Abstract
Chemotherapy is widely used to treat cancer. The toxic effect of conventional chemotherapeutic drugs on healthy cells leads to serious toxic and side effects of conventional chemotherapy. The application of nanotechnology in tumor chemotherapy can increase the specificity of anticancer agents, increase the killing effect of tumors, and reduce toxic and side effects. Currently, a variety of formulations based on nanoparticles (NPs) for delivering chemotherapeutic drugs have been put into clinical use, and several others are in the stage of development or clinical trials. In this review, after briefly introducing current cancer chemotherapeutic methods and their limitations, we describe the clinical applications and advantages and disadvantages of several different types of NPs-based chemotherapeutic agents. We have summarized a lot of information in tables and figures related to the delivery of chemotherapeutic drugs based on NPs and the design of NPs with active targeting capabilities.
Keywords: chemotherapy, nanoparticle, drug delivery system, patient-friendly for oncology
Introduction
Cancer is an important cause of death worldwide.1 At present, chemotherapy is an important method for treating cancer, but traditional chemotherapy preparations have strong toxic and side effects.2 Patients need to be in the hospital for a long time and require strict clinical care to deal with adverse events caused by chemotherapy. The use of nanoparticles (NPs) to deliver chemotherapeutic drugs is expected to change this situation. Nanotechnology has developed rapidly in recent years, and nanoscale materials have unique physical, chemical, and biological properties.3-5 Especially, the use of nanotechnology for drug delivery, diagnosis, imaging, and treatment is of great interest. Nano-oncology, the application of nano-biotechnology in cancer treatment, is currently the most important application area of nanotechnology. The development of NPs chemotherapy drug delivery systems based on nanotechnology can improve the bioavailability of drugs, improve the solubility of drugs, change the biodistribution of chemotherapy drugs, eliminate drug resistance caused by treatment, and reduce nonspecific toxicity.6-8 In particular, it can reduce the side effects of chemotherapy on patients, reduce the adverse events caused by chemotherapy, improve the quality of life of patients, and prolong the survival time.9-13 Several recent studies have shown that nanomaterials can penetrate biofilms and enter cells, tissues, and organs that larger size particles usually cannot penetrate, delivering drugs to locations that are difficult to reach with conventional chemotherapy drugs.14-18 There are currently several formulations based on NPs delivery on the market, and others are at different stages of development. This review will discuss the application of NPs-based drug delivery systems for the delivery of chemotherapy drugs.
Limitations of Current Tumor Chemotherapy
Cancer chemotherapy refers to the use of chemicals to block the growth or kill cancer cells. Chemotherapy of tumors began in the early 20th century. From the first use of nitrogen mustard as a drug for cancer treatment 70 years ago to the current attempt of developing drugs for specific cancer-related targets, researchers from multiple disciplines have joined forces to seek more effective chemotherapeutic drugs.19 At present, chemotherapy has become an important means of treating tumors, especially playing a vital role in the treatment of undetectable cancer microlesions and free cancer cells.
Conventional chemotherapy mainly works by inhibiting mitosis and interfering with DNA synthesis, leading to the death of rapidly growing and dividing cancer cells. The chemotherapeutic agents are usually nontarget toxic and can also damage healthy tissues, especially fast-growing healthy tissues, such as blood cells and digestive tract skin cells, causing serious unintended and adverse side effects.20-22 Side effects of chemotherapy usually include immunosuppression and bone marrow suppression, gastrointestinal discomfort, anemia, fatigue, hair loss, secondary tumors, infertility, cognitive impairment, organ damage, and so on.23-29 Besides, multi-drug resistance (MDR) is another obstacle to chemotherapy. Once tumor cells acquire MDR, the anticancer effect of chemotherapy drugs will be reduced. Multi-drug resistance is the most important cause of cancer chemotherapy failure.30,31 Because conventional chemotherapy has nontarget toxicity and is prone to MDR, it can only extend the patient’s progression-free survival to a certain extent. In some cases, the side effects of chemotherapy seriously reduce the patient’s quality of life and even lead to patient’s death. Therefore, there is a need to develop new formulations for the treatment of cancer, which are less toxic and can provide patients with a better quality of life.
Nanoparticles as Drug Delivery Vehicles
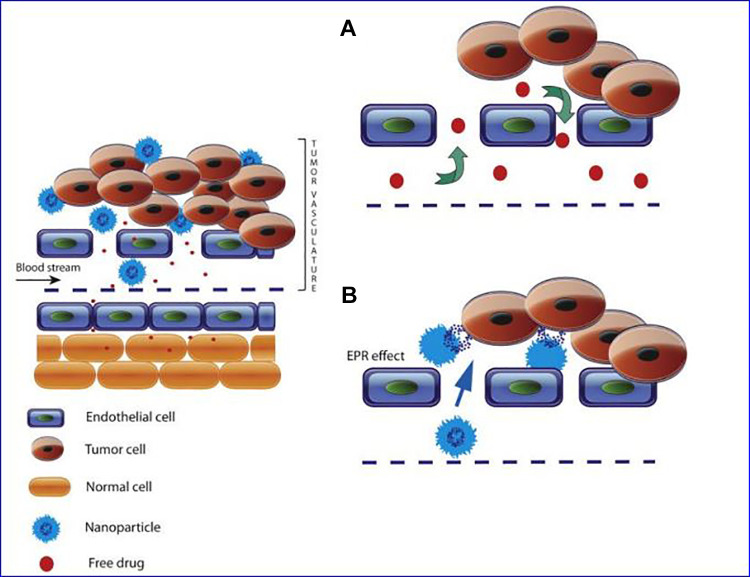
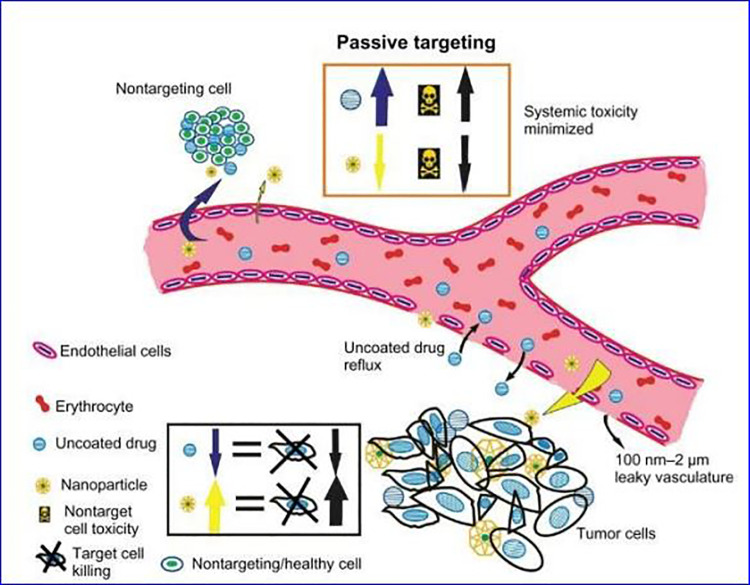
In recent years, the field of nanomedicine has developed rapidly, which will change the diagnosis and treatment of cancer. Nanoparticles are solid colloidal particles that usually have a small particle size (diameter within 10-200 nm). Nanoparticles generally have a large surface area to volume ratio, which allows them to adsorb and contain various types of anticancer agents, such as chemotherapeutic drugs, proteins, DNA, and so on.32-34 Compared to the direct use of free chemotherapy drugs, NPs can deliver chemotherapy drugs with many advantages. Some of them are related to the fact that nano-drugs can improve the solubility of chemotherapeutic drugs and increase the stability of chemotherapeutic drugs; at the same time, intravenous administration of NPs-delivered chemotherapeutic drugs can improve the biodistribution of drugs, extend the circulation time of drugs, and reduce the adverse effects of chemotherapy reaction.35-38 Due to the enhanced permeability and retention (EPR) effect, the use of nanocarriers to deliver chemotherapeutic drugs can preferentially deliver chemotherapeutic drugs to the tumor site, which is because the internal dissection characteristics of solid tumors are different from normal healthy tissues39 as shown in Figure 1. Tumor angiogenesis is tortuous and abnormal, with a gap size of 100 nm ∼2 μm, and most of the lymphatic vessels inside the tumor are folded and compressed. The leaking vasculature and poor lymphatic drainage system inside the solid tumor lead to a pressure difference between the tissue at the center of the tumor and the surrounding tissue. Due to this pressure difference, molecules from about 10 nm to 200 nm preferentially accumulate in the tumor and remain longer. Studies have shown that the retention time of drugs contained in NP is 10 times that of unpackaged drugs, which will eventually return to the vascular system.40-44 Due to the EPR effect, nanocarriers have the ability to passively target tumors. The schematic diagram of passive targeting of NPs is shown in Figure 2.
Figure 1.
Schematic representation of enhanced permeability and retention (EPR) effect. Typical blood vessels from solid tumors contain pores of various sizes that allow the nanoparticles (NPs) and molecules of the drug to enter the interstitium of the tumor tissue. A, The free chemotherapeutic drugs are small in size and reach the tumor site through free diffusion. Only a small amount of drug can reach the tumor site. There is no significant difference in drug concentration between tumor tissues and other healthy tissues. B, The size of the NPs allows them to penetrate the extravascular space through the gap and accumulate inside the tumor, where the carrier releases the drug.41
Figure 2.
Passive targeting of nanoparticles (NPs) to tumor cells. NPs (yellow) can accumulate in the tumor stroma, enhancing the lethality of tumor cells and reducing the systemic toxicity of chemotherapy. Free anticancer drugs (blue) do not have the ability to passively target and will spill over to serious side effects caused by healthy tissues.44
Examples of Nanoparticles used for Chemotherapeutic Drug Delivery
In cancer chemotherapy, nanomedicine has special significance. Table 1 lists some of the NPs that have been used in clinical chemotherapy. In this review, we will discuss the applications, advantages, and limitations of different types of NPs used to deliver chemotherapy drugs.
Table 1.
List of US Food and Drug Administration (FDA)–Approved Nanomedicines for Cancer Treatment.
| Trade name | Description of carrier | Nanoparticle advantage | Indication(s) | Year(s) approved | Ref |
|---|---|---|---|---|---|
| Doxil | Liposomal doxorubicin | Decrease in systemic toxicity of free drug and improved delivery to site of disease | Karposi sarcoma; ovarian cancer; multiple myeloma | 1995 | 45 |
| DaunoXome | Liposomal daunorubicin | Lower systemic toxicity arising from side effects and increased delivery to tumor site | Karposi sarcoma | 1996 | 46 |
| Myocet | Liposomal doxorubicin | Decrease in systemic toxicity of free drug and improved delivery to site of disease | Metastatic breast cancer | 2005 | 47 |
| Onivyde | Liposomal Irinotecan | Lower systemic toxicity arising from side effects and increased delivery to tumor site | Pancreatic cancer | 2015 | 48 |
| DepoCyt | Liposomal cytarabine | Lower systemic toxicity arising from side effects and increased delivery to tumor site | Lymphomatous meningitis | 1996 | 49 |
| Marqibo | Liposomal Vincristine | Lower systemic toxicity arising from side effects and increased delivery to tumor site | Acute lymphoblastic leukemia | 2012 | 50 |
| Abraxane | Albumin-bound paclitaxel nanoparticles | Improved solubility; improved delivery to tumor | Breast cancer; non-small cell lung cancer; pancreatic cancer | 2005 | 51 |
| Eligard | Leuprolide acetate and polymer; PLGH (poly (DL-Lactide-co-glycolide) | Controlled delivery of payload with longer circulation time | Prostate cancer | 2002 | 52 |
Liposomes
Liposomes, illustrated in Figure 3, are phospholipid bilayer vesicles in which drugs can be retained. Liposomes are mainly composed of natural or synthetic phospholipids, have good biocompatibility, are biodegradable, and do not cause immune reactions. Liposomes can deliver both lipophilic and hydrophilic compounds, fat-soluble drugs, and amphiphilic drugs that can be inserted into the liposome bilayer phospholipid membrane, and water-soluble drugs are stored in the aqueous compartment.53 Therefore, liposomes are also regarded as a universal drug carrier that can deliver many different types of drugs.54,55 Liposomes are the most widely used NPs, and they exhibit quite effective capabilities in the following areas: (1) increase the solubility of hydrophobic drugs; (2) improve the biological distribution of chemotherapeutic drugs and the selectivity of therapeutic agents; (3) reduce the cytotoxicity of chemotherapeutic drugs to normal tissues, thereby reducing its toxic side effects; and (4) extend the cycle time of chemotherapeutic drugs and control the release.56-59 In the past few years, many liposome chemotherapeutic agents have observed positive results in the clinic, and some of them have been approved by the European Medicines Agency and the US Food and Drug Administration (FDA) for the treatment of various Kind of cancer. Table 1 lists some of the liposome chemotherapeutic agents approved by the FDA, and there are a variety of anticancer drug-encapsulated liposome preparations at different stages of clinical trials or waiting for approval, as shown in Table 2.
Figure 3.
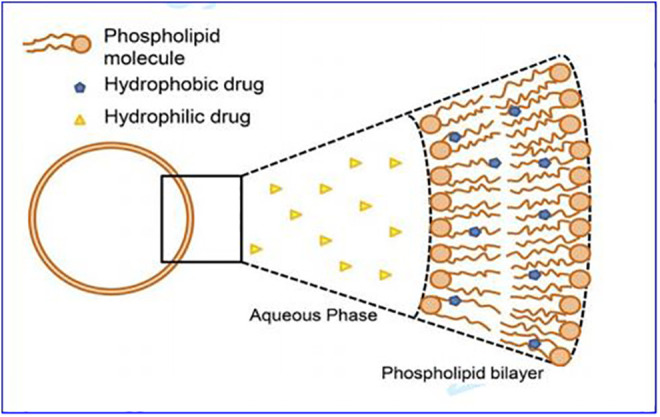
Schematic diagram of liposome structure.55
Table 2.
Liposomal Formulations of Anticancer Drugs in Clinical Trials.
| Product name | Encapsulated drugs | Type of liposomes | Indications | Status | Ref |
|---|---|---|---|---|---|
| Alocrest | Vinorelbine | Optisomes | NSCLC and breast cancers, non-Hodgkin lymphoma, Hodgkin disease | Phase I | 60 |
| ATI-1123 | Docetaxel | Protein-stabilized liposomes | NSCLC, gastric, pancreatic cancer, and soft tissue sarcoma | Phase I | 61 |
| MCC-465 | Doxorubicin | Antibody-conjugated PEGylated liposomes | Stomach cancer | Phase I | 62 |
| NanoVNB | Vinorelbine | PEGylated liposomes | Advanced solid tumors | Phase I | 63 |
| IHL-305 | Irinotecan | PEGylated liposome | Advanced solid tumors | Phase I | 64 |
| EndoTAG-1 | Paclitaxel | Cationic liposomes | Solid tumors | Phase II | 65,66 |
| LEP-ETU | Paclitaxel | Anionic liposomes | Metastatic breast cancer | Phase II | 67 |
| MBP-426 | Oxaliplatin | Tf-conjugated liposomes | Gastric, gastroesophageal, esophageal adenocarcinomas | Phase II | 68 |
| CPX-351 | Cytarabine and daunorubicin (5:1) | Bilamellar liposomes | Acute myeloid leukemia | Phase III | 69,70 |
| Lipoplatin | Cisplatin | PEGylated liposomes | NSCLC, gastric, pancreatic, breast, head and neck cancers | Phase III | 71,72 |
| MM-398 (PEP02) | Irinotecan | PEGylated liposomes | Metastatic pancreatic cancer | Phase III | 73 |
| ThermoDox | Doxorubicin | Lysolipid temperature sensitive liposomes | Hepatocellular carcinoma and breast cancer | Phase III | 74 |
Doxil, the first FDA-approved liposome chemotherapeutic agent.45 Doxil is a PEGylated liposomal DOX formulations. By using polyethylene glycol (PEG) to modify the surface of liposomes, the liposomes can be given stealth properties. Conjugation of PEG to the surface of liposome phospholipid bilayer can reduce the interaction between liposomes with plasma protein through steric hindrance, which will reduce the adsorption of plasma protein to the surface of the liposome. In turn, it can reduce the conditioned effect and clearance of the reticuloendothelial system (RES) on liposomes.75,76 PEGylated liposomes further prolong the circulation time of liposomes and extend their half-life in circulation, therefore increasing the accumulation of liposomes at the tumor site. Doxil has shown highly selective tumor localization (Figure 4) and excellent pharmacokinetic properties in clinical applications.77 Under the condition that the dose of DOX is 50 mg/m2, the area under the curve (AUC) of Doxil is about 300 times that of the free drug. Clearance and volume of distribution are drastically reduced (at least 250-fold and 60-fold, respectively).78 Tumor DOX levels peak between 3 and 7 days after Doxil administration, indicating that tumor cells exposed to the drug are much higher and longer. Compared to the free drug DOX, Doxil not only has a better therapeutic effect but also significantly reduces the side effects of DOX and has better tolerability. The main side effects of DOX include bone marrow suppression, hair loss, vomiting and diarrhea, and tissue damage. DOX has a cumulative dose-dependent cardiotoxicity, and the usual cumulative dose of conventional DOX for chemotherapy is 550 mg/m2.79 In general, Doxil can greatly improve the patient’s daily compliance and particularly important is a significant reduction in cardiac toxicity (compared to standard care), which can increase the cumulative dose, thereby extending the treatment time. However, although Doxil is superior to doxorubicin in overall tolerability, similar to most liposomal formulations, Doxil still observes the side effects observed with 2 atypical standard of care drugs. The first and most important one causes grade 2 or grade 3 desquamative dermatitis, which is called palmar-plantar erythrocyte paresthesia or “hand–foot syndrome.” The second effect is an infusion-related reaction, which is characterized by flushing and shortness of breath. This symptom can be alleviated by slowing down the infusion rate and appropriate medication.45 At present, liposome-encapsulated chemotherapeutic drugs represented by Doxil have been widely used in clinics. With the continuous development of liposome technology, more types of liposome preparations are at different stages of research, such as enzyme-sensitive liposomes, magnetic liposomes, redox-sensitive liposomes, ultrasound-responsive liposomes, and liposomes for photodynamic therapy.
Figure 4.
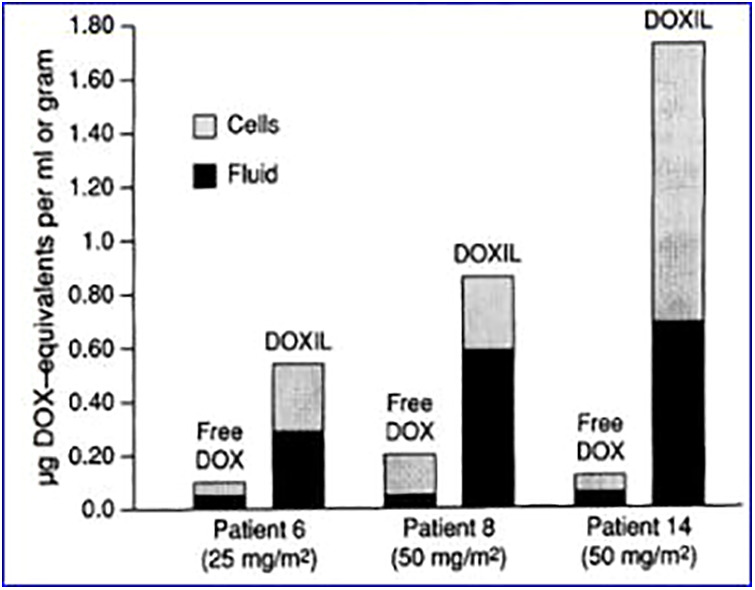
Doxorubicin levels in patients’ tumor biopsies, comparing free DOX and Doxil.77
Protein-Based Nanocarriers
Protein is formed by one or more polypeptide chains with a certain spatial conformation and biological activity. Protein-based NPs have the following advantages for the delivery of tumor chemotherapy drugs: (1) Proteins are endogenous and have excellent biocompatibility. (2) Proteins have biological activity. Protein-based nanoplatforms can inherit this effect without further surface modification, which will greatly simplify the synthesis process. (3) Amino acid residues constituting the basic unit of protein have various functional groups (such as -COOH and -SH) that can be used in combination with chemotherapeutic drugs, which imparts function expansibility to protein.80 With paclitaxel (PTX) preparation (Abraxane) based on human serum albumin (HSA) obtained FDA approval in 2005,81 HSA has been widely studied as a chemotherapeutic drug carrier. Human serum albumin is the main component in serum, consisting of 585 amino acid residues, with a molecular weight of 66.5 kDa. The approximate 3-dimensional shape of HSA can be described as a heart shape82 as shown in Figure 5. Human serum albumin contains 3 homologous domains I, II, and III, and each domain also includes 2 separate subdomains. Its characteristic structure is conducive to the combination of chemotherapeutic drugs.83,84
Figure 5.
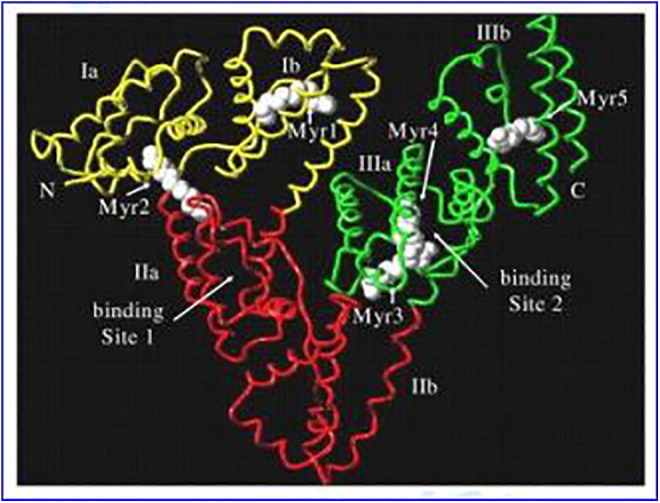
X-ray structure of human serum albumin.85
Abraxane has achieved great clinical success, clinical data show Abraxane treatment results in an improvement in overall response and survival. Compared to the use of PTX, treatment with Abraxane can prolong the average survival time of patients by 2.76 months.86,87 At the same time, Abraxane is better tolerated than PTX. Paclitaxel is a hydrophobic chemotherapeutic drug, and toxic-solubilizing agents such as polyoxyethylene castor oil/PEG-35 castor oil/ethanol are often used to administer the drug.88,89 Polyoxyethylene castor oil can cause severe allergic reactions and even life-threatening hypersensitivity reactions. In order to minimize the risk of hypersensitivity, clinical use of corticosteroids such as dexamethasone is often used to pretreat patients. Even so, 40% of all patients still have mild hypersensitivity reactions, with adverse reactions such as bronchospasm, urticaria, abdominal and limb pain, and so on.41,90 Abraxane eliminates the toxicity associated with polyoxyethylene castor oil to the greatest extent. Patients receiving Abraxane do not need to use corticosteroids in advance to prevent hypersensitivity, and they are easier to administer. The risk of allergic reactions is significantly reduced, making patients less time in hospital and easier to care for. It is worth noting that relevant clinical studies have shown that Abraxane requires a higher dose of 50% than paclitaxel to achieve a better tumor response.86 The cost of treatment with Abraxane is relatively high, which limits its application to a certain extent. In addition to Abraxane, the use of protein-based carriers to deliver other chemotherapeutic drugs, such as rapamycin, doxorubicin, and methotrexate, is also under study and has bright prospects.
Actively Targeted Drug Delivery Nanoparticles
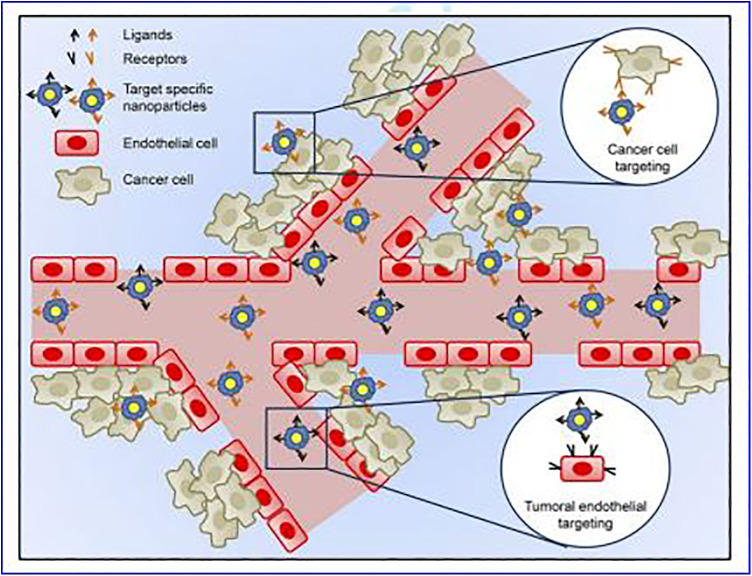
In addition to passive targeting, NPs drug delivery systems can be modified to be more selective for cancer cells through active targeting. In active targeting, specific ligands recognized by cells at the tumor site (Table 3) are coupled to the surface of the NPs, allowing them to interact specifically with tumor cells, as shown in Figure 6. Nanoparticles with active targeting should have the following characteristics: (1) It should be determined that there is a sufficient amount of target on the surface of the tumor cell (overexpression) to provide a good opportunity for targeting NPs to firmly bind to the cancer cell. (2) Targeting ligands are internalized and promote the internalization of the carrier and the anticancer drug combined with the carrier. (3) The specific ligand should not affect its specific binding characteristics and long-term circulating activity in the blood attached to the surface of drug-loaded nanocarriers. (4) The drug released from the carrier inside the tumor or inside the tumor cell should release the therapeutic concentration of the drug and be maintained for a reasonable period.91-93
Table 3.
Specific Cell Surface Moieties Targeted by Nanoparticles for Use in Cancer Therapy.
| Specific cell surface moieties | Targeting ligands | Cancer types | Comments | Ref |
|---|---|---|---|---|
| Folate receptor (FR) | Folate | Human squamous cell oral carcinoma (KB) | In vivo, F-PEG-liposomal UA exhibited greater AUC and half-life than free UA by 6-fold and 9.8-fold, respectively. F-PEG-liposomal UA reduced tumor volume by 55% compared with the control. Animal life span was 56, 47, and 42 days for F-PEG-liposomal UA, PEG-liposomal UA, or free UA, respectively. | 94 |
| Folate receptor (FR) | Folate | Mouse breast cancer cell line (4T1.2) human breast cancer cell line (MCF-7) and drug-resistant cancer cell line (NCI/ADR-RES) | Compared with free DOX and Doxil, FA-coupled DOX-loaded nanomicelle carriers show significantly enhanced tumor suppression with minimal toxicity. Compared with free DOX, the maximum tolerated dose is increased by about 1.5 times | 95 |
| Folate receptor (FR) | Folate | Mouse lymphoma expressing FR (J6456-FR) | In vivo, the DOX level in J6456-FR tumors was 17-fold higher for F-liposomes compared with PEGliposomes. The DOX level was also lower in ascitic fluid (2.25-fold) and plasma fluid (14-fold) when DOX was delivered by F-liposomes compared to PEGliposomes. | 96 |
| Folate receptor (FR) | Folate | Human cervical cancer (HeLa); human lung cancer (A549) | In vitro, FA-albumin NPs have higher cytotoxicity and cell uptake activity than free PTX and non-targeted PTX albumin NPs; in vivo, FA-albumin NPs can accumulate at the tumor site and have the most Good treatment effect. | 97 |
| Transferrin receptor (TfR) | Holo-transferrin | Hepatocellular carcinoma (HepG2) | In vitro, compared with PEG-liposomes, free DOX, Tf-liposomes have higher cytotoxicity; In vivo, Tf-liposomes have the best therapeutic effect. The tumor AUC after 96 hours was Tf-liposome> PEG-liposome> free DOX. |
98 |
| Transferrin receptor (TfR) | Holo-transferrin | Human gastric cancer (MKN45P) | In vivo, the levels of cisplatin in tumor cells increased significantly when treated with Tf-PEG-liposomes compared to non-targeted liposomes, and free cisplatin. The treatment of mice bearing tumor xenografts with TfR-targeted formulation significantly prolonged the survival time of these animals compared to non-targeted liposomes, and free cisplatin. | 99 |
| CD44 receptor | Hyaluronic acid (HA) | Murine mammary (4T1) and human breast cancer (T47D) | In vitro, HA-liposomes have higher cytotoxicity than free PTX and non-targeted PTX liposomes In vivo, HA-liposomes can accumulate at the tumor site and have the best therapeutic effect. |
100 |
| CD44 receptor | Hyaluronic acid (HA) | Mouse colon carcinoma (C-26), human adenocarcinoma (PANC-1) | In vivo, the order of DOX tumor accumulation was HA-liposomes > Doxil > non-targeted liposomes > free DOX. The order was reversed in tumor-free organs. A significant decrease in tumor growth and a marked increase in animal life span were observed across different tumor-types when treated with HA-liposomal DOX compared with the other 3 treatments. | 101 |
| EGFR | Anti-EGFR antibody | Human breast cancer (MDA-MB- 468), human glioblastoma (U87) | In vivo, anti-EGFR-liposomes were internalized more efficiently than non-targeted liposomes (92 vs 5%). Anti-EGFR-liposomes were able to improve the anticancer efficacy of various drugs in mice bearing tumors compared with non-targeted liposomes and free drugs. | 102 |
| HER2 | Anti-HER-2 | Human breast cancer (MCF-7) | In animal models, the cure rate of anti-HER2 immunoliposome-dox reached 50%, and anti-HER2 immunoliposome-dox was also superior to combinations consisting of free MAb plus free dox or free MAb plus liposomal dox. | 103 |
| HER2 | Anti-HER-2 | Human breast cancer (SKBR3) | In vitro, the cytotoxicity of PTX/RAP to immunoliposomes increased, which may be due to increased uptake mediated by HER2 binding; immunoliposomes were better able to control tumor growth in vivo, with tumor volume averages corresponding to 25.27%, 44.38%, and 47.78% of tumor volumes of untreated control, PTX/RAP solution, and control liposomes, respectively. | 104 |
Figure 6.
Active targeting of nanoparticles (NPs) to cancer tumors.55 Active targeting can only occur once passive targeting is completed. In active targeting, specific ligands recognized only by cells at the disease site are coupled on to the surface of nanoparticles, allowing them to interact specifically with these cells. For the treatment of cancer, there are 2 cellular targets in which nanoparticles can be directed to via active targeting, namely, cancer cells, and tumoral endothelium.
Folate-Linked NPs
Folic acid (FA) is a member of the vitamin B family and plays a key role in DNA synthesis and cell proliferation. Human malignancies rely heavily on the biosynthesis of de novo purine and pyrimidine nucleotides. Folic acid plays a key role in the carbon donor of de novo biosynthesis of nucleotides required for DNA replication.105,106 Folic acid receptor (FR) is a tumor marker that binds firmly to its substrate folate. It has been found that FRs are significantly overexpressed on the cell surface of a series of solid tumors including kidney, ovary, lung, bladder, breast, pancreas, and colon.107,108 Studies show that FRs show a high affinity for FA-modified NPs, and FRs internalize FA through receptor-mediated endocytosis.109,110 The study of Ulbrich et al confirmed that the covalent conjugation of FA and HSA NP increases the uptake of cancer cells by NP.111 In the study by Lu et al, the coupling of FA to the surface of DOX-loaded nano-micelle carriers showed higher uptake of DOX by cells in vitro and significantly enhanced antitumor activity with minimal adverse effects in vivo compared to free DOX and liposome Doxorubicin Preparation, Doxil.95
Transferrin-Linked NPs
Transferrin (Tf) is a serum glycoprotein that primarily mediates iron uptake by cells. Transferrin binds to the transferrin receptor (TfR), transports iron into the cell through the blood, and then is internalized by endocytosis mediated by TfR.112 Transferrin is an important protein involved in the regulation of iron homeostasis and cell growth. The high-level expression of TfR in cancer cells may be 100 times higher than the average expression of normal cells, and this receptor is an attractive target for cancer treatment.113,114 Currently, the effectiveness of TfR targeting cancer cells has been studied in vivo and in vitro. In the study by Kobayashi et al, it was found that Tf-conjugated liposome DOX is 3.6 times more cytotoxic than free DOX, showing higher accumulation of cellular DOX.115 In addition, Tf-conjugated NPs have the potential to deliver drugs across the blood–brain barrier. Transferrin-conjugated 5-florouracil 99mTc-DTPA bearing liposomes exhibited 17-fold and 13-fold higher brain uptake compared to free 99mTc-DTPA and non-targeted liposomes.116
Hyaluronic Acid–Linked NPs
Hyaluronic acid (HA) is a natural polyanionic polysaccharide that is the main component of the extracellular matrix and is essential for cell growth, proliferation, and adhesion. The CD44 receptor is a principal cell surface receptor for HA, which overexpressed in many types of cancer, including breast cancer, colorectal cancer, lung cancer, and malignant melanoma.117 CD44 is involved in the regulation of cancer cell proliferation, differentiation, and migration. The interaction of CD44 and HA is also closely related to tumor growth and progression.118 Hyaluronic acid as an active targeting ligand has high specificity, high biocompatibility, low toxicity, and biodegradability making it a cancer-targeting ligand with NPs for chemotherapeutic drugs.119 In the study by Ravar et al, HA electrostatically adsorbed PTX liposomes were prepared. In vitro, compared to free PTX and non-targeted liposomes, HA liposomes are more easily taken up by tumor cells and have stronger cytotoxicity. In vivo, HA liposomes can aggregate more in tumor site and enhance the therapeutic effect of PTX.100 In the study, Zhong et al synthesized a new endosomal pH-activated prodrug micelle based on HA-b-dendritic oligoglycerol; HA-micelles can be highly aggregated at the tumor site (6.19% ID/g at 12-h post injection) with minimal side effects, completely inhibited tumor growth during the 55-day experimental period, and achieved a 100% survival rate.120
Anti-Human Epidermal Growth Factor Receptor 2 Antibody–Labeled NPs
Human epidermal growth factor receptor 2 (HER2) is a tyrosine kinase bound to the surface of cell membranes, which is usually regulated in a complex manner leading to cell growth, survival, and differentiation. Overexpression of HER2 is found in nearly 20% of breast cancers and is associated with poor prognosis.121 Trastuzumab is a humanized monoclonal antibody (mAb) against the HER2 epitope, which can improve the clinical benefit of first-line chemotherapy in patients with metastatic breast cancer that overexpress HER2.122 Previous studies in vivo and in vitro confirmed that PEGylated DOX liposome conjugated anti-HER2 antibody fragments are specific for HER2. Among the 2 HER-2 overexpressing solid tumor cell lines, human breast cancer (SKBR-3) and human gastric cancer (N-87), HER-2 targeted liposomes had 10 to 20 times higher in vitro binding than non-targeted liposomes.123 Park et al studied the pharmacokinetics and therapeutic efficacy of anti-HER2 immunoliposomes containing doxorubicin in animal models. Anti-HER2 immunoliposome-doxorubicin produced enhanced antitumor efficacy through targeted delivery and showed more excellent treatment effect.103
Conclusions
Nano-oncology is a young science. The targeted delivery of drugs through NPs has shown the therapeutic potential to improve the efficacy of cancer treatment compared to traditional chemotherapy and radiation therapy. Passive targeting can accumulate NP in the tumor area by using the EPR effect, prolong the circulation time of drugs in the body, increase the exposure time of tumor cells in cytotoxic drugs, and significantly reduce toxic side effects. The use of NPs to deliver incompatible drugs can significantly improve the solubility of drugs, avoid the use of toxic prosolvents, simplify the administration method, reduce the difficulty in nursing care for patients with chemotherapy, and patients do not even need to be hospitalized, which improves the quality of life of patients. Active targeting uses different molecules overexpressed in tumor cells to design selective NP-based drug delivery systems that recognize specific targets. Nanoparticles with active targeting function can not only accumulate the drug in the tumor site but also be internalized by the target cells, thereby generating high intracellular drug concentration and bypassing MDR. The benefits of active targeted drug delivery systems are expected to be huge compared to equivalent passive targeted drug delivery systems. There is no doubt that nanocarriers, especially NPs-based drug delivery systems, will exist as the main treatment in the future. We can expect the emergence of many NPs for drug delivery applications, which will change the chemotherapy of cancer.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shiyan Dong  https://orcid.org/0000-0003-2912-8895
https://orcid.org/0000-0003-2912-8895
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Kang C, Sun Y, Zhu J, et al. Delivery of nanoparticles for treatment of brain tumor. Curr Drug Metabol. 2016;17(2):745–754. [DOI] [PubMed] [Google Scholar]
- 3. Sun J, Yang Z, Teng L. Nanotechnology and microtechnology in drug delivery systems. Dose-Response. 2020;18(2):155932582090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Z, Chang L, Li W, Xie J. Novel biomaterials and biotechnology for nanomedicine. Euro J BioMed Res. 2015;1:1–2. [Google Scholar]
- 5. Yang Z, Ma Y, Zhao H, Yuan Y, Kim BYS. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;12(2):1590. [DOI] [PubMed] [Google Scholar]
- 6. Sun J, Kormakov S, Liu Y, Huang Y, Wu D, Yang Z. Recent progress in metal-based nanoparticles mediated photodynamic therapy. Molecules. 2018;23(2):1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu C, Chen Z, Hu Y, Rao Z, Wu W, Yang Z. Nanocrystals: the preparation, precise control and application toward the pharmaceutics and food industry. Curr Pharm Design. 2018;24(3):2425–2431. [DOI] [PubMed] [Google Scholar]
- 8. Yang Z, Xie J, Zhu J, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release. 2016;243:160–171. [DOI] [PubMed] [Google Scholar]
- 9. Xie J, Yang Z, Zhou C, Zhu J, Lee RJ, Teng L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol Adv 2016;34:343–353. [DOI] [PubMed] [Google Scholar]
- 10. Chen Z, Chen Z, Zhang A, Hu J, Wang X, Yang Z. Electrospun nanofibers for cancer diagnosis and therapy. Biomat Sci. 2016;4(5):922–932. [DOI] [PubMed] [Google Scholar]
- 11. Zhou C, Yang Z, Teng L. Nanomedicine based on nucleic acids: pharmacokinetic and pharmacodynamic perspectives. Curr Pharm Biotechnol. 2014;15(3):829–838. [DOI] [PubMed] [Google Scholar]
- 12. Meng F, Sun Y, Lee RJ, et al. Folate receptor-targeted albumin nanoparticles based on microfluidic technology to deliver cabazitaxel. Cancers. 2019;11(3):1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan S, Xing H, Fu X, et al. The effect of photothermal therapy on osteosarcoma with polyacrylic acid-coated gold nanorods. Dose Response. 2018;16(3):1559325818789841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zamboni WC. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 2008;13(3):248–260. [DOI] [PubMed] [Google Scholar]
- 15. Yang Z, Shi J, Xie J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Engine. 2020;4(2):69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Z, Ma X, Ma Y, Yang Z, Yuan Y, Liu C. Core/Shell PEGS/HA hybrid nanoparticle via micelle-coordinated mineralization for tumor-specific therapy. ACS Appl Mater Interfaces. 2020;12(10):12109–12119. [DOI] [PubMed] [Google Scholar]
- 17. Hao F, Li Y, Zhu J, et al. Polyethylenimine-based formulations for delivery of oligonucleotides. Curr Med Chem. 2019;26:2264–2284. [DOI] [PubMed] [Google Scholar]
- 18. Yang Z, Yu B, Zhu J, et al. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale. 2014;6(2):9742–9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chabner BA, Roberts TG. Timeline – chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(3):65–72. [DOI] [PubMed] [Google Scholar]
- 20. Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15(4):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmad SS, Reinius MA, Hatcher HM, Ajithkumar TV. Anticancer chemotherapy in teenagers and young adults: managing long term side effects. BMJ. 2016;354(1):i4567. [DOI] [PubMed] [Google Scholar]
- 22. Xie J, Teng L, Yang Z, et al. A polyethylenimine-linoleic acid conjugate for antisense oligonucleotide delivery. BioMed Res Int. 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaikh AY, Shih JA. Chemotherapy-induced cardiotoxicity. Curr Heart Fail Rep. 2012;9(3):117–127. [DOI] [PubMed] [Google Scholar]
- 24. Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22(3):2233–2239. [DOI] [PubMed] [Google Scholar]
- 25. Brydoy M, Fossa SD, Dahl O, Bjoro T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol. 2007;46(3):480–489. [DOI] [PubMed] [Google Scholar]
- 26. Can G, Demir M, Erol O, Aydiner A. A comparison of men and women’s experiences of chemotherapy-induced alopecia. Eur J Oncol Nurs. 2013;17(3):255–260. [DOI] [PubMed] [Google Scholar]
- 27. Vadhan-Raj S. Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents. Semin Hematol. 2009;46(6):S26–S32. [DOI] [PubMed] [Google Scholar]
- 28. Gafter-Gvili A, Fraser A, Paul M, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1(1):CD004386. [DOI] [PubMed] [Google Scholar]
- 29. Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. Jama. 2007;297(11):1207–1215. [DOI] [PubMed] [Google Scholar]
- 30. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Can Lett. 2014;347(6):159–166. [DOI] [PubMed] [Google Scholar]
- 31. Alfarouk KO, Stock CM, Taylor S, et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sha L, Chen Z, Chen Z, Zhang A, Yang Z. Polylactic acid based nanocomposites: promising safe and biodegradable materials in biomedical field. Int J Polym Sci. 2016;2016(3):1–11. [Google Scholar]
- 33. Chen Z, Wu C, Yang Y, et al. Synthesis and drug delivery of mesoporous silica nanoparticles for cancer therapy. Euro J BioMed Res. 2015;1:30–36. [Google Scholar]
- 34. Seno M, Yang X, Yang S, et al. A novel isoquinoline derivative anticancer agent and its targeted delivery to tumor cells using transferrin-conjugated liposomes. Plos One. 2015;10(8):e0136649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3(6):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Z, Zhang A, Wang X, et al. The advances of carbon nanotubes in cancer diagnostics and therapeutics. J Nanomat. 2017;2017(3):1–13. [Google Scholar]
- 37. Chen Z, Zhang A, Yang Z, et al. Application of DODMA and derivatives in cationic nanocarriers for gene delivery. Current Organic Chemistry. 2016;20(17):1813–1839. [Google Scholar]
- 38. Chen Z, Cong M, Hu J, Yang Z, Chen Z. Preparation of functionalized TiO2 nanotube arrays and their applications. Sci Adv Mat. 2016;8(1):1231–1241. [Google Scholar]
- 39. Yu B, Wang X, Zhou C, et al. Insight into mechanisms of cellular uptake of lipid nanoparticles and intracellular release of small RNAs. Pharmaceutical Research. 2014;31(10):2685–2695. [DOI] [PubMed] [Google Scholar]
- 40. Maeda H, Tsukigawa K, Fang J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: next-generation chemotherapeutics and photodynamic therapy—problems, solutions, and prospects. Microcirculation. 2016;23(3):173–182. [DOI] [PubMed] [Google Scholar]
- 41. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: what has been done and the challenges remain ahead. Int J Pharm. 2017;526(4):474–495. [DOI] [PubMed] [Google Scholar]
- 42. Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–419. [DOI] [PubMed] [Google Scholar]
- 43. Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev. 2011;63(3):161–169. [DOI] [PubMed] [Google Scholar]
- 44. Ranganathan R, Madanmohan S, Kesavan A, et al. Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. Int J Nanomed. 2012;7:1043–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barenholz Y. Doxil(R)—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(3):117–134. [DOI] [PubMed] [Google Scholar]
- 46. Krauss AC, Gao X, Li L, et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25(9):2685–2690. [DOI] [PubMed] [Google Scholar]
- 47. Mross K, Niemann B, Massing U, et al. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: an open-label, single-dose study. Cancer Chemother Pharmacol. 2004;54(6):514–524. [DOI] [PubMed] [Google Scholar]
- 48. Drummond DC, Noble CO, Guo Z, et al. Development of a highly stable and targetable nanoliposomal formulation of topotecan. J Control Release. 2010;141(1):13–21. [DOI] [PubMed] [Google Scholar]
- 49. Koller Lucae SKM, Schott H, Schwendener RA. Interactions with human blood in vitro and pharmacokinetic properties in mice of liposomal N-4-octadecyl-1-beta-D-arabinofuranosylcytosine, a new anticancer drug. J Pharmacol Exp Ther. 1997;282(3):1572–1580. [PubMed] [Google Scholar]
- 50. Zhigaltsev IV, Maurer N, Akhong QF, et al. Liposome-encapsulated vincristine, vinblastine and vinorelbine: a comparative study of drug loading and retention. J Control Release. 2005;104(1):103–111. [DOI] [PubMed] [Google Scholar]
- 51. Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23(3):7785–7793. [DOI] [PubMed] [Google Scholar]
- 52. Ohlmann CH, Gross-Langenhoff M. Efficacy and tolerability of leuprorelin acetate (Eligard (R)) in daily practice in Germany: pooled data from 2 prospective, non-interventional studies with 3-or 6-month depot formulations in patients with advanced prostate cancer. UrolInt. 2018;100(2):66–71. [DOI] [PubMed] [Google Scholar]
- 53. Gregoriadis G. Drug entrapment in liposomes. FEBS Lett. 1973;36(3):292–296. [DOI] [PubMed] [Google Scholar]
- 54. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharm. 2015;6(5):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharm Rev. 2016;68(2):701–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maeki M, Kimura N, Sato Y, Harashima H, Tokeshi M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv Drug Deliv Rev. 2018;128:84–100. [DOI] [PubMed] [Google Scholar]
- 57. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tran S, DeGiovanni PJ, Piel B, Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med. 2017;6(3):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. [DOI] [PubMed] [Google Scholar]
- 60. Semple SC, Leone R, Wang J, et al. Optimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activity. J Pharm Sci. 2005;94(5):1024–1038. [DOI] [PubMed] [Google Scholar]
- 61. Mahalingam D, Nemunaitis JJ, Malik L, et al. Phase I study of intravenously administered ATI-1123, a liposomal docetaxel formulation in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;74(6):1241–1250. [DOI] [PubMed] [Google Scholar]
- 62. Hamaguchi T, Matsumura Y, Nakanishi Y, et al. Antitumor effect of MCC-465, pegylated liposomal doxorubicin tagged with newly developed monoclonal antibody GAH, in colorectal cancer xenografts. Cancer Sci. 2004;95(7):608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang SH, Lin CC, Lin ZZ, Tseng YL, Hong RL. A phase I and pharmacokinetic study of liposomal vinorelbine in patients with advanced solid tumor. Invest New Drugs. 2012;30(1):282–289. [DOI] [PubMed] [Google Scholar]
- 64. Infante JR, Keedy VL, Jones SF, et al. Phase I and pharmacokinetic study of IHL-305 (PEGylated liposomal irinotecan) in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;70(5):699–705. [DOI] [PubMed] [Google Scholar]
- 65. Awada A, Bondarenko IN, Bonneterre J, et al. A randomized controlled phase II trial of a novel composition of paclitaxel embedded into neutral and cationic lipids targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann Oncol. 2014;25(4):824–831. [DOI] [PubMed] [Google Scholar]
- 66. Fasol U, Frost A, Buchert M, et al. Vascular and pharmacokinetic effects of EndoTAG-1 in patients with advanced cancer and liver metastasis. Ann Oncol. 2012;23(4):1030–1036. [DOI] [PubMed] [Google Scholar]
- 67. Slingerland M, Guchelaar HJ, Rosing H, et al. Bioequivalence of liposome-entrapped paclitaxel easy-to-use (LEP-ETU) formulation and paclitaxel in polyethoxylated castor oil: a randomized, two-period crossover study in patients with advanced cancer. Clin Ther. 2013;35(12):1946–1954. [DOI] [PubMed] [Google Scholar]
- 68. Van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Adv Drug Deliv Rev. 2013;65:1284–1298. [DOI] [PubMed] [Google Scholar]
- 69. Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine: daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Feldman EJ, Lancet JE, Kolitz JE, et al. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Boulikas T. Clinical overview on Lipoplatin: a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs. 2009;18(8):1197–1218. [DOI] [PubMed] [Google Scholar]
- 72. Stathopoulos GP, Antoniou D, Dimitroulis J, et al. Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol. 2010;21(11):2227–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kang MH, Wang J, Makena MR, et al. Activity of MM-398, nanoliposomal irinotecan (nal-IRI), in Ewing’s family tumor xenografts is associated with high exposure of tumor to drug and high SLFN11 expression. Clin Cancer Res. 2015;21(5):1139–1150. [DOI] [PubMed] [Google Scholar]
- 74. May JP, Li SD. Hyperthermia-induced drug targeting. Expert Opin Drug Deliv. 2013;10(1):511–527. [DOI] [PubMed] [Google Scholar]
- 75. Allen TM, Hansen C, Martin F, Redemann C, Yauyoung A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochimica et biophysica acta. 1991;1066(5):29–36. [DOI] [PubMed] [Google Scholar]
- 76. ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res. 2009;15(6):1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–992. [PubMed] [Google Scholar]
- 78. Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin – review of animal and human studies. Clin Pharmacokinet. 2003;42(3):419–436. [DOI] [PubMed] [Google Scholar]
- 79. Ichikawa Y, Ghanefar M, Bayeva M, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124(2):617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liang K, Chen H. Protein-based nanoplatforms for tumor imaging and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(4):e1616. [DOI] [PubMed] [Google Scholar]
- 81. Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane (R) ABI-007) in the treatment of breast cancer. Int J Nanomed. 2009;4:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. He XM, Carter DC. Atomic-structure and chemistry of human serum-albumin. Nature. 1992;358(1):209–215. [DOI] [PubMed] [Google Scholar]
- 83. Kudarha RR, Sawant KK. Albumin based versatile multifunctional nanocarriers for cancer therapy: fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater Sci Eng C Mater Biol Appl. 2017;81(3):607–626. [DOI] [PubMed] [Google Scholar]
- 84. Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Deliv. 2015;12(2):793–812. [DOI] [PubMed] [Google Scholar]
- 85. Carter DC, Ho JX. Structure of serum-albumin In: Schumaker VN, ed. Advances in Protein Chemistry, Vol 45: Lipoproteins, Apolipoproteins, and Lipases. Elsevier Academic Press Inc; 1994:153–203. [DOI] [PubMed] [Google Scholar]
- 86. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. [DOI] [PubMed] [Google Scholar]
- 87. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122(1):20–30. [DOI] [PubMed] [Google Scholar]
- 88. Liebmann J, Cook JA, Mitchell JB. Cremophor-El, solvent for paclitaxel, and toxicity. Lancet. 1993;342(8884):1428. [DOI] [PubMed] [Google Scholar]
- 89. Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. 2007;6(3):609–621. [DOI] [PubMed] [Google Scholar]
- 90. Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(1-2):179–192. [DOI] [PubMed] [Google Scholar]
- 91. Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(3):1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Biffi S, Voltan R, Bortot B, Zauli G, Secchiero P. Actively targeted nanocarriers for drug delivery to cancer cells. Expert Opin Drug Deliv. 2019;16(1):481–496. [DOI] [PubMed] [Google Scholar]
- 93. Dai LL, Liu JJ, Luo Z, Li MH, Cai KY. Tumor therapy: targeted drug delivery systems. J Mat Chem B. 2016;4(3):6758–6772. [DOI] [PubMed] [Google Scholar]
- 94. Yang G, Yang T, Zhang W, Lu M, Ma X, Xiang G. In vitro and in vivo antitumor effects of folate-targeted ursolic acid stealth liposome. J Agric Food Chem. 2014;62(5):2207–2215. [DOI] [PubMed] [Google Scholar]
- 95. Lu J, Zhao W, Huang Y, et al. Targeted delivery of doxorubicin by folic acid-decorated dual functional nanocarrier. Mol Pharm. 2014;11(11):4164–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shmeeda H, Mak L, Tzemach D, Astrahan P, Tarshish M, Gabizon A. Intracellular uptake and intracavitary targeting of folate-conjugated liposomes in a mouse lymphoma model with up-regulated folate receptors. Mol Cancer Ther. 2006;5(2):818–824. [DOI] [PubMed] [Google Scholar]
- 97. Sun Y, Zhao Y, Teng S, et al. Folic acid receptor-targeted human serum albumin nanoparticle formulation of cabazitaxel for tumor therapy. Int J Nanomedicine. 2019;14:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373(1-2):116–123. [DOI] [PubMed] [Google Scholar]
- 99. Iinuma H, Maruyama K, Okinaga K, et al. Intracellular targeting therapy of cisplatin-encapsulated transferrin-polyethylene glycol liposome on peritoneal dissemination of gastric cancer. Int J Cancer. 2002;99(1):130–137. [DOI] [PubMed] [Google Scholar]
- 100. Ravar F, Saadat E, Gholami M, et al. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J Control Release. 2016;229:10–22. [DOI] [PubMed] [Google Scholar]
- 101. Peer D, Margalit R. Tumor-targeted hyaluronan nanoliposomes increase the antitumor activity of liposomal doxorubicin in syngeneic and human xenograft mouse tumor models. Neoplasia. 2004;6(4):343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mamot C, Drummond DC, Noble CO, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–11638. [DOI] [PubMed] [Google Scholar]
- 103. Park JW, Hong KL, Kirpotin DB, et al. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–1181. [PubMed] [Google Scholar]
- 104. Eloy JO, Petrilli R, Chesca DL, Saggioro FP, Lee RJ, Marchetti JM. Anti-HER2 immunoliposomes for co-delivery of paclitaxel and rapamycin for breast cancer therapy. Eur J Pharm Biopharm. 2017;115:159–167. [DOI] [PubMed] [Google Scholar]
- 105. Raz S, Stark M, Assaraf YG. Folylpoly-gamma-glutamate synthetase: a key determinant of folate homeostasis and antifolate resistance in cancer. Drug Resist Updat. 2016;28(10):43–64. [DOI] [PubMed] [Google Scholar]
- 106. Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012;15(1):183–210. [DOI] [PubMed] [Google Scholar]
- 107. Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat. 2014;17(4-6):89–95. [DOI] [PubMed] [Google Scholar]
- 108. Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13(5):256–262. [DOI] [PubMed] [Google Scholar]
- 109. Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci. 2005;94(4):2135–2146. [DOI] [PubMed] [Google Scholar]
- 110. Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2012;64(2):342–352. [DOI] [PubMed] [Google Scholar]
- 111. Ulbrich K, Michaelis M, Rothweiler F, et al. Interaction of folate-conjugated human serum albumin (HSA) nanoparticles with tumour cells. Int J Pharm. 2011;406(1-2):128–134. [DOI] [PubMed] [Google Scholar]
- 112. Nogueira-Librelotto DR, Codevilla CF, Farooqi A, Rolim CMB. Transferrin-conjugated nanocarriers as active-targeted drug delivery platforms for cancer therapy. Curr Pharm Design. 2017;23(1):454–466. [DOI] [PubMed] [Google Scholar]
- 113. Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121(2):159–176. [DOI] [PubMed] [Google Scholar]
- 114. Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(8):135–146. [DOI] [PubMed] [Google Scholar]
- 115. Kobayashi T, Ishida T, Okada Y, Ise S, Harashima H, Kiwada H. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int J Pharm. 2007;329(1-2):94–102. [DOI] [PubMed] [Google Scholar]
- 116. Soni V, Kohli DV, Jain SK. Transferrin coupled liposomes as drug delivery carriers for brain targeting of 5-florouracil. J Drug Target. 2005;13(4):245–250. [DOI] [PubMed] [Google Scholar]
- 117. Song S, Qi H, Xu J, et al. Hyaluronan-based nanocarriers with CD44-overexpressed cancer cell targeting. Pharm Res. 2014;31(11):2988–3005. [DOI] [PubMed] [Google Scholar]
- 118. Zhao Y, Zhang T, Duan S, Davies NM, Forrest ML. CD44-tropic polymeric nanocarrier for breast cancer targeted rapamycin chemotherapy. Nanomedicine. 2014;10(4):1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mattheolabakis G, Milane L, Singh A, Amiji MM. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target. 2015;23(6):605–618. [DOI] [PubMed] [Google Scholar]
- 120. Zhong Y, Goltsche K, Cheng L, et al. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials. 2016;84:250–261. [DOI] [PubMed] [Google Scholar]
- 121. Hernandez-Blanquisett A, Touya D, Strasser-Weippl K, Ruiz R, St Louis J, Goss P. Current and emerging therapies of HER2-positive metastatic breast cancer. Breast. 2016;29(2):170–177. [DOI] [PubMed] [Google Scholar]
- 122. Sun B, Ranganathan B, Feng SS. Multifunctional poly(D, L-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancer. Biomaterials. 2008;29(3):475–486. [DOI] [PubMed] [Google Scholar]
- 123. Shmeeda H, Tzemach D, Mak L, Gabizon A. Her2-targeted pegylated liposomal doxorubicin: retention of target-specific binding and cytotoxicity after in vivo passage. J Control Release. 2009;136(2):155–160. [DOI] [PubMed] [Google Scholar]