Abstract
Progressive multifocal leukoencephalopathy (PML) is a serious infective disease of the central nervous system that may occur in case of severe immunosuppression or after some treatment for multiple sclerosis (MS) with natalizumab, dimethyl fumarate, and fingolimod. In these case reports, we highlight the importance of differential diagnosis between PML and MS lesions in order to provide rapidly the best treatment option, by discussing the finding of brain (magnetic resonance imaging) MRI suggestive for PML in 2 MS patients, one treated with dimethyl fumarate and the other during natalizumab withdrawal. In both cases, although brain MRI was highly suggestive for PML, the detection of John Cunningham virus-DNA copies in cerebrospinal fluid resulted in negative result. These case reports illustrate the diagnostic process in case of suspected PML, as both patients were diagnosed with suspected PML during a routine brain MRI control, and highlights the importance of providing a strict brain MRI follow-up during dimethyl fumarate treatment, although only a few cases of PML during this therapy have been detected, and during natalizumab suspension phase. In clinical practice, in case of a radiologically suspected case of PML, although not confirmed by the cerebrospinal fluid analysis, the best approach could be to perform a close radiological and clinical monitoring before starting a new MS therapy.
Keywords: multiple sclerosis, progressive multifocal leukoencephalopathy, brain MRI, natalizumab, dimethyl-fumarate, differential diagnosis
Introduction
Progressive multifocal leukoencephalopathy (PML) is a life-threatening infective and demyelinating disease of the central nervous system (CNS), due to the reactivation of polyomavirus John Cunningham virus (JCV).1 PML is usually considered a consequence of immunosuppression,1 following an underlying medical state that affects the immune system directly, or as a result of the use of immunosuppressing medications, thus representing an important concern related to some disease-modifying therapies for multiple sclerosis (MS).1 Several MS treatments have been associated with PML development, including natalizumab, which is responsible for the majority of cases, fingolimod, and dimethyl fumarate.1,2 PML diagnosis requires neurological symptoms, a magnetic resonance imaging (MRI) suggestive of CNS infection, and the presence of JCV-DNA in the cerebrospinal fluid (CSF).3 If the brain MRI is performed in the early PML stages, differentiation from MS lesions can be difficult.4
In this article, we report 2 cases of MS patients showing a radiological picture suggestive for PML, in absence of CSF JCV-DNA positivity.
Case 1
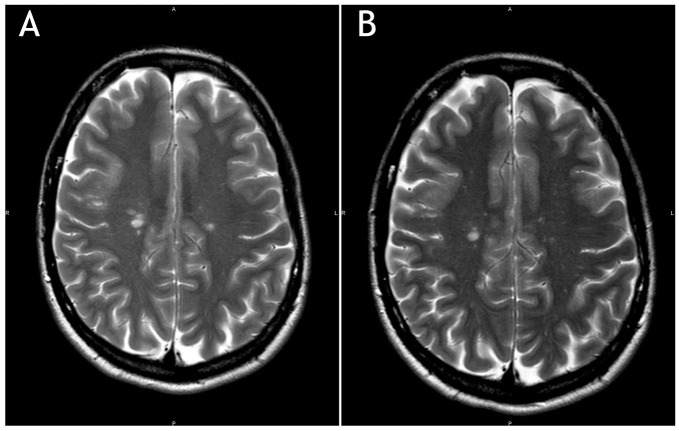
A 42-year-old woman diagnosed with MS in 2003, onset with right optic neuritis, initially treated with interferon-β-1a 44 µg subcutaneously 3 times a week for 9 years, then switched to natalizumab 300 mg intravenously every 28 days, due to high MS activity with more than 2 motor relapses in 12 months and in presence of serum negativity to JCV antibodies. During natalizumab treatment, JCV seroconversion was documented (index = 0.427). After the 24th natalizumab administration, given the PML risk, the patient switched to dimethyl fumarate, after the recommended 3-month washout period. After 17 months of dimethyl fumarate therapy, a routine brain MRI showed few cortico-subcortical hyperintense lesions on T2-weighted images, partly confluent, in the right frontal lobe. One lesion showed contrast enhancement and the findings were suggestive for PML (Figure 1A). Treatment was interrupted and the patient underwent lumbar puncture to perform JCV-DNA with high sensitivity test (10 copies of JCV-DNA in 10 µL of CSF), which resulted negative. The remaining cyto-chemical analysis of CSF was normal. Hematological test showed low lymphocytes level (0.850 × 1000 mg/dL). The brain MRI performed 1 month later showed unmodified findings (Figure 1B), in absence of contrast enhancement. Neurological examination was stable over time. Brain MRIs were shown to 3 different radiology experts in MS with confirmation of a high radiological suspect of PML. During the next months, the stability of the MRI findings along with the negativity of JCV-DNA copies in the CSF led to revision of the diagnosis of PML and consideration of the right frontal lesions as MS disease activity.
Figure 1.
(A) Few cortico-subcortical hyperintense lesions in the right frontal lobe; T2-weighted (T2W). (B) Brain magnetic resonance imaging of the same patient, repeated after 1 month. T2-weighted image.
Case 2
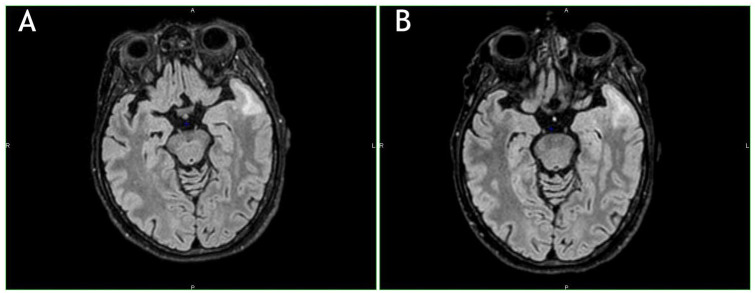
A 61-year-old woman diagnosed with MS in 1995, onset with right arm paresthesia, and then treated with interferon-β-1b 250 µg every other day for 14 years, switched to natalizumab 300 mg intravenously every 28 days for the occurrence of 3 motor and sensitive relapses in 12 months; JCV stratify test was positive (index = 2.584). After 50 natalizumab administrations, considering the high PML risk, the patient decided to stop natalizumab and to switch to fingolimod. Three months after natalizumab discontinuation, and before fingolimod start, a brain MRI showed 2 new small T2 hyperintense lesions in the left frontotemporal pole along with confluent juxta-cortical lesions of the white matter of the left temporal pole and homolateral insula, with patchy, diffuse contrast enhancement (Figure 2A). The patient did not show new neurological signs or symptoms. A further new MRI performed 3 weeks later showed a slight dimensional increase of the confluent temporal lesion (Figure 2B).
Figure 2.
(A) Hyperintense lesions in the left frontotemporal pole along with confluent juxta-cortical lesions of the white matter of the left temporal pole and homolateral insula. T2-FLAIR weighted image. (B) Further new magnetic resonance imaging performed 3 weeks later. Note the slight dimensional increase of the confluent temporal lesion. T2-FLAIR weighted image.
The patient underwent lumbar puncture to perform JCV-DNA with a high sensitivity test (10 copies of JCV-DNA in 10 µL of CSF), which resulted negative. The remaining cyto-chemical analysis of CSF was normal. A month later, a brain MRI was repeated with no modification of the radiological findings and with spectroscopic evidence of slight decrement of NAA concentration along with a slight increase of choline and lactate-lipids peak concentrations. These findings were confirmed as suggestive for PML with immune reconstitution inflammatory syndrome associated. All expert MS neuroradiologists confirmed a high PML suspect. The clinical and radiological findings remained stable over time and the patient remains asymptomatic.
Discussion
PML is associated with various MS therapies; early PML diagnosis is fundamental to prevent further worsening, since asymptomatic PML patients have a better clinical and functional outcome in comparison to symptomatic ones.5
Regardless of differences in etiology and pathogenesis, PML and MS share certain distinctive and similar MRI findings, including the presence of inflammatory demyelinating lesions in the white matter.6 PML usually appears in MRI as one or more areas of hyperintensity on T2/FLAIR (fluid attenuation inversion recovery) sequences in the white matter with a peripheral, bilateral, and asymmetric distribution. Some lesion could have inside a little irregular signal intensity, which can have a punctate microcystic appearance7,8; this probably represents small areas of demyelination in the immediate nearness of infected oligodendrocytes or early immune response within perivascular spaces.9 PML lesions have typically subcortical rather than periventricular location and affect U-fibers. FLAIR sequence has the highest sensitivity in the identification of PML lesions; T2-weighted sequences can show particular features of PML lesions, such as microcysts.10 T1 with and without contrast sequences are useful to determine the degree of demyelination and signs of inflammation, suggestive for immune reconstitution inflammatory syndrome. Diffusion-weighted imaging sequences are used to detect signs of active infection.10 MS lesions have more frequently an ovoid shape with well-circumscribed borders with a size of generally 3 to 5 mm, whereas PML ones are more diffuse with ill-defined margins and larger size10 (see Table 1).
Table 1.
Typical Radiological Findings of MS and Asymptomatic PML.
| MS findings | PML findings | |
|---|---|---|
| Location | Periventricular | Cortical gray matter or juxta-cortical white matter involvement |
| Shape | Ovoid shape with well-circumscribed borders with a size of generally 3-5 mm | Diffuse, confluent irregular, or infiltrative appearance |
Abbreviations: PML, progressive multifocal leukoencephalopathy; MS, multiple sclerosis.
In some cases, JCV-DNA is not detectable in the CSF, as it depends on viral load and on the JCV sensitivity test.1 The gold standard diagnostic tool is a brain biopsy.1,11 However, considering the dangerousness, this procedure is generally not undertaken, unless the PML diagnosis is strictly suspected.11 In our case series, in consideration of the absence of signs and symptoms, brain biopsy was not justified.
At the time there is no specific and effective treatment for PML: the infection outcome depends primarily on the individual’s immune reconstitution ability to respond to JCV.12 The mandatory intervention after PML is suspected highlights the immediate withdrawal of the MS drug.13 Hence, the early diagnosis of PML is crucial since patients, restoring immune functions, could have an improvement in terms of survival and sequelae.12 Both patients were diagnosed with suspected PML during a routine brain MRI control; this highlights the importance to provide a strict brain MRI follow-up during dimethyl fumarate treatment, although only a few cases of PML during this therapy have been detected,1 and during natalizumab suspension phase, prior to starting a new MS treatment. We cannot exclude that both patients had an asymptomatic PML, self-limiting. In the second patient, the contrast enhancement and the washout period could suggest a possible MS reactivation.
In a real-life setting, in a radiologically suspected case of PML, although not confirmed by the CSF analysis, the best approach could be to suspend the treatment that could have caused PML and carry out a close radiological and clinical monitoring before starting a new MS therapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Stefania Federica De Mercanti received travel grants from Sanofi-Genzyme and Novartis. Dario Gned has nothing to disclose. Manuela Matta received travel grants from Biogen and Novartis. Marco Iudicello has nothing to disclose. Emanuele Franchin has nothing to disclose. Marinella Clerico received personal compensations for advisory boards, public speaking, editorial commitments, or travel grants from Biogen Idec, Merck Serono, Fondazione Serono, Novartis, Pomona, Sanofi-Genzyme and Teva.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
References
- 1. Faulkner M. Risk of progressive multifocal leukoencephalopathy in patients with multiple sclerosis Expert Opin Drug Saf. 2015;14:1737-1748. [DOI] [PubMed] [Google Scholar]
- 2. Yukitake M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: a comprehensive review. Clin Exp Neuroimmunol. 2018;9:37-47. [Google Scholar]
- 3. Dong-Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol. 2014;1:755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carruthers RL, Berger J. Progressive multifocal leukoencephalopathy and JC virus-related disease in modern neurology practice. Mult Scler Relat Disord. 2014;3:419-430. [DOI] [PubMed] [Google Scholar]
- 5. Phan-Ba R, Belachew S, Outteryck O, et al. 2012 The earlier, the smaller, the better for natalizumab-associated PML: in MRI vigilance veritas? Neurology. 2012;79:1067-1069. [DOI] [PubMed] [Google Scholar]
- 6. Love S. Demyelinating diseases. J Clin Pathol. 2006;59:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Honce JM, Nagae L, Nyberg E. Neuroimaging of natalizumab complications in multiple sclerosis: PML and other associated entities. Mult Scler Int. 2015;2015:809252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2016;87:117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yousry TA, Pelletier D, Cadavid D, et al. 2012 Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy Ann Neurol. 2012;72:779-787. [DOI] [PubMed] [Google Scholar]
- 10. EL PML. PML in MS learning. Accessed June 19, 2020 https://ms-pml.org
- 11. Chalkley JJ, Berger JR. Progressive multifocal leukoencephalopathy in multiple sclerosis. Curr Neurol Neurosci Rep. 2013;13:408. [DOI] [PubMed] [Google Scholar]
- 12. Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M; Progressive Multifocal Leukeoncephalopathy Consortium. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8:255-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clerico M, Artusi CA, Di Liberto A, et al. Long-term safety evaluation of natalizumab for the treatment of multiple sclerosis. Expert Opin Drug Saf. 2017;16:963-972. [DOI] [PubMed] [Google Scholar]