Abstract
Background:
Previous studies on the systemic immune-inflammation index (SII), which is based on platelet, neutrophil and lymphocyte counts, as a prognostic marker in patients with colorectal cancer (CRC) yielded inconsistent results. The aim of this study was to evaluate the prognostic and clinicopathological role of SII in CRC via meta-analysis.
Methods:
A comprehensive literature survey was performed on PubMed, Web of Science, Embase and the Cochrane Library databases to include studies published up to 6 April 2020. Pooled hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CIs) were computed to estimate the prognostic and clinicopathological value of SII in CRC.
Results:
A total of 12 studies published between 2016 and 2019 were included in our meta-analysis. The combined analysis showed that high SII levels were significantly associated with worse overall survival (OS; HR = 1.61, 95% CI = 1.21–2.13, p = 0.001) and progression-free survival (HR = 1.74, 95% CI = 1.26–2.39, p = 0.001) in CRC. Moreover, elevated SII was also correlated with poor tumor differentiation (OR = 1.60, 95% CI = 1.27–2.02, p < 0.001), presence of distant metastasis (OR = 2.27, 95% CI = 1.10–4.67, p = 0.026), ECOG PS of 1–2 (OR = 1.98, 95% CI = 1.39–2.84, p < 0.001) and tumor size ⩾5 cm (OR = 1.49, 95% CI = 1.18–1.88, p = 0.001). However, high SII was not significantly associated with sex, tumor location, lymph node metastasis, or age in patients with CRC.
Conclusion:
Our meta-analysis indicated that high SII levels predicted poor prognosis in CRC. In addition, an elevated SII was also associated with clinical factors, implying higher malignancy of the disease.
Keywords: colorectal cancer, meta-analysis, prognosis, risk factors, systemic immune-inflammation index
Introduction
Colorectal cancer (CRC) accounts for 10.2% of all newly-diagnosed cancers and 9.2% of all cancer-related deaths worldwide annually.1 Despite previous advances in treatment options, CRC prognosis has not improved substantially.2 The 5-year survival rate remains approximately at 90% with early diagnosis, whereas it falls to 13% when the diagnosis is delayed.3 The incorporation of effective CRC biomarkers into therapeutic strategies could markedly improve outcomes for patients with CRC.4 Many prognostic and predictive markers, including RAS mutational status, BRAF mutations and microsatellite instability, have been used to provide implications for the management of CRC. However, these markers require invasive tests and depend on specific laboratory equipment. Therefore, non-invasive, readily-accessible and cost-effective prognostic indexes are urgently needed to predict prognosis and to evaluate the therapeutic effectiveness in clinical practice for patients with CRC.
Previous evidence has shown that chronic inflammation is extensively involved in CRC development and progression.5 Tumor-associated systemic inflammatory responses involve inflammatory cells and a variety of inflammatory mediators.6 A number of studies have focused on peripheral inflammatory cells and calculated ratios as parameters reflecting the status of immune responses in cancer patients.7 These parameters include the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, C-reactive protein levels and systemic immune-inflammation index (SII). SII is defined as the platelet count × neutrophil count/lymphocyte count, and can be easily derived from daily laboratory tests. Many studies have investigated the prognostic role of SII in patients with CRC, yet the results were inconsistent.8–19 For example, SII was proposed as a significant prognostic factor in several studies,9,13 whereas the correlation between SII and survival outcomes in CRC was not significant in other studies.10,14,17 Therefore, we comprehensively searched for relevant studies in the literature and carried out a quantitative meta-analysis to evaluate the potential of SII as a CRC biomarker.
Materials and methods
Search strategy
The current meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.20 We thoroughly searched the PubMed, Web of Science, Embase and Cochrane Library databases for eligible studies published up until 6 April 2020. The following search phrases were used for literature retrieval: (colonic neoplasms OR colorectal neoplasms OR colon cancer OR rectal cancer OR rectal cancers OR rectal tumor OR colorectal cancer OR CRC OR colorectal tumor OR colorectal carcinoma) AND (systemic immune-inflammatory index OR SII OR systemic-immune-inflammation index OR systemic immune-inflammation index). The detailed search strategies for PubMed are shown in the Supplemental material online. We also manually examined the references of the included articles to identify potential inclusions. Since the data used in this study were extracted from previously published literature, no patient consent or ethical approval was necessary.
Inclusion and exclusion criteria
Studies were included, if (1) CRC diagnosis was histopathologically confirmed in patients, (2) hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) of pretreatment SII with survival outcomes including overall survival (OS) and/or progression-free survival (PFS) were reported, or sufficient data were available to calculate them, or the association between SII and clinicopathological features of CRC was reported, (3) a definite cut-off value of pretreatment SII was determined and (4) full-text articles published in English. Following studies were excluded: (1) reviews, editorials, conference abstracts, letters and case reports, (2) duplicate publications, (3) basic research or animal studies and (4) studies without sufficient data.
Data extraction and quality assessment
Two investigators (MD and YS) independently extracted data from eligible studies. Any disagreements were resolved via discussion with a third investigator (XG). The following information was extracted: name of the first author, year of publication, country of origin, sample size, age of patients, sex distribution, study design, recruitment time, histological type, follow-up, tumor stage, treatment methods, cut-off values of SII, cut-off selection method, survival endpoints and HRs with corresponding 95% CIs. OS and PFS were the primary and secondary endpoints of this meta-analysis, respectively. We evaluated the methodological quality of the included studies using the Newcastle–Ottawa Scale (NOS).21 The NOS is based on patient selection, comparability and outcome of interest. NOS scores range from 0 to 9, and studies with scores higher than 6 are regarded high quality.
Statistical analysis
The pooled HRs and 95% CIs were calculated to estimate the association between SII and OS/PFS in CRC. Statistical heterogeneity among studies was evaluated using Cochran’s Q test22 and Higgins I2 statistics.23 If I2 > 50% or p < 0.10 (indicating significant heterogeneity among studies), the data were combined using a random-effects model. Otherwise, a fixed-effects model was applied. Subgroup analyses were performed based on geographical region, treatment, Tumor Node Metastasis (TNM) stage, sample size, cut-off value, cut-off selection method and NOS score to identify the sources of heterogeneity. ORs and 95% CIs were computed to assess the association between SII and clinicopathological characteristics of patients with CRC. OR, as the effect size for association between SII and clinicopathological factors, was expressed along with 95% CI. ORs >1 with 95% CIs that did not overlap with 1 suggested that a high SII increased the trend of that clinical factor, whereas ORs < 1 with 95% CIs that did not overlap with 1 were indicators of the decreased trend of that clinical factor. Sensitivity analysis was also conducted to evaluate the effect of the individual study data on the HRs of OS and PFS. Potential publication bias was examined using Begg’s and Egger’s tests. All statistical calculations were performed using Stata version 12.0 (Stata Corporation, College Station. TX, USA). p < 0.05 (two-sided) was considered statistically significant.
Results
Search results and study characteristics
A total of 65 records were identified from database searches. Duplicate records were removed to yield 41 studies. After reviewing the titles and abstracts, a further 26 of them were excluded. Subsequently, 15 studies were evaluated by full-text reading, and five of them were discarded for the following reasons: four studies lacked necessary data and one study was a review article. Through an updated literature search, two more eligible studies16,19 were identified. Finally, a total of 12 studies8–19 published between 2016 and 2019 were included in our meta-analysis. The literature search flowchart is shown in Figure 1. The 12 eligible studies recruited a total of 3919 patients, with the number of patients in individual studies ranging from 95 to 1383. Eleven studies were conducted in China9–19 and one in Italy.8 Eleven studies included patients with CRC,8–10,12–19 and one study with colon cancer.11 Eleven studies investigated the relationship between SII and OS,8–15,17–19 eight studies reported the association between SII and PFS,8–10,13–18 and nine studies provided data on the correlation of SII and clinicopathological features in CRC.8–13,15,16,18 The cut-off values ranged from 340 to 1505. Eleven studies were retrospective9–19 and one study was a prospective trial.8 The main characteristics of the 12 included studies are summarized in Table 1. The NOS scores of all studies ranged from 6 to 9, which indicates high quality (NOS scores ⩾6).
Figure 1.
Flow diagram of the study selection process.
Table 1.
Major features of included studies in this meta-analysis.
| Author | Year | Country | Histology | Study period | Study design | Sample size | Age, years Median (range) |
Sex M/F |
TNM stage | Treatment | Cut-off value | Cut-off selection | Follow-up, months | Survival analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passardi | 2016 | Italy | CRC | 2007–2012 | Prospective | 289 | 65.5 | 174/115 | I–IV | Chemotherapy+ targeted therapy | 730 | X-tile software | 36 (1–65) | OS, PFS | 9 |
| Chen | 2017 | China | CRC | 1994–2010 | Retrospective | 1383 | NA | 788/595 | I–IV | Surgical resection | 340 | ROC analysis | NA | OS, PFS | 6 |
| Yang | 2017 | China | CRC | 2009–2015 | Retrospective | 95 | 57 | 58/37 | IV | Chemotherapy+ targeted therapy | 460.66 | Median value | 40 (12–72) | OS, PFS | 7 |
| Tao | 2018 | China | Colon cancer | 2011–2013 | Retrospective | 118 | 60 | 63/55 | I–IV | Surgical resection | 667.75 | Median value | 36 | OS | 8 |
| Xie | 2018 | China | CRC | 2009–2014 | Retrospective | 240 | 59 (18–90) | 157/83 | IV | Surgical resection | 649.45 | Median value | 26.7 (1.1–92.4) | OS | 8 |
| Yang | 2018 | China | CRC | 2010–2015 | Retrospective | 98 | 53 (26–83) | 59/39 | I–IV | Neoadjuvant chemoradiotherapy | 437.72 | Median value | 37 (16.2–93.3) | OS, PFS | 7 |
| Zhou | 2018 | China | CRC | 2007–2015 | Retrospective | 516 | 16–87 | 331/185 | I–IV | Surgical resection | 568.69 | ROC analysis | 21.7 (2.1–118.7) | OS, PFS | 8 |
| Jiang | 2019 | China | CRC | 2010–2017 | Retrospective | 102 | 28–75 | 72/30 | IV | Chemotherapy + targeted therapy | 660.55 | ROC analysis | 33.2 (2.6–94.5) | OS, PFS | 7 |
| Lu | 2019 | China | CRC | 2010–2017 | Retrospective | 182 | 53.5 (15–93) | 95/87 | I–II | Surgical resection | 1505 | ROC analysis | NA | NA | 7 |
| Wang | 2019 | China | CRC | 2002–2016 | Retrospective | 452 | 57 | 289/163 | IV | Surgical resection | 517 | X-tile software | 28 | OS, PFS | 8 |
| Yang | 2019 | China | CRC | 2009–2015 | Retrospective | 220 | 57 | 133/87 | III–IV | Adjuvant chemoradiotherapy | 534.94 | ROC analysis | 23.9 (12–87) | OS, PFS | 7 |
| Zhang | 2019 | China | CRC | 2010–2013 | Retrospective | 224 | 67 (30–89) | 127/97 | I–IV | Surgical resection | 642.2 | Median value | 48 | OS | 8 |
CRC, colorectal cancer; F, female; M, male; NA, not available; NOS, Newcastle–Ottawa Scale; OS, overall survival; PFS, progression-free survival; ROC, receiver-operating characteristics curve
Prognostic impact of SII on OS in CRC patients
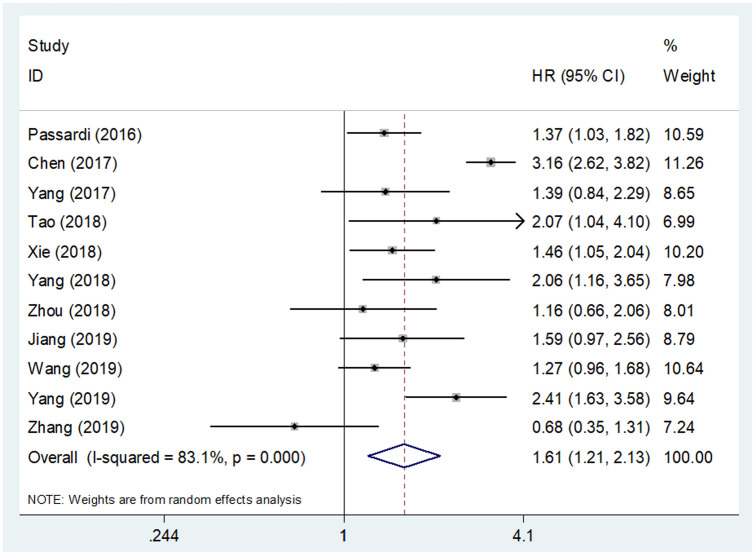
Eleven studies8–15,17–19 provided data from 3737 patients for the OS analysis. A random-effects model was applied due to the significant heterogeneity detected in these data (I2 = 83.1%, p < 0.001, Figure 2 and Table 2). Pooled HR from eligible studies was found to be 1.61, with 95% CI = 1.21–2.13 and p = 0.001 (Figure 2 and Table 2), indicating an association between high SII and poor OS in CRC. Subgroup analysis was also conducted based on the geographical region, treatment procedure, TNM stage, sample size, cut-off value, cut-off selection method and NOS score. High SII was found to be consistently correlated with worse OS irrespective of geographical region, sample size, cut-off value, cut-off selection method or NOS score (Table 2).
Figure 2.
Forest plot of the correlation between systemic immune-inflammation index and overall survival in patients with colorectal cancer.
CI, confidence interval; HR, hazard ratio
Table 2.
Subgroup analysis of pooled HRs and 95% CIs between SII and OS and PFS in colorectal cancer.
| Variables | No. of studies | No. of patients | Effects model | HR (95% CI) | p | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2, % | p | ||||||
| OS | |||||||
| Total | 11 | 3737 | Random | 1.61 (1.21–2.13) | 0.001 | 83.1 | <0.001 |
| Geographical region | |||||||
| China | 10 | 3448 | Random | 1.63 (1.20–2.23) | 0.002 | 83.2 | <0.001 |
| Italy | 1 | 289 | − | 1.37 (1.03–1.82) | 0.030 | − | − |
| Treatment | |||||||
| Surgical resection | 6 | 2933 | Random | 1.50 (0.93–2.42) | 0.097 | 90.0 | <0.001 |
| Chemotherapy + targeted therapy | 3 | 486 | Fixed | 1.42 (1.14–1.76) | 0.002 | 0 | 0.873 |
| Neoadjuvant/adjuvant chemoradiotherapy | 2 | 318 | Fixed | 2.29 (1.66–3.17) | <0.001 | 0 | 0.655 |
| TNM stage | |||||||
| I–IV | 6 | 2628 | Random | 1.61 (0.99–2.61) | 0.056 | 88.1 | <0.001 |
| III–IV | 1 | 220 | − | 2.41 (1.63–3.58) | <0.001 | − | − |
| IV | 4 | 889 | Fixed | 1.38 (1.15–1.66) | <0.001 | 0 | 0.858 |
| Sample size | |||||||
| <230 | 6 | 857 | Random | 1.62 (1.16–2.27) | 0.005 | 58.4 | 0.034 |
| ⩾230 | 5 | 2880 | Random | 1.59 (1.02–2.50) | 0.042 | 91.3 | <0.001 |
| Cut-off value of SII | |||||||
| <550 | 5 | 2248 | Random | 1.96 (1.27–3.04) | 0.002 | 87.6 | <0.001 |
| ⩾550 | 6 | 1489 | Fixed | 1.37 (1.15–1.63) | <0.001 | 24.5 | 0.250 |
| Cut-off selection | |||||||
| X-tile software | 2 | 741 | Fixed | 1.32 (1.08–1.61) | 0.006 | 0 | 0.715 |
| ROC analysis | 4 | 2221 | Random | 2.04 (1.31–3.17) | 0.002 | 81.2 | 0.001 |
| Median value | 5 | 775 | Fixed | 1.45 (1.16–1.80) | 0.001 | 47.7 | 0.105 |
| NOS score | |||||||
| ⩽7 | 6 | 2122 | Random | 1.78 (1.17–2.71) | 0.007 | 83.4 | <0.001 |
| >7 | 5 | 1615 | Fixed | 1.37 (1.17–1.61) | <0.001 | 0 | 0.712 |
| PFS | |||||||
| Total | 8 | 3155 | Random | 1.74 (1.26–2.39) | 0.001 | 84.8 | <0.001 |
| Geographical region | |||||||
| China | 7 | 2866 | Random | 1.87 (1.34–2.61) | <0.001 | 81.6 | <0.001 |
| Italy | 1 | 289 | − | 1.16 (0.90–1.50) | 0.259 | − | − |
| Treatment | |||||||
| Surgical resection | 3 | 2351 | Random | 1.80 (0.99–3.29) | 0.056 | 93.4 | <0.001 |
| Chemotherapy + targeted therapy | 3 | 486 | Fixed | 1.31 (1.07–1.61) | 0.010 | 13.9 | 0.313 |
| Neoadjuvant/adjuvant chemoradiotherapy | 2 | 318 | Fixed | 2.43 (1.53–3.88) | <0.001 | 0 | 0.888 |
| TNM stage | |||||||
| I–IV | 4 | 2286 | Random | 1.90 (1.13–3.21) | 0.016 | 89.9 | <0.001 |
| III–IV | 1 | 220 | − | 2.33 (1.08–5.02) | 0.030 | − | − |
| IV | 2 | 649 | Fixed | 1.34 (1.11–1.61) | 0.002 | 0 | 0.428 |
| Sample size | |||||||
| <230 | 4 | 515 | Fixed | 1.85 (1.41–2.43) | <0.001 | 0 | 0.557 |
| ⩾230 | 4 | 2640 | Random | 1.61 (0.98–2.64) | 0.061 | 93.2 | <0.001 |
| Cut-off value of SII | |||||||
| <550 | 5 | 2248 | Random | 1.94 (1.26–2.99) | 0.003 | 87.4 | <0.001 |
| ⩾550 | 3 | 907 | Fixed | 1.31 (1.06–1.62) | 0.013 | 22.7 | 0.274 |
| Cut-off selection | |||||||
| X-tile software | 2 | 741 | Fixed | 1.20 (1.01–1.42) | 0.034 | 0 | 0.726 |
| ROC analysis | 4 | 2221 | Fixed | 2.49 (2.14–2.91) | <0.001 | 42.3 | 0.158 |
| Median value | 2 | 193 | Fixed | 1.86 (1.30–2.68) | 0.001 | 35.4 | 0.213 |
| NOS score | |||||||
| ⩽7 | 5 | 1898 | Fixed | 2.43 (2.10–2.82) | <0.001 | 46.9 | 0.110 |
| >7 | 3 | 1257 | Fixed | 1.23 (1.05–1.45) | 0.011 | 0 | 0.494 |
CI, confidence interval; HR, hazard ratio; NOS, Newcastle–Ottawa Scale; OS, overall survival; PFS, progression-free survival; ROC, receiver-operating characteristics curve; SII, systemic immune-inflammation index; TNM, Tumor Node Metastasis.
Prognostic role of SII for PFS in CRC
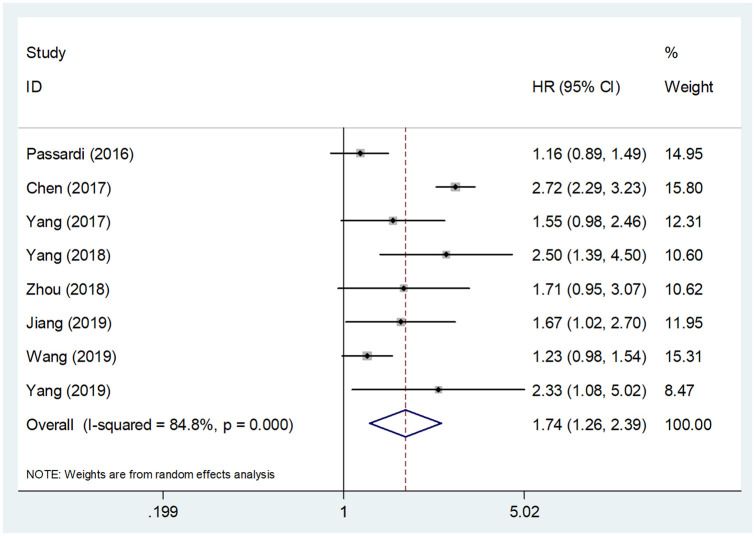
Data for PFS analysis were extracted from eight studies covering 3155 patients.8–10,13–15,17,18 The combined data showed that an elevated SII indicated a worse PFS in CRC (HR = 1.74, 95% CI = 1.26–2.39, p = 0.001) (Figure 3 and Table 2). There was significant heterogeneity among the studies as well (I2 = 84.8%, p < 0.001). Therefore, a random-effects model was adopted. High SII was again associated with poor PFS irrespective of TNM stage, cut-off value, cut-off selection and NOS score. Moreover, high SII also predicted poor PFS in Chinese patients (HR = 1.87, 95% CI = 1.34–2.61, p < 0.001), in patients receiving chemo- and targeted therapy (HR = 1.31, 95% CI = 1.07–1.61, p = 0.010) and in patients receiving neoadjuvant chemoradiotherapy (HR = 2.43, 95% CI = 1.53–3.88, p < 0.001) (Table 2).
Figure 3.
Forest plot of the correlation between systemic immune-inflammation index and progression-free survival in patients with colorectal cancer.
CI, confidence interval; HR, hazard ratio
Correlation between SII and clinicopathological factors in CRC
Nine studies comprising 2727 patients8–13,15,16,18 reported the connection between SII and eight clinicopathological features. The features were as follows: sex (male versus female), tumor differentiation (poor versus moderate/well-differentiated), tumor location (rectum versus colon), distant metastasis (yes versus no), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (1–2 versus 0), lymph node metastasis (yes versus no), age (years) (>60 versus ⩽60) and tumor size (⩾5 cm versus <5 cm) (Table 3 and Figure 4). The pooled results suggested that a high SII prior to treatment was associated with poor tumor differentiation (OR = 1.60, 95% CI = 1.27–2.02, p < 0.001), presence of distant metastasis (OR = 2.27, 95% CI = 1.10–4.67, p = 0.026), ECOG PS of 1–2 (OR = 1.98, 95% CI = 1.39–2.84, p < 0.001) and tumor size ⩾5 cm (OR = 1.49, 95% CI = 1.18–1.88, p = 0.001) (Table 3 and Figure 4). However, the association between high SII and sex (OR = 0.95, 95% CI = 0.80–1.13, p = 0.592), tumor location (OR = 0.76, 95% CI = 0.58–1.03, p = 0.081), lymph node metastasis (OR = 1.25, 95% CI = 0.65–2.41, p = 0.509) or age (OR = 1.16, 95% CI = 0.95–1.43, p = 0.153) was non-significant in patients with CRC (Table 3 and Figure 4).
Table 3.
Correlations of systemic immune-inflammation index and clinicopathological characteristics in patients with colorectal cancer.
| Characteristics | No. of studies | No. of patients | Effects model | OR (95% CI) | p | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2, % | p | ||||||
| Sex, male versus female | 8 | 2529 | Fixed | 0.95 (0.80–1.13) | 0.592 | 12.4 | 0.333 |
| Tumor differentiation, poor versus moderate/well | 7 | 2427 | Fixed | 1.60 (1.27–2.02) | <0.001 | 32.0 | 0.184 |
| Tumor location, rectum versus colon | 6 | 1166 | Fixed | 0.76 (0.58–1.03) | 0.081 | 1.0 | 0.409 |
| Distant metastasis, yes versus no | 4 | 1990 | Random | 2.27 (1.10–4.67) | 0.026 | 84.5 | <0.001 |
| ECOG PS, 1–2 versus 0 | 4 | 702 | Fixed | 1.98 (1.39–2.84) | <0.001 | 0 | 0.442 |
| Lymph node metastasis, yes versus no | 3 | 1721 | Random | 1.25 (0.65–2.41) | 0.509 | 73.9 | 0.022 |
| Age, years, >60 versus ⩽60 | 3 | 1725 | Fixed | 1.16 (0.95–1.43) | 0.153 | 49.5 | 0.138 |
| Tumor size, ⩾5 cm versus <5 cm | 3 | 1721 | Fixed | 1.49 (1.18–1.88) | 0.001 | 7.4 | 0.340 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; OR, odds ratio
Figure 4.
Forest plots of the relationship between systemic immune-inflammation index and clinical factors in colorectal cancer. (A) Sex (male versus female); (B) tumor differentiation (poor versus moderate/well); (C) tumor location (rectum versus colon); (D) distant metastasis (yes versus no); (E) Eastern Cooperative Oncology Group Performance Status (1–2 versus 0); (F) lymph node metastasis (yes versus no); (G) age (years) (>60 versus ⩽60); (H) tumor size (⩾5 cm versus <5 cm).
CI, confidence interval; OR, odds ratio
Sensitivity analysis
We conducted a sensitivity analysis to assess the reliability of pooled HRs of OS and PFS. The overall HR estimates for OS and PFS (Figure 5) were not significantly altered upon sequentially omitting each study from the analysis. Thus, the reliability of the results of this meta-analysis was also confirmed by the sensitivity analysis.
Figure 5.
Sensitivity analysis of the effect of systemic immune-inflammation index on (A) overall survival and (B) progression-free survival in colorectal cancer.
Publication bias
Begg’s funnel and Egger’s tests were used to estimate potential publication bias. As illustrated in Figure 6, there was no significant bias in studies on SII with respect to OS (Begg’s p = 0.938, Egger’s p = 0.089) or PFS (Begg’s p = 0.174, Egger’s p = 0.733).
Figure 6.
Publication bias tested by Begg’s test and Egger’s test. (A) Begg’s test for overall survival, p = 0.938; (B) Egger’s test for overall survival, p = 0.089; (C) Begg’s test for progression-free survival, p = 0.174; (D) Egger’s test for progression-free survival, p = 0.733.
Discussion
To the best of our knowledge, this study is the first meta-analysis exploring the prognostic and clinicopathological impact of SII in patients with CRC. As a novel prognostic parameter, SII can be easily calculated from routine complete blood count tests and reflects the overall status of the immune systems of cancer patients. Previous investigations on the prognostic value of SII in CRC yielded controversial results. For the current meta-analysis, we collected data from 12 studies with 3919 patients to clarify the role of SII in CRC prognosis. Significant prognostic efficiency in different subgroups suggests that high SII was a robust prognostic marker for long-term survival outcomes, including the OS and PFS. Moreover, high SII prior to treatment was related to poor tumor differentiation, presence of distant metastasis, ECOG PS of 1–2 and tumor size of ⩾5 cm. Considering these clinical parameters related to the invasiveness and aggressiveness of malignant tumors, high SII could be a potential marker of disease progression and tumor recurrence possibility. The results of our comprehensive and aggregated analysis demonstrate the effectiveness of SII as an index for CRC prognosis.
The tumor microenvironment has recently attracted increasing attention in the field of cancer immunology. Various inflammatory cells and mediators are important components of the tumor microenvironment.24 SII is based on peripheral lymphocyte, neutrophil and platelet counts. A high SII corresponds to high platelet/neutrophil and (or) low lymphocyte counts. Therefore, an elevated SII indicates a highly-inflammatory tumor microenvironment with infiltrating immune cells.25 The presence of neutrophils in the peripheral blood is generally associated with poor prognosis in patients with cancer.26 Neutrophils can activate endothelial and parenchymal cells and thereby facilitate the metastasis of circulating tumor cells (CTCs).27 Neutrophils also mediate the proliferation and metastasis of cancer cells by secreting inflammatory mediators.28 Moreover, platelets may shield CTCs to protect them from antitumor immune responses, and therefore promote angiogenesis and metastasis of cancer cells.29 Platelets also release a variety of growth factors that enhance cancer cell proliferation in vitro.30 Lymphocytes, especially tumor-infiltrating lymphocytes, further play a pivotal role in the host immune response to malignancy.31 Lymphocytes can induce cytotoxic cell death and inhibit tumor cell proliferation and migration.32 Therefore, SII may also reflect the activation of inflammatory and immune pathways in the host. In addition, the measurements required for the calculation of SII are inexpensive, easily-performed and reproducible, rendering SII a promising marker for CRC prognosis in clinical practice.
The prognostic role of SII in solid tumors has also been investigated in several meta-analyses previously.33,34 A meta-analysis focusing on the use of SII in multiple cancers showed that high SII was associated with poor OS.33 A meta-analysis of data from 2786 lung cancer patients based on seven studies indicated that SII prior to treatment was a prognostic factor for OS.35 Another recent retrospective study showed that baseline SII could serve as an independent prognostic marker for patients with advanced pancreatic cancer.36 The SII was also suggested as a novel independent prognostic factor for predicting OS and PFS in patients with classical Hodgkin lymphoma.37 Here, we demonstrated the prognostic efficiency of SII with respect to OS and PFS in CRC, in line with the findings on other cancer types. In addition, we also demonstrated correlations between high SII and clinical parameters in CRC. Based on our findings and other relevant studies, SII may assist in the cancer prognosis and help develop clinical treatment strategies. For example, hepatocellular carcinoma patients with high SII prior to treatment may benefit more from neoadjuvant chemoradiotherapy, postoperative adjuvant chemoradiotherapy and other cancer-related therapies than patients with low SII.38 SII has also shown good prognostic efficiency in multiple cancers,33,34 which suggests that elevated SII may be a general feature in solid tumors. However, the prognostic value of SII for hematological and other types of cancer, such as gynecological tumors, remains to be investigated. Further investigations focusing on the prognostic impact of SII on diverse cancer types are needed to justify the application of SII in the clinical management of patients with cancer.
Although this study is the first meta-analysis on SII and CRC prognosis to date, there are also several limitations. First, most of the included studies were of retrospective design, which hindered controlling the selection criteria and led to heterogeneity among studies. Second, several HRs were extracted from univariate analyses, and may eventually overestimate effect sizes. Univariate analysis was performed without consideration of confounding factors. Third, SII cut-off values as well as determination methods are not uniform in the included studies, which could cause bias in the results.
In summary, this meta-analysis indicated that higher SII before treatment was correlated with poor OS and PFS in patients with CRC. In addition, high SII was associated with clinical factors implying higher malignancy of the disease. We conclude that SII may play an important role in improving clinical decision-making for CRC. However, larger-scale prospective studies are still needed to validate our results due to the limitations of this study.
Supplemental Material
Supplemental material, Supplementary_material_6 for Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis by Meilian Dong, Yonggang Shi, Jing Yang, Quanbo Zhou, Yugui Lian, Dan Wang, Taoran Ma, Yue Zhang, Yin Mi, Xiaobin Gu and Ruitai Fan in Therapeutic Advances in Medical Oncology
Footnotes
Author contribution: MD, YS, JY, QZ, YL, DW, TM and XG collected and extracted the data and performed quality assessment; MD, YZ, YM, XG, and RF analyzed the data; MD, YS, XG and RF conceived, designed this study and wrote the paper. All authors reviewed the final manuscript. All authors read and approved the final manuscript.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This study was supported by Joint Construction Project of Medical Science and Technology Research Program of Henan Province (No. LHGJ20190019).
ORCID iDs: Yonggang Shi  https://orcid.org/0000-0003-2701-2522
https://orcid.org/0000-0003-2701-2522
Xiaobin Gu  https://orcid.org/0000-0002-4785-1924
https://orcid.org/0000-0002-4785-1924
Ruitai Fan  https://orcid.org/0000-0002-1371-8930
https://orcid.org/0000-0002-1371-8930
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Meilian Dong, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Yonggang Shi, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Jing Yang, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Quanbo Zhou, Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Yugui Lian, Department of Colorectal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Dan Wang, Department of Biotherapy Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Taoran Ma, Department of Education Section, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Yue Zhang, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Yin Mi, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China.
Xiaobin Gu, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Rd, Zhengzhou, Henan 450000, People’s Republic of China.
Ruitai Fan, Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Rd, Zhengzhou, Henan 450000, People’s Republic of China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet 2019; 394: 1467–1480. [DOI] [PubMed] [Google Scholar]
- 3. Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med 2019; 7: 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee MKC, Loree JM. Current and emerging biomarkers in metastatic colorectal cancer. Curr Oncol 2019; 26(Suppl. 1): S7–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol 2016; 17: 230–240. [DOI] [PubMed] [Google Scholar]
- 6. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010; 6: 149–163. [DOI] [PubMed] [Google Scholar]
- 7. Maeda K, Shibutani M, Otani H, et al. Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol 2015; 7: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 2016; 7: 33210–33219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017; 23: 6261–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang J, Guo X, Wang M, et al. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep 2017; 7: 17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tao MY, Wang ZH, Zhang MH, et al. Prognostic value of the systematic immune-inflammation index among patients with operable colon cancer: a retrospective study. Medicine 2018; 97: e13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie QK, Chen P, Hu WM, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med 2018; 16: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Xu H, Guo X, et al. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep 2018; 8: 3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou ZQ, Pang S, Yu XC, et al. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer. Curr Med Sci 2018; 38: 798–808. [DOI] [PubMed] [Google Scholar]
- 15. Jiang JL, Ma T, Xi WQ, et al. Pre-treatment inflammatory biomarkers predict early treatment response and favorable survival in patients with metastatic colorectal cancer who underwent first line cetuximab plus chemotherapy. Cancer Manag Res 2019; 11: 8657–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Y, Xin D, Wang F. Predictive significance of preoperative systemic immune-inflammation index determination in postoperative liver metastasis of colorectal cancer. Onco Targets Ther 2019; 12: 7791–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang YY, Liu ZZ, Xu D, et al. Fibrinogen-Albumin Ratio Index (FARI): a more promising inflammation-based prognostic marker for patients undergoing hepatectomy for colorectal liver metastases. Ann Surg Oncol 2019; 26: 3682–3692. [DOI] [PubMed] [Google Scholar]
- 18. Yang J, Guo XL, Wu T, et al. Prognostic significance of inflammation-based indexes in patients with stage III/IV colorectal cancer after adjuvant chemoradiotherapy. Medicine 2019; 98: e14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang YY, Li WQ, Li ZF, et al. Higher levels of pre-operative peripheral lymphocyte count is a favorable prognostic factor for patients with stage I and II rectal cancer. Front Oncol 2019; 9: 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 22. Cochran W. The combination of estimates from different experiments. Biometrics 1954; 10: 101–129. [Google Scholar]
- 23. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 24. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013; 13: 759–771. [DOI] [PubMed] [Google Scholar]
- 25. Jomrich G, Gruber ES, Winkler D, et al. Systemic Immune-Inflammation Index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg 2020; 24: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16: 431–446. [DOI] [PubMed] [Google Scholar]
- 27. De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 2004; 10: 4895–4900. [DOI] [PubMed] [Google Scholar]
- 28. Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 2010; 16: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015; 126: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int J Cancer 2016; 138: 2078–2087. [DOI] [PubMed] [Google Scholar]
- 31. Mohammed ZM, Going JJ, Edwards J, et al. The role of the tumour inflammatory cell infiltrate in predicting recurrence and survival in patients with primary operable breast cancer. Cancer Treat Rev 2012; 38: 943–955. [DOI] [PubMed] [Google Scholar]
- 32. Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol 2010; 28: 4045–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017; 8: 75381–75388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang R, Chang Q, Meng X, et al. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer 2018; 9: 3295–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Chen B, Wang LJ, et al. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: a meta-analysis. Medicine 2019; 98: e13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang K, Hua YQ, Wang D, et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med 2019; 17: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mirili C, Paydas S, Kapukaya TK, et al. Systemic immune-inflammation index predicting survival outcome in patients with classical Hodgkin lymphoma. Biomark Med 2019; 13: 1565–1575. [DOI] [PubMed] [Google Scholar]
- 38. Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2020; 99: e18571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material_6 for Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis by Meilian Dong, Yonggang Shi, Jing Yang, Quanbo Zhou, Yugui Lian, Dan Wang, Taoran Ma, Yue Zhang, Yin Mi, Xiaobin Gu and Ruitai Fan in Therapeutic Advances in Medical Oncology