Abstract
Zibotentan, an Endothelin-A receptor antagonist, has been used in the treatment of various cardiovascular disorders and neoplasia. Castrated athymic nude mice receiving zibotentan for a preclinical xenograft efficacy study experienced weight loss, gastrointestinal bloat, and the presence of an audible respiratory click. Human side effects have been reported in the nasal cavity, so we hypothesized that the nasal cavity is a target for toxicity in mice receiving zibotentan. Lesions in the nasal cavity predominantly targeted olfactory epithelium in treated mice, and were more pronounced in castrated animals. Minimal lesions were present in vehicle control animals, which suggested possible gavage-related reflux injury. The incidence, distribution, and morphology of lesions suggested direct exposure to the nasal mucosa and a possible systemic effect targeting the olfactory epithelium, driven by a type 2 immune response, with group 2 innate lymphoid cell (ILC2) involvement. Severe nasal lesions may have resulted in recurrent upper airway obstruction, leading to aerophagia and associated clinical morbidity. These data show the nasal cavity is a target of zibotentan when given by gavage in athymic nude mice, and such unanticipated and off-target effects could impact interpretation of research results and animal health in preclinical studies.
INTRODUCTION
Endothelin-1 (ET-1) is a ubiquitous protein synthesized by vascular endothelial cells that acts as a powerful vasoconstrictor to help maintain vascular tone (Yanagisawa et al., 1988), and has significant mitogenic properties on smooth muscle cells (Fujitani et al., 1995, Sugawara et al., 1996, Donckier et al., 2003). As an important regulator of vascular tone, cell proliferation, fibrosis, and inflammation, it has been implicated in the development of various diseases including congestive heart failure, systemic and pulmonary hypertension, renal failure, cancer, cerebrovascular incidents (stroke), and asthma (Mullol et al., 1996, Motte et al., 2006). ET-1 also serves a neuroprotective role, acting as an anti-apoptotic factor for sensory neurons in the olfactory epithelium of the nasal cavity, as well as other neural tissues such as the olfactory bulb (Laziz et al., 2011). ET-1 acts via two distinct G protein-coupled receptors, Endothelin-A (ETA) and Endothelin-B (ETB) (Davenport, 2002). ETA receptor expression on vascular smooth muscle cells, fibroblasts, and cardiomyocytes primarily mediate the vasoconstrictive and proliferative effects of ET-1 while the ETB receptors on vascular smooth muscle and endothelial cells induce endothelial-dependent vasodilatation and clearance of ET-1 from the circulation (Clozel et al., 2013). Endothelin receptors are present in numerous tissues including the lung, heart, and kidney, localized mostly on the smooth muscle of blood vessels, but are also present in nonvascular structures, such as epithelial cells, glia, neurons, and olfactory mucosa (Moller et al., 1999, Davenport, 2002, Motte et al., 2006, Gouadon et al., 2010).
Multiple endothelin receptor antagonists have been evaluated as therapies for a variety of human disorders, including systemic and pulmonary hypertension, congestive heart failure, atherosclerosis, renal failure, and metastatic cancer. ETA receptor antagonists bind selectively to the ETA receptor, inhibiting the vasoconstriction and neuroprotective mechanisms. Currently, FDA approval has been granted for the ETA receptor antagonists ambrisentan, bosentan, and macitentan for the treatment of pulmonary hypertension. Another ETA receptor antagonist, zibotentan, has been recently investigated in human clinical trials for treatment of androgen resistant prostate cancer, metastatic breast cancer, and ovarian cancer, among other cancer types (USNLM, ClinicalTrials.gov). Side effects of zibotentan treatment from early clinical trials largely involved the nasal passage including symptoms such as nasal congestion (James et al., 2010, Miller et al., 2013), sinusitis, dyspnea, upper respiratory tract infection, as well as headache and nausea (Motte et al., 2006). However, data on the specific histologic alterations present in the nasal cavity of humans experiencing these side-effects during dosing is not available. In a preclinical trial assessing the efficacy of zibotentan on prostatic adenocarcinoma in an athymic nude mouse xenograft model, some castrated animals receiving the drug were reported to veterinary staff for marked weight loss and gastrointestinal bloating. Since mice are obligate nasal breathers, upper respiratory lesions can impact normal respiration, leading to aerophagia. Since zibotentan has been shown to cause nasal side effects in humans, and endothelin receptors are expressed in the olfactory mucosa of rats (Gouadon et al., 2010), we hypothesized that the nasal cavity is a target for zibotentan in athymic nude mice receiving the drug. Severe effects in the nasal cavity would support recurrent upper respiratory obstruction as a cause of gastrointestinal bloat and the morbidity observed clinically. To test this hypothesis, the aim of this study was to evaluate the nasal cavity of mice in the preclinical trail for histologic lesions indicative of off-target test article related toxicity.
METHODS
Animals
Mice used for the current pathology study were obtained by the In Vivo Animal Core at the University of Michigan following completion of the in vivo study. Animals included two cohorts of 37 male athymic nude mice [Hsd:Athymic Nude-Foxn1nu] either castrated (n = 19) or having had undergone a sham castration surgery (n = 18). Athymic nude mice were chosen as an immunocompromised mouse model for the original xenograft experiment, in which animals were xenografted with a prostatic adenocarcinoma cell line, then received either 10mg/kg/day zibotentan (ZD4054, AstraZeneca), or vehicle (1% Polysorbate-80) only, administered once daily via oral gavage for 150 days. Doses were volume controlled so each mouse received 100uL of either drug or vehicle via oral gavage, and animals were weighed and weight data collected for analysis 2–3 times weekly through week 19 (132 days). All in vivo experiments were reviewed and approved by the Ann Arbor Veterans Affairs Healthcare System IACUC. Mice were housed four per cage in an AAALAC accredited animal facility. Animals had continued free access to water and food (Forumulab Diet 5008, Purina LabDiet®, St. Louis, MO). Environmental enrichment included BioServ® plastic shelters of various sizes and configurations, and autoclaved empty toilet paper or paper towel tubes. At study termination (week 21) carcasses were received for the current pathology study in 10% neutral buffered formalin from the four experimental groups: castrated zibotentan-treated (Group A, n=10), intact zibotentan-treated (Group B, n=8), castrated vehicle control (Group C, n=9), and intact vehicle control (Group D, n=10) mice.
Histology and Immunohistochemistry
Following fixation for at least 24hrs, the calvarium and associated nasal tissues were decalcified in Immunocal (Fisher Scientific, Waltham MA, cat#NC9044643), then the nasal cavity was trimmed and sectioned based on the standardized Registry of Industrial Toxicology Animal-data (RITA) guidelines to obtain four comprehensive sections focusing on various components of the nasal cavity (Young, 1981, Ruehl-Fehlert et al., 2003, Harkema et al., 2006). Nasal sections were embedded in paraffin, sectioned at a thickness of 4μm, stained with H&E for histopathological evaluation, and examined via light microscopy. A semi-quantitative histological severity grading scale was developed to characterize the severity of each of the nasal cavity lesions identified: 0 – no lesion observed; 1 – minimal; 2 – mild; 3 – moderate; 4 – severe Supplementary Table 2). Since many lesions differ in character, severity grades were established for each reported lesion independently. Each lesion was assigned an individual severity score, and an average severity score was given across all animals within each of the four experimental groups (Table 1).
Table 1.
Lesion incidences within groups and severity scores for histopathologic lesions in zibotentan-treated and vehicle control animals.
| Intact, Vehicle | Castrated, Vehicle | Intact, Treated | Castrated, Treated | |||||
|---|---|---|---|---|---|---|---|---|
| Ave Severity | Group Incidence | Ave Severity | Group Incidence | Ave Severity | Group Incidence | Ave Severity | Group Incidence | |
| Inflammation, chronic-active | 0.6 | 40% | 2.22 | 100% | 2.38 | 100% | 3.7 | 100% |
| NALT hyperplasia | 1 | 70% | 2.33 | 100% | 1.13 | 75% | 2.6 | 100% |
| Olfactory, accumulation, hyaline droplet | 0.5 | 40% | 1.89 | 89% | 2.5 | 100% | 2.9 | 100% |
| Olfactory epithelium, atrophy | 0.7 | 50% | 1.33 | 67% | 2.5 | 100% | 2.6 | 100% |
| Olfactory, metaplasia, respiratory | 0 | 0% | 0.44 | 22% | 0.88 | 50% | 2.4 | 100% |
| Olfactory epithelium, necrosis | 0 | 0% | 0 | 0% | 0.75 | 38% | 1.4 | 70% |
| Olfactory, nerve atrophy | 0.5 | 30% | 1.78 | 78% | 3 | 100% | 4 | 100% |
| Respiratory, accumulation, hyaline droplet | 1.1 | 80% | 2.44 | 89% | 2.25 | 100% | 3.4 | 100% |
| Respiratory epithelium, hyperplasia | 0.7 | 60% | 2.33 | 100% | 1.25 | 88% | 3.1 | 100% |
| Respiratory, metaplasia, squamous | 0 | 0% | 0 | 0% | 0 | 0% | 0.5 | 40% |
| Respiratory epithelium, atrophy | 0.2 | 20% | 0.44 | 22% | 0.75 | 50% | 1.5 | 100% |
| Respiratory epithelium, necrosis | 0 | 0% | 0.78 | 33% | 0.14 | 13% | 0.3 | 30% |
| Nasolacrimal duct, hyperplasia, squamous | 1 | 80% | 1 | 56% | 1.75 | 100% | 1.4 | 60% |
| Luminal protein | 0.6 | 50% | 0.78 | 67% | 1.38 | 75% | 1.8 | 80% |
0 = no lesion; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe
For immunohistochemistry, all nasal sections from two mice from the castrated zibotentan-treated group and two mice from the intact vehicle control group were examined. Slides were deparaffinized and subjected to heat-induced antigen retrieval using a commercial pressure chamber (Biocare Decloaker, Biocare Medical) and buffer (Biocare Diva). Immunostaining was performed using an automated immunohistochemical stainer (Biocare Intellipath FLX®, Biocare Medical, Concord, CA) and included blocking for endogenous peroxidases and non-specific binding (Biocare Rodent Block M). Incubation with primary antibodies goat monoclonal anti-olfactory marker protein (OMP) (1:4000; cat #019–22291544-10001, Wako Chemicals, Richmond VA), rat monoclonal anti-major basic protein (MBP) (1:1000; cat#2000–124, Mayo Clinic, Rochester MN), goat polyclonal anti-chitinase 3-like 3 (CHI3L3, YM1) (1:1500; cat #AF-2446, R&D Systems, Minneapolis MN), goat polyclonal anti-interleukin-33 (IL33) (1:100; cat #AF-3626, R&D Systems, Minneapolis MN), anti-caspase 3 (CASP3) (dilution, catalog number, company, address), rabbit monoclonal anti-CD3 (1:300; cat#RM-9107, Thermo Scientific, Fremont CA), rat monoclonal anti-protein tyrosine phosphatase, receptor type C (PTPRC/B220) (1:200; cat#550286, BD Pharminen, San Jose, CA), and rabbit monoclonal anti-GATA binding protein 3 (GATA3) (1:500; ab199428, Abcam, Cambridge MA) was performed and detection was performed by a biotin-free polymer based commercial detection system (Biocare Univ HRP Polymer) with the chromogen diaminobenzidine (DAB) and a hematoxylin nuclear counterstain. Negative controls were performed with each run using Universal Control (Biocare #NC498, ~1ug/ml), purified mouse and rabbit IgG in buffer with 1% bovine serum albumin (BSA) and preservative, in place of the primary antibody. Additional information about the immunohistochemical protocols and antibody references are included in Supplementary Table 1.
Statistical Analysis
Statistical analysis was performed using R version 3.4.3 or GraphPad Prism 7. A linear mixed effect model regression was used to analyze the effects of drug treatment and castration on weight, a linear mixed model (Cnaan et al., 1997). Model fixed effects included time, castration status (non-castrated vs castrated), and drug treatment status (vehicle vs zibotentan). Nested model random effects included each animal time to weight change within an animal over time. Regression model output including mean regression coefficients and p values are reported in Table 2. Regression analysis was performed in R using the lme4 package (Bates et al., 2015).
Table 2.
Weight gain statistical regression model output.
| Mean regression coefficients | p value | |
|---|---|---|
| Day | 0.065 |
<2e-16 |
| Reproductive status (castration) interaction with Day | −0.010 |
1.39e-07 |
| Drug treatment (zibotentan) interaction with Day | −0.012 |
9.68e-09 |
| Interaction of Drug treatment, Reproductive status, and Day | −0.018 | 2.03e-09 |
Statistically significant values at p < 0.05
Fischer’s exact test was used to determine if the incidence of each histopathologic lesion was statistically different in the different treatment and castration groups (Supplementary Table 3). Incidence was determined as the presence or absence of each pathologic lesion across individuals. Fisher’s exact test was performed on disease incidence for drug treatment (non-treated n = 18, treated n = 18) for all 14 histopathologic lesions. A similar Fisher’s exact test was performed to examine the effect of castration (non-castrated n = 17, castrated n = 19) on incidence of each histopathologic lesion. Fischer’s exact p value and relative risk with 95% confidence interval are reported in Table 3. Relative risk was computed using a Koopman asymptotic score (Koopman, 1984). Results were considered statistically significant when p < 0.05.
Table 3.
Associated p-values for histopathologic lesions correlation with castration and zibotentan treatment.
| Fisher's exact p value for reproductive status | Relative risk (RR) and confidence interval (CI) for reproductive status | Fisher's exact p value for treatment status | Relative risk (RR) and confidence interval (CI) for treatment status | |
|---|---|---|---|---|
| Inflammation, chronic-active | 0.0164* |
1.417 1.229 to 1.552 |
>0.9999 |
1 0.8241 to 1.213 |
| NALT hyperplasia | 0.0404* | 1.308 1.059 to 1.625 |
>0.9999 | 1 0.7411 to 1.349 |
| Olfactory, accumulation, hyaline droplet | 0.0806 |
1.342 0.9965 to 2.038 |
0.0191* |
1.5 1.5 to 1.503 |
| Olfactory epithelium, atrophy | 0.6843 |
1.101 0.7747 to 1.642 |
0.0076* |
1.636 1.612 to 1.819 |
| Olfactory, metaplasia, respiratory | 0.0228* |
2.684 1.174 to 6.935 |
0.0001* |
7 2.62 to 25.5 |
| Olfactory epithelium, necrosis | 0.2742 |
2.088 0.705 to 6.678 |
0.0003* |
∞ 2 to ∞ |
| Olfactory, nerve atrophy | 0.1138 | 1.383 0.9656 to 2.202 |
0.0029* | 1.8 1.771 to 1.866 |
| Respiratory, accumulation, hyaline droplet | >0.9999 |
1.007 0.7931 to 1.308 |
0.4857 |
1.25 0.9171 to 1.267 |
| Respiratory epithelium, hyperplasia | 0.0952 |
1.214 0.9912 to 1.224 |
0.2286 |
1.2 0.9719 to 1.218 |
| Respiratory, metaplasia, squamous | 0.1062 |
∞ 1.045 to ∞ |
0.1039 |
∞ 1 to ∞ |
| Respiratory epithelium, atrophy | 0.2021 |
1.64 0.8095 to 3.597 |
0.0067* |
3.25 1.45 to 8.296 |
| Respiratory epithelium, necrosis | 0.0918 | 5.368 0.9899 to 32.51 |
>0.9999 | 1.333 0.3788 to 4.793 |
| Nasolacrimal duct, hyperplasia, squamous | 0.0438* |
0.6711 0.4318 to 0.9437 |
>0.9999 |
1 0.6733 to 1.485 |
| Luminal protein | 0.7206 | 1.139 0.7248 to 1.874 |
0.4705 | 1.273 0.8126 to 2.098 |
Statistically significant values at p < 0.05
RESULTS
Daily oral gavage of mice with zibotentan results in clinical morbidity in castrated mice
Body weights from the 4 treatment groups (intact vehicle control, intact zibotentan-treated, castrated vehicle control and castrated zibotentan-treated) were monitored during the duration of the study (Figure 1). A linear mixed effects regression model was used to analyze the contribution of time, its interaction with drug treatment, and castration status on weight change. Castrated mice had significantly decreased weight gain over time compared to intact mice (p = 4.39e−07, mean regression coefficient = −0.010, Table 2) when controlled for the individual animal. Similarly, mice treated with zibotentan had significantly decreased weight over time (p = 9.68e-09, mean regression coefficient = −0.012, Table 2). The interaction of castration and zibotentan treatment also significantly decreased body weight (p = 2.03e-09, mean regression coefficient = −0.018, Table 2), suggesting the combination of castration and zibotentan led to greater weight loss than either treatment alone.
Figure 1:
Body weight measurements of zibotentan-treated and vehicle control male castrated and intact male athymic nude mice. A linear mixed effect model regression was used to determine the effect of time, castration controlled for time, and drug treatment controlled for time on weight gain in mice across the various treatments. Castrated animals showed reduced weight gain compared to intact animals over time (p = 1.39e−07). Zibotentan-treated animals showed reduced weight gain compared to vehicle treated animals over time (p = 9.68e−09). Castration and zibotentan combination treatment had also had a significant effect leading to weight decline compared to either treatment alone (p = 2.03e−09). Regression model output can be found in Table 2.
At week 5 of treatment, two castrated mice treated with zibotentan exhibited weight loss of >20% of body weight, necessitating euthanasia. Necropsy and histologic examination were within normal limits aside from gas distension of the small intestine. Four (4/10) other mice in this treatment group had radiographic evidence of significant gas distention involving the stomach and intestines (Figure 2) and an audible respiratory click was observed during inhalation. The mice were treated supportively, zibotentan frequency was reduced from daily to weekday (5 days/week) administration in all experimental groups following the first 5 weeks of initial treatment, and clinical evidence of bloat diminished. While the incidence of bloating remained intermittent throughout the course of the study, bloating was transient and never severe enough to cause significant morbidity necessitating humane euthanasia.
Figure 2:
Radiograph of a castrated zibotentan-treated athymic nude male mouse. There is diffuse gas distention within the stomach, small and large intestines.
Oral gavage resulted in test-article related development of nasal lesions in zibotentan-treated mice
At the end of the study, gross necropsy and histologic examination was performed on all animals. Histologically, lesions were observed at all levels of the nasal cavity, but predominantly affected the olfactory and respiratory epithelium across all groups (Table 1). Predominant lesions in the olfactory mucosa (Figure 3, Supplementary Figure 1) included hyaline droplet accumulation (hyalinosis), necrosis/atrophy, olfactory nerve bundle atrophy in the lamina propria, and respiratory metaplasia of olfactory epithelium. In the respiratory epithelium (Figure 4, Supplementary Figure 2), lesions consisted of hyaline droplet accumulation, necrosis/atrophy, hyperplasia, and squamous metaplasia. In both the olfactory and respiratory epithelium, there was chronic-active inflammation, composed predominantly of lymphocytes, plasma cells, and granulocytes, within the submucosal layers, and there was hyperplasia of nasal-associated lymphoid tissue (NALT). Lesions were most severe in castrated zibotentan-treated animals and reduced in severity in intact zibotentan-treated animals (Table 1). Interestingly, minimal to mild lesions were observed in some vehicle only animals, including hyaline droplet accumulation, olfactory epithelial atrophy, and respiratory epithelial hyperplasia and atrophy, but were more severe in castrated animals than intact animals, suggesting a vehicle effect on the nasal mucosa. Importantly, castrated zibotentan-treated animals showed a high incidence of chronic-active inflammation, which was not present in other groups, and olfactory epithelial necrosis was observed in zibotentan-treated animals, but not in vehicle controls. Additionally, olfactory epithelial necrosis appeared to target the olfactory neuroepithelium, sparing the sustentacular supporting cell layer.
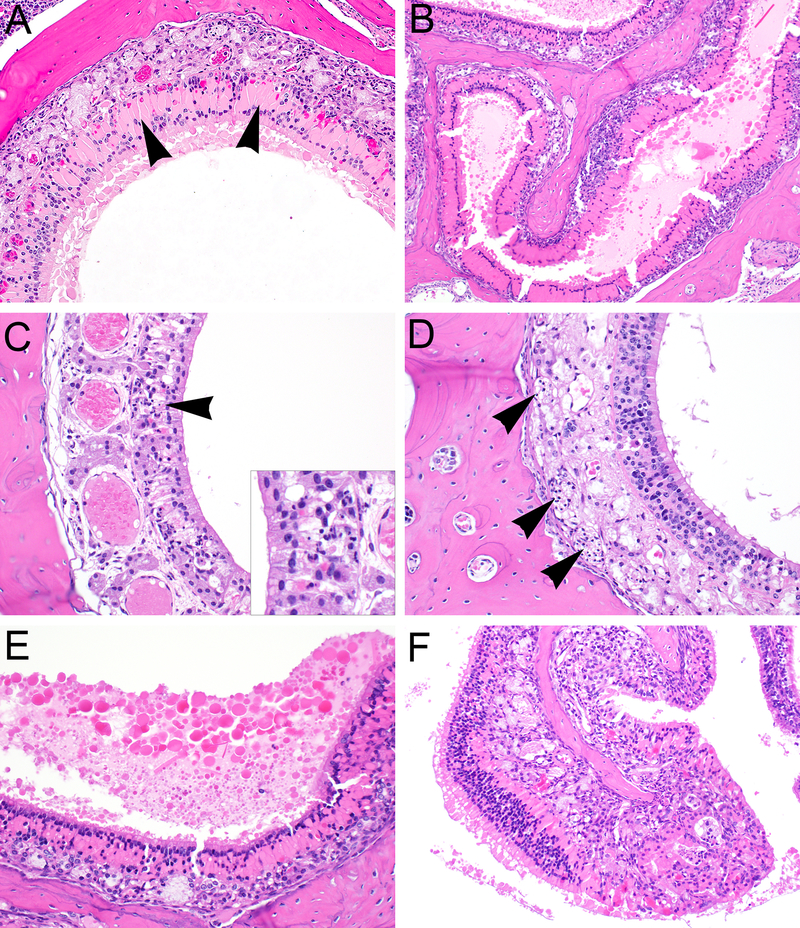
Figure 3.
Characteristic histopathologic features of olfactory epithelial lesions initiated by administration of zibotentan by gavage in male castrated athymic nude mice. Olfactory epithelial hyaline droplet accumulation (arrowheads) within the dorsal meatus at level III (A) and ethmoid turbinates at level IV (B), with diffuse loss of olfactory epithelial layers. (C) Degeneration and necrosis of olfactory epithelium with accompanying cellular debris and apoptotic bodies (arrowheads). (D) Atrophy of olfactory nerves (arrowheads) accompanying severe olfactory epithelial atrophy and necrosis. (E) Accumulation of luminal protein adjacent to areas of severe hyaline droplet accumulation. (F) Severe mixed inflammatory cell infiltrates expanding the submucosa of the ethmoid turbinates, underlying zones of olfactory epithelial hyalinosis.
Figure 4.
Characteristic histopathologic features of respiratory epithelial lesions initiated by administration of zibotentan by gavage in male castrated athymic nude mice. (A) Zones of respiratory epithelial hyaline droplet accumulation along the nasal septum (arrowheads), accompanied by mixed inflammatory infiltrates (B) expanding the submucosa (asterisk). Respiratory epithelial hyperplasia was evidenced by multifocal folding and undulation, or zones of mucosal expansion by numerous proliferative tubular profiles admixed with hyalinosis and inflammatory cell infiltrates (C). Squamous metaplasia (D) was observed as conversion of respiratory epithelium to squamous morphology (arrowheads), often associated with erosion and variable inflammatory infiltrates. Hyperplasia of nasal associated lymphoid tissue (NALT) was characterized by expansion of the submucosa at multiple levels of the nose with multifocal large sheets of well differentiated lymphoid cells (E), compared to normal NALT in control animals typically observed as small foci of lymphocytes within the submucosa (F) of the ventral meatus in level IV.
Zibotentan treatment had a statistically significant association with the development of olfactory nasal lesions
Statistically significant differences in olfactory lesion incidence were observed with respect to zibotentan treatment (Table 3). Compared to vehicle control animals, there were statistically significant increases in olfactory hyaline droplet accumulation, olfactory epithelial atrophy, metaplasia, and necrosis (p < 0.05). Differences in all lesions noted in the olfactory epithelium were statistically significant, while only respiratory epithelial atrophy was significantly increased in zibotentan-treated animals, suggesting most severe lesions targeted to the olfactory epithelium. Lesions associated with castration, irrespective of zibotentan treatment, included chronic-active inflammation, NALT hyperplasia, and squamous hyperplasia of the nasolacrimal duct. There was a general trend of increased lesion severity associated with zibotentan exposure in the olfactory epithelium (Figure 5). The most severe pathology scores were associated with castrated zibotentan-treated animals (Table 1). Less severe and less frequent lesions were observed in intact zibotentan- treated animals. Lesions were present, but to a much less severe degree in castrated vehicle control animals, and minimal but still present in some intact vehicle control animals, suggesting a vehicle effect on the nasal cavity independent from a zibotentan-related effect.
Figure 5.
Scatter plots of severity scores for olfactory epithelial lesions based on statistically significant lesion incidences. Severity scores are elevated in both intact and castrated animals for (A) hyaline droplet accumulation, (B) atrophy, (C) respiratory metaplasia, (D) necrosis, and (E) nerve atrophy in the olfactory epithelium of animals treated with zibotentan compared to vehicle controls.
Lesions in the nasal cavity are associated with a type 2 dominant immune response elicited by IL33 and innate lymphoid cells (ILCs)
We observed a treatment-related effect on the incidence and severity of hyaline droplet accumulation in the respiratory and olfactory epithelium of zibotentan treated mice. Hyalinosis has been associated with a type 2 dominant immune response to injury in the nasal cavity, such as with ozone-induced eosinophilic rhinitis (Kumagai et al., 2016). Given that these athymic nude mice lacked mature B and T cells, we hypothesized that a type 2 dominant immune response driven by innate lymphoid cell type 2 (ILC2) was associated with nasal pathology (Kumagai et al., 2016). Two castrated zibotentan-treated and two intact vehicle control mice were used for immunohistochemical analysis. We confirmed infiltrating lymphocytes were negative for the B- and T-cell markers B220 and CD3 (data not shown), suggesting these cells may be of natural killer (NK) lymphocyte origin (Angelo et al., 2015).
Since respiratory hyalinosis lesions are associated with YM1 (also known as eosinophil chemotactic cytokine (ECF-L) or Chitinase-3 like-3 (CHI3L3)) protein (Owhashi et al., 2000, Ward et al., 2001, Marchesi et al., 2003, Marchesi et al., 2006), we labeled the nasal cavity with anti-YM1/CHI3L3 antibody. Eosinophilic material within olfactory and respiratory epithelial cells in castrated zibotentan-treated was diffusely immunoreactive to antibodies against YM1/CHI3L3, while control animals without hyalinosis in intact vehicle control were negative (Figure 6A–B). Secondly, since eosinophils are commonly recruited in a TH2-driven immune response and are commonly associated with a YM1/CHI3L3 protein response (Owhashi et al., 2000), we assessed the presence of eosinophils using antibodies to Major Basic Protein (MBP), a component of eosinophil granules. Numerous granulocytes within the submucosa of the nasal cavity of castrated zibotentan-treated animals showed cytoplasmic immunoreactivity to anti-MBP antibody, confirming these cells as eosinophils, whereas intact vehicle control animals did not have significant numbers of eosinophils present within the nasal cavity (Figure 6C–D). To confirm necrosis and loss of olfactory neuroepithelium, we labeled the nasal cavity of zibotentan treated and control animals with antibodies to Olfactory Marker Protein (OMP), a widely recognized marker for mature olfactory sensory neurons and not the supporting sustentacular cells (Farbman and Margolis, 1980). Intact vehicle control animals with normal olfactory epithelium showed diffuse nuclear immunoreactivity within the olfactory epithelium, with sustentactular cells showing negative expression. Castrated zibotentan-treated animals had diffuse loss of labeling consistent with global loss of olfactory epithelium with sparing of sustentacular cells (Figure 6E–F). Concurrent with these findings, castrated zibotentan-treated animals showed positive Caspase-3 immunoreactivity in shrunken pyknotic cellular material within olfactory epithelium, as compared to intact vehicle control animals, which did not show similar cellular features (Figure 7A–B).
Figure 6.
Immunohistochemical expression of YM1/CHI3L3, MBP, and OMP in zibotentan-treated and vehicle control male athymic nude mice. In olfactory epithelium of zibotentan-treated mice (A,C,E), there was diffuse positive cytoplasmic immunoreactivity to anti-YM1/CHI3L3 antibody in areas of olfactory epithelial hyalinosis (A), positive immunoreactivity of eosinophils to anti-MBP antibody (C), and diffuse loss of immunoreactivity to anti-OMP antibodies corresponding with loss of olfactory epithelium and nerves (E) compared to vehicle control animals (B, D, F).
Figure 7.
Immunohistochemical expression of Caspase-3, IL33, and GATA3 in zibotentan-treated and vehicle control male athymic nude mice. In olfactory epithelium of zibotentan-treated mice (A,C,E), there was punctate immunoreactivity of scattered apoptotic bodies to anti-Caspase-3 (CSP3) antibodies (A) and diffuse loss of nuclear immunoreactivity to ant-IL33 antibodies within degenerate olfactory epithelium compared to vehicle control animals (B,D). In areas of inflammation, there was multifocal immunoreactivity to anti-GATA3 antibodies (E) compared to vehicle control animals (F).
Since there was marked hyalinosis and widespread olfactory epithelial injury and loss with nerve atrophy in zibotentan treated mice, we investigated the potential role of IL33, an alarmin expressed in the olfactory epithelium and activated during injury to the olfactory epithelium which is involved in promotion of TH2-mediated immune response through induction of innate lymphoid cells (ILCs) and other target cells (Drake and Kita, 2017). In Group A animals, there was diffuse loss of IL33 in areas of olfactory epithelial necrosis (Figure 7C), with retention of IL-33 expression in adjacent zones of atrophied olfactory epithelium and less affected respiratory epithelial cells (Supplementary Figure 3), indicating marked and preferential elaboration of IL-33 from damaged olfactory epithelium. In intact vehicle control animals, there was diffuse nuclear expression of IL33 in intact olfactory epithelium (Figure 7D). Finally, to determine whether or not ILCs were present as a component of these lesions secondary to elaboration of IL33, sections were labeled with the T-lymphocyte marker CD3, the B-lymphocyte marker B220, and GATA3, a transcription factor expressed by ILCs (Hoyler, 2012). While GATA3 is expressed at low levels in all T cells and other ILCs lineages, its expression is high in ILC2 cells, so high GATA3 expression has been reported as a specific characteristic of ILC2 cells (Hoyler, 2012). Consistent with this, in castrated zibotentan-treated animals with inflammatory and hyalinosis lesions, numerous inflammatory cells within the submucosa of the turbinates and nasal septum showed strong positive labeling for GATA3 (Figure 7E–F), consistent with the reported immunophenotype of ILC2s (Hoyler, 2012). Negative and positive control images are available for each antibody in Supplementary Figure 4.
DISCUSSION
Off-target effects of test-article compounds are an important consideration when assessing safety and efficacy of new compounds for use in human populations. In the current study, we have shown that mice treated with zibotentan developed severe lesions within the nasal cavity, an off-target site which led to significant clinical morbidity consistent with data from human clinical trials. Lesions predominantly targeted the olfactory epithelium, leading to promotion of a robust type 2 mediated immune response through elaboration of IL33 and subsequent ILC2 driven immune responses. This response led to significant nasal inflammation and hyalinosis with various degenerative changes in the nasal cavity. Castrated mice treated with zibotentan developed clinical signs of gastrointestinal bloat consistent with observed pathology. According to body weight data, castrated animals in this study had a statistically significant decrease in body weight compared to intact mice, with castrated zibotentan-treated mice having the most severe decline in body weight. It is possible that smaller body weights, combined with more severe nasal cavity lesions in drug-exposed mice, may impact the upper respiratory tract in these animals more significantly than other groups. Mice are obligate nasal breathers and lesions affecting the nasal cavity can result in upper respiratory obstruction (Morgan, 1991, Miyahara et al., 2005, Harkema et al., 2006). As a consequence, upper respiratory obstruction leads to respiratory difficulty or distress and in mice has been shown to lead to aerophagia and excessive accumulation of air in the gastrointestinal tract (Nakajima and Ohi, 1977), similar to our clinical and necropsy observations. Due to these unique anatomic differences, lesions in the nasal cavity of rodents will manifest themselves more quickly and with more severe disease compared to humans. Knowledge of these species-related differences are important when interpreting animal studies in which an unanticipated effect is observed in the nasal cavity that may otherwise be misinterpreted as a drug toxicity and a concern for safety assessment in human populations.
Olfactory epithelial necrosis, nerve atrophy, chronic active inflammation and hyalinosis were prominent features in zibotentan-treated animals compared to controls. The incidence of significant lesions correlated with zibotentan exposure was primarily limited to the olfactory region. This, coupled with the lack of olfactory epithelial necrosis in controls, suggests this region of the nasal cavity was a significant target of drug exposure. The olfactory epithelium is composed of three epithelial cell types: olfactory sensory neurons, the supporting sustentacular cells, and basal cells lining the basal lamina. Sustentacular cells are non-neural cells located in the apical layer of the olfactory epithelium, functioning primarily as structural support (Harkema et al., 2006). These different subpopulations of epithelium in the nose have varying sensitivities to toxic metabolites (Harkema et al., 2006), and the preferential degeneration of olfactory epithelium may suggest that this region was a target of systemic or local test article effects. Results of statistical analysis showed preferential targeting of the olfactory epithelium in terms of lesion incidence related to zibotentan exposure, irrespective of castration, while lesion incidence in the respiratory epithelium was not statistically significant between groups based on drug exposure, except for respiratory epithelial atrophy. We were unable to test for interaction between zibotentan exposure and castration, which is a limitation of the analysis; however, the incidence data used for the Fisher’s exact test comparing presence or absence of lesions based on castration or treatment showed statistically significant differences predominantly across olfactory epithelial lesions as opposed to respiratory epithelium, despite this limitation. The olfactory epithelium is an active site of drug metabolism (some suggest more active than the liver), due to the high content of drug metabolizing enzymes such as NADPH–cytochrome P-450 reductase (Reed et al., 1986, Sarkar, 1992), which are particularly important in the metabolism of inhaled xenobiotics (Bogdanffy, 1990, Harkema et al., 2006). Zibotentan exposure was associated with targeted loss of olfactory epithelium with sparing of surface sustentacular cells, consistent with the role of olfactory epithelium in the detoxification of chemicals in the nasal cavity. Lesions in the olfactory mucosa were thought to be induced by a combination of direct drug exposure to the nasal mucosa secondary to gavage related reflux, an occasional complication of gavage dosing in rodents (Eichenbaum et al., 2011, Damsch et al., 2011b, Damsch et al., 2011a), and systemic exposure of the olfactory epithelium to secondary toxic metabolites.
A direct effect of test article on the nasal mucosa can occur following inhalation or other direct contact of test article with the nasal epithelium, including as a result of gavage-related reflux, an occasional complication of gavage dosing in rats and mice (De Jonghe et al., 2009, Damsch et al., 2011a, Damsch et al., 2011b, Eichenbaum et al., 2011). Evidence for such direct exposure and local effects in the nasal cavity included the audible click noted during respiration, and evidence of involvement in multiple levels and of multiple cell types within the nasal cavity. With direct exposure due to reflux related gavage injury, lesions tend to be ulcerative or necrotizing, predominantly suppurative, located predominantly in the posterior portions of the nasal cavity, and often accompanied with squamous metaplasia and the presence of foreign material such as plant matter (Damsch et al., 2011a, Damsch et al., 2011b). In contrast, lesions secondary to test-article exposure tend to show an exposure-related increase in severity and target specific cell populations such as the olfactory epithelium.
Systemic exposure and hepatic metabolism of certain compounds leads to the generation of toxic metabolites, which reach other tissues including the nose through the systemic circulation, and cause specific toxicity to certain cell populations. Agents administered orally, including phenacetin and hexamethylphosphoramide (HMPA), lead to toxicity and degenerative changes in the olfactory epithelium including necrosis and inflammation (Jeffrey et al., 2006). Systemic administration of a test article may also lead to metabolism and generation of toxic metabolites directly in the nose, without significant hepatic metabolism (Gu et al., 2005), as is observed with formaldehyde, hexamethylphosphoramide, and benzo(a)pyrene (Sarkar, 1992). Evidence supporting systemic exposure included predominant involvement and specific lesions within the olfactory epithelium including olfactory epithelial necrosis and nerve atrophy, more severe pathology in zibotentan-treated animals, and robust hyalinosis, which is unusual for reflux injury. Furthermore, with reflux injury, foreign (feed) material is often observed in the nasal cavity, and lesions tend to be more necrotizing and ulcerative, which was not observed in this study. However, the presence of similar but milder lesions in the nasal cavity of vehicle control mice suggests lesions in all animals were at least in part influenced by gavage-related reflux. In fact, in both vehicle control and zibotentan treated animals, nasal lesions were more frequent and more robust in castrated compared to intact animals, suggesting a significant effect due to castration as well.
The castrated animals were smaller in size compared to intact animals (Figure 1), which may have predisposed them to a higher likelihood of gavage reflux, due to a smaller stomach capacity or issues with handling during the dosing procedure. In addition, sex hormones have been shown to influence drug metabolism in humans (Waxman and Holloway, 2009) and rodents (Shapiro et al., 1995, Clarkson-Jones et al., 2011). For example, androgens repress hepatic monooxygenases in the mouse, which results in increased concentrations of drug metabolizing enzymes in female and castrated males compared to intact male mice. However, rates of excretion of zibotentan following a single oral dose have been shown to be similar between male and female mice (Clarkson-Jones et al., 2011), suggesting that differences in drug metabolic capability likely does not account for differences in lesion severity between castrated and intact animals in this study. In addition, the only lesions that were statistically significantly increased in incidence with respect to castration status were chronic-active inflammation, NALT hyperplasia, and squamous metaplasia of the nasolacrimal duct. The only lesion that was increased in incidence with respect to both castration and zibotentan treatment was squamous metaplasia of the olfactory epithelium.
The histologic lesions and alterations in protein expression in the nasal cavity in zibotentan treated animals are suggestive of a type 2-mediated immune response, a common response in the nasal cavity to various xenobiotics and other chemicals (Hiroi et al., 1998). Damage to olfactory epithelium is associated with elaboration of the alarmin Interleukin-33 (IL-33), which then promotes a TH2-driven inflammatory cascade through binding to its receptor (IL33R/ST2) in the nasal epithelium and on target cells such as innate lymphoid cells (ILCs) (Drake and Kita, 2017). These cells elaborate various type 2 cytokines (IL4, IL5, IL13, etc), which promote eosinophil recruitment and amplification of YM1/CHI3L3 production (Welch et al., 2002, Smithgall et al., 2008, Guo et al., 2015). IL-33 plays an essential role in both innate and adaptive immunity in mucosal organs, and is released rapidly in following cellular damage or tissue injury (Drake and Kita, 2017) allowing ILCs to be sentinels and amplify innate type 2 immune responses from epithelium-derived signals (McKenzie et al., 2014, Guo et al., 2015, Halim, 2016). ILCs are a group of lymphocyte-like innate immune cells with morphological characteristics of lymphoid cells, yet lacking the recombination activating gene (RAG), which renders them without antigen specific T or B cell receptors (Spits and Cupedo, 2012). Several distinct groups of ILCs with phenotypes that have differential functions within the innate immune response have been identified (Spits and Cupedo, 2012). Type 2 ILCs (ILC2) are characteristic of type 2 type immune responses and an innate source of type 2 cytokines that can express type 2 immune responses independently of TH2 cells (Halim, 2016). ILC2s are dependent on the transcription factors RORα, GATA3, and TCF-1 and secrete type 2 cytokines including interleukin (IL)-4, IL-5, IL-9, and IL-13 which alter CD4+ T cells to develop a TH2 phenotype (Hoyler, 2012, Drake et al., 2014, Mirchandani et al., 2014, Kim and Artis, 2015). ILC2s have been found to have a critical role in the initiation of the TH2 response to antigens exposed through mucosal routes, and in efficient memory TH2 cell responses, with production of the cytokine CCL17 from dendritic cells dependent on IL-33-induced ILC2 activation (Gold et al., 2014, Halim et al., 2016).
A limitation of the current study is the lack of demonstration of the presence of other groups of ILCs (ILC1, ILC3). While demonstration of ILC2 subtypes of cells is generally more straightforward than other subtypes, ILC1 and ILC3 subtypes require complex panels of markers to definitively identify these subtypes of ILCs. However, while GATA3 is present in low levels in all T cells, there is high expression of this marker in ILC2s, and given that other innate lymphocyte lineages (i.e., RORgt+ ILCs and NK cells) express only low amounts of GATA-3, high GATA-3 expression can be said to be a specific characteristic of ILC2s (Hoyler, 2012). However, since documentation of the presence of other ILC subsets was not performed, we can only state that ILC2 cells may be in part mediating these effects. Another limitation was the lack of demonstration of IL33R/ST2 and ET-A receptors in the olfactory epithelium to inform on specific targeting of olfactory neurons as opposed to sustentacular cells. Although we were unable to optimize antibodies to these targets, the histology indicates degeneration and loss of the multinuclear olfactory layer, with preservation of the single superficial layer of sustentacular cells remaining in affected animals (Figure 6F). Further studies would be warranted to determine the distribution of these targets within the olfactory epithelium.
As the mice used in this study were athymic nude mice from a xenograft study, a functional T-lymphocyte population was not present and thus these animals are incapable of mounting an effective T-cell response. However, recent studies have shown that in mice deficient in T and B cells are still able to mount a type 2 cytokine response and eosinophilia following experimental administration of IL-33 (Drake and Kita, 2017). Also, studies in a number of airway disorders have shown that IL-33/ST2-pathway-mediated ILC2 activation produces large amounts of type 2 cytokines, especially IL-5 and IL-13, leading to exacerbated TH2-mediated inflammation (Liao et al., 2015, Kumagai et al., 2016, Morikawa et al., 2017, Poposki et al., 2017). Interestingly, it has recently been shown ILC2 development is hormone-dependent; intact male mice have decreased populations of mature and progenitor ILC2 cells, suggesting that ILC2 populations are partially androgen dependent. Therefore, males may be less susceptible to allergic airway inflammation and have less severe IL-33–mediated lung inflammation than females (Laffont et al., 2017b, Laffont et al., 2017a). If ILC2 populations are indeed in some part dependent on androgen expression, this may partially explain the exacerbation of effects in castrated males compared to intact animals, due to an increased IL33-mediated response in the nasal cavity in this study.
An additional limitation of this study was the lack of evaluation of multiple timepoints throughout the pathogenesis of nasal lesions secondary to compound exposure. Ideally, evaluation of the nasal cavity at multiple timepoints along the course of treatment would have provided additional mechanistic information about the pathogenesis of the lesion, and including recovery groups would have helped inform on whether or not these lesions are reversible, and by what mechanism. However, since this study utilized animals from an ongoing xenograft cancer therapeutic study, the option to evaluate the nasal cavity at multiple timepoints along the course of treatment was not available. Further studies are warranted to better understand the pathogenesis of these lesions over time, and the role that ILC2 cells play in promotion of nasal olfactory epithelial damage due to endothelin-A receptor antagonist exposure.
CONCLUSIONS
In the current study, zibotentan exposure by oral gavage induced lesions in the nasal cavity, predominantly targeting olfactory epithelium, which were more severe in castrated animals. The incidence, distribution, and morphology of lesions may have been produced by both systemic and direct exposure of the drug to the nasal mucosa, and were likely influenced by gavage-related reflux injury since milder and more infrequent lesions were also observed in vehicle control animals. Olfactory epithelial damage may have been promoted in part by IL33-mediated promotion of an ILC2 dependent, type 2 immune response in the nasal cavity, leading to severe inflammatory changes that produced recurrent upper airway obstruction, periodic obstructive respiration and aerophagia, ultimately culminating in gastrointestinal ileus and associated morbidity observed clinically. Such unanticipated and off-target effects can significantly impact both animal welfare as well as interpretation of research results, and both appropriate knowledge of species differences between rodents and humans and better understanding of mechanisms behind these test-article effects is critical in proper interpretation of preclinical data as well as maintaining appropriate standards in animal care in research.
Supplementary Material
Supplementary Figure 1: Olfactory hyalinosis (left column, arrowheads) and olfactory epithelial atrophy and nerve atrophy (right column, arrowheads) in Group B (intact zibotentan-treated), Group C (castrated vehicle control), and Group D (intact vehicle-control) animals. Note normal thickness of olfactory epithelium and unaffected nerve fibers (arrowhead) in Group D panel.
Supplementary Figure 2: Respiratory hyalinosis (left column, arrowheads) and respiratory epithelial hyperplasia (right column, arrowheads) in Group B (intact zibotentan-treated), Group C (castrated vehicle control), and Group D (intact vehicle control) animals.
Supplementary Figure 3: Interleukin-33 labeling in residual intact olfactory epithelial cells (arrowheads) adjacent to zones of marked atrophy and degeneration, with sparing of the superficial single layer of sustentacular cells (arrows).
Supplementary Figure 4: Control images for immunohistochemical labeling. Negative controls and positive controls (inset) for olfactory marker protein (A, olfactory epithelium), major basic protein (B, bone marrow), YM1/CHI3L3 (C, olfactory epithelium), IL33 (D, olfactory epithelium), Caspase-3 (E, lymph node), and GATA3 (nasal inflammatory infiltrate negative control, mammary gland positive control).
Acknowledgements
This work was supported by a Department of Veterans Affairs Merit Review Award (I01 BX001370 to G.A.C.) and by the Michigan Integrative Musculoskeletal Health Core Center (NIH P30 AR069620). The authors would like to thank Wendy Rosebury-Smith and Kathy Toy from the In Vivo Animal Core at University of Michigan for histology and immunohistochemistry expertise, and Amy Porter and Kathy Joseph from the Investigative Histology Laboratory at Michigan State University for immunohistochemistry expertise. The authors would also like to thank Dr. Keith Shockley of the Biostatistics and Computational Biology Branch of the National Institute of Environmental Health Sciences for helpful discussions and biostatistics expertise.
REFERENCES
- Angelo LS, Banerjee PP, Monaco-Shawver L, Rosen JB, Makedonas G, Forbes LR, Mace EM and Orange JS (2015). Practical NK cell phenotyping and variability in healthy adults. Immunologic research, 62, 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, Walker SC (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Bogdanffy MS (1990). Biotransformation enzymes in the rodent nasal mucosa: the value of a histochemical approach. Environmental health perspectives, 85, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson-Jones JA, Kenyon AS and Tomkinson HK (2011). The disposition and metabolism of zibotentan (ZD4054): an oral-specific endothelin A receptor antagonist in mice, rats and dogs. Xenobiotica; the fate of foreign compounds in biological systems, 41, 784–796. [DOI] [PubMed] [Google Scholar]
- Clozel M, Maresta A and Humbert M (2013). Endothelin receptor antagonists. Handbook of experimental pharmacology, 218, 199–227. [DOI] [PubMed] [Google Scholar]
- Cnaan A, Laird NM and Slasor P (1997). Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Statistics in medicine, 16, 2349–2380. [DOI] [PubMed] [Google Scholar]
- Damsch S, Eichenbaum G, Looszova A, Lammens L, Feyen B, Van den Bulck K, Knight E, Kelley M and Tonelli A(2011a). Unexpected Nasal Changes in Rats Related to Reflux after Gavage Dosing. [DOI] [PubMed]
- Damsch S, Eichenbaum G, Tonelli A, Lammens L, Van den Bulck K, Feyen B, Vandenberghe J, Megens A, Knight E and Kelley M (2011b). Gavage-related reflux in rats: identification, pathogenesis, and toxicological implications (review). Toxicologic pathology, 39, 348–360. [DOI] [PubMed] [Google Scholar]
- Davenport AP (2002). International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacological reviews, 54, 219–226. [DOI] [PubMed] [Google Scholar]
- De Jonghe S, Lammens L, Raoof A, Steemans K, Broeckaert F, Verbeeck J, Van Goethem F, and Hanton G (2009). Lethal rhinitis/sinusitis in rodents by aspiration of formulation in gavage studies: Importance of evaluation of the nose. Poster presented at the 6th European Congress of Toxicologic Pathology, Edinburgh/Scotland, 2008. Exp Toxicol Pathol, 61, 410. [Google Scholar]
- Donckier JE, Michel L, Van Beneden R, Delos M and Havaux X (2003). Increased expression of endothelin-1 and its mitogenic receptor ETA in human papillary thyroid carcinoma. Clinical endocrinology, 59, 354–360. [DOI] [PubMed] [Google Scholar]
- Drake LY, Iijima K and Kita H (2014). Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy, 69, 1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake LY and Kita H (2017). IL-33: biological properties, functions, and roles in airway disease. Immunological reviews, 278, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum G, Damsch S, Looszova A, Vandenberghe J, Van den Bulck K, Roels K, Megens A, Knight E, Hillsamer V, Feyen B, Kelley MF, Tonelli A and Lammens L (2011). Impact of gavage dosing procedure and gastric content on adverse respiratory effects and mortality in rat toxicity studies. Journal of applied toxicology : JAT, 31, 342–354. [DOI] [PubMed] [Google Scholar]
- Farbman AI and Margolis FL (1980). Olfactory marker protein during ontogeny: Immunohistochemical localization. Developmental Biology, 74, 205–215. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Ninomiya H, Okada T, Urade Y and Masaki T (1995). Suppression of endothelin-1-induced mitogenic responses of human aortic smooth muscle cells by interleukin-1 beta. The Journal of clinical investigation, 95, 2474–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, Takei F and McNagny KM (2014). Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. The Journal of allergy and clinical immunology, 133, 1142–1148. [DOI] [PubMed] [Google Scholar]
- Gouadon E, Meunier N, Grebert D, Durieux D, Baly C, Salesse R, Caillol M and Congar P (2010). Endothelin evokes distinct calcium transients in neuronal and non-neuronal cells of rat olfactory mucosa primary cultures. Neuroscience, 165, 584–600. [DOI] [PubMed] [Google Scholar]
- Gu J, Cui H, Behr M, Zhang L, Zhang Q-Y, Yang W, Hinson JA and Ding X (2005). In Vivo Mechanisms of Tissue-Selective Drug Toxicity: Effects of Liver-Specific Knockout of the NADPH-Cytochrome P450 Reductase Gene on Acetaminophen Toxicity in Kidney, Lung, and Nasal Mucosa. Molecular pharmacology, 67, 623–630. [DOI] [PubMed] [Google Scholar]
- Guo L, Huang Y, Chen X, Hu-Li J, Urban JF Jr. and Paul WE (2015). Innate immunological function of TH2 cells in vivo. Nature immunology, 16, 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY (2016). Group 2 innate lymphoid cells in disease. International immunology, 28, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG and McKenzie AN (2016). Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nature immunology, 17, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema JR, Carey SA and Wagner JG (2006). The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicologic pathology, 34, 252–269. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M and Kiyono H (1998). Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. European journal of immunology, 28, 3346–3353. [DOI] [PubMed] [Google Scholar]
- Hoyler T (2012). The transcription factor GATA3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity, 37, 634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Caty A, Payne H, Borre M, Zonnenberg BA, Beuzeboc P, McIntosh S, Morris T, Phung D and Dawson NA (2010). Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized Phase II trial. BJU international, 106, 966–973. [DOI] [PubMed] [Google Scholar]
- Jeffrey AM, Iatropoulos MJ and Williams GM (2006). Nasal cytotoxic and carcinogenic activities of systemically distributed organic chemicals. Toxicologic pathology, 34, 827–852. [DOI] [PubMed] [Google Scholar]
- Kim BS and Artis D (2015). Group 2 innate lymphoid cells in health and disease. Cold Spring Harbor perspectives in biology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman PAR (1984). Confidence Intervals for the Ratio of Two Binomial Proportions. Biometrics, 40, 513–517. [PubMed] [Google Scholar]
- Kumagai K, Lewandowski R, Jackson-Humbles DN, Li N, Van Dyken SJ, Wagner JG and Harkema JR (2016). Ozone-Induced Nasal Type 2 Immunity in Mice Is Dependent on Innate Lymphoid Cells. American journal of respiratory cell and molecular biology, 54, 782–791. [DOI] [PubMed] [Google Scholar]
- Laffont S, Blanquart E and Guery JC (2017a). Sex Differences in Asthma: A Key Role of Androgen-Signaling in Group 2 Innate Lymphoid Cells. Frontiers in immunology, 8, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont S, Blanquart E, Savignac M, Cénac C, Laverny G, Metzger D, Girard J-P, Belz GT, Pelletier L, Seillet C and Guéry J-C (2017b). Androgen signaling negatively controls group 2 innate lymphoid cells. The Journal of experimental medicine, 214, 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laziz I, Larbi A, Grebert D, Sautel M, Congar P, Lacroix MC, Salesse R and Meunier N (2011). Endothelin as a neuroprotective factor in the olfactory epithelium. Neuroscience, 172, 20–29. [DOI] [PubMed] [Google Scholar]
- Liao B, Cao PP, Zeng M, Zhen Z, Wang H, Zhang YN, Hu CY, Ma J, Li ZY, Song J, Liu JX, Peng LY, Liu Y, Ning Q and Liu Z (2015). Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy, 70, 1169–1180. [DOI] [PubMed] [Google Scholar]
- McKenzie ANJ, Spits H and Eberl G (2014). Innate lymphoid cells in inflammation and immunity. Immunity, 41, 366–374. [DOI] [PubMed] [Google Scholar]
- Marchesi F, Minucci S, Pelicci PG, Gobbi A and Scanziani E (2006). Immunohistochemical detection of Ym1/Ym2 chitinase-like lectins associated with hyalinosis and polypoid adenomas of the transitional epithelium in a mouse with acute myeloid leukemia. Veterinary pathology, 43, 773–776. [DOI] [PubMed] [Google Scholar]
- Marchesi F, Monestiroli SV, Capillo M, Gobbi A, Minucci S, Pelicci PG and Scanziani E (2003). Eosinophilic crystals as a distinctive morphologic feature of a hyaline droplet nephropathy in a mouse model of acute myelogenous leukaemia. Journal of veterinary medicine. A, Physiology, pathology, clinical medicine, 50, 103–107. [DOI] [PubMed] [Google Scholar]
- Miller K, Moul JW, Gleave M, Fizazi K, Nelson JB, Morris T, Nathan FE, McIntosh S, Pemberton K and Higano CS (2013). Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate cancer and prostatic diseases, 16, 187–192. [DOI] [PubMed] [Google Scholar]
- Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ and Liew FY (2014). Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. Journal of immunology (Baltimore, Md. : 1950), 192, 2442–2448. [DOI] [PubMed] [Google Scholar]
- Miyahara S, Miyahara N, Takeda K, Joetham A and Gelfand EW (2005). Physiologic assessment of allergic rhinitis in mice: role of the high-affinity IgE receptor (FcepsilonRI). The Journal of allergy and clinical immunology, 116, 1020–1027. [DOI] [PubMed] [Google Scholar]
- Moller S, Uddman R, Cardell LO, Fernstrom G and Edvinsson L (1999). Expression of endothelin A- and B-receptors in human nasal mucosa. Acta oto-laryngologica, 119, 708–711. [DOI] [PubMed] [Google Scholar]
- Morgan KT (1991). Approaches to the identification and recording of nasal lesions in toxicology studies. Toxicologic pathology, 19, 337–351. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Fukuoka A, Matsushita K, Yasuda K, Iwasaki N, Akasaki S, Fujieda S and Yoshimoto T (2017). Activation of group 2 innate lymphoid cells exacerbates and confers corticosteroid resistance to mouse nasal type 2 inflammation. International immunology, 29, 221–233. [DOI] [PubMed] [Google Scholar]
- Motte S, McEntee K and Naeije R (2006). Endothelin receptor antagonists. Pharmacology & therapeutics, 110, 386–414. [DOI] [PubMed] [Google Scholar]
- Mullol J, Baraniuk JN, Logun C, Benfield T, Picado C and Shelhamer JH (1996). Endothelin-1 induces GM-CSF, IL-6 and IL-8 but not G-CSF release from a human bronchial epithelial cell line (BEAS-2B). Neuropeptides, 30, 551–556. [DOI] [PubMed] [Google Scholar]
- Nakajima K and Ohi G (1977). Aerophagia induced by the nasal obstruction on experimental animals. Jikken dobutsu. Experimental animals, 26, 149–159. [DOI] [PubMed] [Google Scholar]
- Owhashi M, Arita H and Hayai N (2000). Identification of a novel eosinophil chemotactic cytokine (ECF-L) as a chitinase family protein. The Journal of biological chemistry, 275, 1279–1286. [DOI] [PubMed] [Google Scholar]
- Poposki JA, Klingler AI, Tan BK, Soroosh P, Banie H, Lewis G, Hulse KE, Stevens WW, Peters AT, Grammer LC, Schleimer RP, Welch KC, Smith SS, Conley DB, Raviv JR, Karras JG, Akbari O, Kern RC and Kato A (2017). Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immunity, inflammation and disease, 5, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CJ, Lock EA and De Matteis F (1986). NADPH: cytochrome P-450 reductase in olfactory epithelium. Relevance to cytochrome P-450-dependent reactions. The Biochemical journal, 240, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehl-Fehlert C, Kittel B, Morawietz G, Deslex P, Keenan C, Mahrt CR, Nolte T, Robinson M, Stuart BP and Deschl U (2003). Revised guides for organ sampling and trimming in rats and mice--part 1. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie, 55, 91–106. [PubMed] [Google Scholar]
- Sarkar MA (1992). Drug metabolism in the nasal mucosa. Pharmaceutical research, 9, 1–9. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Agrawal AK and Pampori NA (1995). Gender differences in drug metabolism regulated by growth hormone. The international journal of biochemistry & cell biology, 27, 9–20. [DOI] [PubMed] [Google Scholar]
- Smithgall MD, Comeau MR, Park Yoon B-R, Kaufman D, Armitage R and Smith DE (2008). IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK Cells. International immunology, 20, 1019–1030. [DOI] [PubMed] [Google Scholar]
- Spits H and Cupedo T (2012). Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annual review of immunology, 30, 647–675. [DOI] [PubMed] [Google Scholar]
- Sugawara F, Ninomiya H, Okamoto Y, Miwa S, Mazda O, Katsura Y and Masaki T (1996). Endothelin-1-induced mitogenic responses of Chinese hamster ovary cells expressing human endothelinA: the role of a wortmannin-sensitive signaling pathway. Molecular pharmacology, 49, 447–457. [PubMed] [Google Scholar]
- U.S. National Library of Medicine, ClinicalTrials.gov (n.d.). Retrieved April 16, 2018 from: https://clinicaltrials.gov/ct2/results?term=zibotentan&Search=Search
- Ward JM, Yoon M, Anver MR, Haines DC, Kudo G, Gonzalez FJ and Kimura S (2001). Hyalinosis and Ym1/Ym2 Gene Expression in the Stomach and Respiratory Tract of 129S4/SvJae and Wild-Type and CYP1A2-Null B6,129 Mice. The American journal of pathology, 158, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ and Holloway MG (2009). Sex Differences in the Expression of Hepatic Drug Metabolizing Enzymes. Molecular pharmacology, 76, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR and Glass CK (2002). TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. The Journal of biological chemistry, 277, 42821–42829. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K and Masaki T (1988). A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature, 332, 411–415. [DOI] [PubMed] [Google Scholar]
- Young JT (1981). Histopathologic examination of the rat nasal cavity. Fundamental and applied toxicology : official journal of the Society of Toxicology, 1, 309–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Olfactory hyalinosis (left column, arrowheads) and olfactory epithelial atrophy and nerve atrophy (right column, arrowheads) in Group B (intact zibotentan-treated), Group C (castrated vehicle control), and Group D (intact vehicle-control) animals. Note normal thickness of olfactory epithelium and unaffected nerve fibers (arrowhead) in Group D panel.
Supplementary Figure 2: Respiratory hyalinosis (left column, arrowheads) and respiratory epithelial hyperplasia (right column, arrowheads) in Group B (intact zibotentan-treated), Group C (castrated vehicle control), and Group D (intact vehicle control) animals.
Supplementary Figure 3: Interleukin-33 labeling in residual intact olfactory epithelial cells (arrowheads) adjacent to zones of marked atrophy and degeneration, with sparing of the superficial single layer of sustentacular cells (arrows).
Supplementary Figure 4: Control images for immunohistochemical labeling. Negative controls and positive controls (inset) for olfactory marker protein (A, olfactory epithelium), major basic protein (B, bone marrow), YM1/CHI3L3 (C, olfactory epithelium), IL33 (D, olfactory epithelium), Caspase-3 (E, lymph node), and GATA3 (nasal inflammatory infiltrate negative control, mammary gland positive control).