Abstract
Aflatoxin B1 (AFB1) is reported to elicit adverse reproductive outcomes in animals. Gallic acid (GA) is known to exhibit antioxidant and inflammatory bioactivities. The impact of GA on AFB1-facilitated reproductive dysfunction is nonexistent in literature. This investigation elucidated GA protective effect on AFB1-induced reproductive toxicities in rats, exposed for 28 consecutive days to AFB1 (75 µg/kg), or co-treated with GA (20 or 40 mg/kg) body weight. AFB1 significantly (p < 0.05) reduced testicular function biomarkers, serum hormonal levels, and functional sperm characteristics in experimental animals. GA abated AFB1-induced increases (p < 0.05) in lipid peroxidation and reactive oxygen and nitrogen species, suppressed myeloperoxidase, interleukin-1β, nitric oxide, and tumor necrosis factor-α levels—inflammatory biomarkers—in testes, epididymis, and hypothalamus. Furthermore, GA improved antioxidant defenses and alleviated reduction in interleukin-10, caspase-3 activation, and histological variations in epididymis, testes, and hypothalamus of rats dosed with AFB1. Conclusively, GA enhanced reproductive function in AFB1-exposed rats by modulating inflammatory, oxidative stress, and apoptosis mediators.
Impact statement
Infertility resulting from reproductive deficiency can be stressful. Exposure to aflatoxin B1, a dietary mycotoxin prevalent in improperly stored grains, is reported to elicit reproductive insufficiencies and infertility. We, therefore, examined the likely beneficial effect of gallic acid (GA) a phytochemical, recognized to exhibit in vitro and in vivo pharmacological bioactivities against oxidative stress and related inflammatory damages in rats, since AFB1 toxicities are predicated on oxidative epoxide formation, in a bid to proffer new evidence to advance the field of nutriceutical application from plant-derived chemopreventive agents. Our findings will advance the field of chemoprevention by presenting data absent in the literature on GA. Our results demonstrate further evidence for GA conferred protection against AFB1-mediated histological lesions in testes, epididymis, and hypothalamus of treated rats; suppresses oxidative damages, relieved inflammatory and apoptotic responses, restored sperm functional characteristics, and hormonal levels relevant for reproductive integrity and function.
Keywords: Aflatoxin B1, gallic acid, reproductive deficits, oxidative stress, inflammation, caspase-3
Introduction
The increasing rate of deterioration in the reproductive health of both animals and humans is of great concern globally. Epidemiological studies recently indicated that there are almost 186 million infertile patients in the world, of which male infertility cases are more than half.1,2 The contributory role of environmental pollutants to reproductive dysfunction is well documented.3,4 Humans and wildlife are frequently exposed to toxic chemicals from industries and diet. Exposure to dietary toxins, including mycotoxins, is of significant health concern, owing to their involvement in carcinogenesis, hepatotoxicity, reproductive damage, and suppression of growth and immunity.5 Aflatoxin B1 (AFB1, Figure 1(a)) is a toxic secondary metabolite of Aspergillus flavus and Aspergillus parasiticus species, which occurs naturally in food crops, namely corns, ground-nuts, millet, and wheat due to poor storage and favorable hot and humid climates.6,7
Figure 1.
Chemical structures of AFB1 (a) and gallic acid (b).
Epidemiological data, mainly in developing countries, revealed that approximately 4.5 billion persons are at danger of persistent AFB1 exposure via tainted food crops.8,9 Indeed, AFB1 metabolite known as aflatoxin M1 (AFM1) is frequently found in milk, samples of cord blood, and colostrum in pregnant women, which signify the possibility of prenatal contact to AFB1 in humans.10–12 Aflatoxins were reportedly detected in semen samples (40%) obtained from infertility clinics amid a higher chance of sperm anomaly compared with fecund men living within a locality in Nigeria.13 Moreover, data from experimental animals have demonstrated that AFB1 may be an essential risk factor for male infertility.14,15 Earlier animal studies revealed that acute and chronic AFB1 exposure induced testicular damage disrupted endocrine function and spermatogenesis, leading to impaired fertility.16,17 Elucidated mechanisms associated with AFB1-mediated reproductive toxicity include suppression of androgen biosynthetic proteins (17 β-hydroxysteroid dehydrogenase 3, 3β-hydroxysteroid dehydrogenase, and steroidogenic acute regulator), induction of oxidative stress, inhibition of antioxidant defense systems, germ cell apoptosis and autophagy.18–20 Thus, the potential chemopreventive or chemotherapeutic agents to assuage the deleterious effects of AFB1 in the exposed population are warranted.
Gallic acid (3, 4, 5-trihydroxybenzoic acid; GA) (Figure 1(b)) occurs naturally as a low molecular triphenolic acid in several plants. It is produced as a secondary metabolite via the shikimic acid pathway in plants. GA is abundant in several commercially available beverages, namely red wine, pomegranate juice, coffee, and green tea. GA has been documented to elicit numerous critical biological activities, namely antioxidant, anti-inflammatory, anticancer, antidiabetic, and antimicrobial activities.21,22 Moreover, GA finds application industrially as an additive to food, thereby precluding oxidation/rancidity of oils and fats.23 GA is demonstrated to defend against cyclophosphamide-mediated reproductive toxicity,24,25 radiation26 and type I diabetes.27 GA was demonstrated to inhibit aflatoxin biosynthesis in Aspergillus flavus,28 and GA is a direct inhibitor of several CYP450s that facilitate the intrahepatic activation of AfB1,29,30 one of the mechanisms of GA-mediated detoxification of AFB1 could be through a direct inhibition of these CYP450s.
Nevertheless, there is a gap in literature regarding the effect of GA on AFB1 toxicity and the reproductive system. We hypothesized that GA, owing to its intrinsic biological activities, may mitigate oxidative stress and inflammation to abrogate AFB1-induced reproductive damage. The present investigating is aimed at elucidating the possible effects of GA on AFB1-mediated toxicity in the reproductive function and system of exposed adult rats.
Materials and methods
Chemicals
Aflatoxin B1 (AFB1) (≥98%), gallic acid (≥95%), thiobarbituric acid (TBA); 5,5-dithio-bis-2-nitrobenzoic acid (DTNB); trichloroacetic acid (TCA); epinephrine; and CDNB -1-chloro-2,4-dinitrobenzene procured from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). ELISA-Enzyme-Linked Immunosorbent Assay plates for the spectrophotometric microplate estimation of caspase 3 (CASP3) activity, interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-10 (IL-10) levels were supplied by E-labscience Biotech, Beijing, China.
Maintenance of experimental animals
Fifty adult Wistar rats (sex: male; age nine weeks old, weight: 161 ± 7 g) were sourced from the University of Ibadan, Department of Veterinary Medicine, primate colony, Ibadan, Nigeria for the purpose of this experimentation. Experimental rats were housed in polycarbonated cages in a standard rodent experimental vivarium and maintained under a cycle of 12 h—dark and light photoperiod. The animals were acclimatized for one week while being feed with standard rodent pellets (Ladokun™ Feeds, Ibadan, Nigeria) and with free access to clean drinking water, before the experimentation. All protocols and care of experimental animals were done with adherence to the authorized guidelines Ethical use of small laboratory animal in experimentation Committee University of Ibadan. Furthermore, we complied with the National Institute of Health “Guide for the Care and Use of Laboratory Animals” in all experimentation.
Research plan
Following the acclimatization period, experimental animals were sorted randomly into five groups of 10 animals each. They were treated as detailed below for 28 consecutive days per os (p.o.) or gavage (orally):
Group 1: Control—treated with corn oil (2 mL/kg) alone p.o.
Group 2: Gallic acid (GA) alone—treated with GA:40 mg/kg p.o.
Group 3: Aflatoxin B1 (AFB1)—treated with AFB1 (75 µg/kg) alone p.o.
Group 4: AFB1 + GA1—treated with AFB1 (75 µg/kg +GA at 20 mg/kg) p.o.
Group 5: AFB1 + GA2—treated with AFB1 (75 µg/kg +GA at 40 mg/kg) p.o.
AFB1 and GA doses utilized for this study were chosen based on previous studies.31,32 Earlier studies revealed that commonly consumed corn (maize) reportedly had the highest levels of AFB1. Specifically, studies in Croatia, Pakistan, and the Democratic Republic of Congo evidenced maximum AFB1 levels to be 2072, 1405.3, and 1401.45 μg/kg, respectively.33–35 The estimated AFB1 daily intake during chronic exposure ranged from 48.4 µg to 77.4 µg in humans.31,36 AFB1 and GA were separately reconstituted in corn oil before administration to experimental animals. In groups that received AFB1 and GA, AFB1 was administered 30 min prior to dosing of rats with GA. Terminal sacrifice of experimental animal occurred on day 29, after the animals were weighed—to obtain final body weights—and blood collected via retro-orbital venous plexus into vials without anticoagulant. Subsequently, the serum prepared from clotted blood via centrifugation (4°C; 3000g; 10 min) were probed for the assessment of hormones relevant in reproduction. Thereafter, experimental rats were sacrificed under anesthesia—light ether. The testis, epididymis, and hypothalamus were instantly removed, weight recorded, and processed for histological and biochemical evaluations.
Assessment of sperm characteristics and reproductive hormones
Sperm progressive motility was estimated in line with established method.37 Briefly, epididymal sperm isolated from the cauda epididymis were released onto a sterile glass slide after surgical incision. The sperm was consequently diluted with 2.9% sodium citrate dehydrate solution pre-warmed to 37°C, and enclosed with a coverslip on a slide. Ten microscopic fields were observed (×200 magnification) using a microscope (phase-contrast) to assess motility. The spermatozoa motility was estimated by recording the quantity of each progressive sperm, non-progressive, and immotile sperm in the same field of view. Data were expressed as progressive sperm motility in percentages. The viability and morphological aberrations of the sperm were estimated using precise stain in line with established procedure,38 diluted epididymal sperm suspension obtained from the cauda epididymis (1 drop of sperm + 10 droplets of sodium citrate dihydrate), and mixed with staining solution containing eosin B (0.2 g) plus fast green (0.6 g) in ethyl alcohol, and distilled water in a ratio of 1:2 and maintained for one min at 37°C. A small drop of the final solution was finely spread on a slide, air-dried, and observed with a Leica light microscope (DM 500-Leica, Germany) for counting. Spermatozoa’s—400 counts—from each rat were tallied and allotted to various classes of morphological abnormalities attributable to sperm. Viability of sperm was estimated with eosin (1%) and nigrosin (5%) stains in sodium citrate dihydrate solution. Epididymal sperm count was calculated following standard method.39 Testicular sperm count and daily sperm production were evaluated in line with standard method.40 Using sperm obtained after mincing caudal epididymis in normal saline, the resultant suspension was filtered through a nylon mesh. Sperm aliquot (5 µL) was mixed with a diluent (95 µL) containing: formalin (0.35%) + NaHCO3(5%) + trypan blue (0.25%). Subsequently, diluted sperm (10 µL) was pipetted into the grove of a hemocytometer and left to settle for 5 min in a moist compartment to avoid dehydration and counted in the Neubauer chamber (Deep 1/10 m; LAB ART, Munich, Germany) and a Leica light microscope (Magnification: ×400). Assessment of reproductive hormones was done using appropriate precoated ELISA 96-well microplates (E-labscience, Beijing, China) for FSH (E-R0391), LH (E-R0026), testosterone (E-R0033), and prolactin (E-R0052) in line with manufacturers’ manual. All hormonal analyses were completed simultaneously to reduce the inter-assay difference. The sensitivities of LH, FSH, testosterone, and prolactin were 0.41 ng, 0.35 ng, 0.39 ng, and 0.11 ng, respectively. In contrast, the intra-assay coefficients of variations were 2.3%, 3.1%, 3.2%, and 2.6% for LH, FSH, testosterone, and prolactin, respectively.
Assessment of biomarker enzymes of testicular function
Moreover, marker enzymes activities of testicular function were assessed in the supernatant of the testes to assess further the effect of GA on AFB1-stimulated toxicity. Briefly, the testicular activity of glucose-6-phosphate dehydrogenase (G6PD) was assayed using nicotinamide adenine dinucleotide phosphate and glucose-6-phosphate as substrates as earlier reported.41,42 Testicular acid phosphatase (ACP) and alkaline phosphatase (ALP) activities were assayed following established practice based on the degradation of p-nitrophenyl-phosphate in acid and alkaline milieu, respectively.43,44 Also, testicular lactate dehydrogenase-X (LDH-X) activity was assessed by a standard technique that depended on the inter-change of lactate and pyruvate.45
Biochemical analyses
The testes, epididymis, and hypothalamus of the experimental animals were discretely homogenized in a Tris-HCl buffer (50 mM; pH 7.4). The resulting homogenate was centrifuged (12,000g; 15 min) to get the supernatant that was subsequently utilized for biochemical assays. The quantity of protein in the samples was assessed using bovine serum albumin as standard, according to Bradford.46
Assessment of antioxidant status in the testes, epididymis, and hypothalamus
Superoxide dismutase (SOD) activity was analyzed by the method of Misra and Fridovich.47 Briefly, sample aliquots were added to 0.05 M carbonate buffer (2.5 ml; pH 10.2) followed by adrenaline (0.3 ml). The absorbance (at 480 nm) increases was recorded every 30 s for 150 s. The activity of catalase (CAT) was assayed for as earlier described by Clairborne,48 CAT splits hydrogen peroxide resulting in a loss of absorbance at 240 nm. The changes in absorbance was recorded each minute for 5 min. Activities of glutathione peroxidase (GPx) following Rotruck et al.,49 protocol, using standard laboratory reagents, reduced glutathione (GSH), H2O2 and sample were reconstituted to form the reacting mixture. The reaction mixture was further made up to 2.0 mL with the addition of distilled water and incubated (37°C; 3 min). Subsequently, the reaction was stopped by the addition of TCA, centrifuged, and the residual GSH was determined from the supernatant obtained by adding disodium hydrogen phosphate, and 5,5″-dithio-bis-2-nitrobenzoic acid (DTNB). Finally, the absorbance (412 nm) was measured spectrophotometrically. The values of GPx activity were obtained and expressed in µmoles per mg protein. Glutathione-S-transferase (GST) was assayed as earlier described by Habig et al.50 Briefly, 0.1 mL of 1-chloro-2,4-dinitrobenzene (CDNB) was mixed with 1.7 mL of phosphate buffer to constitute the reaction mixture and for 5 min, incubated at 37°C. The mixture was then primed with the test samples (50 µL), and the absorbance (340 nm) was monitored for 5 min and recorded. Similarly constituted reaction mixture without the samples containing enzyme served as a reference blank. GST-specific activity was determined as GSH/CDNB conjugate formed in µmoles per min per milligram protein. Levels of reactive oxygen and nitrogen species (RONS) were estimated based on the capacity of RONS to oxidize 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) to 2′,7′-dichlorofluorescin (DCF).51,52 Quickly, the reaction admixture (sample (10 µL) + potassium phosphate buffer (0.1 M; pH 7.4; 150 µL) + distilled water (35 µL) + DCFH-DA (5 µL)) was prepared with marginal exposure to air. DCF fluorescence emission ensuing from DCFH-DA oxidation was evaluated (10 min; at 30 s gaps) at the following wavelengths: emission (525 nm) and excitation (488 nm). The readings were obtained with the aid of M384 SpectraMax™ Multi-modal plate reader. The levels of GSH was biochemically estimated using methods earlier described by Jollow et al.,53 using standard reagents following deproteination, samples were incubated with 5,5″-dithio-bis-2-nitrobenzoic acid (DTNB), and the absorbance at 412 nm was obtained by spectrophotometrically proportional to GSH level and expressed in µmoles/g tissue. Lipid peroxidation (LPO) was assayed as described by Farombi et al.54 Briefly, samples were mixed with Tris-KCl buffer-containing trichloroacetic acid (TCA). Subsequently, thiobarbituric acid (TBA) was added to each sample suspended in a hot water bath for 45 min. The reaction was then cooled, centrifuged, and the absorbance of the resultant supernatant was taken at 532 nm, against a reference blank. All biochemical analyses, apart from SOD and CAT activities, were done with the aid of a plate reader—Multimodal M384 SpectraMax™ (Molecular Devices, San Jose, USA).
Assessment of caspase-3 activity and inflammatory biomarkers
The level of nitric oxide (NO) was assayed using Griess reagent as earlier described by Green et al.55 Briefly, equal volumes of sample and Griess’ reagent were mixed together and allowed to stand for 15 min at 25°C and the absorbance obtained at 540 nm. The level of total nitrite was calculated from the absorbance obtained from a standard solution and the results expressed in Units per mg protein. Activity of myeloperoxidase (MPO) was assessed as earlier described by Granell et al.,56 by monitoring the oxidation of O-dianisidine in the presence of H2O2 catalyzed by myeloperoxidase at 470 nm. The levels of interleukin-1β (IL-1β), interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α) as well as caspase-3 activity in the hypothalamus, testes, and epididymis were evaluated by means of ELISA Kits (E-labscience, Beijing, China). All readings were obtained with the aid of a plate reader—Multimodal M384 SpectraMax™.
Microscopic assessment
Microscopic evaluation of the testes, epididymis, and hypothalamus was performed as earlier described by Bancroft.57 Quickly, the tissue samples were fixed using Bouin’s solution, dehydrated, and embedded in paraffin. Using a microtome, 5 µm slices were sectioned from the paraffin-embedded tissues. Sliced tissue sections were layered on treated glass slides and stained using hematoxylin-eosin stains. The stained slides were coded before examination by a pathologist using a Leica microscope (DM-500, Germany) and a digital camera (Leica ICC50 E, Germany).
Statistical analyses
Analysis of the results was done by One-way analysis of variance (ANOVA) and posthoc test (Bonferroni) with the aid of Prism version 8.3.0 for Mac (GraphPad Software, La Jolla, California, USA, www.graphpad.com). The assay was performed in duplicates and the results expressed as mean ± S.D. We considered value of p < 0.05 to be statistically significant.
Results
Gallic acid improved the body weight gain and relative organ weight of the epididymis and testes in AFB1-treated animals
Experimental animal body weight gain and relative organ weight of the epididymis, testes, and hypothalamus of control, GA alone, AFB1 only, and AFB1 and GA are shown in Table 1. The rats treated with AFB1alone demonstrated significant (p < 0.05) reduction in body weight gain and relative organ weight—epididymis, testes, and hypothalamus—matched with the control animals. Conversely, these alterations were effectively reversed to conditions similar to control in animals co-administered with AFB1 and GA at 20 and 40 mg/kg body weight.
Table 1.
Effect of gallic acid on the body weight gain and organo-somatic indices of testes and epididymis in AFB1-treated rats.
| Control | AFB1 alone | GA alone | AFB1 + GA1 | AFB1 + GA2 | |
|---|---|---|---|---|---|
| Final body weight (g) | 187.84 ± 2.02 | 178.57 ± 2.11 | 182.83 ± 2.06 | 180.48 ± 2.73 | 184.14 ± 2.16 |
| Initial body weight (g) | 163.52 ± 1.26 | 165.98 ± 1.38 | 162.11 ± 1.64 | 163.96 ± 2.05 | 165.97 ± 1.08 |
| Body weight gain (g) | 22.38 ± 1.26 | 13.61 ± 1.08a | 20.75 ± 1.25 | 17.12 ± 1.07b | 18.86 ± 1.02b |
| Hypothalamus | 0.32 ± 0.01 | 0.29 ± 0.02 | 0.31 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.01 |
| Testes | 2.52 ± 0.17 | 1.81 ± 0.15a | 2.49 ± 0.13 | 2.19 ± 0.09b | 2.44 ± 0.23b |
| Epididymis | 1.24 ± 0.03 | 0.72 ± 0.08a | 1.25 ± 0.08 | 1.12 ± 0.02b | 1.21 ± 0.05b |
Note: Values are expressed as mean ± S.D. for 10 rats per group.
AFB1: Aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: Gallic acid at 40 mg/kg.
aValues differ significantly from control (p < 0.05).
bValues differ significantly from AFB1 alone (p < 0.05).
Gallic acid alleviated AFB1-induced deficits in reproductive hormones and enzyme biomarker of testicular function in treated animals
Figure 2 displays GA effect on serum concentrations of reproductive hormones- FSH, LH, and testosterone-, also the marker enzymes of testicular function in AFB1-treated rats. When compared with the control rats, exposure to AFB1 alone elicited a significant reduction in serum testosterone, FSH, and LH levels with a simultaneous reduction in the activities of G6PD, LDH, ACP, and ALP of the testicule, although co-administration alongside GA (20 and 40 mg/kg body weight) significantly abrogated AFB1-mediated decreases in enzyme biomarker activities of testicular function and deficit in serum hormone levels relative to animals dosed with AFB1 only.
Figure 2.
Effect of gallic acid on serum hormonal levels and marker enzymes of testicular function in AFB1-exposed rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg; FSH: follicle stimulating hormone; LH: luteinizing hormone; ACP: acid phosphatase; ALP: alkaline phosphatase; LDH: lactate dehydrogenase.
Gallic acid improved spermatogenesis and functional parameters in AFB1-treated animals
The role of GA on the testicular sperm count, functional parameters, and daily production in AFB1-exposed animals are presented in Figure 3. Exposure to AFB1 alone evidently diminished spermatogenesis and testicular sperm count in the experimental animals in contrast with the control group. Besides, sperm functional parameters, namely epididymal sperm motility, and count were reduced (p < 0.05), while morphological defects of the sperm were markedly increased in animals dosed with AFB1 only. GA co-treatment notably abrogated AFB1-mediated decreases in sperm parameters, testicular sperm production, and number compared with animals treated with AFB1 only.
Figure 3.
Effect of gallic acid on sperm parameters in AFB1-exposed rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg.
Gallic acid enhanced antioxidant status in the epididymis, testes, and hypothalamus of AFB1-treated animals
The effects of GA on RONS level and antioxidant enzyme activities in AFB1-dosed rats are presented in Figures 4 to 6. Exposure to AFB1 alone caused marked decreases in GPx, GST, SOD, and CAT activities, and GSH level, but increased (p < 0.05) RONS level in the epididymis, testes, and hypothalamus of animals when relative to the control. However, GA co-treatment enhanced (p < 0.05) antioxidant enzymes activity and decreased RONS level in the epididymis, testes, and hypothalamus of animals compared to AFB1 alone-treated animals.
Figure 4.
Effect of gallic acid on SOD and CAT activities in the testes, epididymis, and hypothalamus of rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg; CAT: catalase; SOD: superoxide dismutase.
Figure 5.
Effect of gallic acid on GPx and GST activities in the testes, epididymis, and hypothalamus of AFB1-exposed rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg; GPx: glutathione peroxidase; GST: glutathione-S-transferase.
Figure 6.
Effect of gallic acid on GSH and RONS levels in the testes, epididymis, and hypothalamus of AFB1-exposed rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg; GSH: glutathione; RONS: reactive oxygen and nitrogen species.
Gallic acid suppressed inflammatory biomarkers in AFB1-treated animals
The effects of GA on inflammatory biomarkers in animals dosed with AFB1 are presented in Figures 7 to 9. Administration of AFB1 alone significantly elevated TNF-α, NO, and IL-1β levels among MPO activity in the epididymis, testes, and hypothalamus of the treated animals in comparison with the control. Moreover, AFB1 alone-treated rats exhibited a marked decrease in anti-inflammatory cytokine IL-10 in comparison with control. GA (20 and 40 mg/kg) co-treatment diminished (p < 0.05) NO, TNF-α, and IL-1β levels in addition to MPO activity with concomitant increase in IL-10 level in treated animals relative to AFB1-only group.
Figure 7.
Effect of gallic acid on MPO activity and NO level in the testes, epididymis, and hypothalamus of AFB1-exposed rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg; NO: nitric oxide; MPO: myeloperoxidase.
Figure 8.
Effect of gallic acid on TNF-α level in the testes, epididymis, and hypothalamus of AFB1-exposed rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg.
Figure 9.
Effect of gallic acid on IL-1β and IL-10 levels in the testes, epididymis, and hypothalamus of AFB1-exposed rats. The values are expressed as mean ± SD for ten rats per group. *: Values differ significantly from control (p < 0.05). **: Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg.
Gallic acid inhibited biomarkers of lipid peroxidation and apoptosis in AFB1-treated rats
The effect of GA on apoptosis and lipid peroxidation biomarkers in AFB1-exposed animals are shown in Figure 10. Administration of AFB1 alone elevated (p < 0.05) MDA, a biomarker of LPO, and caspase-3 activity, a killer protease in the apoptotic cascade, in the epididymis, testes, and hypothalamus of the treated animals relative to control. Moreover, co-administration of GA at 20 and 40 mg/kg diminished (p < 0.05) LPO level and caspase-3 activity in treated animals compared with the AFB1 alone group.
Figure 10.
Effect of gallic acid on LPO level and caspase-3 activity in the testes, epididymis, and hypothalamus of AFB1-exposed rats. The values are expressed as mean ± SD for 10 rats per group. *Values differ significantly from control (p < 0.05). **Values differ significantly from AFB1 alone (p < 0.05). (A color version of this figure is available in the online journal.)
AFB1: aflatoxin B1 at 75 µg/kg; GA1: gallic acid at 20 mg/kg; GA2: gallic acid at 40 mg/kg; LPO: lipid peroxidation. (A color version of this figure is available in the online journal.)
Gallic acid alleviated AFB1-induced histopathological lesions in rats
Histological characteristics of the testes, epididymis, and hypothalamus from the experimental animals using a light microscope are presented in Figure 11. Control and GA only treated groups exhibited typical histological structure of the epididymis, testes, and hypothalamus. Evident vacuolation (red star) and an extended area of tubular necrosis (black arrow) of the seminiferous tubules, degeneration of the epididymis depicted by focal area of necrotic tubules (black arrows) with inadequate sperm in the lumen. In contrast, mild neuronal degeneration (red arrow) in the hypothalamus was detected in rats exposed to AFB1 alone. However, animals co-treated with AFB1 and GA (20 and 40 mg/kg) exhibited typical architecture of the testes, epididymis, and hypothalamus similar to control.
Figure 11.
Photomicrographs of hypothalamus, epididymis, and testes of rats. Histology of the control and GA alone-treated rats appears typical. AFB1 alone-exposed rats showing mild neuronal degeneration in the hypothalamus (red arrow), the seminiferous tubules of the testes appeared necrotic (black arrow) with severe atrophy (red star). In contrast, the epididymis showed a focal area of necrotic tubules (black arrows) with insufficient sperm in the lumen. The testes, epididymis, and hypothalamus of rats co-exposed to AFB1 and gallic acid showed typical histology architecture comparable to control. Mag: ×400. (A color version of this figure is available in the online journal.)
Discussion
The occurrence of harmful food contaminants usually has a widespread impact because of the extensive trading of agricultural produce in the world.58 Exposure to AFB1 is a global health concern due to its grave toxicity and carcinogenicity in both humans and animals.59,60 Reproductive toxicity is a significant extra-hepatic effect of AFB1. The male factor has been reported to account for 50% of infertility cases in the world.1 Data from previous investigations have implicated AFB1 as a critical risk factor for male infertility.13,14 The present study showed that GA effectively mitigated reproductive dysfunction related to AFB1 exposure in male rats. The significant reductions in the body weight gain and relative organ weight—testes, and epididymis—in animals dosed with AFB1 as such connote evident harmfulness, and interference with metabolic functions in exposed animals. The reduction in testicular and epididymal weights in rats dosed with AFB1 as such implies organ atrophy. However, the restoration weight and relative weights gain—body, testes, and epididymis—in AFB1 and GA (20 and 40 mg/kg) animals bare the beneficial role of GA in the metabolic activities and health status of the reproductive organs in the experimental rats.
The maintenance of reproductive hormonal levels and spermatogenesis in mammals is mainly regulated by the hypothalamic-pituitary-testicular axis.61 Dosing with AFB1 in this study elicited significant reductions of pituitary hormones levels in the serum, specifically LH, FSH, and prolactin as well as in the gonadal hormone (i.e. testosterone), thus indicating the harmful effect of this toxin on the male reproductive axis. The reduction in serum testosterone level signifies the subduing effect of AFB1 on testicular steroidogenesis, previously associated with AFB1 toxic effect on the Leydig cells.18,19 However, the restoration of serum LH, FSH, prolactin, and testosterone levels in animals co-treated with AFB1 and GA (20 and 40 mg/kg) clearly showed the protecting effect of GA against AFB1-mediated endocrine deficits in the experimental rats.
Moreover, the adverse consequence of AFB1 exposure was evident from the activities of enzymes necessary for spermatogenesis in the testicule. The reduction in testicular LDH and G6PD activities in rats exposed to AFB1 alone signifies impairment in the metabolic pathway of lactate and biosynthesis of nicotinamide adenine dinucleotide phosphate (NADPH), respectively; both are necessary for the spermatogenic cells in rats. Also, decreased activities of ALP and ACP observed in AFB1 alone-treated rats signify an inhibitory effect of AFB1 on the phosphorylative role of ALP and ACP in the usage of glucose by germ cells during spermatogenesis. The restoration of testicular activities of G6PD, LDH, ALP, and ACP in rats dosed with both AFB1 and GA (20 and 40 mg/kg) indicates the protecting effect of GA against AFB1-mediated testicular germ cells toxicity.
The present study evidenced that exposure to AFB1 alone elicited harmful effects on the epididymis—responsible for the storage, transport, and maturity of sperm—produced by the testes.61 Also, AFB1-induced impairment in the testicular function of spermatogenesis is evident by the marked reduction in the testicular daily sperm production and sperm count. In contrast, its spermatotoxicity effect associated with a decrease in epididymal sperm count and motility. The marked elevation in the sperm defects, precisely the sperm mid-piece which houses the mitochondria necessary for adenosine triphosphate (ATP) production and the sperm tail where structural mechanisms directly involved in motility resides, may be associated with the observed decrease in sperm motility in rats exposed to AFB1 alone. The restoration in the spermatogenesis and sperm characteristics following co-exposure to AFB1 and GA (20 and 40 mg/kg) indicates the protective effect of GA against AFB1-mediated epididymal toxicity in the treated animals.
To further define the mechanisms associated with the protective effects of GA on AFB1-mediated male reproductive toxicity, the assessment of biochemical indices, namely inflammation, apoptosis, and oxidative stress, were explored in the testes, epididymis, and hypothalamus of experimental animals. The enzymatic and non-enzymatic antioxidant defense systems are responsible for the maintenance of redox status and subdual of oxidative stress in the cells. Hence, the marked elevation in the testicular, epididymal, and hypothalamic LPO and RONS levels in AFB1 alone-dosed animals signifies that the AFB1 increased RONS generation which suppressed the antioxidant capacity and consequently induced oxidative-RONS-mediated injury in the rats. Reductions in SOD, CAT and GSH-dependent enzymes (GST and GPx) activities and GSH levels in AFB1 alone-treated animals signify the inhibition of their antioxidant function which is related to the cellular RONS buildup and oxidative damage in the epididymis, testes, and hypothalamus of the rats. The current data substantiate earlier reports on AFB1-mediated stimulation of oxidative stress testes of rats,15,16 although decreases in MDA and RONS levels with a simultaneous increase in the antioxidant enzymes activities in rats co-treated with AFB1 and GA are associated with the antioxidant effect of GA previously reported.62
The TNF-α regulation of cytokine production during inflammatory response reportedly triggers the generation of NO via nitric oxide synthase pathway in the cell. Elevation in the cellular NO level is associated with nitrosative stress, which reportedly injures cellular proteins, nucleic acids, and lipids following the diminution of antioxidant defense systems.63 In the current study, exposure to AFB1 alone evidently increased NO, TNF-α, and IL-1β levels aside from MPO activity, whereas it decreased anti-inflammatory cytokine, IL-10 level in the epididymis, testes, and hypothalamus of the treated rats. This observation signifies the induction of inflammation. Uncontrolled production of pro-inflammatory cytokines for example IL-1β and TNF-α in the testes is harmful to spermatogenesis and is well known to cause male infertility.64 Hence, the decrease in the TNF-α, NO, and IL-1β levels along with MPO in epididymis, testes, and hypothalamus following co-exposure to GA denotes that it occasioned anti-inflammatory mechanism to abate AFB1-induced testicular toxicity.
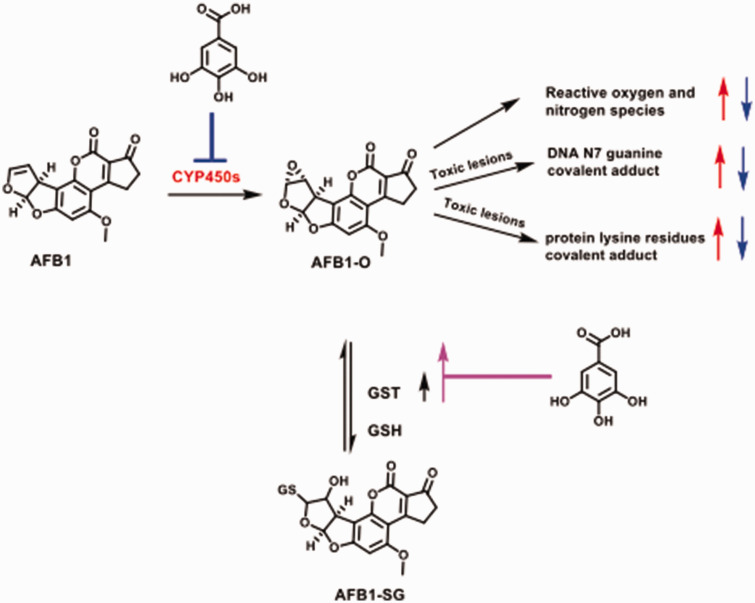
Caspase-3 activity evidently increases in testicular, epididymal, and hypothalamus of rats dosed with AFB1 alone indicates activation of this critical downstream executioner protease in the programmed cell death pathway.65 Caspase-3 activity was reduced in animals co-treated with AFB1 and GA (20 and 40 mg/kg) indicating inhibition of caspase-3 activity and, thus, the anti-apoptotic role of GA in the experimental rats. The testicular, epididymal, and hypothalamic lesions associated with AFB1 exposure are attributable directly or indirectly to elevated levels of RONS and induction of apoptosis and inflammation in the treated animals. The protective effect of GA (20 and 40 mg/kg) on the histology of investigated tissues is evidence in the remarkable drop in lesions seen in rats co-treated with AFB1 and GA. The preservation of these histological structures corroborates the biochemical findings of the beneficial consequences of GA on AFB1-induced male reproductive dysfunction in rats. The proposed mechanism by which GA modulates reproductive toxicity induced by AFB1 in rats is depicted in Figure 12.
Figure 12.
Plausible mechanisms of GA-mediated amelioration of AFB1-induced injury in rats’ testis hypothalamic-pituitary-testicular axis. Note that glutathione S-transferase (GST), known to detoxify AFB1-O,70 here was used as a model to illustrate the consequence of induction of Phase II drug metabolizing enzymes by GA. Red arrow indicates upregulation, blue arrow indicates downregulation, black arrow indicates basal level and purple arrow indicates induction. (A color version of this figure is available in the online journal.)
GSH: glutathione.
Conclusively, the GA-mediated amelioration of AFB1-induced deficits in the hypothalamic-pituitary-testicular axis in experimental rats is associated with its inherent anti-inflammatory, antioxidant, and anti-apoptotic properties. GA has broader protective role to safeguard against AFB1 toxicity,66 and causes damage to cellular macromolecules. Previous reports in the literature suggest that GA is a direct inhibitor of several CYP450s that facilitate the intrahepatic activation of AFB1,29,30 and there is evidence suggesting that GA and its esters can induce Phase II drug metabolizing enzymes.67 Therefore, it is possible that GA could mediate the detoxification of AFB1 by inhibiting AFB1 activation29 and scavenging AFB1-exo-8,9-epoxide (AFB1-O), associated in AFB1 carcinogenesis68–70 as depicted in Figure 12. Additional in-depth studies are necessary to precisely elucidate the mechanism(s) of GA-mediated detoxification of AFB1. Our current data strongly suggest that GA represents a prospective beneficial agent to mitigate reproductive toxicity related to aflatoxicosis in man.
Authors’ contribution
All authors partook in the design, interpretation, analysis of the data generated from the study. SEO, IAA, OEF, and AKO conceptualized the experiments; APA: carried out the research and preliminary data analysis. SEO, IAA supervised the investigation, EOF proof check data for error. The article was written, and revised by: SEO, IAA, and AKO.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors privately funded this research by the author’s contribution and received no external grant from funding agencies in the commercial, not-for-profit, or public sectors.
ORCID iD
Solomon E Owumi https://orcid.org/0000-0002-4973-0376
References
- 1.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015; 21:411–26 [DOI] [PubMed] [Google Scholar]
- 2.Yan X, Dong L, Liu Y, Yang F, Tan K, Li J, Chang D, Yu X. Effects of physical exercises on semen quality and reproductive outcomes in male infertility: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019; 98:e17494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guvvala PR, Ravindra JP, Selvaraju S. Impact of environmental contaminants on reproductive health of male domestic ruminants: a review. Environ Sci Pollut Res Int 2020; 27:3819–36 [DOI] [PubMed] [Google Scholar]
- 4.Di Nisio A, Foresta C. Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility. Reprod Biol Endocrinol 2019; 17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: prevention and detoxification in foods. Foods 2020; 9:137 [ 10.3390/foods9020137] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adedara IAO, SE, Uwaifo AO, Farombi EO. Aflatoxin B1 and ethanol co-exposure induces hepatic oxidative damage in mice. Toxicol Ind Health 2010; 26:717–24 [DOI] [PubMed] [Google Scholar]
- 7.Peles F, Sipos P, Gyori Z, Pfliegler WP, Giacometti F, Serraino A, Pagliuca G, Gazzotti T, Pocsi I. Adverse effects, transformation and channeling of aflatoxins into food raw materials in livestock. Front Microbiol 2019; 10:2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alshannaq AF, Gibbons JG, Lee MK, Han KH, Hong SB, Yu JH. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci Rep 2018; 8:16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamid AS, Tesfamariam IG, Zhang Y, Zhang ZG. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention. Oncol Lett 2013; 5:1087–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdulrazzaq YM, Osman N, Ibrahim A. Fetal exposure to aflatoxins in the United Arab Emirates. Ann Trop Paediatr 2002; 22:3–9 [DOI] [PubMed] [Google Scholar]
- 11.Elaridi J, Bassil M, Kharma JA, Daou F, Hassan HF. Analysis of aflatoxin M1 in breast milk and its association with nutritional and socioeconomic status of lactating mothers in Lebanon. J Food Prot 2017; 80:1737–41 [DOI] [PubMed] [Google Scholar]
- 12.Awaisheh SS, Rahahleh RJ, Algroom RM, Al-Bakheit AA, Al-Khaza’leh JM, Al-Dababseh BA. Contamination level and exposure assessment to aflatoxin M1 in Jordanian infant milk formulas. Ital J Food Saf 2019; 8:8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibeh IN, Uraih N, Ogonar JI. Dietary exposure to aflatoxin in human male infertility in Benin City, Nigeria. Int J Fertil Menopausal Stud 1994; 39:208–14 [PubMed] [Google Scholar]
- 14.Komsky-Elbaz A, Saktsier M, Roth Z. Aflatoxin B1 impairs sperm quality and fertilization competence. Toxicology 2018; 393:42–50 [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Cao Z, Yao Q, Ji Q, Zhang J, Li Y. Mitochondrial damage are involved in aflatoxin B1-induced testicular damage and spermatogenesis disorder in mice. Sci Total Environ 2020; 701:135077. [DOI] [PubMed] [Google Scholar]
- 16.Cao Z, Shao B, Xu F, Liu Y, Li Y, Zhu Y. Protective effect of selenium on aflatoxin B1-induced testicular toxicity in mice. Biol Trace Elem Res 2017; 180:233–8 [DOI] [PubMed] [Google Scholar]
- 17.Supriya C, Reddy PS. Prenatal exposure to aflatoxin B1: developmental, behavioral, and reproductive alterations in male rats. Naturwissenschaften 2015; 102:26. [DOI] [PubMed] [Google Scholar]
- 18.Adedara IA, Nanjappa MK, Farombi EO, Akingbemi BT. Aflatoxin B1 disrupts the androgen biosynthetic pathway in rat Leydig cells. Food Chem Toxicol 2014; 65:252–9 [DOI] [PubMed] [Google Scholar]
- 19.Chen XL, C, Chen Y, Ni C, Chen X, Zhang L, Xu X, Chen M, Ma X, Zhan H, Xu A, Ge R, Guo X. Aflatoxin B1 impairs Leydig cells through inhibiting AMPK/mTOR-mediated autophagy flux pathway. Chemosphere 2019; 233:261–72 [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Cao Z, Zhang J, Ji Q, Li Y. Aflatoxin B1 promotes autophagy associated with oxidative stress-related PI3K/AKT/mTOR signaling pathway in mice testis. Environ Pollut 2019; 255:113317. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes FH, Salgado HR. Gallic acid: review of the methods of determination and quantification. Crit Rev Anal Chem 2016; 46:257–65 [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Moneim A, El-Twab SMA, Yousef AI, Reheim ESA, Ashour MB. Modulation of hyperglycemia and dyslipidemia in experimental type 2 diabetes by gallic acid and p-coumaric acid: the role of adipocytokines and PPARgamma. Biomed Pharmacother 2018; 105:1091–7 [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishnan SA, Angayarkanni N. Basics in biochemistry for professional nursing. New Delhi, India: BI Publications Private Limited, 2007, pp.24–32 [Google Scholar]
- 24.Oyagbemi AA, Omobowale TO, Saba AB, Adedara IA, Olowu ER, Akinrinde AS, Dada RO. Gallic acid protects against cyclophosphamide-induced toxicity in testis and epididymis of rats. Andrologia 2016; 48:393–401 [DOI] [PubMed] [Google Scholar]
- 25.Mehraban Z, Ghaffari Novin M, Golmohammadi MG, Sagha M, Ziai SA, Abdollahifar MA, Nazarian H. Protective effect of gallic acid on testicular tissue, sperm parameters, and DNA fragmentation against toxicity induced by cyclophosphamide in adult NMRI mice. Urol J 2020; 17:78–85 [DOI] [PubMed] [Google Scholar]
- 26.Saygin M, Asci H, Ozmen O, Cankara FN, Dincoglu D, Ilhan I. Impact of 2.45 GHz microwave radiation on the testicular inflammatory pathway biomarkers in young rats: the role of gallic acid. Environ Toxicol 2016; 31:1771–84 [DOI] [PubMed] [Google Scholar]
- 27.Yigitturk G, Acara AC, Erbas O, Oltulu F, Yavasoglu NUK, Uysal A, Yavasoglu A. The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomed Pharmacother 2017; 87:240–6 [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Zhi QQ, Li JY, Keller NP, He ZM. The antioxidant gallic acid inhibits aflatoxin formation in Aspergillus flavus by modulating transcription factors FarB and CreA. Toxins (Basel) 2018; 10:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Athukuri BL, Neerati P. Enhanced oral bioavailability of metoprolol with gallic acid and ellagic acid in male wistar rats: involvement of CYP2D6 inhibition. Drug Metab Pers Ther 2016; 31:229–34 [DOI] [PubMed] [Google Scholar]
- 30.Vijayakumar TM, Kumar RM, Agrawal A, Dubey GP, Ilango K. Comparative inhibitory potential of selected dietary bioactive polyphenols, phytosterols on CYP3A4 and CYP2D6 with fluorometric high-throughput screening. J Food Sci Technol 2015; 52:4537–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian G, Wang F, Tang L, Massey ME, Mitchell NJ, Su J, Williams JH, Phillips TD, Wang JS. Integrative toxicopathological evaluation of aflatoxin B(1) exposure in F344 rats. Toxicol Pathol 2013; 41:1093–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garud MS, Kulkarni YA. Gallic acid attenuates type I diabetic nephropathy in rats. Chem Biol Interact 2018; 282:69–76 [DOI] [PubMed] [Google Scholar]
- 33.Firdous S, Ashfaq A, Khan SJ, Khan N. Aflatoxins in corn and rice sold in Lahore, Pakistan. Food Addit Contam Part B Surveill 2014; 7:95–8 [DOI] [PubMed] [Google Scholar]
- 34.Pleadin J, Sokolović M, Perši N, Zadravec M, Jaki V, Vulić A. Contamination of maize with deoxynivalenol and zearalenone in Croatia. Food Control 2012; 28:94–8 [Google Scholar]
- 35.Kamika I, Ngbolua K-T-N, Tekere M. Occurrence of aflatoxin contamination in maize throughout the supply chain in the democratic republic of Congo. Food Control 2016; 69:292–6 [Google Scholar]
- 36.Groopman JD, Zhu JQ, Donahue PR, Pikul A, Zhang LS, Chen JS, Wogan GN. Molecular dosimetry of urinary aflatoxin-DNA adducts in people living in Guangxi autonomous region, People’s Republic Of China. Cancer Res 1992; 52:45–52 [PubMed] [Google Scholar]
- 37.Zemjanis R. Collection and evaluation of semen. 2nd ed Baltimore, MD: William and Wilkins Company, Waverly Press, Inc, 1970 [Google Scholar]
- 38.Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci 1970; 53:227–32 [DOI] [PubMed] [Google Scholar]
- 39.Worlf Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed New York: Cambridge University Press, 1999. [Google Scholar]
- 40.Blazak WF, Trienen TK, Juniewicz PE. Application of testicular sperm head counts in the assessment of male reproductive toxicity In: Chapin RE, Heindel J. (eds) Methods in toxicology: Male reproductive toxicology. San Diego: Academic Press, 1993;86–94 [Google Scholar]
- 41.Wolf BH, Weening RS, Schutgens RB, van Noorden CJ, Vogels IM, Nagelkerke NJ. Detection of glucose-6-phosphate dehydrogenase deficiency in erythrocytes: a spectrophotometric assay and a fluorescent spot test compared with a cytochemical method. Clin Chim Acta 1987; 168:129–36 [DOI] [PubMed] [Google Scholar]
- 42.Salihu M, Ajayi BO, Adedara IA, Farombi EO. 6-Gingerol-rich fraction prevents disruption of histomorphometry and marker enzymes of testicular function in carbendazim-treated rats. Andrologia 2017; 49(10). doi:10.1111/and.12782 [DOI] [PubMed] [Google Scholar]
- 43.Malymy MH, Horecker BL. Alkaline phosphatase. In: Methods in enzymology. New York: Academic Press, 1966. 639–642
- 44.Vanha-Perttula T. Acid phosphatases of the rat testis in experimental conditions. Acta Endocrinol 1973; 72:376–90 [DOI] [PubMed] [Google Scholar]
- 45.Vassault A. Lactate dehydrogenase. UV-method with pyruvate and NADH In: Bergmeyer HU. (ed.) Methods of enzymatic analysis. 3rd ed New York: Plenum, 1993, pp.118–25. [Google Scholar]
- 46.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–54 [DOI] [PubMed] [Google Scholar]
- 47.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972; 247:3170–5 [PubMed] [Google Scholar]
- 48.Clairborne A. Catalase activity In: A.R Greenwald, (ed.) Handbook of methods for oxygen radical research. Boca Raton, FL: CRC Press, 1995;283–284 [Google Scholar]
- 49.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973; 179:588–90 [DOI] [PubMed] [Google Scholar]
- 50.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249:7130–9 [PubMed] [Google Scholar]
- 51.Owumi SE, Dim UJ. Manganese suppresses oxidative stress, inflammation and caspase-3 activation in rats exposed to chlorpyrifos. Toxicol Rep 2019; 6:202–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Severiano F, Santamaria A, Pedraza-Chaverri J, Medina-Campos ON, Rios C, Segovia J. Increased formation of reactive oxygen species, but no changes in glutathione peroxidase activity, in striata of mice transgenic for the Huntington’s disease mutation. Neurochem Res 2004; 29:729–33 [DOI] [PubMed] [Google Scholar]
- 53.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974; 11:151–69 [DOI] [PubMed] [Google Scholar]
- 54.Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron – a Garcinia kola seed extract. Food Chem Toxicol 2000; 38:535–41 [DOI] [PubMed] [Google Scholar]
- 55.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982; 126:131–8 [DOI] [PubMed] [Google Scholar]
- 56.Granell S, Gironella M, Bulbena O, Panes J, Mauri M, Sabater L, Aparisi L, Gelpi E, Closa D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit Care Med 2003; 31:525–30 [DOI] [PubMed] [Google Scholar]
- 57.Bancroft JD. Theory and practice of histological techniques. 6th ed. China: Churchill Livingston/Elsevier, 2008 [Google Scholar]
- 58.IARC. Monographs on aflatoxins. In: Cancer IAfRC editor. International Agency for Research on Cancer: IARC Press, 2000, pp.171–300
- 59.Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. Aflatoxins: a global concern for food safety, human health and their management. Front Microbiol 2016; 7:2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HJR, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J Agric Food Chem 2017; 65:7034–51 [DOI] [PubMed] [Google Scholar]
- 61.Adedara IA, Subair TI, Ego VC, Oyediran O, Farombi EO. Chemoprotective role of quercetin in manganese-induced toxicity along the brain-pituitary-testicular axis in rats. Chem Biol Interact 2017; 263:88–98 [DOI] [PubMed] [Google Scholar]
- 62.Thompson MC. Handbook on gallic acid: natural occurrences, antioxidant properties and health implications. United States: Nova Science Publishers Inc, 2013 [Google Scholar]
- 63.Taysi S, Tascan AS, Ugur MG, Demir M. Radicals, oxidative/nitrosative stress and preeclampsia. Mini Rev Med Chem 2019; 19:178–93 [DOI] [PubMed] [Google Scholar]
- 64.Fijak MP, A, Hedger MP, Nicolas N, Bhushan S, Michel V, Tung KSK, Schuppe HC, Meinhardt A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: how do rodent models inform clinical practice? Hum Reprod Update 2018; 24:416–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savitskaya MA, Onishchenko GE. Mechanisms of apoptosis. Biochemistry Mosc 2015; 80:1393–405 [DOI] [PubMed] [Google Scholar]
- 66.Choubey S, Goyal S, Varughese LR, Kumar V, Sharma AK, Beniwal V. Probing gallic acid for its broad spectrum applications. Mini Rev Med Chem 2018; 18:1283–93 [DOI] [PubMed] [Google Scholar]
- 67.Ow YY, Stupans I. Gallic acid and gallic acid derivatives: effects on drug metabolizing enzymes. Curr Drug Metab 2003; 4:241–8 [DOI] [PubMed] [Google Scholar]
- 68.Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol 1994; 34:135–72 [DOI] [PubMed] [Google Scholar]
- 69.Sotomayor RE, Sahu S, Washington M, Hinton DM, Chou M. Temporal patterns of DNA adduct formation and glutathione S-transferase activity in the testes of rats fed aflatoxin B1: a comparison with patterns in the liver. Environ Mol Mutagen 1999; 33:293–302 [PubMed] [Google Scholar]
- 70.Jowsey IR, Jiang Q, Itoh K, Yamamoto M, Hayes JD. Expression of the aflatoxin B1-8,9-epoxide-metabolizing murine glutathione S-transferase A3 subunit is regulated by the Nrf2 transcription factor through an antioxidant response element. Mol Pharmacol 2003; 64:1018–28 [DOI] [PubMed] [Google Scholar]