 0.3 | 83 | 2099 | trans-ascaridol glycol * | 4.6 | ||
| 37 | 1602 | MHDO | t | 84 | 2104 | viridiflorol | 0.2 | ||
| 38 | 1608 | β-pinone | 1.3 | 85 | 2115 | HMCH | 3.4 | ||
| 39 | 1608 | β-copaene | 0.4 | 86 | 2144 | spathulenol | 1.6 | 1.9 | |
| 40 | 1611 | terpinen-4-ol | 7.4 | 2.6 | 87 | 2179 | tetradecanol | 0.7 | |
| 41 | 1612 | β-caryophyllene | t | 9.5 | 88 | 2184 | cis-p-menth-3-en-1,2-diol | 9.1 | |
| 42 | 1613 | β-cedrene | 0.4 | 89 | 2187 | T-cadinol | 0.6 | ||
| 43 | 1638 | CMEOL | 1.1 | 90 | 2209 | T-muurolol | 0.8 | ||
| 44 | 1661 | alloaromadendrene | 0.6 | 91 | 2219 | torreyol | 0.2 | ||
| 45 | 1668 | (Z)-β-farnesene | 0.6 | 92 | 2255 | α-cadinol | 1.5 | 1.9 | |
| 46 | 1670 | trans-pinocarveol | 0.6 | 93 | 2260 | alismol | 0.1 | ||
| 47 | 1683 | trans-verbenol | 1.6 | 94 | 2329 | trans-sobrerol * | 1.7 |
a The data are presented as relative % by weight for each component isolated from H. perforatum flowers and leaves. RRI was calculated based on retention of n-alkanes; %, calculated from flame ionization detector data. Trace amounts (t) were present at <0.1%. * Tentatively identified using Wiley and MassFinder mass spectra libraries and published RRI. All other compounds were identified by comparison with co-injected standards. Abbreviations: CMEOL, cis-p-menth-2-en-1-ol; HMCH, 4-hydroxy-4-methyl-cyclohex-2-enone; MBOL, 2-methyl-3-buten-2-ol; MDCB, 3-methoxy-2,3-dimethylcyclobutene; MHDO, 6-methyl-3,5-heptadien-2-one; RRI, relative retention index; TMEOL, trans-p-menth-2-en-1-ol.
Table 3.
Summary of the chemical compositions of HEOFl and HEOLv.
| Total (%) | 71.3 | 97.2 |
|---|---|---|
| Monoterpene hydrocarbons (%) | 3.6 | 27.9 |
| Oxygenated monoterpenes (%) | 49.2 | 3.0 |
| Sesquiterpene hydrocarbons (%) | 0 | 52.9 |
| Oxygenated sesquiterpenes (%) | 3.1 | 10.3 |
| Miscellaneous compounds (%) | 15.4 | 3.1 |
The chemical composition of H. perforatum essential oils obtained from aerial parts of the plant has been shown previously to vary according to the collection period and location of the plants collected [44]. Comparison of the chemical profiles of HEOFl and HEOLv with those reported previously from other locations showed that they all contained common sesquiterpene constituents, such as β-caryophyllene and germacrene D, and indicated similarities with H. perforatum essential oils from Lithuania [45]. For example, the concentrations of β-caryophyllene and caryophyllene oxide in essential oils from leaves were higher than those from flowers, whereas dodecanol, spathulenol, viridiflorol, carotol, and tetradecanol were present in higher quantities in flowers from H. perforatum collected in Lithuania [45,46]. Based on these compounds, H. perforatum essential oils from Lithuania were classified into three chemotypes: β-caryophyllene, caryophyllene oxide, and germacrene D [45,46]. Similarly, analysis of the chemical composition of the essential oils from flower, leaf, and stems of H. perforatum collected in Serbia revealed that the highest concentration of non-terpene compounds was found in the flower and stem essential oils, while high concentrations of sesquiterpenes were characteristic of leaf essential oils [44]. Finally, essential oils isolated from H. perforatum collected in Uzbekistan have been reported to contain β-caryophyllene as their main constituent [47].
3.2. Effect of the Essential Oils and Their Components on Neutrophil Functional Responses
Essential oils and their components have been reported previously to modulate intracellular Ca2+ flux and inhibit cell migration [24,25,26]. We screened Hypericum essential oils for neutrophil immunomodulatory activity and evaluated the effects of HEOFl and HEOLv and selected compounds on neutrophil activation.
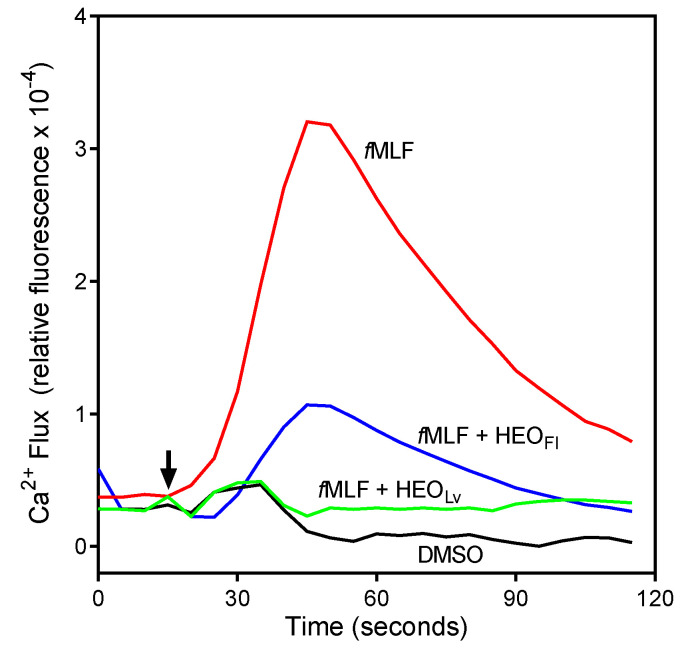
As shown in Table 4, both HEOFl and HEOLv inhibited intracellular Ca2+ flux in fMLF activated neutrophils, although HEOLv was ~5-fold more potent than HEOFl. A representative time course for the inhibition of fMLF-stimulated Ca2+ flux by HEOFl and HEOLv (25 µg/mL each) is shown in Figure 2. We next considered the effects of individual constituent compounds on neutrophil Ca2+ mobilization in an effort to identify the active component(s). Previously, we analyzed the effect of a number of these same compounds on neutrophil Ca2+ flux, including 16 compounds that we found here to comprise 24.0% of HEOFl and 29.6% of HEOLv [24,26]. These data are included in Table 4 for reference. As shown in Table 4, β-pinene, sabinene, and γ-terpinene, which represent 11.3% of HEOLv, were found previously to have no effect on neutrophil Ca2+ mobilization and thus are likely not involved in the inhibitory effects of Hypericum essential oils. In contrast, we found previously that 6-methyl-3,5-heptadien-2-one (MHDO) inhibited neutrophil Ca2+ flux, although it is present in only trace amounts in HEOFl (<1.0%). Thus, it is possible that MHDO contributes to the inhibition observed with HEOFl treatment, but it is more likely that there are other inhibitory compounds in HEOFl. Unfortunately, pure samples of the main compounds in HEOFl, such as 3-methoxy-2,3-dimethylcyclobutene (MDCB, 9.8%), cis-p-menth-3-en-1,2-diol (9.1%), and 4-hydroxy-4-methyl-cyclohex-2-enone (HMCH, 3.4%), are not commercially available for testing. In HEOFl, most of the unidentified compounds are oxygenated constituents, which we could not identify by MS data alone, and their relative amounts were <0.5% except for a few at ~1.0%. Since we identified 71.3% of the HEOFl components, the unidentified active components may also be present in the remaining 28.7% of unknown compounds, and further investigation will be needed to identify these components.
Table 4.
Biological activity of HEOFl and HEOLv and their commercially available constituent compounds in human neutrophils.
| Essential Oil or Pure Compound |
Composition (%) | Ca2+ Flux a | Chemotaxis b | ROS Production c | |
|---|---|---|---|---|---|
| IC50 (μg/mL) | |||||
| HEOFl | 11.3 ± 1.8 | 5.7 ± 1.8 | 9.5 ± 0.9 | ||
| HEOLv | 2.3 ± 0.4 | 5.2 ± 1.1 | 1.2 ± 0.4 | ||
| HEOFl | HEOLv | IC50 (μM) | |||
| Caryophyllene oxide | 0 | 1.2 | N.A. d | N.A. d | N.A. d |
| p-Cymen-8-ol | 2.9 | 0.1 | N.A. d | N.A. d | N.A. d |
| p-Cymene | 0.5 | 1.2 | N.A. d | N.A. d | N.A. d |
| Myrcene | 0 | 2.1 | N.A. d | N.A. d | N.A. d |
| α-Terpinene | 0 | 0.5 | N.A. d | N.A. d | N.A. d |
| Limonene | 0 | 0.5 | N.A. d | N.A. d | N.A. d |
| Myrtenol | 2.7 | 0 | N.A. d | N.A. | N.A. |
| (E/Z)-β-Ocimene | 0 | 4.7 | N.A. d | N.A. d | N.A. d |
| α-Pinene | 2.2 | 4.9 | N.A. d | N.A. d | N.A. d |
| (1S)-(-)-β-Pinene | 0.9 | 4.6 | N.A. d | 22.7 ± 2.6 d | N.A. d |
| (±)-Sabinene | 0 | 5.6 | N.A. d | 37.4 ± 4.3 d | N.A. d |
| (−)-Terpinen-4-ol | 7.4 | 2.6 | N.A. d | N.A. | N.A. |
| γ-Terpinene | 0 | 1.1 | N.A. | 32.5 ± 4.6 d | N.A. |
| α-Terpineol | 6.1 | 0 | N.A. d | N.A. | N.A. |
| Terpinolene | 0 | 0.3 | N.A. d | N.A. d | N.A. d |
| (-)-Linalool | 1.3 | 0.2 | N.A. d | N.A. d | N.A. d |
| MHDO | < 0.1 | 0 | 8.2 ± 2.5 d | 3.6 ± 0.5 d | 2.8 ± 0.4 d |
| Germacrene D | 0 | 25.7 | 0.51 ± 0.08 | 5.4 ± 2.3 | 9.9 ± 1.9 |
| Geraniol | 1.4 | < 0.1 | N.A. | N.A. | 50.1 ± 3.2 |
| α-Humulene | 0 | 1.2 | 0.31 ± 0.06 | 12.0 ± 3.4 | 2.2 ± 0.8 |
| β-Caryophyllene | < 0.1 | 9.5 | 0.33 ± 0.02 | 17.6 ± 5.7 | 2.6 ± 0.9 |
a Inhibition of neutrophil Ca2+ flux induced by 5 nM fMLF. b Inhibition of neutrophil chemotaxis toward 0.5 nM fMLF. c Inhibition of neutrophil ROS production induced by 200 nM PMA. N.A.: no activity was observed, even at the highest concentration tested (50 µM). d Previously reported data [48,49]. IC50 values are presented as the mean ± S.D. of three independent experiments, as described in Section 2.
Figure 2.
Effect of HEOLv and HEOFl on fMLF-induced Ca2+ mobilization in human neutrophils. Human neutrophils were pretreated for 10 min with 25 µg/mL of the indicated essential oil or 1% DMSO (negative control), followed by stimulation with 5 nM fMLF (indicated by arrow), and Ca2+ flux was monitored for the indicated times. The data are from one experiment that is representative of three independent experiments.
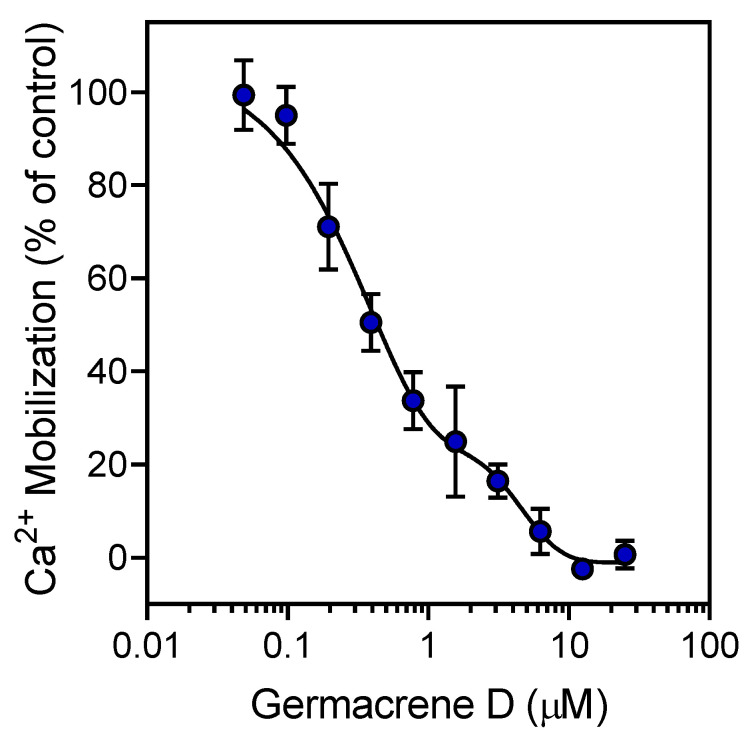
We also evaluated the effect of the sesquiterpenes germacrene D, α-humulene (also known as α-caryophyllene), and β-caryophyllene, which are principal components of HEOLv, and the monoterpenoid geraniol, a minor component of both HEOFl and HEOLv, on neutrophil Ca2+ mobilization induced by fMLF. As shown in Table 4, geraniol had no effect on fMLF-stimulated Ca2+ flux in human neutrophils. In contrast, all three sesquiterpenes potently inhibited fMLF-stimulated Ca2+ mobilization, with IC50 values in the sub-micromolar range. A representative concentration-dependent response for the inhibition of fMLF-induced neutrophil Ca2+ mobilization by germacrene D is shown in Figure 3.
Figure 3.
Inhibition of neutrophil Ca2+ mobilization by germacrene D. Human neutrophils were treated with the indicated concentrations of germacrene D or 1% DMSO (negative control) for 10 min. The cells were activated by 5 nM fMLF, and intracellular Ca2+ flux was monitored as described. The data are presented as the mean ± S.D. (N = 3) from one experiment that is representative of three independent experiments.
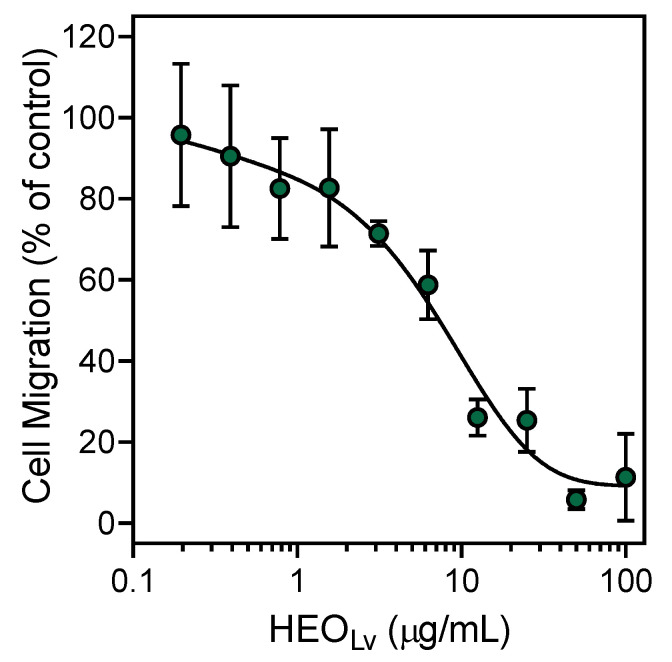
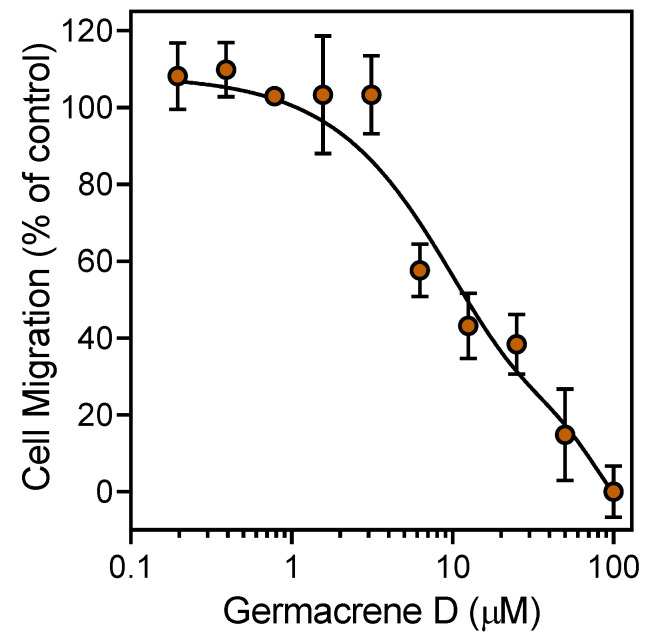
Various essential oils and their components have been reported previously to inhibit cell migration [24,50,51]. We found that pretreatment with HEOFl or HEOLv for 30 min concentration-dependently attenuated fMLF-induced neutrophil chemotaxis with IC50 values of 5.7 and 5.2 µg/mL, respectively (Table 4). In this case, the inhibitory effect of HEOLv was approximately the same as that of HEOFl. A representative concentration-dependent response for the inhibition of neutrophil chemotaxis by HEOLv is shown in Figure 4. In our previous studies [24,26], we found that pretreatment with β-pinene, sabinene, and γ-terpinene inhibited neutrophil migration with IC50 values of 23.9, 39.1, and 32.5 µM, respectively. These active compounds compose 8.3% and 11.3% of HEOFl and HEOLv, respectively. We also tested other commercially available components of the essential oils, including geraniol; myrtenol; terpinen-4-ol; α-terpineol; and the sesquiterpenes germacrene D, α-humulene, and β-caryophyllene, and found that only these three sesquiterpenes inhibited neutrophil migration, whereas the other compounds tested were inactive (Table 4). A representative concentration-dependent response for the inhibition of neutrophil chemotaxis by germacrene D is shown in Figure 5.
Figure 4.
Inhibition of neutrophil chemotaxis by HEOLv. Neutrophil migration toward 1 nM fMLF was measured, as described in Section 2. The data are presented as the mean ± S.D. (N = 3) from one experiment that is representative of two independent experiments.
Figure 5.
Inhibition of neutrophil chemotaxis by germacrene D. Neutrophil migration toward 1 nM fMLF was measured, as described in Section 2. The data are presented as the mean ± S.D. (N = 3) from one experiment that is representative of two independent experiments.
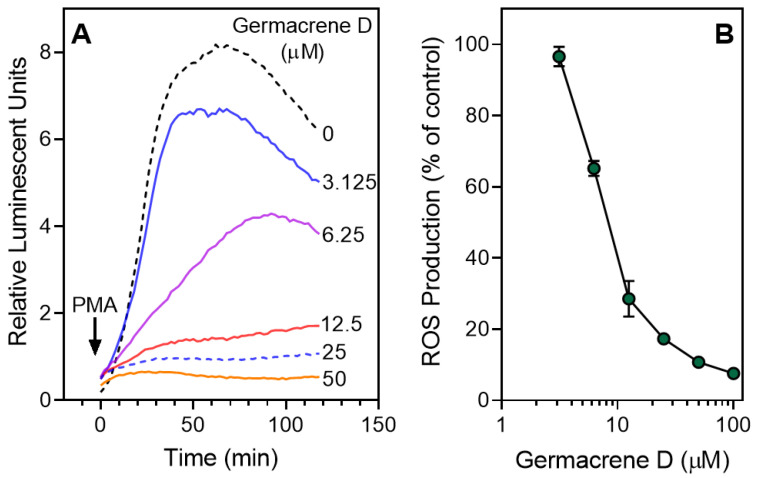
Several essential oils have been reported to modulate ROS production by neutrophils [26,52,53]. Thus, we evaluated the effect of HEOFl and HEOLv on PMA-induced ROS production by human neutrophils and found that, similar to their effects on Ca2+ mobilization and chemotaxis, Hypericum essential oils inhibited ROS production, with HEOLv being ~8-fold more potent than HEOFl. We also evaluated geraniol; myrtenol; terpinen-4-ol; α-terpineol; γ-terpinene; and the sesquiterpenes germacrene D, α-humulene, and β-caryophyllene in the same test-system and found that only the three sesquiterpenes inhibited ROS production in neutrophils, with IC50 values in the micromolar range (Table 3). As examples, representative concentration-dependent responses for inhibition of PMA-induced ROS production in human neutrophils treated by germacrene D are shown in Figure 6. Note also that none of the essential oils, monoterpenes, or sesquiterpenes that we evaluated directly activated ROS production by human neutrophils. Although various essential oils have been reported to modulate ROS production and Ca2+ mobilization in neutrophils [24,26,52,53], this is the first study to report the neutrophil immunomodulatory effects of essential oils isolated from Hypericum species, as well as the selected sesquiterpenes found in HEOLv.
Figure 6.
Inhibition of PMA-stimulated neutrophil ROS production by germacrene D. (A) Neutrophils were treated with 1% DMSO (negative control) or the indicated concentrations of germacrene D. After 10 min of preincubation, the cells were activated with 200 nM PMA (indicated by an arrow), and ROS production was monitored using an L-012-amplified assay system. (B) Relative integrated luminescence (120 min) is shown as the luminescence ratio normalized to background (1% DMSO) and plotted against germacrene D concentrations. The data are presented as the mean ± S.D. (N = 3) from one experiment. For both panels, a representative experiment from three independent experiments is shown.
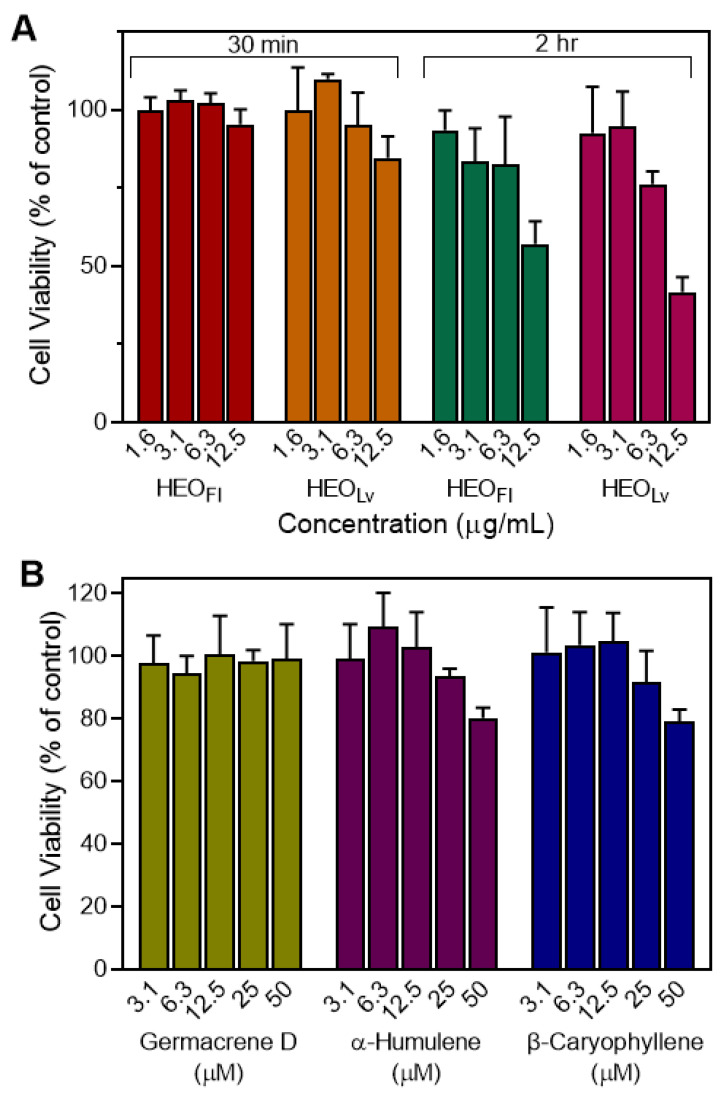
To ensure that the results regarding inhibition of neutrophil functional activity (Ca2+ flux, cell migration, and ROS production) were not significantly influenced by potential toxicity, we evaluated cytotoxicity of HEOFl, HEOLv, germacrene D, α-humulene, and β-caryophyllene at various concentrations in HL-60 cells. As shown in Figure 7A, HEOFl and HEOLv had minimal cytotoxicity during a 30-min incubation. After 2 h, HEOFl and HEOLv exhibited some cytotoxic effects at 12.5 µg/mL. On the other hand, HEOLv demonstrated inhibitory activity in all three cell-based assays, and HEOFl inhibited chemotaxis assay at much lower concentrations (1–5 µg/mL, see Table 4). In addition, the inhibitory effects of HEOFl and HEOLv on neutrophil ROS production were observed during the first 30 min after PMA activation. Thus, it is unlikely that the inhibition of neutrophil responses by Hypericum essential oils was due to cytotoxicity at the concentrations and time periods tested. Furthermore, analysis of the pure sesquiterpenes showed that they had little or no cytotoxicity at all concentrations when tested over a 2 h incubation time (Figure 7B), again indicating that the inhibition of neutrophil responses by germacrene D, α-humulene, and β-caryophyllene was not due to cytotoxicity.
Figure 7.
Cytotoxicity of HEOLv, HEOFl, and selected sesquiterpenes. HL-60 cells were preincubated with HEOLv or HEOFl for 30 min and 2 h (A) or with the indicated pure compounds for 2 h (B), and cell viability was analyzed as described. The data are presented as the mean ± S.D. (N = 3) from one experiment that is representative of two independent experiments.
Although some essential oils and their components were previously identified as HNE inhibitors [48,49], evaluation of HEOFl; HEOLv; and the sesquiterpenes germacrene D, α-humulene, and β-caryophyllene showed that they did not inhibit HNE, even at high tested concentrations (up to 50 μg/mL for the essential oils and 50 μM for the pure sesquiterpenes, data not shown).
Clearly, HEOLv was most potent essential oil in our biological screening. Thus, we focused our biological evaluation on the major compounds in HEOLv that had not been investigated previously (i.e., the sesquiterpene compounds). Germacrene D, β-caryophyllene, α-humulene, and bicyclogermacrene are the main sesquiterpenes, representing 38.9% of HEOLv. Although the germacrene D receptor was identified in neuronal cells of insects [54], biological activity of this compound in mammalian cells is completely unknown. In contrast, β-caryophyllene and α-humulene have been investigated in terms of their biological activity. For example, β-caryophyllene is a type 2 cannabinoid (CB2) receptor agonist and has been reported to inhibit α-glucosidase [55,56]. It also has anti-inflammatory activity in vitro and in vivo [57,58,59]. The reported immunomodulatory effects of β-caryophyllene include inhibition of microglial cells, CD4+ and CD8+ T lymphocytes, and expression of proinflammatory cytokines [59]. In addition, β-caryophyllene was reported to exert neuroprotective effects by modulating the expression of inflammatory mediators [60,61]. Furthermore, this sesquiterpene was reported to induce tumor cell apoptosis [62], prevent attachment of monocytic THP-1 cells to endothelial cells in vitro [63], and impair Mycobacterium bovis (BCG)-induced neutrophil accumulation in mouse pleurisy [64]. Although there are no direct data regarding inhibitory effects of this compound on ROS production, the caryophyllene-related sesquiterpenoids rumphellols A and B were reported to inhibit ROS production in human neutrophils [65].
α-Humulene is an isomer of β-caryophyllene, and these two compounds are often present as a mixture in some plants [66,67]. α-Humulene has been reported to inhibit nuclear factor (NF)-κB and activating protein (AP)-1 activity, the expression of P-selectin, and the increased mucus secretion in the lung in experimental airway allergic inflammation [68]. Both α-humulene and β-caryophyllene have been reported to inhibit lipopolysaccharide-induced NF-κB activation and neutrophil migration, although only α-humulene had the ability to prevent the production of the proinflammatory cytokines tumor necrosis factor (TNF) and interleukin (IL)-1β in a model of acute inflammation in rat paw [69]. α-Humulene and β-caryophyllene have also been reported to have acaricidal activities against Dermatophagoides farinae and D. pteronyssinus [70]. Finally, these sesquiterpenes inhibited cytochrome P4503A activity in rats and in human hepatic microsomes in vitro [71]. Thus, it is clear that the sesquiterpene compounds found in H. perforatum essential oils have a number of biological activities, including the neutrophil immunomodulatory activity reported here.
3.3. Identification of Potential Protein Targets for Selected Sesquiterpenes
Although we have not performed enantiomeric investigation of the main active sesquiterpenes of H. perforatum oils, (−)-β-caryophyllene was reported to be the most commonly found form of β-caryophyllene in many essential oils [72]. This enantiomeric form was also reported in essential oils of Hypericum species, including H. perforatum [73]. In nature, germacrene D occurs in two enantiomer forms, although the (−)-enantiomer is the most prevalent one found in higher plants [54,74]. Likewise, the (+)-configuration of bicyclogermacrene is the most common enantiomer in higher plants [75,76]. Thus, we performed reverse pharmacophore mapping on α-humulene and the enantiomeric structures of (−)-β-caryophyllene, (−)-germacrene D, and (+)-bicyclogermacrene to identify their potential biological targets. PharmMapper compared a large database of pharmacophore patterns with our test compounds and generated target information, including normalized fitness scores and pharmacophoric characteristics.
As shown in Table 5, PharmMapper analysis indicated that among the eight top ranked potential targets, three targets were common for all sesquiterpenes and included serum albumin, aldo-keto reductase family 1 member C2 (AKR1C2), and mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MAPKAPK2 or MK2). Bone morphogenetic protein 2 (BMP-2) was a common target for (−)-β-caryophyllene, α-humulene, and bicyclogermacrene. Apolipoprotein A-II (ApoA-II) and kinesin-like protein KIF11 were potential targets for (−)-β-caryophyllene, (−)-germacrene D, and bicyclogermacrene. Steroid sulfatase was a target for (−)-β-caryophyllene, (−)-germacrene D, and α-humulene. Proviral integration Moloney virus kinase (PIM1) and caspase-7 were potential targets for (−)-germacrene D, α-humulene, and bicyclogermacrene. Integrin α-L (CD11a) was a target for (−)-β-caryophyllene, and thyroid hormone receptor β (THRB) was a potential target for α-humulene only.
Table 5.
Identification of potential protein targets of (−)-β-caryophyllene, (−)-germacrene D, α-humulene, and bicyclogermacrene.
| Rank | PDB ID | Target Name | Fit Score | Rank | PDB ID | Target Name | Fit Score |
|---|---|---|---|---|---|---|---|
| (−)-β-Caryophyllene | α-Humulene | ||||||
| 1 | 1XDD | Integrin α-L | 0.998 | 1 | 2P3G | MAPKAPK2 | 0.989 |
| 2 | 1REU | BMP-2 | 0.987 | 2 | 3BMP | BMP-2 | 0.987 |
| 3 | 2P3G | MAPKAPK2 | 0.981 | 3 | 1J96 | AKR1C2 | 0.981 |
| 4 | 1J96 | AKR1C2 | 0.981 | 4 | 1SHJ | Caspase-7 | 0.961 |
| 5 | 1L6L | ApoA-II | 0.957 | 5 | 2O65 | PIM1 | 0.925 |
| 6 | 1P49 | Steroid sulfatase | 0.954 | 6 | 1P49 | Steroid sulfatase | 0.908 |
| 7 | 1E7E | Serum albumin | 0.919 | 7 | 2PIN | THRB | 0.885 |
| 8 | 2PG2 | KIF11 | 0.919 | 8 | 1E7A | Serum albumin | 0.880 |
| ( −)-Germacrene D | Bicyclogermacrene | ||||||
| 1 | 1E7A | Serum albumin | 0.999 | 1 | 1REU | BMP-2 | 0.998 |
| 2 | 1SHJ | Caspase-7 | 0.990 | 2 | 2O65 | PIM1 | 0.993 |
| 3 | 1P49 | Steroid sulfatase | 0.977 | 3 | 1E7E | Serum albumin | 0.991 |
| 4 | 2O65 | PIM1 | 0.969 | 4 | 1J96 | AKR1C2 | 0.990 |
| 5 | 2PG2 | KIF11 | 0.958 | 5 | 2P3G | MAPKAPK2 | 0.988 |
| 6 | 1L6L | ApoA-II | 0.951 | 6 | 2PG2 | KIF11 | 0.920 |
| 7 | 2P3G | MAPKAPK2 | 0.948 | 7 | 1L6L | ApoA-II | 0.913 |
| 8 | 1J96 | AKR1C2 | 0.924 | 8 | 1SHJ | Caspase-7 | 0.913 |
Abbreviations: AKR1C2, aldo-keto reductase family 1 member C2; BMP-2, bone morphogenetic protein 2; KIF11, kinesin-like protein KIF11; MAPKAPK2, mitogen-activated protein kinase-activated protein kinase 2; THRB, thyroid hormone receptor β; ApoA-II, apolipoprotein A-II; PDB ID, the 4-character unique identifier of every entry in the Protein Data Bank.
Among these potential protein targets, only CD11a, MAPKAPK2, and PIM1 could explain the direct inhibitory effect of HEOLv and its primary sesquiterpenes on human neutrophil functional activities, including inhibition of ROS production and chemotaxis. Indeed, neutrophil arrest and migration involves integrin α-L (CD11a) [77]. Upon activation of p38 MAPK, MAPKAPK2 binds to p38 MAPK, leading to phosphorylation of Hsp27, Akt, and Cdc25, which are involved in regulation of various essential cellular functions [78]. In support of this idea, MAPKAPK2−/− neutrophils generated less O2−, and both NADPH-oxidase activation and p47phox phosphorylation were decreased [79]. PIM kinases have been reported to promote cell migration and invasion [80], and participation of PIM1 in p22phox-dependent signaling also was reported [81].
Since MAPKAPK2 and PIM1 could interfere with NADPH-oxidase activation and suppress phagocyte migration and ROS production, we evaluated the binding affinity of pure β-caryophyllene, α-humulene, and germacrene D toward these two kinases using KINOMEscan but did not find any binding activity.
It should be noted that β-caryophyllene oxide was completely inactive in human neutrophils. We also conducted PharmMapper analysis for this compound and found that CD11a (fit score = 0.998) was its best ranked potential protein target. Other potential protein targets for β-caryophyllene oxide are KIF11 (fit score = 0.980), AKR1C2 (0.979), BMP-2 (0.966), MAPKAPK2 (0.965), steroid sulfatase (0.936), caspase-7 (0.930), and PIM1 (0.923). Thus, because CD11a is a potential target for both β-caryophyllene oxide and β-caryophyllene, this protein is unlikely to be a relevant target for β-caryophyllene in human neutrophils.
Using the SwissADME online tool [31], we calculated the most important physicochemical parameters for the sesquiterpenes, including β-caryophyllene oxide (Table 6), and found that the compounds are very similar to each other in terms of many ADME properties. Nevertheless, they differed noticeably in topological polar surface area (tPSA) and Log P. These descriptors are usually related to the capacity of molecules to cross cellular membranes [82]. For example, it was reported earlier that compounds with LogP > 4 and TPSA < 40 Å2 had optimal antimycobacterial activity [83]. Thus, it is possible that the inactivity of β-caryophyllene oxide in human neutrophils could be explained by low cell membrane permeability, as this compound is less lipophilic and more polar than the investigated sesquiterpenes of purely hydrocarbon nature.
Table 6.
Physicochemical properties of (−)-germacrene D, bicyclogermacrene, α-humulene, (−)-β-caryophyllene, and β-caryophyllene oxide.
| Property | Germacrene D | Bicyclo- germacrene |
α-Humulene | β-Caryophyllene | β-Caryophyllene Oxide |
|---|---|---|---|---|---|
| Formula | C15H24 | C15H24 | C15H24 | C15H24 | C15H24O |
| M.W. | 204.35 | 204.35 | 204.35 | 204.35 | 220.35 |
| Heavy atoms | 15 | 15 | 15 | 15 | 16 |
| Fraction Csp3 | 0.60 | 0.73 | 0.60 | 0.73 | 0.87 |
| Rotatable bonds | 1 | 0 | 0 | 0 | 0 |
| H-bond acceptors | 0 | 0 | 0 | 0 | 1 |
| H-bond donors | 0 | 0 | 0 | 0 | 0 |
| MR | 70.68 | 68.78 | 70.42 | 68.78 | 68.27 |
| tPSA | 0.00 | 0.00 | 0.00 | 0.00 | 12.53 |
| Log P | 4.30 | 4.15 | 4.26 | 4.24 | 3.68 |
Abbreviations: M.W., molecular weight (g/mol); MR, molar refractivity; tPSA, topological polar surface area (Å2); Log P, lipophilicity (consensus Log Po/w).
4. Conclusions
We report here that essential oils isolated from leaves of H. perforatum contain a high amount of sesquiterpenes and that these essential oils are potent inhibitors of human neutrophil functional responses. Moreover, the essential oil constituents germacrene D, β-caryophyllene, and α-humulene were also potent inhibitors of fMLF-induced Ca2+ mobilization, chemotaxis, and ROS production by human neutrophils. Thus, our data provide a molecular basis to explain at least part of the beneficial therapeutic effects of essential oils from H. perforatum and suggest that suppression of neutrophils by the essential oil components of this plant might have anti-inflammatory effects. Future studies are now in progress to evaluate the potential of Hypericum essential oils as therapeutic remedies for various disorders with immune and/or inflammatory mechanisms, as well as to determine molecular targets of their active components.
Author Contributions
I.A.S. and M.T.Q. conceived and designed the project. I.A.S., G.Ö., T.Ö., and L.N.K. performed the experiments. A.I.K. conducted molecular modeling. I.A.S., G.Ö., T.Ö., L.N.K., A.I.K., and M.T.Q. analyzed and interpreted the data. I.A.S., G.Ö., A.I.K., and M.T.Q. drafted and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by National Institutes of Health IDeA Program Grants GM115371 and GM103474, USDA National Institute of Food and Agriculture Hatch project 1009546, the Montana State University Agricultural Experiment Station, and the Tomsk Polytechnic University Competitiveness Enhancement Program.
Conflicts of Interest
The authors declare no competing financial interest.
References
- 1.Zhang R., Ji Y., Zhang X., Kennelly E.J., Long C. Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020;254:112686. doi: 10.1016/j.jep.2020.112686. [DOI] [PubMed] [Google Scholar]
- 2.Velingkar V.S., Gupta G.L., Hegde N.B. A current update on phytochemistry, pharmacology and herb-drug interactions of Hypericum perforatum. Phytochem. Rev. 2017;16:725–744. doi: 10.1007/s11101-017-9503-7. [DOI] [Google Scholar]
- 3.Marrelli M., Statti G., Conforti F., Menichini F. New potential pharmaceutical applications of Hypericum Species. Mini-Rev. Med. Chem. 2016;16:710–720. doi: 10.2174/1389557515666150709105844. [DOI] [PubMed] [Google Scholar]
- 4.Galeotti N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017;200:136–146. doi: 10.1016/j.jep.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Dogan S., Gokalsin B., Senkardes I., Dogan A., Sesal N.C. Anti-quorum sensing and anti-biofilm activities of Hypericum perforatum extracts against Pseudomonas aeruginosa. J. Ethnopharmacol. 2019;235:293–300. doi: 10.1016/j.jep.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz U., Kaya H., Turan M., Bir F., Sahin B. Investigation the effect of Hypericum perforatum on corneal alkali burns. Cutan. Ocul. Toxicol. 2019;38:356–359. doi: 10.1080/15569527.2019.1622560. [DOI] [PubMed] [Google Scholar]
- 7.Wise K., Selby-Pham S., Bennett L., Selby-Pham J. Pharmacokinetic properties of phytochemicals in Hypericum perforatum influence efficacy of regulating oxidative stress. Phytomedicine. 2019;59:152763. doi: 10.1016/j.phymed.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Kandilarov I.K., Zlatanova H.I., Georgieva-Kotetarova M.T., Kostadinova I.I., Katsarova M.N., Dimitrova S.Z., Lukanov L.K., Sadakov F. Antidepressant effect and recognition memory improvement of two novel plant extract combinations—Antistress I and anti-stress II on rats subjected to a model of mild chronic stress. Folia Med. 2018;60:110–116. doi: 10.1515/folmed-2017-0073. [DOI] [PubMed] [Google Scholar]
- 9.Crockett S.L. Essential Oil and Volatile Components of the Genus Hypericum (Hypericaceae) Nat. Prod. Commun. 2010;5:1493–1506. doi: 10.1177/1934578X1000500926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hostettmann K., Wolfender J.-L. St. John’s Wort and its active principles in depression and anxiety. In: Müller W.E., editor. Phytochemistry. Springer Science & Business Media; Basel, Switzerland: 2005. pp. 5–20. [Google Scholar]
- 11.Belwal T., Devkota H.P., Singh M.K., Sharma R., Upadhayay S., Joshi C., Pande V. Nonvitamin and Nonmineral Nutritional Supplements. Academic Press; London, UK: 2019. St. John’s Wort (Hypericum perforatum) pp. 415–432. [Google Scholar]
- 12.de Cassia da Silveira E.S.R., Andrade L.N., Dos Reis Barreto de Oliveira R., de Sousa D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules. 2014;19:1459–1480. doi: 10.3390/molecules19021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry N.B., Anderson R.E., Brennan N.J., Douglas M.H., Heaney A.J., McGimpsey J.A., Smallfield B.M. Essential oils from dalmatian sage (Salvia officinalis L.): Variations among individuals, plant parts, seasons, and sites. J. Agric. Food Chem. 1999;47:2048–2054. doi: 10.1021/jf981170m. [DOI] [PubMed] [Google Scholar]
- 14.Marcetic M.D., Milenkovic M.T., Lakusic D.V., Lakusic B.S. Chemical Composition and Antimicrobial Activity of the Essential Oil and Methanol Extract of Hypericum aegypticum subsp. webbii (Spach) N. Robson. Chem. Biodivers. 2016;13:427–436. doi: 10.1002/cbdv.201500119. [DOI] [PubMed] [Google Scholar]
- 15.Zorzetto C., Sanchez-Mateo C.C., Rabanal R.M., Lupidi G., Petrelli D., Vitali L.A., Bramucci M., Quassinti L., Caprioli G., Papa F., et al. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium) Fitoterapia. 2015;100:95–109. doi: 10.1016/j.fitote.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Akhbari M., Batooli H., Mozdianfard M. Comparative study of composition and biological activities of SDE prepared essential oils from flowers and fruits of two Hypericum species from central Iran. Nat. Prod. Res. 2012;26:193–202. doi: 10.1080/14786419.2010.534994. [DOI] [PubMed] [Google Scholar]
- 17.Toker Z., Kizil G., Ozen H.C., Kizil M., Ertekin S. Compositions and antimicrobial activities of the essential oils of two Hypericum species from Turkey. Fitoterapia. 2006;77:57–60. doi: 10.1016/j.fitote.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Maggi F., Cecchini C., Cresci A., Coman M.M., Tirillini B., Sagratini G., Papa F., Vittori S. Chemical composition and antimicrobial activity of the essential oils from several Hypericum taxa (Guttiferae) growing in central Italy (Appennino Umbro-Marchigiano) Chem. Biodivers. 2010;7:447–466. doi: 10.1002/cbdv.200900091. [DOI] [PubMed] [Google Scholar]
- 19.Beutler B. Innate immunity: An overview. Mol. Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher S., Steffy K., Averett D. Masked oral prodrugs of toll-like receptor 7 agonists: A new approach for the treatment of infectious disease. Curr. Opin. Investig. Drugs. 2006;7:702–708. [PubMed] [Google Scholar]
- 22.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol. Pharmacol. 2007;71:1061–1074. doi: 10.1124/mol.106.033100. [DOI] [PubMed] [Google Scholar]
- 23.Reshetnikov V., Hahn J., Maueroder C., Czegley C., Munoz L.E., Herrmann M., Hoffmann M.H., Mokhir A. Chemical tools for targeted amplification of reactive oxygen species in neutrophils. Front. Immunol. 2018;9:1827. doi: 10.3389/fimmu.2018.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schepetkin I.A., Kushnarenko S.V., Ozek G., Kirpotina L.N., Sinharoy P., Utegenova G.A., Abidkulova K.T., Ozek T., Baser K.H., Kovrizhina A.R., et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016;64:7156–7170. doi: 10.1021/acs.jafc.6b03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozek G., Schepetkin I.A., Utegenova G.A., Kirpotina L.N., Andrei S.R., Ozek T., Baser K.H.C., Abidkulova K.T., Kushnarenko S.V., Khlebnikov A.I., et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017;101:1361–1371. doi: 10.1189/jlb.3A1216-518RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schepetkin I.A., Kushnarenko S.V., Ozek G., Kirpotina L.N., Utegenova G.A., Kotukhov Y.A., Danilova A.N., Ozek T., Baser K.H., Quinn M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food Chem. 2015;63:4999–5007. doi: 10.1021/acs.jafc.5b01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozek G., Ishmuratova M., Tabanca N., Radwan M.M., Goger F., Ozek T., Wedge D.E., Becnel J.J., Cutler S.J., Can Baser K.H. One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 2012;35:650–660. doi: 10.1002/jssc.201100950. [DOI] [PubMed] [Google Scholar]
- 28.Karaman M.W., Herrgard S., Treiber D.K., Gallant P., Atteridge C.E., Campbell B.T., Chan K.W., Ciceri P., Davis M.I., Edeen P.T., et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 29.Giovannoni M.P., Cantini N., Crocetti L., Guerrini G., Iacovone A., Schepetkin I.A., Vergelli C., Khlebnikov A.I., Quinn M.T. Further modifications of 1H-pyrrolo[2,3-b]pyridine derivatives as inhibitors of human neutrophil elastase. Drug Dev. Res. 2019;80:617–628. doi: 10.1002/ddr.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X., Ouyang S., Yu B., Liu Y., Huang K., Gong J., Zheng S., Li Z., Li H., Jiang H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010;38:W609–W614. doi: 10.1093/nar/gkq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rancic A., Sokovic M., Vukojevic J., Simic A., Marin P., Duletic-Lausevic S., Djokovic D. Chemical composition and antimicrobial activities of essential oils of Myrrhis odorata (L.) Scop, Hypericum perforatum L. and Helichrysum arenarium (L.) Moench. J. Essent. Oil Res. 2005;17:341–345. doi: 10.1080/10412905.2005.9698925. [DOI] [Google Scholar]
- 33.Ghasemi Pirbalouti A., Fatahi-Vanani M., Craker L., Shirmardi H. Chemical composition and bioactivity of essential oils of Hypericum helianthemoides, Hypericum perforatum and Hypericum scabrum. Pharm. Biol. 2014;52:175–181. doi: 10.3109/13880209.2013.821663. [DOI] [PubMed] [Google Scholar]
- 34.Đorđević A.S. Chemical composition of Hypericum perforatum L. essential oil. Adv. Technol. 2015;4:64–68. [Google Scholar]
- 35.Yüce E. Analysis of the Essential Oils of two Hypericum species (H. lanuginosum var. lanuginosum Lam. and H. perforatum L.) from Turkey. Hacettepe J. Biol. Chem. 2016;44:29–34. doi: 10.15671/HJBC.20164417564. [DOI] [Google Scholar]
- 36.Parchin R.A., Ebadollahi A. Biological activities of Hypericum perforatum L. essential oil against red flour beetle, Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae) J. Entomol. 2016;13:91–97. [Google Scholar]
- 37.Morshedloo M.R., Nabizadeh M., Akramian M., Yazdani D. Characterization of the volatile oil compositions from Hypericum perforatum L. shoot cultures in different basal media. Azarian J. Agric. 2017;4:7–11. [Google Scholar]
- 38.Saleh B. Volatile constituents of three Hypericum (Hypericaceae) species using GC-MS analysis. Int. J. Pharm. Life Sci. 2019;10:6396–6405. [Google Scholar]
- 39.Zeliou K., Koui E.M., Papaioannou C., Koulakiotis N.S., Iatrou G., Tsarbopoulos A., Papasotiropoulos V., Lamari F.N. Metabolomic fingerprinting and genetic discrimination of four Hypericum taxa from Greece. Phytochemistry. 2020;174:112290. doi: 10.1016/j.phytochem.2020.112290. [DOI] [PubMed] [Google Scholar]
- 40.Bergonzi M.C., Bilia A.R., Gallori S., Guerrini D., Vincieri F.F. Variability in the content of the constituents of Hypericum perforatum L. and some commercial extracts. Drug Dev. Ind. Pharm. 2001;27:491–497. doi: 10.1081/DDC-100105173. [DOI] [PubMed] [Google Scholar]
- 41.Cirak C., Bertoli A., Pistelli L., Seyis F. Essential oil composition and variability of Hypericum perforatum from wild populations of northern Turkey. Pharm. Biol. 2010;48:906–914. doi: 10.3109/13880200903311136. [DOI] [PubMed] [Google Scholar]
- 42.Morshedloo M.R., Ebadi A., Maggi F., Fattahi R., Yazdani D., Jafari M. Chemical characterization of the essential oil compositions from Iranian populations of Hypericum perforatum L. Ind. Crop. Prod. 2015;76:565–573. doi: 10.1016/j.indcrop.2015.07.033. [DOI] [Google Scholar]
- 43.Morshedloo M.R., Ebadi A., Fatahi Moghaddam M.R., Yazdani D. Essential oil composition, total phenol compounds and antioxidant activity of Hypericum perforatum L. extract collected from North of Iran. J. Med. Plants. 2012;1:218–226. [Google Scholar]
- 44.Smelcerovic A., Spiteller M., Ligon A.P., Smelcerovic Z., Raabe N. Essential oil composition of Hypericum L. species from Southeastern Serbia and their chemotaxonomy. Biochem. Syst. Ecol. 2007;35:99–113. doi: 10.1016/j.bse.2006.09.012. [DOI] [Google Scholar]
- 45.Radusiene J., Judzentiene A., Bernotiene G. Essential oil composition and variability of Hypericum perforatum L. growing in Lithuania. Biochem. Syst. Ecol. 2005;33:113–124. doi: 10.1016/j.bse.2004.06.010. [DOI] [Google Scholar]
- 46.Mockute D., Bernotiene G., Judzentiene A. The essential oils with dominant germacrene D of Hypericum perforatum L. growing wild in Lithuania. J. Essent. Oil Res. 2008;20:128–131. doi: 10.1080/10412905.2008.9699973. [DOI] [Google Scholar]
- 47.Baser K.H.C., Ozek T., Nuriddinov H.R., Demirci A.B. Essential oils of two Hypericum species from Uzbekistan. Chem. Nat. Compd. 2002;38:54–57. doi: 10.1023/A:1015781715535. [DOI] [Google Scholar]
- 48.Fraternale D., Flamini G., Ascrizzi R. In Vitro anticollagenase and antielastase activities of essential oil of Helichrysum italicum subsp. italicum (Roth) G. Don. J. Med. Food. 2019;22:1041–1046. doi: 10.1089/jmf.2019.0054. [DOI] [PubMed] [Google Scholar]
- 49.Laothaweerungsawat N., Sirithunyalug J., Chaiyana W. Chemical compositions and anti-skin-ageing activities of Origanum vulgare L. essential oil from tropical and Mediterranean region. Molecules. 2020;25:1101. doi: 10.3390/molecules25051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fachini-Queiroz F.C., Kummer R., Estevao-Silva C.F., Carvalho M.D., Cunha J.M., Grespan R., Bersani-Amado C.A., Cuman R.K. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid. Based Complementary Altern. Med. 2012;2012:657026. doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danielli L.J., de Souza T.J.T., Maciel A.J., Ferrao M.F., Fuentefria A.M., Apel M.A. Influence of monoterpenes in biological activities of Nectandra megapotamica (Spreng.) Mez essential oils. Biomolecules. 2019;9:112. doi: 10.3390/biom9030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basile A., Senatore F., Gargano R., Sorbo S., Del Pezzo M., Lavitola A., Ritieni A., Bruno M., Spatuzzi D., Rigano D., et al. Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J. Ethnopharmacol. 2006;107:240–248. doi: 10.1016/j.jep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Cosentino M., Luini A., Bombelli R., Corasaniti M.T., Bagetta G., Marino F. The essential oil of bergamot stimulates reactive oxygen species production in human polymorphonuclear leukocytes. Phytother. Res. 2014;28:1232–1239. doi: 10.1002/ptr.5121. [DOI] [PubMed] [Google Scholar]
- 54.Stranden M., Liblikas I., Konig W.A., Almaas T.J., Borg-Karlson A.K., Mustaparta H. (-)-Germacrene D receptor neurones in three species of heliothine moths: Structure-activity relationships. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003;189:563–577. doi: 10.1007/s00359-003-0434-y. [DOI] [PubMed] [Google Scholar]
- 55.Yan D.W., Huang C.D., Zheng H.H., Zhao N., Feng X.L., Ma S.J., Zhang A.L., Zhang Q. Meroterpene-Like α-glucosidase inhibitors based on biomimetic reactions starting from β-caryophyllene. Molecules. 2020;25:260. doi: 10.3390/molecules25020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gertsch J., Leonti M., Raduner S., Racz I., Chen J.Z., Xie X.Q., Altmann K.H., Karsak M., Zimmer A. β-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA. 2008;105:9099–9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Ascola A., Irrera N., Ettari R., Bitto A., Pallio G., Mannino F., Atteritano M., Campo G.M., Minutoli L., Arcoraci V., et al. Exploiting curcumin synergy with natural products using quantitative analysis of dose-effect relationships in an experimental in vitro model of osteoarthritis. Front. Pharmacol. 2019;10:1347. doi: 10.3389/fphar.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian X., Liu H., Xiang F., Xu L., Dong Z. β-Caryophyllene protects against ischemic stroke by promoting polarization of microglia toward M2 phenotype via the TLR4 pathway. Life Sci. 2019;237:116915. doi: 10.1016/j.lfs.2019.116915. [DOI] [PubMed] [Google Scholar]
- 59.Alberti T.B., Barbosa W.L.R., Vieira J.L.F., Raposo N.R.B., Dutra R.C. (-)-β-Caryophyllene, a CB2 receptor-selective phytocannabinoid, suppresses motor paralysis and neuroinflammation in a murine model of multiple sclerosis. Int. J. Mol. Sci. 2017;18:691. doi: 10.3390/ijms18040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang H.J., Kim J.M., Lee J.C., Kim W.K., Chun H.S. Protective Effect of β-Caryophyllene, a Natural bicyclic sesquiterpene, against cerebral ischemic injury. J. Med. Food. 2013;16:471–480. doi: 10.1089/jmf.2012.2283. [DOI] [PubMed] [Google Scholar]
- 61.Machado K.D.C., Islam M.T., Ali E.S., Rouf R., Uddin S.J., Dev S., Shilpi J.A., Shill M.C., Reza H.M., Das A.K., et al. A systematic review on the neuroprotective perspectives of β-caryophyllene. Phytother. Res. 2018;32:2376–2388. doi: 10.1002/ptr.6199. [DOI] [PubMed] [Google Scholar]
- 62.Amiel E., Ofir R., Dudai N., Soloway E., Rabinsky T., Rachmilevitch S. β-Caryophyllene, a compound isolated from the biblical balm of Gilead (Commiphora gileadensis), is a selective apoptosis inducer for tumor cell lines. Evid. Based Complementary Altern. Med. 2012;2012:872394. doi: 10.1155/2012/872394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z., Yang C.F., Dai X.L., Ao Y., Li Y.M. Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicol. Appl. Pharm. 2017;329:326–333. doi: 10.1016/j.taap.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Andrade-Silva M., Correa L.B., Candea A.L.P., Cavalher-Machado S.C., Barbosa H.S., Rosas E.C., Henriques M.G. The cannabinoid 2 receptor agonist β-caryophyllene modulates the inflammatory reaction induced by Mycobacterium bovis BCG by inhibiting neutrophil migration. Inflamm. Res. 2016;65:869–879. doi: 10.1007/s00011-016-0969-3. [DOI] [PubMed] [Google Scholar]
- 65.Chung H.M., Wang W.H., Hwang T.L., Chen J.J., Fang L.S., Wen Z.H., Wang Y.B., Wu Y.C., Sung P.J. Rumphellols A and B, new caryophyllene sesquiterpenoids from a Formosan gorgonian coral, Rumphella antipathies. Int. J. Mol. Sci. 2014;15:15679–15688. doi: 10.3390/ijms150915679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Legault J., Dahl W., Debiton E., Pichette A., Madelmont J.C. Antitumor activity of balsam fir oil: Production of reactive oxygen species induced by α-humulene as possible mechanism of action. Planta Med. 2003;69:402–407. doi: 10.1055/s-2003-39695. [DOI] [PubMed] [Google Scholar]
- 67.Ouattara Z.A., Boti J.B., Ahibo C.A., Bekro Y.A., Casanova J., Tomi F., Bighelli A. Composition and chemical variability of Ivoirian Polyalthia oliveri leaf oil. Chem. Biodivers. 2016;13:293–298. doi: 10.1002/cbdv.201500066. [DOI] [PubMed] [Google Scholar]
- 68.Rogerio A.P., Andrade E.L., Leite D.F.P., Figueiredo C.P., Calixto J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene α-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009;158:1074–1087. doi: 10.1111/j.1476-5381.2009.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medeiros R., Passos G.F., Vitor C.E., Koepp J., Mazzuco T.L., Pianowski L.F., Campos M.M., Calixto J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007;151:618–627. doi: 10.1038/sj.bjp.0707270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh M.S., Yang J.Y., Kim M.G., Lee H.S. Acaricidal activities of β-caryophyllene oxide and structural analogues derived from Psidium cattleianum oil against house dust mites. Pest Manag. Sci. 2014;70:757–762. doi: 10.1002/ps.3608. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen L.T., Mysliveckova Z., Szotakova B., Spicakova A., Lnenickova K., Ambroz M., Kubicek V., Krasulova K., Anzenbacher P., Skalova L. The inhibitory effects of β-caryophyllene, β-caryophyllene oxide and α-humulene on the activities of the main drug-metabolizing enzymes in rat and human liver in vitro. Chem. Biol. Interact. 2017;278:123–128. doi: 10.1016/j.cbi.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Schipilliti L., Bonaccorsi I., Sciarrone D., Dugo L., Mondello L., Dugo G. Determination of petitgrain oils landmark parameters by using gas chromatography-combustion-isotope ratio mass spectrometry and enantioselective multidimensional gas chromatography. Anal. Bioanal. Chem. 2013;405:679–690. doi: 10.1007/s00216-012-6031-6. [DOI] [PubMed] [Google Scholar]
- 73.Saroglou V., Marin P.D., Rancic A., Veljic M., Skaltsa H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007;35:146–152. doi: 10.1016/j.bse.2006.09.009. [DOI] [Google Scholar]
- 74.Stranden M., Borg-Karlson A.K., Mustaparta H. Receptor neuron discrimination of the germacrene D enantiomers in the moth Helicoverpa armigera. Chem. Senses. 2002;27:143–152. doi: 10.1093/chemse/27.2.143. [DOI] [PubMed] [Google Scholar]
- 75.Nishimura K., Shinoda N., Hirose Y. A New Sesquiterpene, bicyclogermacrene. Tetrahedron Lett. 1969;10:3097–3100. doi: 10.1016/S0040-4039(01)88358-1. [DOI] [Google Scholar]
- 76.Konig W.A., Rieck A., Saritas Y., Hardt I.H., Kubeczka K.H. Sesquiterpene hydrocarbons in the essential oil of Meum athamanticum. Phytochemistry. 1996;42:461–464. doi: 10.1016/0031-9422(96)00006-4. [DOI] [Google Scholar]
- 77.Dixit N., Kim M.H., Rossaint J., Yamayoshi I., Zarbock A., Simon S.I. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J. Immunol. 2012;189:5954–5964. doi: 10.4049/jimmunol.1201638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qian F., Deng J., Wang G., Ye R.D., Christman J.W. Pivotal Role of mitogen-activated protein kinase-activated protein kinase 2 in inflammatory pulmonary diseases. Curr. Protein Pept. Sci. 2016;17:332–342. doi: 10.2174/1389203716666150629121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun L., Wu Q., Nie Y., Cheng N., Wang R., Wang G., Zhang D., He H., Ye R.D., Qian F. A Role for MK2 in enhancing neutrophil-derived ROS production and aggravating liver ischemia/reperfusion injury. Front. Immunol. 2018;9:2610. doi: 10.3389/fimmu.2018.02610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bialopiotrowicz E., Gorniak P., Noyszewska-Kania M., Pula B., Makuch-Lasica H., Nowak G., Bluszcz A., Szydlowski M., Jablonska E., Piechna K., et al. Microenvironment-induced PIM kinases promote CXCR4-triggered mTOR pathway required for chronic lymphocytic leukaemia cell migration. J. Cell. Mol. Med. 2018;22:3548–3559. doi: 10.1111/jcmm.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woolley J.F., Naughton R., Stanicka J., Gough D.R., Bhatt L., Dickinson B.C., Chang C.J., Cotter T.G. H2O2 production downstream of FLT3 is mediated by p22phox in the endoplasmic reticulum and is required for STAT5 signalling. PLoS ONE. 2012;7:e34050. doi: 10.1371/journal.pone.0034050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giner B., Lafuente C., Lapena D., Errazquin D., Lomba L. QSAR study for predicting the ecotoxicity of NADES towards Aliivibrio fischeri. Exploring the use of mixing rules. Ecotoxicol. Environ. Saf. 2020;191:110004. doi: 10.1016/j.ecoenv.2019.110004. [DOI] [PubMed] [Google Scholar]
- 83.Kishk S.M., McLean K.J., Sood S., Smith D., Evans J.W.D., Helal M.A., Gomaa M.S., Salama I., Mostafa S.M., de Carvalho L.P.S., et al. Design and synthesis of imidazole and triazole pyrazoles as Mycobacterium tuberculosis CYP121A1 inhibitors. ChemistryOpen. 2019;8:995–1011. doi: 10.1002/open.201900227. [DOI] [PMC free article] [PubMed] [Google Scholar]