Abstract
X-ray radiotherapy has been widely used in the treatment of cervical cancer, a common gynecologic malignant tumor. However, the therapeutic efficacy tends to be indistinctive. One major reason for this is amplification of the dihydrofolate reductase (DHFR) gene, which causes an increase in DHFR activity and attenuation of the treatment effect. To solve this problem, we synthesized a series of DHFR inhibitors derived from methotrexate (MTX) analogues as radiotherapy sensitizers. Activity screening revealed that compound 2a exerted the best inhibitory effect toward DHFR activity. In combination with X-ray radiotherapy (4 Gy), 2a showed much more prominent antiproliferative activity on cervical cancer cells than 2a or X-rays alone and revealed higher selectivity and radiosensitization than MTX. In vitro experiments showed that 2a + X-rays significantly induced cell apoptosis, as revealed by the increase in the Sub-G1 population and activation of caspase 3, 8, and 9. The in vivo antitumor effect demonstrated that in the presence of X-rays, 2a effectively suppressed tumor growth and did not cause obvious side effects. In conclusion, as a DHFR inhibitor, 2a successfully reversed the radioresistance problem induced by radiotherapy and greatly promoted the therapeutic effect. This is a promising candidate for tumor treatment that deserves further research and development. This study clearly demonstrates that DHFR inhibitors could be developed as promising radiosensitizers in the treatment of cervical cancer and that further research to improve their activity and potential in future clinical use is deserved.
Keywords: DHFR inhibitor, radiotherapy, sensitizers, gene amplification
Cervical cancer is a common cancer among female patients with genital diseases. Radiotherapy has become one of the most important treatments applied in all stages.1 However, radiotherapy tends to trigger amplification of the dihydrofolate reductase (DHFR) gene,2−4 which enhances the DHFR activity. DHFR is a critical enzyme for the synthesis of thymidine, which is the precursor of DNA. Enhancement of the DHFR activity promotes DNA replication of tumor cells, resulting in attenuation of the treatment effect. Thus, if the DHFR activity is inhibited, radiotherapy effects would be improved to varying degrees.5,6
Different types of tumor cells show different forms of response to ionizing radiation. For instance, lymphoma responds pretty well,7 whereas HeLa cells are not sensitive to ionizing radiation.8 One of the mechanisms of radiotherapy is that the hydrolytic dissociation by high-energy rays forms reactive oxygen species (ROS), which oxidize, attack, and damage DNA bases and cellular structure, resulting in tumor cell death.9 The DNA replication and repair processes require a sufficient supply of bases. Once the synthesis of bases is impeded, DNA replication and repair are blocked.10 DHFR inhibitors, such as methotrexate (MTX), mainly impede the synthesis of thymidine, leading to blocking of DNA synthesis. Thus, in the presence of X-rays, the use of MTX analogues as sensitizers is hopeful to improve the treatment efficacy because of their inhibitory effects toward DHFR.11−14
Deriving from the parent structure (2,4-diaminopteridine) of MTX, a series of 2,4-diaminopteridine analogues were synthesized as DHFR inhibitors in this work. Their structures are shown in Figure 1A, and their anticancer activities in the presence or absence of X-rays (4 Gy) were compared. In the screening of inhibitory activity toward DHFR, compound 2a showed the highest activity among the synthetic compounds. Meanwhile, 2a-induced cell death and ROS-mediated mitochondrial dysfunction in the presence of X-rays and the in vivo effects on HeLa cells were also investigated. This study clearly demonstrates that these synthetic DHFR inhibitors deserve further investigation as promising radiosensitizers in clinical therapy for cervical cancer to improve their treatment efficacy.
Figure 1.
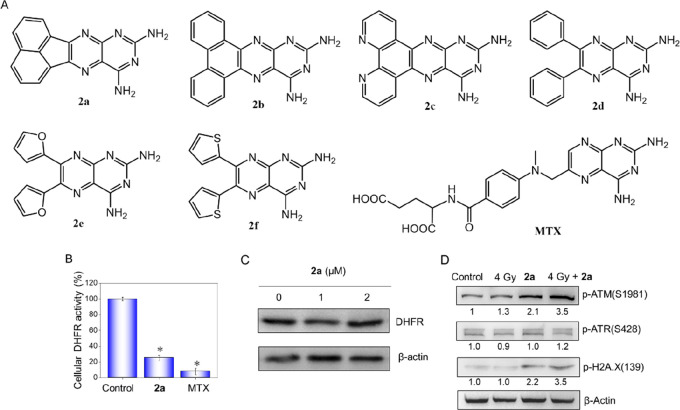
Structures of the synthetic 2,4-diaminopteridine derivatives and inhibitory effects of 2a on intracellular DHFR activity and enhancement on X-ray-induced DNA damage. (A) Structures of the synthetic 2,4-diaminopteridine derivatives and MTX. (B) DHFR activities in lysates of HeLa cells treated with 2a at a concentration of 2 μM. (C) Protein levels of DHFR from cell lysates analyzed by Western blotting. (D) Efficient activation of ATM and H2A.X by 2a and X-rays after DNA replication stress. After treatment for 48 h, cells were harvested, lysed, and subjected to Western blotting.
Synthesis
Compounds 2a–f (Figure 1A) were prepared according to Scheme S1. The general principle for the synthetic approach to 2,4-diaminopteridine derivatives was based on the condensation of pyrimidine-2,4,5,6-tetraamine with different substituted biacetyls in refluxing EtOH. Their structures were confirmed by NMR and MS analyses (Figures S1–S17).
In Vitro Antiproliferative Activities of 2,4-Diaminopteridine Derivatives in Combination with X-rays against HeLa Cells
The antiproliferative activities of the 2,4-diaminopteridine derivatives alone and in combination with X-rays (4 Gy) against HeLa cells were measured, and the results are shown in Figure S18. The IC50’s of synthetic 2,4-diaminopteridine derivatives toward HeLa cancer cells and Ect1/E6E7 normal cells are shown in Table 1. In general, we identified MTX > 2a > 2f > 2c > 2e > 2d > 2b in terms of IC50. Moreover, in combination with X-rays, the antiproliferative activities of all of the compounds except 2b improved to varying degrees. It is worth noting that in the presence of X-rays, 2a (0.64 μM) surpassed MTX (0.82 μM), with a sensitivity enhancement ratio (SER) of 3.3, which is larger than that of MTX (1.8).
Table 1. Cytotoxic Activities of 2,4-Diaminopteridine Derivatives on HeLa Cancer Cells and Ect1/E6E7 Normal Cells with (4 Gy) or without (0 Gy) X-rays by MTT Assay.
| IC50 (μM) |
|||||||
|---|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2d | 2e | 2f | MTX | |
| HeLa + 0 Gy | 2.1 ± 0.2 | >80 | 9.8 ± 0.3 | 32.6 ± 1.2 | 24.5 ± 1.2 | 4.6 ± 0.3 | 1.5 ± 0.2 |
| HeLa + 4 Gy | 0.64 ± 0.03 | >80 | 6.3 ± 0.2 | 14.8 ± 0.4 | 14.6 ± 0.8 | 2.3 ± 0.2 | 0.82 ± 0.08 |
| SERa | 3.3 ± 0.2 | NA | 1.6 ± 0.1 | 2.20 ± 0.2 | 1.7 ± 0.1 | 2.0 ± 0.4 | 1.8 ± 0.3 |
| Ect1/E6E7 + 0 Gy | 6.9 ± 0.3 | >80 | 20.0 ± 1.0 | 59.3 ± 1.6 | 40.1 ± 1.6 | 10.9 ± 1.8 | 2.9 ± 0.3 |
| Ect1/E6E7 + 4 Gy | 3.1 ± 0.2 | >80 | 13.9 ± 1.0 | 38.1 ± 1.9 | 19.8 ± 1.2 | 5.3 ± 0.3 | 1.8 ± 0.2 |
| SERb | 2.2 ± 0.1 | NA | 1.4 ± 0.2 | 1.6 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.2 | 1.6 ± 0.3 |
| SI0c | 3.3 ± 0.4 | NA | 2.0 ± 0.1 | 1.8 ± 0.1 | 1.6 ± 0.1 | 2.5 ± 0.3 | 1.9 ± 0.2 |
| SI4d | 4.8 ± 0.5 | NA | 2.2 ± 0.1 | 2.6 ± 0.1 | 1.4 ± 0.1 | 2.3 ± 0.4 | 2.2 ± 0.1 |
Sensitivity enhancement ratio (SER) = IC50(HeLa + 0 Gy)/IC50(HeLa + 4 Gy).
SER = IC50 (Ect1/E6E7 + 0 Gy)/IC50 (Ect1/E6E7 + 4 Gy).
Safe index for 0 Gy (SI0) = (Ect1/E6E7 + 0 Gy)/(HeLa + 0 Gy).
Safe index for 4 Gy (SI4) = (Ect1/E6E7 + 4 Gy)/(HeLa + 4 Gy).
On the other hand, the cytotoxic activities of the diaminopteridine derivatives against Ect1/E6E7, a normal ectocervix epithelial cell line, were also determined. The results displayed that MTX exhibits high toxicity toward Ect1/E6E7 cells with IC50’s of 2.9 μM (+0 Gy) and 1.8 μM (+4Gy). However, the toxicity of 2a against Ect1/E6E7 was lower than that of MTX, with IC50’s of 6.9 and 3.1 μM, respectively. Similarly, the other diaminopteridines possessed a certain extent of selectivity, which were reflected by the safe indexes (SIs) with X-rays (4 Gy) (SI4) or without X-rays (SI0). Especially, the SI4 of 2a was 4.8, which was more than 2-fold larger compared with MTX. These results led to the conclusion that 2a demonstrated apparent sensitization in the presence of X-rays (4 Gy) against HeLa cell lines and possessed a certain extent of selectivity.
Evaluation of In Vitro DHFR Inhibitory Activities of 2a–f
After investigating the cytotoxic activities of 2,4-diaminopteridine derivatives, we next wanted to determine whether there were similar discrepancies in the inhibitory effects of 2a–f on DHFR activity. The DHFR inhibitory activities of 2a–f along with MTX as a comparison were determined using a DHFR assay kit (Sigma-Aldrich, S0340); the results are presented in Figure S19, and the IC50 values are presented in Table 2. Although 2a, 2b, and 2c possess a similar planar structure, they showed significantly different inhibitory activities against DHFR. 2a exhibited the best inhibitory efficacy among the six compounds, with an IC50 of 0.81 μM, followed by 2f (IC50 = 1.43 μM). 2c had moderate inhibitory activity, with an IC50 of 5.8 μM. It can thus be seen that the inhibitory activities of 2a, 2f, and 2c corresponded well with their antiproliferative activities. On the other hand, 2b, with a similar planar structure as 2a, exhibited the worst activity, with IC50 > 50 μM. Besides, 2d and 2e had similar structures with rotatable substituent groups at the 6- and 7-position and showed relatively poor activities against DHFR, with IC50’s of 13.6 and 10.4 μM, respectively. Together, our results showed that 2a has the most excellent inhibitory activity against DHFR among the six compounds.
Table 2. IC50 Values for Inhibition of DHFR Activity by Compounds 2a–f.
| compound | IC50 (μM) |
|---|---|
| 2a | 0.81 ± 0.10 |
| 2b | >50 |
| 2c | 5.8 ± 0.36 |
| 2d | 13.6 ± 0.59 |
| 2e | 10.4 ± 0.44 |
| 2f | 1.43 ± 0.19 |
| MTX | 0.083 ± 0.005 |
2a Inhibits DHFR Activity and Activates ATM and H2A.X in Response to DNA Replication Stress
As the leading drug, 2a was used to evaluate the inhibitory effects on intracellular DHFR on account of its best inhibitory effect against DHFR in vitro among the six synthetic derivatives. Meanwhile, MTX was used as a positive control. The results showed that intracellular DHFR activity was obviously inhibited by 2a at the cytotoxic concentrations of 10 μM (76% inhibition compared with the control) (Figure 1B). However, a more apparent inhibitory effect on DFHR can be seen for MTX (91% inhibition). This corresponded well with the IC50 values against HeLa cells. Therefore, the prominent antiproliferative activity of 2a was mainly attributed to the inhibitory effect on DHFR. Western blot analyses (Figure 1C) indicated that there was obvious change in protein levels of DHFR in cells treated with 2a, demonstrating that the decline of intracellular DHFR activity was regulated by 2a and not associated with downregulation of DHFR protein levels.
DHFR is indispensable for the synthesis of nucleic acids. Therefore, the inhibition of DHFR activity gives rise to DNA replication stress. Persistent replication stress results in phosphorylation of DNA-damage-dependent kinases such as ATR, ATM, and DNA-PK. Western blotting results (Figure 1D) showed that no distinct phosphorylation of ATR can be seen in the treatment groups compared with the control group. However, an obvious increase in ATM phosphorylation following treatment with 2a or X-rays alone can be observed. Meanwhile, the phosphorylation level of ATM increased much more significantly in the 2a + X-rays group than in the groups with 2a or X-rays alone. Furthermore, we also examined the level of γ-H2A.X, which is the phosphorylated form of H2A.X produced by ATR, ATM, and DNA-PK. Meanwhile, γ-H2A.X is essential for checkpoint-related cell-cycle arrest and DNA repair resulting from ionizing radiation or chemotherapeutic agent, which leads to rapid phosphorylation of H2A.X at Ser139. Our results showed that X-rays alone did not cause H2A.X phosphorylation but that 2a alone or combined with X-rays resulted in distinct phosphorylation of γ-H2A.X. This demonstrates that phosphorylation of ATM and H2A.X plays a critical role in the response of 2a-induced DNA replication stress.
2a Combines with X-rays to Promote Apoptosis of HeLa Cells
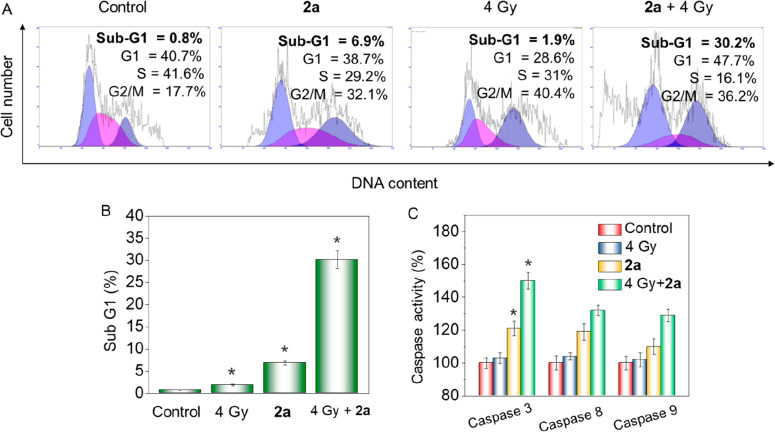
Anticancer drugs typically exert their cytotoxic effects by triggering cell-cycle arrest or apoptosis in susceptible cells.15,16 To understand the mechanism of drug action induced by 2a in the absence or presence of X-rays, cell-cycle assays were performed. Figure 2A shows that X-rays alone caused apparent G2/M phase arrest in HeLa cells from 17.7% (control) to 40.4%. Besides, cells treated with 2a showed a moderate level of G2/M arrest accompanied by a rise in the Sub-G1 peak, accounting for 6.9%, demonstrating that apoptosis is involved in 2a-triggered cell death. Interestingly, cells treated with 2a and X-rays together exerted a remarkable synergistic effect, as the Sub-G1 peak shot up to 30.2% (Figure 2B), indicating a marked increase of apoptosis. These data showed that apoptosis was mainly involved in the synergistic action of 2a and X-rays on HeLa cells.
Figure 2.
2a combines with X-rays to promote apoptosis of HeLa cells. (A) Flow cytometry analysis indicated the distribution of cell cycle in HeLa cells. Cells were treated with 2a (1 μM) without or with X-rays (4 Gy) for 48 h. (B) Percentages of Sub-G1 phase population. (C) Activities of caspase 8, 9, and 3 on HeLa cells after treatment with 2a (1 μM) without or with X-rays (4 Gy) for 12 h. Values are shown as mean ± SD for three independent experiments. Bars are statistically different at *p < 0.05.
The caspase cascade system is closely related to the intracellular apoptosis signals, such as signal induction, transduction, and amplification.17,18 Since apoptosis-inducing effects and anticancer activity of X-rays were significantly amplified by 2a, detection of caspase activity was subsequently conducted. As shown in Figure 2C, the activation of caspase 8, 9, and 3 by X-rays alone was limited, whereas they were effectively activated to various degrees by 2a (caspase 3 > caspase 8 > caspase 9). Moreover, the activation was apparently improved by the addition of 2a to X-rays, and the improvement was more distinct for caspase 3 and 8, from 121% to 150% and 110% to 129%, respectively. These data demonstrated that 2a was able to activate caspase 8 and caspase 9, which gave rise to the activation of executor caspase 3 and resulted in apoptosis. The activation was apparently improved and apoptosis was obviously promoted when 2a was used in combination with X-rays.
Synergistic Effect of 2a and X-rays Improved Mitochondrial Dysfunction and ROS Generation
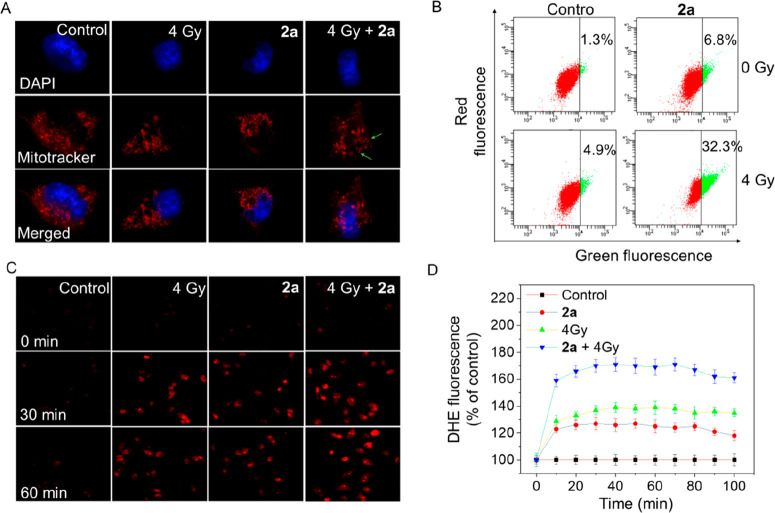
Mitochondrial dysfunction has been demonstrated to be closely related to cellular apoptosis and plays a vitally important in the apoptotic pathway.19,20 Mitochondria are typically strewn through the whole cytoplasm, and the structures are mostly long, tubular, or filamentous. Mitochondrial fission has vital implications in stress response and apoptosis. Herein, the change in mitochondrial morphology was also studied. As shown in Figure 3A, treatment of cells with X-rays or 2a alone gave rise to slight mitochondrial fission. Moreover, the fission became significantly apparent when cells were treated with the combination of 2a and X-rays (4 Gy).
Figure 3.
Synergistic effect of 2a and X-rays on induction of mitochondrial dysfunction and ROS generation. (A) Fluorescent micrographs of mitochondrial fission induced by 2a (1 μM) in the absence or presence of X-rays (4 Gy). The photomicrographs were detected using Mitotracker and DAPI costaining. (B) Flow cytometry analysis of the changes in ΔΨm on HeLa cells treated with 2a (1 μM) without or with X-ray treatment. (C) Fluorescence images of intracellular ROS detected by DHE probe on HeLa cells. (D) Fluorescence microplate readings for intracellular ROS measured by DHE probe on HeLa cells.
The loss of mitochondrial membrane potential (MMP) has been proved to be an early event during the apoptotic process.21 To evaluate the role of the MMP in 2a-treated cells, the status of ΔΨm was studied by JC-1 flow cytometric analysis (Figure 3B). Our results indicated that cells treated with 2a (1 μM) or X-rays (4 Gy) alone displayed only limited depletion of ΔΨm (6.8% and 4.9%, respectively; the green fluorescence represents the proportion of loss of ΔΨm). However, the combination of 2a and X-rays induced a significant rise in the proportion of depolarized mitochondria (up to 32.3%), demonstrating that in the presence of X-rays, a low 2a concentration gave rise to rapid dissipation of ΔΨm compared with 2a alone. These data showed that mitochondrial dysfunction contributed to 2a-induced apoptosis on HeLa cells and that the dysfunction was apparently amplified in the presence of X-rays.
Reactive oxygen species (ROS) is a collective term for various oxygen-containing reactive and short-lived molecules.22 Ionizing radiation has been shown to typically generate ROS in a variety of cell types.23 ROS-induced oxidative injury causes DNA damage (mutations), protein oxidation, and lipid peroxidation. Under the circumstance of redox imbalance, cells react rapidly to give a plethora of biological responses. Rapid responses include cell-cycle-specific growth arrest, gene transcription, activation of signaling pathways, and repair of damaged DNA, which determine cell fates such as necroses, senesce, apoptosis, and proliferation. Therefore, we investigated ROS generation in HeLa cells by the use of the fluorescent probe dihydroethidium (DHE). Chemically reduced and acetylated forms of DHE are nonfluorescent. Upon reaction with superoxide anions, DHE forms a red fluorescent product (2-hydroxyethidium). Oxidation of these probes can be detected by monitoring the increase in fluorescence using a microplate reader or fluorescence microscope.
The fluorescence microscopy results (Figure 3C) showed that HeLa cells pretreated with X-rays (4 Gy) or 2a alone and then incubated with DHE for 30 min turned red, demonstrating the generation of a certain level of ROS. This fluorescence still remained in the next 30 min. Notably, the brightness of the DHE probe became higher when cells were treated with 2a and X-rays (4 Gy) together at the same time point. Meanwhile, microplate reader results (Figure 3D) showed that exposing HeLa cells to X-rays (4 Gy) alone triggered a 150% increase of ROS generation compared with nonirradiated cells. Treatment with 2a alone caused only a 132% increase in ROS. Interestingly, the ROS level of 2a-treated cells increased significantly in the presence of X-rays, rising to 205%. These results revealed that the synergistic effect of X-rays and 2a in inducing extensive accumulation of ROS is important and mediates the apoptosis of HeLa cells.
2a and X-rays Synergistically Inhibit the Migration of HeLa Cells
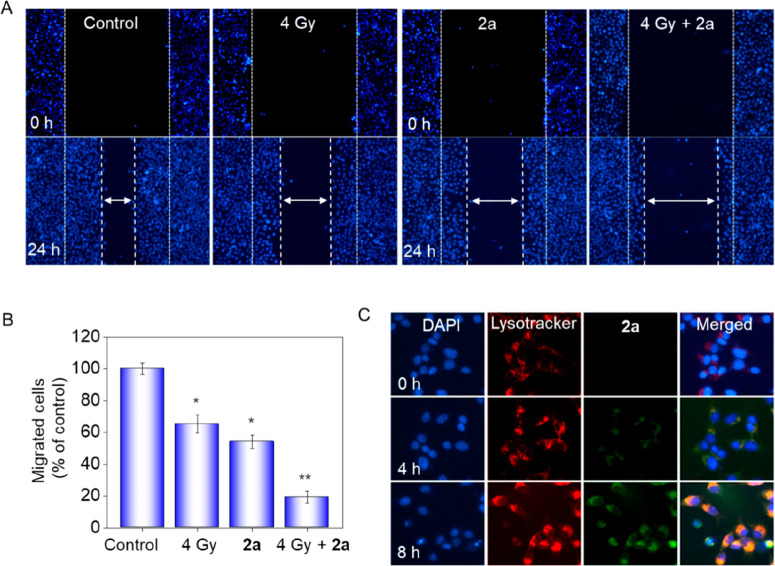
On many occasions, tumor metastasis is the major cause of higher death rates.24 Tumor cells tend to spread from the original site to adjacent sites and form secondary tumors. Metastasis occurs via immigration and infiltration of the original tumor to distant organs and tissue. For most clinical drugs, prevention of metastatic diseases becomes rather important beyond killing the tumor cells in the therapeutic process. Thus, a wound-healing migration assay was performed on HeLa cells. Figure 4A shows that the average cell migration velocity was reduced by X-rays or 2a alone (2a > X-rays) in comparison with the control, indicating that the ability of cell movement was suppressed. Notably, combination treatment of 2a and X-rays resulted in a significant decrease in the spreading rate. The level of cell migration into the wound scratch was quantified and is shown in Figure 4B. To be specific, X-rays and 2a alone suppressed 35% and 46% of migration of HeLa cells, respectively. The migration was suppressed up to 81% when cells were treated with X-rays and 2a together.
Figure 4.
Synergistic inhibition of migration by 2a and X-rays and cellular localization of 2a in HeLa cells. (A) Cancer cell migration was inhibited by treatment with 2a in the absence or presence of X-rays (4 Gy). Wound closure was recorded under phase-contrast microscopy at the indicated times. (B) Quantitative analysis of the migrated cells by manual counting. Values are expressed as mean ± SD of three independent experiments. (C) Intracellular trafficking of 2a in individual HeLa cells. The cells were pretreated with 2a (20 μM) in a 2 cm dish and then stained with LysoTracker 2 h before the point in time and DAPI 30 min before the point in time, and then the fluorescence was captured under a fluorescence microscope.
On the basis of the fluorescence spectrum of 2a, in which the maximum emission wavelength is about 510 nm, the fluorescence color should be green. To further confirm intracellular localization, DAPI and LysoTracker (red) were used to stain the nucleus and lysosomes, respectively. As shown in Figure 4C, after incubation of 2a for 4 h, the green fluorescence of 2a was well-overlapped with the red fluorescence, whereas this overlap was not so apparent in the next 4 h, demonstrating that 2a accumulated in lysosomes at first and then diffused into the cytoplasm.
In Vivo Anticancer Activity of 2a and in Combination with X-rays
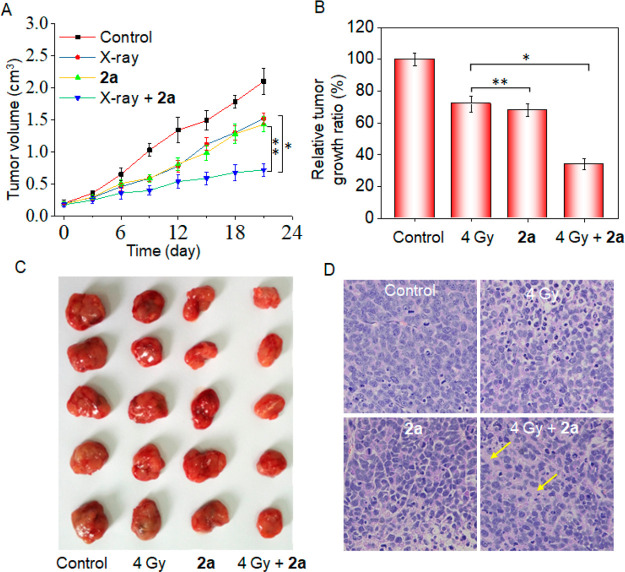
The therapeutic efficacy of compound 2a in the absence or presence of X-rays was also measured in HeLa-tumor-bearing nude mice. Nude mice bearing HeLa tumors were treated with 2a through tail intravenous injection every 3 days during the 21-day period at a dose of 4 mg/kg. As shown in Figure 5A, X-rays exerted a certain degree of inhibitory effect on tumor growth, as demonstrated by the decreased growth in tumor volume in comparison with the control group (Figure 5B). 2a, which had obvious antiproliferative activity compared with X-rays in vitro, exerted similar inhibitory effects on tumor growth throughout the 21-day period. It is worth noting that when 2a was combined with X-rays, their inhibitory effect on tumor growth was significantly improved, as the relative tumor growth ratio decreased to 32%. This was confirmed by the tumor photographs from different groups (Figure 5C). In addition, histological section analysis (Figure 5D) also displayed that the combination treatment induced much more remarkable tumor destruction than X-rays or 2a alone. These data indicated that in combination with X-rays, 2a significantly inhibited tumor growth.
Figure 5.
In vivo anticancer activity of 2a and X-rays. (A) Changes in tumor volume of 2a-treated HeLa xenografts on nude mice in the presence or absence of X-rays. (B) Relative tumor growth rates. (C) Tumor photographs collected from different groups. (D) H&E staining in tumor tissue sections from different groups of mice.
In Vivo Toxicity of 2a
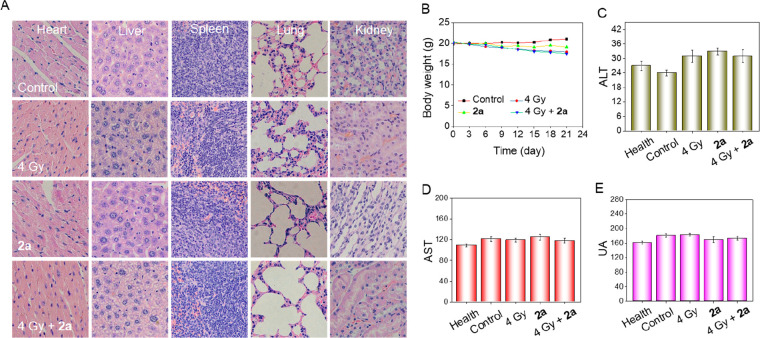
Folic acid analogues such as methotrexate and trimethoprem typically have potential hepatotoxicity (fatty liver disease and even cirrhosis), renal toxicity, and neurovirulence.25 To evaluate the systemic toxicities of compound 2a in vivo, mice were euthanized 24 h after the last injection. Hematoxylin and eosin (H&E) staining was conducted on major organs, and the blood was collected for biochemistry analysis. The results showed that the heart, liver, lungs, kidneys, and spleen all witnessed no evident damage after irradiation with X-rays or treatment of 2a (4 mg/kg), as reflected by the observation that X-ray-irradiated or 2a-treated mice did not show a significant difference with control group (Figure 6A). Meanwhile, the synergistic effect of X-rays and 2a together also showed no apparent damage to these organs.
Figure 6.
Acute toxicity evaluation of 2a. (A) H&E staining sections of main organs from nude mice after treatment with 2a in the absence or presence of X-rays. (B) Record of the body weight over 21 days. (C–E) Blood biochemistry analyses of ALT (C), AST (D), and UA (E) in mice after different treatments.
Drug toxicity typically brings about a variation in body weight (mostly weight loss). Figure 6B shows the records of body weight throughout the treatment period between the first day and the 21st day. In the control group, there was a slight increase in body weight after 21 days of treatment, and there was not much change in 2a-treated mice. X-rays, with similar tumor inhibitory rate compared with 2a, resulted in moderate weight loss. However, in the presence of X-rays, the therapeutic effect of 2a was greatly improved but did not bring about further apparent weight loss compared with X-rays alone.
Furthermore, blood biochemistry analysis, including liver function indicators (alanine aminotransferase (ALT) and aspartate aminotransferase (ALT)) and kidney function indicators (blood urea nitrogen (BUN) and uric acid (UA)) showed that 2a-treated or X-ray-irradiated nude mice as well as mice that received the combination treatment of X-rays and 2a did not exhibit acute or further adverse effects in the liver and kidney, indicating that the therapy did not mess up the normal function of the liver (Figure 6C,D) and kidneys (Figures 6E and S20). This was different from most classical folic acid analogues, which typically induce different levels of liver and kidney toxicity.26 Even in combination with X-rays, 2a posed no apparent side effects on nude mice. These data indicated the remarkable tumoricidal efficacy and limited side effects of compound 2a in the presence of X-rays and its clinical potential and promising application prospects.
We next investigated the cellular uptake of 2a and MTX. The results showed that the cellular uptake rate of 2a was much higher than that of MTX (Figure S21), indicating that the increased antiproliferative effects could be due to the increased cellular uptake rate.
Furthermore, the pharmacokinetic parameters, including elimination-phase half-life period of medicine (t1/2β), area under the concentration versus time curve from 0 to 48 h (AUC0–48h), maximum concentration observed (Cmax), clearance of medicine (Cl), and mean retention time (MRT) were also tested. These results demonstrated the moderate blood circulation of 2a.
Conclusions
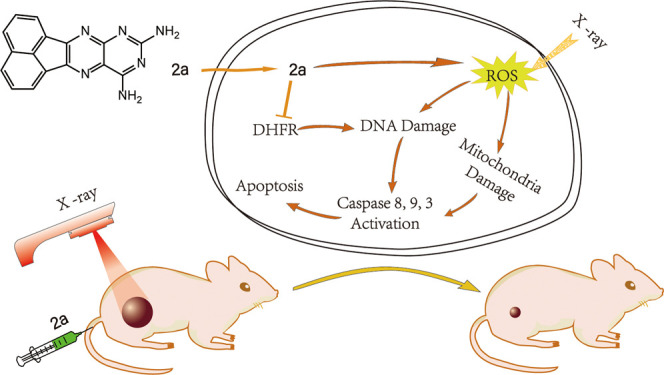
Amplification of the DHFR gene may make radiotherapy for cervical cancer indistinctive. As a consequence, the increase in DHFR activity causes attenuation of the treatment effect. In this study, we synthesized a series of DHFR inhibitors derived from methotrexate analogues as radiotherapy sensitizers to reverse the radiotherapy resistance in cervical cancer. Our results revealed the following: (i) among the compounds synthesized, the leading compound 2a exerted the best inhibitory effects toward the activity of DHFR; (ii) the combination of 2a and X-ray radiotherapy exerted much stronger effects in killing cervical cancer cells than 2a or X-rays alone, with higher selectivity and radiosensitization activity than MTX; (iii) the combination of X-rays and 2a effectively caused cell death by triggering cell apoptosis through activation of caspase 8, 9, and 3 and ROS-mediated mitochondria dysfunction; (iv) in combination with X-rays, 2a notably suppressed the migration capacity of HeLa cells; and (v) in the presence of X-rays, 2a effectively suppressed tumor growth and did not cause obvious side effects in vivo. In conclusion, as a DHFR inhibitor, 2a successfully reversed the radioresistance of cervical cancer cells and greatly promoted the therapeutic effect. The combination of the DHFR inhibitor and radiotherapy proved to be a promising strategy in cervical cancer therapy.
Acknowledgments
This work was supported by National Natural Science Foundation of China (21877049), the Major Program for Tackling Key Problems of Industrial Technology in Guangzhou (201902020013), the Dedicated Fund for Promoting High-Quality Marine Economic Development in Guangdong Province (GDOE-2019-A31), the Guangzhou Key Laboratory of Molecular and Functional Imaging for Clinical Translation (Project 201905010003), the Science and Technology Program of Guangzhou (202002030170), and the Guangdong Natural Science Foundation (2017A030310409).
Glossary
Abbreviations
- DHFR
dihydrofolate reductase
- ROS
reactive oxygen species
- DHE
dihydroethidium
- MTX
methotrexate
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- UA
uric acid
- BUN
blood urea nitrogen
- t1/2β
elimination phase, half-life period of medicine
- AUC0–48h
area under the concentration versus time curve from 0 to 48 h
- Cmax
maximum concentration observed
- Cl
clearance of medicine
- MRT
mean retention time
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00105.
Synthetic experimental details, related spectroscopic data for the compounds, and biological assay protocols (PDF)
Author Contributions
∥ Y.L. and D.Z. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Moelle U.; Mathewos A.; Aynalem A.; Wondemagegnehu T.; Yonas B.; Begoihn M.; Addissie A.; Unverzagt S.; Jemal A.; Thomssen C.; Vordermark D.; Kantelhardt E. J. Cervical Cancer in Ethiopia: The Effect of Adherence to Radiotherapy on Survival. Oncologist 2018, 23 (9), 1024–1032. 10.1634/theoncologist.2017-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit P. T.; Ridings G. R.; Coin J. W.; Williams G. R.; Mitchell D. Jr.; Boles G. W. Methotrexate and radiation in the treatment of patients with cancer. Cancer Res. 1964, 24, 1524–1533. [PubMed] [Google Scholar]
- Lustig R. A.; DeMare P. A.; Kramer S. Adjuvant methotrexate in the radiotherapeutic management of advanced tumors of the head and neck. Cancer 1976, 37 (6), 2703–2708. . [DOI] [PubMed] [Google Scholar]
- Hahn P.; Nevaldine B.; Morgan W. F. X-ray induction of methotrexate resistance due to dhfr gene amplification, Somat. Somatic Cell Mol. Genet. 1990, 16 (5), 413–423. 10.1007/BF01233191. [DOI] [PubMed] [Google Scholar]
- Gresty K. J.; Gray K.-A.; Bobogare A.; Wini L.; Taleo G.; Hii J.; Cheng Q.; Waters N. C. Genetic mutations in Plasmodium falciparum and Plasmodium vivax dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS) in Vanuatu and Solomon Islands prior to the introduction of artemisinin combination therapy. Malar. J. 2014, 13, 402. 10.1186/1475-2875-13-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi V.; Giovannini L.; Santucci M.; Cichero E.; Costi M. P.; Naesens L.; Giordanetto F.; Tonelli M. Synthesis, biological evaluation and molecular modeling of novel azaspiro dihydrotriazines as influenza virus inhibitors targeting the host factor dihydrofolate reductase (DHFR). Eur. J. Med. Chem. 2018, 155, 229–243. 10.1016/j.ejmech.2018.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline V.; Remouchamps V.; Isnardi V.; Vander Borght T. Multimodality imaging using PET/CT ((18)F)-fluorodeoxyglucose for radiotherapy field delineation of localized Hodgkin lymphoma. Cancer Radiother 2018, 22 (5), 384–392. 10.1016/j.canrad.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Shi D.; Liang Z.; Zhang C.; Zhang H.; Liu X. The effect of surgery on the survival status of patients with locally advanced cervical cancer after radiotherapy/chemoradiotherapy: a meta-analysis. BMC Cancer 2018, 18 (1), 308. 10.1186/s12885-018-4232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.; Chen Y.; Shi J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119 (8), 4881–4985. 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- Trucco L. D.; Mundra P. A.; Hogan K.; Garcia-Martinez P.; Viros A.; Mandal A. K.; Macagno N.; Gaudy-Marqueste C.; Allan D.; Baenke F.; Cook M.; McManus C.; Sanchez-Laorden B.; Dhomen N.; Marais R. Ultraviolet radiation-induced DNA damage is prognostic for outcome in melanoma. Nat. Med. 2019, 25 (2), 221–224. 10.1038/s41591-018-0265-6. [DOI] [PubMed] [Google Scholar]
- Ng H. L.; Ma X.; Chew E. H.; Chui W. K. Design, Synthesis, and Biological Evaluation of Coupled Bioactive Scaffolds as Potential Anticancer Agents for Dual Targeting of Dihydrofolate Reductase and Thioredoxin Reductase. J. Med. Chem. 2017, 60 (5), 1734–1745. 10.1021/acs.jmedchem.6b01253. [DOI] [PubMed] [Google Scholar]
- Cammarata M.; Thyer R.; Lombardo M.; Anderson A.; Wright D.; Ellington A.; Brodbelt J. S. Characterization of trimethoprim resistant E. coli dihydrofolate reductase mutants by mass spectrometry and inhibition by propargyl-linked antifolates. Chem. Sci. 2017, 8 (5), 4062–4072. 10.1039/C6SC05235E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveridge E. J.; Behiry E. M.; Guo J.; Allemann R. K. Evidence that a ’dynamic knockout’ in Escherichia coli dihydrofolate reductase does not affect the chemical step of catalysis. Nat. Chem. 2012, 4 (4), 292–7. 10.1038/nchem.1296. [DOI] [PubMed] [Google Scholar]
- Burns D. D.; Teppang K. L.; Lee R. W.; Lokensgard M. E.; Purse B. W. Fluorescence Turn-On Sensing of DNA Duplex Formation by a Tricyclic Cytidine Analogue. J. Am. Chem. Soc. 2017, 139 (4), 1372–1375. 10.1021/jacs.6b10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madungwe N. B.; Feng Y.; Lie M.; Tombo N.; Liu L.; Kaya F.; Bopassa J. C. Mitochondrial inner membrane protein (mitofilin) knockdown induces cell death by apoptosis via an AIF-PARP-dependent mechanism and cell cycle arrest. Am. J. Physiol Cell Physiol 2018, 315 (1), C28–C43. 10.1152/ajpcell.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Lin W.; He L.; Chen T. Radiosensitive core/satellite ternary heteronanostructure for multimodal imaging-guided synergistic cancer radiotherapy. Biomaterials 2020, 226, 119545. 10.1016/j.biomaterials.2019.119545. [DOI] [PubMed] [Google Scholar]
- Sallmyr A.; Matsumoto Y.; Roginskaya V.; Van Houten B.; Tomkinson A. E. Inhibiting Mitochondrial DNA Ligase IIIalpha Activates Caspase 1-Dependent Apoptosis in Cancer Cells. Cancer Res. 2016, 76 (18), 5431–41. 10.1158/0008-5472.CAN-15-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.; Huang G.; Liu H.; Sang C.; Liu X.; Chen T. Highly bioactive zeolitic imidazolate framework-8-capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Science Advances 2020, 6, eaay9751 10.1126/sciadv.aay9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C.; Ciccotosto G. D.; Cappai R.; Wang Y.; Tang S.; Hoyer D.; Schneider E. K.; Velkov T.; Xiao X. Rapamycin Confers Neuroprotection against Colistin-Induced Oxidative Stress, Mitochondria Dysfunction, and Apoptosis through the Activation of Autophagy and mTOR/Akt/CREB Signaling Pathways. ACS Chem. Neurosci. 2018, 9 (4), 824–837. 10.1021/acschemneuro.7b00323. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Fu Y.; Li M.; Jiang D.; Kutyreff C. J.; Engle J. W.; Lan X.; Cai W.; Chen T. Chirality-Driven Transportation and Oxidation Prevention by Chiral Selenium Nanoparticles. Angew. Chem., Int. Ed. 2020, 59, 4406–4414. 10.1002/anie.201910615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. G.; Choi B. K.; Kim Y. H.; Oh H. S.; Park S. H.; Bae Y. S.; Kwon B. S. The repopulating cancer cells in melanoma are characterized by increased mitochondrial membrane potential. Cancer Lett. 2016, 382 (2), 186–194. 10.1016/j.canlet.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Yang W.; Tao Y.; Wu Y.; Zhao X.; Ye W.; Zhao D.; Fu L.; Tian C.; Yang J.; He F.; Tang L. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10 (1), 1076. 10.1038/s41467-019-09046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. T.; Kim M. J.; Kang Y. H.; Choi S. Y.; Lee J. H.; Choi J. A.; Kang C. M.; Cho C. K.; Kang S.; Bae S.; Lee Y. S.; Chung H. Y.; Lee S. J. Phytosphingosine in combination with ionizing radiation enhances apoptotic cell death in radiation-resistant cancer cells through ROS-dependent and-independent AIF release. Blood 2005, 105 (4), 1724–33. 10.1182/blood-2004-07-2938. [DOI] [PubMed] [Google Scholar]
- Liang Y.; Huang W.; Zeng D.; Huang X.; Chan L.; Mei C.; Feng P.; Tan C. H.; Chen T. Cancer-targeted design of bioresponsive prodrug with enhanced cellular uptake to achieve precise cancer therapy. Drug Delivery 2018, 25 (1), 1350–1361. 10.1080/10717544.2018.1477862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y.; De Castro S. C.; Savery D.; Copp A. J.; Greene N. D. Nucleotide precursors prevent folic acid-resistant neural tube defects in the mouse. Brain 2013, 136 (9), 2836–41. 10.1093/brain/awt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Ke J.; Zhou X. E.; Yi W.; Brunzelle J. S.; Li J.; Yong E. L.; Xu H. E.; Melcher K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500 (7463), 486–9. 10.1038/nature12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.