Abstract
Dermatologists are ideally suited to manage the various cutaneous sequelae of graft-versus-host disease (GVHD) outlined in part I of this review. However, the complexity of the patient with GVHD, including comorbidities, potential drug interactions related to polypharmacy, and the lack of evidence-based treatment guidelines, are significant challenges to optimizing patient care. In this section, we will provide an outline for the role of the dermatologist in a multispecialty approach to caring for patients with GVHD.
Keywords: cyclosporine, extracorporeal photopheresis, graft-versus-host disease, hematopoietic cell transplantation, imatinib mesylate, mycophenolate mofetil, phototherapy, tacrolimus, rituximab
TOPICALLY DIRECTED THERAPY OF SKIN DISEASE
Key points
GVHD predominately affecting the epidermis is amenable to topical therapy
Topical steroids and calcineurin inhibitors are useful for short-term treatment of localized GVHD
Sclerotic skin involvement and fascial disease often need systemic therapy
Localized acute cutaneous graft-versus-host disease (GVHD) and chronic cutaneous GVHD primarily involving the epidermis may respond well to mid- to high-potency topical steroids, but the requirement for long-term use frequently necessitates the transition to other topical agents or the use of systemic treatments.1 Alternative topical agents include tacrolimus2,3 and pimecrolimus.4 Choi and Nghiem3 described a response to tacrolimus 0.1% ointment in 13 of 18 patients with chronic cutaneous GVHD; however, all patients eventually required additional treatment to control their skin disease. Topical calcineurin inhibitors (CNIs) may be particularly useful for sites at high risk of skin atrophy—particularly the lips and intertriginous surfaces—but are more poorly tolerated at areas of very active skin involvement with erosions.1 The long-term side effects of topical immunomodulators in the setting of GVHD are unknown. Children and patients with significant skin breakdowns may be at risk of reaching significant systemic drug levels.5 Bland emollients may be helpful for patients with ichthyosiform and xerotic skin changes. Anecdotal use of topical retinoids and hydroquinone has been reported in the setting of periocular lichen planus—like GVHD, but clinical studies supporting their use in GVHD is lacking.1 Additional general skin care recommendations are provided in Table I, including the importance of photoprotection to minimize flares of GVHD (Fig 1).
Table I.
Guidelines for dermatologic evaluation and treatment
| Evaluate for areas of skin breakdown/portals of infection |
| Evaluate for potential skin malignancy;prompt treatment of premalignant lesions |
| Review new medications and potential skin adverse reactions (eg, sulfamethoxasole/trimethoprim or voriconazole) |
| Monitor for early signs of skin/subcutaneous tissue sclerosis, rippling, joint range of motion limitations, and joint contractures |
| Inquire about vulvovaginal symptoms in females |
| Skin and nail care recommendations |
| Moisturizing, nonscented soaps |
| Frequent emollients: thick petrolatum-based ointments |
| Avoid tight fitting/abrasive clothing;wear cool, breathable fabrics |
| Avoid antihistamines in patient with concurrent oral/ ocular sicca symptoms |
| Keep nails trimmed/filed to prevent breakage and pain |
| Clear nail lacquer can be used as a nail hardener |
| Patient education |
| Risk of skin cancer elevated in patients with GVHD;risk is potentiated by iatrogenic immunosuppression and/or phototherapy treatment |
| UV exposure may induce a flare of GVHD or exacerbate drug-induced photosensitivity |
| Avoid outdoor activities during peak hours of UV radiation (10 am-4 pm) |
| Liberal use of broad spectrum sunblock on photoexposed surfaces |
| Use broad-brimmed hat, long sleeves, or UV-protective clothing, and/or use of laundry additive to increase UV protective factor of clothing (SunGuard, Phoenix Brands LLC, Stamford, CT) |
| Reinforce importance of self-skin examination |
| Advise patients on early signs of sclerotic chronic GVHD (darkening or tightening of skin at waistband or brassiere line, skin thickening, rippling/dimpling of skin, decreasing joint range of motion, and joint contractures) |
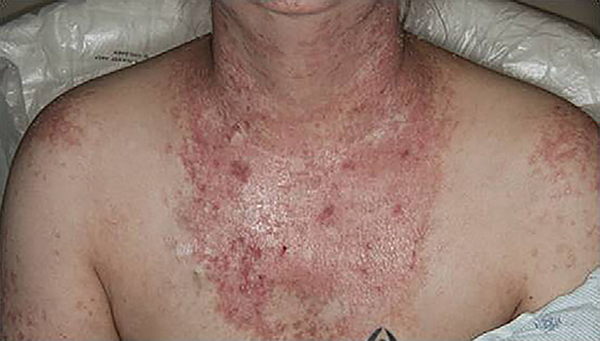
Fig 1.
The sudden onset of erythematous papules and plaques followed unprotected sun exposure; biopsy confirmed the diagnosis of graft-versus-host disease.
Treatment of contractures
Sclerotic skin involvement and fascial disease affecting the joints should be treated aggressively with systemic management and physical therapy—ideally before contracture formation to preserve range of motion and functional mobility. Establishing baseline joint range of motion early in the course of sclerotic disease is also helpful to determine subsequent therapeutic response or disease progression. Physical interventions include occupational therapy, massage, heat therapies (hot pack, ultrasound, or paraffin bath), whirlpool bath, and stretching and strengthening exercises.6 Restoration of range of motion, strength, mobility, and pain relief are the goals of physical therapy, and involvement of physical medicine and occupational therapy specialists is an important aspect of the multidisciplinary management of patients with sclerotic manifestations.1
Wound management
Skin erosions and ulceration in the chronic GVHD setting are complicated by chronic illness, poor nutrition, compromised skin barrier function, and concurrent immunosuppressive therapy. Primary and secondary infection at sites of skin breakdown should be evaluated by bacterial, viral, mycobacterial, and fungal cultures when appropriate (Fig 2). Patients with chronic GVHD are at risk for both common and opportunistic infections, and comprehensive guidelines for infectious disease prophylaxis have been published.7 Other potential causes of skin breakdown, including vasculitis, bullous drug reaction, chronic neuropathy, cutaneous malignancy, and metastatic disease, should be considered if wounds are not responding to appropriate treatment.
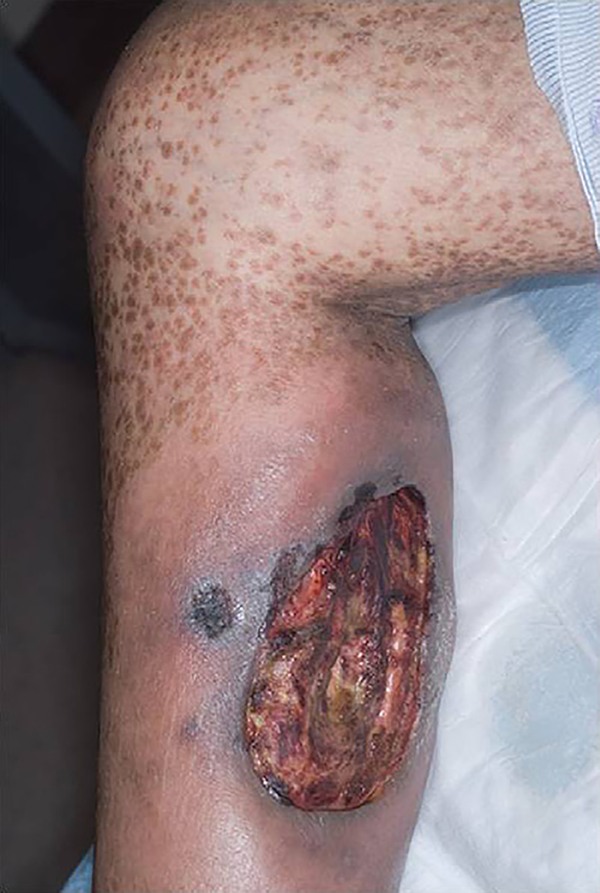
Fig 2.
Opportunistic infection. This ulcer started on the sclerotic skin of the lower extremities. The biopsy specimen and culture confirmed the etiology to be invasive Aspergillosis.
Superficial skin erosions at sites of recurrent minor trauma can be treated with protective film coverings. Full-thickness wounds overlying sclerotic skin are often slow to heal, but may respond to topical wound adjuvants, including hyaluronic acid, collagen, fibroblast, keratinocyte, and platelet-derived growth factor products. Hyperbaric oxygen therapy has been used for scleroderma-related ulceration.8 Recalcitrant wounds are optimally managed in the setting of a dedicated wound care clinic and may require plastic surgery evaluation.1 Berg et al9 described successful allogeneic skin grafting of a chronic GVHD—associated scalp ulceration using donor skin from the original allogeneic hematopoietic cell transplantation (HCT) donor.
TOPICAL THERAPY OF ORAL AND GENITAL DISEASE
Key points
Systemic absorption has been reported in patients who apply topical tacrolimus to mucosal surfaces and extra precaution is warranted in patients on concurrent treatment with systemic tacrolimus
Vulvovaginal involvement may lead to significant morbidity and sexual dysfunction, but may go undiagnosed unless the physician asks about signs/symptoms of disease activity
The recommended first-line treatment for oral GVHD is high-potency topical corticosteroids (fluocinonide gel 0.05%, clobestasol gel 0.05%, or dexamethasone 0.05% mouth wash). Corticosteroid rinses may be swished in the mouth for 4 to 6 minutes four to six times per day.1 Tacrolimus ointment has been used with some success.10 However, systemic absorption may occur, and obtaining a serum drug level after initiating treatment is reasonable in patients treated with concomitant systemic tacrolimus to avoid systemic toxicity.11 The short-term use of topical cyclosporine and azathioprine solution may be useful, but require pharmacy compounding.12 Generalized oral disease or isolated lesions that do not respond to topical therapy may require systemic management. Sicca symptoms may be managed with salivary stimulants (eg, sugar-free gum) and sialogogue therapy (cevimeline and pilocarpine).1 Medications that can exacerbate sicca symptoms (eg, antihistamines, selective serotonin reuptake inhibitors, and tricyclic antidepressants) should be avoided if possible.
Although genital involvement is usually managed in the gynecologic setting, early detection as part of a thorough dermatologic examination may facilitate both diagnosis and treatment. Genital erosions and fissures associated with chronic vulvovaginal disease may be treated with clobetasol proprionate ointment nightly, which should be tapered to a maintenance regimen of two to three times weekly. Topical CNIs may also benefit mild to moderate disease.13 Topical estrogen cream, estrogen ring, or oral replacement therapy is recommended in woman who do not have a contraindication to hormone therapy.14 Limited vaginal scarring/synechiae can be treated with dilators or manual lysing. Severe vaginal scarring may require surgical intervention.14 Involvement of the male genitalia is likely an underrecognized manifestation of chronic GVHD15 and may be treated with topical corticosteroids and topical CNIs after infectious etiologies like herpetic or yeast infections are excluded. Sexual dysfunction in the posttransplant setting is multifactorial, and may be precipitated by depression, fatigue, loss of libido, body image issues, and skin and vulvovaginal disease. The accurate diagnosis and treatment of genital involvement is an important step toward improving sexual function. Referral to appropriate counseling with the goal of a slow return to intimacy may be helpful for both the patient and his/her sexual partner.1
ULTRAVIOLET RADIATION THERAPY
Key points
Many types of phototherapy have been reported to benefit chronic graft-versus-host disease in small case series
Psoralen plus ultraviolet A light and ultraviolet A-l phototherapies appear to be superior options for the treatment of sclerotic skin manifestations
Careful evaluation for concurrent photosensitizing medications or autoantibodies is warranted before the initiation of phototherapy
Skin cancer may preclude the use of ultraviolet radiation therapy in the treatment of graft-versus-host disease
After allogeneic HCT, patients are counseled to avoid ultraviolet radiation (UVR), not only because it may be associated with a GVHD flare16 but also because it imparts an increased risk of skin malignancy. 17 Nevertheless, ultraviolet light phototherapy is efficacious in the treatment of some cases of established cutaneous GVHD. Extensive experience with ultraviolet light phototherapy for the treatment of other inflammatory dermatoses18,19 prompted the successful use of psoralen plus ultraviolet A phototherapy (PUVA) in 1985 to treat lichen planus—like cutaneous GVHD.20 Possible targets of UVR are Langerhans cells, which may be depleted or altered, affecting their capacity to present antigens,21 and keratinocytes, which release immunosuppressive cytokines.19,22–24 PUVA,20,25 PUVA bath photochemotherapy,26 ultraviolet B light,27 narrowband UVB (NB-UVB),28 and ultraviolet A-1 (UVA-1)29 phototherapies have all shown efficacy in small series or case reports. Although epidermal (and particularly the lichen planus—like GVHD phenotypes) may respond to UVB and NB-UVB, deep sclerotic changes do not. PUVA (and particularly UVA-1) have proven useful in some cases of treatment of sclerotic plaques.29–31 However, while UVR may be an attractive option for patients with chronic cutaneous GVHD, there is no evidence that it is efficacious for internal organ involvement. UVR should be considered in patients in whom the addition of additional systemic immunosuppression poses a high risk of infection or interference with a graft-versus-tumor (GVT) response. Modification of the UVR dose is important in patients who are taking photosensitizing medications, and treatment may be particularly problematic in patients who have autoantibodies, particularly antinuclear antibodies or anti-Sjögren antibodies. In addition, the potential benefit of ultraviolet light therapy must be weighed against the elevated risk of cutaneous malignancy in immunocompromised patients, particularly those with actinic damage or a history of ionizing radiation. Voriconazole, an oral antifungal agent that is commonly used to prevent and treat invasive fungal infections in this population, is associated with phototoxicity and may predispose susceptible individuals to squamous cell carcinoma (SCC)32’33 and melanoma.34
Surveillance for secondary cutaneous tumors
Conditioning regimens and prolonged immunosuppression increases the risk of secondary malignancies of the skin including SCC, basal cell carcinoma, and melanoma.17,35–37,38 The long-term risk of melanoma in pediatric patients receiving HCT may be associated with a conditioning regimen using high doses of alkylating drugs.36 Patient age, actinic exposure, skin phototype, and previous ionizing radiation are additional risk factors in the development of nonmelanoma skin cancers after HCT39’40 (Fig 3).
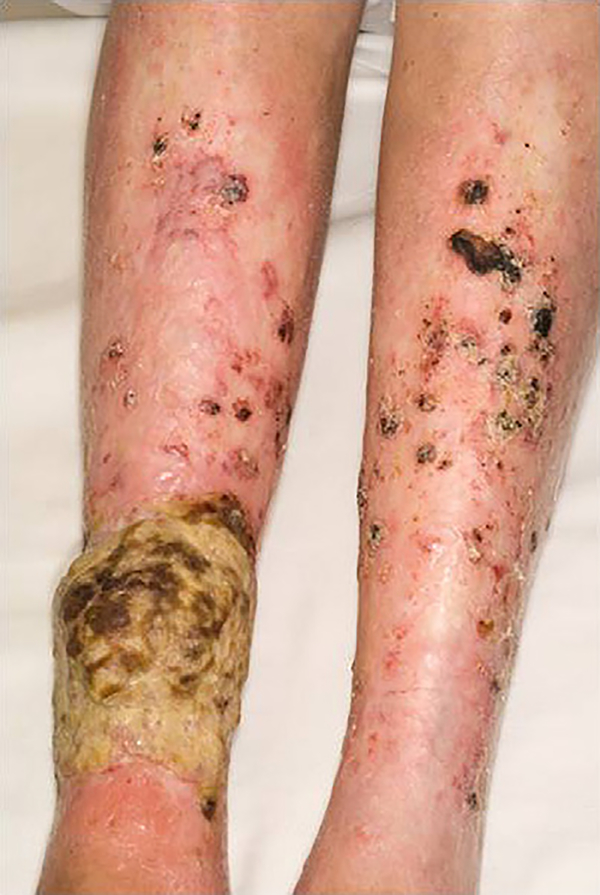
Fig 3.
Nonmelanoma skin cancer. This large squamous cell carcinoma started on the sclerotic skin of the right lower extremity. Biopsy specimens of several of the other erosions on both legs revealed less deeply invasive squamous cell carcinoma.
Benign vascular tumors can develop on severely sclerotic skin41–43 (Fig 4) and may require a biopsy specimen to rule out malignancy. Epidermodysplasia verucciformis (EV)—like lesions associated with human papillomavirus types 8 and 20 have been reported in the setting of GVHD and are linked to other EV-related haplotypes.44 Recently, human papillomavirus was detected in multiple eccrine poromas in a patient with chronic GHVD, but not in his normal-appearing skin.45
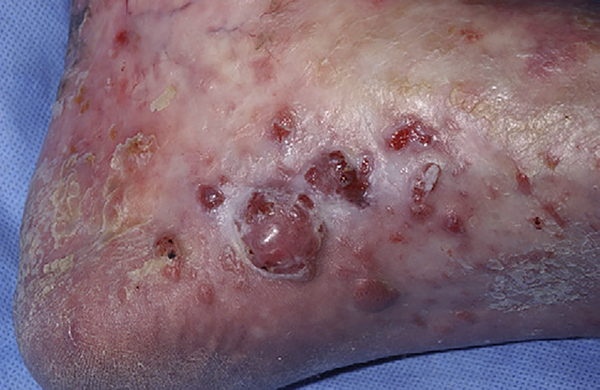
Fig 4.
Multiple benign vascular tumors are present on the foot of a patient with long-standing sclerotic-type chronic graft-versus-host disease.
SYSTEMIC THERAPY
Key points
Systemic treatment requires knowledge of the patient’s comorbidities, other graftversus-host disease organ system involvement, and infection risk, among other factors
Systemic corticosteroids are the mainstay of acute graft-versus-host disease
Sclerotic skin involvement, particularly fascial disease, should prompt aggressive treatment before the development of skin contractures and functional disability
The initiation of systemic treatment should be made in consultation with the primary transplant team or with a transplant clinician experienced in the management of patients with GVHD. The decision to treat topically, systemically, or with other physical modalities (eg, extracorporeal photopheresis [ECP] or phototherapy) requires assessment of the subtype of skin involvement, the potential for long-term morbidity (eg, contractures resulting from joint restriction), the presence of other active mucosal or internal organ involvement, and the patient’s underlying health, particularly infection status and risk of malignancy relapse. Systemic therapy is often considered in the setting of high-risk features, severe individual organ involvement, or the failure to achieve symptom control with topical or organsupportive care. Three risk factors predictive of poor outcomes in patients with newly diagnosed chronic GVHD are progressive onset chronic GVHD, a platelet count <100,000 mm3, and pulmonary involvement (bronchiolitis obliterans).46–50 In addition, patients who have >3 organs involved by GVHD, >50% skin involvement, or develop chronic GVHD while taking ≥0.5 mg/kg of steroids should be considered for initiation of systemic therapy.47'51,52
Acute GVHD therapy and GVHD prophylaxis
Systemic corticosteroids remain the standard initial treatment of extensive acute GVHD, but even with the prompt initiation of therapy, response may be suboptimal. Less than 50% of patients with acute GVHD will sustain a durable response after initial therapy and will require secondary treatment.53 Unfortunately, the use of very high-dose corticosteroid therapy or the concurrent use of a second immunosuppressive agent has not led to improved responses.54–57 The outcome for patients with steroid-refractory GVHD is poor, with a mortality rate approaching 70%, and no therapy has been shown to improve survival.58 The primary causes of death among patients with refractory acute GVHD are organ failure and infection related to poor immune reconstitution. For these reasons, GVHD prevention through improved prophylaxis strategies has been a primary focus of acute GVHD management.
The combination of cyclosporine (CSA) and methotrexate (MTX) has been shown to improve survival in the prophylactic setting.59 CSA is a cyclic polypeptide that prevents T cell activation by inhibiting interleukin-2 production and expression. While effective as GVHD prophylaxis, CSA has significant toxicities, including hypertension, nephrotoxicity, hypomagnesemia, tremors, seizures, anorexia, hypertrichosis, and gingival hyperplasia.60 CSA is usually initiated intravenously 1 to 2 days before stem cell infusion and converted to oral dosing when tolerated.
Tacrolimus is a macrolide lactone that closely resembles CSA in mechanism of action, spectrum of toxicities, and pharmacologic interactions. In a randomized study, the prophylactic regimen of tacrolimus and MTX was superior to CSA and MTX for the prevention of grade II to IV acute GVHD.61 However, a higher death rate was observed in patients with advanced disease who received tacrolimus, possibly related to its use at serum levels above those currently recommended. The implications for this finding are unclear, and CSA and tacrolimus are generally viewed as equivalent when used for GVHD prophylaxis.
Chronic GVHD systemic therapy
Determining therapeutic response in patients with chronic GVHD, particularly those with multisystem involvement, can be extremely challenging. Many chronic GVHD clinical reports represent small, unblinded therapeutic trials in combination with other immunosuppressive therapies, often in patients with refractory disease. Therefore, patients with chronic GVHD who require systemic treatment—particularly those with refractory disease—should be considered for entry on a clinical trial.62 For patients who are not eligible for a clinical trial or are receiving therapy at a center for which no trial is available, standard initial therapy is the initiation of 1 mg/kg of corticosteroids with or without a CNI. Continuation of a CNI in patients who develop chronic GVHD on CNI therapy or restarting a CNI for those who developed symptoms after being tapered off is an area of controversy. A randomized study of prednisone with or without cyclosporine in patients with extensive chronic GVHD found a decrease in avascular necrosis (a steroid-related complication) in patients randomized to CSA and steroids; however, there was no benefit in transplantrelated mortality, discontinuation of immunosuppressive therapy, or need for secondary GVHD therapy.63 In fact, survival without recurrence was statistically lower for patients randomized to two drugs when compared to those who received steroids alone, leading to the conclusion that the continuation of a CNI may impair immune reconstitution and lead to increased mortality.63
Similar results were observed in a randomized trial of mycophenolate mofetil (MMF) compared to placebo in combination with steroids (95% of patients) and a CNI (80% of patients). This study was closed after interim analysis determined no difference in treatment success as initial therapy for chronic GVHD between the two arms (defined as resolution of reversible GVHD manifestations or withdrawal of immunosuppressive therapy within 2 years of enrollment). In addition, patients randomized to the MMF arm had a trend toward a higher risk for death (primarily because of an increased rate of infection and relapse).64 These two trials highlight the importance of randomized trials in assessing efficacy and evaluating competing risks such as relapse and treatment-related mortality.
CNIs have been shown to inhibit regulatory T cells (Tregs) through the nonspecific inhibition of interleukin-2, which may in turn negatively impact the development of immune tolerance.65 By contrast, recent interest in the role of Tregs in chronic GVHD has led to the development of a multicenter trial sponsored by the Blood and Marrow Clinical Trials Network evaluating two GVHD therapies believed to promote Treg expansion: sirolimus and ECP (www.clinicaltrials.gov, trial NCT01106833). In order to test whether the so-called “Treg permissive therapy” improves outcomes for patients with recently diagnosed chronic GVHD, two “Treg permissive” strategies (ECP/sirolimus/prednisone and sirolimus/prednisone) are being compared to a CNI-based regimen (sirolimus/prednisone/CNI) in parallel, randomized phase II trials. The most promising Treg permissive therapy based on the phase II studies will then be compared to sirolimus/prednisone/CNI in a phase III trial. This trial opened in 2010 and has a target accrual of 500 patients for the phase II and III portions of the study. In addition to studying these two Treg permissive strategies, validation of the National Institutes of Health consensus response assessment, formal quality of life assessments, and evaluation of chronic GVHD biomarkers are major secondary goals for this important trial.
ACUTE AND CHRONIC GRAFT-VERSUSHOST DISEASE: SECOND-LINE THERAPY
Key points
For patients who fail treatment with systemic corticosteroids, there is no single proven therapy that is superior
Published clinical reports often combine sclerotic and nonsclerotic disease, leading to difficulty in determining cutaneous responses
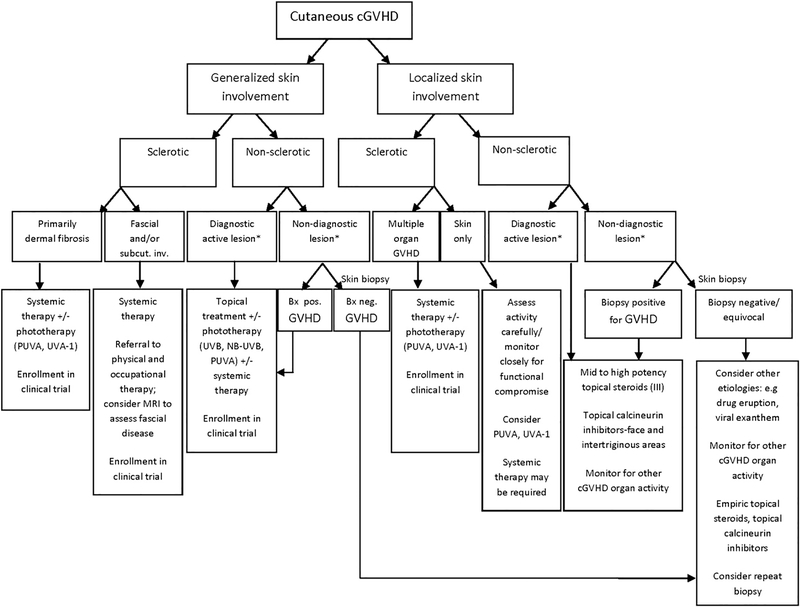
No single second-line therapy has shown superiority for corticosteroid refractory chronic GVHD. Individual treatment choices are often made on the basis of institutional experience, phase II trials, or retrospective analyses, which do not always offer uniform criteria for response assessment or details about the severity of GHVD. A 2009 Consensus Conference on Clinical Practice in Chronic GVHD aimed to summarize the current evidence for second-line treatment.66 Because the evidence and majority of treatment options are sparse, the strength of most recommendations fell into category C (ie, the evidence for efficacy is insufficient to support for or against, or the evidence might not outweigh adverse consequences or cost). These recommendations are outlined by Wolff et al66 and will not be extensively reviewed here. In addition, the following agents are primarily recommended for use in collaboration with an experienced transplant physician and, if possible, in the clinical trial setting. A diagnostic and treatment algorithm for the dermatologist is outlined in Fig 5.
Fig 5.
Algorithm for the diagnosis and management of chronic graft-versus-host disease of the skin. Asterisk indicates that the definition of diagnostic skin involvement is based on National Institutes of Health consensus criteria.
Extracorporeal photopheresis
Key points
Extracorporeal photopheresis is a timeintensive procedure that is not readily available at all medical centers, but has shown efficacy for some patients with cutaneous graft-versus-host disease
Limitations to the use of extracorporeal photopheresis include the cost, time commitment, and risk of line infection or venous thrombosis
ECP is the process of leukopheresis, followed by ex vivo photoactivation with 8-methoxypsoralen and UVA and reinfusion of the buffy coat. Although the mechanism of action of ECP in the treatment of both acute and chronic GVHD is not fully understood, it may induce apoptosis, inhibit proinflammatory cytokine production, increase antiinflammatory cytokine production, reduce the stimulation of effector T cells, and induce donor-derived Tregs.67–69 The potential efficacy of this treatment has been shown in patients with nonsclerotic and sclerotic skin manifestations in several uncontrolled series.70,71 In a review of 71 patients with chronic GVHD treated with ECP, Couriel et al71 reported a cutaneous response of 59%, including a 67% response in patients with sclerotic skin changes. In contrast, in a multicenter, randomized phase II, single-blinded (observer) study of ECP versus conventional therapy for refractory chronic cutaneous GHVD, a significant difference in total skin score was not observed between treatment groups.72 However, a greater percentage of patients receiving ECP in this study achieved a 25% reduction in total skin score and a ≥ 50% reduction in steroid dose. The percentage of patients who achieved a complete or partial response was also higher in the ECP group. ECP has been used successfully for eosinophilic fasciitis unrelated to GVHD73 and GVHD-related fasciitis.74’75 It also appears to have efficacy in extra cutaneous manifestations of chronic GVHD; however, validated outcome measures and standardization of optimal treatment intervals and duration of therapy are needed. The best responses to ECP have been observed in skin, mucous membrane, eye, hepatic, and lung chronic GHVD.71,76 ECP is often an attractive option for patients with steroid-refractory disease, but is a time-consuming procedure that requires a dedicated apheresis center, which is not available at all medical facilities. Treatment is commonly administered on 2 consecutive days each week for 2 to 3 months followed by a subsequent taper schedule, with the patient continually reevaluated throughout the therapeutic course.77 Adverse effects are usually sporadic and mild, including fluid shifts and blood pressure issues. Serious adverse events, such as thrombosis and infection, may occur if a central venous catheter is required for apheresis access.
Mammalian target of rapamycin inhibitors
Key points
Mammalian target of rapamycin inhibitors are immunosuppressive agents that may have beneficial effects on chronic graftversus-host disease through the maintenance of regulatory T cells
Drug interactions and hyperlipidemia are frequent issues associated with the use of mammalian target of rapamycin inhibitors
Sirolimus and evorolimus inhibit T cells via the mammalian target of rapamycin (mTOR) pathway. In addition, mTOR inhibition has been shown to decrease collagen mRNA stability and inhibit platelet-derived and fibroblast growth factors.78 As described earlier, animal studies suggest that sirolimus promotes the development and maintenance of Tregs, and there is therefore considerable interest in this class of agents in chronic GVHD. A recent retrospective study in patients with sclerotic chronic GVHD found a 76% response rate with either sirolimus or evorolimus given in combination with corticosteroids.79 These results were comparable to retrospective studies describing a response rate ranging from 63% to 81%.80,81 The role of concurrent therapy with a CNI in patients taking an mTOR inhibitor is yet to be determined; however, there is some evidence of comparable efficacy when an mTOR inhibitor was given without a CNI.79 mTOR inhibitors have a significantly longer half-life than CNIs, and regular monitoring of trough levels is required. In addition, mTOR inhibitors are associated with numerous drug interactions because of metabolism through cytochrome p450 3A4. Renal toxicity and thrombotic microangiopathy are two of the most serious adverse effects. The risk of renal toxicity is further increased when combined with a CNI. Hyperlipidemia is also common, mandating close monitoring of lipid levels.
Imatinib mesylate
Key points
Imatinib mesylate is a chrome graft-versushost disease salvage therapy that is thought to work via the inhibition of profibrotic pathways rather than as an immunosuppressive agent
Preliminary clinical studies have shown improvement in some patients with sclerotic skin manifestations
Imatinib mesylate is a multikinase inhibitor with activity against bcr-abl, c-kit, platelet-derived growth factor receptor (PDGFR), and other kinases. The drug is approved for the treatment of Philadelphia chromosome-positive chronic myelogenous and acute lymphoblastic leukemia and in other conditions associated with c-kit or PDGF-associated mutations. Initial interest in this drug for the treatment of sclerotic-type chronic GVHD stemmed from its putative antifibrotic effect via PDGFR inhibition. PDGF signaling activates transforming growth factor-beta,82 a potent profibrotic cytokine, capable of stimulating collagen production, abrogating metalloproteinase activity, and sensitizing fibroblasts to a constitutive activated state via autocrine signaling.83 In addition, Italian researchers reported stimulatory antibodies directed against PDGFR in patients with chronic GVHD and in patients with systemic sclerosis. 84,85 Together with the established safety profile for the drug in the setting of chronic myelogenous leukemia, this has led to significant interest in the use of imatinib mesylate for the treatment of fibrotic manifestations of chronic GVHD, with reported responses of 50% to 79% in two small, recent series.86,87 However, to date, the detection of PDGFR antibodies in systemic sclerosis and sclerotic-type chronic GVHD has not been replicated by other research groups,88 and the administration of imatinib before the onset of GVHD does not appear to eliminate the risk of developing skin sclerosis.89 The mechanism of action of imatinib therefore, remains unclear, and other mechanisms, including T cell inhibition90 and the inhibition of fibrosis via “nonclassic” pathways downstream of transforming growth factor-beta, such as cellular Abelson, may be relevant.83 Clinical trials are underway to determine efficacy, tolerability, and optimum dosing of imatinib, as well as correlative biologic studies to better understand its potential as an antifibrotic agent. The role of the “second-generation” tyrosine kinase inhibitors (nilotinib and dasatinib) in the prophylaxis and treatment of GVHD also remains to be determined.91,92
Other systemic therapies
Key points
Limited data are available to support the use of anti—tumor necrosis factor-alpha therapy in chronic graft-versus-host disease
The use of anti—tumor necrosis factor-alfa therapy in the post—allogeneic transplant setting has been associated with invasive fungal infections
Etanercept93–95 and inflixamab96’97 have been used for the treatment of acute GHVD, particularly in patients with steroid-refractory gastrointestinal disease; however, clinical experience in chronic disease is limited.94,98 Enthusiasm for these agents in the treatment of GVHD has been tempered by the significant risk of invasive fungal infections.97,99 Thalidomide and its newer derivatives, such as lenalidomide, also block tumor necrosis factor—alfa and other cytokines and impede angiogenesis, the expression of adhesion molecules, and nuclear factor-κβ activity,100,101 but the evidence supporting their use is limited.
Alefacept
Alefacept is a fusion protein approved for the treatment of psoriasis consisting of the extracellular CD2-binding portion of the human leukocyte function antigen-3 linked to the Fc portion of immunoglobulin Gl. Shapira et al102 described 12 patients with chronic GVHD (11 with skin involvement) treated at higher doses than those typically used for psoriasis (15 mg [pediatric] and 30 mg [adults] by weekly intramuscular injection). After a median of 8 weeks (8 injections), subjective marked or moderate responses were reported in 5 of 12 patients.102 The drug was generally well tolerated; however, one patient developed SCC of the lip during the follow-up period—a potentially significant finding, because alefacept is associated with malignancy risk that may be compounded in the setting of concurrent GVHD-related immunosuppression.
Rituximab
Rituximab, an anti-CD20 monoclonal antibody, targets B cells, which, as previously discussed, are thought to play a role in the pathogenesis of GVHD.103–109 When used in the preceding 6 months before an allogeneic HCT in patients with B-cell lymphoma, rituximab has been associated in a retrospective analysis with a reduced incidence of acute GVHD.110 Response rates have varied in the treatment of chronic GVHD, but few complete responses have been reported. Because of the small numbers of patients in previous series, a metaanalysis was recently performed of rituximab for the treatment of steroid-refractory chronic GVHD.111 Based on seven evaluable studies (3 prospective and 4 retrospective; total number of patients, 111), cutaneous response rates ranged from 13% to 100% (oral disease, 0–83%; hepatic disease, 0–66%; and lung disease, 0–38%). Rituximab facilitated a reduction in corticosteroid doses in some patients105 and was well tolerated with minimal treatment-related morbidity (primarily infusion reactions and infectious complications). Rituximab is most commonly dosed at 375 mg/m2 weekly for 4 weeks; however, a lower dose and frequency of rituximab may produce a comparable overall response.112
Miscellaneous agents
Mesenchymal stem cells (MSCs) are pluripotent cells that are distinct from hematopoietic stem cells, and have been used to treat steroid-refractory acute GVHD.113–115 The immunomodulatory mechanism of MSCs is unclear, but may occur through the induction of Tregs.116 In contrast to hematopoietic cells, MSCs can be derived from haploidentical or even third-party unmatched donors without inducing immunogenicity. In a 2008 study, 39 of 55 (71%) participants with steroid-resistant acute GVHD sustained a complete or partial response to MSC infusion.115 More recently, a response to MSCs was reported in four patients with sclerotic GVHD-related skin disease without discernable adverse effects.117 Whether MSCs offer a therapeutic benefit for patients with acute or chronic GVHD will require more extensive studies. In case reports, small, uncontrolled trials, or retrospective evaluation, systemic retinoids,118 MTX,119 hydroxychloroquine,120 pentostatin,121 and clofazimine122 have been used as additional second-line treatment options in chronic GVHD.76,123
THE ROLE OF DERMATOLOGY IN GRAFTVERSUS-HOST DISEASE CLINICAL RESEARCH AND MULTIDISCIPLINARY MANAGEMENT
Key points
Numerous barriers have hampered the development of better evidence-based therapies for chronic graft-versus-host disease
Dermatologists can play a key role in patient management through active participation in clinical trials, research investigation, and the development of improved cutaneous outcome measures
Despite the fact that cutaneous involvement is often a primary endpoint in chronic GVHD clinical trials, the polymorphic nature of the disease, problems with study design, and the lack of validated outcome measures have impeded the development of evidence-based treatment guidelines. Chronic GVHD is also a relatively “rare” condition and, therefore, expertise is typically limited to centers that perform allogeneic HCT. However, by the time of chronic GVHD onset, patients have often returned from the transplant center to live in their home community and may be unwilling to travel to enter a clinical trial. As discussed earlier, no single salvage therapy has yet proven superior in patients who fail corticosteroids; enrollment in clinical trials is therefore recommended for all patients who require salvage therapy.62 Development of a chronic GVHD clinical trials network is one important recent effort underway to facilitate recruitment into clinical trials. Ideally, clinical trials would use an array of validated organ-specific outcome measures, including skin assessment, by specialists experienced with the disease (eg, dermatology, ophthalmology, and oral medicine specialists). Unfortunately, this requires tremendous resources and coordination, which is not feasible in many settings. A single response measurement tool that may be used by nonspecialists and that focuses on the most important and common chronic GVHD manifestations has been proposed (Table II).77 Validation of this response tool is ongoing; however, this tool provides only a gross estimate of cutaneous disease activity based on affected body surface area of sclerotic and nonsclerotic skin epidermal involvement. Therefore, an acknowledged need remains for more quantifiable measures of skin sclerosis.77,124
Table II.
Clinician-assessed and patient-reported chronic graft-versus-host disease assessment
| Measurement | Assessor | |
|---|---|---|
| Skin | ||
| Erythematous rash | % BSA | C |
| Moveable sclerosis | % BSA | C |
| Nonmoveable sclerosis/fasciitis | % BSA | C |
| Ulcer | Largest diameter | C |
| Pruritus | 0–10 | P |
| Eyes | ||
| Bilateral Schirmer tear test | Mean of both eyes | C |
| Main ocular symptom | 0–10 scale | P |
| Mouth | ||
| Erythema | Total score 0–15 | C |
| Lichen planus/hyperkeratosis | C | |
| Mucoceles | C | |
| Oral pain/dryness/sensitivity | 0–10 scale | P |
| Hematology | ||
| Platelet count | No. per μL | C |
| Eosinophils | % | C |
| Gastrointestinal | ||
| Upper GI symptoms | 0–3 | C |
| Esophageal symptoms | 0–3 | C |
| Diarrhea | 0–3 score | C |
| Liver | ||
| Total serum bilirubin | mg/dL | C |
| ALT, alkaline phosphatase | U/L | C |
Adapted from Pavletic et al.77
ALT, Alanine aminotransferase; BSA, body surface area; C, clinician; GI, gastrointestinal; P, patient.
The need for a validated skin outcome measure for chronic GVHD is highlighted by the randomized multicenter ECP trial described earlier that found that the median change in total skin score (TSS) in ECP-treated patients (14.5%) was not superior to patients who did not receive the treatment (8.5%).72 The TSS is a numerical scoring system proposed by Greinix et al125 in which a numeric score is derived from body surface area assessment and variable skin features (1 = alopecia and dyspigmentation; 2 = lichenoid plaques or skin thickening, moveable sclerosis; 3 = thickened skin with limited motion, but moveable; and 4 = hidebound, unmovable skin). The intra- and interobserver reliability of this system has been shown to be reasonable in a small validation study of TSS.125 In the ECP study,72 skin scorers were blinded to the treatment assignment; however, the unblinded investigator assessment detected a significant improvement in the ECP-treated group (p < .001). Several potential factors may explain the discrepancy between the TSS and investigator assessment. First, the primary endpoint of the study (12 weeks) may have been too soon to detect significant improvement in skin sclerosis by TSS. An understanding of the biology of collagen remodeling—specifically, differences in expected response times between epidermal and sclerotic repair—should be considered in clinical trial design. Second, the discrepancy between the TSS and unblinded assessments suggests that either the TSS tool is not sensitive to change (eg, by the inclusion of features of GVHD skin damage that are unlikely to change in response to treatment, such as alopecia or dyspigmentation) or that significant investigator bias may have been present.126
This study underscores the need for a scoring system that shows clinically meaningful responsiveness to change for all chronic GVHD skin manifestations (eg, epidermal involvement, dermal sclerosis, and fasciitis). The use of validated tools taken from systemic sclerosis trials is a helpful starting point; however, clinical differences between systemic sclerosis and the sclerotic manifestations of GVHD must be appreciated. For instance, modified Rodnan scoring, a validated method of assessment for systemic sclerosis127 that has been appropriated in GVHD clinical trials, is based on palpation of the skin and is weighted towards acral involvement. In contrast, the fingers and toes are infrequently affected in sclerotic GVHD. Rodnan scoring is also inadequate to assess changes in the deep subcutaneous fat and fascia.77 Other objective tools should be considered, particularly in trials in which skin sclerosis and fascial involvement is a primary outcome measure. These include imaging (ultrasound26,128 and magnetic resonance imaging129,130) and devices that measure tissue hardness, such as durometry.131 In patients with joint involvement, range of motion assessment at the sites of joint contracture75 provides an another avenue to assess clinically meaningful improvement.
In conclusion, skin involvement is a significant problem for many patients with acute and chronic GHVD. The lack of a “criterion standard” diagnostic test, especially in chronic GVHD, makes it imperative that dermatologists be aware of its presentation in its many forms, the natural history of the disease, and the many variables that are factored into treatment decisions. The psychological impact of chronic GVHD can be devastating, and studies have consistently shown diminished physical, social, and sexual functioning in affected patients.132 Anxiety, psychosocial disturbances, and length of depression episodes are also more severe.133 Cutaneous involvement contributes to struggles with body image and impaired functional performance, as do problems with infection and secondary tumors. It is not yet known why certain patients develop particular skin changes, but dermatologists play an integral role in the multidisciplinary team needed to care for their problems.
CAPSULE SUMMARY.
Treatment of graft-versus-host disease requires close collaboration with the transplant team and an understanding of the complex medical issues facing patients with the disease.
Prompt treatment of skin involvement may decrease the risk of skin breakdown, contracture formation, and permanent disability.
Optimal care of the patient with chronic graft-versus-host disease involves consideration of topical therapies, phototherapy, and systemic management, along with surveillance for skin infection and cutaneous malignancy.
Abbreviations used
- CNI
calcineurin inhibitor
- CSA
cyclosporine
- ECP
extracorporeal photopheresis
- EV
epidermodysplasia verucciformis
- GVHD
graft-versus-host disease
- GVT
graft-versus-tumor
- HCT
hematopoietic cell transplantation
- MMF
mycophenolate mofetil
- MSC
mesenchymal stem cell
- mTOR
mammalian target of rapamycin
- MTX
methotrexate
- NB-UVB
narrowband ultraviolet B light phototherapy
- PUVA
psoralen plus ultraviolet A phototherapy
- PDGFR
platelet-derived growth factor receptor
- SCC
squamous cell carcinoma
- TSS
total skin score
- UVA-1
ultraviolet A-l
- UVR
ultraviolet radiation
- Tregs
T-regulatory cells
- TSS
total skin score
Footnotes
Supported in part by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research.
Reprints not available from the authors.
REFERENCES
- 1.Couriel D, Carpenter PA, Cutler C, Bolahos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-host Disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 2006;12:375–96. [DOI] [PubMed] [Google Scholar]
- 2.Elad S, Or R, Resnick I, Shapira MY. Topical tacrolimus—a novel treatment alternative for cutaneous chronic graft-versus-host disease. Transpl Int 2003;16:665–70. [DOI] [PubMed] [Google Scholar]
- 3.Choi CJ, Nghiem P. Tacrolimus ointment in the treatment of chronic cutaneous graft-vs-host disease: a case series of 18 patients. Arch Dermatol 2001;137:1202–6. [DOI] [PubMed] [Google Scholar]
- 4.Schmook T, Kraft J, Benninghoff B, Nindl I, Roewert J, Ulrich C, et al. Treatment of cutaneous chronic graft-versus-host disease with topical pimecrolimus. Bone Marrow Transplant 2005;36:87–8. [DOI] [PubMed] [Google Scholar]
- 5.Neuman DL, Farrar JE, Moresi JM, Vogelsang GB, Higman MA. Toxic absorption of tacrolimus [corrected] in a patient with severe acute graft-versus-host disease. Bone Marrow Transplant 2005;36:919–20. [DOI] [PubMed] [Google Scholar]
- 6.Choi IS, Jang IS, Han JY, Kim JH, Lee SG. Therapeutic experience on multiple contractures in sclerodermoid chronic graft versus host disease. Support Care Cancer 2009;17:851–5. [DOI] [PubMed] [Google Scholar]
- 7.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009;15: 1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markus YM, Bell MJ, Evans AW. Ischemic scleroderma wounds successfully treated with hyperbaric oxygen therapy. J Rheumatol 2006;33:1694–6. [PubMed] [Google Scholar]
- 9.Berg JO, Vindelov L, Schmidt G, Drzewiecki KT. Allogeneic split-skin grafting in stem cell transplanted patients. J Plast Reconstr Aesthet Surg 2008;61:1512–5. [DOI] [PubMed] [Google Scholar]
- 10.Eckardt A, Starke O, Stadler M, Reuter C, Hertenstein B. Severe oral chronic graft-versus-host disease following allogeneic bone marrow transplantation: highly effective treatment with topical tacrolimus. Oral Oncol 2004;40: 811–4. [DOI] [PubMed] [Google Scholar]
- 11.Conrotto D, Carrozzo M, Ubertalli AV, Gandolfo S, Giaccone L, Boccadoro M, et al. Dramatic increase of tacrolimus plasma concentration during topical treatment for oral graft-versus-host disease. Transplantation 2006;82:1113–5. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JB, Nantel S, Sheoltch SM. Topical azathioprine in the combined treatment of chronic oral graft-versus-host disease. Bone Marrow Transplant 2000;25:683–7. [DOI] [PubMed] [Google Scholar]
- 13.Spiryda LB, Laufer MR, Soiffer RJ, Antin JA. Graft-versus-host disease of the vulva and/or vagina: diagnosis and treatment. Biol Blood Marrow Transplant 2003;9:760–5. [DOI] [PubMed] [Google Scholar]
- 14.Turner ML, Stratton P. Gynecological manifestations of chronic graft versus host disease. In: Vogelsang G, Pavletic S, editors. Chronic graft versus host disease: interdisciplinary management. Cambridge (UK): Cambridge University Press; 2009. pp. 207–15. [Google Scholar]
- 15.Yared J, Gojo I, Akpek G. Gians penis involvement: an under-recognized manifestation of chronic GVHD. Bone Marrow Transplant doi: 10.1038/bmt.2011.209. Published online November 7, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Kitajima T, Imamura S. Graft-versus-host reaction enhanced by ultraviolet radiation. Arch Dermatol Res 1993;285:499–501. [DOI] [PubMed] [Google Scholar]
- 17.Murphy GM. Ultraviolet radiation and immunosuppression. Br J Dermatol 2009;161(suppl 3):90–5. [DOI] [PubMed] [Google Scholar]
- 18.Ozawa M, Ferenczi K, Kikuchi T, Cardinale I, Austin LM, Coven TR, et al. 312-nanometer ultraviolet B light (narrow-band UVB) induces apoptosis of T cells within psoriatic lesions. J Exp Med 1999;189:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kripke ML, Morison WL, Parrish JA. Systemic suppression of contact hypersensitivity in mice by psoralen plus UVA radiation (PUVA). J Invest Dermatol 1983;81:87–92. [DOI] [PubMed] [Google Scholar]
- 20.Hymes SR, Morison WL, Farmer ER, Walters LL, Tutschka PJ, Santos GW. Methoxsalen and ultraviolet A radiation in treatment of chronic cutaneous graft-versus-host reaction. J Am Acad Dermatol 1985;12:30–7. [DOI] [PubMed] [Google Scholar]
- 21.Ashworth J, Kahan MC, Breathnach SM. PUVA therapy decreases HLA-DR+ CDIa+ Langerhans cells and epidermal cell antigen-presenting capacity in human skin, but flow cytometrically-sorted residual HLA-DRT CDIa+ Langerhans cells exhibit normal alloantigen-presenting function. Br J Dermatol 1989;120:329–39. [DOI] [PubMed] [Google Scholar]
- 22.Kim TY, Kripke ML, Ullrich SE. Immunosuppression by factors released from UV-irradiated epidermal cells: selective effects on the generation of contact and delayed hypersensitivity after exposure to UVA or UVB radiation. J Invest Dermatol 1990;94:26–32. [DOI] [PubMed] [Google Scholar]
- 23.Kang K, Hammerberg C, Meunier L, Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J Immunol 1994;153:5256–64. [PubMed] [Google Scholar]
- 24.Grundmann-Kollmann M, Martin H, Ludwig R, Klein S, Boehncke WH, Hoelzer D, et al. Narrowband UV-B phototherapy in the treatment of cutaneous graft versus host disease. Transplantation 2002;74:1631–4. [DOI] [PubMed] [Google Scholar]
- 25.Vogelsang GB, Wolff D, Altomonte V, Farmer E, Morison WL, Corio R, et al. Treatment of chronic graft-versus-host disease with ultraviolet irradiation and psoralen (PUVA). Bone Marrow Transplant 1996;17:1061–7. [PubMed] [Google Scholar]
- 26.Leiter U, Kaskel P, Krahn G, Gottlober P, Bunjes D, Peter RU, et al. Psoralen plus ultraviolet-A-bath photochemotherapy as an adjunct treatment modality in cutaneous chronic graft versus host disease. Photodermatol Photoimmunol Photomed 2002;18:183–90. [DOI] [PubMed] [Google Scholar]
- 27.Enk CD, Elad S, Vexler A, Kapelushnik J, Gorodetsky R, Kirschbaum M. Chronic graft-versus-host disease treated with UVB phototherapy. Bone Marrow Transplant 1998;22: 1179–83. [DOI] [PubMed] [Google Scholar]
- 28.Brazzelli V, Grasso V, Muzio F, Moggio E, Zecca M, Locatelli F, et al. Narrowband ultraviolet B phototherapy in the treatment of cutaneous graft-versus-host disease in oncohaematological paediatric patients. Br J Dermatol 2010;162: 404–9. [DOI] [PubMed] [Google Scholar]
- 29.Grundmann-Kollmann M, Behrens S, Gruss C, Gottlober P, Peter RU, Kerscher M. Chronic sclerodermic graft-versus-host disease refractory to immunosuppressive treatment responds to UVA1 phototherapy. J Am Acad Dermatol 2000; 42:134–6. [DOI] [PubMed] [Google Scholar]
- 30.Ziemer M, Thiele JJ, Gruhn B, Eisner P. Chronic cutaneous graft-versus-host disease in two children responds to UVA1 therapy: improvement of skin lesions, joint mobility, and quality of life. J Am Acad Dermatol 2004;51:318–9. [DOI] [PubMed] [Google Scholar]
- 31.Wetzig T, Sticherling M, Simon JC, Hegenbart U, Niederwieser D, Al-Ali HK. Medium dose long-wavelength ultraviolet A (UVA1) phototherapy for the treatment of acute and chronic graft-versus-host disease of the skin. Bone Marrow Transplant 2005;35:515–9. [DOI] [PubMed] [Google Scholar]
- 32.Cowen EW, Nguyen JC, Miller DD, McShane D, Arron ST, Prose NS, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol 2010;62:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vadnerkar A, Nguyen MH, Mitsani D, Crespo M, Pilewski J, Toyoda Y, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transplant 2010;29:1240–4. [DOI] [PubMed] [Google Scholar]
- 34.Miller DD, Cowen EW, Nguyen JC, McCalmont TH, Fox LP. Melanoma associated with long-term voriconazole therapy: a new manifestation of chronic photosensitivity. Arch Dermatol 2010;146:300–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolb HJ, Socie G, Duell T, Van Lint MT, Tichelli A, Apperley JF, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med 1999;131:738–44. [DOI] [PubMed] [Google Scholar]
- 36.Andreani V, Richard MA, Blaise D, Gouvernet J, Grob JJ. Naevi in allogeneic bone marrow transplantation recipients: the effect of graft-versus-host disease on naevi. Br J Dermatol 2002;147:433–41. [DOI] [PubMed] [Google Scholar]
- 37.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med 1997;336:897–904. [DOI] [PubMed] [Google Scholar]
- 38.Sloand EM, Pfannes L, Ling C, Feng X, Jasek M, Calado R, et al. Graft-versus-host disease: role of inflammation in the development of chromosomal abnormalities of keratinocytes. Biol Blood Marrow Transplant 2010;16:1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol 2006;24:1119–26. [DOI] [PubMed] [Google Scholar]
- 40.Szeto CH, Shek TW, Lie AK, Au WY, Yuen AP, Kwong YL. Squamous cell carcinoma of the tongue complicating chronic oral mucosal graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Am J Hematol 2004;77:200–2. [DOI] [PubMed] [Google Scholar]
- 41.Garnis S, Billick RC, Srolovitz H. Eruptive vascular tumors associated with chronic graft-versus-host disease. J Am Acad Dermatol 1984;10:918–21. [DOI] [PubMed] [Google Scholar]
- 42.Soo JK, Mortimer PS. Eruptive angiomas associated with graft-versus-host disease. Br J Dermatol 2006;154:376–8. [DOI] [PubMed] [Google Scholar]
- 43.Adamski H, Le Gall F, Cartron L, Dauriac C, Lancien G, Wechsler J, et al. Eruptive angiomatous lesions associated with graft-versus-host disease. Br J Dermatol 2003;149:667–8. [DOI] [PubMed] [Google Scholar]
- 44.Kunishige JH, Hymes SR, Madkan V, Wyatt AJ, Uptmore D, Lazar AJ, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol 2007;57(5 suppl):S78–80. [DOI] [PubMed] [Google Scholar]
- 45.Diamantis ML, Richmond HM, Rady PL, Tyring SK, Cutlan JE, Torres-Cabala C, et al. Detection of human papillomavirus in multiple eccrine poromas in a patient with chronic graft-vs-host disease and immunosuppression. Arch Dermatol 2011;147:120–2. [DOI] [PubMed] [Google Scholar]
- 46.Wingard JR, Piantadosi S, Vogelsang GB, Farmer ER, Jabs DA, Levin LS, et al. Predictors of death from chronic graft-versus-host disease after bone marrow transplantation. Blood 1989;74:1428–35. [PubMed] [Google Scholar]
- 47.Akpek G, Zahurak ML, Piantadosi S, Margolis J, Doherty J, Davidson R, et al. Development of a prognostic model for grading chronic graft-versus-host disease. Blood 2001;97: 1219–26. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan KM, Witherspoon RP, Storb R, Weiden P, Flournoy N, Dahlberg S, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood 1988;72:546–54. [PubMed] [Google Scholar]
- 49.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 2003;9:657–66. [DOI] [PubMed] [Google Scholar]
- 50.Arora M, Klein JP, Weisdorf DJ, Hassebroek A, Flowers ME, Cutler CS, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood 2011;117:6714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-Simon JA, Encinas C, Silva F, Arcos MJ, Díez-Campelo M, Sánchez-Guijo FM, et al. Prognostic factors of chronic graft-versus-host disease following allogeneic peripheral blood stem cell transplantation: the National Institutes Health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biol Blood Marrow Transplant 2008;14:1163–71. [DOI] [PubMed] [Google Scholar]
- 52.Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood 2004;104:3501–6. [DOI] [PubMed] [Google Scholar]
- 53.Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood 1991;77:1821–8. [PubMed] [Google Scholar]
- 54.Van Lint MT, Uderzo C, Locasciulli A, Majolino I, Scime R, Locatelli F, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood 1998;92:2288–93. [PubMed] [Google Scholar]
- 55.Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 2009;114: 511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cragg L, Blazar BR, Defor T, Kolatker N, Miller W, Kersey J, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant 2000;6:441–7. [DOI] [PubMed] [Google Scholar]
- 57.Couriel DR, Saliba R, de Lima M, Giralt S, Andersson B, Khouri I, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant 2009;15:1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 1990;75: 1024–30. [PubMed] [Google Scholar]
- 59.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986;314:729–35. [DOI] [PubMed] [Google Scholar]
- 60.Rossi SJ, Schroeder TJ, Hariharan S, First MR. Prevention and management of the adverse effects associated with immunosuppressive therapy. Drug Saf 1993;9:104–31. [DOI] [PubMed] [Google Scholar]
- 61.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998;92:2303–14. [PubMed] [Google Scholar]
- 62.Bhushan V, Collins RH Jr. Chronic graft-vs-host disease. JAMA 2003;290:2599–603. [DOI] [PubMed] [Google Scholar]
- 63.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 2002;100:48–51. [DOI] [PubMed] [Google Scholar]
- 64.Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood 2009; 113:5074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 2006; 108:390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVFID. Biol Blood Marrow Transplant 2010;16:1611–28. [DOI] [PubMed] [Google Scholar]
- 67.Wolff D, Schleuning M, von Flarsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2011;17:1–17. [DOI] [PubMed] [Google Scholar]
- 68.Peritt D. Potential mechanisms of photopheresis in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006;12:7–12. [DOI] [PubMed] [Google Scholar]
- 69.Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood 2008;112:1515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seaton ED, Szydlo RM, Kanfer E, Apperley JF, Russell-Jones R. Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood 2003;102: 1217–23. [DOI] [PubMed] [Google Scholar]
- 71.Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood 2006;107:3074–80. [DOI] [PubMed] [Google Scholar]
- 72.Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood 2008;112:2667–74. [DOI] [PubMed] [Google Scholar]
- 73.Romano C, Rubegni P, De Aloe G, Stanghellini E, D'Ascenzo G, Andreassi L, et al. Extracorporeal photochemotherapy in the treatment of eosinophilic fasciitis. J Eur Acad Dermatol Venereol 2003;17:10–3. [DOI] [PubMed] [Google Scholar]
- 74.Patel AR, Avila D, Malech HL, Pavletic SZ, Yao L, Cowen EW. Rippled skin, fasciitis, and joint contractures. J Am Acad Dermatol 2008;59:1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sbano P, Rubegni P, De Aloe GB, Guidi S, Fimiani M. Extracorporeal photochemotherapy for treatment of fasciitis in chronic graft-versus-host disease. Bone Marrow Transplant 2004;33:869–70. [DOI] [PubMed] [Google Scholar]
- 76.Marshall SR. Technology insight: ECP for the treatment of GvHD—can we offer selective immune control without generalized immunosuppression? Nat Clin Pract Oncol 2006;3:302–14. [DOI] [PubMed] [Google Scholar]
- 77.Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006; 12:252–66. [DOI] [PubMed] [Google Scholar]
- 78.Park J, Ha H, Ahn HJ, Kang SW, Kim YS, Seo JY, et al. Sirolimus inhibits platelet-derived growth factor-induced collagen synthesis in rat vascular smooth muscle cells. Transplant Proc 2005;37:3459–62. [DOI] [PubMed] [Google Scholar]
- 79.Jedlickova Z, Burlakova I, Bug G, Baurmann H, Schwerdtfeger R, Schleuning M. Therapy of sclerodermatous chronic graft-versus-host disease with mammalian target of rapamycin inhibitors. Biol Blood Marrow Transplant 2011;17:657–63. [DOI] [PubMed] [Google Scholar]
- 80.Couriel DR, Saliba R, Escalon MP, Hsu Y, Ghosh S, Ippoliti C, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol 2005;130:409–17. [DOI] [PubMed] [Google Scholar]
- 81.Jurado M, Vallejo C, Perez-Simon JA, Brunet S, Ferra C, Balsalobre P, et al. Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol Blood Marrow Transplant 2007;13:701–6. [DOI] [PubMed] [Google Scholar]
- 82.Schultz K. Pathophysiology of chronic graft-versus-host disease. In: Vogelsang G, Pavletic S, editors. Chronic graft versus host disease: interdisciplinary management. Cambridge (UK): Cambridge University Press; 2009. pp. 17–30. [Google Scholar]
- 83.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med 2010;152:159–66. [DOI] [PubMed] [Google Scholar]
- 84.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 2006;354: 2667–76. [DOI] [PubMed] [Google Scholar]
- 85.Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graftversus-host disease. Blood 2007;110:237–41. [DOI] [PubMed] [Google Scholar]
- 86.Magro L, Mohty M, Catteau B, Coiteux V, Chevallier P, Terriou L, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood 2009;114: 719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Olivieri A, Locatelli F, Zecca M, Sanna A, Cimminiello M, Raimondi R, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood 2009; 114:709–18. [DOI] [PubMed] [Google Scholar]
- 88.Dragun D, Distler JH, Riemekasten G, Distler O. Stimulatory autoantibodies to platelet-derived growth factor receptors in systemic sclerosis: what functional autoimmunity could learn from receptor biology. Arthritis Rheum 2009;60:907–11. [DOI] [PubMed] [Google Scholar]
- 89.Nakasone H, Kanda Y, Takasaki H, Nakaseko C, Sakura T, Fujisawa S, et al. Prophylactic impact of imatinib administration after allogeneic stem cell transplantation on the incidence and severity of chronic graft versus host disease in patients with Philadelphia chromosome-positive leukemia. Leukemia 2010;24:1236–9. [DOI] [PubMed] [Google Scholar]
- 90.Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 2005;105:2473–9. [DOI] [PubMed] [Google Scholar]
- 91.Breccia M, Cannella L, Stefanizzi C, Carotti A, Santopietro M, Alimena G. Efficacy of dasatinib in a chronic myeloid leukemia patient with disease molecular relapse and chronic GVHD after haploidentical BMT: an immunomodulatory effect? Bone Marrow Transplant 2009;44:331–2. [DOI] [PubMed] [Google Scholar]
- 92.Pulanic D, Cowen EW, Baird K, Bishop MR, Pavletic SZ. Development of severe sclerotic chronic GVHD during treatment with dasatinib. Bone Marrow Transplant 2010;45:1469–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uberti JP, Ayash L, Ratanatharathorn V, Silver S, Reynolds C, Becker M, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant 2005;11:680–7. [DOI] [PubMed] [Google Scholar]
- 94.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol 2007;82:45–52. [DOI] [PubMed] [Google Scholar]
- 95.Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pidala J, Kim J, Field T, McBride A, Kharfan-Dabaja M, Perkins J, et al. Infliximab for managing steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant 2009;15:1116–21. [DOI] [PubMed] [Google Scholar]
- 97.Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood 2004;104:649–54. [DOI] [PubMed] [Google Scholar]
- 98.Chiang KY, Abhyankar S, Bridges K, Godder K, Henslee-Downey JP. Recombinant human tumor necrosis factor receptor fusion protein as complementary treatment for chronic graft-versus-host disease. Transplantation 2002;73:665–7. [DOI] [PubMed] [Google Scholar]
- 99.Marty FM, Lee SJ, Fahey MM, Alyea EP, Soiffer RJ, Antin JH, et al. Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-Candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: a cohort study. Blood 2003;102: 2768–76. [DOI] [PubMed] [Google Scholar]
- 100.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS Jr. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem 2001;276: 22382–7. [DOI] [PubMed] [Google Scholar]
- 101.Lepper ER, Smith NF, Cox MC, Scripture CD, Figg WD. Thalidomide metabolism and hydrolysis: mechanisms and implications. Curr Drug Metab 2006;7:677–85. [DOI] [PubMed] [Google Scholar]
- 102.Shapira MY, Abdul-Hai A, Resnick IB, Bitan M, Tsirigotis P, Aker M, et al. Alefacept treatment for refractory chronic extensive GVHD. Bone Marrow Transplant 2009;43:339–43. [DOI] [PubMed] [Google Scholar]
- 103.Ratanatharathorn V, Ayash L, Reynolds C, Silver S, Reddy P, Becker M, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant 2003;9:505–11. [DOI] [PubMed] [Google Scholar]
- 104.Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, Sanders CJ, van den Tweel JG, Verdonck LF. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy-refractory chronic graft-versus-host disease. Blood 2004;104:2603–6. [DOI] [PubMed] [Google Scholar]
- 105.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versushost disease. Blood 2006;108:756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mohty M, Marchetti N, EI-Cheikh J, Faucher C, Furst S, Blaise D. Rituximab as salvage therapy for refractory chronic GVHD. Bone Marrow Transplant 2008;41:909–11. [DOI] [PubMed] [Google Scholar]
- 107.Teshima T, Nagafuji K, Henzan H, Miyamura K, Takase K, Hidaka M, et al. Rituximab for the treatment of corticosteroid-refractory chronic graft-versus-host disease. Int J Hematol 2009;90:253–60. [DOI] [PubMed] [Google Scholar]
- 108.Okamoto M, Okano A, Akamatsu S, Ashihara E, Inaba T, Takenaka H, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia 2006;20:172–3. [DOI] [PubMed] [Google Scholar]
- 109.Zaja F, Bacigalupo A, Patriarca F, Stanzani M, Van Lint MT, Fili C, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant 2007;40:273–7. [DOI] [PubMed] [Google Scholar]
- 110.Ratanatharathorn V, Logan B, Wang D, Horowitz M, Uberti JP, Ringden O, et al. Prior rituximab correlates with less acute graft-versus-host disease and better survival in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation. Br J Haematol 2009;145:816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2009;15:1005–13. [DOI] [PubMed] [Google Scholar]
- 112.von Bonin M, Oelschlagel U, Radke J, Stewart M, Ehninger G, Bornhauser M, et al. Treatment of chronic steroid-refractory graft-versus-host disease with low-dose rituximab. Transplantation 2008;86:875–9. [DOI] [PubMed] [Google Scholar]
- 113.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004;363:1439–41. [DOI] [PubMed] [Google Scholar]
- 114.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006;81:1390–7. [DOI] [PubMed] [Google Scholar]
- 115.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579–86. [DOI] [PubMed] [Google Scholar]
- 116.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3 + regulatory T cells. Stem Cells 2008;26:212–22. [DOI] [PubMed] [Google Scholar]
- 117.Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, et al. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant 2010;16:403–12. [DOI] [PubMed] [Google Scholar]
- 118.Marcellus DC, Altomonte VL, Farmer ER, Horn TD, Freemer CS, Grant J, et al. Etretinate therapy for refractory sclerodermatous chronic graft-versus-host disease. Blood 1999;93: 66–70. [PubMed] [Google Scholar]
- 119.de Lavallade H, Mohty M, Faucher C, Furst S, EI-Cheikh J, Blaise D. Low-dose methotrexate as salvage therapy for refractory graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Haematologica 2006;91:1438–40. [PubMed] [Google Scholar]
- 120.Gilman AL, Schultz KR. Treatment of chronic GVHD. Bone Marrow Transplant 2000;26:460–2. [DOI] [PubMed] [Google Scholar]
- 121.Jacobsohn DA, Chen AR, Zahurak M, Piantadosi S, Anders V, Bolanos-Meade J, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versushost disease. J Clin Oncol 2007;25:4255–61. [DOI] [PubMed] [Google Scholar]
- 122.Lee SJ, Wegner SA, McGarigle CJ, Bierer BE, Antin JH. Treatment of chronic graft-versus-host disease with clofazimine. Blood 1997;89:2298–302. [PubMed] [Google Scholar]
- 123.Greinix H, Antin J. Salvage therapy in chroinic graft versus host disease. In: Vogelsang G, Pavletic S, editors. Chronic graft versus host disease. Cambridge (UK): Cambridge University Press; 2009. pp. 134–45. [Google Scholar]
- 124.Jacobsohn DA, Rademaker A, Kaup M, Vogelsang GB. Skin response using NIH consensus criteria vs Hopkins scale in a phase II study for steroid-refractory chronic GVHD. Bone Marrow Transplant 2009;44:813–9. [DOI] [PubMed] [Google Scholar]
- 125.Greinix HT, Pohlreich D, Maalouf J, Soukup P, Supper V, Kalhs P, et al. A single-center pilot validation study of a new chronic GVHD skin scoring system. Biol Blood Marrow Transplant 2007;13:715–23. [DOI] [PubMed] [Google Scholar]
- 126.Jacobsohn DA. Shedding some LIGHT on chronic GVHD. Blood 2008;112:2593–4. [DOI] [PubMed] [Google Scholar]
- 127.Merkel PA, Clements PJ, Reveille JD, Suarez-Almazor ME, Valentini G, Furst DE. Current status of outcome measure development for clinical trials in systemic sclerosis. Report from OMERACT 6. J Rheumatol 2003;30:1630–47. [PubMed] [Google Scholar]
- 128.Kerscher M, Volkenandt M, Gruss C, Reuther T, von Kobyletzki G, Freitag M, et al. Low-dose UVA phototherapy for treatment of localized scleroderma. J Am Acad Dermatol 1998;38:21–6. [DOI] [PubMed] [Google Scholar]
- 129.Clark J, Yao L, Pavletic SZ, Krumlauf M, Mitchell S, Turner ML, et al. Magnetic resonance imaging in sclerotic-type chronic graft-vs-host disease. Arch Dermatol 2009;145:918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oda K, Nakaseko C, Ozawa S, Nishimura M, Saito Y, Yoshiba F, et al. Fasciitis and myositis: an analysis of muscle-related complications caused by chronic GVHD after allo-SCT. Bone Marrow Transplant 2009;43:159–67. [DOI] [PubMed] [Google Scholar]
- 131.Merkel PA, Silliman NP, Denton CP, Furst DE, Khanna D, Emery P, et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum 2008;59:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Worel N, Biener D, Kalhs P, Mitterbauer M, Keil F, Schulenburg A, et al. Long-term outcome and quality of life of patients who are alive and in complete remission more than two years after allogeneic and syngeneic stem cell transplantation. Bone Marrow Transplant 2002;30:619–26. [DOI] [PubMed] [Google Scholar]
- 133.Beanlands HJ, Lipton JH, McCay EA, Schimmer AD, Elliott ME, Messner HA, et al. Self-concept as a "BMT patient", illness intrusiveness, and engulfment in allogeneic bone marrow transplant recipients. J Psychosom Res 2003;55:419–25. [DOI] [PubMed] [Google Scholar]