Abstract
Organic solute transporter (OST) α/β is a key bile acid transporter expressed in various organs, including the liver under cholestatic conditions. However, little is known about the involvement of OSTα/β in bile acid-mediated drug-induced liver injury (DILI), a major safety concern in drug development. This study investigated whether OSTα/β preferentially transports more hepatotoxic, conjugated, primary bile acids and to what extent xenobiotics inhibit this transport. Kinetic studies with OSTα/β-overexpressing cells revealed that OSTα/β preferentially transported bile acids in the following order: taurochenodeoxycholate > glycochenodeoxycholate > taurocholate > glycocholate. The apparent half-maximal inhibitory concentrations for OSTα/β-mediated bile acid (5 µM) transport inhibition by fidaxomicin, troglitazone sulfate, and ethinyl estradiol were: 210, 334, and 1050 µM, respectively, for taurochenodeoxycholate; 97.6, 333, and 337 µM, respectively, for glycochenodeoxycholate; 140, 265, and 527 µM, respectively, for taurocholate; 59.8, 102, and 117 µM, respectively, for glycocholate. The potential role of OSTα/β in hepatocellular glycine-conjugated bile acid accumulation and cholestatic DILI was evaluated using sandwich-cultured human hepatocytes (SCHH). Treatment of SCHH with the farnesoid X receptor agonist chenodeoxycholate (100 µM) resulted in substantial OSTα/β induction, among other proteomic alterations, reducing glycochenodeoxycholate and glycocholate accumulation in cells+bile 4.0- and 4.5-fold, respectively. Treatment of SCHH with troglitazone and fidaxomicin together under cholestatic conditions resulted in increased hepatocellular toxicity compared with either compound alone, suggesting that OSTα/β inhibition may accentuate DILI. In conclusion, this study provides insights into the role of OSTα/β in preferential disposition of bile acids associated with hepatotoxicity, the impact of xenobiotics on OSTα/β-mediated bile acid transport, and the role of this transporter in SCHH and cholestatic DILI.
Keywords: B-CLEAR, DILI, hepatotoxicity, OSTα/β, targeted proteomics
Organic solute transporter (OST) α/β, a bidirectional heteromeric transporter localized on the basolateral membrane of hepatic, intestinal, and kidney epithelial cells, is important in the homeostasis of bile acids and other steroid hormones (Ballatori et al., 2008; Frankenberg et al., 2006; Rao et al., 2008; Sultan et al., 2018; Wang et al., 2001). OSTα/β-mediated transport is driven by the substrate-concentration gradient on either side of the cell membrane (Ballatori et al., 2005; Malinen et al., 2018). Hepatic OSTα/β expression is significantly increased in obstructive cholestasis (Chai et al., 2015) and primary biliary cholangitis (Boyer et al., 2006; Malinen et al., 2018), suggesting that this transporter facilitates excretion of bile acids in cholestatic liver disease, a condition in which bile flow from the liver to the duodenum is impaired. It was recently discovered that OSTα/β is overexpressed in patients with nonalcoholic steatohepatitis (Malinen et al., 2018), a disease characterized by elevated serum bile acid concentrations (Ferslew et al., 2015), whereas a link between a genetic defect in OSTα/β and cholestasis has also been reported (Sultan et al., 2018).
Bile acids are derived from hepatic cholesterol metabolism and are important amphipathic molecules used by the body to enhance the absorption of fat and fat-soluble vitamins, and aid the elimination of lipids and xenobiotics (Hofmann, 1999). Bile acids act as steroid hormones and are ligands for the farnesoid X receptor (FXR), a nuclear receptor that plays vital roles in regulating the expression of proteins involved in bile acid homeostasis, and the metabolism of glucose and lipids (Arab et al., 2017). Primary bile acids are synthesized in the liver and can be conjugated with glycine (Byrne et al., 2002; Tagliacozzi et al., 2003) or taurine (Alvaro et al., 1986a,b; Mizuta et al., 1999; Pellicoro and Faber, 2007) prior to biliary excretion by the bile salt export pump (BSEP). Secondary bile acids are formed in the intestine by gut bacteria-mediated deconjugation, dehydroxylation, epimerization, and oxidation of primary bile acids (Wahlstrom et al., 2016). The majority of bile acids (> 90%) excreted into bile are reabsorbed and returned to the liver via enterohepatic circulation, which is mediated by transporters; de novo bile acid synthesis plays a smaller role, indicating that efficient hepatic and intestinal transport processes are essential to maintain bile acid homeostasis (Barrasa et al., 2013; Hofmann, 1977, 1984; Ørntoft et al., 2017).
Due to detergent-like properties, the lipophilicity of bile acids has been associated with hepatotoxicity (Attili et al., 1986). Dysregulation of bile acid disposition, particularly by drug-mediated inhibition of hepatic efflux transporters, can lead to hepatocellular accumulation of toxic bile acid species resulting in liver damage (Dawson et al., 2012; Köck et al., 2014; Morgan et al., 2013; Perez and Briz, 2009). This is an important mechanism of drug-induced liver injury (DILI), a major safety issue in drug development and a primary reason that approved drugs are withdrawn from the market (Onakpoya et al., 2016; Perez and Briz, 2009; Temple and Himmel, 2002). The International Transporter Consortium has acknowledged the potential importance of OSTα/β-dependent drug interactions (Kenna et al., 2018; Zamek-Gliszczynski et al., 2018). Although several xenobiotics are known to inhibit OSTα/β-mediated transport (Malinen et al., 2018, 2019; Seward et al., 2003; Wang et al., 2001), thus far very little is known about the potential role of OSTα/β in bile acid-mediated DILI. A comprehensive analysis of various bile acids as OSTα/β substrates has recently been performed, showing that OSTα/β has low affinity but high capacity for transport of numerous bile acids (Suga et al., 2019). To further understand the role of OSTα/β in human health, disease and DILI, it is vital to evaluate OSTα/β-bile acid interactions in the presence of xenobiotics and in OSTα/β-expressing human hepatocytes.
To fill gaps in the understanding of hepatocellular OSTα/β-mediated bile acid transport, this study focused on some of the most prevalent, conjugated, primary bile acids in human plasma (glycochenodeoxycholate [GCDCA], glycocholate [GCA], taurochenodeoxycholate [TCDCA] (Schadt et al., 2016), and taurocholate [TCA]) as OSTα/β substrates. The relatively lipophilic GCDCA and TCDCA have been associated with hepatotoxicity, whereas the more hydrophilic GCA and TCA are considered less toxic (Chatterjee et al., 2014; Galle et al., 1990; Murakami et al., 2020; Woolbright et al., 2014). This study evaluated preferential OSTα/β-mediated transport of these bile acids, quantified the hepatocellular accumulation of GCDCA and GCA when OSTα/β was induced, and assessed the impact of OSTα/β inhibition on OSTα/β-mediated bile acid transport and cholestatic hepatocellular toxicity.
MATERIALS AND METHODS
Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, Missouri), unless specified otherwise. GCA, TCA (Chem-Impex International, Wood Dale, Illinois), GCDCA and TCDCA were used as unlabeled, putative substrates for bile acid transport studies. Radiolabeled and deuterated compounds were purchased from PerkinElmer Life Sciences (Boston, Massachusetts) and Toronto Research Chemicals (Toronto, Ontario, Canada), respectively. [14C]-GCA (46.3–51.6 mCi/mmol, radiochemical purity > 97%), [3H]-TCA (9.74 Ci/mmol, radiochemical purity > 97%), GCDCA and TCDCA were used as probe substrates in cell line transport studies, whereas d5-GCA and d7-GCDCA were used as probe substrates in sandwich-cultured human hepatocytes (SCHH) transport studies.
Culture of OSTα/β-overexpressing cells
Flp-In 293 cells stably overexpressing human OSTα and OSTβ (named OSTab cells), and mock-transfected Flp-In 293 cells (named Mock cells) were generated as described previously (Malinen et al., 2018). The cell lines were cultured in high glucose Dulbecco’s Modified Eagle’s Medium (catalog number: 11960044) supplemented with 1% (v/v) GlutaMAX, 100 U/ml penicillin and 100 µg/ml streptomycin from Thermo Fisher Scientific (Waltham, Massachusetts), and with 10% (v/v) fetal bovine serum (EMD Millipore, Billerica, Massachusetts). Culture medium was renewed every 2–3 days. Cells were maintained at 37°C and 5% CO2, and subcultured once a week. Trypan blue exclusion was used to determine cell viability.
Bile acid transport studies using OSTα/β-overexpressing cells
OSTab and Mock cells were used to study uptake of [14C]-GCA, [3H]-TCA, GCDCA, and TCDCA. To assess differences in time-dependence of OSTab- and Mock-mediated transport of bile acids, uptake was assessed at designated time points (5, 10, 15, and 30 s; 1, 2, 5, and 10 min). Differences in concentration-dependent OSTα/β-mediated transport of bile acids were evaluated at designated concentrations (5, 10, 25, 50, 100, 250, 500 µM). In addition, to evaluate substrate-dependent inhibition of bile acid uptake (5 µM), three OSTα/β inhibitors: fidaxomicin (FDX; Cayman Chemical Company, Ann Arbor, Michigan), troglitazone sulfate (TS, the sulfate metabolite of troglitazone [TGZ]; Toronto Research Chemicals), and ethinyl estradiol (EE; Cayman Chemical Company) were studied at concentrations of 0.125, 0.5, 2.5, 10, 40, 100, 200 µM; apparent half-maximal inhibitory concentrations (IC50,app) were determined. These OSTα/β inhibitors were identified previously from a panel of DILI-associated xenobiotics and inhibitors of other transporters using dehydroepiandrosterone sulfate (DHEAS) as an OSTα/β probe substrate (Malinen et al., 2019). All transport studies in OSTab and Mock cells were performed on three separate days (ie, n = 3). On each day, experiments were conducted in triplicate wells.
Two days before uptake experiments, OSTab and Mock cells were seeded at a concentration of 5.0 × 105 viable cells/well in 24-well Corning BioCoat Poly-d-Lysine Multiwell Plates (Thermo Fisher Scientific). Cells were washed and preincubated for 10 min at 37°C with modified extracellular fluid buffer (ECF, pH 7.4) containing 125 mM potassium chloride and no sodium chloride, as described previously (Malinen et al., 2018, 2019). For inhibition studies, an OSTα/β inhibitor was included in the preincubation phase. Following the preincubation phase, cells were incubated with bile acid substrate in ECF for designated times (various incubation times for time-dependence studies were used as described above, and a single incubation time [30 s] within the linear range of the initial uptake vs time profile was used for concentration-dependence and inhibition studies). After substrate incubation, the reaction was stopped by two washes with ECF at 4°C. Samples were stored at −20°C until analysis. Substrate-associated radioactivity accumulated in cells was extracted from cells by incubating the thawed plates for 20 min on a plate shaker with lysis buffer, consisting of 0.005% (v/v) antifoam A (Sigma), and 0.5% (v/v) Triton-X100 in phosphate buffered saline. Radioactivity was analyzed using a Bio-Safe II counting cocktail (RPI Corp, Mt Prospect, Illinois) and a Tri-Carb 3100TR low activity liquid scintillation analyzer (PerkinElmer, Inc). Bile acid substrates from nonradioactive samples were extracted from cells by incubating the thawed plates for 20 min on a plate shaker with 70% methanol and 30% water (v/v) containing an internal standard (IS; 100 nM), which was followed by liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis. The amount of substrate taken up by cells per time interval was normalized based on total protein present in a cell culture well treated with lysis buffer using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific) per the manufacturer instructions.
SCHH studies
Transporter certified cryopreserved human hepatocytes isolated from one Caucasian female, one Asian male, and two Caucasian male donors (Lots MLM, WWQ, and WID obtained from BioIVT [Durham, North Carolina], and HU8246 obtained from Gibco [Gaithersburg, Maryland]; age range, 22–71 years; body mass index range, 22.0–26.9) were used for SCHH experiments. After thawing, hepatocytes were seeded on BioCoat collagen-coated plates (Corning, Durham, North Carolina) at a density of 4.0–4.5 × 105 viable cells/well in a 24-well format for bile acid accumulation studies, and at 5.6–6.3 × 104 cells/well in a 96-well format (Corning) for cytotoxicity experiments. Cell viability was determined by trypan blue exclusion. The following day, cells were overlaid with 0.25 mg/ml Matrigel (Corning) in QualGro culture medium, resulting in SCHH. Each subsequent day, culture medium was replaced with fresh medium. After 48 h of culture, SCHH in the 24-well format were treated every 24 h for the next 72 h (until the start of the bile acid accumulation study) with 100 µM chenodeoxycholate (CDCA; at a final dimethyl sulfoxide [DMSO] concentration [v/v] of 0.1%) to induce OSTα/β protein in SCHH (Guo et al., 2018) or 0.1% (v/v) DMSO as the control condition. OSTα/β expression is low in healthy human hepatocytes under basal conditions in vivo and in vitro, but under cholestatic conditions or following treatment of SCHH with a potent FXR agonist (eg, CDCA), OSTα/β is induced substantially (Guo et al., 2018; Jackson et al., 2016; Krattinger et al., 2016).
Bile acid uptake studies in SCHH
SCHH were washed with standard Hank’s Balanced Salt Solution (HBSS; preserving tight junction integrity) or Ca2+-free HBSS (disrupting tight junctions and allowing contents in the bile canaliculi to be washed into the medium), followed by a 10-min preincubation with standard HBSS or Ca2+-free HBSS at 37°C (B-CLEAR technology [U.S. patent 6,780,580], BioIVT) (Liu et al., 1999a,b). Then, SCHH were exposed to 2.5 µM d7-GCDCA or 2.5 µM d5-GCA in standard HBSS for 10 min to assess differences in accumulation of a relatively lipophilic versus a more hydrophilic glycine-conjugated, primary bile acid in DMSO- and CDCA-treated SCHH. Deuterated substrates were used to distinguish administered bile acids from endogenous hepatocellular bile acids. Transport was terminated by washing SCHH with ice cold buffer, after which the plates were stored at −80°C to enhance cell lysis for extraction of the bile acid substrate from cells prior to LC-MS/MS analysis. d5-GCA and d7-GCDCA were extracted from SCHH by incubating the thawed plates for 20 min on a plate shaker with 70% methanol and 30% water (v/v) containing an IS. To also evaluate the impact of CDCA treatment on the biliary excretion of d5-GCA and d7-GCDCA, the biliary excretion index (BEI) was calculated as the difference between the amount of compound accumulated in cells and total accumulation in cells and bile, relative to total accumulation of compound in cells and bile. Bile acid uptake studies in SCHH were performed in triplicate for one hepatocyte donor (MLM) and in duplicate for another donor (HU8246).
LC-MS/MS analysis of bile acids
LC-MS/MS with an API 6500 triple quadrupole instrument mass spectrometer (Sciex, Framingham, Massachusetts) coupled to an Agilent 1260 LC or 1290 Infinity LC system and autosampler (Agilent Technologies, Santa Clara, California) was used to measure cell lysate concentrations of bile acids in transport studies. Concentrations of GCDCA and TCDCA (with d7-GCDCA and d5-TCDCA as the IS, respectively) were determined in cell line transport studies as described previously (Notenboom et al., 2018). Using comparable methods, concentrations of d5-GCA (with d7-GCDCA as IS) and d7-GCDCA (with d5-GCA as IS) were analyzed in SCHH transport studies. The LC-MS/MS apparatus utilized Analyst 1.6.3 software (Sciex) and Analyst device driver software (Agilent Technologies). Compounds were separated using an AQUASIL C18 analytical column (3 μm; 50 × 2.1 mm, Thermo Scientific, Waltham, Massachusetts). The aqueous mobile phase (A) consisted of 0.1% formic acid in water, and the organic mobile phase (B) 0.1% formic acid in acetonitrile. The elution gradient was as follows: 0 min 70% A/30% B, 2–2.5 min 20% A/80% B, and 2.6–4 min 70% A/30% B with a flow rate of 0.6 ml/min and column temperature of 40°C. The injection volume was 10 µl. Negative electrospray ionization was achieved using nitrogen as a desolvation gas with the ionization voltage set at −4.5 kV. The source temperature was set at 500°C. The multiple reaction monitoring (MRM) transitions used in the MS/MS detection are described in Supplementary Table 1. Calibration curves were prepared in extraction buffer (70% methanol and 30% water [v/v]) containing IS for quantification of the bile acid concentrations. MultiQuant 2.1 software (Sciex) was used to perform peak integration and data analysis for LC-MS/MS results.
Quantitative targeted absolute proteomics analysis
SCHH treated for 72 h with CDCA or DMSO were harvested from duplicate or triplicate cell culture wells, after which the ProteoExtract Native Membrane Protein Extraction Kit (Calbiochem, San Diego, California) was used to extract membrane proteins per the manufacturer instructions. Protein samples (20 µg), as previously described (Khatri et al., 2019; Morse et al., 2020), were digested with trypsin. Added stable isotope labeled peptide standards, purchased from JPT Peptide Technologies (Berlin, Germany), were used to calculate drug and bile acid metabolizing enzyme and transporter concentrations. Analysis was by nanoLC-MS/MS (Khatri et al., 2019; Morse et al., 2020). Human liver microsome samples were used as positive controls for the majority of transporters and metabolizing enzymes (data not shown). Tryptic peptides and corresponding MRM transitions that were analyzed are listed in Supplementary Table 2, and were processed as reported previously (Khatri et al., 2019). For the purposes of data analysis, protein concentrations between the lower limit of quantification of 0.1 pmol/mg protein and 0.02 pmol/mg protein were used as calculated, whereas concentrations < 0.02 pmol/mg protein were imputed as 0.02 pmol/mg protein, as previously described (Khatri et al., 2019).
Hepatocellular toxicity assays
The C-DILI assay (BioIVT), a novel, mechanism-based in vitro platform developed to predict cholestatic DILI (Jackson and Brouwer, 2019; Jackson et al., 2018), was used to evaluate cholestatic hepatocellular toxicity associated with test compounds of interest, per the manufacturer instructions. After four days of culturing hepatocytes in a 96-well format, cells were treated for 24 h with compounds of interest in C-DILI Sensitization Medium (creating conditions that sensitize hepatocytes to the cholestatic hepatotoxic potential of a test compound) or C-DILI Standard Medium (control condition), both obtained from BioIVT. Solvent concentrations across conditions were kept constant, and did not exceed 0.25% (v/v) DMSO contributed by test compound stock solutions. TGZ (75 µM), a thiazolidinedione that was withdrawn from the market after reports of severe hepatotoxicity (Isley, 2003), was used as a positive control for cholestatic hepatocellular toxicity per the manufacturer instructions (BioIVT). Although all conditions were tested at least in triplicate for one hepatocyte donor (WWQ), all conditions except TGZ (75 µM) were tested at least in duplicate for a second donor (WID), due to limited availability of hepatocytes. The CytoTox-ONE Homogeneous Membrane Integrity Assay (Promega, Madison, Wisconsin) was used as a fluorescent method for quantifying extracellular lactate dehydrogenase (LDH) released from plasma membrane-damaged hepatocytes. Per the manufacturer instructions, the kit relies on a LDH-mediated enzymatic reaction that results in the formation of resorufin, the fluorescence of which is measurable with an excitation wavelength of 560 nm and an emission wavelength of 590 nm. In addition, the CellTiter-Glo Luminescent Cell Viability Assay (Promega) was used as a luminescent method for quantifying hepatocellular adenosine triphosphate (ATP), indicative of metabolically active cells. Per the manufacturer instructions, a luciferase-catalyzed oxygenation reaction of luciferin in the presence of ATP, oxygen and Mg2+ produced the luminescence that was used to quantify the ATP content.
After 24-h treatment of SCHH with compounds of interest, 100 µl of the cell culture supernatant was transferred to a black opaque 96-well plate (Thermo Fisher Scientific). Subsequently, 100 µl of LDH reagent was mixed with the supernatant for 10 min, followed by the addition of stop solution, after which the fluorescence was recorded using a BioTek Cytation 3 instrument. After discarding the remaining supernatant from the 96-well culture plate, 100 µl of ATP reagent was added per well and mixed for 2 min on an orbital shaker, after which the supernatant was transferred to a black opaque 96-well plate. After a 10-min incubation, luminescence was measured with a BioTek Cytation 3 instrument.
Western blotting
The ProteoExtract Native Membrane Protein Extraction Kit (Calbiochem), was used to extract membrane proteins from SCHH. Total protein from the membrane fraction was determined using the Pierce BCA Protein Assay kit. Protein (12 µg) in extraction buffer containing 10% (v/v) of 0.5 M Pierce dithiothreitol (DTT; Thermo Fisher Scientific) and 25% (v/v) NuPAGE LDS Sample Buffer (Novex, Carlsbad, California) was loaded onto a precasted NuPAGE 4%–12% Bis-Tris gel. Electrophoresis was performed with NuPAGE 2-(N-morpholino) ethanesulfonic acid (MES) sodium dodecyl sulfate running buffer containing 500 μl of NuPAGE antioxidant solution (Thermo Fisher Scientific) in the center chamber. Proteins were transferred using NuPAGE transfer buffer (Novex) onto a polyvinylidene difluoride (PVDF) membrane overnight at 4°C and 15 V. Membranes were washed using Tris-buffered saline, 0.1% (v/v) Tween 20 (TBST) and blocked for 1 h using TBST containing 5% (w/v) nonfat dry milk (Bio-Rad, Hercules, California). Membranes were incubated overnight at 4°C with rabbit-anti-OSTα (ab103442, Abcam, 1:250 dilution) or rabbit-anti-OSTβ (HPA008533, Sigma-Aldrich, 1:150 dilution) primary antibody in TBST containing 5% (w/v) bovine serum albumin (BSA). OSTα and OSTβ were evaluated on separate membranes, while Na+/K+ ATPase (using a rabbit-anti-Na+/K+ ATPase primary antibody, ab185065, 1:5000 in TBST with 5% [w/v] BSA for 1 h at room temperature) was used as the loading control and, thus, probed on the same blot as OSTα or OSTβ. After washing with TBST, the PVDF membrane was incubated with secondary horseradish peroxidase-anti-rabbit antibody for 2 h at room temperature, followed by additional washing. The membrane was subsequently incubated with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) for 5 min, after which the proteins were visualized using a ChemiDoc XRS+ imaging system with Image Lab software (Bio-Rad).
Data analysis
Linear regression was performed on the initial uptake versus time profile to obtain the initial uptake rate (slope) and nonspecific binding (y-intercept) of conjugated bile acids in OSTab cells. In concentration-dependent studies, the Mock cell-mediated uptake data were subtracted from OSTab cell-mediated uptake data, and linear regression was performed on the resulting velocity versus substrate concentration data to derive the slope ± SD approximating the OSTα/β-mediated uptake clearance [CLuptake = Vmax/(Km+C)] of each bile acid, where C represents the concentration of the bile acid, and Vmax and Km represent the Michaelis-Menten parameters for the maximum velocity of transport and the Michaelis constant, respectively, for each bile acid substrate. In inhibition studies, Mock cell-mediated uptake data were subtracted from OSTab cell-mediated uptake data, followed by normalization based on vehicle control (DMSO) data. Nonlinear (least squares) regression, with normalized bile acid uptake relative to control (decreasing from 100% to 0% as inhibitor concentrations increase) and a standard slope (ie, a Hill slope of −1.0) was used to approximate the IC50,app and corresponding SD for each inhibitor-bile acid substrate pair, described by the following equation:
The half-maximal inhibitory concentrations are labeled as IC50,app to emphasize that in some cases, the uptake of the tested bile acid was inhibited < 50% relative to control in the evaluated inhibitor concentration range (which was not increased due to solubility limits). SCHH data were analyzed using 2-way analysis of variance followed by a Bonferroni’s multiple comparisons statistical test. This statistical test was also used for log10-transformed quantitative targeted absolute proteomics (QTAP) data. All statistical tests were performed using GraphPad Prism version 8.2.0 for Windows, GraphPad Software (San Diego, California).
RESULTS
Time-dependent Bile Acid Transport Studies Using OSTab Cells
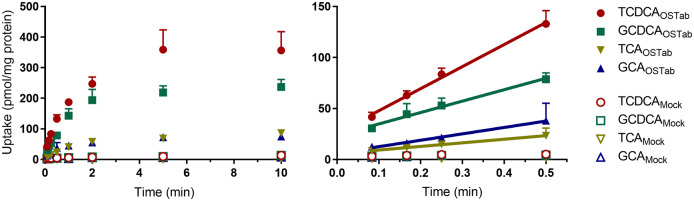
Using OSTab cells and Mock cells, the time-dependence of OSTα/β-mediated transport of four conjugated, primary bile acids (TCDCA, GCDCA, TCA, GCA, Figure 1) was assessed. The relatively lipophilic TCDCA and GCDCA were preferentially transported by OSTα/β compared with the more hydrophilic TCA and GCA. Mock cells showed very little uptake of these bile acids. Bile acid uptake in OSTab cells was within the linear range over the first 30 s of the initial uptake versus time profiles; thus, the 30-s time point was selected for subsequent concentration-dependence and inhibition studies. The initial uptake rate and y-intercept, presumably the nonspecific binding component, of these bile acids in OSTab cells are presented in Table 1.
Figure 1.
Time-dependent uptake of taurochenodeoxycholate (TCDCA; 5 µM), glycochenodeoxycholate (GCDCA; 5 µM), taurocholate (TCA; 5 µM), and glycocholate (GCA; 5 µM). Transport studies were performed using 5.0 × 105 viable cells/well in modified extracellular fluid buffer, as described previously (Malinen et al., 2018, 2019). Solid and open symbols represent OSTab- and Mock-mediated bile acid uptake, respectively. Data are presented as mean ± SD (n = 3, in triplicate), as described in the Materials and Methods section. Only the SD in the upper direction is shown for clarity. Uptake data points from Mock cells that are not visible are hidden behind the TCDCA data corresponding to Mock cells. Abbreviations: Mock cells, mock-transfected cells; OSTab cells, OSTα/β-overexpressing cells.
Table 1.
Initial Uptake Kinetics of Conjugated, Primary Bile Acids in OSTab Cells
| Conjugated Bile Acid | Initial Uptake Rate ± SD (pmol/min/mg Protein) | y-Intercept ± SD (pmol/mg Protein) |
|---|---|---|
| TCDCA | 216.2 ± 10.0 | 26.2 ± 2.9 |
| GCDCA | 111.8 ± 8.2 | 23.7 ± 2.4 |
| TCA | 35.3 ± 0.5 | 5.8 ± 0.2 |
| GCA | 62.7 ± 3.0 | 6.4 ± 0.9 |
The initial uptake rate (slope) and y-intercept of conjugated bile acid species (5 µM) in OSTab cells were determined by performing linear regression on the initial uptake over the first 30 s (Figure 1).
Abbreviations: GCA, glycocholate; GCDCA, glycochenodeoxycholate; OSTab cells, OSTα/β-overexpressing cells; TCA, taurocholate; TCDCA, taurochenodeoxycholate.
Concentration-dependent Bile Acid Transport Studies Using OSTab Cells
Based on the concentration-dependence study, the initial velocity of transport (ie, uptake rate) by OSTα/β for the relatively lipophilic bile acids TCDCA and GCDCA was greater than for the more hydrophilic bile acids TCA and GCA (Figure 2). The uptake rate was linear in the studied concentration range (5–500 µM) for all four conjugated bile acids, precluding the determination of the Michaelis constant (Km) and the maximum velocity of transport (Vmax). However, the slopes of these velocity versus concentration plots were used to approximate OSTα/β-mediated CLuptake of each bile acid, and to rank-order the preferential OSTα/β-mediated CLuptake of bile acid substrates: TCDCA > GCDCA > TCA > GCA.
Figure 2.
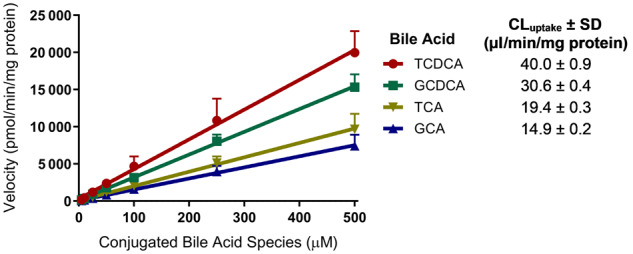
Concentration-dependent uptake rate (at substrate concentrations of 5–500 µM) of taurochenodeoxycholate (TCDCA), glycochenodeoxycholate (GCDCA), taurocholate (TCA), and glycocholate (GCA) by OSTα/β. Solid symbols represent the rate of bile acid uptake (30 s) after subtracting uptake in Mock cells from uptake in OSTab cells. Data are presented as mean ± SD (n = 3, in triplicate), as described in the Materials and Methods section. Only the SD in the upper direction is shown for clarity. Linear regression using GraphPad Prism version 8.2.0 was used to derive the slope ± SD approximating OSTα/β-mediated uptake clearance [CLuptake = Vmax/(Km+C)] of conjugated bile acid species. Abbreviations: Mock cells, mock-transfected cells; OSTab cells, OSTα/β-overexpressing cells.
Bile Acid Transport Inhibition Studies Using OSTab Cells
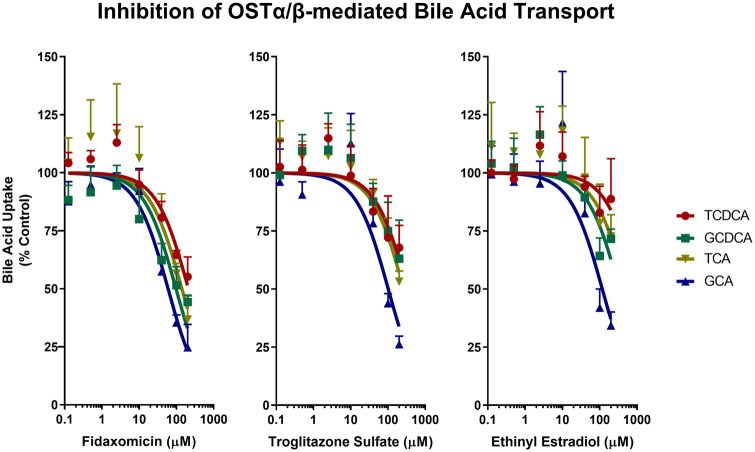
The effect of three OSTα/β inhibitors (FDX, TS, and EE) on OSTα/β-mediated bile acid transport (5 µM) was examined (Figure 3). The IC50,app point estimates for the three inhibitors using any of the four evaluated bile acids as substrate was in the order: FDX < TS < EE (Table 2; individual plots of inhibition of OSTα/β-mediated bile acid uptake are shown in Supplementary Figure 1). Based on nonoverlapping 95% confidence intervals (CIs) across inhibitors, FDX was the most potent inhibitor of OSTα/β-mediated transport of the evaluated bile acids. However, when comparing TS and EE as inhibitors of bile acid transport mediated by OSTα/β, only the 95% CIs for the taurine-conjugated bile acid substrates (ie, TCDCA and TCA) did not overlap.
Figure 3.
Inhibition of OSTα/β-mediated uptake of taurochenodeoxycholate (TCDCA; 5 µM), glycochenodeoxycholate (GCDCA; 5 µM), taurocholate (TCA; 5 µM), and glycocholate (GCA; 5 µM) by fidaxomicin, troglitazone sulfate, and ethinyl estradiol over a concentration range of 0.125–200 µM. Solid symbols represent bile acid uptake after subtracting uptake in Mock cells from uptake in OSTab cells, followed by vehicle control normalization. Data represent mean ± SD (n = 3, in triplicate), as described in the Materials and Methods section. Only the SD in the upper direction is shown. The apparent half-maximal inhibitory concentration and corresponding 95% confidence interval for each bile acid substrate were determined by nonlinear regression using GraphPad Prism version 8.2.0 (Table 2). Abbreviations: Mock cells, mock-transfected cells; OSTab cells, OSTα/β-overexpressing cells.
Table 2.
Inhibition of OSTα/β-mediated Uptake of Conjugated, Primary Bile Acids
| IC50,app [95% CI] (µM) |
|||
|---|---|---|---|
| Conjugated Bile Acid | Fidaxomicin | Troglitazone Sulfate | Ethinyl Estradiol |
| TCDCA | 210 [179, 249] | 334 [254, 455] | 1050 [670, 2050] |
| GCDCA | 97.6 [78.3, 122] | 333 [250, 460] | 337 [260, 451] |
| TCA | 140 [107, 186] | 265 [214, 335] | 527 [357, 866] |
| GCA | 59.8 [51.5, 69.4] | 102 [82.4, 127] | 117 [90.1, 154] |
The apparent half-maximal inhibitory concentration (IC50,app) and corresponding 95% confidence interval (CI) for each conjugated bile acid substrate (5 µM) were determined using an OSTα/β inhibitor concentration range of 0.125–200 µM and nonlinear regression (Figure 3). The half-maximal inhibitory concentrations are labeled as IC50,app to emphasize that several of these values were extrapolated beyond the tested inhibitor concentration range due to solubility limits.
Abbreviations: GCA, glycocholate; GCDCA, glycochenodeoxycholate; TCA, taurocholate; TCDCA, taurochenodeoxycholate.
Bile Acid Uptake and Proteomic Analyses in CDCA-treated SCHH
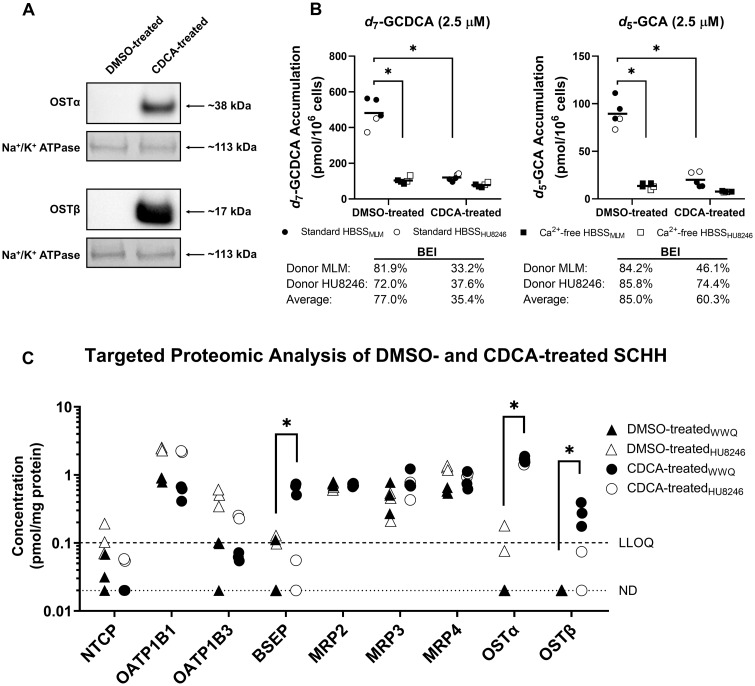
In separate studies with human hepatocytes, Western blot analysis revealed that 72-h treatment of SCHH with CDCA (100 µM) resulted in increased protein expression of both OSTα/β subunits compared with vehicle (DMSO) control (Figure 4A). To provide additional insight into CDCA-mediated alterations at the protein level, QTAP analysis was performed for bile acid transporters and metabolizing enzymes (Figure 4C, Supplementary Figure 2, and Table 3). CDCA treatment of SCHH induced OSTα (p < .0001), OSTβ (p = .0003), and BSEP (p = .0130), with corresponding increases in protein concentrations of ≥51-, 8.2-, and 6.6-fold. In addition, protein concentrations of the efflux transporter multidrug resistance-associated protein (MRP) 3 trended higher in CDCA-treated SCHH, although differences failed to reach statistical significance. Protein concentrations of the uptake transporters sodium taurocholate cotransporting polypeptide (NTCP), and organic anion transporting polypeptides (OATPs) 1B1 and 1B3 trended lower in CDCA-treated SCHH, but differences were not statistically significant. Furthermore, cytochrome P450 (CYP) 3A4 showed a notable decrease in protein concentrations, whereas there was a trend toward increased protein concentrations of sulfotransferase (SULT) 1A1 and most uridine 5′-diphospho-glucuronosyltransferase (UGT) enzymes in these cells.
Figure 4.
Accumulation of two glycine-conjugated bile acid species in sandwich-cultured human hepatocytes (SCHH) treated for 72 h with 100 µM CDCA to induce OSTα/β, or with DMSO (control). A, Protein expression of the OSTα/β subunits in DMSO- and CDCA-treated SCHH (donor WWQ) was assessed by Western blot. Both proteins were analyzed separately on different membranes, while Na+/K+ ATPase was evaluated as the loading control on the same membrane. B, Using SCHH from two donors (MLM, closed symbols, in triplicate wells; HU8246, open symbols, in duplicate wells), uptake studies (10 min) using B-CLEAR methodology as described in the Materials and Methods section were performed in standard HBSS (circles) or Ca2+-free HBSS (squares) with d7-glycochenodeoxycholate (d7-GCDCA, 2.5 µM) or d5-glycocholate (d5-GCA, 2.5 µM) as probe substrates. Horizontal bars denote mean values. C, Quantitative targeted absolute proteomics analysis was performed on membrane protein (from duplicate or triplicate cell culture wells) extracted from two SCHH donors (WWQ, closed symbols; HU8246, open symbols) treated with DMSO (triangles) or CDCA (circles). In this panel, data for bile acid transporters are presented. See Supplementary Figure 2 for metabolizing enzyme data. For the purpose of analysis, protein concentrations between the LLOQ of 0.1 pmol/mg protein and 0.02 pmol/mg protein were used as calculated, whereas concentrations < 0.02 pmol/mg protein were imputed as 0.02 pmol/mg protein, as described previously (Khatri et al., 2019). In B and C, technical replicates for each donor are presented, and 2-way analysis of variance followed by a Bonferroni’s multiple comparisons statistical test was performed. *p < .05. Abbreviations: BEI, biliary excretion index; BSEP, bile salt export pump; CDCA, chenodeoxycholate; DMSO, dimethyl sulfoxide; GCA, glycocholate; GCDCA, glycochenodeoxycholate; HBSS, Hank’s Balanced Salt Solution; LLOQ, lower limit of quantitation; MRP, multidrug resistance-associated protein; Na+/K+ ATPase, sodium-potassium adenosine triphosphatase; ND, not detected; NTCP, sodium taurocholate cotransporting polypeptide; OATP, organic anion transporting polypeptide; OST, organic solute transporter.
Table 3.
Effect of CDCA Treatment on Protein Levels of Bile Acid Transporters and Metabolizing Enzymes in Sandwich-cultured Human Hepatocytes (SCHH)
| Transporter | Log10 Ratio | Fold Change | Metabolizing Enzyme | Log10 Ratio | Fold Change |
|---|---|---|---|---|---|
| Upregulated proteins | |||||
| OSTα | 1.7 | 51 | SULT1A1 | 0.29 | 1.9 |
| OSTβ | 0.91 | 8.2 | UGT1A1 | 0.19 | 1.5 |
| BSEP | 0.82 | 6.6 | UGT2A3 | 0.13 | 1.3 |
| MRP3 | 0.20 | 1.6 | UGT2B4 | 0.13 | 1.3 |
| MRP4 | 0.040 | 1.1 | UGT2B15 | 0.11 | 1.3 |
| MRP2 | 0.0037 | 1.0 | UGT1A3 | 0.064 | 1.2 |
| UGT1A4 | 0.038 | 1.1 | |||
| SULT2A1 | 0.026 | 1.1 | |||
| UGT2B7 | 0.023 | 1.1 | |||
| UGT1A6 | 0.021 | 1.0 | |||
| Downregulated proteins | |||||
| NTCP | −0.32 | 0.48 | CYP3A4 | −0.30 | 0.51 |
| OATP1B3 | −0.17 | 0.68 | UGT1A9 | −0.16 | 0.70 |
| OATP1B1 | −0.10 | 0.79 | SULT1A3 | −0.065 | 0.86 |
| UGT2B17 | −0.039 | 0.91 | |||
Quantitative targeted absolute proteomics analysis was performed on membrane protein (from duplicate or triplicate cell culture wells) extracted from two SCHH donors (WWQ and HU8246). The average fold change (relative to DMSO treatment) in protein levels across both donors was calculated using the protein concentrations shown in Figure 4C and Supplementary Figure 2.
Abbreviations: BSEP, bile salt export pump; CDCA, chenodeoxycholate; CYP, cytochrome P450; DMSO, dimethyl sulfoxide; MRP, multidrug resistance-associated protein; NTCP, sodium taurocholate cotransporting polypeptide; OATP, organic anion transporting polypeptide; OST, organic solute transporter; SULT, sulfotransferase; UGT, uridine 5′-diphospho-glucuronosyltransferase.
Accumulation of d5-GCA and d7-GCDCA in SCHH treated with CDCA (100 µM) was performed using B-CLEAR methodology to evaluate the potential impact of OSTα/β and other proteins on the hepatobiliary disposition of two prevalent primary glycine-conjugated bile acids. CDCA treatment of SCHH reduced the accumulation of d7-GCDCA and d5-GCA in cells+bile (standard HBSS buffer) 4.0- and 4.5-fold, respectively, compared with DMSO-treated SCHH (Figure 4B). On average, the BEI decreased from 77.0% to 35.4% for d7-GCDCA and from 85.0% to 60.3% for d5-GCA when SCHH were treated with CDCA.
Evaluating OSTα/β Inhibitor-mediated Hepatocellular Toxicity in SCHH
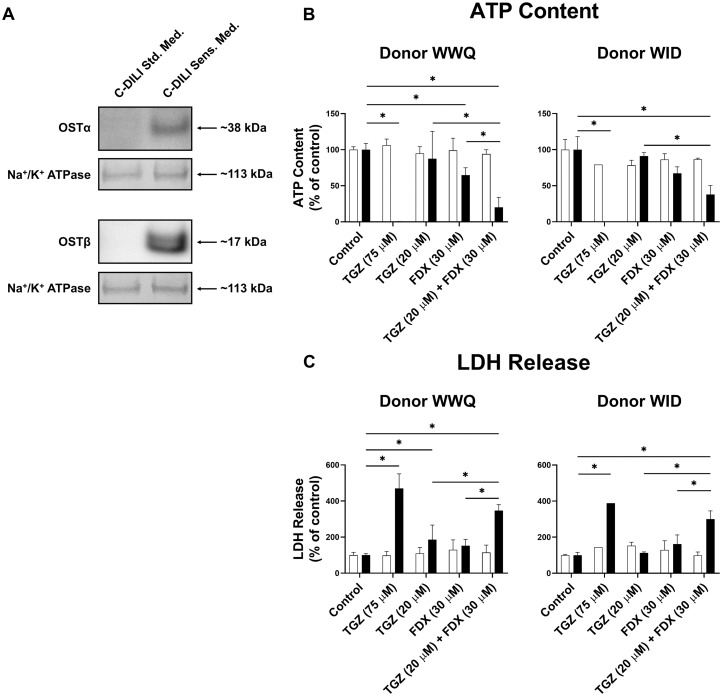
The cholestatic hepatotoxic potential of the most potent inhibitor of OSTα/β-mediated bile acid transport in this study (ie, FDX) was assessed in SCHH using the C-DILI assay. Western blot analysis showed that 24-h treatment of SCHH with C-DILI Sensitization Medium, which sensitizes hepatocytes to the cholestatic hepatotoxic potential of a test compound, resulted in induction of both OSTα/β protein subunits compared to treatment with C-DILI Standard Medium (Figure 5A). Toxicity was observed as a decrease in ATP content (Figure 5B) and increased LDH release into the medium (Figure 5C) in SCHH treated for 24 h with C-DILI Sensitization Medium using TGZ (75 µM) as the positive control. In contrast, 20 µM TGZ exhibited hardly any hepatotoxic effect, as expected, because of the lower concentration relative to the positive control. The OSTα/β inhibitor FDX (30 µM) caused some toxicity (decrease in ATP) in the C-DILI Sensitization Medium when administered alone. The combination of TGZ (20 µM) and FDX (30 µM) resulted in a statistically significant increase in hepatocellular toxicity compared with administration of either of these drugs alone.
Figure 5.
Cholestatic hepatocellular toxicity in sandwich-cultured human hepatocytes (SCHH) using OSTα/β inhibitors. A, Protein expression of the OSTα/β subunits in C-DILI Standard Medium (Std. Med.)-treated and C-DILI Sensitization Medium (Sens. Med.)-treated SCHH (donor WWQ) was assessed by Western blot. Both proteins were analyzed separately on different membranes, while Na+/K+ ATPase was evaluated as the loading control on the same membrane. B and C, SCHH from two donors (WWQ and WID) were treated for 24 h with DMSO (control) or compound(s) dissolved in C-DILI Std. Med. (white bars) or C-DILI Sens. Med. (black bars), the latter of which creates conditions that sensitize hepatocytes to the cholestatic hepatotoxic potential of a test compound. Toxicity was assessed by measuring ATP content (B) and LDH release (C). Technical replicates for each donor are presented. Using SCHH from donor WWQ, all conditions were tested at least in triplicate wells. Using SCHH from donor WID, all conditions except TGZ (75 µM) were tested at least in duplicate wells. In B and C, 2-way analysis of variance followed by a Bonferroni’s multiple comparisons statistical test was performed. *p < .05. Abbreviations: ATP, adenosine triphosphate; FDX, fidaxomicin; LDH, lactate dehydrogenase; Na+/K+ ATPase, sodium-potassium adenosine triphosphatase; OST, organic solute transporter; TGZ, troglitazone.
DISCUSSION
Although a key role for OSTα/β is implicated in health and a variety of bile acid-related diseases, little is known about how this transporter may be involved in DILI. OSTα/β is a bidirectional transporter and primarily functions as an efflux transporter in vivo (Dawson et al., 2010; Malinen et al., 2018). Based on CLuptake values from this study, OSTα/β preferentially transports relatively lipophilic GCDCA and TCDCA compared with more hydrophilic GCA and TCA (Figure 2). This suggests that OSTα/β serves to protect hepatocytes from toxic bile acids. Furthermore, the taurine conjugates (TCDCA and TCA) showed preferential OSTα/β-mediated CLuptake compared with their glycine-conjugated counterparts (GCDCA and GCA), indicating that OSTα/β preferentially transports taurine-conjugated species. These data suggest that the substrate binding site of OSTα/β may have differential affinity for more lipophilic compared with relatively hydrophilic bile acids, and for taurine compared with glycine functional groups. The TCDCA > GCDCA > TCA > GCA rank order of transport is consistent with a recently published analysis of bile acid transport using OSTα/β-overexpressing Madin-Darby canine kidney (MDCK)-II cells (Suga et al., 2019). Using initial velocity versus substrate concentration data from Suga et al. (2019), OSTα/β-mediated CLuptake of the even more lipophilic glycodeoxycholate (GDCA), taurodeoxycholate (TDCA), glycolithocholate (GLCA), and taurolithocholate (TLCA) can be approximated. These CLuptake estimations (not shown) further support preferential OSTα/β-mediated bile acid transport of more lipophilic compared with more hydrophilic bile acids, and taurine- compared with glycine-conjugated OSTα/β-mediated bile acid transport: TLCA > GLCA > TDCA(∼TCDCA) > GDCA(∼GCDCA). In addition to these findings for human OSTα/β, Ballatori et al. (2005) studied murine OSTα/β using MDCK cells expressing both OSTα/β and the apical sodium-dependent bile acid transporter. These data suggested that murine OSTα/β also transports taurine-conjugated bile acids more efficiently compared with glycine-conjugated bile acids. Protein structural data explaining the preference of OSTα/β for taurine- over glycine-conjugated bile acids, and more lipophilic over more hydrophilic bile acids is lacking, and warrants further investigation. Additional insights into the substrate binding site of OSTα/β may be gathered from previous reports demonstrating that the sulfated but not the unconjugated forms of dehydroepiandrosterone and pregnenolone were OSTα/β substrates (Ballatori et al., 2008; Fang et al., 2010), and that docetaxel, but not paclitaxel, was transported by OSTα/β (Schwarz, 2012).
In the inhibition studies using a fixed substrate concentration of 5 µM, it was not possible to calculate IC50,app values with high confidence, as shown by the relatively wide 95% CIs, due to low inhibition even at 200 µM. Despite the low confidence in IC50,app values, these data show that the selection of the probe substrate in OSTα/β-based inhibition studies has a substantial impact on IC50,app values corresponding to a particular xenobiotic, which has not been shown previously for this transporter. TCA is used often as a prototypical bile acid substrate in transport inhibition studies, and utilized to predict the effect of a xenobiotic on the transport of bile acids in general. Compared with the IC50,app point estimate derived using TCA as the probe substrate in OSTα/β inhibition studies (Table 2), the values obtained with EE as the inhibitor using GCA or TCDCA as the probe substrate could be up to 5-fold lower or 2-fold higher, respectively (an order of magnitude difference overall). Interestingly, EE was the weakest of the three studied inhibitors for each of the evaluated bile acids based on IC50,app point estimates, although previously EE was shown to be the strongest individual inhibitor of OSTα/β-mediated DHEAS uptake (Malinen et al., 2019), which further emphasizes the importance of substrate-dependence in inhibition studies.
While OSTα/β-overexpressing cells are ideally suited to study OSTα/β-mediated bile acid transport in isolation, SCHH with canalicular network formation represent a physiologically relevant model to study the hepatobiliary disposition of bile acids. This study quantified the accumulation of two glycine-conjugated, primary bile acids in SCHH under basal conditions (ie, minor OSTα/β expression) and when OSTα/β was induced after 72-h CDCA treatment. In CDCA-treated SCHH, d7-GCDCA and d5-GCA accumulation in cells+bile and the BEI values were reduced (Figure 4B). Transcriptomic and Western blot analyses were performed previously to analyze the effects of CDCA treatment in SCHH (Guo et al., 2018; Jackson et al., 2016; Krattinger et al., 2016). Results from the larger-scale quantitative proteomic analysis conducted in this study (Table 3) support previous findings that OSTα/β is the most highly induced bile acid transporter measured in SCHH following 100 µM CDCA treatment. Interestingly, although CDCA treatment of SCHH increased BSEP protein levels on average, the BEI values of both d7-GCDCA and d5-GCA were decreased. The observed reduction in BEI is likely due to the markedly decreased accumulation of d7-GCDCA and d5-GCA in SCHH, which can be explained by increased (OSTα/β-mediated) basolateral efflux of bile acids, and possibly by decreased uptake and/or increased metabolism. Decreased protein levels of the uptake transporters NTCP, OATP1B1, and OATP1B3, which preferentially transport GCDCA over GCA (Suga et al., 2017), could reduce bile acid accumulation and alter hepatobiliary disposition of bile acids. Aside from transporters, CYP3A4, SULTs (eg, SULT2A1) and UGTs (eg, UGT1A1, -1A3, -2B4, -2B7) are involved in bile acid disposition (Alnouti, 2009; Barnabas and Chapman, 2012; Beilke et al., 2009; Trottier et al., 2006). CDCA-induced alterations in these metabolizing enzymes could lead to functional changes in bile acid oxidation, sulfation, and/or glucuronidation, and the resulting metabolites may undergo preferential basolateral or biliary transport due to differential transporter affinity.
The observed trend in the hepatic disposition of d7-GCDCA and d5-GCA following CDCA treatment of SCHH (Figure 4B) is similar to recently published data on d8-TCA (Guo et al., 2018) in which an important role of OSTα/β-mediated basolateral efflux of d8-TCA was demonstrated. The increased intrinsic basolateral efflux clearance of d8-TCA was attributed, at least partially, to higher levels of OSTα/β because CDCA treatment significantly upregulated OSTα/β, but not the basolateral efflux transporters MRP3 and MRP4 that can also efflux TCA (Guo et al., 2018). Considering the induction of OSTα/β and that GCDCA and GCA are substrates of OSTα/β, it is highly plausible that CDCA treatment increased the intrinsic basolateral efflux clearance of d7-GCDCA and d5-GCA. However, a more extensive investigation characterizing uptake and efflux over time combined with mechanistic modeling to assess biliary and basolateral efflux clearance values would be necessary to draw further conclusions.
Additional studies were conducted to demonstrate the importance of OSTα/β in protecting human hepatocytes from bile acid-mediated DILI. For these studies, FDX served as a useful inhibitor of OSTα/β-mediated bile acid transport in vitro even though plasma concentrations of this orally administered, large (1058 g/mol) macrocyclic antibiotic in humans do not reach IC50,app values in the range evaluated in this study (Sears et al., 2012). Previously it was shown that in TGZ (10 µM)-treated SCHH (30–120 min), accumulation of the metabolite TS was extensive, with intracellular concentrations ranging from 136 to 160 µM (Lee et al., 2010). This is within the range of IC50,app values (102–334 µM) of TS for OSTα/β-mediated bile acid transport estimated in this study. Using the C-DILI assay, the combination of FDX and TGZ (at a concentration lower than when serving as the positive control for cholestatic hepatotoxicity [20 vs 75 µM, respectively]) in sensitization conditions increased cholestatic hepatocellular toxicity compared with administration of either drug alone (Figs. 5B and 5C). Sensitization conditions lead to FXR activation (eg, FGF19 and OSTβ mRNA elevations) (Jackson and Brouwer, 2019; Jackson et al., 2018), and OSTα/β protein levels were induced by the Sensitization Medium used in the C-DILI assay (Figure 5A). Because FDX, TGZ, and the generated TS metabolite can all inhibit OSTα/β (Malinen et al., 2019), these results suggest that OSTα/β inhibition may accentuate DILI. It is important to note that sensitization conditions may impact alternative hepatotoxicity-promoting pathways. Although FDX inhibits MRP3 in inside-out membrane vesicles (Ali et al., 2017), this inhibitory effect may be negligible in whole-cell systems, such as SCHH, where poorly permeable compounds cannot access intracellular binding sites. Interactions of FDX with hepatic bile acid uptake transporters (eg, NTCP, OATPs) on the extracellular side of the plasma membrane have not been studied, but it is unlikely that inhibition of bile acid uptake would exacerbate bile acid-mediated hepatotoxicity.
In conclusion, the results of the present studies suggest that OSTα/β has a preference to transport relatively lipophilic over more hydrophilic bile acids, and that xenobiotics have different effects on this transport. The results also support a protective role for OSTα/β in the hepatocyte in cholestatic conditions. Furthermore, the model systems used in this study provide insights into the potential role of OSTα/β in cholestatic DILI, and lend support for evaluating OSTα/β interactions during drug development, especially when there is a concern regarding cholestatic DILI liability.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the support provided by the Biomarkers Mass Spectrometry sub-core at UNC funded in part by NIEHS grant P30ES010126. In addition, we thank Dr Melina M. Malinen for generously sharing the OSTab and Mock cells used in this study, and Dr Philip C. Smith for his expert advice regarding the QTAP analysis.
FUNDING
National Institutes of Health under award numbers F31DK120196 from the National Institute of Diabetes and Digestive and Kidney Diseases (J.J.B.) and R35GM122576 from the National Institute of General Medical Sciences (K.L.R.B.); Sigrid Jusélius Foundation (N.S.). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the National Institutes of Health.
DECLARATION OF CONFLICTING INTERESTS
Dr Kim L.R. Brouwer is a coinventor of the sandwich-cultured hepatocyte technology for quantification of biliary excretion (B-CLEAR®) and related technologies, which have been licensed exclusively to Qualyst Transporter Solutions, acquired by BioIVT. B-CLEAR® is covered by U.S. patent 6,780,580 and other U.S. and international patents both issued and pending. All other coauthors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
J.J.B. and K.L.R.B. participated in research design; J.J.B., J.B., N.S., and J.K.F. conducted experiments; J.J.B., J.B., N.S., J.K.F., and K.L.R.B. performed data analysis; J.J.B., J.B., N.S., J.K.F., and K.L.R.B. wrote or contributed to the writing of the manuscript.
REFERENCES
- Ali I., Welch M. A., Lu Y., Swaan P. W., Brouwer K. L. R. (2017). Identification of novel MRP3 inhibitors based on computational models and validation using an in vitro membrane vesicle assay. Eur. J. Pharm. Sci. 103, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouti Y. (2009). Bile acid sulfation: A pathway of bile acid elimination and detoxification. Toxicol. Sci. 108, 225–246. [DOI] [PubMed] [Google Scholar]
- Alvaro D., Angelico M., Cantafora A., Di Biase A., De Santis A., Bracci F., Minervini G., Ginanni Corradini S., Attili A. F., Capocaccia L. (1986. a). Biliary secretion of phosphatidylcholine and its molecular species in cholecystectomized T-tube patients: Effects of bile acid hydrophilicity. Biochem. Med. Metab. Biol. 36, 125–135. [DOI] [PubMed] [Google Scholar]
- Alvaro D., Angelico M., Cantafora A., Di Biase A., Gaeta G. B., Ginanni Corradini S., Tripodi M. F., Attili A. F., Utili R. (1986. b). Influence of tauroursodeoxycholic and taurodeoxycholic acids on hepatic metabolism and biliary secretion of phosphatidylcholine in the isolated rat liver. Biochim. Biophys. Acta 878, 216–224. [DOI] [PubMed] [Google Scholar]
- Arab J. P., Karpen S. J., Dawson P. A., Arrese M., Trauner M. (2017). Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 65, 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attili A. F., Angelico M., Cantafora A., Alvaro D., Capocaccia L. (1986). Bile acid-induced liver toxicity: Relation to the hydrophobic-hydrophilic balance of bile acids. Med. Hypotheses 19, 57–69. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Christian W. V., Lee J. Y., Dawson P. A., Soroka C. J., Boyer J. L., Madejczyk M. S., Li N. (2005). OSTalpha-OSTbeta: A major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42, 1270–1279. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Fang F., Christian W. V., Li N., Hammond C. L. (2008). OSTalpha-OSTbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabas A., Chapman R. W. (2012). Primary sclerosing cholangitis: Is any treatment worthwhile? Curr. Gastroenterol. Rep. 14, 17–24. [DOI] [PubMed] [Google Scholar]
- Barrasa J. I., Olmo N., Lizarbe M. A., Turnay J. (2013). Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol. In Vitro 27, 964–977. [DOI] [PubMed] [Google Scholar]
- Beilke L. D., Aleksunes L. M., Holland R. D., Besselsen D. G., Beger R. D., Klaassen C. D., Cherrington N. J. (2009). Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice. Drug Metab. Dispos. 37, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. L., Trauner M., Mennone A., Soroka C. J., Cai S. Y., Moustafa T., Zollner G., Lee J. Y., Ballatori N. (2006). Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1124–1130. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Strautnieks S. S., Mieli-Vergani G., Higgins C. F., Linton K. J., Thompson R. J. (2002). The human bile salt export pump: Characterization of substrate specificity and identification of inhibitors. Gastroenterology 123, 1649–1658. [DOI] [PubMed] [Google Scholar]
- Chai J., Feng X., Zhang L., Chen S., Cheng Y., He X., Yang Y., He Y., Wang H., Wang R., et al. (2015). Hepatic expression of detoxification enzymes is decreased in human obstructive cholestasis due to gallstone biliary obstruction. PLoS One 10, e0120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Bijsmans I. T., van Mil S. W., Augustijns P., Annaert P. (2014). Toxicity and intracellular accumulation of bile acids in sandwich-cultured rat hepatocytes: Role of glycine conjugates. Toxicol. In Vitro 28, 218–230. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Hubbert M. L., Rao A. (2010). Getting the most from OST: Role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism. Biochim. Biophys. Acta 1801, 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S., Stahl S., Paul N., Barber J., Kenna J. G. (2012). In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab. Dispos. 40, 130–138. [DOI] [PubMed] [Google Scholar]
- Fang F., Christian W. V., Gorman S. G., Cui M., Huang J., Tieu K., Ballatori N. (2010). Neurosteroid transport by the organic solute transporter OSTalpha-OSTbeta. J. Neurochem. 115, 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferslew B. C., Xie G., Johnston C. K., Su M., Stewart P. W., Jia W., Brouwer K. L. R., Barritt A. S., 4th. (2015). Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig. Dis. Sci. 60, 3318–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg T., Rao A., Chen F., Haywood J., Shneider B. L., Dawson P. A. (2006). Regulation of the mouse organic solute transporter alpha-beta, OSTalpha-OSTbeta, by bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G912–922. [DOI] [PubMed] [Google Scholar]
- Galle P. R., Theilmann L., Raedsch R., Otto G., Stiehl A. (1990). Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology 12, 486–491. [DOI] [PubMed] [Google Scholar]
- Guo C., LaCerte C., Edwards J. E., Brouwer K. R., Brouwer K. L. R. (2018). Farnesoid X receptor agonists obeticholic acid and chenodeoxycholic acid increase bile acid efflux in sandwich-cultured human hepatocytes: Functional evidence and mechanisms. J. Pharmacol. Exp. Ther. 365, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F. (1977). The enterohepatic circulation of bile acids in man. Clin. Gastroenterol. 6, 3–24. [PubMed] [Google Scholar]
- Hofmann A. F. (1984). Chemistry and enterohepatic circulation of bile acids. Hepatology 4, 4S–14. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F. (1999). The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 159, 2647–2658. [DOI] [PubMed] [Google Scholar]
- Isley W. L. (2003). Hepatotoxicity of thiazolidinediones. Expert Opin. Drug Saf. 2, 581–586. [DOI] [PubMed] [Google Scholar]
- Jackson J. P., Brouwer K. R. (2019). The C-DILI assay: An integrated in vitro approach to predict cholestatic hepatotoxicity. Methods Mol. Biol. 1981, 75–85. [DOI] [PubMed] [Google Scholar]
- Jackson J. P., Freeman K. M., Friley W. W., St. Claire R. L., Black C., Brouwer K. R. (2016). Basolateral efflux transporters: A potentially important pathway for the prevention of cholestatic hepatotoxicity. Appl. In Vitro Toxicol. 2, 207–216. [Google Scholar]
- Jackson J. P., Freeman K. M., St. Claire R. L., Black C. B., Brouwer K. R. (2018). Cholestatic drug induced liver injury: A function of bile salt export pump inhibition and farnesoid X receptor antagonism. Appl. In Vitro Toxicol. 4, 265–279. [Google Scholar]
- Kenna J. G., Taskar K. S., Battista C., Bourdet D. L., Brouwer K. L. R., Brouwer K. R., Dai D., Funk C., Hafey M. J., Lai Y., et al. (2018). Can bile salt export pump inhibition testing in drug discovery and development reduce liver injury risk? An international transporter consortium perspective. Clin. Pharmacol. Ther. 104, 916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri R., Fallon J. K., Rementer R. J. B., Kulick N. T., Lee C. R., Smith P. C. (2019). Targeted quantitative proteomic analysis of drug metabolizing enzymes and transporters by nano LC-MS/MS in the sandwich cultured human hepatocyte model. J. Pharmacol. Toxicol. Methods 98, 106590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck K., Ferslew B. C., Netterberg I., Yang K., Urban T. J., Swaan P. W., Stewart P. W., Brouwer K. L. R. (2014). Risk factors for development of cholestatic drug-induced liver injury: Inhibition of hepatic basolateral bile acid transporters multidrug resistance-associated proteins 3 and 4. Drug Metab. Dispos. 42, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger R., Bostrom A., Lee S. M. L., Thasler W. E., Schioth H. B., Kullak-Ublick G. A., Mwinyi J. (2016). Chenodeoxycholic acid significantly impacts the expression of miRNAs and genes involved in lipid, bile acid and drug metabolism in human hepatocytes. Life Sci. 156, 47–56. [DOI] [PubMed] [Google Scholar]
- Lee J. K., Marion T. L., Abe K., Lim C., Pollock G. M., Brouwer K. L. R. (2010). Hepatobiliary disposition of troglitazone and metabolites in rat and human sandwich-cultured hepatocytes: Use of Monte Carlo simulations to assess the impact of changes in biliary excretion on troglitazone sulfate accumulation. J. Pharmacol. Exp. Ther. 332, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., LeCluyse E. L., Brouwer K. R., Gan L. S., Lemasters J. J., Stieger B., Meier P. J., Brouwer K. L. R. (1999. a). Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am. J. Physiol. 277, G12–21. [DOI] [PubMed] [Google Scholar]
- Liu X., LeCluyse E. L., Brouwer K. R., Lightfoot R. M., Lee J. I., Brouwer K. L. R. (1999. b). Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 289, 1592–1599. [PubMed] [Google Scholar]
- Malinen M. M., Ali I., Bezençon J., Beaudoin J. J., Brouwer K. L. R. (2018). Organic solute transporter OSTalpha/beta is overexpressed in nonalcoholic steatohepatitis and modulated by drugs associated with liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 314, G597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen M. M., Kauttonen A., Beaudoin J. J., Sjöstedt N., Honkakoski P., Brouwer K. L. R. (2019). Novel in vitro method reveals drugs that inhibit organic solute transporter alpha/beta (OSTalpha/beta). Mol. Pharm. 16, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta K., Kobayashi E., Uchida H., Ogino Y., Fujimura A., Kawarasaki H., Hashizume K. (1999). Cyclosporine inhibits transport of bile acid in rats: Comparison of bile acid composition between liver and bile. Transplant. Proc. 31, 2755–2756. [DOI] [PubMed] [Google Scholar]
- Morgan R. E., van Staden C. J., Chen Y., Kalyanaraman N., Kalanzi J., Dunn R. T. 2nd, Afshari C. A., Hamadeh H. K. (2013). A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol. Sci. 136, 216–241. [DOI] [PubMed] [Google Scholar]
- Morse B. L., Kolur A., Hudson L. R., Hogan A. T., Chen L. H., Brackman R. M., Sawada G. A., Fallon J. K., Smith P. C., Hillgren K. M. (2020). Pharmacokinetics of organic cation transporter 1 (OCT1) substrates in OCT1/2 knockout mice and species difference in hepatic OCT1-mediated uptake. Drug Metab. Dispos. 48, 93–105. [DOI] [PubMed] [Google Scholar]
- Murakami K., Tenge V. R., Karandikar U. C., Lin S. C., Ramani S., Ettayebi K., Crawford S. E., Zeng X. L., Neill F. H., Ayyar B. V., et al. (2020). Bile acids and ceramide overcome the entry restriction for GII.3 human norovirus replication in human intestinal enteroids. Proc. Natl. Acad. Sci. U.S.A. 117, 1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notenboom S., Weigand K. M., Proost J. H., van Lipzig M. M. H., van de Steeg E., van den Broek P. H. H., Greupink R., Russel F. G. M., Groothuis G. (2018). Development of a mechanistic biokinetic model for hepatic bile acid handling to predict possible cholestatic effects of drugs. Eur. J. Pharm. Sci. 115, 175–184. [DOI] [PubMed] [Google Scholar]
- Onakpoya I. J., Heneghan C. J., Aronson J. K. (2016). Worldwide withdrawal of medicinal products because of adverse drug reactions: A systematic review and analysis. Crit. Rev. Toxicol. 46, 477–489. [DOI] [PubMed] [Google Scholar]
- Ørntoft N. W., Munk O. L., Frisch K., Ott P., Keiding S., Sørensen M. (2017). Hepatobiliary transport kinetics of the conjugated bile acid tracer 11C-CSar quantified in healthy humans and patients by positron emission tomography. J. Hepatol. 67, 321–327. [DOI] [PubMed] [Google Scholar]
- Pellicoro A., Faber K. N. (2007). Review article: The function and regulation of proteins involved in bile salt biosynthesis and transport. Aliment. Pharmacol. Ther. 26, 149–160. [DOI] [PubMed] [Google Scholar]
- Perez M. J., Briz O. (2009). Bile-acid-induced cell injury and protection. World J. Gastroenterol. 15, 1677–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Haywood J., Craddock A. L., Belinsky M. G., Kruh G. D., Dawson P. A. (2008). The organic solute transporter alpha-beta, OSTalpha-OSTbeta, is essential for intestinal bile acid transport and homeostasis. Proc. Natl. Acad. Sci. U.S.A. 105, 3891–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt H. S., Wolf A., Pognan F., Chibout S. D., Merz M., Kullak-Ublick G. A. (2016). Bile acids in drug induced liver injury: Key players and surrogate markers. Clin. Res. Hepatol. Gastroenterol. 40, 257–266. [DOI] [PubMed] [Google Scholar]
- Schwarz U. I. (2012). The bile acid transporter organic solute transporter (OST) alpha-beta is also an intestinal drug transporter. In Intestinal and Hepatic Drug Transporters and Their Role in the Disposition of Lipid-lowering Drugs, pp. 81–112. Electronic Thesis and Dissertation Repository: The University of Western Ontario, London, Ontario, Canada.
- Sears P., Crook D. W., Louie T. J., Miller M. A., Weiss K. (2012). Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with clostridium difficile infection. Clin. Infect. Dis. 55(Suppl. 2), S116–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seward D. J., Koh A. S., Boyer J. L., Ballatori N. (2003). Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J. Biol. Chem. 278, 27473–27482. [DOI] [PubMed] [Google Scholar]
- Suga T., Yamaguchi H., Ogura J., Mano N. (2019). Characterization of conjugated and unconjugated bile acid transport via human organic solute transporter alpha/beta. Biochim. Biophys. Acta Biomembr. 1861, 1023–1029. [DOI] [PubMed] [Google Scholar]
- Suga T., Yamaguchi H., Sato T., Maekawa M., Goto J., Mano N. (2017). Preference of conjugated bile acids over unconjugated bile acids as substrates for OATP1B1 and OATP1B3. PLoS One 12, e0169719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan M., Rao A., Elpeleg O., Vaz F. M., Abu-Libdeh B., Karpen S. J., Dawson P. A. (2018). Organic solute transporter-beta (SLC51B) deficiency in two brothers with congenital diarrhea and features of cholestasis. Hepatology 68, 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliacozzi D., Mozzi A. F., Casetta B., Bertucci P., Bernardini S., Di Ilio C., Urbani A., Federici G. (2003). Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: A simple and rapid one-step method. Clin. Chem. Lab. Med. 41, 1633–1641. [DOI] [PubMed] [Google Scholar]
- Temple R. J., Himmel M. H. (2002). Safety of newly approved drugs: Implications for prescribing. JAMA 287, 2273–2275. [DOI] [PubMed] [Google Scholar]
- Trottier J., Milkiewicz P., Kaeding J., Verreault M., Barbier O. (2006). Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol. Pharm. 3, 212–222. [DOI] [PubMed] [Google Scholar]
- Wahlstrom A., Sayin S. I., Marschall H. U., Backhed F. (2016). Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50. [DOI] [PubMed] [Google Scholar]
- Wang W., Seward D. J., Li L., Boyer J. L., Ballatori N. (2001). Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc. Natl. Acad. Sci. U.S.A. 98, 9431–9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright B. L., Li F., Xie Y., Farhood A., Fickert P., Trauner M., Jaeschke H. (2014). Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol. Lett. 228, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski M. J., Taub M. E., Chothe P. P., Chu X., Giacomini K. M., Kim R. B., Ray A. S., Stocker S. L., Unadkat J. D., Wittwer M. B., et al. (2018). Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin. Pharmacol. Ther. 104, 890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.