Abstract
Neurodegenerative diseases are commonly generated by intracellular accumulation of misfolded/aggregated mutated proteins. These abnormal protein aggregates impair the functions of mitochondria and induce oxidative stress, thereby resulting in neuronal cell death. In turn, neuronal damage induces chronic inflammation and neurodegeneration. Thus, reducing/eliminating these abnormal protein aggregates is a priority for any anti-neurodegenerative therapeutic approach. Although several antibodies against mutated neuronal proteins have been already developed, how to efficiently deliver them inside the target cells remains an unmet issue. Extracellular vesicles/exosomes incorporating intrabodies against the pathogenic products would be a tool for innovative therapeutic approaches. In this review/perspective article, we identify and describe the major molecular targets associated with neurodegenerative diseases, as well as the antibodies already developed against them. Finally, we propose a novel targeting strategy based on the endogenous engineering of extracellular vesicles/exosomes constitutively released by cells of the central nervous system.
Keywords: neurodegenerative disease therapy, extracellular vesicles/exosomes, intrabodies
1. Introduction
Several neurodegenerative diseases (NDs) arise from cell degeneration induced by the accumulation of misfolded/mutated proteins. Among NDs generated by protein mutations, many disorders are caused by polyglutamine extension, a consequence of the genetic expansion of the CAG trinucleotide repeat. These include Huntington’s disease, spinobulbar muscular atrophy, spinocerebellar ataxia, Machado–Joseph disease, and α-synucleinopathies [1]. Parkinson’s disease (PD), among α-synucleinopathies, is associated with the formation of α-synuclein aggregates referred to as Lewy bodies [2]. In Alzheimer’s disease (AD), both extracellular Amyloid beta (Aβ) plaques and intracellular fibrillary tangles of Tau protein accumulate over time leading to potent neurotoxic effects [3]. Similarly, aggregated/misfolded mutated Cu/Zn superoxide dismutase (SOD1) associate with amyotrophic lateral sclerosis (ALS) [4].
1.1. Intrabodies
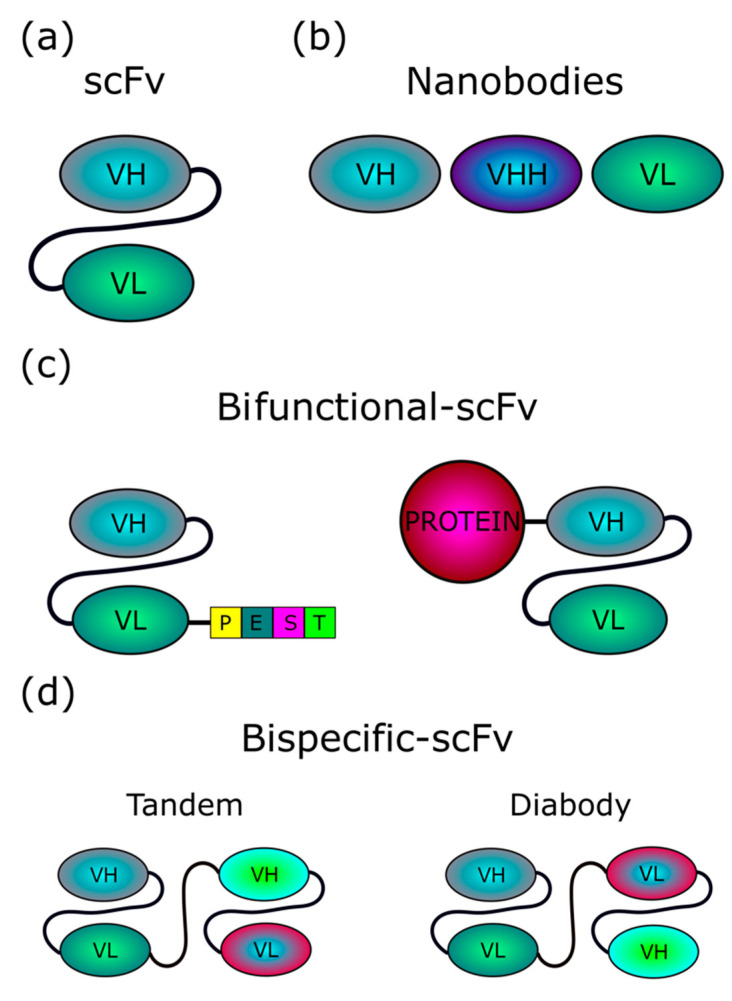
To specifically target such abnormal protein aggregates, a number of antibody fragments that are active intracellularly, called intrabodies, have been recently developed (Table 1). To become intrabodies, in general, antibody portions are specifically modified for localization in intracellular compartments, such as the cytoplasm, the nucleus, mitochondria, or the endoplasmic reticulum (ER) of cells that express them. Intrabodies include single-chain fragment variable antibodies (scFvs). In fact, the antibody Fv region, which is responsible for antibody specificity and binding to a target, can be expressed in the absence of other immunoglobulin domains, and further engineered for the design of therapeutic molecules (Figure 1) [5]. Notably, scFv intrabodies encompass both variable heavy (VH) and light (VL) chains of an antibody, connected by a flexible hinge (Figure 1) [6], and are capable of effectively binding their target antigens. Several other antibody or scFv derivatives against NDs have been developed to be used as intrabodies, such as bifunctional scFvs and bispecific scFvs, as well as camelid VHH, VH, or VL single domain antibodies (nanobodies), which may be more stable, more soluble in the intracellular reducing environment, and have easier access to hidden epitopes than scFvs (Figure 1) [7]. However, despite the proposition of new strategies, the efficient intracellular expression/delivery of these intrabodies remains an unsolved issue.
Table 1.
Intrabodies against neurodegenerative diseases.
| Alzheimer’s Disease Target: β-amyloid Protein | ||||
| Antigen | Species | Intrabody | Experimental Phase | Reference(s) |
| Aβ1–42 | scFv | scFvAβIB | Preclinical in mice | [8] |
| Aβ | scFv | Aβ-scFv | Preclinical in mice | [9] |
| Parkinson’s Disease Target: α-synuclein Protein | ||||
| Antigen | Species | Intrabody | Experimental Phase | Reference(s) |
| Oligomeric α-synuclein | Human scFv | D5 | Preclinical in mice | [10,11,12,13] |
| Oligomeric α-synuclein | Human scFv | 10H | Preclinical in mice | [10,12,14] |
| Pan-specific α-synuclein | Human scFv | D10 | Preclinical in mice | [10,12,15] |
| Hydrophobic non-amyloid component (NAC) of α-synuclein, AA53–95 | Human nanobody (VH) | VH14 (NAC14) | Preclinical in rats | [10,16,17] |
| α-synuclein AA53–87 | Human scFv | NAC32 | In vitro in cell culture | [17] |
| Fibrillar α-synuclein | Human scFv | 6E | In vitro in cell culture | [11,18] |
| α-synuclein, AA118–131 | Camelid nanobody (VHH) | Nbsyn2 | In vitro in cell culture | [16,19] |
| α-synuclein, AA137-140 | Camelid nanobody (VHH) | Nbsyn87 | Preclinical in rats | [16,19,20] |
| Huntington’s Disease Target: Huntingtin (HTT) Protein | ||||
| Antigen | Species | Intrabody | Experimental Phase | Reference(s) |
| HTT-N17 (PolyQ N-term AA1-17) | Human scFv | scFvC4 | Preclinical in mice | [11,21,22,23,24,25,26,27,28,29,30] |
| HTT-N20 (PolyQ N-term, AA1-20) | Human VL | VL12.3 | Preclinical in mice | [22,31,32,33,34,35] |
| HTT-polyQ | Mouse scFv | MW1 | In vitro in cell culture | [36,37] |
| HTT-polyQ | Mouse scFv | MW2 | In vitro in cell culture | [36,37] |
| HTT-PRR | Mouse scFv | MW7 | Ex vivo in brain tissue | [33,36,37] |
| HTT-PRR | Human VL | Happ1 | Preclinical in mice | [33,34] |
| HTT-PRR | Human VL | Happ3 | Ex vivo in brain tissue | [33] |
| HTT-Exon1 | Mouse scFv | EM48 | Preclinical in mice | [38] |
| HTT-PRR | Human scFv | INT41 | Preclinical in mice | [39] |
| Fibrillar mHTT | Human scFv | 6E | In vitro in cell culture | [18,21] |
| Amyotrophic Lateral Sclerosis (ALS) Disease Target: SOD1 Protein | ||||
| Antigen | Species | Intrabody | Experimental Phase | Reference(s) |
| SOD1 | Human scFv | B1 | Preclinical in mice | [40,41] |
| SOD1 | Human scFv | B12 | Preclinical in mice | [40,41] |
| G93A human SOD1 | Mouse scFv | D3H5 | Preclinical in mice | [42] |
| Prion Disorder Target: Prion Protein (PrP) | ||||
| Antigen | Species | Intrabody | Experimental Phase | Reference(s) |
| Cellular PrP | Mouse scFv | 8H4 | Preclinical in mice | [43,44] |
| Cellular PrP | Mouse scFv | 8F9 | In vitro in cell culture | [43] |
| Cellular PrP | Camelid nanobody (VHH) | Nb_PrP_01 | In vitro in crystallography studies | [45,46] |
| Cellular PrP AA123–125, 164–170, and 174–185 | Camelid nanobody (VHH) | Nb484 | In vitro in crystallography studies | [46] |
Figure 1.
Structure of intrabodies. (a) scFvs (~30 kDa) consist of variable regions of heavy (VH) and light (VL) chains of an antibody, joined by a flexible linker, which is usually a 15-aa (Gly4Ser)3 sequence. Both orientations, VH-linker-VL or VL-linker-VH, are possible. (b) Single domain intrabodies (~15 kDa), also termed nanobodies, consist of one single variable domain, and are referred to as VH (or VHH, for nanobodies based on naturally occurring camelid heavy-chain only antibodies), or VL, depending on the variable region included. (c) Bifunctional scFvs can be designed by coupling, at either the N- or C-terminus, an scFv with either a protein domain (for instance, PEST degron) able to redirect the scFv-bound target to cellular clearance machinery, or a whole protein, such as the Nef variant (~27 kDa) we propose to exploit in our exosome-based intrabody delivery strategy. (d) Bispecific scFvs include two scFvs with different specificity, fused either in tandem or head-to-tail (diabody).
1.2. Extracellular Vesicles
All cells constitutively release lipid bi-layered vesicles of various sizes and with different biogenesis, named extracellular vesicles (EVs). EVs are classified as microvesicles/ectosomes (50–1000 nm) and exosomes (50–200 nm) [47]. Whereas the former shed directly from the plasma membrane, exosomes are nanovesicles that originate intracellularly upon inward invagination of endosome membranes, accumulation of intraluminal vesicles (ILVs) in multivesicular bodies (MVBs), trafficking towards the plasma membrane, and release in the extracellular milieu [48].
Exosomes play many relevant roles in CNS, both in health and in disease. For instance, oligodendrocytes release exosomes loaded with components of the myelin sheaths, likely in response to glutamate secreted by neurons [49]. Also, EVs from microglia stimulate neuron synaptic activity by increasing the sphingolipid metabolism and astrocytes release EVs loaded with synapsin I, i.e., a protein that induces neurite formation and survival in neurons [50]. On the other hand, EVs upload and transmit many CNS pathogenetic products in NDs, like Aβ peptides, α-synuclein, SOD-1, as well as the altered prion protein PrPSC [51,52].
In this review, the genetic/molecular bases of most common NDs are summarized. In addition, an overview of the characterization of scFvs and other intrabodies against functional targets of a number of NDs is provided, as well as current strategies for their intracellular expression/delivery. Finally, the potential and future implementation of a novel approach based on the endogenous engineering of EVs/exosomes that we are developing for the delivery of scFvs into target cells is also described.
2. Genetic Basis of Neurodegenerative Diseases
2.1. Alzheimer’s Disease
AD is the most widespread cause of dementia [53]. Although the onset of disease mostly occurs after 65 years, an AD subtype (i.e., early-onset AD) can affect younger people [54]. In this case also, molecular signatures of AD are the accumulation of misfolded protein aggregates, which can self-propagate in a prion-like manner, leading to neurotoxicity and cell death [55]. In particular, AD brains accumulate amyloid plaques formed by Aβ(1-42) fibrils generated by the cleavage of the amyloid plaques by both β- and γ-secretases [3,55]. In addition, hyperphosphorylated Tau protein, which normally stabilizes neuronal microtubules, aggregates thereby producing neurofibrillary tangles affecting the intracellular transport machinery of neurons [3]. Both familial and early-onset AD associate with genetic alterations, in particular localized in the amyloid precursor protein, presenilin 1, and presenilin 2 [56].
2.2. Parkinson’s Disease
PD is the second most diffuse neurodegenerative pathology after Alzheimer’s [57]. Although the mechanisms underlying the disease pathogenesis remain largely unknown, a number of genetic signatures characterize disease onset. For instance, the A53T mutation in α-synuclein was the first amino acid substitution associated with familial PD to be discovered [58]. Afterward, additional mutations within the α-synuclein N-terminus have been identified. In its physiologic form, this protein forms intracellular fibrils. When mutated, it becomes the major component of Lewy bodies, i.e., macromolecular aggregates having toxic effects on dopaminergic neurons as a consequence of the altered intracellular signaling and defective intracellular trafficking leading, for instance, to autophagy suppression [59,60].
Mutations associated with the death of dopaminergic neurons and PD have been identified in additional six genes, i.e., Parkin, vacuolar protein sorting-35, glucocerebrosidase, PTEN-induced putative kinase, leucine-rich repeat kinase 2, and DJ-1 [61]. Whatever the role possibly played by each mutated product, it is now accepted that either single-or multi-gene mutations are involved in PD pathogenesis, thereby representing potential targets for therapy.
2.3. Huntington’s Disease
At least nine human NDs are generated by the expansion of the CAG nucleotide repeats, forming an abnormal polyglutamine extension of neuron proteins [62]. Among these, Huntington’s disease (HD) is an autosomal dominant ND generated by the CAG expansion in the first exon of the Huntingtin gene resulting in a protein with an abnormally long polyglutamine sequence [63]. The N-terminal polyglutamine expansion leads to abnormal protein aggregation and neuron intracellular inclusions ultimately having a cytotoxic effect on neurons [64].
2.4. Amyotrophic Lateral Sclerosis
ALS is a fatal neurodegenerative disorder characterized by the progressive loss of motor neurons, ultimately leading to paralysis and death [65]. To date, nearly 200 mutations have been described to be associated with familial ALS [66]. Similarly to other NDs, superoxide dismutase 1 (SOD1) toxic effects are at least in part due to the generation of intracellular aggregates of misfolded protein. The most common mutations found in the SOD1 gene are D90A, A4V, and G93A. More recently, mutations in more than 20 genes have been linked to both familial and sporadic ALS, including C9orf72, TARDBP (TDP43), and FUS [67].
2.5. Prion Disorders
A misfolded form of the prion protein (PrP) is at the basis of a number of human NDs, including Creutzfeldt–Jakob disease, Kuru, as well as spongiform encephalitis and scrapie in bovines and sheep, respectively. Native PrP (PrPC) is expressed in all tissues, but particularly in the CNS [68]. Misfolded PrPC (PrPSc) is infectious and able to catalyze the conversion of PrPC into PrPSc, and is, therefore, responsible for both transmission and pathogenesis of the disease [55,69,70]. PrPSc aggregates generate amyloid fibers that heavily affect nervous functions [55].
3. Intrabodies against ND Targets
Intrabodies can be specifically designed, for instance, to accumulate in the cell where they are expressed, by not including a secretory signal. Alternatively, they can be engineered to be either released extracellularly for bystander cells uptake or uploaded as cargoes into extracellular vesicles, depending on the strategy to be developed for their therapeutic application.
The rationale behind the use of antibodies and, more specifically, intrabodies as an approach against NDs becomes clear when considering the particular pathogenesis, with intracellularly accumulated misfolded proteins, which might be targeted in order to accelerate their turnover, block post-translational pathogenic modifications or cleavage, and/or modify their subcellular compartmentalization.
3.1. Antibody Targets for Alzheimer’s Disease
Phase III clinical trials with at least four anti-Aβ antibodies failed to improve AD symptoms possibly because of the advanced disease stage of the treated patients [71,72]. Nonetheless, in the future, with early treatment, both early-diagnosed and genetic AD cases might benefit from this approach. In fact, passive immunotherapy with nanobodies showed some promise in preclinical studies, as their small size may permit them to access brain tissue more effectively than conventional antibody-based therapies. Moreover, the nanobody technology may allow the combination of multiple functions into the same molecule and nanobodies are considered safer than full-size antibodies, which may cause a number of serious adverse effects [73].
To date, a nanobody specific to Aβ fibrils selected from a phage display a fully synthetic library of camelid VHH (heavy chain variable region)-domains [74] and other anti-Aβ intrabodies have been described [8,9]. In particular, an AAV-delivered scFv intrabody was reported to decrease AD pathology in a mouse model for AD, both at the molecular and cognitive levels [9].
Anti-Aβ scFv can prevent fibril aggregation in a cell-free system, and toxicity in neuronal cells [75,76]. Furthermore, scFv intrabodies specific for a cleavage site in the Aβ precursor protein prevented the generation of Aβ [77]. These observations indicate that Aβ is produced intracellularly, before accumulating outside the cell, thus the formation process and intra- and extracellular accumulation may be effectively targeted by intrabodies. The binding of scFvs or nanobodies to extracellular Aβ may also be beneficial to prevent deposition, plaque formation, and pathogenesis, although, as such, these would not be truly considered intrabodies [78].
Interestingly, a bispecific scFv construct both blocking the cleavage site of β-secretase and increasing α-secretase cleavage showed promise in a mouse model [79]. Such scFv was delivered through AAV gene therapy to the liver and, being engineered to cross the blood–brain barrier (BBB), exerted its function upon entering target cells from the outside. Long-term studies will be necessary to determine whether a similar bispecific intrabody-based approach can overcome the issue of irreversible changes that may occur during the intrabody-antigen dissociation phase, as also reported for other NDs (see below).
3.2. Antibody Targets for Parkinson’s Disease
Misfolded α-synuclein accumulates inside and outside the cell and causes PD progression. For this reason, despite the small size and the partially unfolded structure, both intracellular and extracellular α-synuclein monomers have been considered as therapeutic targets, in particular the non-amyloid component (NAC) hydrophobic interaction region, which is critical for misfolding and pathogenic aggregation of α-synuclein [17,80,81]. Efforts to generate antibodies specific to the NAC region in vivo were unsuccessful, likely because this domain is not exposed within the folded protein. Therefore, the search for therapeutic molecules shifted to the screening of libraries of antibody fragments with peptides derived from α-synuclein NAC. Intrabodies initially selected from a yeast surface-display library of human scFvs were quite disappointing, as those identified as the best binders in vitro, when tested in a cell-based assay, resulted not protective, thus proving dysfunctional. This was due to modifications undergone in the intracellular reducing environment, possibly leading to insolubility, and/or to other reasons related to proteostatic stress in cells affected by the disease. The lack of availability of cognate epitope in the context of the protein target in vivo would also account for the observed unsatisfactory results. In some instances, intrabody modifications to obtain a bispecific molecule, such as a proteasome targeting signal, augmented overall protein solubility thanks to an increase in negative charge, thus improving intrabody performance in vivo [10,16].
Full-length α-synuclein protein was also used in an immunization and phage-display library production/selection approach that resulted in the identification of a pool of VHH nanobodies. Among these, Nbsyn2 and Nbsyn87 were characterized and found specific for an α-synuclein C-terminus [19]. This domain of α-synuclein can undergo post-translational modifications that are involved in pathogenic protein misfolding [82], therefore, it is a potential therapeutic target. Yet, only Nbsyn87 proved able to interfere with mutant α-synuclein aggregation in vitro, whereas Nbyn2, specific for a more C-terminal and longer peptide epitope, did not [83].
When both Nbsyn87 camelid VHH and VH14 human VH nanobodies were fused to the C-terminal of mouse ornithine decarboxylase (mODC) PEST degron (a proline, aspartate or glutamate, serine, and threonine motif that modulates protein degradation), α-synuclein aggregation was decreased, its clearance was augmented, and α-synuclein overexpression-induced toxicity in ST14A cells was reduced. Nbsyn87 showed the strongest α-synuclein reduction, whereas VH14 conferred the best survival benefits to the cells [16]. In an in vivo model of viral gene therapy of α-synuclein overexpression in rats [20], both PEST-fused Nbsyn87 and VH14 nanobodies showed protective effects against pathological α-synuclein aggregation, mostly in terms of a reduction in phosphorylated Serine-129 α-synuclein. VH14-PEST nanobody was somewhat more protective than Nbsyn87-PEST, possibly because the latter induced localized inflammation that may have affected overall protection. Initial studies have also been conducted with other scFvs and nanobodies, which were tested against oligomeric, protofibrillar, and fibrillar forms of α-synuclein in vitro [84,85].
3.3. Antibody Targets for Huntington’s Disease
The approach based on intrabodies is reasonably feasible also in HD, since abnormally folded polyQ protein fragments accumulate inside the cell. Thus, it is conceivable to target and prevent the early stages of the disease by altering the initial misfolding of the expanded polyQ tract and its subcellular compartmentalization. In particular, it would be of therapeutic relevance to contrast its accumulation in cell nuclei and interaction with other abnormal proteins, as well as promoting its turnover [86,87]. Based on this rationale, specific anti-HTT intrabodies (scFvs and single-domain antibodies) were initially selected from phage or yeast surface-display libraries, as well as from hybridoma cell lines to target the N-terminal fragment of the mutant HTT (mHTT) protein, presenting an extended, misfolded polyQ. Unfortunately, these anti-fibrillar intrabodies stabilized and enlarged the size of pathogenic fibrils, and this approach turned out to increase their toxicity [18,36]. An alternative strategy has been therefore explored, aimed at targeting both the N- and the prolin-rich C-terminal domains flanking the polyQ, in order to alter its context and neutralize mHTT toxicity.
In line with this, scFvC4 was the first anti-HTT intrabody selected from sub-pools of a naïve human spleen phage-display library against the unmodified HTT N-terminal 1–17 peptide [25]. It was proven able to preferentially disaggregate pathogenic mHTT Exon1 fibrils [27] rather than the full-length wild type HTT, thus leaving the latter able to exert its normal functions in vivo [88].
Selected candidate intrabodies were tested as transgenes in fly and mouse models with some promising results [26,30,89], yet the effect of treatment with scFvC4 diminished over time because of the formation of large insoluble complexes, which escape mHTT Exon1 clearance by intrabodies. This effect was likely due to PolyQ becoming rapidly fibrillar during the brief periods of dissociation of the intrabody. Therefore, newer approaches aim at developing bifunctional intrabodies, which facilitate the turnover of targeted proteins before complex dissociation occurs, by exploiting cellular clearance mechanisms to rapidly target bound intrabody–antigen complexes [21,90,91]. In such intrabodies, for instance, the scFvC4 C-terminus is coupled with the mODC PEST degron to redirect intrabody–antigen complexes to the proteasome [21]. Alternatively, bifunctional anti-Huntingtin intrabodies have been developed by fusing heat shock protein 70 (HSC70) protein binding motifs (KFERQ and VKKDQ) to polyQ binding protein (QBP1), derived from a monoclonal antibody specific to expanded polyglutamine repeats [92], to direct mHTT to the chaperone-mediated autophagy (CMA) lysosomal degradation pathway [90,91]. Further studies will be necessary to optimize the design of bifunctional antibodies taking into account general stability, delivery strategies, and safety.
Another anti-N-terminus intrabody was selected against HTT AA1-20 from the non-immune human yeast surface-display library and engineered as a single domain intrabody (VL)12.3, which had shown full binding activity [31]. In vivo studies in mouse models [33] showed controversial results, with VL12.3 administration either improving animal behavior and decreasing neuropathology or actually inducing a slight acceleration of the disease. The latter effect was likely due to high antigen–antibody complexes in nuclei of transduced cells [33].
Several monoclonal antibodies and intrabodies specific to the PolyQ C-terminal region, which encompasses a polyproline (PolyP)-rich region involved in the modulation of PolyQ aggregation were also investigated [93,94]. In particular, an scFv derived from PolyP-specific MW7 monoclonal antibody proved able to reduce mHTT aggregation and to enhance cell survival in an in vitro HD model [36]. In addition, anti-proline-rich region single-domain VL intrabodies Happ1 and Happ3 obtained from a non-immune human recombinant scFV phage library [33] were able to prevent aggregation and toxicity at lower doses than scFvMW7 [33]. All three intrabodies were capable of increasing mHTT Exon1 clearance in HEK293 cells, possibly by keeping their bound target soluble and available for regular turnover processes.
An additional anti-PolyQ C-terminus intrabody, INT41, showed some promise in short-term studies in both in vitro and in vivo HD models, in terms of phenotype amelioration, decrease of protein aggregation, and cognition improvement. Longer-term experiments will be required to assess whether such improvements last over time. Promising results were also obtained with another monoclonal antibody-derived scFv (SCFV48), binding a more C-terminal epitope, which counteracted the cytoplasmic, yet not the nuclear, toxicity of the mHTT Exon 1 in HEK293 cells [38].
3.4. Antibody Targets for Amyotrophic Lateral Sclerosis
ALS candidate intrabody targets SOD1, C9orf72, and TDP43 are being evaluated in preclinical studies. In particular, phage-display library-selected anti-SOD1 scFvs, administered intravenously in the G93A mutant SOD1 mouse model of ALS, displayed expression in motor neurons and astrocytes and had beneficial effects [40,41]. A monoclonal antibody-derived scFv (D3H5), specific for misfolded SOD1, administered by intrathecal injection, also induced delayed disease onset and prolonged survival in G93A mutant SOD1 mice in a dose-response manner, proportional to D3H5 scFv levels in spinal cord motor neurons [42].
Another monoclonal antibody-derived scFv, specific for the TDP-43 RNA recognition motif 1 (RRM1) domain, proved protective against TDP-43 proteinopathy both in cell cultures and upon intrathecal/intracranial delivery [95]. Despite the absence of fused degron sequences, a slight increase in target protein turnover was also reported. Both intracellular and secreted scFv taken-up by cells from the outside are likely to contribute to counteracting pathological inflammation.
3.5. Targets for Prion Disorders
Although PrP misfolding is not correlated with known genetic mutations, the different conformations of native compared to pathogenic altered protein allowed the isolation of antibodies specifically recognizing PrPSc. In particular, 8H4- and 8F9-scFvs, directed against different PrP epitopes, were targeted to the secretory compartment of mammalian cells by ER retention signal KDEL-tagging. In transmissible spongiform encephalopathy (TSE), these scFvs were able to prevent the conversion of normal PrPC to pathological isoforms and avoid the accumulation of the misfolded protein, therefore diminishing its infectivity [43,44]. In vivo studies also showed that anti-PrP KDEL-fused intrabodies can prevent scrapie infectivity in mice [44]. On the other hand, crystallographic studies based on anti-PrP scFvs and nanobodies also helped characterize the bovine PrP unstructured domain [45,46,96].
4. Current Strategies for Intrabody Delivery into Cells
4.1. Extracellular Vesicles/Exosomes as a Tool for scFv Delivery
EV manipulation is now considered a rather promising approach for the delivery of drugs and biologically active macromolecules. Stability, biocompatibility, and capacity to cross the BBB are significant advantages of EV-based delivery strategies. Consistently, the use of EVs has been successful for the delivery of small molecules, micro-, non-coding, and messenger RNAs, and proteins [97].
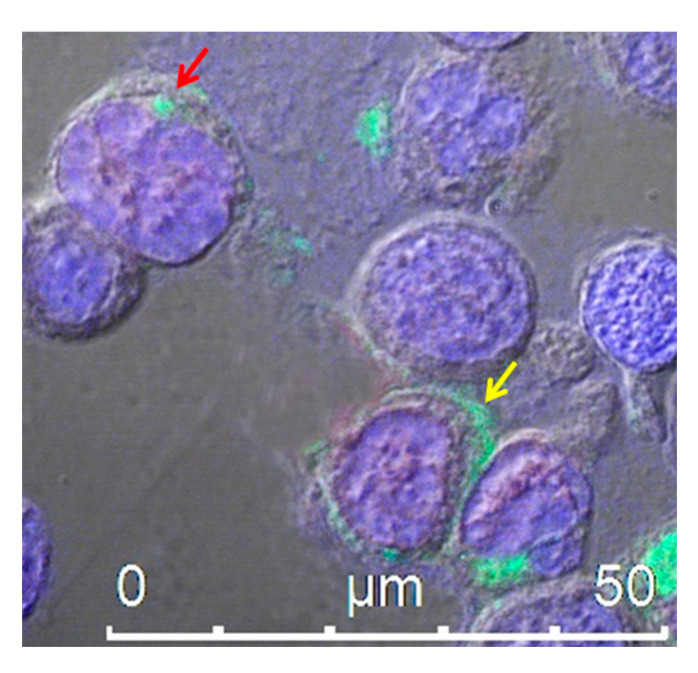
Proteins can be uploaded by EVs either as constituents of the limiting membrane or incorporated into the lumen, as described for human immunodeficiency virus (HIV)-1 Nef protein [98]. We identified a Nef mutant (referred to as Nefmut), which is incorporated into EVs/exosomes at very high levels even when fused with foreign proteins, meanwhile lacking all the biological activities of the wild-type counterpart [99,100]. These unique features allowed us to envision and obtain the proof-of-concept for a novel strategy for intracellular scFv delivery, based on EVs engineered by Nefmut fused at its C-terminus with an scFv. In this conformation, the scFv incorporated into EVs remains protected from modifier/degradation factors present in the extracellular milieu. In the cell, the Nefmut/scFv fusion product mostly localizes at the plasma membrane and in intracytoplasmic vesicle aggregates (Figure 2). Using an scFv binding the oncogenic HPV16-E7 fused with Nefmut, we demonstrated that the scFv moiety is functional upon delivery in target cells [101]. Hence, engineering EVs with scFvs has the potential to be exploited as a novel therapeutic approach to target intracellular pathogenic factors in NDs. Nonetheless, in view of a translation into the clinic, severe technical hindrances still need to be solved, including the effective delivery of sufficient amounts of engineered exosomes at CNS, industrial manufacturing, cost of production, and storage. To overcome most part of these hurdles, we propose a strategy through which the engineered exosomes are produced by CNS cells of the patient (Figure 3).
Figure 2.
Confocal microscope analysis of HEK293T cells transfected with an expression vector encoding Nefmut fused to an scFv, labeled with an anti-Nef monoclonal antibody and Alexa 488-conjugated anti-mouse IgG antibody. The picture shows intracellular localization of the fusion protein product in intracytoplasmic vesicle aggregates (red arrow) and at the plasma membrane (yellow arrow). DAPI (blue fluorescence) was used to highlight cell nuclei. Scale bar is shown.
Figure 3.
Exosome model for delivering intrabodies against NDs. The model we propose relies on Nefmut-based endogenously engineered extracellular vesicles (EVs), produced in vivo by cells expressing vectors encoding a Nefmut-scFv fusion intrabody specific for the ND molecular target of interest. Such a bifunctional intrabody may bind its intracellular target both in the DNA-expressing cells and in bystander cells, upon release and cell-to-cell transfer of Nefmut-scFv-engineered EVs, by exploiting Nefmut ability to be uploaded into exosomes in large amounts. To enhance the targeting of cells that are relevant to ND pathogenesis, EVs could be doubly engineered with an additional fusion product encompassing a membrane-associated protein and a protein domain specifically able to bind CNS cellular targets, such as, for instance, the RVG-29 peptide, which binds nicotinic AchR.
4.2. Endogenously Engineered Exosomes: Potential and Further Development
As an alternative to the in vitro production of Nefmut-based engineered EVs, we developed an in vivo method to engineer the EVs constitutively released by muscle cells [102]. This strategy relies on the intracellular expression of a DNA vector expressing the product of the fusion between Nefmut and the gene of interest. By entering target cells, these engineered EVs allow the intracellular delivery of their cargo. When Nefmut is fused with sequences coding for an scFv, intracellular antigens can be targeted both in DNA vector expressing cells and in bystanders, through cell-to-cell transfer of scFv-engineered EVs (Figure 3).
To attain the highest concentration of anti-ND therapeutic exosomes in the relevant tissue (CNS), at least two conditions should be met: i) engineered exosomes not encountering physiological barriers, which may limit their diffusion, except the plasma membrane of target cells, and ii) therapeutic exosomes preferably entering cell types relevant to disease pathogenesis. As they can spontaneously cross the BBB, exosome surface engineering with the RVG peptide, i.e., a 29-amino acid peptide derived from the neurotropic rabies virus glycoprotein binding the nicotinic acetylcholine receptor (AchR), could increase their delivery to CNS [103,104,105] and may be exploited when adapting our intrabody delivery strategy to ND treatment.
4.3. Delivery using Gene Therapy
Antibody fragments can be delivered as genes in the CNS thanks to advancements made in the past few years in the field of gene therapy for the nervous system, which include clinical trials for gene replacement and FDA-approved gene therapy for spinal muscular atrophy [106]. Although the expression of genes of interest in the CNS has been achieved by several methods, the use of viral vectors still appears to be the best way for in vivo transduction of nervous cells. Vectors derived from adenoviruses, herpesviruses, and lentiviruses have proven useful to transduce CNS cells. However, because of their safety profile and genetic simplicity, vectors derived from adeno-associated viruses (AAVs) can be considered the best option for the delivery of genes of interest in CNS [107,108]. AAVs are small, non-enveloped virions of about 20 nm in diameter, with an icosahedral protein capsid containing an approximately 4.8 Kb linear single-stranded DNA. AVVs belong to the Parvoviridae family and depend on the coinfection of helper viruses (adenovirus, herpesvirus) for replication in host cells. In their absence, AAVs may stably integrate into the host cell, albeit at a relatively low frequency, and remain quiescent. Both wild-type AAVs and recombinant AAVs (rAAVs) used for gene therapy do not cause any known associated pathologies and cause a very mild immune response [109].
AAV-delivered transgene expression is essentially permanent in non-dividing cells. In fact, several studies showed that they are expressed for more than six months in the mouse brain [110] and can persist in other tissues for at least six years in primates [111]. Importantly, a gene therapy trial has shown that the therapeutic effects of AAV-delivered transgenes can persist for at least 10 years in the human brain [112].
Recombinant AAVs can be engineered to target different cell types and tissues. Several variant capsids have been described with potential for delivering intrabodies in neurodegenerative diseases [113,114,115], with AAV9 being the best characterized for intrathecal administration. By gene therapy, intrabodies can be delivered directly to the brain or be administered in the periphery with a viral vector capable of entering the brain to target specific cells. For all these characteristics, AAVs are also a good candidate for delivery in the CNS of the product of the fusion between Nefmut and an anti-ND scFv.
The therapeutic effect of engineered EVs emerging from transduced neuronal cells would also benefit from precise cell targeting. This might be easily achieved by co-expressing with the Nefmut-scFv fusion protein, another chimeric product including portions of proteins that strongly associate with exosome membranes, such as the transmembrane domain of platelet-derived growth factor receptor (PDGF-R) or lactadherin C1-C2 domains, and ligands that specifically bind the cell type of interest. For instance, with glycoprotein CD24 being a potential marker of cell populations affected in PD [116], the engineering of exosomes with both a membrane protein fused to a humanized anti-CD24 scFv and Nefmut-anti-ND scFv would ensure precise targeting of the therapeutic scFv to CD24-expressing cells.
With these premises, we feel that the combination of gene-therapy and EV-based nanotechnologies may offer an unprecedented opportunity to counteract still untreatable NDs.
5. Conclusions
EVs/exosomes are considered a smart tool for drug delivery. Stability, biocompatibility, stealth ability in biologic fluids, as well as the documented capacity to cross the BBB are significant advantages of EV-based delivery systems. However, at present, the use of exosomes in therapy is difficult because of a number of yet unresolved issues, such as the efficiency of exosome engineering, cost-effectiveness, safety, and biodistribution/pharmacokinetics. The strategy we developed would overcome at least part of these difficulties. Furthermore, engineering EVs with ligands of specific cell receptors is feasible and would represent a critical step forward towards a precise intervention against ND mediators.
Apart from our proposal, the rapid evolution towards even more sophisticated and reliable EV-based nanotechnologies combined with the antibody-based technologies holds much promise to fight still untreatable diseases, included NDs, tumors, and infectious diseases.
Funding
This work was supported by the grant PGR00810 from Ministero degli Affari Esteri e della Cooperazione Internazionale, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.La Spada A.R., Paulson H.L., Fischbeck K.H. Trinucleotide repeat expansion in neurological disease. Ann. Neurol. 1994;36:814–822. doi: 10.1002/ana.410360604. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011;1 doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravits J.M., La Spada A.R. ALS motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology. 2009;73:805–811. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo A.-Y., Zhu Q., Marasco W.A. Therapeutic Antibodies. Springer; Berlin/Heidelberg, Germany: 2008. Intracellular antibodies (intrabodies) and their therapeutic potential; pp. 343–373. [DOI] [PubMed] [Google Scholar]
- 6.Huston J.S., Margolies M.N., Haber E. Antibody binding sites. Adv. Protein Chem. 1996;49:329–450. doi: 10.1016/s0065-3233(08)60493-3. [DOI] [PubMed] [Google Scholar]
- 7.Marschall A.L.J., Dübel S. Antibodies inside of a cell can change its outside: Can intrabodies provide a new therapeutic paradigm? Comput. Struct. Biotechnol. J. 2016;14:304–308. doi: 10.1016/j.csbj.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudol K.L., Mastrangelo M.A., Narrow W.C., Frazer M.E., Levites Y.R., Golde T.E., Federoff H.J., Bowers W.J. Generating differentially targeted amyloid-β specific intrabodies as a passive vaccination strategy for Alzheimer’s disease. Mol. Ther. 2009;17:2031–2040. doi: 10.1038/mt.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan D.A., Mastrangelo M.A., Narrow W.C., Sullivan M.A., Federoff H.J., Bowers W.J. AB-directed single-chain antibody delivery via a serotype-1 AAV vector improves learning behavior and pathology in alzheimer’s disease mice. Mol. Ther. 2010;18:1471–1481. doi: 10.1038/mt.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi S.N., Butler D.C., Messer A. Proceedings of the MAbs. Volume 4. Taylor & Francis; Abingdon, UK: 2012. Fusion to a highly charged proteasomal retargeting sequence increases soluble cytoplasmic expression and efficacy of diverse anti-synuclein intrabodies; pp. 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kvam E., Sierks M.R., Shoemaker C.B., Messer A. Physico-chemical determinants of soluble intrabody expression in mammalian cell cytoplasm. Protein Eng. Des. Sel. 2010;23:489–498. doi: 10.1093/protein/gzq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer B., Williams S., Rockenstein E., Valera E., Xin W., Mante M., Florio J., Adame A., Masliah E., Sierks M.R. α-synuclein conformational antibodies fused to penetratin are effective in models of Lewy body disease. Ann. Clin. Transl. Neurol. 2016;3:588–606. doi: 10.1002/acn3.321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Valera E., Spencer B., Fields J.A., Trinh I., Adame A., Mante M., Rockenstein E., Desplats P., Masliah E. Combination of alpha-synuclein immunotherapy with anti-inflammatory treatment in a transgenic mouse model of multiple system atrophy. Acta Neuropathol. Commun. 2017;5:2. doi: 10.1186/s40478-016-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emadi S., Kasturirangan S., Wang M.S., Schulz P., Sierks M.R. Detecting Morphologically Distinct Oligomeric Forms of α-Synuclein. J. Biol. Chem. 2009;284:11048–11058. doi: 10.1074/jbc.M806559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C., Emadi S., Sierks M.R., Messer A. A human single-chain Fv intrabody blocks aberrant cellular effects of overexpressed α-synuclein. Mol. Ther. 2004;10:1023–1031. doi: 10.1016/j.ymthe.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Butler D.C., Joshi S.N., De Genst E., Baghel A.S., Dobson C.M., Messer A. Bifunctional anti-non-amyloid component α-Synuclein nanobodies are protective in situ. PLoS ONE. 2016;11:e0165964. doi: 10.1371/journal.pone.0165964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch S.M., Zhou C., Messer A. An scFv intrabody against the nonamyloid component of alpha-synuclein reduces intracellular aggregation and toxicity. J. Mol. Biol. 2008;377:136–147. doi: 10.1016/j.jmb.2007.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvam E., Nannenga B.L., Wang M.S., Jia Z., Sierks M.R., Messer A. Conformational targeting of fibrillar polyglutamine proteins in live cells escalates aggregation and cytotoxicity. PLoS ONE. 2009;4:e5727. doi: 10.1371/journal.pone.0005727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilliams T., El-Turk F., Buell A.K., O’Day E.M., Aprile F.A., Esbjörner E.K., Vendruscolo M., Cremades N., Pardon E., Wyns L. Nanobodies raised against monomeric α-synuclein distinguish between fibrils at different maturation stages. J. Mol. Biol. 2013;425:2397–2411. doi: 10.1016/j.jmb.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee D., Bhatt M., Butler D., De Genst E., Dobson C.M., Messer A., Kordower J.H. Proteasome-targeted nanobodies alleviate pathology and functional decline in an α-synuclein-based Parkinson’s disease model. NPJ Parkinson’s Dis. 2018;4:1–10. doi: 10.1038/s41531-018-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler D.C., Messer A. Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments. PLoS ONE. 2011;6:e29199. doi: 10.1371/journal.pone.0029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler D.C., Snyder-Keller A., De Genst E., Messer A. Differential nuclear localization of complexes may underlie in vivo intrabody efficacy in Huntington’s disease. Protein Eng. Des. Sel. 2014;27:359–363. doi: 10.1093/protein/gzu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Genst E., Chirgadze D.Y., Klein F.A.C., Butler D.C., Matak-Vinković D., Trottier Y., Huston J.S., Messer A., Dobson C.M. Structure of a Single-Chain Fv Bound to the 17 N-Terminal Residues of Huntingtin Provides Insights into Pathogenic Amyloid Formation and Suppression. J. Mol. Biol. 2015;427:2166–2178. doi: 10.1016/j.jmb.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hathorn T., Snyder-Keller A., Messer A. Nicotinamide improves motor deficits and upregulates PGC-1α and BDNF gene expression in a mouse model of Huntington’s disease. Neurobiol. Dis. 2011;41:43–50. doi: 10.1016/j.nbd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecerf J.-M., Shirley T.L., Zhu Q., Kazantsev A., Amersdorfer P., Housman D.E., Messer A., Huston J.S. Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:4764–4769. doi: 10.1073/pnas.071058398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLear J.A., Lebrecht D., Messer A., Wolfgang W.J. Combinational approach of intrabody with enhanced Hsp70 expression addresses multiple pathologies in a fly model of Huntington’s disease. FASEB J. 2008;22:2003–2011. doi: 10.1096/fj.07-099689. [DOI] [PubMed] [Google Scholar]
- 27.Miller T.W., Zhou C., Gines S., MacDonald M.E., Mazarakis N.D., Bates G.P., Huston J.S., Messer A. A human single-chain Fv intrabody preferentially targets amino-terminal huntingtin fragments in striatal models of Huntington’s disease. Neurobiol. Dis. 2005;19:47–56. doi: 10.1016/j.nbd.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Murphy R.C., Messer A. A single-chain Fv intrabody provides functional protection against the effects of mutant protein in an organotypic slice culture model of Huntington’s disease. Mol. Brain Res. 2004;121:141–145. doi: 10.1016/j.molbrainres.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Snyder-Keller A., McLear J.A., Hathorn T., Messer A. Early or late-stage anti-N-terminal Huntingtin intrabody gene therapy reduces pathological features in B6.HDR6/1 mice. J. Neuropathol. Exp. Neurol. 2010;69:1078–1085. doi: 10.1097/NEN.0b013e3181f530ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfgang W.J., Miller T.W., Webster J.M., Huston J.S., Thompson L.M., Marsh J.L., Messer A. Suppression of Huntington’s disease pathology in Drosophila by human single-chain Fv antibodies. Proc. Natl. Acad. Sci. USA. 2005;102:11563–11568. doi: 10.1073/pnas.0505321102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colby D.W., Chu Y., Cassady J.P., Duennwald M., Zazulak H., Webster J.M., Messer A., Lindquist S., Ingram V.M., Wittrup K.D. Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond-free single-domain intracellular antibody. Proc. Natl. Acad. Sci. USA. 2004;101:17616–17621. doi: 10.1073/pnas.0408134101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colby D.W., Garg P., Holden T., Chao G., Webster J.M., Messer A., Ingram V.M., Wittrup K.D. Development of a Human Light Chain Variable Domain (VL) Intracellular Antibody Specific for the Amino Terminus of Huntingtin via Yeast Surface Display. J. Mol. Biol. 2004;342:901–912. doi: 10.1016/j.jmb.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 33.Southwell A.L., Khoshnan A., Dunn D.E., Bugg C.W., Lo D.C., Patterson P.H. Intrabodies binding the proline-rich domains of mutant huntingtin increase its turnover and reduce neurotoxicity. J. Neurosci. 2008;28:9013–9020. doi: 10.1523/JNEUROSCI.2747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Southwell A.L., Ko J., Patterson P.H. Intrabody Gene Therapy Ameliorates Motor, Cognitive, and Neuropathological Symptoms in Multiple Mouse Models of Huntington’s Disease. J. Neurosci. 2009;29:13589–13602. doi: 10.1523/JNEUROSCI.4286-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Southwell A.L., Bugg C.W., Kaltenbach L.S., Dunn D., Butland S., Weiss A., Paganetti P., Lo D.C., Patterson P.H. Perturbation with Intrabodies Reveals That Calpain Cleavage Is Required for Degradation of Huntingtin Exon 1. PLoS ONE. 2011;6:e16676. doi: 10.1371/journal.pone.0016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoshnan A., Ko J., Patterson P.H. Effects of intracellular expression of anti-huntingtin antibodies of various specificities on mutant huntingtin aggregation and toxicity. Proc. Natl. Acad. Sci. USA. 2002;99:1002–1007. doi: 10.1073/pnas.022631799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko J., Ou S., Patterson P.H. New anti-huntingtin monoclonal antibodies: Implications for huntingtin conformation and its binding proteins. Brain Res. Bull. 2001;56:319–329. doi: 10.1016/S0361-9230(01)00599-8. [DOI] [PubMed] [Google Scholar]
- 38.Wang C.-E., Zhou H., McGuire J.R., Cerullo V., Lee B., Li S.-H., Li X.-J. Suppression of neuropil aggregates and neurological symptoms by an intracellular antibody implicates the cytoplasmic toxicity of mutant huntingtin. J. Cell Biol. 2008;181:803–816. doi: 10.1083/jcb.200710158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaro I.A., Henderson L.A. An Intrabody Drug (rAAV6-INT41) Reduces the Binding of N-Terminal Huntingtin Fragment(s) to DNA to Basal Levels in PC12 Cells and Delays Cognitive Loss in the R6/2 Animal Model. J. Neurodegener. Dis. 2016;2016:7120753. doi: 10.1155/2016/7120753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghadge G.D., Kay B.K., Drigotas C., Roos R.P. Single chain variable fragment antibodies directed against SOD1 ameliorate disease in mutant SOD1 transgenic mice. Neurobiol. Dis. 2019;121:131–137. doi: 10.1016/j.nbd.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Ghadge G.D., Pavlovic J.D., Koduvayur S.P., Kay B.K., Roos R.P. Single chain variable fragment antibodies block aggregation and toxicity induced by familial ALS-linked mutant forms of SOD1. Neurobiol. Dis. 2013;56:74–78. doi: 10.1016/j.nbd.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel P., Kriz J., Gravel M., Soucy G., Bareil C., Gravel C., Julien J.-P. Adeno-associated Virus–mediated Delivery of a Recombinant Single-chain Antibody Against Misfolded Superoxide Dismutase for Treatment of Amyotrophic Lateral Sclerosis. Mol. Ther. 2014;22:498–510. doi: 10.1038/mt.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardinale A., Filesi I., Vetrugno V., Pocchiari M., Sy M.-S., Biocca S. Trapping prion protein in the endoplasmic reticulum impairs PrPC maturation and prevents PrPSc accumulation. J. Biol. Chem. 2005;280:685–694. doi: 10.1074/jbc.M407360200. [DOI] [PubMed] [Google Scholar]
- 44.Vetrugno V., Cardinale A., Filesi I., Mattei S., Sy M.-S., Pocchiari M., Biocca S. KDEL-tagged anti-prion intrabodies impair PrP lysosomal degradation and inhibit scrapie infectivity. Biochem. Biophys. Res. Commun. 2005;338:1791–1797. doi: 10.1016/j.bbrc.2005.10.146. [DOI] [PubMed] [Google Scholar]
- 45.Abskharon R.N.N., Soror S.H., Pardon E., El Hassan H., Legname G., Steyaert J., Wohlkonig A. Crystallization and preliminary X-ray diffraction analysis of a specific VHH domain against mouse prion protein. Acta Cryst. F. 2010;66:1644–1646. doi: 10.1107/S1744309110042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abskharon R.N.N., Giachin G., Wohlkonig A., Soror S.H., Pardon E., Legname G., Steyaert J. Probing the N-Terminal β-Sheet Conversion in the Crystal Structure of the Human Prion Protein Bound to a Nanobody. J. Am. Chem. Soc. 2014;136:937–944. doi: 10.1021/ja407527p. [DOI] [PubMed] [Google Scholar]
- 47.Van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 48.Schorey J.S., Cheng Y., Singh P.P., Smith V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fröhlich D., Kuo W.P., Frühbeis C., Sun J.-J., Zehendner C.M., Luhmann H.J., Pinto S., Toedling J., Trotter J., Krämer-Albers E.-M. Multifaceted effects of oligodendroglial exosomes on neurons: Impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janas A.M., Sapoń K., Janas T., Stowell M.H.B., Janas T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim. Biophys. Acta (BBA) - Biomembr. 2016;1858:1139–1151. doi: 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Jan A.T., Malik M.A., Rahman S., Yeo H.R., Lee E.J., Abdullah T.S., Choi I. Perspective Insights of Exosomes in Neurodegenerative Diseases: A Critical Appraisal. Front. Aging Neurosci. 2017;9:317. doi: 10.3389/fnagi.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ugalde C.L., Finkelstein D.I., Lawson V.A., Hill A.F. Pathogenic mechanisms of prion protein, amyloid-β and α-synuclein misfolding: The prion concept and neurotoxicity of protein oligomers. J. Neurochem. 2016;139:162–180. doi: 10.1111/jnc.13772. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A., Singh A., Ekavali A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Mendez M.F. Early-Onset Alzheimer Disease. Neurol. Clin. 2017;35:263–281. doi: 10.1016/j.ncl.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jucker M., Walker L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nikolac Perkovic M., Pivac N. Genetic Markers of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019;1192:27–52. doi: 10.1007/978-981-32-9721-0_3. [DOI] [PubMed] [Google Scholar]
- 57.Tysnes O.-B., Storstein A. Epidemiology of Parkinson’s disease. J. Neural. Transm. (Vienna) 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 58.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 59.Sato H., Arawaka S., Hara S., Fukushima S., Koga K., Koyama S., Kato T. Authentically phosphorylated α-synuclein at Ser129 accelerates neurodegeneration in a rat model of familial Parkinson’s disease. J. Neurosci. 2011;31:16884–16894. doi: 10.1523/JNEUROSCI.3967-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karampetsou M., Ardah M.T., Semitekolou M., Polissidis A., Samiotaki M., Kalomoiri M., Majbour N., Xanthou G., El-Agnaf O.M.A., Vekrellis K. Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice. Sci. Rep. 2017;7:16533. doi: 10.1038/s41598-017-15813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng X.-S., Geng W.-S., Jia J.-J., Chen L., Zhang P.-P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018;10:109. doi: 10.3389/fnagi.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGinty R.J., Mirkin S.M. Cis- and Trans-Modifiers of Repeat Expansions: Blending Model Systems with Human Genetics. Trends Genet. 2018;34:448–465. doi: 10.1016/j.tig.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caron N.S., Wright G.E., Hayden M.R. Huntington Disease. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington; Seattle, WA, USA: 1993. [PubMed] [Google Scholar]
- 64.Juenemann K., Weisse C., Reichmann D., Kaether C., Calkhoven C.F., Schilling G. Modulation of mutant huntingtin N-terminal cleavage and its effect on aggregation and cell death. Neurotox. Res. 2011;20:120–133. doi: 10.1007/s12640-010-9227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swinnen B., Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014;10:661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- 66.Abel O., Powell J.F., Andersen P.M., Al-Chalabi A. ALSoD: A user-friendly online bioinformatics tool for amyotrophic lateral sclerosis genetics. Hum. Mutat. 2012;33:1345–1351. doi: 10.1002/humu.22157. [DOI] [PubMed] [Google Scholar]
- 67.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., Van Den Berg L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 2017;3:1–19. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 68.Peralta O.A., Eyestone W.H. Quantitative and qualitative analysis of cellular prion protein (PrP(C)) expression in bovine somatic tissues. Prion. 2009;3:161–170. doi: 10.4161/pri.3.3.9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aguzzi A., Haass C. Games played by rogue proteins in prion disorders and Alzheimer’s disease. Science. 2003;302:814–818. doi: 10.1126/science.1087348. [DOI] [PubMed] [Google Scholar]
- 70.Prusiner S.B. Neurodegenerative diseases and prion, Shattuck lecture. N. Engl. J. Med. 2001;344:1516–1526. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 71.Huang L.-K., Chao S.-P., Hu C.-J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020;27:18. doi: 10.1186/s12929-019-0609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mullard A. Anti-amyloid failures stack up as Alzheimer antibody flops. Nat. Rev. Drug Discov. 2019;18:327. doi: 10.1038/d41573-019-00064-1. [DOI] [PubMed] [Google Scholar]
- 73.Foster J.K., Verdile G., Bates K.A., Martins R.N. Immunization in Alzheimer’s disease: Naïve hope or realistic clinical potential? Mol. Psychiatry. 2009;14:239–251. doi: 10.1038/mp.2008.115. [DOI] [PubMed] [Google Scholar]
- 74.Habicht G., Haupt C., Friedrich R.P., Hortschansky P., Sachse C., Meinhardt J., Wieligmann K., Gellermann G.P., Brodhun M., Götz J. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Aβ protofibrils. Proc. Natl. Acad. Sci. USA. 2007;104:19232–19237. doi: 10.1073/pnas.0703793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu R., McAllister C., Lyubchenko Y., Sierks M.R. Proteolytic antibody light chains alter beta-amyloid aggregation and prevent cytotoxicity. Biochemistry. 2004;43:9999–10007. doi: 10.1021/bi0492354. [DOI] [PubMed] [Google Scholar]
- 76.Liu R., Yuan B., Emadi S., Zameer A., Schulz P., McAllister C., Lyubchenko Y., Goud G., Sierks M.R. Single chain variable fragments against beta-amyloid (Abeta) can inhibit Abeta aggregation and prevent abeta-induced neurotoxicity. Biochemistry. 2004;43:6959–6967. doi: 10.1021/bi049933o. [DOI] [PubMed] [Google Scholar]
- 77.Paganetti P., Calanca V., Galli C., Stefani M., Molinari M. beta-site specific intrabodies to decrease and prevent generation of Alzheimer’s Abeta peptide. J. Cell Biol. 2005;168:863–868. doi: 10.1083/jcb.200410047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller T.W., Messer A. Intrabody applications in neurological disorders: Progress and future prospects. Mol. Ther. 2005;12:394–401. doi: 10.1016/j.ymthe.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 79.He P., Xin W., Schulz P., Sierks M.R. Bispecific Antibody Fragment Targeting APP and Inducing α-Site Cleavage Restores Neuronal Health in an Alzheimer’s Mouse Model. Mol. Neurobiol. 2019;56:7420–7432. doi: 10.1007/s12035-019-1597-z. [DOI] [PubMed] [Google Scholar]
- 80.Periquet M., Fulga T., Myllykangas L., Schlossmacher M.G., Feany M.B. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J. Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giasson B.I., Murray I.V., Trojanowski J.Q., Lee V.M. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J. Biol. Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 82.Eliezer D. The mysterious C-terminal tail of alpha-synuclein: Nanobody’s guess. J. Mol. Biol. 2013;425:2393–2396. doi: 10.1016/j.jmb.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 83.El-Turk F., Newby F.N., De Genst E., Guilliams T., Sprules T., Mittermaier A., Dobson C.M., Vendruscolo M. Structural effects of two camelid nanobodies directed to distinct C-terminal epitopes on α-synuclein. Biochemistry. 2016;55:3116–3122. doi: 10.1021/acs.biochem.6b00149. [DOI] [PubMed] [Google Scholar]
- 84.Bhatt M.A., Messer A., Kordower J.H. Can intrabodies serve as neuroprotective therapies for Parkinson’s disease? Beginning thoughts. J. Parkinson’s Dis. 2013;3:581–591. doi: 10.3233/JPD-130252. [DOI] [PubMed] [Google Scholar]
- 85.De Genst E., Messer A., Dobson C.M. Antibodies and protein misfolding: From structural research tools to therapeutic strategies. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014;1844:1907–1919. doi: 10.1016/j.bbapap.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 86.Gupta S., Colby D.W. Protein misfolding detected early in pathogenesis of transgenic mouse model of Huntington disease using amyloid seeding assay. J. Biol. Chem. 2012;287:9982–9989. doi: 10.1074/jbc.M111.305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller T.W., Messer A. Gene Therapy of the Central Nervous System: From Bench to Bedside. Academic Press; Amsterdam, The Netherlands: Boston, MA, USA: 2006. Gene therapy for CNS diseases using Intrabodies; pp. 133–150. [Google Scholar]
- 88.Cattaneo E., Zuccato C., Tartari M. Normal huntingtin function: An alternative approach to Huntington’s disease. Nat. Rev. Neurosci. 2005;6:919–930. doi: 10.1038/nrn1806. [DOI] [PubMed] [Google Scholar]
- 89.Bortvedt S.F., McLear J.A., Messer A., Ahern-Rindell A.J., Wolfgang W.J. Cystamine and intrabody co-treatment confers additional benefits in a fly model of Huntington’s disease. Neurobiol. Dis. 2010;40:130–134. doi: 10.1016/j.nbd.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cuervo A.M., Wong E. Chaperone-mediated autophagy: Roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bauer P.O., Goswami A., Wong H.K., Okuno M., Kurosawa M., Yamada M., Miyazaki H., Matsumoto G., Kino Y., Nagai Y., et al. Harnessing chaperone-mediated autophagy for the selective degradation of mutant huntingtin protein. Nat. Biotechnol. 2010;28:256–263. doi: 10.1038/nbt.1608. [DOI] [PubMed] [Google Scholar]
- 92.Popiel H.A., Burke J.R., Strittmatter W.J., Oishi S., Fujii N., Takeuchi T., Toda T., Wada K., Nagai Y. The Aggregation Inhibitor Peptide QBP1 as a Therapeutic Molecule for the Polyglutamine Neurodegenerative Diseases. J. Amino Acids. 2011;2011:265084. doi: 10.4061/2011/265084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin Z.-H., Wang Y., Sapp E., Cuiffo B., Wanker E., Hayden M.R., Kegel K.B., Aronin N., DiFiglia M. Huntingtin bodies sequester vesicle-associated proteins by a polyproline-dependent interaction. J. Neurosci. 2004;24:269–281. doi: 10.1523/JNEUROSCI.1409-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rockabrand E., Slepko N., Pantalone A., Nukala V.N., Kazantsev A., Marsh J.L., Sullivan P.G., Steffan J.S., Sensi S.L., Thompson L.M. The first 17 amino acids of Huntingtin modulate its sub-cellular localization, aggregation and effects on calcium homeostasis. Hum. Mol. Genet. 2007;16:61–77. doi: 10.1093/hmg/ddl440. [DOI] [PubMed] [Google Scholar]
- 95.Pozzi S., Thammisetty S.S., Codron P., Rahimian R., Plourde K.V., Soucy G., Bareil C., Phaneuf D., Kriz J., Gravel C., et al. Virus-mediated delivery of antibody targeting TAR DNA-binding protein-43 mitigates associated neuropathology. J. Clin. Invest. 2019;129:1581–1595. doi: 10.1172/JCI123931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luginbühl B., Kanyo Z., Jones R.M., Fletterick R.J., Prusiner S.B., Cohen F.E., Williamson R.A., Burton D.R., Plückthun A. Directed Evolution of an Anti-prion Protein scFv Fragment to an Affinity of 1 pM and its Structural Interpretation. J. Mol. Biol. 2006;363:75–97. doi: 10.1016/j.jmb.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 97.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McNamara R.P., Costantini L.M., Myers T.A., Schouest B., Maness N.J., Griffith J.D., Damania B.A., MacLean A.G., Dittmer D.P. Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses. MBio. 2018;9 doi: 10.1128/mBio.02344-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lattanzi L., Federico M. A strategy of antigen incorporation into exosomes: Comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles. Vaccine. 2012;30:7229–7237. doi: 10.1016/j.vaccine.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 100.D’Aloja P., Santarcangelo A.C., Arold S., Baur A., Federico M. Genetic and functional analysis of the human immunodeficiency virus (HIV) type 1-inhibiting F12-HIVnef allele. J. Gen. Virol. 2001;82:2735–2745. doi: 10.1099/0022-1317-82-11-2735. [DOI] [PubMed] [Google Scholar]
- 101.Ferrantelli F., Arenaccio C., Manfredi F., Olivetta E., Chiozzini C., Leone P., Percario Z., Ascione A., Flego M., Di Bonito P., et al. The Intracellular Delivery of Anti-HPV16 E7 scFvs Through Engineered Extracellular Vesicles Inhibits the Proliferation of HPV-Infected Cells. Int. J. Nanomed. 2019;14:8755–8768. doi: 10.2147/IJN.S209366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Bonito P., Chiozzini C., Arenaccio C., Anticoli S., Manfredi F., Olivetta E., Ferrantelli F., Falcone E., Ruggieri A., Federico M. Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation. Int. J. Nanomed. 2017;12:4579–4591. doi: 10.2147/IJN.S131309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar P., Wu H., McBride J.L., Jung K.-E., Kim M.H., Davidson B.L., Lee S.K., Shankar P., Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 104.Cui G., Guo H., Li H., Zhai Y., Gong Z., Wu J., Liu J., Dong Y., Hou S., Liu J. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing. 2019;16:10. doi: 10.1186/s12979-019-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 106.Mullard A. FDA approves SMA gene therapy. Nat. Rev. Drug Discov. 2019;18:488. doi: 10.1038/d41573-019-00101-z. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H., Yang B., Mu X., Ahmed S.S., Su Q., He R., Wang H., Mueller C., Sena-Esteves M., Brown R., et al. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol. Ther. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merkel S.F., Andrews A.M., Lutton E.M., Mu D., Hudry E., Hyman B.T., Maguire C.A., Ramirez S.H. Trafficking of adeno-associated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. J. Neurochem. 2017;140:216–230. doi: 10.1111/jnc.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naso M.F., Tomkowicz B., Perry W.L., Strohl W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klein R.L., Muir D.F., King M.A., Peel A.L., Zolotukhin S., Möller J.C., Krüttgen A., Heymach J.V., Muzyczka N., Meyer E.M. Long-term actions of vector-derived nerve growth factor or brain-derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience. 1999;90:815–821. doi: 10.1016/S0306-4522(98)00537-5. [DOI] [PubMed] [Google Scholar]
- 111.Rivera V.M., Gao G., Grant R.L., Schnell M.A., Zoltick P.W., Rozamus L.W., Clackson T., Wilson J.M. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 112.Leone P., Shera D., McPhee S.W.J., Francis J.S., Kolodny E.H., Bilaniuk L.T., Wang D.-J., Assadi M., Goldfarb O., Goldman H.W., et al. Long-Term Follow-Up After Gene Therapy for Canavan Disease. Sci. Transl. Med. 2012;4:165ra163. doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Challis R.C., Ravindra Kumar S., Chan K.Y., Challis C., Beadle K., Jang M.J., Kim H.M., Rajendran P.S., Tompkins J.D., Shivkumar K., et al. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 2019;14:379–414. doi: 10.1038/s41596-018-0097-3. [DOI] [PubMed] [Google Scholar]
- 114.Huang Q., Chan K.Y., Tobey I.G., Chan Y.A., Poterba T., Boutros C.L., Balazs A.B., Daneman R., Bloom J.M., Seed C., et al. Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS ONE. 2019 doi: 10.1371/journal.pone.0225206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hudry E., Vandenberghe L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron. 2019;101:839–862. doi: 10.1016/j.neuron.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stott S.R.W., Hayat S., Carnwath T., Garas S., Sleeman J.P., Barker R.A. CD24 expression does not affect dopamine neuronal survival in a mouse model of Parkinson’s disease. PLoS ONE. 2017;12:e0171748. doi: 10.1371/journal.pone.0171748. [DOI] [PMC free article] [PubMed] [Google Scholar]