Abstract
Amino acids are thought to have a key role in the processes contributing to overreaching development through their metabolic properties and neuronal functions. In the present study, the effects of 10-week military training on the concentrations of 19 amino acids were investigated. Plasma amino acid concentrations were measured at rest from 53 healthy male conscripts on weeks 1, 4, 7, and 9 of their military service. Conscripts were classified as overreached and non-overreached. Overreaching classification was based on fulfilling at least three of five criteria: greater than 5% decrease in maximal oxygen uptake, increased rating perceived exertion (RPE), and decreased lactate-RPE ratio in submaximal marching test, admitting feeling overloaded and both increased scores in fatigue and decreased scores in vigor in the Profile of Mood States Adolescents. Eight conscripts (15%) were classified as overreached; their glutamine–glutamate ratio and alanine and arginine levels were significantly lower (P < 0.05) and glutamate concentration significantly higher (P < 0.05) in comparison to their non-overreached counterparts. The levels of arginine increased (P < 0.05) and tryptophan (P < 0.001) decreased in both groups throughout the study. The tyrosine concentration increased in non-overreached but, in contrast, remained at the same level in overreached individuals (P < 0.05). The results suggest that alterations in the levels of three metabolically important amino acids, alanine, glutamate and arginine, and the possibly neuroactive tyrosine and tryptophan might explain some of the physical and psychological symptoms of overreaching. The present study also confirms the potential use of glutamine–glutamate ratio as a tool for detecting overreaching.
Impact statement
The diagnosis of overtraining syndrome and overreaching poses a great challenge. Military training aims at improving the physical performance of the conscripts, but an excessive training load could also lead to overreaching. This study of Finnish conscripts provides new insights into the pathophysiology of overreaching and overtraining through amino acids concentrations. In addition to confirming the possible use of plasma glutamine/glutamate concentration to indicate and predict overreaching, we made a novel finding, i.e. low alanine and arginine concentrations might have a role in performance decrement and fatigue related to overreaching. Moreover, this study is the first to show the possible association between amino acids with putative neuronal properties and overreaching. Thus, the present findings might help to detect and prevent overreaching and offer a reliable diagnostic approach. In order to avoid overreaching, military training should be planned more periodically and individually, especially during the first four weeks of military service.
Keywords: Overreaching, overtraining, military training, amino acids, glutamine–glutamate ratio, metabolism
Introduction
A primary target of basic military training (BT) programs is to develop physical fitness, strength and endurance of the conscripts within a short time to enable them to perform combat-specific tasks. Imbalanced excessive training and recovery, however, can lead to overreaching (OR), a short-term decrement of performance, which may take several days or even weeks for recovery.1 A prolonged imbalance, in turn, may lead to the so-called overtraining syndrome (OTS), a long-term decrease in performance despite continuous training, from which recovery may take months or years.1
Despite intensive research, the mechanism underlying the development of OTS and OR has not yet been clarified nor have any reliable and specific biomarkers been identified. However, both OTS and OR may also include several individually varying physiological and psychological symptoms.1 Amino acids are involved in many crucial biochemical processes, i.e. brain function and various metabolic pathways including protein turnover and energy metabolism during exercise. Therefore, they could be associated with the symptoms of OTS and OR. The glutamine–glutamate (Gln/Glu) ratio has been reported to decline in overreached athletes,2–4 whereas increased glutamate (Glu) levels in OR may reflect excessive training stress, while a decreased glutamine (Gln) level has been associated with a deteriorated capacity of work5 and an impaired immune system.3,4,6 Nevertheless, not all studies have reported altered Glu3,4 and Gln concentrations in overreached athletes3,4,6 nor found a link between Gln and immune depression.7,8 On the other hand, the branched chain amino acids (BCAAs) leucine, isoleucine, and valine are known to compete with tryptophan for the same carrier in the blood–brain barrier and furthermore, these energy yielding BCAAs are utilized when exercising. Hence, a lower BCAA concentration has been suggested to increase tryptophan intake to the brain during exercise. Tryptophan is a precursor of the neurotransmitter serotonin, and therefore, its excess could be involved in the development of centrally mediated fatigue related to OR.9,10 Thus, strenuous training evoked alterations in amino acid metabolism may trigger the development of OR.
There is a paucity of knowledge on whether changes in the levels of amino acids can be involved in OR. Military training provides a relevant model to study OR and OTS because the conscripts represent a healthy homogenous age group undergoing strenuous training programs while having a controlled diet and an active lifestyle. Moreover, intolerance of conscripts to BT represents a major challenge to military training and therefore the mechanisms underlying this condition need to be clarified. Therefore, the aim of this study was to investigate the effect of military training on the concentrations of 19 plasma amino acid in overreached (OR) and non-overreached (nOR) conscripts during the BT period and to evaluate the role of amino acids as a tool for indicating OR.
Materials and methods
Subjects
Finnish male conscripts (N = 53, age 19.6 ± 0.3 years) volunteered to take part in the study. They were a part of 60 participants who had been selected according to specific criteria including willingness and not having any musculoskeletal and cardiorespiratory disorders.11 All the conscripts were informed of the study design and experiments, gave their written informed consent, and were given permission to withdraw from the study at any point. The study was approved by the Finnish Defence Forces and the Ethical Committees of the University of Jyväskylä and the Kainuu region of Finland. This study was funded by the Finnish Ministry of Education, Finnish Cultural Foundation, Polar Electro Oy and the Scientific Advisory Board for Defence.
Military training protocol
Finnish conscripts performed a standard eight-week BT period and a consequent two weeks of their specialized military training in wintertime where the outdoor temperatures varied between −30 and +1°C. Military service included gradually increasing physical exercise from 2 h per day to approximately 4 h at the end of the study consisting of both sports-related endurance and strength type of physical training and military-related physical training such as combat training and other forms of military training including shooting, and standard military education.11 Conscripts marched to the dining rooms four times a day (approximately 5 km in total) to consume standard military meals and performed other daily living activities as described previously.11 The dietary intake and energy balance have been assessed in a previous study, where the reported intake indicated that daily carbohydrate consumption in relation to the total reported energy intake was 58 ± 4% (5.2 ± 1.6 g·kg−1), fat 27 ± 4% (1.1 ± 0.4 g·kg−1), protein 14 ± 2% (1.3 ± 0.4 g·kg−1), and alcohol 0.3 ± 0.9% (0.01 ± 0.04 g·kg−1).12 During the study period, there were four marches with battle equipment and an overnight combat training exercise.
Study protocol
To measure the symptoms of overtraining, conscripts performed physical and psychological tests, which have been utilized in previous studies investigating overtraining or training adaptation among endurance and strength athletes and soldiers, since military training is a combination of various stressors.11 These tests involved performance tests such as a submaximal marching test and a maximal treadmill test, Profile of Mood States Adolescents (POMS-A) questionnaire,13 an inquiry of upper respiratory infections (URTI), and direct question on fatigue and assessment of Rating Perceived Exertion (RPE). Blood samples were also drawn and analyzed. The overall study protocol is presented in Table 1.
Table 1.
Research protocol during the first 10 weeks of the military training.
|
Week |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| VO2max test | X | X | X | X | ||||||
| Submaximal marching test | X | X | X | X | ||||||
| POMS | X | X | X | X | X | X | ||||
| Questionnaire | X | X | X | |||||||
| Inquiry about symptoms of URTI | X | X | X | X | X | X | ||||
| Blood samples | X | X | X | X | ||||||
Conscripts performed a 45-min-submaximal marching test with battle equipment on an outdoor track at the speed of their 70% maximal workload to determine their general aerobic performance in a military environment. This was calculated by using the American College of Sports Medicine estimation formula for maximal oxygen uptake (VO2max) in running and taking into account the 20-kg combat gear.14 The marching test was chosen since it represented the daily routine well and was available for a large group of soldiers. RPE was assessed three times, every 15 min using a 6-to 20-point scale, and their averaged value was utilized in the further analyses. The maximal treadmill test for evaluating VO2max, in turn, included a specific warming up, walking, and running at pre-determined velocities, and a gradually increasing speed and inclination of the treadmill until the point of exhaustion.14 RPE was measured just before each increase in the workload. Blood lactate was determined 1 min after the completion of the exercise from fingertip blood using a lactate analyzer (LactatePro, Arkray, Japan).Lactate-RPE ratio being determined subsequently. Pulmonary ventilation and respiratory gas exchange data were measured by a breath-by-breath method (Jaeger Oxygen Pro, VIASYS Healthcare GmbH, Hoechberg, Germany). Heart rate was recorded at 5-s intervals (Polar810i, Polar Electro Oy, Kempele, Finland). The maximal treadmill tests and submaximal tests were performed four times in total (Table 1). The exercise protocol remained the same during the entire study.
Physical activity
The subjects reported their physical activity (PA) during one month before military service by a self-administered short version of international physical activity questionnaire (IPAQ) covering the previous seven days.15 IPAQ outcome and physical activity were reported as minutes per week calculated in five different categories (1) Light PA: daily minutes × days per week with walking × 3.3 MET. (2) Moderate PA: daily minutes × days per week with moderate-intensity physical activity × 4.0 MET. (3) Vigorous PA: daily minutes × days per week with physical, vigorous activity × 8.0 MET. (4) Moderate to vigorous PA (ModVig PA): Moderate PA + Vigorous PA. (5) Total PA: Light PA + Moderate PA + Vigorous PA.
POMS-A questionnaire
On weeks 1, 2, 4, 7, 8, and 9, the conscripts filled in a POMS-A questionnaire for evaluating their mood state by answering questions about it over the past week as well as the current day by using a scale from 1 to 5 (1 refers to not at all and 5 extremely). Points were summed up and mood scores determined for each conscript. Conscripts were also directly asked “Do you feel mentally or physically overloaded?” on weeks 7, 8, and 9.
Inquiry about URTIs and sickness-related absences
Conscripts filled in a form enquiring about the symptoms of upper respiratory track infections (URTI) at baseline and on weeks 4, 6, 7, 8, and 9. Their responses were rated from 0 to 5 and a maximum sum of points was 55 on each survey week. Points were added up, and cumulative sums for weeks 2, 4, 7, 8, and 9 were created to represent the total burden of URTIs per week. Sickness-related absences were defined as an attendance/not-attendance in service, because of illnesses or injuries examined by physician, and as previously reported,11 the average number of sickness absences was three days; the main reason for sickness absence was URTI (71%), while musculoskeletal disorders (13%) and other disorders (13%) were the second most common reasons with digestion disorders accounting for the remainder of the sick leaves. In this study, sickness absence days were added up and divided into tertiles (from 0 to 2 days, from 3 to 6 days, and from 7 to 18 days).
Anthropometric measurements and fitness level
Body composition measurements of body mass (BM), fat-free mass (FFM), fat mass (FM), and percentage of body fat (F%) were determined using an eight-point bioelectrical impedance (Inbody 720, Biospace Co. Ltd, Seoul, Korea). For each subject, the measurements were performed between 6 a.m. and 7 a.m. after an overnight fast and after voiding, with no exercise for 12 h before the test. Conscripts wore T-shirts and trousers during the measurements. Height was measured by using a wall-mounted stadiometer and rounded to the nearest 0.5 cm.
Blood samples
Blood samples were drawn from an antecubital vein after an overnight fast (10 h) each time in the seated position, between 6:30 and 7:30 a.m. The first sample was taken on day 7, followed by measurements on weeks 4, 7, and 9. Fingertip lactate samples were taken before and after the submaximal exercise between 9 and 12 a.m. after standard food ingestion as described in the previous study.11 After protein precipitation, the concentrations of free amino acids in plasma were determined by reversed phase high performance liquid chromatography (RPHPLC). The HPLC system included a Quaternary Gradient Pump unit, PU-2089 Plus, an Intelligent AutosamplerAS-2057 Plus, Intelligent Fluorescence Detector, FP-2020 by Jasco, and data processing software Jasco Chrompass as previously described. Briefly, plasma samples were extracted with acetonitrile and derivatized to form fluorescent o-phthalaldehyde conjugates. Individual amino acids were separated on a reversed phase Zorbax C18 column (3.0 mm × 150 mm × 3.5 μm, Agilent Technologies, Santa Clara, CA) and detected at wavelengths 338 nm for excitation and 455 nm for emission.16
Overreaching classification
Conscripts were classified as overreached (OR) and non-overreached (nOR) according to five criteria from which they had to fulfill at least three in order to be classified as OR to ensure the most accurate classification. Since there are no exact diagnostic tools to identify either OR or OTS and the symptoms may vary among individuals, the OR criteria were chosen based on the literature regarding accepted physiological changes in OR athletes or soldiers. According to the literature, the most typical symptoms related to OR are performance decline with a reported increase in perceived exertion,17–20 fatigue,1,19,21 and mood changes1,22,23 and these signs were taken into consideration in the classification. Table 2 presents the descriptive data between the nOR and OR conscripts on week 1 of the present study.
Table 2.
The descriptive statistics of non-OR and OR conscripts at week 1.
| Non-overreached | Overreached | P | |
|---|---|---|---|
| Total physical activity (MET · min · wk−1)a | 3607 ± 3403 | 7808 ± 11026 | 0.40 |
| VO2max (mL · kg−1 · min−1) | 43.0 ± 6.5 | 45.0 ± 9.4 | 0.57 |
| Height (cm) | 177.6 ± 6.7 | 176.6 ± 6.1 | 0.58 |
| Body mass (kg) | 77.9 ± 12.9 | 76.9 ± 18.3 | 0.85 |
| BMI (kg/m2) | 24.7 ± 3.8 | 24.5 ± 4.4 | 0.70 |
| Fat mass (kg) | 15.1 ± 8.3 | 17.1 ± 11.6 | 0.95 |
| Fat-free mass (kg) | 62.8 ± 8.5 | 59.8 ± 7.7 | 0.36 |
| Body fat percentage | 18.7 ± 1.1 | 20.6 ± 3.1 | 0.65 |
aTotal PA: Light PA + Moderate PA + Vigorous PA reported by international physical activity questionnaire (IPAQ). P Significance of differences between groups.
Criterion 1 was a decreased VO2max ranging from greater than 5%19 of the lowest value on weeks 1 or 5 to the lowest value on weeks 7 or 9. Criterion 2 was an increase greater than 1.0 in mean RPE during the sub-maximal marching test17–19 from the lowest value on weeks 1 or 4 until weeks 7 and 9. Criterion 3 was a decrease greater than 0.27 mmol in the lactate-RPE ratio. Two times the standard deviation was set as a threshold since it was clearly different compared to the values from healthy athletes, and the literature illustrates similar changes in OR athletes.24 Criterion 4 was feeling mentally or physically overloaded on weeks 7, 8, and 9. Criterion 5 was a decrease greater than 1.0 in vigor in POMS-A from the highest score on weeks 1, 2, or 4 until weeks 7, 8, and 9 and an increase greater than 1.0 in fatigue from the lowest score on weeks 1, 2, or 4 until weeks 7, 8, and 9. Both criteria had to be valid and the change had to remain during the following weeks until the end of the 10-week study period. In order to avoid the possible impact of individual mental stress caused by the change to a military environment, the baseline value was taken from the lowest value in the early weeks. Only vigor and fatigue were considered in our study since they have been reported to clearly change in overreached conditions, while the other negative moods have increased or presented no change.4,19,25,26 We did not take into account URTIs as a criterion of OR since the military environment poses a high risk for even otherwise healthy adults to develop respiratory illnesses,27,28 and possible epidemics in wintertime could lead to an overestimation of OR among conscripts.
Statistical analysis
Statistical analyses were performed using IBM Statistics SPSS 20. To determine the possible differences in the descriptive statistics on week 1 between the OR and nOR, an independent sample T-test was performed if normality was evident. Total physical activity, height, fat mass, and body mass index (BMI) were not normally distributed and, therefore Mann–Whitney U test was used. Pearson’s χ2 was performed to evaluate possible differences between the groups in the fitness level and in the three-point scale of sickness absences. Possible differences in the scores of the URTI inquiry between nOR and OR were tested by Mann–Whitney U test as data were skewed. Generalized linear mixed model was used to identify significant differences, for the effect of training between the groups and training × group interactions. The least significance difference (LSD) post hoc test was used to examine the differences between the groups on a specific week if a main effect of group on any specific amino acid was detected. In order to fulfill the assumption of normally distributed residuals, glutamate was log transformed. The estimates of the mixed model (E) are reported. Significance was set at P < 0.05.
Results
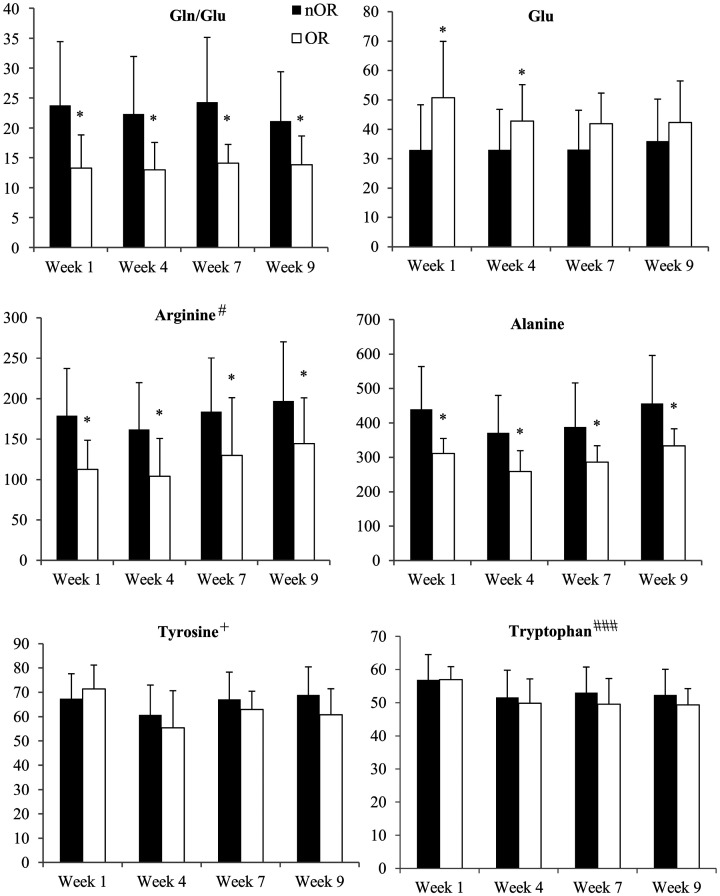
Out of a total of 53 conscripts, 8 (15%) were classified as OR with the remaining 45 designated as nOR. Criterion 1 was met by 47% of the conscripts, while criteria 2, 3, 4, 5 were met by 16%, 20%, 49%, and 18%, respectively. There were no statistical differences in anthropometric measurements nor in the level of activity between nOR and OR at the beginning of the study (Table 2). The response rate for URTI inquiry was 100%, 96%, 87%, 68%, and 51%, on weeks 2, 4, 7, 8, and 9, respectively. All conscripts reported URTI symptoms during BT. There was no significant difference in the symptoms of URTI between the groups apart from week 8 when the OR individuals had a significantly higher cumulative score in the URTI inquiry (P < 0.05). Sickness absence data were available for 98% of the conscripts; 38% of the conscripts had sickness absence of 0 to 2 days, 34% of 3 to 6 days, and 26% of 7 to 18 days. When compared to nOR, OR conscripts also had significantly more sickness absence days in the subscale 7–18 days (P < 0.05). There was a main effect of group for Gln/Glu ratio, and levels of Gln, alanine, and arginine (P < 0.05) (Figure 1). Compared to nOR, Gln/Glu, and the levels of alanine and arginine were significantly lower in OR (E−10.1 ± 4.3, −119.1 ± 49.1, −65.0 ± 25.0, respectively) during the entire 10-week study period (P < 0.05) (Figure 1). Moreover, the Glu concentration was higher (E 0.2 ± 0.1, P < 0.05) in OR than in nOR during the first four weeks of military training (Figure 1). The arginine level also increased in both groups (E 4.6 ± 2.5) (P < 0.05). There were no significant changes in the amounts of Gln or BCAAs among nOR and OR individuals over the study period. The tyrosine concentration exhibited a group × training interaction with no change in OR but an increase in nOR (E 1.4 ± 0.6, P < 0.05) on weeks 4, 7, and 9 compared to the measurement at day 7. Tryptophan levels decreased (E −1.2 ± 0.4, P < 0.001) throughout the study period in both groups. There were no significant changes in the concentrations of the other amino acids (Table 3).
Figure 1.
Mean ± SD concentration at rest for significantly changed amino acids, i.e. glutamine–glutamate ratio (Gln/Glu) and levels of glutamate (Glu), arginine, alanine, tyrosine, and tryptophan in non-overreached (nOR) and overreached (OR) conscripts at weeks 1, 4, 7, and 9 of the military service. Significant difference between groups *P < 0.05. Significant change due to exercise #P < 0.05, ###P < 0.001. Group × training interaction +P < 0.05.
Table 3.
Descriptive statistics of non-significantly changed amino acids at rest (nmol/mL) among nOR and OR conscripts during the first nine weeks of the military service.
| Week 1 | Mean ± SD | Week 4 | Mean ± SD | Week 7 | Mean ± SD | Week 9 | Mean ± SD | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asparagine | nOR | OR | 85.8 ± 25.3 | 66.1 ± 19.3 | 82.4 ± 25.4 | 60.4 ± 21.8 | 88.2 ± 25.6 | 65.2 ± 20.9 | 86.5 ± 26.8 | 69.4 ± 16.0 | 0.27 |
| Aspartate | nOR | OR | 4.1 ± 2.2 | 6.0 ± 3.7 | 4.0 ±1.7 | 4.8 ± 2.4 | 4.0 ± 1.9 | 4.7 ± 1.7 | 4.7 ± 1.7 | 6.1 ± 3.0 | 0.073 |
| Glutamine | nOR | OR | 641.8 ± 72.7 | 588.0 ± 52.6 | 622.8 ± 80.7 | 559.0 ± 76.7 | 675.8 ± 80.8 | 637.3 ± 94.2 | 646.1 ± 86.7 | 600.7 ± 39.8 | 0.12 |
| Glycine | nOR | OR | 257.5 ± 92.8 | 240.6 ± 19.3 | 250.2 ± 83.7 | 220.7 ± 35.0 | 264.7 ± 88.8 | 254.8 ± 53.4 | 271.8 ±105.7 | 294.5 ± 62.1 | 0.19 |
| Histidine | nOR | OR | 73.2 ± 10.3 | 76.1 ± 4.8 | 73.1 ± 7.9 | 72.8 ± 13.3 | 72.7 ± 8.9 | 72.9 ± 13.9 | 71.4 ± 10.2 | 74.9 ± 7.3 | 0.44 |
| Isoleucine | nOR | OR | 55.5 ± 8.9 | 59.2 ± 9.8 | 54.2 ±8.0 | 60.4 ± 10.6 | 53.6 ± 8.1 | 55.8 ± 9.2 | 54.9 ± 9.6 | 52.9 ± 6.5 | 0.094 |
| Leusine | nOR | OR | 151.5 ± 21.7 | 158.0 ± 13.5 | 150.7 ± 21.9 | 152.8 ± 18.1 | 153.0 ± 18.0 | 153.5 ± 23.9 | 154.5 ± 23.0 | 145.1 ± 21.6 | 0.36 |
| Lysine | nOR | OR | 146.9 ± 23.9 | 146.6 ± 24.8 | 134.3 ± 25.6 | 122.4 ± 35.5 | 151.9 ± 24.4 | 144.6 ± 44.6 | 148.4 ± 29.9 | 150.2 ± 35.7 | 0.65 |
| Methionine | nOR | OR | 29.0 ± 3.9 | 28.7 ± 3.4 | 25.9 ± 4.6 | 21.3 ± 3.2 | 29.7 ± 4.3 | 28.6 ± 3.9 | 28.4 ± 5.8 | 24.6 ± 3.2 | 0.59 |
| Phenylalanine | nOR | OR | 59.6 ± 7.8 | 60.3 ± 2.7 | 57.4 ± 7.2 | 52.0 ± 5.8 | 60.5 ± 6.4 | 60.1 ± 7.1 | 62.3 ± 8.7 | 54.4 ± 8.6 | 0.61 |
| Serine | nOR | OR | 80.7 ± 15.9 | 84.9 ± 13.8 | 77.6 ± 16.5 | 74.9 ± 12.0 | 83.9 ± 16.9 | 80.2 ± 12.2 | 81.9 ± 20.1 | 86.6 ± 15.5 | 0.79 |
| Taurine | nOR | OR | 37.1 ± 7.8 | 39.0 ± 11.2 | 36.2 ± 7.4 | 34.7 ±8.6 | 33.8 ± 6.7 | 33.9 ±5.8 | 60.7 ± 9.1 | 37.8 ± 9.9 | 0.91 |
| Treonine | nOR | OR | 59.4 ± 8.0 | 56.2 ± 9.4 | 55.6 ± 10.7 | 50.2 ± 7.3 | 60.8 ± 11.8 | 52.3 ± 12.3 | 58.8 ± 12.4 | 56.8 ± 13.0 | 0.33 |
| Valine | nOR | OR | 217.9 ± 34.9 | 218.7 ± 18.3 | 216.0 ± 35.5 | 217.8 ± 34.8 | 213.2 ± 26.2 | 205.7 ± 25.7 | 226.2 ± 40.4 | 214.7 ± 14.9 | 0.83 |
Note: The data are presented in the form of mean ± SD. P Significance of changes between groups.
Discussion
As far as we are aware, the present study is the first to have evaluated the role of 19 amino acids in overreaching. The present study provides further evidence that the Gln/Glu ratio is a reliable tool for evaluating overreaching, mainly due to significant increase in Glu in OR. One notable new finding was the result that alanine, arginine, and tyrosine might be involved in overreaching. In addition, military training, which includes not only endurance and strength-type of training but also other stressors like sleep deprivation, negative energy balance, as well as stressful environmental factors like working, living and sleeping outdoors and, occasionally, a restricted social life, seems to have an impact on tryptophan and arginine concentrations.
As there are no specific diagnostic criteria for OR and the existing classifications have not been clinically validated, we created a 5-scale categorization based on the literature regarding typical findings on overreaching and overtraining. It is, however, recognized that several markers may indicate OR.1 Therefore, in an attempt to ensure the most accurate classification, we required that at least three of five criteria had to be valid in order for an individual to be classified as OR. The selected criteria values were clearly outside the normal range and represented the following changes in OR. Criterion 1, decrease in VO2max, which is the most important and most widely applied parameter as a critical criterion of overtraining, and criterion 4, feelings of being mentally or physically overloaded, were fulfilled by almost every second conscript, while the rest of the fulfilled criteria represented approximately a prevalence of OR (15%) among conscripts. Since all conscripts had reported symptoms of URTI, the observed decrease in VO2max in nOR might be an effect of mild or recent URTI. As military training consists of specified training programs combined with environmental stressors, conscripts are under pressure to learn new combat training skills and feelings of fatigue are subjective, almost half of the conscripts might have felt themselves mentally or physically overloaded, although classical OR might not have been present.
Interestingly, we also observed differences in the levels of alanine, in the Gln/Glu ratio, as well as in arginine and Glu levels between the groups on the 7th day of military training when OR was not expected to be present. However, the OR conscripts had reported higher but non-significant, physical activity levels compared to nOR before starting their military service. Although the VO2max level slightly differed between groups, an elevated physical activity level (even if statistically non-significantly) might be a predisposing factor for developing OR later during BT and might have an impact on the results measured during the 1st week. Alterations in amino acid profiles may represent early biochemical changes related to the onset of OR, although OR was not yet clinically evident. On the other hand, an altered amino acid profile might further aggravate OR through the impacts of these changes in amino acid levels on several metabolic, neuronal, and hormonal pathways which will be discussed in the following paragraphs.
Previously, several studies have suggested that a lower Gln/Glu ratio may be a promising marker for overreaching due to either lower Gln or higher Glu.2–4 Smith and Norris5 even proposed overreaching to occur when Gln/Glu would be less than 3.58 but higher ratios were observed in the present study. The differences might be due to the different analytical methods, since in our study, amino acids were analyzed by HPLC while a colorimetric assay was used in the study of Smith and Norris.5 Nevertheless, also in the present study, the Gln/Glu ratio was significantly reduced in OR thus confirming the results from previous studies that the Gln/Glu ratio may be beneficial for evaluating OR.
In some studies, the Gln concentration has been observed to decrease in overreached athletes8,29,30 and this has been proposed to reflect a response to the training volume,5 representing a negative effect of exercise stress,29 which may also result in overload training-induced impairment of the immune system. Monocytes and lymphocytes require Gln for their activity, and therefore a lower Gln concentration could explain the more common URTIs in OR.6,31,32 We also detected that the OR conscripts had more symptoms of URTI by week 8 than their nOR counterparts. The difference disappeared on the 9th week; however, the response rate also declined which may have affected the results. Nevertheless, we did not observe any difference of Gln levels between OR and nOR which is in agreement with other studies.3,4 Therefore, we did not find any association of low glutamine levels with URTIs in OR. However, URTIs may have contributed to the development of OR and exposed the OR conscripts to a higher likelihood of sickness absence compared with nOR.
Consistent with our results, earlier studies have reported a significant increase in Glu levels in OR humans4,5 and rats33 due to excessive training stress. Therefore, as there was no difference in Gln levels between nOR and OR, this indicates that the lower Gln/Glu ratio in OR is probably due to increased Glu levels although marginal and insignificant changes in Gln may alter this ratio. The increase in Glu concentration may precede OTS rather than being specific for OTS since in the present study there was no difference between OR and nOR in the post hoc tests on the weeks 7 and 9. In support of this proposal, Coutts et al.3 reported that Glu levels tended to increase in response to intensive training but did not detect a significant difference between OR and nOR. In addition, our findings agree with Smith and Norris5 suggesting that the Glu level could potentially be used to evaluate an individual’s tolerance to training and risk of OR. Thus, our study indicates that the ability to cope with training load was mostly impaired in the first four weeks of basic military training period.
There is little in the current literature regarding the effect of training on the plasma arginine concentrations. However, exercise stimulates the production of nitric oxide,34 while arginine has an important role as its precursor.35,36 A recent study demonstrated that aerobic exercise training decreased arginine concentrations but increased nitric oxide generation in a heterogeneous population of subjects.37 In contrast to this observation, we found that military training increased arginine levels in both the OR and nOR groups. Thus, the possible underlying mechanism may not be related solely to the nitric oxide metabolism. The conflicting results may also be due to the characteristics of the subjects and the different type and intensity of the training program as military training is a combination of strength and endurance training, which might differentially affect the arginine levels. Moreover, arginine also has multiple functions in the body and an increased arginine concentration may be associated with several exercise adaptations including the synthesis of creatine,38 enhanced ammonia removal during exercising,39 and increased growth hormone secretion40 and release.41
Furthermore, we detected a difference in the arginine concentrations between nOR and OR. Arginine is a conditionally essential amino acid and under stressful conditions, including serious illnesses and intensive physical activity, there is elevated utilization of arginine for physiological processes.42,43 Thus, the increased utilization rate of arginine might be a possible response to stressful conditions and this could account for the difference in OR, even though a training response was detected in both groups. Arginine metabolism might be involved in the development of OR and contribute to some of the symptoms via metabolic pathways.
Interestingly, we found a reduced alanine concentration in OR. Alanine has a central role in energy metabolism during exercise through its role in the glucose-alanine cycle, which is a crucial step of gluconeogenesis in the liver.44 In support of this mechanism, previous studies have reported that the plasma alanine concentration increases in response to acute exercise44–46 but decreases if it is prolonged.47 Nevertheless, since alanine is closely related to energy metabolism and we found a decreased alanine concentration in OR, it is possible to postulate that OR is linked with an increased gluconeogenic demand. Furthermore, there is a consensus that OR represents a maladaptation of the hypothalamic-pituitary-adrenal axis (HPA)1 indicating that there might be a hypersensitivity of the pituitary subsequently followed by insensitivity and a blunted hormone response.48,49 Thus, in the early stages of OR, there might be elevated cortisol and catecholamine responses as a result of abnormalities in the HPA axis, leading to increased gluconeogenesis which can also be connected with the lower alanine levels. In addition, we previously reported a non-significant negative energy balance among the present conscripts,12 which can be a consequence of strenuous military training with high-energy expenditure and the lack of sleep and nutritional deprivation. Energy deficiency is a known risk factor for OR1 and might be associated with alterations in alanine levels.
According the central fatigue theory, the changes in tryptophan, which is a precursor of serotonin, could explain the exhaustion related to OR through increased serotonin synthesis in the brain. It is known that free tryptophan competes with BCAAs for the same carrier to pass through the blood–brain barrier and, on the other hand, with fatty acids for albumin. Thus, exercise-induced energy metabolism has been suggested to favor the transport of free tryptophan to the brain as the utilization of BCAAs and fatty acids increases. This, in turn, could elevate the rate of serotonin synthesis in the brain, leading to a feeling of fatigue which is a characteristic symptom of OR.9,10,50 In fact, both mechanisms would lead to increased unbound tryptophan concentrations in the plasma. In contrast, we did not observe any significant increase in tryptophan in OR nor any significant changes in the levels of BCAAs. Thus, tryptophan does not seem to be a sensitive marker for the early changes of OR.
Although we did not observe any increase in tryptophan levels in OR, we found that its levels did decline in both groups throughout the training. Acute exercise has been reported to decrease the tryptophan levels in trained athletes.51,52 However, as far as we are aware, there are only two studies, which have investigated the effect of chronic exercise on tryptophan levels among healthy subjects53 or athletes.54 Melancon et al.53 found that plasma tryptophan availability was elevated by 30 and 60 min of acute exercise after 16-week aerobic exercise training among 60 aged men.53 However, free tryptophan availability measured during the bouts of acute exercise was attenuated following training. The researchers suggested this occurred possibly due to a lower serotonergic and sympathetic response due to regular exercising.53 Thus, a similar process might explain the decreased tryptophan levels in our study. Additionally, a randomized double-blinded placebo-controlled trial of a probiotic among trained athletes found that tryptophan levels were lower after 12 weeks of normal winter training in the placebo group. The placebo group also experienced more upper respiratory infections than the probiotic group indicating that tryptophan may have a possible role in the regulation of immune system, although confounding factors could not be ruled out in that trial.54 Endurance training has also been reported to cause adaptations to the kynurenine pathway and in the activity of indoleamine 2,3-dioxygenase, which is the enzyme responsible for the breakdown of tryptophan.55 Activation of kynurenine pathway and decreased tryptophan levels were also reported during acute exercise.52 Therefore, the reduction in the tryptophan levels observed in the present study could also be a result of intense training activating the kynurenine pathway and might contribute to the high prevalence of symptoms of URTI among conscripts.
Tyrosine is a precursor of the neurotransmitter dopamine, which takes part in excitatory neurotransmission in the reward-motivation system of the brain and has an important role in motivation, reward, memory, and attention.56 A reduction of the dopamine concentration in the brain as a result of prolonged exercise has been suggested to be linked with the onset of central fatigue based on the studies conducted with rats57–59 or humans.60,61 Already in the 1990s, Davis and Bailey56 proposed the central fatigue theory of BCAAs and serotonin, and postulated that a high serotonin-dopamine ratio could be related to impaired physical performance, tiredness, and onset of fatigue, whereas a low ratio was associated with improved performance as a consequence of maintenance of motivation and arousal through dopaminergic neuronal pathways. Furthermore, previous studies have also shown that long-term exercise modifies the activity of the dopaminergic system in patients with Parkinson disease.62–64 Thus, the observed increase in tyrosine levels throughout training in nOR might be a physiological response to the alterations of neuronal pathways in the dopaminergic system, whereas in OR, the exercise-induced plasticity might be disrupted due to pathophysiological changes in brain neurotransmitter levels which also lead to a sensation of fatigue.
Tyrosine is also a precursor of the neurotransmitter noradrenaline, which plays a role in learning and memory, attention, arousal, anxiety, and pain.65 Studies performed with noradrenaline reuptake inhibitors have demonstrated that the noradrenergic system decreases performance rather than improving it in humans.66–68 Although the earlier studies have not reported any improvement in physical performance after tyrosine supplementation,69–73 some trials suggest that ingestion of tyrosine can enhance working memory, cognitive tasks, and tolerance to stress under stressed situations, such as military operations74,75 or sleep deprivation,76,77 through adrenergic pathways. Consequently, our findings of no change in tyrosine in OR, in contrast to an increase in nOR throughout training, may reflect a maladaptation to the increased exercise stress. The increased tyrosine levels in nOR, in turn, might also represent a physiological adaptation in the conscripts to the challenges posed by stressful military conditions resulting in better cognitive functioning.
The determination of OR and OTS is challenging and, therefore our findings should be considered with caution. Due to logistic reasons, we were unable to take the baseline blood samples on day 1 with the first samples being taken on day 7, which might slightly affect the present results. Furthermore, OR is a consequence of complex set of stressors and individually altered symptoms are also affected by other factors including environmental and social circumstances, which makes it challenging to detect even using a variety of criteria. Furthermore, amino acids form a complex metabolic network which might be changed not only on strenuous exercise but also by many other factors such as nutrition, therefore the possible clarification of the mechanism behind the pathophysiology of OR and OTS still remains unclear.
Conclusion
In conclusion, the present study indicates that two metabolically active amino acids, alanine and arginine, might be predisposing factors for OR due to early observed decrease in their levels in OR. In addition, changes in the levels of the neuronal precursors, tryptophan, and tyrosine might be involved in the pathophysiology of OR although it does seem that tryptophan might not be a sensitive marker for early changes of OR. Moreover, an increase in the arginine concentration in response to military training may be indicative of a positive training adaptation. The results of our study also confirm the possible use of the Gln/Glu ratio as a tool for evaluating OR due to significant increase in the Glu concentration which may reflect the exercise-induced fatigue and OR. However, further investigations will be needed with a determination of other biochemical parameters, which might allow a clear interpretation of the changes in the levels of the amino acids. Moreover, because approximately 15% of conscripts were classified as OR, more attention should be paid to the individual response to military training, for example to have more tolerable training intensities, especially during the first four weeks of BT.
ACKNOWLEDGEMENTS
We would like to thank the Finnish Ministry of Education, Finnish Cultural Foundation, Polar Electro Oy and the Scientific Advisory Board for Defense for their financial support of the research.
Authors’ contributions
MMT-T and HK designed the study, MMT-T performed tests and collected the data. VK analyzed the concentrations of amino acids in blood. JNI, RJ, MA, ALTU, and MMT-T chose and reviewed the OR criteria based on the literature. JNI and RJ performed the statistical analysis. JNI wrote the manuscript and all the authors participated in reviewing it.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Finnish Ministry of Education; Finnish Cultural Foundation; Polar Electro Oy; and the Scientific Advisory Board for Defense. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
ORCID iD
Mustafa Atalay https://orcid.org/0000-0001-8999-1426
References
- 1.Meeusen R, Duclos M, Foster C, Fry A, Gleeson M, Nieman D, Raglin J, Rietjens G, Steinacker J, Urhausen A; European College of Sport Science; American College of Sports Medicine. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc 2013; 45:186–205. [DOI] [PubMed] [Google Scholar]
- 2.Coutts A, Reaburn P, Piva TJ, Murphy A. Changes in selected biochemical, muscular strength, power, and endurance measures during deliberate overreaching and tapering in rugby league players. Int J Sports Med 2007; 28:116–24 [DOI] [PubMed] [Google Scholar]
- 3.Coutts AJ, Reaburn P, Piva TJ, Rowsell GJ. Monitoring for overreaching in rugby league players. Eur J Appl Physiol 2007; 99:313–24 [DOI] [PubMed] [Google Scholar]
- 4.Halson SL, Lancaster GI, Jeukendrup AE, Gleeson M. Immunological responses to overreaching in cyclists. Med Sci Sports Exerc 2003; 35:854–61 [DOI] [PubMed] [Google Scholar]
- 5.Smith DJ, Norris SR. Changes in glutamine and glutamate concentrations for tracking training tolerance. Med Sci Sports Exerc 2000; 32:684–9 [DOI] [PubMed] [Google Scholar]
- 6.Walsh NP, Blannin AK, Robson PJ, Gleeson M. Glutamine, exercise and immune function. Links and possible mechanisms. Sports Med 1998; 26:177–91 [DOI] [PubMed] [Google Scholar]
- 7.Gabriel HH, Urhausen A, Valet G, Heidelbach U, Kindermann W. Overtraining and immune system: a prospective longitudinal study in endurance athletes. Med Sci Sports Exerc 1998; 30:1151–7 [DOI] [PubMed] [Google Scholar]
- 8.Mackinnon LT, Hooper SL. Plasma glutamine and upper respiratory tract infection during intensified training in swimmers. Med Sci Sports Exerc 1996; 28:285–90 [DOI] [PubMed] [Google Scholar]
- 9.Newsholme EA, Blomstrand E. Branched-chain amino acids and Central fatigue. J Nutr 2006; 136:274S–6S [DOI] [PubMed] [Google Scholar]
- 10.Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Brain neurotransmitters in fatigue and overtraining. Appl Physiol Nutr Metab 2007; 32:857–64 [DOI] [PubMed] [Google Scholar]
- 11.Tanskanen MM, Kyröläinen H, Uusitalo AL, Huovinen J, Nissilä J, Kinnunen H, Atalay M, Häkkinen K. Serum sex hormone-binding globulin and cortisol concentrations are associated with overreaching during strenuous military training. J Strength Cond Res 2011; 25:787–97 [DOI] [PubMed] [Google Scholar]
- 12.Tanskanen M, Uusitalo AL, Häkkinen K, Nissilä J, Santtila M, Westerterp KR, Kyröläinen H. Aerobic fitness, energy balance, and body mass index are associated with training load assessed by activity energy expenditure. Scand J Med Sci Sports 2009; 19:871–8 [DOI] [PubMed] [Google Scholar]
- 13.Terry PC, Lane AM, Lane HJ, Keohane L. Development and validation of a mood measure for adolescents. J Sports Sci 1999; 17:861–72 [DOI] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine. Guidelines for exercise testing and prescription. 6th ed Baltimore: Lippincott Williams & Wilkins, 2001, pp. 68,117,303 [Google Scholar]
- 15.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35:1381–95 [DOI] [PubMed] [Google Scholar]
- 16.Mero A, Leikas A, Knuutinen J, Hulmi JJ, Kovanen V. Effect of strength training session on plasma amino acid concentration following oral ingestion of leucine, BCAAs or glutamine in men. Eur J Appl Physiol 2009; 105:215–23 [DOI] [PubMed] [Google Scholar]
- 17.Decroix L, Lamberts RP, Meeusen R. Can the lamberts and lambert submaximal cycle test reflect overreaching in professional cyclists? Int J Sports Physiol Perform 2018; 13:23–8 [DOI] [PubMed] [Google Scholar]
- 18.Siegl A, M, Kosel E, Tam N, Koschnick S, Langerak NG, Skorski S, Meyer T, Lamberts RP. Submaximal markers of fatigue and overreaching; implications for monitoring athletes. Int J Sports Med 2017; 38:675–82 [DOI] [PubMed] [Google Scholar]
- 19.Halson SL, Bridge MW, Meeusen R, Busschaert B, Gleeson M, Jones DA, Jeukendrup AE. Time course of performance changes and fatigue markers during intensified training in trained cyclists. J Appl Physiol 2002; 93:947–56 [DOI] [PubMed] [Google Scholar]
- 20.Uusitalo AL. Overtraining: making a difficult diagnosis and implementing targeted treatment. Phys Sportsmed 2001; 29:35–50 [DOI] [PubMed] [Google Scholar]
- 21.Budgett R. Fatigue and underperformance in athletes: the overtraining syndrome. Br J Sports Med 1998; 32:107–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kentta G, Hassmen P, Raglin JS. Training practices and overtraining syndrome in Swedish age-group athletes. Int J Sports Med 2001; 22:460–5 [DOI] [PubMed] [Google Scholar]
- 23.Matos NF, Winsley RJ, Williams CA. Prevalence of nonfunctional overreaching/overtraining in young English athletes. Med Sci Sports Exerc 2011; 43:1287–94 [DOI] [PubMed] [Google Scholar]
- 24.Garcin M, Fleury A, Billat V. The ratio HLa: RPE as a tool to appreciate overreaching in young high-level middle-distance runners. Int J Sports Med 2002; 23:16–21 [DOI] [PubMed] [Google Scholar]
- 25.Cadegiani FA, Kater CE. Body composition, metabolism, sleep, psychological and eating patterns of overtraining syndrome: results of the EROS study (EROS-PROFILE). J Sports Sci 2018; 36:1902–10 [DOI] [PubMed] [Google Scholar]
- 26.Comotto S, Bottoni A, Moci E, Piacentini MF. Analysis of session-RPE and profile of mood states during a triathlon training camp. J Sports Med Phys Fitness 2015; 55:361–7 [PubMed] [Google Scholar]
- 27.Korzeniewski K, Nitsch-Osuch A, Konior M, Lass A. Respiratory tract infections in the military environment. Respir Physiol Neurobiol 2015; 209:76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan MA, Christian RS, Wohlrabe J. Handwashing and respiratory illness among young adults in military training. Am J Prev Med 2001; 21:79–83 [DOI] [PubMed] [Google Scholar]
- 29.Rowbottom DG, Keast D, Goodman C, Morton AR. The haematological, biochemical and immunological profile of athletes suffering from the overtraining syndrome. Eur J Appl Physiol Occup Physiol 1995; 70:502–9 [DOI] [PubMed] [Google Scholar]
- 30.Parry-Billings M, Budgett R, Koutedakis Y, Blomstrand E, Brooks S, Williams C, Calder PC, Pilling S, Baigrie R, Newsholme EA. Plasma amino acid concentrations in the overtraining syndrome: possible effects on the immune system. Med Sci Sports Exerc 1992; 24:1353–8 [PubMed] [Google Scholar]
- 31.Castell LM, Poortmans JR, Newsholme EA. Does glutamine have a role in reducing infections in athletes? Europ J Appl Physiol 1996; 73:488–90 [DOI] [PubMed] [Google Scholar]
- 32.Kingsbury KJ, Kay L, Hjelm M. Contrasting plasma free amino acid patterns in elite athletes: association with fatigue and infection. Br J Sports Med 1998; 32:25,32; discussion 32–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hohl R, Ferraresso RL, De Oliveira RB, Lucco R, Brenzikofer R, De Macedo DV. Development and characterization of an overtraining animal model. Med Sci Sports Exerc 2009; 41:1155–63. [DOI] [PubMed] [Google Scholar]
- 34.Moncada S. Nitric oxide in the vasculature: physiology and pathophysiology. Ann N Y Acad Sci 1997; 811:60–7; discussion 67–9 [DOI] [PubMed] [Google Scholar]
- 35.Wu G, Morris SM., Jr., Arginine metabolism: nitric oxide and beyond. Biochem J 1998; 336:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand 1998; 162:401–9 [DOI] [PubMed] [Google Scholar]
- 37.Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino K. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr 2017; 60:180–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 2007; 27:241–61 [DOI] [PubMed] [Google Scholar]
- 39.Schaefer A, Piquard F, Geny B, Doutreleau S, Lampert E, Mettauer B, Lonsdorfer J. L-arginine reduces exercise-induced increase in plasma lactate and ammonia. Int J Sports Med 2002; 23:403–7 [DOI] [PubMed] [Google Scholar]
- 40.Wideman L, Weltman JY, Hartman ML, Veldhuis JD, Weltman A. Growth hormone release during acute and chronic aerobic and resistance exercise: recent findings. Sports Med 2002; 32:987–1004 [DOI] [PubMed] [Google Scholar]
- 41.Kanaley JA. Growth hormone, arginine and exercise. Curr Opin Clin Nutr Metab Care 2008; 11:50–4 [DOI] [PubMed] [Google Scholar]
- 42.Zhou M, Martindale RG. Arginine in the critical care setting. J Nutr 2007; 137:1687S–92S [DOI] [PubMed] [Google Scholar]
- 43.Morris SM., Jr., Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 2009; 157:922–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felig P, Wahren J. Amino acid metabolism in exercising man. J Clin Invest 1971; 50:2703–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Einspahr KJ, Tharp G. Influence of endurance training on plasma amino acid concentrations in humans at rest and after intense exercise. Int J Sports Med 1989; 10:233–6 [DOI] [PubMed] [Google Scholar]
- 46.Brodan V, Kuhn E, Pechar J, Tomkova D. Changes of free amino acids in plasma of healthy subjects induced by physical exercise. Eur J Appl Physiol Occup Physiol 1976; 35:69–77 [DOI] [PubMed] [Google Scholar]
- 47.Refsum HE, Gjessing LR, Stromme SB. Changes in plasma amino acid distribution and urine amino acids excretion during prolonged heavy exercise. Scand J Clin Lab Invest 1979; 39:407–13 [DOI] [PubMed] [Google Scholar]
- 48.Buyse L, Decroix L, Timmermans N, Barbe K, Verrelst R, Meeusen R. Improving the diagnosis of nonfunctional overreaching and overtraining syndrome. Med Sci Sports Exerc 2019; 51:2524–30 [DOI] [PubMed] [Google Scholar]
- 49.Meeusen R, Nederhof E, Buyse L, Roelands B, de Schutter G, Piacentini MF. Diagnosing overtraining in athletes using the two-bout exercise protocol. Br J Sports Med 2010; 44:642–8 [DOI] [PubMed] [Google Scholar]
- 50.Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Central fatigue: the serotonin hypothesis and beyond. Sports Med 2006; 36:881–909 [DOI] [PubMed] [Google Scholar]
- 51.Areces F, Gonzalez-Millan C, Salinero JJ, Abian-Vicen J, Lara B, Gallo-Salazar C, Ruiz-Vicente D, Del Coso J. Changes in serum free amino acids and muscle fatigue experienced during a Half-Ironman triathlon. PLoS One 2015; 10:e0138376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strasser B, Geiger D, Schauer M, Gatterer H, Burtscher M, Fuchs D. Effects of exhaustive aerobic exercise on Tryptophan-Kynurenine metabolism in trained athletes. PLoS One 2016; 11:e0153617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melancon MO, Lorrain D, Dionne IJ. Changes in markers of brain serotonin activity in response to chronic exercise in senior men. Appl Physiol Nutr Metab 2014; 39:1250–6 [DOI] [PubMed] [Google Scholar]
- 54.Strasser B, Geiger D, Schauer M, Gostner JM, Gatterer H, Burtscher M, Fuchs D. Probiotic supplements beneficially affect Tryptophan-Kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, Double-Blinded, Placebo-Controlled trial. Nutrients 2016; 8:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, Kamandulis S, Ruas JL, Erhardt S, Westerblad H, Andersson DC. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol 2016; 310:C836–40 [DOI] [PubMed] [Google Scholar]
- 56.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc 1997; 29:45–57 [DOI] [PubMed] [Google Scholar]
- 57.Gerald MC. Effects of (+)-amphetamine on the treadmill endurance performance of rats. Neuropharmacology 1978; 17:703–4 [DOI] [PubMed] [Google Scholar]
- 58.Heyes MP, Garnett ES, Coates G. Central dopaminergic activity influences rats ability to exercise. Life Sci 1985; 36:671–7 [DOI] [PubMed] [Google Scholar]
- 59.Zheng X, Hasegawa H. Administration of caffeine inhibited adenosine receptor agonist-induced decreases in motor performance, thermoregulation, and brain neurotransmitter release in exercising rats. Pharmacol Biochem Behav 2016; 140:82–9 [DOI] [PubMed] [Google Scholar]
- 60.Roelands B, Hasegawa H, Watson P, Piacentini MF LD, Buyse, Schutter G, Meeusen RR. The effects of acute dopamine reuptake inhibition on performance. Med Sci Sports Exerc 2008; 40:879–85 [DOI] [PubMed] [Google Scholar]
- 61.Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol 2005; 565:873–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer SS, Mortimer JA, Webster DD, Bistevins R, Dickinson GL. Exercise therapy for parkinson’s disease. Arch Phys Med Rehabil 1986; 67:741–5 [DOI] [PubMed] [Google Scholar]
- 63.Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil 2007; 88:1154–8 [DOI] [PubMed] [Google Scholar]
- 64.Sacheli MA, Neva JL, Lakhani B, Murray DK, Vafai N, Shahinfard E, English C, McCormick S, Dinelle K, Neilson N, McKenzie J, Schulzer M, McKenzie DC, Appel-Cresswell S, McKeown MJ, Boyd LA, Sossi V, Stoessl AJ. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov Disord 2019; 34:1891–900 [DOI] [PubMed] [Google Scholar]
- 65.Meeusen R, Roelands B. Fatigue: is it all neurochemistry? Eur J Sport Sci 2018; 18:37–46 [DOI] [PubMed] [Google Scholar]
- 66.Roelands B, Goekint M, Heyman E, Piacentini MF, Watson P, Hasegawa H, Buyse L, Pauwels F, De Schutter G, Meeusen R. Acute norepinephrine reuptake inhibition decreases performance in normal and high ambient temperature. J Appl Physiol 2008; 105:206–12 [DOI] [PubMed] [Google Scholar]
- 67.Klass M, Duchateau J, Rabec S, Meeusen R, Roelands B. Noradrenaline reuptake inhibition impairs cortical output and limits endurance time. Med Sci Sports Exerc 2016; 48:1014–23 [DOI] [PubMed] [Google Scholar]
- 68.Klass M, Roelands B, Levenez M, Fontenelle V, Pattyn N, Meeusen R, Duchateau J. Effects of noradrenaline and dopamine on supraspinal fatigue in well-trained men. Med Sci Sports Exerc 2012; 44:2299–308 [DOI] [PubMed] [Google Scholar]
- 69.Struder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K. Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Horm Metab Res 1998; 30:188–94 [DOI] [PubMed] [Google Scholar]
- 70.Chinevere TD, Sawyer RD, Creer AR, Conlee RK, Parcell AC. Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance. J Appl Physiol 2002; 93:1590–7 [DOI] [PubMed] [Google Scholar]
- 71.Sutton EE, Coill MR, Deuster PA. Ingestion of tyrosine: effects on endurance, muscle strength, and anaerobic performance. Int J Sport Nutr Exerc Metab 2005; 15:173–85 [DOI] [PubMed] [Google Scholar]
- 72.Tumilty L, Davison G, Beckmann M, Thatcher R. Failure of oral tyrosine supplementation to improve exercise performance in the heat. Med Sci Sports Exerc 2014; 46:1417–25 [DOI] [PubMed] [Google Scholar]
- 73.Watson P, Enever S, Page A, Stockwell J, Maughan RJ. Tyrosine supplementation does not influence the capacity to perform prolonged exercise in a warm environment. Int J Sport Nutr Exerc Metab 2012; 22:363–73 [DOI] [PubMed] [Google Scholar]
- 74.Owasoyo JO, Neri DF, Lamberth JG. Tyrosine and its potential use as a countermeasure to performance decrement in military sustained operations. Aviat Space Environ Med 1992; 63:364–9 [PubMed] [Google Scholar]
- 75.Deijen JB, Wientjes CJ, Vullinghs HF, Cloin PA, Langefeld JJ. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res Bull 1999; 48:203–9 [DOI] [PubMed] [Google Scholar]
- 76.Dollins AB, Krock LP, Storm WF, Wurtman RJ, Lieberman HR. L-tyrosine ameliorates some effects of lower body negative pressure stress. Physiol Behav 1995; 57:223–30 [DOI] [PubMed] [Google Scholar]
- 77.Neri DF, Wiegmann D, Stanny RR, Shappell SA, McCardie A, McKay DL. The effects of tyrosine on cognitive performance during extended wakefulness. Aviat Space Environ Med 1995; 66:313–9 [PubMed] [Google Scholar]