Abstract
There is a growing need to understand the potential neurotoxicity of organophosphate flame retardants (OPFRs) and plasticizers because use and, consequently, human exposure, is rapidly expanding. We have previously shown in rats that developmental exposure to the commercial flame retardant mixture Firemaster 550 (FM 550), which contains OPFRs, results in sex-specific behavioral effects, and identified the placenta as a potential target of toxicity. The placenta is a critical coordinator of fetal growth and neurodevelopment, and a source of neurotransmitters for the developing brain. We have shown in rats and humans that flame retardants accumulate in placental tissue, and induce functional changes, including altered neurotransmitter production. Here, we sought to establish if OPFRs (triphenyl phosphate and a mixture of isopropylated triarylphosphate isomers) alter placental function and fetal forebrain development, with disruption of tryptophan metabolism as a primary pathway of interest. Wistar rat dams were orally exposed to OPFRs (0, 500, 1000, or 2000 μg/day) or a serotonin (5-HT) agonist 5-methoxytryptamine for 14 days during gestation and placenta and fetal forebrain tissues collected for analysis by transcriptomics and metabolomics. Relative abundance of genes responsible for the transport and synthesis of placental 5-HT were disrupted, and multiple neuroactive metabolites in the 5-HT and kynurenine metabolic pathways were upregulated. In addition, 5-HTergic projections were significantly longer in the fetal forebrains of exposed males. These findings suggest that OPFRs have the potential to impact the 5-HTergic system in the fetal forebrain by disrupting placental tryptophan metabolism.
Keywords: endocrine disruptors, endocrine toxicology, flame retardants, metabolome, neurotoxicity, developmental, neurotoxicology, neurotransmitter, developmental toxicity, prenatal, reproductive and developmental toxicology, developmental/teratology
Chemical flame retardants (FRs) have long been suspected of contributing to rising rates of neurodevelopmental disorders, with the now phased out polybrominated diphenyl ethers (PBDEs) specifically linked to deficits in memory and cognitive function (Bennett et al., 2016; Chen et al., 2014; Hoffman et al., 2017; Messer, 2010). Replacement brominated flame retardants (BFRs) have also been shown to have toxic effects, including effects on brain and behavior (Bailey and Levin, 2015; Baldwin et al., 2017; Jarema et al., 2015; Quevedo et al., 2019; Rock et al., 2018), and are environmentally problematic because of their persistence, thermostability, and lipophilicity. Consequently, production and application of organophosphate flame retardants (OPFRs), as an alternative FR class, has rapidly increased, with global consumption jumping from 100 000 tons in 1992 to 680 000 tons in 2015 (van der Veen and de Boer, 2012; Wang et al., 2015a; Wei et al., 2015). Although these alternatives show less bioaccumulation than the PBDEs (Hou et al., 2016), likely due to their rapid metabolism (Cooper et al., 2011; Hou et al., 2016; Van den Eede et al., 2013a), their potential toxicity has not been studied to the same extent. Neurotoxicity is a significant concern because structurally related compounds with an organophosphorous backbone, including organophosphate pesticides, have been shown in a variety of model systems to affect brain development, and are suspected developmental neurotoxicants in humans (Bjorling-Poulsen et al., 2008; Carr et al., 2014; Dishaw et al., 2011; Jarema et al., 2015; Timofeeva et al., 2008a,b).
Firemaster 550 (FM 550) is a commercial FR mixture comprised both BFRs and OPFRs, that rapidly became one of the most commonly used FRs for baby products and residential furniture (Stapleton et al., 2009, 2011, 2012). We have previously reported evidence of behavioral effects in rats following developmental exposure, and identified multiple pathways by which gestational FM 550 exposure may impact the placenta and developing forebrain (Baldwin et al., 2017; Rock et al., 2018). The present studies were conducted to assess the degree to which an OPFR mixture can impact neurodevelopmental outcomes by altering placental function and disrupting neurotransmitter (NT) signaling between the placenta and developing brain.
Due to their broad application as FRs and plasticizers in paints, glue, lacquers, and other industrial products, OPFRs have become ubiquitous environmental contaminants, with human exposure primarily occurring through inhalation, inadvertent ingestion of indoor dust, and dermal absorption (Kim et al., 2019; Stapleton et al., 2008; van der Veen and de Boer, 2012). Although the present studies focused on the OPFRs present in FM 550, numerous other OPFRs are present in isomeric and commercial mixtures with other classes of FRs (Behl et al., 2015). Approximately 54% of FM 550 is made up of organophosphate components, including triphenyl phosphate (TPHP also known as TPP) and a mixture of isopropylated triarylphosphate isomers (ITPs) (Phillips et al., 2017) (Table 1). TPHP is a high production volume chemical (10–50 million lbs/year), used as a plasticizer in products like polyvinyl chloride and circuit boards for decades (World Health Organization & International Programme on Chemical Safety, 1991), and has recently been found in nail polish (Mendelsohn et al., 2016). Consequently, TPHP has become one of the most abundant and frequently detected OPFRs in indoor dust globally (Abdallah and Covaci, 2014; Brommer et al., 2012; Coelho et al., 2016; Stapleton et al., 2009; Tajima et al., 2014). Biomonitoring studies have shown nearly ubiquitous detection of diphenyl phosphate (DPHP), the primary metabolite of TPHP, in urine samples (Butt et al., 2014; Cequier et al., 2015; Dodson et al., 2014; Hoffman et al., 2014; Meeker et al., 2013; Van den Eede et al., 2013b, 2015). Fewer studies have looked at the prevalence of the ITPs, as this isomeric mixture was not fully characterized until 2017 (Phillips et al., 2017). There is a growing body of literature, however, showing that exposure in the United States is common (Hammel et al., 2016). For example 1 study found detectable levels of mono-isopropylphenyl phenyl phosphate (mono-IPPPP), which is a confirmed metabolite of the ITPs, in 98%–100% of urine samples (Butt et al., 2014; Phillips et al., 2018). Accumulating evidence of widespread exposure highlights the pressing need to understand the potential neurotoxicity of replacement FRs like TPHP and the ITPs.
Table 1.
Summary of Chemicals in Organophosphate Flame Retardant Mixture and Detection in Maternal Serum and Material/Fetal Placenta
| Chemical Name (Cas No.) | Structure | Mass Fraction (w/w) in Mixturea | Exposure | Dam Serum(ng/ml) | Maternal Placenta(ng/g ww) |
Fetal Placenta (ng/g ww) |
||
|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | |||||
| TPHP(26040-51-7) |
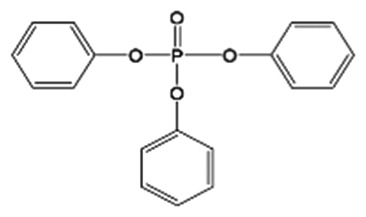
|
44.6 ± 0.9% | Control | <MDL | <MDL | <MDL | 11.1 ± 2.6 (1) | 5.9 ± 0.2 (1) |
| High | <MDL | 19.0 ± 3.2 (1) | 18.9 ± 3.5 (1) | <MDL | <MDL | |||
| 2IPPDPP(28108-99-8; 93925-53-2; 64532-94-1) |
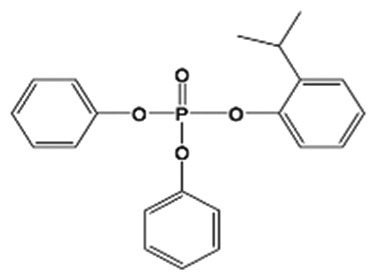
|
26.9 ± 1.1% | Control | <MDL | <MDL | <MDL | 3.3 ± 0.5 (1) | <MDL |
| High | <MDL | 7.0 ± 1.2 (1) | 7.8 ± 1.0 (2) | 3.1 ± 0.3 (2) | 3.7 ± 0.2 (2) | |||
| 3IPPDPP |
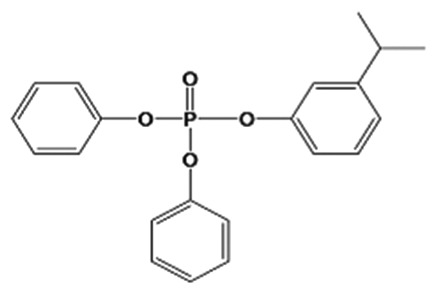
|
0.3 ± 0.1% | Control | <MDL | <MDL | <MDL | <MDL | <MDL |
| High | <MDL | <MDL | <MDL | <MDL | <MDL | |||
| 4IPPDPP |
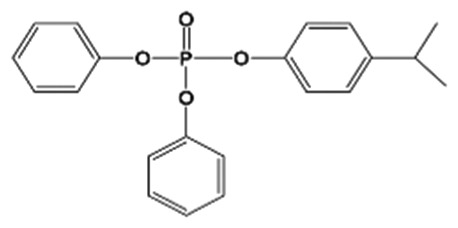
|
4.9 ± 0.8% | Control | 0.4 ± 0.2 | <MDL | <MDL | 2.1 ± 0.3 (1) | <MDL |
| High | 6.5 ± 1.6 | 7.5 ± 1.3 (4) | 10.2 ± 1.8 (4) | 6.8 ± 0.9 (4) | 7.8 ± 0.7 (4) | |||
| 24DIPPDPP |
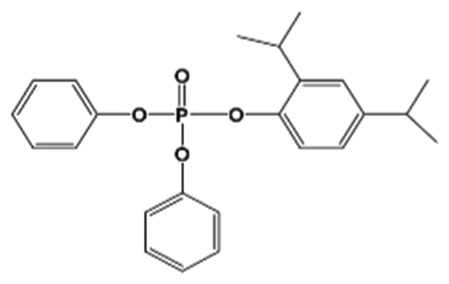
|
7.2 ± 0.9% | Control | 2.2 ± 1.3 | <MDL | <MDL | <MDL | <MDL |
| High | 14.8 ± 3.4 | <MDL | <MDL | <MDL | <MDL | |||
| B2IPPPP(69500-29-4) |
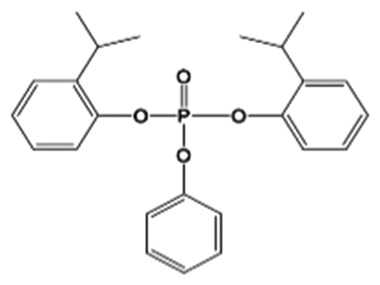
|
11.1 ± 0.9% | Control | NA | <MDL | <MDL | 2.0 ± 0.4 (1) | <MDL |
| High | NA | 4.6 ± 0.8 (1) | 4.7 ± 0.6 (2) | 2.1 ± 0.3 (2) | 2.7 ± 0.4 (3) | |||
| B3IPPPP |
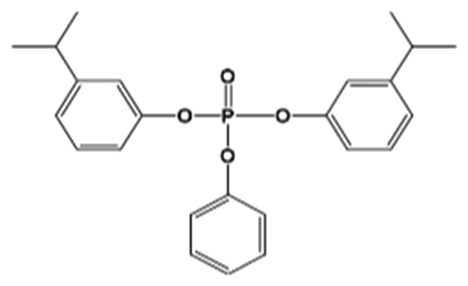
|
0.8 ± 0.2% | Control | 0.1 ± 0.07 | <MDL | <MDL | <MDL | <MDL |
| High | 0.3 ± 0.06 | 4.2 ± 0.8 (1) | 5.2 ± 1.1 (3) | 4.6 ± 0.8 (4) | 4.9 ± 0.7 (4) | |||
| B4IPPPP |
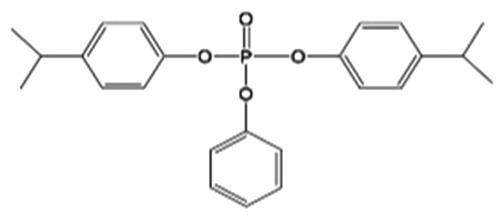
|
1.1 ± 0.3% | Control | <MDL | <MDL | <MDL | <MDL | <MDL |
| High | <MDL | <MDL | <MDL | <MDL | <MDL | |||
| T3IPPP(72668-27-0) |
|
0.1 ± 0.05% | Control | <MDL | <MDL | <MDL | <MDL | <MDL |
| High | <MDL | <MDL | <MDL | <MDL | <MDL | |||
Values represent the mean ± SEM. Numbers in parentheses indicate number of samples that were > MDL, n = 4.
Abbreviation: MDL, minimum detection level.
aFrom Phillips et al. (2017).
In a recent study by the National Toxicology Program, TPHP was identified as a “high priority” chemical meriting additional testing due to evidence of developmental and neurotoxic properties comparable with the BFRs it was meant to replace (Behl et al., 2015). Additional studies in zebrafish have shown that acute or developmental exposure to OPFRs, including TPHP, can interfere with locomotor behavior (Jarema et al., 2015; Shi et al., 2018). Mechanistic data are limited but indicate that OPFRs likely impact neurodevelopment via multiple mechanisms, including the disruption of endocrine and NT signaling (Belcher et al., 2014; Kojima et al., 2013; Liu et al., 2012, 2013b; Shi et al., 2018). For example, a zebrafish study identified that TPHP can inhibit acetylcholinesterase (AChE) activity and disrupt other NTs, including Gamma-Aminobutyric Acid . In addition, the expression of genes involved in neurodevelopmental processes such as cytoskeleton regulation, axon growth, and neuron maturation, was also altered (Shi et al., 2018). We have recently shown that FM 550 can alter serotonin (5-HT) levels in the developing rat forebrain (Rock et al., 2018). 5-HT has also been shown to be a target of the organophosphate pesticide chlorpyrifos (Aldridge et al., 2005; Slotkin and Seidler, 2005, 2007, 2008), which is structurally similar. Therefore, we hypothesized that disruption of 5-HT signaling may be a critical mechanism by which TPHP and ITPs can impact brain development and behavior.
In addition to the brain, the placenta was also of primary interest because it is a critical coordinator of fetal growth and development, including neurodevelopment. A highly active endocrine organ, the placenta is responsible for the transfer and synthesis of hormones, monoamines, and other signaling factors required for proper brain development (Bonnin et al., 2011; Bonnin and Levitt, 2011; Konkel, 2016; Nugent and Bale, 2015). For example, the fetal side of the placenta serves as a critical and primary source of 5-HT for the developing fetal forebrain from gestational day (GD) 10 to 15; a time frame that precedes the organization and function of the brain’s serotonergic network (Bonnin et al., 2011; Bonnin and Levitt, 2011). Placental function is environmentally responsive and sex-specific, with placental sex (XX vs XY) determining many aspects of placental development and function (Bale, 2016; Konkel, 2016; Nugent and Bale, 2015). Such plasticity is believed to play a role in sex-specific fetal programing, including neural changes that may contribute to sex differences in risk of neurodevelopmental disorders. One well-studied example is the programmatic effects of prenatal stress, which results in male-specific placental inflammation and offspring hyperactivity (Bronson and Bale, 2014). Notably, maternal inflammation has been shown to disrupt forebrain development via increased placental 5-HT output to the fetal brain (Goeden et al., 2016). In our previous work in Wistar rats, we found that FM 550 accumulates in placental tissue and can alter various aspects of placental function, including endocrine, inflammatory, and neurotransmitter signaling (Baldwin et al., 2017) suggesting it may adversely impact neurodevelopment (Rock et al., 2018) and later in life socioemotional behaviors (Baldwin et al., 2017; Gillera et al., 2019). We have yet to identify which of the chemical classes in the FM 550 mixture (BFR vs OPFR) contributes to the adverse phenotypes. The present studies focused on the OPFRs, with the hypothesis that placental NT production, particularly 5-HT and other tryptophan metabolites, and forebrain serotonergic innervation would be disrupted.
We employed a combination of hypothesis-generating (RNA-sequencing [RNA-seq] and untargeted metabolomics) and targeted (quantitative real-time PCR [qRT-PCR] and targeted NT analysis) approaches to examine Wistar rat placentas following gestational exposure to 500, 1000, and 2000 μg OPFRs/kg/day (TPHP and ITPs, Table 1). The global 5-HT agonist 5-methoxytryptamine (5-MT) (5 mg/kg/day) was included as a positive control for fetal 5-HT disruption. Development of the 5-HTergic system in fetal Wistar rat brains was also examined using immunohistochemistry.
MATERIALS AND METHODS
Animals
Animal care, maintenance, and experimental protocols met the standards of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and use of Laboratory Animals” and were approved by the North Carolina State University (NCSU) Institutional Animal Care and Use Committee (IACUC). A supervising veterinarian approved and monitored all procedures throughout the duration of the project. For each aim, Wistar rats were obtained from Charles River (Raleigh, North Carolina) and bred in house as indicated in rooms with 12 h:12 h light, dark cycles at 25°C and 45%–60% average humidity in the AAALAC approved Biological Resource Facility at NCSU. As in our prior studies (Patisaul et al., 2009, 2012), and in accordance with recommended practices for endocrine disrupting chemical (EDC) research (Hunt et al., 2009; Li et al., 2008; Richter et al., 2007), all animals were housed in conditions specifically designed to minimize unintended EDC exposure including use of glass water bottles with metal sippers, soy-free diet, woodchip bedding, and thoroughly washed polysulfone caging. The ARRIVE (Animal Research: Reporting of In Vivo Experiments) Guidelines Checklist for Reporting Animal Research was used in the construction of this manuscript with all elements met (Kilkenny et al., 2010). The ARRIVE guidelines were developed in collaboration with the scientific community as part of an NC3Rs (National Centre for the Replacement Refinement and Reduction of Animals in Research) initiative to improve the standard of reporting of research using animals.
Dosing Prep
All animals were orally dosed using a concentrated ITP-mixture ethanol-based solution (approximately 100 mg/ml) prepared in the Stapleton Lab. The commercial mixture was weighed using a Mettler Toledo A21 Comparator microbalance and dissolved in 100% ethanol to make the concentrated stock. This concentrated stock contained the same mixture and percent composition of TPHP and ITPs used in a prior study conducted in the Stapleton Lab (Phillips et al., 2017). The chemical components included TPHP (Cas No. 26040-51-7), 2-isopropylphenyl diphenyl phosphate (2IPPDPP; Cas No. 28108-99-8; 93925-53-2; 64532-94-1), 3-isopropylphenyl diphenyl phosphate (3IPPDPP), 4-isopropylphenyl diphenyl phosphate (4IPPDPP), 2,4-diisopropylphenyl diphenyl phosphate (24DIPPDPP), bis(2-isopropylphenyl) phenyl phosphate (B2IPPPP; Cas No. 69500-29-4), bis(3-isopropylphenyl) phenyl phosphate (B3IPPPP), bis(4-isopropylphenyl) phenyl phosphate (B4IPPPP), and tris(3-isopropylphenyl) phosphate (T3IPPP; Cas No. 72668-27-0; Table 1). The Patisaul Lab then prepared each dosing solution (0 , 25 , 50 , and 100 mg/ml) by diluting the appropriate amount of concentrated stock with 100% ethanol. These solutions were coded to blind dosing and subsequent testing. The global 5-HT agonist, 5-MT was used as a positive control for chemical disruption of 5-HT signaling in the fetal brain because gestational exposure has been found to alter brain neurochemistry (including 5-HT terminal density) and behavior in rats (Shemer et al., 1988, 1991), and prairie voles (Martin et al., 2012). 5-MT was purchased from Sigma-Aldrich (5-MT: Cas No. 608-07-1, 97% purity) dissolved in ethanol to obtain the target dose of 5 mg/kg bw/day. All solutions were stored in glass scintillation vials, wrapped in foil, and then stored at 4°C until use.
Animal Husbandry and Exposure
Adult Wistar rats were obtained from Charles River at PND 70–90 in batches referred to as cohorts (cohort 1: n = 20 females and 10 males, PND 70; cohort 2: n = 10 females, PND 70; cohort 3: n = 40 females and 10 males, PND 90; cohort 4: n = 10 females, PND 90). Two dams were paired with a single male during diestrus (as determined by vaginal cytology) to ensure they reached the proestrus/estrus (peak sexual receptivity) stage while with a male. After 72 h or detection of a sperm plug, males were removed, and dams housed individually. GD 0 was defined 24 h after separation or detection of a sperm plug. Dams were weighed daily to monitor weight gain as an indicator of pregnancy. If dams were not successfully impregnated dosing was terminated. These dams were incorporated in the next cohort as part of the same dose group they were assigned originally after about a 2-week period where they were not being dosed. Therefore, the number of dams being dosed as part of cohort 1 was 20, cohort 2 was 13, cohort 3 was 43, and cohort 4 was 13. Of the females being dosed 12 were successfully impregnated in cohort 1, 10 in cohort 2, 36 in cohort 3, and 12 in cohort 4. Animals were maintained on Teklad 2020 (phytoestrogen-free) diet, then paired and monitored for the presence of a sperm plug. Exposure was once per day for 14 days across GDs 1–14. At the time of dosing 20 µl of each dosing solution was pipetted onto ¼ of a soy-free food treat pellet (apple flavored AIN-76A Rodent Diet Test Tabs, Test Diet, Richmond, Indiana) as we have done previously (Patisaul et al., 2013) resulting in the following exposure groups (animals randomly assigned; each cohort contained animals in each exposure group): control (0 µg OPFRs), 500 µg OPFRs, 1000 µg OPFRs, 2000 µg OPFRs, and 1.25 mg 5-MT. The food treat was administered once the solution was completely dry and consumption was monitored to ensure the dam ate the entire treat. Dams were not dosed by individual weight but rather by an average colony (all female) weight of 250 g at conception producing exposures of approximately 0, 2 (low), 4 (medium), and 8 (high) mg/kg bw/day OPFRs and 5 mg/kg bw/day 5-MT (relative exposure likely decreased slightly across the 10-day window as body weight increased, on average, 61 g across pregnancy). This method reduces handling and, consequently, possible confounding by prenatal stress (Charil et al., 2010).
Tissue Collection
Sacrifice on GD 14 was performed 4 h after final dosing (12:00 h ± 60 min) to control for time postexposure and time of collection. All dams and fetuses were weighed and sacrificed by CO2 asphyxiation and rapid decapitation. From each litter, half of the fetal heads were flash frozen whereas the other half were drop-fixed in 4% paraformaldehyde. A single paw was collected from each fetus to determine sex via PCR as previously described (Baldwin et al., 2017; Poletti et al., 1997). Fetal age was assigned using Witschi, Thelier, and Carnegie criteria as guides (Hill). Placentas were collected, dissected, and flash frozen on powdered dry ice. Placental dissection consisted of separating the decidua, myometrium, and mesometrium (referred to as the maternal side of the placenta) from the trophospongium and labyrinth zone (referred to as the fetal side of the placenta). When litters were large (greater than 12) a single placenta was placed in 10% neutral buffered formalin for gross histopathological assessment. Placentas, 4 from the control group (2 of each sex), 4 from the 2 mg/kg OPFR group (2 of each sex), and 5 from the 8 mg/kg OPFR group (3 males and 2 females) were sent to the Histology Laboratory and the associated Anatomic Pathology Core at the NCSU College of Veterinary Medicine for pathological analysis (no gross pathology was identified) and confirmation of gestational age.
Most litters were confirmed to be between GDs 13 and 14, with some falling outside of this range. Because placental functions change dramatically over the course of gestation, to maximize consistency, we only used placentas from litters of the correct gestational age. Frozen placentas were cryosectioned (Leica CM1900, Nubloch, Germany) at an orientation that produced a cross-section of each layer, and sliced to a depth approximately one-third of the way through the tissue, where we could see distinct layers, including the junctional and labyrinth zone, as well as the maternal arterial channel as reference landmarks for tissue collection (Figure 1). Then two 1.25-mm punches were collected and stored in separate tubes at −80°C. A single punch was used for RNA extractions for RNA-seq and qRT-PCR. Majority of placentas used for gene expression analysis underwent dissection to isolate the fetal side. However, some punches were collected from whole placentas. For RNA-seq this included whole placentas from the 0 mg/kg OPFR (1 male) and the 8 mg/kg OPFR (1 female, 1 male) dose groups. For qRT-PCR this included whole placentas from the 0 mg/kg OPFR (1 female, 1 male), 2 mg/kg OPFR (2 female, 3 male), 8 mg/kg OPFR (2 female, 1 male), and 5-MT (1 female, 1 male) dose groups. The remaining placental tissue, after slicing and collecting punches, was reserved for NT analysis.
Figure 1.
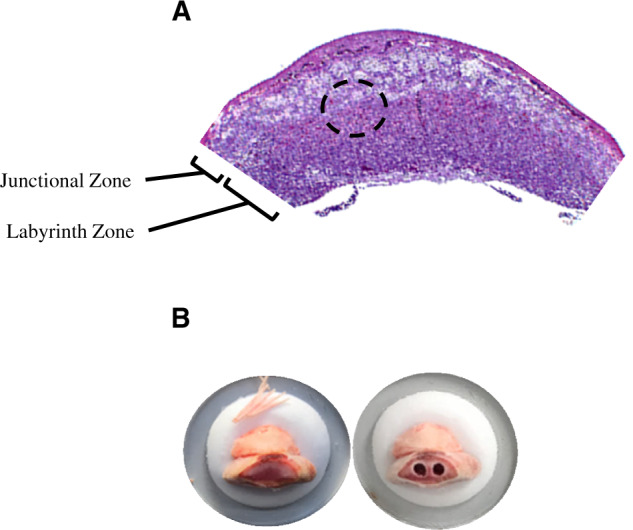
A, A representative hematoxylin and eosin stained placental section depicting location of the junctional and labyrinth zones. The dotted circle indicates the region where the micropunches were obtained. B, Representative images of a placenta mounted on the cryostat before and after two 1.25-mm micropunches were collected.
Only placentas that underwent dissection to isolate the fetal side were used for NT analysis.
Serum Processing and OPFR Analysis
Serum samples were processed according to Butt et al. (2016). Samples (approximately 1 ml) were allowed to thaw and then were spiked with the internal standard 13C-TPHP (Wellington Laboratories). Deionized water (1 ml) and formic acid (0.5 ml) were added to deproteinate the serum. Samples were vortexed and subsequently sonicated for 15 min. Serum extracts were purified in 2 rounds of solid phase extraction (SPE). The first SPE purification step used Waters Oasis PRiME HLB extraction cartridges (500 mg, 6 cc). Briefly, cartridges were conditioned with 5 ml of dichloromethane, 5 ml of methanol, and 5 ml of deionized water. After samples were loaded, the cartridges were washed with 5 ml deionized water and vacuumed to dryness. Analytes were eluted from the cartridges using 10 ml of 1:1 dichloromethane/ethyl acetate. Extracts were then concentrated to near dryness under a gentle stream of nitrogen and reconstituted in 1 ml of hexane. The second SPE purification step used Waters Sep-Pak Ca Certified Silica cartridges (1 g, 6 cc). Briefly, cartridges were conditioned with 10 ml of hexane and sample extracts were loaded. Analytes were eluted from the cartridges using 10 ml of 1:1 hexane/ethyl acetate. Samples were concentrated under a gentle stream of nitrogen, reconstituted in hexane, and spiked with the recovery standard dTPHP (Sigma-Aldrich). Analytes were quantified using previously described gas chromatography/mass spectrometry methods and 6-point calibration curves (Phillips et al., 2017). Average recovery of 13C-TPHP was 97 ± 11%.
Placenta Processing for OPFR Analysis
Dissected placentas from litters younger than GD 14 (GD 12–13.5) were sent to the Stapleton Lab to quantify OPFR levels in the fetal and maternal sides of the placenta (n = 4 per sex per group, control and 2000 µg OPFRs only). Approximately 100 mg of maternal placental tissue and approximately 200 mg of fetal placental tissue were ground in 3 g of sodium sulfate and spiked with internal stand 13C-TPHP (Wellington Laboratories). The tissues were extracted by sonication in 1:1 hexane:dichloromethane and purified using Florisil SPE cartridges (SUPELCO Supelclean ENVI 6 ml 0.5 g). Briefly, cartridges were conditioned with 5 ml of methanol and 3 ml of hexane. The sample was loaded onto the cartridge and eluted with 8 ml of hexane (fraction 1) followed by 10 ml of ethyl acetate (fraction 2). Fraction 2 was blown down to near dryness under a gentle stream of nitrogen, reconstituted in 0.5 ml of hexane and spiked with recovery standard dTPHP (Sigma-Aldrich). Analytes were quantified using the same methods for serum analysis.
Placental RNA-seq
As an initial hypothesis-generating approach, placental punches from the fetal side (5 per sex per group, from vehicle and 8 mg/kg OPFRs) underwent RNA-seq. The experimental design and analysis were developed in consultation with the NCSU Genomic Sciences Laboratory (GSL). RNA extraction was performed with the Qiagen RNEasy Miniprep kit according to the manufacturer protocol (Qiagen, Cat. 74134). RNA quality was determined using an Agilent 2100 Bioanalyzer and all samples, for both tissue types, had an RNA integrity number (RIN) = 10. Sequencing libraries were prepared as described previously (Puzianowska-Kuznicka et al., 2006), using NEBNext Ultra Directional RNA Library Prep kit and NEBNext Poly(A) mRNA Magnetic Isolation Module (catalogs E7420 and E7490; New England Biolabs), to be compatible with Illumina sequencing. Isolation, heat fragmentation, and priming were performed according to manufacturer’s instructions. cDNA synthesis was followed by purification and size selection. Finally, library clean-up was performed used AMPure XP beads (Beckman Coulter Genomics, Cat. A63881) and quality was assessed using the Agilent 2100 Bioanalyzer. Sequencing was performed using a 125-bp single-end protocol on a single lane of an Illumina HiSeq2500 sequencer. Approximately, 33 million uniquely mapped reads were generated per placental library. The transcriptome data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE148266 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE148266).
RNA-seq Data Processing
Data analysis for placenta RNA-seq was performed in collaboration with the Bioinformatics Core of the NCSU Center for Human Health and Environment. Briefly, quality control (QC) of read data was evaluated with FastQC. Alignment was performed using STAR short read aligner (Wise et al., 2016) to Rattus norvegicus (rn6) reference genome. The number of reads mapped to genome features was determined using htseq-count script from HTSeq (Anders et al., 2015) python package and count data was imported to R statistical computing environment (R Foundation for Statistical Computing, 2015) to normalize count data, estimate dispersion, and fit a negative binomial model for each gene, using an extended model matrix considering the main effects of both sex and treatment as well as the interaction term.
Initially, genes that had no counts in more than 2/3 of the replicate samples were excluded from the analysis. Data normalization based on dispersion and differential analysis were conducted using DESeq2 (Deverman and Patterson, 2009) package. We fitted a generalized linear model (∼Gender + Dose) between the expression count, Gender (M, F) and Dose (Control, High). Finally, differential expression analysis was preformed between Female versus Male and high versus control dose. The overall mean of the normalized counts, the log2(fold change), the p value, and the adjusted p value (p adj, also known as q values), adjusted for multiple testing using the Benjamini-Hochberg False Discovery Rate (Benjamini and Hochberg, 1995), were calculated.
Quantitative Real-time PCR
Analysis was performed on RNA from fetal side placenta punches in all 5 treatment groups: control (8 female, 6 male; 4 litters), 2 mg/kg OPFR (9 per sex; 5 litters), 4 mg/kg OPFR (11 per sex; 6 litters), 8 mg/kg OPFR (10 per sex; 5 litters), and 5-MT (9 female, 8 male; 5 litters). A set of preidentified genes of interest were selected for qRT-PCR based on previous studies highlighting inflammatory (interleukin-6: Il6), sex steroid signaling (estrogen receptor α: Esr1), stress (hydroxysteroid 11-beta dehydrogenase 2: Hsd11b2), and NT pathways as potential targets of FM 550 (Rock et al., 2018). More specifically, we assessed changes in the expression of genes involved in the transport (solute carrier family 6 member 4: Slc6a4), synthesis (dopa decarboxylase: Ddc, tryptophan hydroxylase 1: Tph1), and metabolism of 5-HT (monoamine oxidase a: Maoa). Because 5-HT synthesis is dependent on the availability of tryptophan (Trp) we also quantified the expression of enzymes related to Trp metabolism (tryptophan 2,3-dioxygenase: Tdo2, indoleamine 2,3-dioxygenase 1 & 2: Ido1 & Ido2) and kynurenine (Kyn) metabolism (kynurenine 3-monooxygenase: Kmo). Finally, we followed up on 1 specific gene of interest identified by our RNA-seq data, superoxide dismutase 2 (Sod2). RNA extractions were performed as described above for RNA-seq. RNA concentrations were normalized to 20 ng/μl prior to cDNA synthesis. cDNA synthesis was performed using high-capacity RNA-to-cDNA kits (Applied Biosystems, Cat. 4387406) according to the manufacturer instructions. Incubation for reverse transcriptase reactions was 60 min at 37°C, 5 min at 95°C, and the cDNA stored at −20°C until use. A QuantStudio 6 Flex Real-Time PCR System and TaqMan probes were used for qRT-PCR as previously detailed (Puzianowska-Kuznicka et al., 2006). Triplicate reactions were run as well as negative controls (no template present) for each TaqMan assay. A house keeping gene (18 s rRNA) was used to normalize CT values for differences in starting concentrations of cDNA. Relative changes in expression were determined using the Livak ΔΔCT method (Livak and Schmittgen, 2001).
Neurotransmitter Analysis—Relative and Targeted
The relative abundance of several Kyn pathway metabolites was first measured in the fetal portion of placentas (4 per sex per dose group, from vehicle and high dose), including Trp, Kyn, kynurenic acid (Kyna), xanthurenic acid (Xan), 5-HT, and 5-hydroxyindole acetic acid (5-HIAA), to explore which might be vulnerable to exposure and identify which would be appropriate for further analysis. Placenta samples were transferred to 2.0 ml Eppendorf tubes and 100 μl of acidified mobile phase and 500 μl of cold methanol were added prior to homogenization using an Omni tissue homogenizer with disposable tips. After about 10 s of homogenization, the samples were allowed to sit at −20°C for 30 min before centrifugation for 15 min (4°C, 3000 × g) for protein precipitation. The supernatant was removed and transferred to a weighed 1.5 ml Eppendorf tube and taken to dryness on a SpeedVac. The samples were then resuspended in mobile phase A based on extract weight (30 mg/ml) and centrifuged for 15 min (4°C, 3000 × g). The supernatant was transferred directly to an LCMS autosampler vial for analysis. QC samples were prepared by pooling and mixing equal volumes of each sample. The QC sample and blank samples were injected at regular intervals throughout the sequence.
For quantification of the targeted pathway, a 1 mg/ml stock solution of each of 6 Kyn pathway metabolites (Kyn, Trp, Kyna, Xan, 5-HT, and 5-HIAA) was prepared using a 1:1:2 MeOH/H2O/DMSO mixture acidified with 0.1% formic acid. These individual stocks were combined and diluted to achieve a 1000 ng/ml working standard solution of the Kyn pathway mixture. Seven calibration standards ranging from 7 to 500 ng/ml were prepared by serially diluting the working standard solution. Chromatographic separation was achieved using a Waters Acquity BEH C18 column (2.1 × 100 mm) and a linear gradient of 1%–100% acetonitrile with 0.1% formic acid (flow rate 350 μl/min, 2 μl injections in duplicate). High-resolution mass spectrometry data was acquired using a Thermo Orbitrap ID-X mass spectrometer in positive mode (spray voltage 3.5 kV, vaporizer temperature 350°C) with a mass range of m/z 70–1000 and a dwell time of 0.6 s based on the chromatographic peak width of 6 s allowing 10 scans across each peak for accuracy in quantification. MS1 data were collected with a resolution of 120 000 and an AGC target of 2.0e5. MS2 data was collected using a prioritized ddMS2 method. The first order scan priority was guided by a targeted list of metabolites in the Kyn pathway (AGC target 1.0e5, resolution 30 K, and fixed HCD collision energy of 30). The second order scan priority provided MS2 data for other metabolites in the extracts for global metabolite profiling (AGC target 1.0e5, resolution 30 K, and stepped HCD collision energy of 10, 35, 50).
Targeted Neurotransmitter Data Processing
Peak integration and quantification were performed in TraceFinder 2.3. Individual standard curves for each of the 6 Kyn pathway metabolites were constructed using extracted ion chromatogram peak areas from MS1 data and the slope of each curve was calculated using a linear curve fit. The concentrations of the Kyn pathway metabolites in the study samples were calculated in an identical manner relative to the regression line. Calibration curves for each of the metabolites had R2 values ranging from .9941 to .9997 for the linear range of 7–500 ng/ml.
Untargeted Neurotransmitter Data Processing
Preprocessing and annotation in CD 3.0
Raw data files were uploaded into Compound Discoverer 3.0 (Thermo Fisher Scientific) and processed using a workflow to find and identify differences between samples. This workflow performed retention time alignment, unknown compound detection, and compound grouping across all samples. Elemental compositions were predicted for all compounds and chemical background was hidden using blank samples. Compound annotations were assigned using ddMS2 data in comparison with the mzCloud database (Thermo Fisher Scientific) as well as molecular formula comparisons with selected ChemSpider databases (KEGG, HMDB, Mass Bank) which were further ranked using the mzLogic algorithm (Thermo Fisher Scientific).
Compound annotations
A total of 22 458 compounds were identified in the data set. Compounds were filtered to exclude background resulting in 17 031 compounds of which 5931 were annotated in Compound Discoverer. This subset was further filtered to only include compounds that were mapped to a pathway in Metabolika, resulting in 92 compounds. Manual curation of these 92 compounds to remove duplicate and erroneous annotations resulted in 68 compounds.
Statistical analysis in MetaboAnalyst
The filtered data set (68 compounds) with peak areas per file was formatted for further statistical analysis in MetaboAnalyst (Yida et al., 2015). Sample normalization, data transformation, and data scaling were set to normalization by sum, log transformation, and pareto scaling, respectively. Results were analyzed using univariate (t test/ANOVA, Fold Change, Volcano Plot, Correlation Analysis) and multivariate (principal component analysis [PCA], partial least squares discriminant analysis [PLS-DA]) statistics as well as by hierarchical clustering (Heatmap).
Pathway analysis in MetaboAnalyst
All 68 compounds selected for statistical analysis as well as a subset of 20 compounds that were highlighted in the top 15 features using both PLS-DA variable importance in projections (VIP) score was analyzed using the Pathway Analysis tool in MetaboAnalyst using the R. norvegicus reference library and a Fishers’ exact test for over-representation analysis.
Fetal brain immunohistochemistry
Fixed fetal heads were cryosectioned (Leica CM1900) into 3 serial sets of 20 µm sagittal sections, mounted on Superfrost Plus slides (Fisher, Pittsburgh, Pennsylvania), and stored at −80°C. Immunolabeling of 5-HT, Netg1a and HuC/D was consistent with what we have done previously in floating sections using antibodies validated previously by us or others and listed in the Antibody Registry (http://antibodyregistry.org, last accessed February 18, 2020) (Patisaul et al., 2008). All slides were brought to room temperature and allowed to dry for 15 min. Then slides were placed in room temperature methanol for 10 min, followed by 3 10-min rinses in KPBS. A PAP pen was used to create a hydrophobic barrier around the perimeter of each slide for incubation. Slices were preincubated overnight in 2% donkey serum (Colorado Serum Co, Denver, Colorado) and 0.3% Triton X-100 in 500 μl KPBS (LKPBS) per slide in a humidity-controlled chamber at 4°C. The LKPBS was gently poured off, then the slides were incubated in a humidity-controlled chamber at 4°C in a 500 μl cocktail of primary antibodies directed against 5-HT (rabbit anti-5-HT, 1:2000; Immunostar, Hudson, Wisconsin), Netg1a (goat anti-Netrin-G1a, 1:1000; R&D Systems, Minneapolis, Minnesota), and HuC/D (mouse anti-HuC/HuD 16A11, 1:500, Invitrogen, Carlsbad, California) in LKPBS. HuC/D is a neuronal marker and was used to confirm all 5-HT cell bodies were neuronal (Patisaul et al., 2008; Polston et al., 2004). Netg1a positive axonal projections are not only sensitive to 5-HT disruption and thus a critical endpoint but also served as an anatomical landmark. All slides underwent 6 10-min washes in KPBS, then incubated for 1 h at 4°C in a cocktail of secondary antibodies (Alexa-Fluor donkey anti-rabbit 488, Alexa-Fluor donkey anti-goat 555, Alexa-Fluor donkey anti-mouse 647; Invitrogen) each diluted to 1:200 in LKPBS. The slides were rinsed for 10 min in KPBS, counterstained with Hoechst 33258 (Invitrogen), and finally underwent a series of 5 10-min washes in KPBS before being coverslipped with VWR micro cover glass (VWR, Radnor, Pennsylvania) using a standard glycerol mountant made with potassium bicarbonate.
Confocal Microscopy and Image Analysis
A developing rat atlas (Paxinos and Ashwell, 2018) was used to verify neuroanatomical landmarks, and to ensure that only GD 14 fetal heads were analyzed. 5-HT, Netg1a, and HuC/D triple-immunofluorescent label was visualized in all exposure groups: control (6♀, 7♂), 2 mg/kg OPFR (5♀, 5♂), 4 mg/kg OPFR (4♀, 5♂), 8 mg/kg OPFR (6♀, 6♂), and 5-MT (8♀, 8♂). Anatomical identification of a midline section corresponding figure 67 sagittal slice 3 in the brain atlas (Paxinos and Ashwell, 2018) was made in each animal for quantification of 5-HTergic and Netg1a positive projections. Identification was made by using a cluster of dorsal thalamic neurons that were Netg1a-immunoreactive (Bonnin et al., 2011), which takes on a distinct fan shape close to the midline, whose projections originate in the prethalamus and extend dorsally toward the thalamus and hippocampus. Similarly, a single brain section per animal was selected corresponding to figure 66 E14 sagittal 2, to quantify 5-HT cell bodies, Immunolabeling of all triple-labeled sections was visualized using a Leica DM6 B epifluorescent microscope. Leica Application Suite X software measurement tool was used to determine the length of 5-HTergic projections, from the base of the Netg1a-immunoreactive cluster. Length was also determined for the Netg1a-immunoreactive fibers and to assess the spread of 5-HTergic cell bodies.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 8 (La Jolla, California) with statistical significance set at α ≤ .05. Potential litter effects were accounted for either in the experimental design (by testing only 1 animal per sex per group) or in the statistical analysis (by including litter as a covariate). Accumulation of OPFRs in the placenta was analyzed by 2-way ANOVA with chemical component and sex as biological variables, followed by Sidak post hoc tests to compare accumulation of individual components in (1) male fetal side versus female fetal side, (2) male maternal side versus female maternal side, (3) and male versus female whole placenta. Two-way ANOVA followed by Sidak post hoc test, with chemical and placental compartment (fetal vs maternal) as the 2 factors, was used to determine if accumulation in the fetal and maternal side was different regardless of sex. Finally, a paired t test was used to compare the total burden of OPFRs in exposed placentas between male- and female-associated placentas from the same litter. Because equal variance was not found for all endpoints, gene expression was analyzed using nonparametric approaches. Placental gene expression (qRT-PCR) data was first analyzed using a 2-tailed exact Mann-Whitney U test with males and females binned together to compare exposure groups to controls. The data were then examined within sex and a 2-tailed exact Mann-Whitney U test used to identify sex differences between male and female controls, and exposure-related effects. Similarly, placental neurotransmitter levels were examined using a 2-tailed exact Mann-Whitney U test to look for baseline sex differences, comparing male and female controls, and exposure-related effects. Fetal forebrain projection and cell body measurements in the control groups were first analyzed by an unpaired 2-tailed t test to test for a sex difference. Possible exposure effects were probed by 1-way ANOVA followed by Dunnett’s multiple comparisons test. Female 5-HTergic projections appeared to have a dose response even though the ANOVA did not reveal a significant effect of dose. Thus, a Brown-Forsyth test was conducted to confirm equality of group variances, and then a test for linear trend was conducted as the post hoc analysis.
RESULTS
Accumulation of OPFR Components in Placenta
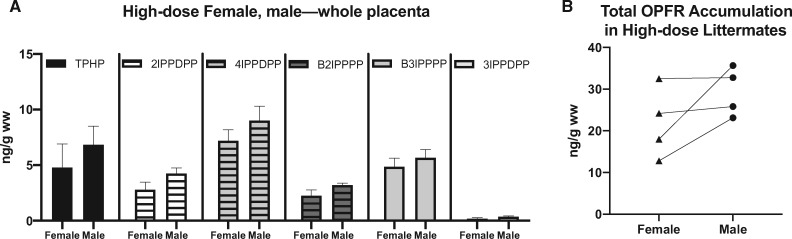
Only tissues from the high-dose group (2000 µg) were examined. A greater number of OPFR analytes were detected in placental tissue (5/9) than in dam serum (3/9) (Table 1). Two components were detected in both dam serum and placental tissue, 4IPPDPP and B3IPPPP, with levels consistently higher in the placenta. In dam serum 24DIPPDPP was also detected and, at an average concentration of 14.8 ± 3.4 ng/ml, was the most abundant of 3. TPHP, 2IPPDPP, 4IPPDPP, B2IPPPP, and B3IPPPP were observed in both the maternal and fetal sides of the placenta. In control placentas, levels were either below the minimum detection level (MDL) or very low in comparison with placentas exposed to 8 mg/kg OPFR, with the exception of TPHP on the fetal side of the placenta and 2IPPDPP on the fetal side of control female placentas. Although detection of any analyte in the controls was unexpected, for TPHP and 2IPPDPP they were only found in a single sample. We cannot account for this exposure, but because OPFRs are so ubiquitous, unintended exogenous exposure from an unidentified source is possible. Placental accumulation was highest for TPHP, with levels reaching up to 19.0 ± 3.2 ng/g ww on the maternal side of female-associated placentas. Frequency of detection in placenta samples was greatest for 4IPPDPP, with all high-dose placentas showing detectable levels on both the fetal and maternal side. No other metabolite was detected in all samples.
Sex differences in accumulation were considered plausible because we have previously shown that components of FM 550 accumulate to a greater degree in male-associated whole placentas than female (Baldwin et al., 2017). No significant effect of sex was observed for OPFR levels on the maternal side or the fetal side of the placenta. Analysis of the whole placenta also revealed a significant effect of sex (F(1, 36) = 4.4, p ≤ .04) on OPFR levels, however post hoc analysis did not reveal a significant effect of sex for any of the individual OPFR components (Figure 2A). A significant difference in OPFR levels was also found for the maternal versus the fetal side (F(1, 70) = 44.9, p ≤ .0001). When examining the individual components, post hoc analysis revealed a significant difference in accumulation of TPHP (p ≤ .0001) and 2IPPDPP (p ≤ .02) with levels higher on the maternal side. We also examined the total burden of OPFRs in exposed placentas and although there was not a significant difference between male- and female-associated placentas from the same litter, total burden was suggestively higher in male-associated placentas (Figure 2B).
Figure 2.
A, Levels of individual chemicals in whole placenta separated by sex. Graph depicts mean ± SEM. B, Total burden of OPFRs in whole placenta from the 8 mg/kg OPFR group separated by sex. Lines connect littermates. Abbreviations: OPFR, organophosphate flame retardant; TPHP, triphenyl phosphate.
Impact of Prenatal OPFR Exposure on Placental Gene Expression—RNA-seq
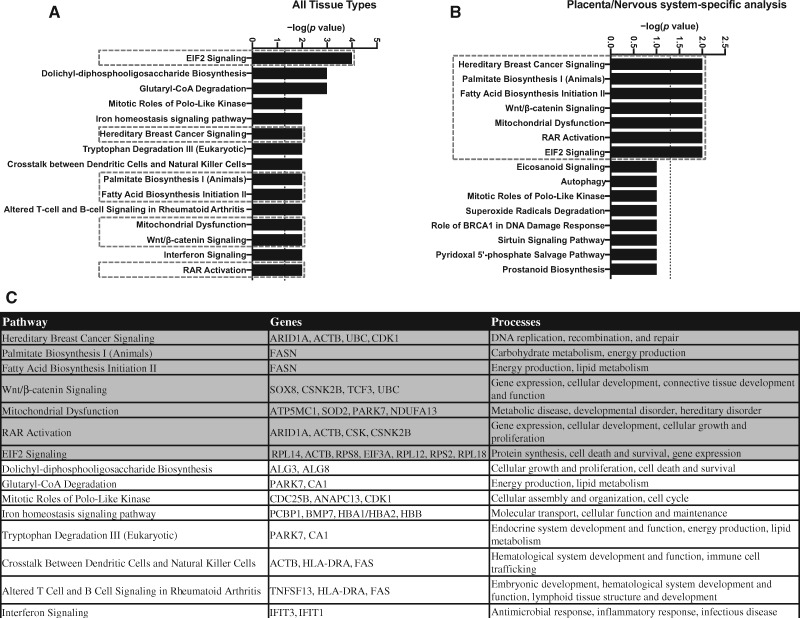
RNA-seq of the placental transcriptome was performed on the micropunches from the fetal side and analyzed as a hypothesis-generating approach to identify possible modes of action. Unfortunately, 1 female-associated placenta sample from the 8 mg/kg OPFR dose group had to be excluded for technical reasons. RNA-seq results were first analyzed by sex, but lack of separation in an unsupervised PCA prohibited this approach and revealed reasonable separation for only the female-associated placenta samples. Therefore, pathway analysis was only conducted on female-associated placenta data. Differential expression of 79 genes, at a p adjusted value of .05, was found in female-associated placental tissue from dams exposed to 8 mg/kg OPFR. Ingenuity Pathway Analysis (QIAGEN) software was used to identify potential pathways perturbed by OPFR exposure. To maximize rigor and reproducibility, several different iterations were employed using the full gene list with a p adjusted value of .1 as a cutoff; no cutoff value was implemented for fold-change. The first iteration included annotations from all tissue types (Figure 3A), whereas the second iteration included annotations from only placenta and nervous system tissue types (Figure 3B) because we were specifically interested in how changes in placental function may impact the brain. Numerous pathways were identified in both analyses and are enriched with genes that play roles in energy production as well as cellular growth and development (Figure 3C).
Figure 3.
The top 15 canonical pathways identified by Ingenuity Pathway Analysis (IPA) analysis using annotations for all tissue types (A) and only the annotations specific to the placenta/nervous system (B). The dotted boxes indicate pathways identified in both analyses. C, Genes enriched in the pathways identified using annotations from all tissue types, and the processes they coordinate, with pathways identified by both IPA analyses highlighted in gray.
Impact of Prenatal OPFR Exposure on Placental Gene Expression—qRT-PCR
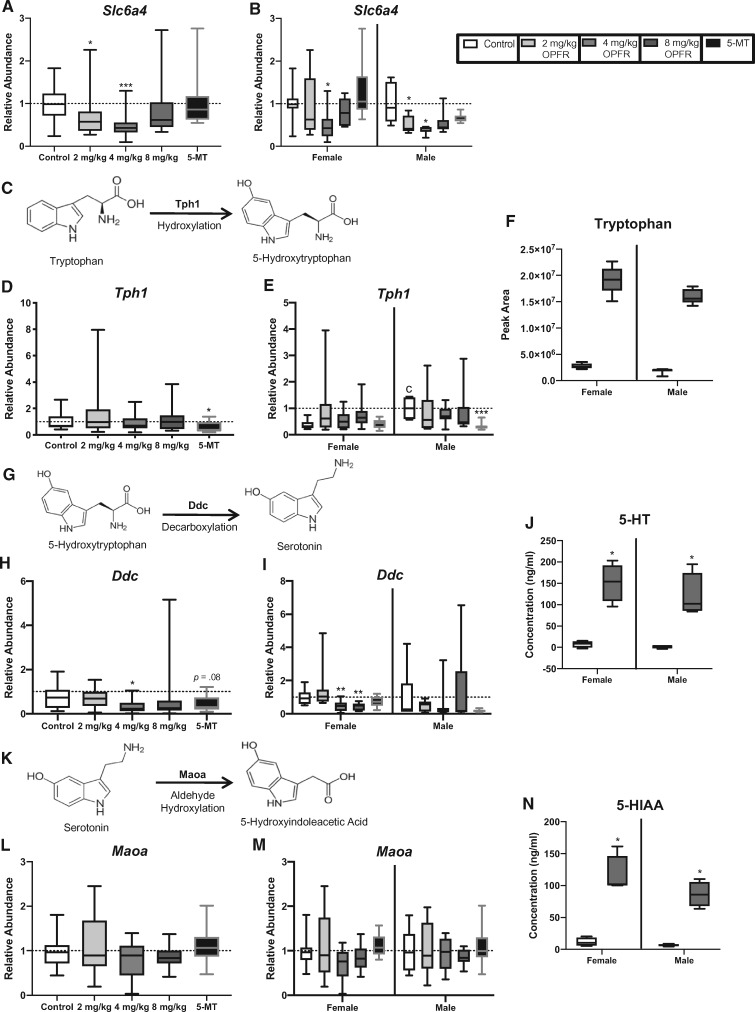
Follow-up analyses on genes of interest in the fetal side micropunches by qRT-PCR to validate RNA-seq findings, used a larger sample size (n = 8–11 per sex per group) and considered sex as a biological variable. It is well established that there are numerous sex differences between male- and female-associated placentas, including differences in gene expression (Bale, 2016). Therefore, to assess baseline sex differences, the data were first normalized to the control values for a single sex, in this case males. When analyzed for baseline sex differences only one of the genes showed a significant difference in expression between control males and females. Relative abundance of Tph1 was found to be significantly higher in control male-associated placentas than in female-associated placentas (U = 4, p ≤ .01; Figure 5E).
Figure 5.
Effects of prenatal organophosphate flame retardant (OPFR) exposure on the relative abundance of genes involved in the transport, synthesis, and metabolism of serotonin (5-HT) and levels of tryptophan, 5-HT and 5-hydroxyindole acetic acid (5-HIAA). A and B, Slc6a4, the 5-HT transporter, was lower in the 2 mg/kg and 4 mg/kg OPFR dose when sexes were combined for analysis, and in male-associated placentas when separated by sex. In female-associated placentas levels were only lower in the 4 mg/kg OPFR dose group. (D and E) Expression of Tph1, enzyme that converts tryptophan to 5-hydroxytryptophan (5-HTP) (C), was significantly lower in the 5-methoxytryptamine (5-MT) group in both the combined analysis and in male-associated placentas when separated by sex. E, Tph1 also showed a significant sex difference in the unexposed controls, with male-associated placentas having greater expression than female-associated placentas (cp ≤ .05). (H) Expression of Ddc, the enzyme that converts 5-HTP to 5-HT (G), was lower in the 4 mg/kg OPFR group in the combined analysis, whereas a suggestive decrease was observed in the 5-MT animals. I, When separated by sex, lower expression of Ddc was observed in the 4 mg/kg and 8 mg/kg females. L and M. Expression of Maoa, the enzyme that converts 5-HT to 5-HIAA (K), was unaffected. F, Relative abundance of tryptophan was higher in placentas exposed to 8 mg/kg OPFR compared with unexposed placentas, with male-associated placentas showing consistently higher levels than female-associated placentas. J and N, Quantitative analysis of 5-HT and 5-HIAA showed a significant increase in the concentration of these neuroactive metabolites in male and female placentas exposed to 8 mg/kg OPFR. Graphs depict median with whiskers from minimum to maximum (*p ≤ .05, **p ≤ .01, ***p ≤ .001).
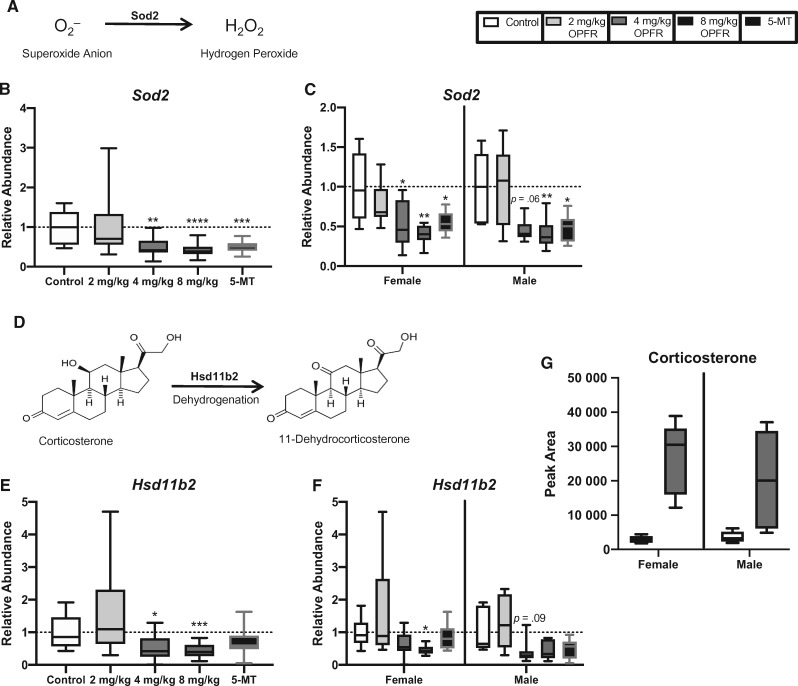
Expression of Sod2 was significantly altered by OPFR exposure (H = 31.5, p ≤ .0001), with both the 4 mg/kg OPFR and 8 mg/kg OPFR dose groups showing a significant reduction compared with controls (p ≤ .001; p ≤ .0001; Figure 4B). This relationship was also found when female- and male-associated placentas analyzed separately (H = 16.8, p ≤ .0008; H = 14.4, p ≤ .003). Reduced Sod2 was found at both the 4 mg/kg OPFR and 8 mg/kg OPFR dose for female (p ≤ .04; p ≤ .002) and at the highest dose (8 mg/kg OPFR) for male-associated placentas (p ≤ .006; Figure 4C). Although a significant reduction in expression was not observed for 4 mg/kg OPFR male-associated placentas, a suggestive decrease was found (p = .06). Expression of Sod2 was also reduced by treatment with 5-MT (U = 36, p ≤ .001), in both female- and male-associated placentas (U = 12, p ≤ .02; U = 6, p ≤ .02; Figure 4C).
Figure 4.
Effects of prenatal organophosphate flame retardant (OPFR) exposure on placental genes responsible for protection against oxidative stress and exposure to corticosterone. (B and C) Sod2, the enzyme responsible for the inactivation of superoxide anion (A), was downregulated in the 4 mg/kg OPFR, 8 mg/kg OPFR, and 5-MT groups when males and females were grouped for analysis, as well as when they were analyzed within sex. (E) Similarly, Hsd11b2, the enzyme responsible for the inactivation of corticosterone (D), was downregulated in the 4 mg/kg OPFR and 8 mg/kg OPFR groups when sexes were combined. F, Expression of Hsd11b2 was downregulated in the female 8 mg/kg OPFR group, whereas males showed only a suggestive decrease in expression at the 4 mg/kg OPFR dose. G, Relative abundance of corticosterone appeared to be elevated in both female- and male-associated placentas exposed to 8 mg/kg OPFR, with males showing a greater exposure-related increase than females. Graphs depict median with whiskers from minimum to maximum (*p ≤ .05, **p ≤ .01, ***p ≤ .001, ****p ≤ .0001).
Relative abundance of Hsd11b2 was significantly impacted by OPFR exposure (H = 24.8, p ≤ .0001), with both the 4 mg/kg OPFR and 8 mg/kg OPFR dose groups having significantly lower expression (p ≤ .011; p ≤ .003; Figure 4E). When stratified by sex, expression of Hsd11b2 was altered by exposure in both females and males (H = 13.2, p ≤ .004; H = 13.2, p ≤ .004). At the highest dose (8 mg/kg OPFR), female-associated placentas showed a significant reduction in expression (p ≤ .011), whereas males at the 4 mg/kg OPFR dose showed a suggestive decrease (p = .09; Figure 4F).
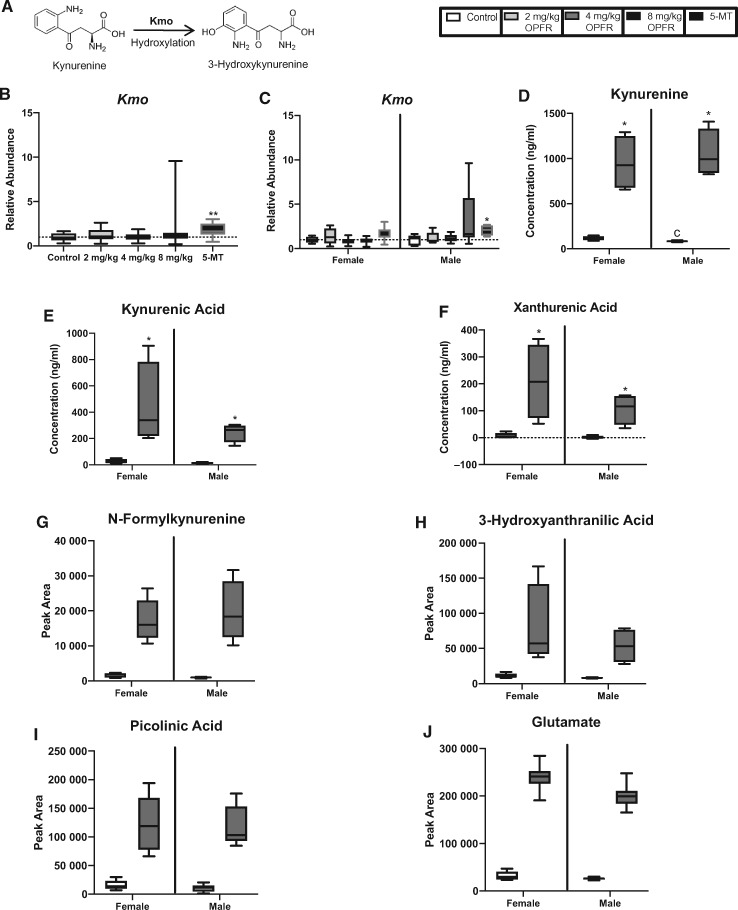
Expression of Tph1 and Maoa were unchanged, but relative abundance of Ddc was significantly altered by exposure to OPFRs (H = 9.9, p ≤ .02) with the 4 mg/kg OPFR dose group showing a decrease in relative abundance compared with controls (p ≤ .05; Figure 5H). When stratified by sex, expression of Ddc was significantly impacted by OPFR exposure (H = 18.9, p ≤ .0003), with female-associated placentas exposed in the 4 mg/kg OPFR and 8 mg/kg OPFR dose groups showing a significant reduction in the relative abundance of Ddc (p ≤ .03; p ≤ .02; Figure 5I). Expression of Ddc also appeared to be lower in the 5-MT group, but did not reach statistical significance (U = 70, p = .08) in the combined analysis (Figure 5I). Although not impacted by OPFR exposure, expression of Tph1 was significantly altered by 5-MT treatment (U = 58, p ≤ .05; Figure 5D). When stratified by sex, treatment with 5-MT lead to a significant reduction in the expression of Tph1 in male-associated placentas (U = 1, p ≤ .008; Figure 5E). Relative abundance of the 5-HT transporter, Slc6a4, was significantly impacted by OPFR exposure (H = 15.9, p ≤ .001; Figure 5A) with expression in both the 2 mg/kg OPFR and 4 mg/kg OPFR groups significantly lower than in unexposed controls (0 mg/kg OPFR; p ≤ .05; p ≤ .0004; Figure 5A). When separated by sex this relationship held true for both female- and male-associated placentas (H = 7.7, p ≤ .05; H = 9.4, p ≤ .03). Female-associated placentas showed a significant decrease in Slc6a4 at the lowest dose (p ≤ .03), whereas male-associated placentas showed a significantly lower relative abundance at both the 2 mg/kg OPFR and 4 mg/kg OPFR dose compared with controls (p ≤ .04; p ≤ .01; Figure 5B). Kmo, which is responsible for metabolizing Kyn, was not altered by OPFR exposure but was significantly impacted by treatment with 5-MT (U = 41, p ≤ .002; Figure 6B), which only held for male-associated placentas when analyzed by sex (U = 6, p ≤ .02; Figure 6C).
Figure 6.
Effects of prenatal organophosphate flame retardant (OPFR) exposure on the expression of a gene that metabolizes kynurenine as well as the relative abundance of kynurenine and its metabolites. (B and C) Expression of Kmo, which converts kynurenine to 3-hydroxykynurenine (A), was not impacted by OPFR exposure but significantly lower in the 5-methoxytryptamine (5-MT) animals in the combined analysis as well as in male-associated placentas when separated by sex. D–F, Quantitative analysis of kynurenine, kynurenic acid, and xanthurenic acid showed a significant increase in the concentration of these neuroactive metabolites in male and female placentas exposed to 8 mg/kg OPFR. G–J, Relative abundance of N-formylkynurenine, 3-hydroxyanthranilic acid, picolinic acid, and glutamate were all higher in placentas exposed to 8 mg/kg OPFR compared with unexposed placentas, with male-associated placentas showing consistently higher levels than female–associate placentas. Graphs depict median with whiskers from minimum to maximum (*p ≤ .05, **p ≤ .01).
Impact of Prenatal OPFR Exposure on Placental Neurotransmitters—Targeted Analysis
Relative abundance of Trp, 5-HT, 5-HIAA, N-formylkynurenine (N-formylKyn), Kyn, Kyna, 3-hydroxy anthranilic acid, Xan, picolinic acid, L-glutamate, corticosterone, and progesterone were all elevated in response to OPFR exposure. Similarly, targeted quantification of the selected Kyn pathway metabolites (Figure 7) revealed significant upregulation of all measured metabolites (Kyn, Kyna, Xan, Ser, HIAA) in exposed animals (Figs. 5 and 6 depict results from the targeted analysis for these 5 metabolites, and relative abundance for the others). Trp was also measured and determined to be upregulated, however the concentrations in the exposed samples far exceeded the upper limit of quantification in the calibration curves generated so the concentrations were unable to be extrapolated (data not shown).
Figure 7.
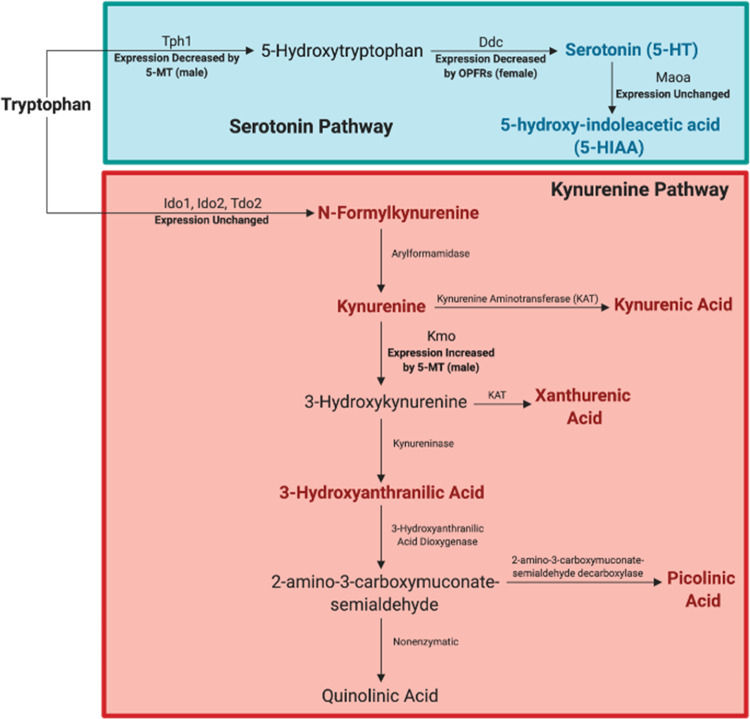
Summary of observed organophosphate flame retardant (OPFR) effects on metabolites and genes involved in the 5-HT and kynurenine metabolic pathways. Metabolites elevated by exposure are bolded and colored according to the pathway of interest (red for kynurenine and green for 5-HT). Outcomes, changes in expression, and sex-specific effects, are also depicted. Created with BioRender.com.
For the metabolites of interest, we first analyzed for baseline sex differences. Only the concentration of Kyn was found to be significantly higher in control female-associated placentas than in male-associated placentas (U = 1, p ≤ .05; Figure 6D). Quantitative analysis of 5-HT, 5-HIAA, Kyn, Kyna, and Xan showed a significant increase in the concentration of all of these neuroactive metabolites in placentas exposed to 8 mg/kg OPFR when compared with unexposed placentas from same sex (Figs. 5J, 5N and 6D–F). For example, 5-HT reached 194 ng/ml in male-associated, and 203 ng/ml in female-associated placentas, which translates into a fold increase in approximately 63-fold in males and 20-fold in females (U = 0, p ≤ .03; Figure 5J). 5-HTs metabolite, 5-HIAA, also showed a significant increase in exposed placentas with levels in female-associated placentas being 10 times higher and male-associated placentas 13 times higher than unexposed same sex controls (U = 0, p ≤ .03; Figure 5N). A similar response was seen for Kyn, where levels were off the curve in 8 mg/kg OPFR exposed placentas of both sexes representing an approximately 8- to 12-fold increase over the same sex controls (U = 0, p ≤ .03; Figure 6D). Kyna, was also elevated in exposed placentas of both sexes with approximately a 17-fold change in male- and female-associated placentas (U = 0, p ≤ .03; Figure 6E). Xan, a metabolite that was below the detection limit in unexposed placentas reached 156 and 366 ng/ml in OPFR exposed male- and female-associated placentas, respectively (U = 0, p ≤ .03; Figure 6F). Because Trp is the precursor to both the 5-HT and Kyn pathway, changes in gene expression and relative abundance of metabolites for both pathways are summarized in Figure 7.
Impact of Prenatal OPFR Exposure on Placental Neurotransmitters—Untargeted Metabolomics
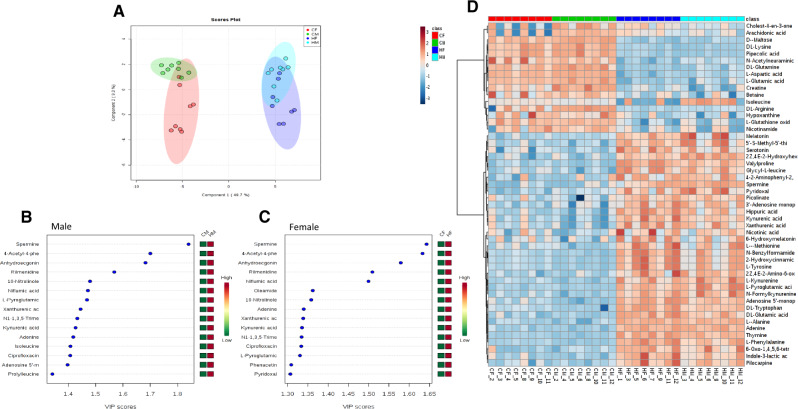
The LC-HRMS metabolomics data was also analyzed in an untargeted manner to identify other pathways that may be perturbed by exposure. The data set was filtered down to 68 compounds confidently annotated in Compound Discoverer (molecular formula/isotope ratio match and MS/MS match). PCA revealed distinct grouping of the conditions with minimal sex differences (Figure 8A). PLS-DA indicated distinct grouping and was used to rank features based on the variable VIP score which indicated that the polyamine spermine was the most important variable in distinguishing differences among the 4 conditions (Figs. 8B and 8C). Several of the Kyn pathway metabolites (Kyna, Xan, N-formylKyn, melatonin) and other features of note included aspartic acid, glutamine, pyroglutamic acid, glutamic acid, and adenine were identified in the top 15 features of importance as well as by hierarchical clustering (Figs. 8B–D).
Figure 8.
Principal component analysis scores plot (A), variable importance in projections (VIP) plots (B and C), and hierarchical clustering heatmap (D) from MetaboAnalyst. Abbreviations: CF, control female; CM, control male; HF, high-dose female; HM, high-dose male.
Impact of Prenatal OPFR Exposure on Development of 5-HTergic Projections
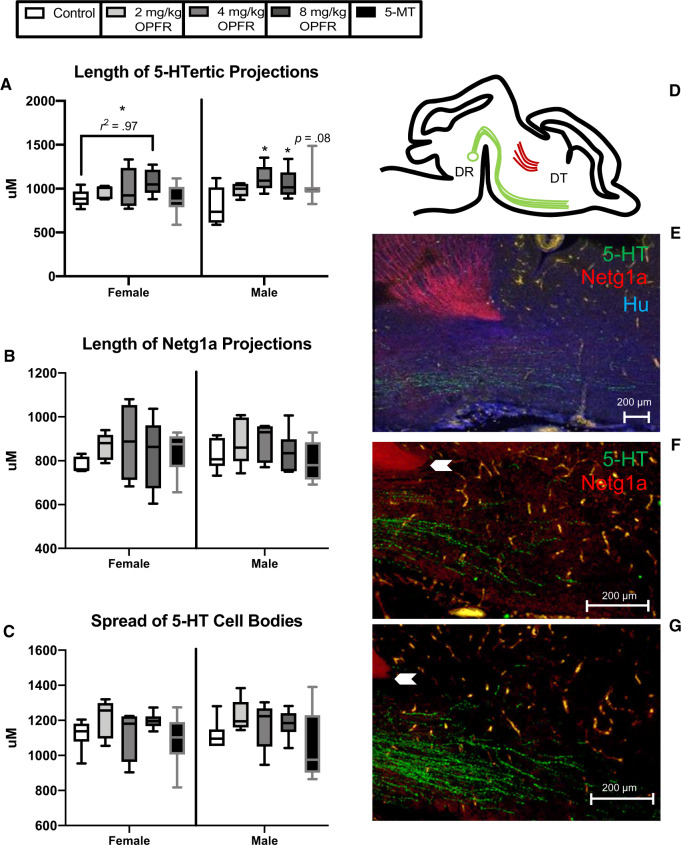
Length of 5-HTergic projections, a cluster of Netg1a-immunoreactive neurons, and the spread of 5-HTergic cell bodies was assessed in GD 14 fetal brains. No significant sex differences were detected for any endpoint when female and male controls were compared. OPFR exposure did not significantly alter the length of Netg1a projections or the spread of 5-HTergic cell bodies. In female brains, 5-HTergic projections were not significantly impacted by OPFR exposure, however a significant linear trend was detected (F(1, 17) = 4.5, p ≤ .05, R2 = .97; Figure 9A). Length of 5-HTergic projections was significantly altered by OPFR exposure in male offspring (F(3, 19) = 3.6, p ≤ .03), with both the 4 mg/kg OPFR and 8 mg/kg OPFR dose groups showing a significant increase in length of projections (p ≤ .007; p ≤ .02; Figure 9A). A suggestive increase in length of 5-HTergic projections was observed in male brains exposed to 5-MT (p = .08; Figure 9A).
Figure 9.
Effects of gestational organophosphate flame retardant (OPFR) exposure on the extension of serotonin (5-HT) and thalamocortical immunolabeled axons in the GD 14 brain. A, Length of 5-HTergic projections was greater in the forebrains of males exposed to 4 mg/kg and 8 mg/kg OPFR, whereas only a suggestive increase was observed in the 5-MT group. A significant linear trend was observed for 5-HT fiber length in female offspring. (B) No significant effect of exposure or sex was found for Netg1a length or (C) the spread of 5-HT cell bodies across the emerging dorsal raphe. (D) Representative diagram and (E) image of thalamocortical axons and 5-HT immunolabeled fibers with the neuronal marker Hu, which was used to confirm all 5-HT-labeled cells were neuronal, and identify key landmarks. (F and G) The point at which length measurements were obtained is indicated by the white arrow with length visibly less in the male controls (F) than the 8 mg/kg OPFR males (G). Graphs depict median with whiskers from minimum to maximum (*p ≤ .05). Abbreviations: DR, dorsal raphe; DT, dorsal thalamus.
DISCUSSION
This study supports and expands upon our previous findings that gestational exposure to FM 550 alters placental function, by identifying Trp metabolism and the neurotransmitter 5-HT as particularly vulnerable to OPFRs. Placental OPFR levels were not sex-specific; an outcome that differs from our prior study that identified sex specific TPHP placental accumulation following gestational exposure to FM 550 (Baldwin et al., 2017). However, when examining total placental burden of OPFR components, male-associated placentas appeared to have been more heavily exposed, which may contribute to the male-biased exposure-related phenotypes observed, such as the length of 5-HTergic projections in fetal brain. Pathway analysis indicated that gestational OPFR exposure may disrupt aspects of placental energy production, cellular development, and protein synthesis; all of which could negatively impact the health and development of the placenta, fetus, and fetal brain. Most significantly, exposure-related increases in placental Trp, 5-HT, 5-HIAA, Kyn, and several other Kyn metabolites were observed in placentas of both sexes. Untargeted metabolomics also indicated possible perturbation of the alanine, aspartate, and glutamate metabolism pathways. The observed increase in neuroactive metabolites in the placenta was associated with a concomitant increase in the length of 5-HTergic projections in the fetal forebrain. This phenotype was more pronounced in male offspring. Collectively, our data suggest that there may be a causal and sex-specific link between OPFR-induced placental disruption and the fetal brain, particularly the development of the 5-HTergic system.
The data consistently show that the synthesis and metabolism of placental 5-HT was altered as a result of OPFR exposure. Neurotransmitter analysis revealed an increase in the relative abundance of Trp (the precursor to 5-HT), 5-HT, and its primary metabolite, 5-HIAA, in placentas exposed to the highest dose (2000 µg OPFR). It is unclear whether these changes in 5-HT are indicative of changes in maternal blood levels or placentally derived 5-HT. The 5-HT transporter, Slc6a4, is highly expressed in both human and rodent placentas (Viau et al., 2009) and has been localized to the villous trophoblasts where it is thought to regulate the amount of 5-HT taken up from maternal circulation (Viau et al., 2009). Alternatively, it has recently been shown that the fetal side of the placenta may be the sole source of 5-HT for the developing forebrain up until approximately GD 15 (Bonnin et al., 2011; Bonnin and Levitt, 2011). Regardless, OPFR-induced changes in placental Trp metabolism and 5-HT synthesis during the mid-gestational window could result in altered neurodevelopment.
Expression of Slc6a4 was significantly decreased in exposed female- and male-associated placentas, suggesting OPFR exposure could affect the storage and transfer of 5-HT to the fetus. To better assess placental 5-HT output, the expression of enzymes involved in 5-HT synthesis and metabolism, including Tph1, Ddc, and Maoa was also examined. None were observed to be altered in male-associated placentas, but expression of Ddc was significantly reduced in female-associated placentas. Ddc is responsible for converting the intermediate, 5-hydroxytryptophan, to 5-HT. Decreased expression may be the placenta’s response to high levels of 5-HT. These phenotypes were not recapitulated with the 5-HT agonist, 5-MT, however male-associated placentas exposed to 5-MT had decreased Tph1 expression. Importantly, although we did not see major effects of OPFR exposure on the expression of these enzymes, a change in enzyme activity cannot be ruled out, and may be more biologically meaningful. Follow-up studies focusing specifically on placental 5-HT synthesis and metabolism should include assessment of the activity levels of these enzymes. Furthermore, prenatal stress has been shown to increase the fraction of free Trp in maternal blood, which was associated with increased levels of Trp, 5-HT, and 5-HIAA in the fetal brain (Peters, 1990). Thus, it is possible that the observed increases in placental Trp are of maternal blood origin. Because several of the phenotypes observed in this study, including elevated levels of Trp, appear in models of prenatal stress (Notarangelo and Schwarcz, 2016), follow-up work should also assess effects of OPFR exposure on the maternal hypothalamic-pituitary-adrenal axis.
Other neuroactive metabolites were also increased in the OPFR exposed placentas. Previously, we found that FM 550 increased the expression of tryptophan 2,3-diooxygenase (Tdo2) and indoleamine 2,3-diooxygenase (Ido2), which break down tryptophan; diverting it away from 5-HT synthesis and toward Kyn. In this study Tryptophan Degradation III was identified as an enriched pathway in the RNA-seq data set when analyzed without filters (annotations from all tissue types included). Although, there were no significant changes in Tdo2, Ido2, or Ido1 expression in the OPFR exposed placentas, levels of Kyn and its metabolites Kyna and Xan were significantly elevated. Relative abundance of other Kyn metabolites, including N-formylkynurenine, 3-hydroxy anthranilic acid, and picolinic acid, was also elevated. These metabolites all act differently in the brain, with some being neuroprotective (Kyn and picolinic acid) and others having neurotoxic properties. In particular, exposure to quinolinic acid (QA) can result in glutamate driven excitotoxicity, through activation of N-methyl-D-aspartate receptors, oxidative stress, and neuroinflammation (Lugo-Huitron et al., 2013). The enzyme Kmo has been implicated in enhanced risk of neurotoxicity because it elevates production of QA and its neurotoxic precursor 3-hydroxykynurenine (3-HK) (Parrott et al., 2016). Relative abundance of QA or 3-HK in OPFR exposed placentas was unchanged, and expression of Kmo was only elevated by 5-MT exposure. However, increased levels of Kyn in the placenta could signify overexposure of the fetal brain to Kyn and its neuroactive metabolites. A recent study in mice showed that Kyn is able to cross the placenta to a greater degree than Kyna, and leads to a subsequent increase in 3-HK in the fetal brain (Goeden et al., 2017).
In our previous study, gestational FM 550 exposure significantly decreased fetal forebrain 5-HT turnover, as determined by the ratio of 5-HIAA:5-HT; an effect we concluded to be indicative of reduced 5-HT activity in the fetal brain. 5-HT plays an instrumental role in many aspects of brain development including synaptogenesis, cell proliferation and migration, myelination, and differentiation (Gaspar et al., 2003; Kepser and Homberg, 2015). Development and targeting of 5-HTergic axons occurs during the gestational window in which placentally derived 5-HT is required. Changes in placental output of 5-HT are associated with blunting of 5-HT axonal outgrowth in the mouse fetal forebrain (Goeden et al., 2016). Therefore, 5-HT signaling between the placenta and fetal forebrain may be essential for the establishment of the 5-HTergic system itself. Here we examined the length of 5-HT projections and spread of 5-HT cell bodies, as well as the development of thalamocortical axons, which are known to be 5-HT sensitive. Exposure to the top 2 OPFR doses increased length of male 5-HTergic projections; a phenotype that was at least partly recapitulated by 5-MT. The 5-HTergic system has been implicated in a variety of neurological disorders (Charnay and Leger, 2010), including autism spectrum disorder where hyperserotonemia is suspected to be an endophenotype (Tanaka et al., 2018). Hyperinnervation of the developing forebrain by 5-HTergic projections may be a critical mechanism by which OPFR exposure impacts brain physiology and behavior. We have previously observed changes in 5-HT-dependent behaviors, including anxiety and hyperactivity, in prairie vole and Wistar rat offspring exposed to FM 550 (Baldwin et al., 2017; Gillera et al., 2019). Ongoing work is seeking to establish whether or not this is attributable to the OPFRs.
RNA-seq revealed other possible exposure-related pathway changes. Clear separation of dose groups (control vs high) was observed for female-associated placentas thus canonical pathways were identified in females only using 2 approaches; first with no annotation filters (all tissue types) and second by applying annotations from only placenta and nervous system tissues. There was a significant amount of overlap with 7 of the 15 pathways identified in common. Notably, activation of the retinoic acid receptor (RAR) was highlighted in both of these analyses, which has been shown to form heterodimers with retinoid X receptor (RXR) and regulate the transcription of genes involved in endocrine and metabolic regulation as well as fetal development (Lefebvre et al., 2010; Nakanishi, 2008). We have previously shown that gestational exposure to FM 550 can perturb both the farnesoid X receptor (FXR)/RXR and liver X receptor (LXR)/RXR pathways (Rock et al., 2018). Therefore, RXR and the nuclear receptors it forms complexes with, especially RAR, FXR, and LXR, may be particularly susceptible to OPFRs.
Mitochondrial dysfunction was also identified as an important pathway altered by OPFR exposure in all of the untargeted approaches. This finding is consistent with recent in vivo and in vitro work that highlighted OPFR-induced mitochondrial dysfunction and oxidative stress as potential mechanisms leading to cytotoxicity and endocrine disruption (Chen et al., 2015; Yu et al., 2019). Mitochondria are fundamental to energy production, metabolism, and response to oxidative stress (Holland et al., 2017). Oxidative stress has been identified as a potential risk factor in the pathophysiology of placenta-related disorders, including preeclampsia (PE) and intrauterine growth restriction (IUGR). Over the course of pregnancy, the placenta upregulates antioxidant enzymes for several reasons, including protection of the fetus and the placenta itself. For example, mRNA expression of various antioxidant enzymes, such as Sod2, increase in their expression to protect the placenta from oxidative stress in an increasingly oxygenated environment (Jones et al., 2010; Roland et al., 2010). Within the mitochondrial dysfunction pathway, genes perturbed by OPFR exposure included ATP synthase membrane subunit c locus 1 (Atp5mc1), parkinsonism-associated deglycase (Park7), NADH:ubiquinone oxidoreductase subunit A13 (Ndufa13), and Sod2. Although the RNA-seq data set showed an increase in the expression of Sod2, by 1.5-fold, qRT-PCR actually showed a significant decrease in both female- and male-associated placentas, emphasizing the necessity for replication and assessment of enzyme activity.
Untargeted metabolomics identified the sialic acid N-acetylneuraminic acid as potentially affected, a metabolite that has been linked to inflammatory response and oxidative stress (Yida et al., 2015). Similarly, upregulation of aromatic amino acids (Phe and Tyr) and related metabolites (2-hydroxcinnamic acid) was also observed. Both nucleotide metabolic pathways and amino acid metabolic pathways have previously been shown to be disrupted by exposure to arsenic, cadmium, and bisphenol analogues (Sarma et al., 2018; Wang et al., 2015b; Yue et al., 2019). Overall, these findings suggest that placentas exposed to OPFRs may be susceptible to the effects of oxidative stress which may play a role in functional changes similar to phenotypes observed with PE and IUGR.
Exposure to high levels of glucocorticoids can elevate production of reactive oxygen species leading to oxidative stress (Bjelakovic et al., 2007). In both sexes, but particularly males, corticosterone was elevated in the 2000 µg OPFR groups, whereas expression of the enzyme responsible for inactivating corticosterone, Hsd11b2, was significantly reduced. Upregulation of Hsd11b2 in the placenta is believed to be an adaptive response to protect the fetus against increased levels of maternal glucocorticoids (Benediktsson et al., 1997), and decreased expression has been observed in several models of prenatal stress (Jensen Pena et al., 2012; Mairesse et al., 2007; O’Donnell et al., 2012). The programmatic effects of prenatal stress on neurodevelopment likely stem from increased exposure to maternal glucocorticoids in utero. Therefore, overexposure to corticosterone may be a potential mechanism by which OPFR exposure alters aspects of brain development and behavior and warrants further investigation.
Other mechanisms relevant to OPFR exposure and neurodevelopmental outcomes include disruption of thyroid hormone (TH) and acetylcholine (ACh) signaling. TH plays a vital role in fetal development, particularly brain development, and disruption of maternal and placental TH regulation can result in adverse pregnancy outcomes including severe neural and cognitive deficiencies in the offspring (Chan et al., 2009). Exposure to FRs, including OPFRs, can disrupt many aspects of TH function including transport, synthesis, and metabolism (Kim et al., 2015; Liu et al., 2013a; Patisaul et al., 2013; Rock et al., 2018; Ruis et al., 2019). Activity of the enzyme AChE, which regulates availability of ACh, has been used as a biomarker of neurotoxicant exposure (Souza et al., 2005) and appears to be inhibited by OPFR exposure, including TPHP (Shi et al., 2018). Disruption of AChE activity in both brain and placental tissue may have significant implications for fetal brain development (Lips et al., 2005). Although not addressed in this study, these mechanisms should be considered in future investigations.
CONCLUSIONS
The placenta plays a critical role in fetal health and development, including neurodevelopment. Multiple lines of evidence have shown that placental dysfunction, as a result of prenatal stress or infection, can lead to significant changes in brain development and behavior (Bronson and Bale, 2016; Goeden et al., 2016). However, very little is known about how exposure to environmental contaminants may alter aspects of placental function important for brain development. The data reported herein indicate that altered placental function, may be a route by which OPFRs can disrupt brain development and alter later-in-life behaviors. More specifically, disruption of neuroactive metabolites, including the NT 5-HT, was found in conjunction with overgrowth of 5-HTergic projections in the fetal forebrain. Although there is some overlap between this study and changes in placental function as a result of FM 550 exposure, including increased levels of placental 5-HT, there are some notable differences. For example, with FM 550 exposure we saw numerous changes in gene expression related to endocrine and inflammatory signaling that were not observed in this study. This provides some preliminary mechanistic evidence for each FR class, with the BFRs contributing to endocrine disruption and inflammation and the OPFRs perturbing neurotransmitter signaling in the placenta. Although a sex difference in the placental accumulation of individual OPFRs was not observed, the sum OPFR concentration trended higher in males. This study used real-world complex mixtures, but independent examination of individual components could further mechanistic understanding. Greater resolution on changes in 5-HT signaling between the placenta and fetal brain would also be beneficial and may be obtainable in future studies by measuring levels of Trp and 5-HT in the placenta as well as maternal and fetal blood. Our results provide novel evidence that gestational exposure to OPFRs can impact physiological endpoints in the rat placenta and development of the 5-HTergic system in the fetal forebrain.
ACKNOWLEDGMENTS
We would like to thank Dr David Baltzegar, and the rest of the NCSU GSL staff for their assistance with cDNA library preparation and sequencing. We also appreciate the support of the Biological Resources Facility at NCSU for all of the animal care and husbandry.
FUNDING
National Institute of Environmental Health Sciences (F31ES02900-01 to K.D.R., R56ES022957 to H.B.P., T32ES007046 and P30ES025128 to NCSU, R01ES028110 to H.B.P., and R01ES016099 to H.M.S).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Abdallah M. A., Covaci A. (2014). Organophosphate flame retardants in indoor dust from Egypt: Implications for human exposure. Environ. Sci. Technol. 48, 4782–4789. [DOI] [PubMed] [Google Scholar]
- Aldridge J. E., Levin E. D., Seidler F. J., Slotkin T. A. (2005). Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 113, 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P. T., Huber W. (2015). HTSeq—A python framework to work with high-throughput sequencing data. Bioinformatics (Oxford) 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Levin E. D. (2015). Neurotoxicity of Firemaster 550® in zebrafish (Danio rerio): Chronic developmental and acute adolescent exposures. Neurotoxicol. Teratol. 52, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin K. R., Phillips A. L., Horman B., Arambula S. E., Rebuli M. E., Stapleton H. M., Patisaul H. B. (2017). Sex specific placental accumulation and behavioral effects of developmental Firemaster® 550 exposure in Wistar rats. Sci. Rep. 7, 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T. L. (2016). The placenta and neurodevelopment: Sex differences in prenatal vulnerability. Dialogues Clin. Neurosci. 18, 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M., Hsieh J.-H., Shafer T. J., Mundy W. R., Rice J. R., Boyd W. A., Freedman J. H., Hunter E. S., Jarema K. A., Padilla S., et al. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52, 181–193. [DOI] [PubMed] [Google Scholar]
- Belcher S. M., Cookman C. J., Patisaul H. B., Stapleton H. M. (2014). In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture fm 550 and its triarylphosphate and brominated components. Toxicol. Lett. 228, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson R., Calder A. A., Edwards C. R., Seckl J. R. (1997). Placental 11 beta-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. (Oxf.) 46, 161–166. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300. [Google Scholar]
- Bennett D., Bellinger D. C., Birnbaum L. S., Bradman A., Chen A., Cory-Slechta D. A., Engel S. M., Fallin M. D., Halladay A., Hauser R., et al. (2016). Project TENDR: Targeting environmental neuro-developmental risks the TENDR consensus statement. Environ. Health Perspect. 124, A118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G., Beninati S., Pavlovic D., Kocic G., Jevtovic T., Kamenov B., Saranac L. J., Bjelakovic B., Stojanovic I., Basic J. (2007). Glucocorticoids and oxidative stress. J. Basic Clin. Physiol. Pharmacol. 18, 115–127. [DOI] [PubMed] [Google Scholar]
- Bjorling-Poulsen M., Andersen H. R., Grandjean P. (2008). Potential developmental neurotoxicity of pesticides used in Europe. Environ. Health 7, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A., Goeden N., Chen K., Wilson M. L., King J., Shih J. C., Blakely R. D., Deneris E. S., Levitt P. (2011). A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A., Levitt P. (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommer S., Harrad S., Van den Eede N., Covaci A. (2012). Concentrations of organophosphate esters and brominated flame retardants in German indoor dust samples. J. Environ. Monit. 14, 2482–2487. [DOI] [PubMed] [Google Scholar]
- Bronson S. L., Bale T. L. (2014). Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson S. L., Bale T. L. (2016). The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt C. M., Congleton J., Hoffman K., Fang M., Stapleton H. M. (2014). Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 48, 10432–10438. [DOI] [PubMed] [Google Scholar]
- Butt C. M., Miranda M. L., Stapleton H. M. (2016). Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2,4,6-TBP, EH-TBB, and BEH-TEBP in human serum. Anal. Bioanal. Chem. 408, 2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R. L., Graves C. A., Mangum L. C., Nail C. A., Ross M. K. (2014). Low level chlorpyrifos exposure increases anandamide accumulation in juvenile rat brain in the absence of brain cholinesterase inhibition. Neurotoxicology 43, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E., Sakhi A. K., Marce R. M., Becher G., Thomsen C. (2015). Human exposure pathways to organophosphate triesters—A biomonitoring study of mother-child pairs. Environ. Int. 75, 159–165. [DOI] [PubMed] [Google Scholar]
- Chan S. Y., Vasilopoulou E., Kilby M. D. (2009). The role of the placenta in thyroid hormone delivery to the fetus. Nat. Clin. Pract. Endocrinol. Metab. 5, 45–54. [DOI] [PubMed] [Google Scholar]
- Charil A., Laplante D. P., Vaillancourt C., King S. (2010). Prenatal stress and brain development. Brain Res. Rev. 65, 56–79. [DOI] [PubMed] [Google Scholar]
- Charnay Y., Leger L. (2010). Brain serotonergic circuitries. Dialogues Clin. Neurosci. 12, 471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A., Yolton K., Rauch S. A., Webster G. M., Hornung R., Sjodin A., Dietrich K. N., Lanphear B. P. (2014). Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: The home study. Environ. Health Perspect. 122, 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Jin Y., Wu Y., Liu L., Fu Z. (2015). Exposure of male mice to two kinds of organophosphate flame retardants (OPFRs) induced oxidative stress and endocrine disruption. Environ. Toxicol. Pharmacol. 40, 310–318. [DOI] [PubMed] [Google Scholar]
- Coelho S. D., Sousa A. C. A., Isobe T., Kim J. W., Kunisue T., Nogueira A. J. A., Tanabe S. (2016). Brominated, chlorinated and phosphate organic contaminants in house dust from Portugal. Sci. Total Environ. 569–570, 442–449. [DOI] [PubMed] [Google Scholar]
- Cooper E. M., Covaci A., van Nuijs A. L., Webster T. F., Stapleton H. M. (2011). Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 401, 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman B. E., Patterson P. H. (2009). Cytokines and CNS development. Neuron 64, 61–78. [DOI] [PubMed] [Google Scholar]
- Dishaw L. V., Powers C. M., Ryde I. T., Roberts S. C., Seidler F. J., Slotkin T. A., Stapleton H. M. (2011). Is the pentabde replacement, tris(1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 256, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. E., Van den Eede N., Covaci A., Perovich L. J., Brody J. G., Rudel R. A. (2014). Urinary biomonitoring of phosphate flame retardants: Levels in California adults and recommendations for future studies. Environ. Sci. Technol. 48, 13625–13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P., Cases O., Maroteaux L. (2003). The developmental role of serotonin: News from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Gillera S. E. A., Marinello W. P., Horman B. M., Phillips A. L., Ruis M. T., Stapleton H. M., Reif D. M., Patisaul H. B. (2019). Sex-specific effects of perinatal Firemaster® 550 (FM 550) exposure on socioemotional behavior in prairie voles. Neurotoxicol. Teratol. 106840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeden N., Notarangelo F. M., Pocivavsek A., Beggiato S., Bonnin A., Schwarcz R. (2017). Prenatal dynamics of kynurenine pathway metabolism in mice: Focus on kynurenic acid. Dev. Neurosci. 39, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeden N., Velasquez J., Arnold K. A., Chan Y., Lund B. T., Anderson G. M., Bonnin A. (2016). Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. J. Neurosci. 36, 6041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel S. C., Hoffman K., Webster T. F., Anderson K. A., Stapleton H. M. (2016). Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ. Sci. Technol. 50, 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Daniels J. L., Stapleton H. M. (2014). Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ. Int. 63, 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Webster T. F., Sjodin A., Stapleton H. M. (2017). Toddler’s behavior and its impacts on exposure to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 27, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland O. J., Hickey A. J. R., Alvsaker A., Moran S., Hedges C., Chamley L. W., Perkins A. V. (2017). Changes in mitochondrial respiration in the human placenta over gestation. Placenta 57, 102–112. [DOI] [PubMed] [Google Scholar]
- Hou R., Xu Y., Wang Z. (2016). Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 153, 78–90. [DOI] [PubMed] [Google Scholar]
- Hunt P. A., Susiarjo M., Rubio C., Hassold T. J. (2009). The bisphenol a experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 81, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarema K. A., Hunter D. L., Shaffer R. M., Behl M., Padilla S. (2015). Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 52, 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen Pena C., Monk C., Champagne F. A. (2012). Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One 7, e39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. L., Mark P. J., Lewis J. L., Mori T. A., Keelan J. A., Waddell B. J. (2010). Antioxidant defenses in the rat placenta in late gestation: Increased labyrinthine expression of superoxide dismutases, glutathione peroxidase 3, and uncoupling protein 2. Biol. Reprod. 83, 254–260. [DOI] [PubMed] [Google Scholar]
- Kepser L. J., Homberg J. R. (2015). The neurodevelopmental effects of serotonin: A behavioural perspective. Behav. Brain Res. 277, 3–13. [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W. J., Cuthill I. C., Emerson M., Altman D. G. (2010). Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 8, e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jung J., Lee I., Jung D., Youn H., Choi K. (2015). Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat. Toxicol. 160, 188–196. [DOI] [PubMed] [Google Scholar]
- Kim U. J., Wang Y., Li W., Kannan K. (2019). Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ. Int. 125, 342–349. [DOI] [PubMed] [Google Scholar]
- Kojima H., Takeuchi S., Itoh T., Iida M., Kobayashi S., Yoshida T. (2013). In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314, 76–83. [DOI] [PubMed] [Google Scholar]
- Konkel L. (2016). Lasting impact of an ephemeral organ: The role of the placenta in fetal programming. Environ. Health Perspect. 124, A124129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P., Benomar Y., Staels B. (2010). Retinoid X receptors: Common heterodimerization partners with distinct functions. Trends Endocrinol. Metab. 21, 676–683. [DOI] [PubMed] [Google Scholar]
- Li A. A., Baum M. J., McIntosh L. J., Day M., Liu F., Gray L. E. Jr (2008). Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology 29, 504–519. [DOI] [PubMed] [Google Scholar]
- Lips K. S., Bruggmann D., Pfeil U., Vollerthun R., Grando S. A., Kummer W. (2005). Nicotinic acetylcholine receptors in rat and human placenta. Placenta 26, 735–746. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang Q., Liang K., Liu J., Zhou B., Zhang X., Liu H., Giesy J. P., Yu H. (2013. a). Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat. Toxicol. 128–129, 147–157. [DOI] [PubMed] [Google Scholar]
- Liu X., Ji K., Choi K. (2012). Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 114–115, 173–181. [DOI] [PubMed] [Google Scholar]
- Liu X., Ji K., Jo A., Moon H. B., Choi K. (2013. b). Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat. Toxicol. 134–135, 104–111. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lugo-Huitron R., Ugalde Muniz P., Pineda B., Pedraza C. J., Rios C., Perez-de la Cruz V. (2013). Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairesse J., Lesage J., Breton C., Breant B., Hahn T., Darnaudery M., Dickson S. L., Seckl J., Blondeau B., Vieau D., et al. (2007). Maternal stress alters endocrine function of the feto-placental unit in rats. Am. J. Physiol. Endocrinol. Metab. 292, E1526–1533. [DOI] [PubMed] [Google Scholar]
- Martin M. M., Liu Y., Wang Z. (2012). Developmental exposure to a serotonin agonist produces subsequent behavioral and neurochemical changes in the adult male prairie vole. Physiol. Behav. 105, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Cooper E. M., Stapleton H. M., Hauser R. (2013). Urinary metabolites of organophosphate flame retardants: Temporal variability and correlations with house dust concentrations. Environ. Health Perspect. 121, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn E., Hagopian A., Hoffman K., Butt C. M., Lorenzo A., Congleton J., Webster T. F., Stapleton H. M. (2016). Nail polish as a source of exposure to triphenyl phosphate. Environ. Int. 86, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer A. (2010). Mini-review: Polybrominated diphenyl ether (PBDE) flame retardants as potential autism risk factors. Physiol. Behav. 100, 245–249. [DOI] [PubMed] [Google Scholar]
- Nakanishi T. (2008). Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. J. Toxicol. Sci. 33, 269–276. [DOI] [PubMed] [Google Scholar]
- Notarangelo F. M., Schwarcz R. (2016). Restraint stress during pregnancy rapidly raises kynurenic acid levels in mouse placenta and fetal brain. Dev. Neurosci. 38, 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent B. M., Bale T. L. (2015). The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front. Neuroendocrinol. 39, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K. J., Bugge Jensen A., Freeman L., Khalife N., O’Connor T. G., Glover V. (2012). Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology 37, 818–826. [DOI] [PubMed] [Google Scholar]
- Parrott J. M., Redus L., Santana-Coelho D., Morales J., Gao X., O'Connor J. C. (2016). Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 6, e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B., Fortino A. E., Polston E. K. (2008). Sex differences in serotonergic but not {gamma}-aminobutyric acidergic (GABA) projections to the rat ventromedial nucleus of the hypothalamus. Endocrinology 149, 397–408. [DOI] [PubMed] [Google Scholar]
- Patisaul H. B., Roberts S. C., Mabrey N., McCaffrey K. A., Gear R. B., Braun J., Belcher S. M., Stapleton H. M. (2013). Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: An exploratory assessment. J. Biochem. Mol. Toxicol. 27, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B., Sullivan A. W., Radford M. E., Walker D. M., Adewale H. B., Winnik B., Coughlin J. L., Buckley B., Gore A. C. (2012). Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One 7, e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H. B., Todd K. L., Mickens J. A., Adewale H. B. (2009). Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 30, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Ashwell K. (2018). Atlas of the Developing Rat Nervous System. 4th ed. Academic Press, San Diego.
- Peters D. A. (1990). Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: A possible mechanism by which stress influences brain development. Pharmacol. Biochem. Behav. 35, 943–947. [DOI] [PubMed] [Google Scholar]
- Phillips A. L., Hammel S. C., Hoffman K., Lorenzo A. M., Chen A., Webster T. F., Stapleton H. M. (2018). Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ. Int. 116, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. L., Hammel S. C., Konstantinov A., Stapleton H. M. (2017). Characterization of individual isopropylated and tert-butylated triarylphosphate (ITP and TBPP) isomers in several commercial flame retardant mixtures and house dust standard reference material SRM 2585. Environ. Sci. Technol. 51, 13443–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti A., Celotti F., Rumio C., Rabuffetti M., Martini L. (1997). Identification of type 1 5alpha-reductase in myelin membranes of male and female rat brain. Mol. Cell. Endocrinol. 129, 181–190. [DOI] [PubMed] [Google Scholar]
- Polston E. K., Gu G., Simerly R. B. (2004). Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience 123, 793–803. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M., Pietrzak M., Turowska O., Nauman A. (2006). Thyroid hormones and their receptors in the regulation of cell proliferation. Acta Biochim. Pol. 53, 641–650. [PubMed] [Google Scholar]
- Quevedo C., Behl M., Ryan K., Paules R. S., Alday A., Muriana A., Alzualde A. (2019). Detection and prioritization of developmentally neurotoxic and/or neurotoxic compounds using zebrafish. Toxicol. Sci. 168, 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Foundation for Statistical Computing. (2015). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: Available at: http://www.R-project.org/. [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. (2007). In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. D., Horman B., Phillips A. L., McRitchie S. L., Watson S., Deese-Spruill J., Jima D., Sumner S., Stapleton H. M., Patisaul H. B. (2018). EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain. Endocr. Connect. 7, 305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland L., Beauchemin D., Acteau G., Fradette C., St-Pierre I., Bilodeau J. F. (2010). Effects of labor on placental expression of superoxide dismutases in preeclampsia. Placenta 31, 392–400. [DOI] [PubMed] [Google Scholar]
- Ruis M. T., Rock K. D., Hall S. M., Horman B., Patisaul H. B., Stapleton H. M. (2019). PBDEs concentrate in the fetal portion of the placenta: Implications for thyroid hormone dysregulation. Endocrinology 160, 2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization & International Programme on Chemical Safety. (1991). Triphenyl Phosphate/Published Under the Joint Sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. World Health Organization, Geneva.
- Sarma S. N., Saleem A., Lee J. Y., Tokumoto M., Hwang G. W., Man Chan H., Satoh M. (2018). Effects of long-term cadmium exposure on urinary metabolite profiles in mice. J. Toxicol. Sci. 43, 89–100. [DOI] [PubMed] [Google Scholar]
- Shemer A. V., Azmitia E. C., Whitaker-Azmitia P. M. (1991). Dose-related effects of prenatal 5-methoxytryptamine (5-MT) on development of serotonin terminal density and behavior. Brain Res. Dev. Brain Res. 59, 59–63. [DOI] [PubMed] [Google Scholar]
- Shemer A., Whitaker-Azmitia P. M., Azmitia E. C. (1988). Effects of prenatal 5-methoxytryptamine and parachlorophenylalanine on serotonergic uptake and behavior in the neonatal rat. Pharmacol. Biochem. Behav. 30, 847–851. [DOI] [PubMed] [Google Scholar]
- Shi Q., Wang M., Shi F., Yang L., Guo Y., Feng C., Liu J., Zhou B. (2018). Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Aquat. Toxicol. 203, 80–87. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Seidler F. J. (2005). The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Brain Res. Dev. Brain Res. 158, 115–119. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Seidler F. J. (2007). Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: Critical periods for regional and sex-selective effects. Reprod. Toxicol. 23, 421–427. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Seidler F. J. (2008). Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: Chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol. Appl. Pharmacol. 233, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M. S., Magnarelli G. G., Rovedatti M. G., Cruz S. S., De D. A. (2005). Prenatal exposure to pesticides: Analysis of human placental acetylcholinesterase, glutathione S-transferase and catalase as biomarkers of effect. Biomarkers 10, 376–389. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Allen J. G., Kelly S. M., Konstantinov A., Klosterhaus S., Watkins D., McClean M. D., Webster T. F. (2008). Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 42, 6910–6916. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Klosterhaus S., Eagle S., Fuh J., Meeker J. D., Blum A., Webster T. F. (2009). Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Klosterhaus S., Keller A., Ferguson P. L., van Bergen S., Cooper E., Webster T. F., Blum A. (2011). Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 45, 5323–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Sharma S., Getzinger G., Ferguson P. L., Gabriel M., Webster T. F., Blum A. (2012). Novel and high volume use flame retardants in US couches reflective of the 2005 pentabde phase out. Environ. Sci. Technol. 46, 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S., Araki A., Kawai T., Tsuboi T., Ait Bamai Y., Yoshioka E., Kanazawa A., Cong S., Kishi R. (2014). Detection and intake assessment of organophosphate flame retardants in house dust in Japanese dwellings. Sci. Total Environ. 478, 190–199. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Sato A., Kasai S., Hagino Y., Kotajima-Murakami H., Kashii H., Takamatsu Y., Nishito Y., Inagaki M., Mizuguchi M., et al. (2018). Brain hyperserotonemia causes autism-relevant social deficits in mice. Mol. Autism 9, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O. A., Roegge C. S., Seidler F. J., Slotkin T. A., Levin E. D. (2008. a). Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol. Teratol. 30, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O. A., Sanders D., Seemann K., Yang L., Hermanson D., Regenbogen S., Agoos S., Kallepalli A., Rastogi A., Braddy D., et al. (2008. b). Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 77, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede N., Heffernan A. L., Aylward L. L., Hobson P., Neels H., Mueller J. F., Covaci A. (2015). Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int. 74, 1–8. [DOI] [PubMed] [Google Scholar]
- Van den Eede N., Maho W., Erratico C., Neels H., Covaci A. (2013. a). First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 223, 9–15. [DOI] [PubMed] [Google Scholar]
- Van den Eede N., Neels H., Jorens P. G., Covaci A. (2013. b). Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 1303, 48–53. [DOI] [PubMed] [Google Scholar]
- van der Veen I., de Boer J. (2012). Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. [DOI] [PubMed] [Google Scholar]
- Viau M., Lafond J., Vaillancourt C. (2009). Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies. Reprod. Biomed. Online 19, 207–215. [DOI] [PubMed] [Google Scholar]
- Wang R., Tang J., Xie Z., Mi W., Chen Y., Wolschke H., Tian C., Pan X., Luo Y., Ebinghaus R. (2015. a). Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, north China. Environ. Pollut. 198, 172–178. [DOI] [PubMed] [Google Scholar]
- Wang X., Mu X., Zhang J., Huang Q., Alamdar A., Tian M., Liu L., Shen H. (2015. b). Serum metabolomics reveals that arsenic exposure disrupted lipid and amino acid metabolism in rats: A step forward in understanding chronic arsenic toxicity. Metallomics 7, 544–552. [DOI] [PubMed] [Google Scholar]
- Wei G. L., Li D. Q., Zhuo M. N., Liao Y. S., Xie Z. Y., Guo T. L., Li J. J., Zhang S. Y., Liang Z. Q. (2015). Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 196, 29–46. [DOI] [PubMed] [Google Scholar]
- Wise L. M., Sadowski R. N., Kim T., Willing J., Juraska J. M. (2016). Long-term effects of adolescent exposure to bisphenol A on neuron and glia number in the rat prefrontal cortex: Differences between the sexes and cell type. Neurotoxicology 53, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yida Z., Imam M. U., Ismail M., Ismail N., Ideris A., Abdullah M. A. (2015). High fat diet-induced inflammation and oxidative stress are attenuated by N-acetylneuraminic acid in rats. J. Biomed. Sci. 22, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Yin H., Peng H., Lu G., Liu Z., Dang Z. (2019). OPFRs and BFRs induced A549 cell apoptosis by caspase-dependent mitochondrial pathway. Chemosphere 221, 693–702. [DOI] [PubMed] [Google Scholar]
- Yue S., Yu J., Kong Y., Chen H., Mao M., Ji C., Shao S., Zhu J., Gu J., Zhao M. (2019). Metabolomic modulations of HepG2 cells exposed to bisphenol analogues. Environ. Int. 129, 59–67. [DOI] [PubMed] [Google Scholar]