Abstract
Objectives:
Tests for gustatory function have become increasingly important in diagnosis and treatment of patients with taste disorders. While caffeine and quinine hydrochloride solutions have been used for global testing of bitter perception, only quinine has been used to test regional bitter perception by means of taste strips. The aim of the present study was to validate caffeine impregnated taste strips as an alternative to quinine taste strips for assessment of regional bitter perception.
Methods:
A total of 46 healthy volunteers (mean age/range, 23/19-27 years) were included in this study. Quinine and caffeine impregnated taste strips were pairwise presented at different parts of the tongue. Perceived intensity and hedonic dislike were evaluated using labeled magnitude scales. Additionally, gustatory function was assessed using the taste strips test and overall sense of taste was rated using visual analog scales.
Results:
Assessment of gustatory function demonstrated scores within the normogeusic range in most included subjects (mean/SD, 13.1/2.5). Notably, equally concentrated quinine and caffeine impregnated taste strips placed on different regions of the tongue did not lead to significant differences in perceived intensity or hedonic dislike, whereas quinine and caffeine impregnated taste strips of different concentrations placed on the same region on the tongue led to significant differences of perceived intensity and hedonic dislike. Furthermore, no correlation was found between self-assessment of gustatory function and taste strips scores.
Conclusion:
Caffeine seems to be a valid bitter compound for regional testing using taste strips and may be used alternatively to quinine.
Keywords: taste strips, bitter, caffeine, quinine, tongue, intensity
Introduction
Disorders of the chemical senses are frequently encountered by otolaryngologists. While olfactory disorders are relatively common and affect up to 20% of the general population,1-3 taste disorders are more likely to remain in the background with a prevalence of only 5%.2,4 The main causes of taste disorders are idiopathic reasons, head traumas, infections of the upper respiratory tract, exposure to toxic substances, iatrogenic causes (eg, operations and irradiation), and drug-induced.5
Disparities in the prevalence of smell and taste disorders are also reflected in the number of test methods available. While constant progress is made in the field of olfactory test methods, tests for gustatory function are still limited.6 Gustatory function can be examined by using whole mouth (global) or lateralized (regional) tests. Global testing may be performed using sweet, sour, salty, and bitter solutions,7 whereas in for example, ear surgery (due to possible effects on the chorda tympani nerve8) regional tests should be preferred. The taste strips (further termed “TS”) test is widely used and renowned for its durability, transportability, and practicability allowing regional testing of gustatory function. It uses filter papers impregnated with specific taste solutions.9 Further extensions of the taste strips test include self-testing, umami testing, and testing of the basic tastes (eg, sweet, sour, salty, and bitter) with up to six different concentrations.10-12 Furthermore, it has been shown that paradigm selection (forced choice or non-forced choice, respectively) may not be as trivial, potentially leading to differences in bitter taste scores.13
Taste receptors, primarily located on specific cells gathered in taste buds on the human tongue have also been identified at various extra-oral sites of the human body such as the respiratory or gastrointestinal system.14-16 Recently, a connection has been shown between the activity of bitter receptors and various diseases affecting the upper respiratory tract including obstructive lung diseases17 and chronic rhinosinusitis,16,18,19 which underpins the need of research on bitter perception to further understand the exact pathophysiology behind those diseases. Especially, sweet and bitter taste receptors are of importance, as they are believed to belong to a complex system of pathogen recognition.16,18
One of the best-known bitter substances is caffeine, which has established broad acceptance through various beverages consumed on a daily base.20 Quinine hydrochloride (further termed “quinine”), by contrast, is less well known, even though it has been used as a drug for centuries.21 It is best-known as an anti-malarial medication,21 but also gained popularity as a medication for self-induced abortion.22 Considering the lack of willingness of subjects to participate in medical studies,23 specifically regarding additional barriers in vulnerable populations such as pregnant women24 or children (parental permission),25 there is a need to improve participation rates. However, while both caffeine and quinine solutions have been used for global testing of bitter perception, only quinine, not caffeine, has been used to test regional bitter perception by means of TS. Hence, there is a need to assess new compounds for regional bitter perception testing, which rely on widely accepted substances and in consequence can find broad acceptance and wide distribution.
In the present work, we assessed the validity of caffeine impregnated TS as an alternative to quinine TS to test regional bitter perception. The aim was to compare perceived intensity and hedonic dislike for different concentrations of caffeine and quinine TS at variable parts of the tongue.
Methods
Ethical Statement
The study was performed at the Medical University of Vienna, Department of Otorhinolaryngology according to the Declaration of Helsinki on biomedical research involving human subjects. Healthy volunteers were recruited through our outpatient clinic. The study protocol was approved by the local ethics committee (no. 360/2006). Participants were thoroughly informed about the study and written informed consent was obtained prior to all tests.
Participants
The study included a total of 46 healthy volunteers (25 males, 21 females, mean age ± SD/range, 23.0 ± 1.9/19-27). We excluded subjects with current or past conditions that might be related to gustatory dysfunction (head traumas, infections of the upper respiratory tract, exposure to toxic substances, middle ear surgeries and intake of daily medication which could affect gustatory function).26 Young subjects were preferably recruited for this study to ensure a homogenous group of participants.27 All participants were instructed not to eat, drink or brush their teeth 1 hour prior to the study. Furthermore, a complete Ear, Nose, and Throat examination was performed.
Taste Strips
The TS test (Burghart, Wedel, Germany) is a well-established test for the assessment of gustatory performance.8,9,11 It consists of 16 paper strips with a length of 8 cm and an area of 2 cm² impregnated with four concentrations of sucrose (sweet: 0.4; 0.2; 0.1; 0.05 g/ml), citric acid (sour: 0.3; 0.165; 0.09; 0.05 g/ml), sodium chloride (salty: 0.25; 0.1; 0.04; 0.016 g/ml) or quinine (bitter: 0.006; 0.0024; 0.0009; 0.0004 g/ml) solutions (all solved in distilled water). Additionally, two paper strips without taste were included in the test procedure. The TS were presented in increasing concentrations following a pseudo-randomized order and placed on the middle of the tongue. Subsequently, subjects were asked to close the mouth and select one of five possible answers on a form (sweet, sour, salty, bitter, and no taste; non-forced choice paradigm). The sum of correctly identified impregnated TS was compared with normative data, which allowed the distinction between normogeusia and hypogeusia using the 10th percentile as the cut-off point. Maximal reachable score was 16 with hypogeusia defined as a TS score <9.
Bitter Compounds
Two concentrations of quinine impregnated TS and two concentrations of caffeine impregnated TS were manufactured according to the manufacturing process for TS: filter papers with a length of 8 cm and an area to be impregnated of 2 cm² were soaked in solutions mentioned below and dried on a slowly rotating wheel.9 Quinine (Department of Pharmacy, University Erlangen-Nürnberg) and caffeine (Department of Pharmacy, University Erlangen-Nürnberg) were dissolved in distilled water. Following concentrations were used: for quinine, high concentration: 0.0004 g/ml (HQ) and low concentration: 0.00024 g/ml (LQ); for caffeine, high concentration: 0.02 g/ml (HC) and low concentration: 0.003 g/ml (LC). Additionally, two concentrations of quinine and caffeine solutions were manufactured for whole mouth (global) liquid testing for comparison with quinine and caffeine TS. Quinine and caffeine were dissolved in distilled water. Following concentrations were used: for quinine, high concentration: 0.0015 g/ml (WHQ) and low concentration: 0.0002 g/ml (WLQ); for caffeine, high concentration: 0.02 g/ml (WHC) and low concentration: 0.003 g/ml (WLC).
Self-Assessment of Gustatory Function
Prior to gustatory testing, subjects were asked to rate gustatory function (“How would you rate your sense of taste, eg, sweet, sour, salty, bitter?”) on a visual analog scale ranging from 1 to 10 (1 = left hand end: very bad; 10 = right hand end: perfect gustatory function).
Assessment of Bitter Intensity and Hedonic Dislike
Labeled magnitude scales were used to assess intensity and hedonic dislike. Six descriptors were placed along a vertical line scale with a length of 10 cm following the ideas of Green et al28 (measured from bottom to top in mm): (i) 1.5 mm, barely detectable/uncomfortable, (ii) 6 mm weakly detectable/uncomfortable, (iii) 17 mm moderately detectable/uncomfortable, (iv) 35 mm strongly detectable/uncomfortable, (v) 53 mm very strongly detectable/uncomfortable, and (vi) 100 mm strongest imaginable perception/uncomfortable imageable perception.
Test Procedure
Prior to bitter testing, subjects were instructed to rate intensity and hedonic dislike after presentation of each bitter substance. Two equally concentrated bitter TS were placed symmetrically on the anterior part of the tongue followed by two new TS of the same concentration placed on the posterior part of the tongue (Figure 1). The TS were held at their position for 30 seconds and a pause of 30 seconds was made between each exchange of TS. The mouth was rinsed with distilled water during that interval. Bitter TS were presented according to the following sequence: LC, LQ, HC, and HQ. Subsequent whole mouth testing was performed using 5 ml of bitter solutions starting with WLQ, followed by WLC, WHQ, and WHC. The mouth was rinsed with distilled water after each bitter solution and an interval of 30 seconds was adhered.
Figure 1.

Placement of bitter TS for testing.
Abbreviations: A, anterior part of the tongue; P, posterior part of the tongue.
Statistical Analysis
Statistical analysis and graphical visualization were performed using GraphPrism 8.1.2 (GraphPad Software, Inc., La Jolla, CA). Data normality was assessed using Shapiro–Wilk test. Group differences were tested using Mann–Whitney test. Correlation analysis was performed using Spearman correlation. A P-value <.05 was required for statistical significance.
Results
TS Scores Within Normogeusic Range
Forty-three subjects achieved scores within the range of normogeusia, whereas three subjects achieved scores within the range of (mild) hypogeusia. Subjects of the hypogeusic group correctly identified at least two out of four bitter concentrations. Mean ± standard deviation (range) of taste strip scores were 13.1 ± 2.5 (7-16).
Equally Concentrated Bitter TS at Different Regions of the Tongue did Not Lead to Significant Differences in Perceived Intensity or Hedonic Dislike
Median (interquartile range) intensity and hedonic dislike for quinine and caffeine impregnated TS of the same concentration at the anterior and posterior part of the tongue are displayed in Tables 1 and 2. Subsequent statistical analysis for differences in perceived intensity and hedonic dislike of bitter TS with the same concentration placed on the anterior and posterior part of the tongue revealed no significant differences (all P > .05, Mann–Whitney-U-test; Tables 1 and 2). Since region of the tongue did not seem to affect perceived intensity and hedonic dislike of equally concentrated quinine and caffeine TS, it would be interesting to know whether there might be an association between intensity and hedonic dislike.
Table 1.
Median (IQR) Perceived Intensity for Equally Concentrated Caffeine and Quinine TS Placed on Different Parts of the Tongue.
| Type | Impregnation | Part | Concentration | Median (IQR) | P-Value |
|---|---|---|---|---|---|
| Intensity | Caffeine | Anterior | Low | 9 (2-18) | |
| Intensity | Caffeine | Posterior | Low | 10 (2-14.3) | .97 |
| Intensity | Quinine | Anterior | Low | 14.5 (7.5-35.3) | |
| Intensity | Quinine | Posterior | Low | 16 (6.1-29.3) | .58 |
| Intensity | Caffeine | Anterior | High | 25.5 (11.8-43.5) | |
| Intensity | Caffeine | Posterior | High | 23.5 (10.8-42.3) | .80 |
| Intensity | Quinine | Anterior | High | 35 (20.8-54) | |
| Intensity | Quinine | Posterior | High | 32 (17.8-54.8) | .59 |
Table 2.
Median (IQR) Perceived Hedonic Dislike for Equally Concentrated Caffeine and Quinine TS Placed on Different Parts of the Tongue.
| Type | Impregnation | Region | Concentration | Median (IQR) | P-Value |
|---|---|---|---|---|---|
| Hedonic dislike | Caffeine | Anterior | Low | 2.3 (1-9) | |
| Hedonic dislike | Caffeine | Posterior | Low | 3.3 (1-8) | .75 |
| Hedonic dislike | Quinine | Anterior | Low | 6.8 (2-18) | |
| Hedonic dislike | Quinine | Posterior | Low | 5.3 (1.9-22.5) | .53 |
| Hedonic dislike | Caffeine | Anterior | High | 11 (6-34.3) | |
| Hedonic dislike | Caffeine | Posterior | High | 15 (4.5-36.3) | .94 |
| Hedonic dislike | Quinine | Anterior | High | 21.5 (8.6-49.3) | |
| Hedonic dislike | Quinine | Posterior | High | 20 (10-49.8) | .89 |
Significant Correlations between Perceived Intensity and Hedonic Dislike for All Equally Concentrated Bitter Compounds Presented at the Same Part on the Tongue
Statistical analysis revealed moderate to strong correlations between hedonic dislike and perceived intensity for all equally concentrated bitter TS placed on the anterior and posterior part of the tongue (correlation coefficients between 0.65 and 0.80; all Spearman correlation). Perceived intensity and hedonic dislike also showed strong correlations for all bitter solutions in global testing (correlation coefficients between 0.67 and 0.85; all Spearman correlation).
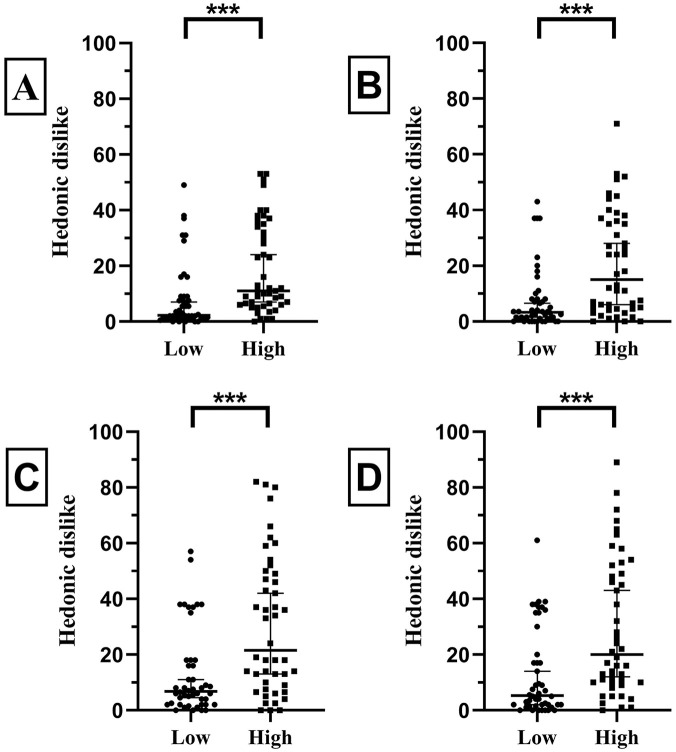
Bitter TS of Different Concentrations Placed on the Same Part of the Tongue Led to Significant Differences in Perceived Intensity and Hedonic Dislike
Perceived intensity and hedonic dislike for quinine and caffeine impregnated TS placed on the anterior and posterior part the tongue are displayed in Figures 2 and 3. Statistical analysis revealed significant differences for perceived intensity and hedonic dislike for both quinine and caffeine impregnated TS of different concentrations placed on the same region of the tongue (all P < .001, Mann–Whitney-U-test; Figures 2 and 3). Quinine and caffeine solutions of different concentrations in global testing also showed significant differences in perceived intensity and hedonic dislike: for intensity, mean WLC and WHC (18.5 and 45.0, P < .001), mean WLQ and WHQ (36.0 and 74.5, P < .0001); for hedonic dislike, mean WLC and WHC (16.5 and 30.0, P = .0018), mean WLQ and WHQ (39.5 and 72.0, P = .0004; all Mann–Whitney-U-test).
Figure 2.
Perceived intensity for caffeine and quinine TS of different concentrations placed on the same part of the tongue. (A) Caffeine TS placed on the anterior part of the tongue. (B) Caffeine TS placed on the posterior part of the tongue. (C) Quinine TS placed on the anterior part of the tongue. (D) Quinine TS placed on the posterior part of the tongue.
Abbreviation: Low, low concentration; High, high concentration.
***=P < .001, Mann–Whitney-U-test. The middle horizontal lines mark the median values and the lower and upper horizontal lines represent the 95% confidence intervals.
Figure 3.
Perceived hedonic dislike for caffeine and quinine TS of different concentrations placed on the same part of the tongue. (A) Caffeine TS placed on the anterior part of the tongue. (B) Caffeine TS placed on the posterior part of the tongue. (C) Quinine TS placed on the anterior part of the tongue. (D) Quinine TS placed on the posterior part of the tongue.
Abbreviation: Low, low concentration; High, high concentration.
***=P < .001, Mann–Whitney-U-test. The middle horizontal lines mark the median values and the lower and upper horizontal lines represent the 95% confidence intervals.
Non-Significant Correlation between Self-Assessment of Gustatory Function and TS Scores
Mean ± SD (range) self-assessment of gustatory function was 7.2 ± 1.4 (4-10). Forty-one of 43 normogeusic subjects rated gustatory function ≥ 5. Correlation analysis revealed no significant correlation between VAS and TS scores (Spearman rank correlation, r46 = 0.15, P = .32).
Discussion
Although several substances have been proposed and used for whole mouth gustatory testing of bitter perception, only quinine has been used for TS so far. Here, we showed that caffeine impregnated taste strips demonstrate the same properties as quinine impregnated TS. Both bitter compounds were evaluated at the anterior and posterior part of the tongue, showing comparable results both in intensity and hedonic dislike. In addition, higher concentrations resulted in significant higher perceived intensity and hedonic dislike. Moreover, perceived intensity and hedonic dislike showed a strong correlation. Furthermore, self-assessment of gustatory function showed a non-significant correlation with achieved TS scores in our tested cohort of young and healthy adults.
The role of the part of the tongue on perceived intensity has been described previously for all five basic tastes presented at threshold and suprathreshold concentrations.29,30 Perceived intensity for bitter and umami stimuli, presented at suprathreshold concentrations were shown to be higher, when presented at the posterior part of the tongue, compared to the anterior part.30 Interestingly, equally concentrated quinine and caffeine TS placed on different parts of the tongue did not lead to significant differences in ratings of intensity or hedonic dislike in our cohort. One reason for this finding might be explained by the number of taste buds on the dorsal surface of the oral part of the tongue, which seemed to be distributed fairly regular apart from the tip of the tongue.31
When it comes to the association between intensity and hedonic dislike for caffeine and quinine impregnated TS, as well as solutions during regional and global testing, we were able to confirm previous findings showing moderate to strong correlations between perceived intensity and hedonic dislike during bitter testing.32,33 From an evolutionary point of view, bitter perception has emerged as one of the most important cornerstones in the prevention of toxic substances.34-36 The natural aversion to bitter substances and preference for sweetness, but also the increased sensitivity of pregnant women to bitter substances is well known,37-40 explaining why an increase in bitter concentration may also lead to even stronger aversion.
In regard to caffeine for regional bitter testing using TS, we were also able to demonstrate that TS of different concentrations placed at the same part of the tongue led to differences in ratings of intensity and hedonic dislike. The difference in perceived intensity is one of the basic requirements for components in order to be used as TS, since during the initial validation process, the highest concentration was chosen to be detected by nearly all and the lowest only by approximately 50% of tested subjects. Additionally, regional testing of caffeine and quinine TS by means of the same concentration showed the same distinct properties, which is a further indicator of comparability. The wide range of inter-individually perceived intensities as well as hedonic dislike to caffeine and quinine TS and solutions might also reflect the individual sensitivity to bitter substances that has already been extensively studied.41-43 However, it should be noted that this study included mainly young and healthy subjects. Concerning age-related decrease of gustatory function,44 further studies including older subjects are needed to raise normative values for different age groups.
As mentioned in the introduction, caffeine has established broad acceptance through various beverages across all age groups,20,45 whereas quinine is less well known and was primarily used as a drug.21 The use of caffeine as bitter compound for regional testing of gustatory function might be of special interest for studies including vulnerable populations, such as pregnant women or children,24,25 in order to improve participation rates and in cases of self-reported hypersensitivity to quinine or quinine-containing beverages.46,47
Regarding self-assessment of gustatory function, we could confirm previous findings, showing that the majority of our cohort of healthy subjects rated gustatory function with at least normal (defined as VAS ≥ 5), even though no significant correlation could be found between self-assessment and TS scores.4,8,9,11 Interestingly, similar results could be found between self-assessment of olfactory perception and scores achieved with the Sniffin’ Sticks test.48,49 Landis et al also demonstrated no significant correlation between self-assessment of olfactory perception and test scores prior to testing, whereas self-assessment after the test procedure showed a significant correlation with achieved test results.50 Likewise, self-assessment of gustatory function prior to testing in patients with taste disorder also did not seem to reflect achieved test results. However, self-assessment of gustatory function seems to be accurate in detecting patients with no taste disorder.51
Conclusion
In summary, caffeine seems to be a valid bitter compound for regional testing using TS and may be used alternatively or in addition to quinine. No differences were found between perceived intensity and hedonic dislike for equally concentrated quinine and caffeine impregnated TS placed on different parts of the tongue. Assessment of four different caffeine TS concentrations for bitter testing and further studies including awareness about gustatory function and self-assessment scores will be the subject of future investigations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: David T. Liu  https://orcid.org/0000-0001-6948-737X
https://orcid.org/0000-0001-6948-737X
Gerold Besser  https://orcid.org/0000-0003-3168-7477
https://orcid.org/0000-0003-3168-7477
References
- 1. Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the skövde population-based study. Laryngoscope. 2004;114(4):733-737. [DOI] [PubMed] [Google Scholar]
- 2. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255(8):1121-1126. [DOI] [PubMed] [Google Scholar]
- 3. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307-2312. [DOI] [PubMed] [Google Scholar]
- 4. Welge-Lüssen A, Dörig P, Wolfensberger M, Krone F, Hummel T. A study about the frequency of taste disorders. J Neurol. 2011;258(3):386-392. [DOI] [PubMed] [Google Scholar]
- 5. Fark T, Hummel C, Hähner A, Nin T, Hummel T. Characteristics of taste disorders. Eur Arch Otorhinolaryngol. 2013;270(6):1855-1860. [DOI] [PubMed] [Google Scholar]
- 6. Rimmer J, Hellings P, Lund VJ, et al. European position paper on diagnostic tools in rhinology. Rhinology. 2019;57(suppl S28):1-41. [DOI] [PubMed] [Google Scholar]
- 7. Henkin RI, Bartter FC. Studies on olfactory thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids and of serum sodium concentration. J Clin Invest. 1966;45(10):1631-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mueller CA, Khatib S, Temmel AF, Baumgartner WD, Hummel T. Effects of cochlear implantation on gustatory function. Ann Otol Rhinol Laryngol. 2007;116(7):498-501. [DOI] [PubMed] [Google Scholar]
- 9. Mueller C, Kallert S, Renner B, et al. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips.” Rhinology. 2003;41(1):2-6. [PubMed] [Google Scholar]
- 10. Wolf A, Varga L, Wittibschlager L, Renner B, Mueller CA. A self-administered test of taste function using “Taste Strips.” Int Forum Allergy Rhinol. 2016;6(4):362-366. [DOI] [PubMed] [Google Scholar]
- 11. Mueller CA, Pintscher K, Renner B. Clinical test of gustatory function including umami taste. Ann Otol Rhinol Laryngol. 2011;120(6):358-362. [DOI] [PubMed] [Google Scholar]
- 12. Wolf A, Illini O, Uy D, Renner B, Mueller CA. A new extension to the “Taste Strips” test. Rhinology. 2016;54(1):45-50. [DOI] [PubMed] [Google Scholar]
- 13. Besser G, Prassl A, Mueller CA, Renner B. Testing gustatory function using either a forced-choice or a non-forced-choice paradigm - does it make a difference? Rhinology. 2019;57(5):385-391. [DOI] [PubMed] [Google Scholar]
- 14. Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069-15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100(15):8981-8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med (Berl). 2014;92(12):1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nayak AP, Shah SD, Michael JV, Deshpande DA. Bitter taste receptors for asthma therapeutics. Front Physiol. 2019;10:e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee RJ, Cohen NA. Role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2015;15(1):14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolf A, Renner B, Tomazic PV, Mueller CA. Gustatory function in patients with chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2018;127(4):229-234. [DOI] [PubMed] [Google Scholar]
- 20. Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2015;13(1):71-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shanks GD. Historical review: problematic malaria prophylaxis with quinine. Am J Trop Med Hyg. 2016;95(2):269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dannenberg AL, Dorfman SF, Johnson J. Use of Quinine for Self-Induced Abortion. South Med J. 1983;76(7):846-849. [DOI] [PubMed] [Google Scholar]
- 23. Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. J Heal Soc Policy. 2000;12(2):23-43. [DOI] [PubMed] [Google Scholar]
- 24. Frew PM, Saint-Victor DS, Isaacs MB, et al. Recruitment and retention of pregnant women into clinical research trials: an overview of challenges, facilitators, and best practices. Clin Infect Dis. 2014;59(suppl 7):S400-S407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004;364(9436):803-811. [DOI] [PubMed] [Google Scholar]
- 26. Doty RL, Shah M, Bromley SM. Drug-induced taste disorders. Drug Saf. 2008;31(3):199-215. [DOI] [PubMed] [Google Scholar]
- 27. Gudziol H, Hummel T. Normative values for the assessment of gustatory function using liquid tastants. Acta Otolaryngol. 2007;127(6):658-661. [DOI] [PubMed] [Google Scholar]
- 28. Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “labeled magnitude scale” for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323-334. [DOI] [PubMed] [Google Scholar]
- 29. Collings VB. Human taste response as a function of locus of stimulation on the tongue and soft palate. Percept Psychophys. 1974;16(1):169-174. [Google Scholar]
- 30. Feeney EL, Hayes JE. Regional differences in suprathreshold intensity for bitter and umami stimuli. Chemosens Percept. 2014;7(3-4):147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng LH, Robinson PP. The distribution of fungiform papillae and taste buds on the human tongue. Arch Oral Biol. 1991;36(8):583-589. [DOI] [PubMed] [Google Scholar]
- 32. Bossola M, Cadoni G, Bellantone R, et al. Taste intensity and hedonic responses to simple beverages in gastrointestinal cancer patients. J Pain Symptom Manage. 2007;34(5):505-512. [DOI] [PubMed] [Google Scholar]
- 33. Trant AS, Serin J, Douglass HO. Is taste related to anorexia in cancer patients? Am J Clin Nutr. 1982;36(1):45-58. [DOI] [PubMed] [Google Scholar]
- 34. Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56(6):1217-1227. [DOI] [PubMed] [Google Scholar]
- 35. Lindemann B. Taste reception. Physiol Rev. 1996;76(3):719-766. [DOI] [PubMed] [Google Scholar]
- 36. Breslin PA. An evolutionary perspective on food and human taste. Curr Biol. 2013;23(9):R409-R418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973;4:254-278. [PubMed] [Google Scholar]
- 38. Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444(7117):288-294. [DOI] [PubMed] [Google Scholar]
- 39. Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25(1):53-74. [DOI] [PubMed] [Google Scholar]
- 40. Kölble N, Hummel T, von Mering R, Huch A, Huch R. Gustatory and olfactory function in the first trimester of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;99(2):179-183. [DOI] [PubMed] [Google Scholar]
- 41. Yokomukai Y, Cowart BJ, Beauchamp GK. Individual differences in sensitivity to bitter-tasting substances. Chem Senses. 1993;18(6):669-681. [Google Scholar]
- 42. Reed DR, Zhu G, Breslin PA, et al. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet. 2010;19(21):4278-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hansen JL, Reed DR, Wright MJ, Martin NG, Breslin PA. Heritability and genetic covariation of sensitivity to PROP, SOA, quinine HCl, and caffeine. Chem Senses. 2006;31(5):403-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaneda H, Maeshima K, Goto N, Kobayakawa T, Ayabe-Kanamura S, Saito S. Decline in taste and odor discrimination abilities with age, and relationship between gustation and olfaction. Chem Senses. 2000;25(3):331-337. [DOI] [PubMed] [Google Scholar]
- 45. Temple JL. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev. 2009;33(6):793-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 10th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2015. [Google Scholar]
- 47. Liles NW, Page EE, Liles AL, Vesely SK, Raskob GE, George JN. Diversity and severity of adverse reactions to quinine: a systematic review. Am J Hematol. 2016;91(5):461-466. [DOI] [PubMed] [Google Scholar]
- 48. Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 1996;34(4):222-226. [PubMed] [Google Scholar]
- 49. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. “Sniffin’ sticks.” Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39-52. [DOI] [PubMed] [Google Scholar]
- 50. Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. Ratings of overall olfactory function. Chem Senses. 2003;28(8):691-694. [DOI] [PubMed] [Google Scholar]
- 51. Soter A, Kim J, Jackman A, Tourbier I, Kaul A, Doty RL. Accuracy of self-report in detecting taste dysfunction. Laryngoscope. 2008;118(4):611-617. [DOI] [PubMed] [Google Scholar]