Abstract
Nitric oxide is an important inflammation mediator with a recognized role in the development of different cancers. Gliomas are primary tumors of the central nervous system with poor prognosis, and the expression of the inducible nitric oxide synthase correlates with the degree of malignancy, changes in vascular reactivity, and neo-angiogenesis. Therefore, targeting the nitric oxide biosynthesis appears as a potential strategy to impair glioma progression. In the present work a set of aryl and amido-aryl acetamidine derivatives were synthesized to obtain new potent and selective inducible nitric oxide synthase inhibitors with improved physicochemical parameters with respect to the previously published molecules. Compound 17 emerged as the most promising inhibitor and was evaluated on C6 rat glioma cell line, showing antiproliferative effects and high selectivity over astrocytes.
Keywords: acetamidine, cancer, inducible nitric oxide synthase, inhibitor, synthesis, glioma
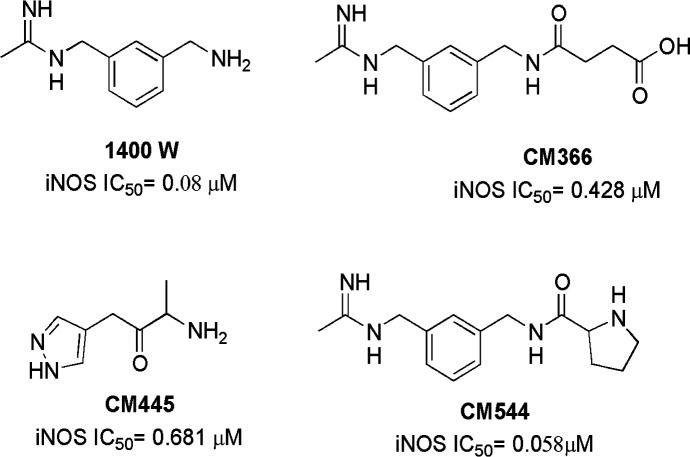
Inflammation is widely recognized as a key factor in the development of different human cancers, due to its role in tumorigenesis, disease progression, and metastasis.1 An important inflammation mediator is the free radical nitric oxide (NO), which is typically produced in inflammatory and epithelial cells and is biosynthesized by NO synthase (NOS). This is a family of three enzyme isoforms, two of which are constitutive (namely the neuronal NOS or nNOS, and the endothelial NOS or eNOS), and one of which is inducible (iNOS), with this last one being extensively expressed during inflammation. In chronic inflammation, the sustained generation of NO by iNOS tends to increase the levels of reactive oxygen species (ROS) and nitrogen species (RNS), which are responsible for the damage of a wide variety of biomolecules, such as nucleic acids, proteins, and lipids.2−4 The overexpression of iNOS occurs in different malignancies, including melanoma, breast and ovarian cancer, and gliomas.5−7 These last are primary tumors of the central nervous system with poor prognosis, and iNOS expression correlates with the degree of their malignancy, changes in vascular reactivity, and neo-angiogenesis, prompting disease progression.8 Interestingly, it has been observed that the treatment with iNOS inhibitors in mice implanted with human glioma xenografts reduces tumor volumes,9 as well as ameliorates photodynamic therapy effects.10 Importantly, the iNOS overexpression is also associated with chemoresistance, with NO being implicated in the adaptive responses of tumoral cells.7,11−15 The iNOS isoenzyme is a homodimer, and each subunit contains an oxygenase domain and a reductase domain, and dimerization is necessary for the catalytic activity. Different molecules able to inhibit this isoform have been disclosed so far both from academia and industry, such as heterocyclic amino acids, amidinoamino acids, cyclic amidines, imidazopyridines, etc.16−20 iNOS inhibitors can act with different mechanisms, i.e. they can be substrate analogues, dimerization inhibitors, cofactor analogues, or down regulators of enzyme expression. Some of these compounds have also been investigated as antiglioma agents, with encouraging results.21 However, no iNOS inhibitor has been approved for clinical applications, and actually there is a need for new molecules with improved pharmacodynamics and pharmacokinetics. Moreover, due to the important physiological roles of constitutive isoforms, and especially of eNOS, it is important to disclose iNOS inhibitors showing a high degree of isoform selectivity. In the past years, different acetamidine derivatives have been reported able to give potent and selective iNOS inhibitors. These molecules show an acetamidine group or a bioisoster able to give a bidentate hydrogen bonding with a conserved l-Glu residue into the iNOS catalytic site, spaced through an aromatic or alkyl chain from a moiety containing polar, ionizable groups (Figure 1).22−26
Figure 1.
Chemical structure of some iNOS inhibitors.
W1400 was investigated as a promising agent against glioma stem cells, although due to its unfavorable pharmacokinetic profile, it does not pass the brain blood barrier.27,28 Very recently, compound CM544 has revealed antiproliferative and cytotoxic effects against C6 rat glioma cells without impairing astrocytes viability, and being able to compromise glioma cells chemoresistance.29 However, also CM544 is a quite polar molecule with poor pharmacokinetics, being effective on C6 rat glioma cells only at high concentrations. Considering the need for more potent and lipophilic iNOS inhibitors, in the present work a new small set of acetamidine-aryl derivatives was designed, aiming to obtain new potent and selective iNOS inhibitors with improved molecular physicochemical properties to be investigated as antiglioma agents (Figure 2).
Figure 2.
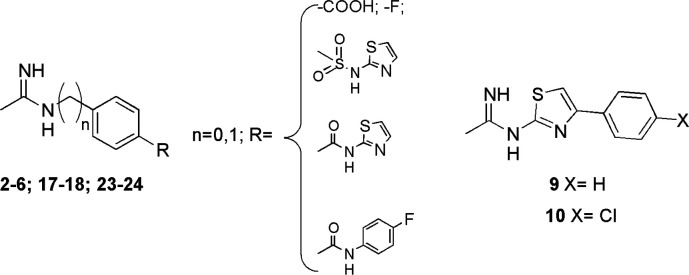
General structures of the designed compounds.
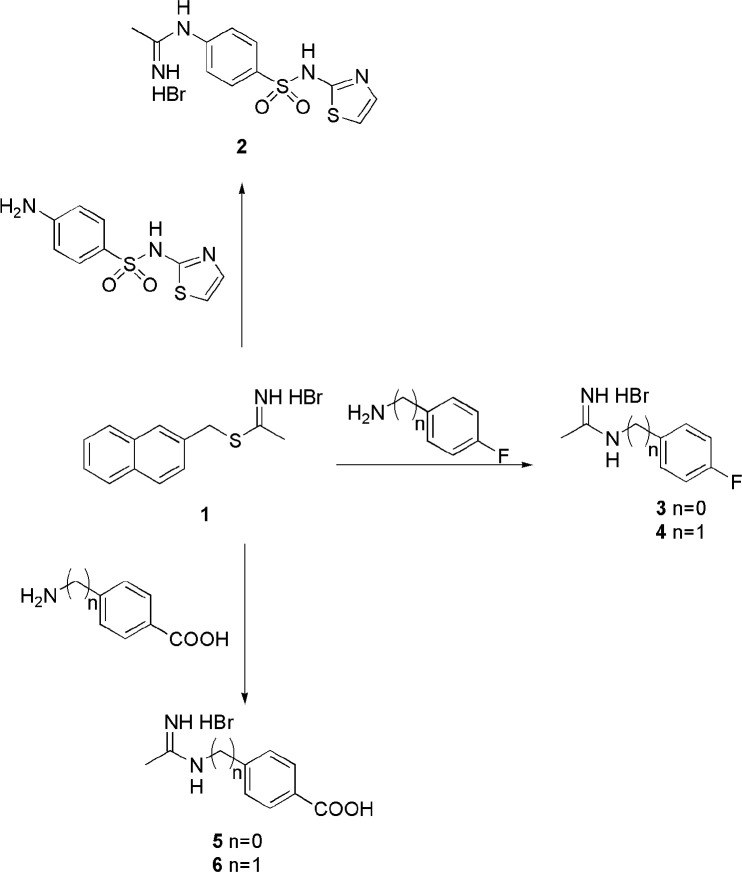
In these compounds, the pharmacophoric acetamidine group is connected to a hydrophobic aromatic tail by a phenyl, or benzyl, or thiazolyl group, which were introduced with the aim of increase molecular lipophilicity. Differently from the previously reported iNOS inhibitors, in the designed molecules the aromatic substituents are in the para position instead of in the meta one, since this structural modification contributes to increase lipophilicity (Table S1 in the Supporting Information). Moreover, the thiazole moiety was introduced into the new molecules since it was previously investigated as an iNOS inhibitor,30 and it is recurrently included in anticancer compounds.31,32 Compounds 2–6 were synthesized according to Scheme 1, by reacting the appropriate aniline or amine derivative with the S-naphtyl-thioacetimidate hydrobromide 1, which was prepared as previously reported.33
Scheme 1. Synthesis of Compounds 2–6.
Conditions: TEA, DMF, or EtOH abs., 24 h, r.t.
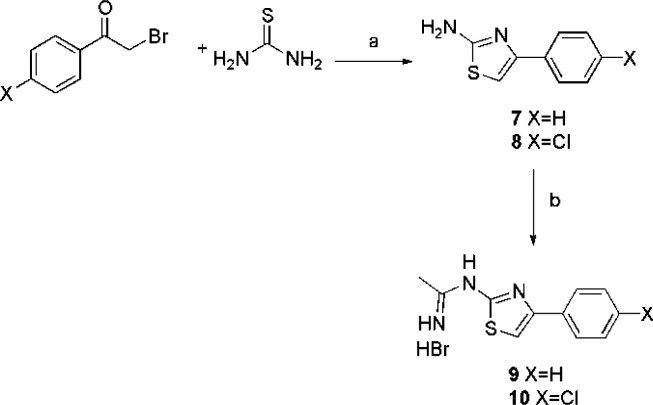
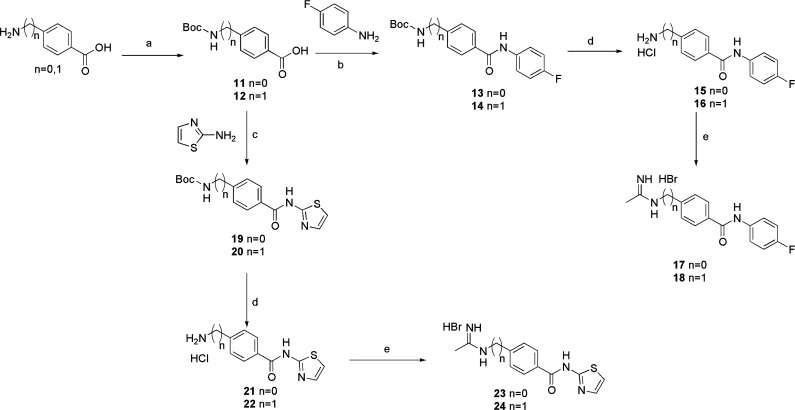
As for molecules 9 and 10, they were synthesized as reported in Scheme 2. The appropriate phenacyl bromide was reacted with thiourea, and the isolated 2-aminothiazole derivatives 7 and 8 were then reacted with compound 1, to obtain the desired acetamidines. Finally, in Scheme 3 the synthesis of molecules 17, 18, 23, and 24 is reported. In the first step, the amino group of the 4-aminomethylbenzoic acid or p-aminobenzoic acid was protected by means of the benzyloxycarbonyl group, obtaining intermediates 11 and 12. These latter were then coupled with the p-fluoroaniline by means of isobutylchloroformate, to give intermediates 13 and 14, or with 2-aminothiazole by means of EDC hydrochloride, obtaining intermediates 19 and 20.
Scheme 2. Synthesis of Compounds 9 and 10.
Reagents and conditions. (a) EtOH abs; b.t. 2 h; (b)1, TEA, EtOH abs., r.t., 5 days.
Scheme 3. Synthesis of Compounds 17, 18, 23, and 24.
Reagents and conditions: (a) di-tert-butyl-dicarbonate, dioxane, NaOH 1 N, from 0 °C to r.t., 24 h; (b) isobutylchloroformate, DMF dry, N2, −15 °C to r.t., 24 h; (c) EDC HCl, DMAP, DMF dry, N2, 0 °C, 24 h; (d) HCl 4 N, dioxane, r.t. 24 h; (e) 1, CHCl3, b.t. 24–48 h.
All of them were subsequently deprotected with hydrochloric acid in dioxane, obtaining the corresponding ammonium salts 15, 16, 21, and 22. These latter, after treatment with NaOH 1 N, were then reacted with compound 1, affording the desired target molecules 17, 18, 23, and 24. The synthesized compounds were then evaluated for their in vitro NOS inhibitory activities by measuring the conversion of l-arginine to l-citrulline, using a HPLC-fluorimetric method34 (Table 1).
Table 1. NOS Inhibition% Given by Compounds 2–6, 9, 10, 17, 18, 23, and 24.
| %inhibition
@ 1 μMa |
% inhibition @ 10 μMa | ||
|---|---|---|---|
| Cpd | iNOS | nNOS | eNOSa |
| 2 | 81 ± 0.35 | 47 ± 0.23 | 3 ± 0.01 |
| 3 | 85 ± 0.58 | 10 ± 0.06 | 0 ± 0.01 |
| 4 | 93 ± 0.27 | 0 ± 0.07 | 5 ± 0.02 |
| 5 | 86 ± 0.25 | 14 ± 0.08 | 62 ± 0.21 |
| 6 | 70 ± 0.23 | 0 ± 0.03 | 4 ± 0.05 |
| 9 | 100 ± 0.55 | 2 ± 0.08 | 14 ± 0.12 |
| 10 | 100 ± 0.44 | 13 ± 0.24 | 4 ± 0.02 |
| 17 | 100 ± 0.76 | 0 ± 0.03 | 0 ± 0.01 |
| 18 | 87 ± 0.33 | 97 ± 0.21 | 0 ± 0.01 |
| 23 | 84 ± 0.24 | 96 ± 0.11 | 0 ± 0.01 |
| 24 | 100 ± 0.69 | 0 ± 0.01 | 0 ± 0.01 |
Values given are mean ± SD of experiments performed in triplicate.
In order to screen the activity of the synthesized molecules toward the three NOS isoforms, the percent inhibition of iNOS and nNOS at 1 μM compound and the eNOS percent inhibition at 10 μM compound were at first evaluated. This difference in compound concentration was used to have a preliminary evaluation of molecule selectivity with respect to eNOS. In general, all the synthesized compounds were able to inhibit iNOS, although best results were obtained from molecules 9, 10, 17, and 24, which gave 100% iNOS inhibition.
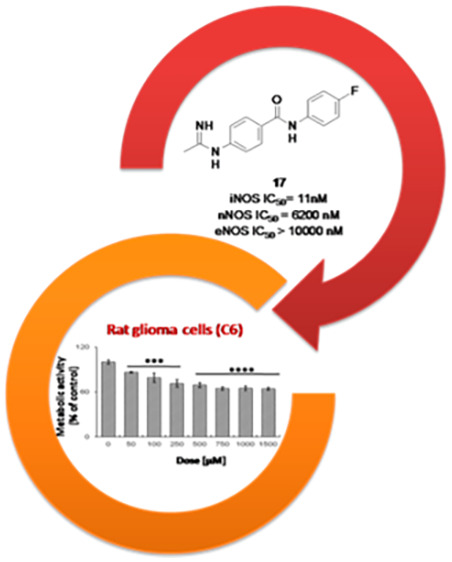
These molecules showed also some selectivity with respect to the constitutive isoforms. In particular, an important effect on the iNOS/nNOS isoform selectivity seems to be related to the acetamidine distance from the aromatic linker: indeed, the couples of molecules 3 and 4, 5 and 6, 17 and 18, and 23 and 24 are homologues, differing only for the presence of a phenyl or benzyl group, and only 4, 6, 17, and 24 are selective toward iNOS. On the other side, this structural feature does not seem to affect activity toward eNOS, although 2–10 gave some inhibition of this isoform, therefore failing selectivity requirements. Moreover, since compounds 18 and 23 fully inhibited also nNOS, molecules 17 and 24 emerged as the most promising iNOS inhibitors from this preliminary evaluation. On the basis of its better calculated physicochemical profile (Table 2), compound 17 was selected for further biological studies. Indeed, it appears more lipophilic (cLogP = 2.07 and cLogD7.4 = 0.91) with respect to 24 (cLogP = 1.15 and cLogD7.4 = 0.15), displaying a smaller topological polar surface area (64.98 vs 77.87) and a higher solubility (4.19 g/L vs 2.89 g/L). Therefore, IC50 of 17 toward human iNOS, nNOS, and eNOS were evaluated (Table 3), confirming a high potency of action (hiNOS IC50 11 nM), as well as an excellent degree of selectivity, especially with respect to eNOS (>900-fold).
Table 2. Calculated Physicochemical Parameters of Compounds 17 and 24.
| Cpd | cLogP | cLogD7,4 | TPSA | Solubility (g/L) |
|---|---|---|---|---|
| 17 | 2.07 | 0.91 | 64.98 | 4.19 |
| 24 | 1.15 | 0.15 | 77.87 | 2.89 |
Table 3. Potency and Selectivity of Compound 17 against Human NOS.
| IC50 (μM)a |
Selectivity |
||||
|---|---|---|---|---|---|
| Cpd | hiNOS | hnNOS | heNOS | nNOS/iNOS | eNOS/iNOS |
| 17 | 0.011 ± 0.02 | 6.2 ± 0.08 | >10 | 563 | >900 |
| 1400W | 0.080 ± 0.02 | 7.8 ± 0.04 | 304 ± 2 | 72 | 3800 |
Values given are mean ± SD of experiments performed in triplicate at seven different concentrations.
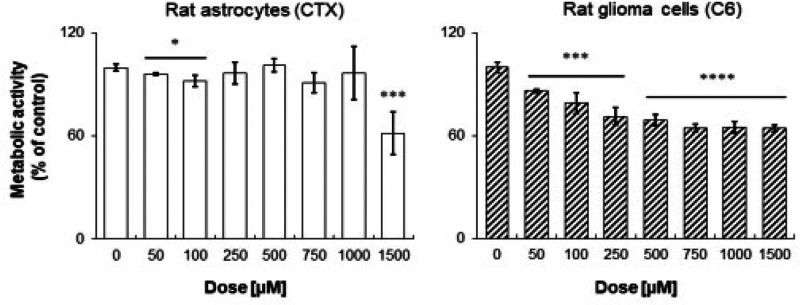
Subsequently, the potential activity of 17 as an antiglioma agent was evaluated (Figure 3). To demonstrate the biological activity and selectivity of compound 17, the metabolic activity of both rat astrocytes (CTX) and glioma cells was measured through the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay after a 24 h exposure. As for rat astrocytes, compound 17 is ineffective, with the percentages of cell metabolic activity being comparable to the one of control (0 μM) up to 1000 μM (Figure 3, left graph). A significant decrease in rat astrocyte metabolizing capacity is registered only when 1500 μM of compound 17 is administered. Contrariwise, compound 17 significantly affects C6 rat glioma cell metabolic activity in a dose-dependent manner, starting from a dose of 50 μM (86.45%) up to 750 μM (64.86%) (Figure 3, right graph). After that, cell metabolic activity remains stationary, being assessed at 65.22% and 64.42% for 1000 and 1500 μM compound 17, respectively. The flattening of metabolic activity starting from a dose of 500 μM could be ascribed to Nrf2-related chemoresistance mechanisms, which have been reported on the C6 rat glioma cell line in our previous work29 and in accordance with recent studies.35,36 However, comparing these results with those previously obtained from the investigation on CM544, Temozolamide (TMZ), which is the only drug approved for the glioma palliative treatment, and 1400W,26,29 it appears that 17 gives considerably potentiated antiproliferative effects even after a short exposure time (24 h) (Figure S1 in the Supporting Information). In more detail, TMZ at the dose of 500 μM inhibits C6 cell proliferation only for 12%, whereas compound 17 for 31%. Notably CM544 and 1400W are ineffective at the same dose, starting to inhibit cell proliferation only at higher concentrations, as previously reported. Therefore, it appears that 17, being more potent and lipophilic than CM544 and 1400W, is able to better impair glioma cells proliferation, also with respect to the reference TMZ treatment.
Figure 3.
Cell metabolic activity of rat astrocytes and rat glioma cells in the presence of increasing concentrations of compound 17. Data shown are the means ± SD of three replicates and are expressed as percentages of untreated cultures (set as 100%) (*p < 0.05; ***p < 0.005; ****p < 0.001).
In order to deepen into the biological effects of 17, cytotoxicity occurrence was afterward evaluated by means of LDH release on rat glioma cultures after 24 h (Figure 4). The percentage of LDH release slightly increases in a dose-dependent manner at lower concentrations with respect to untreated cultures (13.49%), being assessed at 22.48% when compound 17 is administered at 250 μM. At higher doses (500–1000 μM range), LDH release is significantly raised up to 27%. The dose-dependent increase of LDH let us hypothesize that the antiproliferative effect of 17 could be related to its cytotoxicity.
Figure 4.
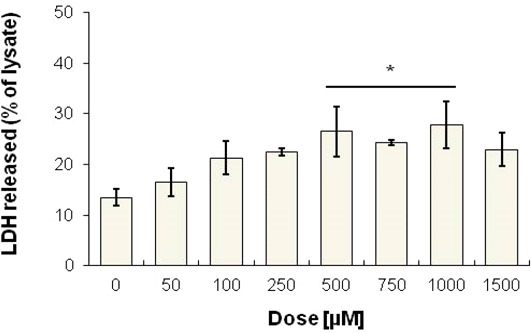
Cytotoxicity occurrence in rat glioma cells in the presence of increasing concentrations of compound 17. Data shown are the means ± SD of three replicates and are expressed as percentages of the untreated cell lysate (set as 100%) (*p < 0.05).
In conclusion, a new set of acetamidine derivatives was synthesized as selective iNOS inhibitors. Among these molecules, compound 17 emerged as the most interesting one, demonstrating high potency of action against the human iNOS (IC50 = 11 nM) and an excellent profile of selectivity with respect to the constitutive isoforms. The effectiveness of this molecule was then evaluated on C6 rat glioma cells, obtaining encouraging antiproliferative and cytotoxic effects, notably ameliorated with respect to our previous findings on less potent and more polar iNOS inhibitors, as well as compared to the standard drug TMZ. The present study, while supporting knowledge on the important role of iNOS in glioma progression, points to the potential therapeutic value of selective and potent iNOS inhibitors against this type of cancer.
Glossary
Abbreviations
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- DMF
dimethylformamide
- TEA
triethylamine
- EDC HCl
N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride
- DMAP
4-(dimethylamino)pyridine
- IC50
half maximal inhibitory concentration
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00285.
General methods and materials; synthetic procedures for compounds 2–6, 9, 10, 17, 18, 23, and 24; compounds chemical characterization; procedures for the NOS assay, the MTT assay, and the LDH assay; Table S1; Figure S1; statistical analysis (PDF)
The present work was supported by University “G. d’Annunzio” of Chieti-Pescara local grants: FAR Maccallini 2018, FAR Cataldi 2019; FAR Amoroso2019.
The authors declare no competing financial interest.
Supplementary Material
References
- Greten R.; Grivennikov S. I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S. P.; Hofseth L. J.; Harris C. C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- Wiseman H.; Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala P. K.; Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001, 2, 149–156. 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Akhavan-Sigari R.; Gaab M. R.; Rohde V.; Brandis A.; Tezval H.; Abili M.; von Eckardstein K.; Ostertag H. Expression of vascular endothelial growth factor receptor 2 (VEGFR-2), inducible nitric oxide synthase (iNOS), and Ki-M1P in skull base chordoma: a series of 145 tumors. Neurosurg. Rev. 2014, 37, 79–88. 10.1007/s10143-013-0495-5. [DOI] [PubMed] [Google Scholar]
- Switzer C. H.; Glynn S. A.; Ridnour L. A.; Cheng R. Y.; Vitek M. P.; Ambs S.; Wink D. A. Nitric oxide and protein phosphatase 2A provide novel therapeutic opportunities in ER-negative breast cancer. Trends Pharmacol. Sci. 2011, 32, 644–651. 10.1016/j.tips.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila-González D.; Choi D. S.; Rosato R. R.; Granados-Principal S. M.; Kuhn J. G.; Li W. F.; Qian W.; Chen W.; Kozielski A. J.; Wong H.; Dave B.; Chang J. C. Pharmacological inhibition of NOS activates ASK1/JNK pathway augmenting docetaxel-mediated apoptosis in triple-negative breast cancer. Clin. Cancer Res. 2018, 24, 1152–1162. 10.1158/1078-0432.CCR-17-1437. [DOI] [PubMed] [Google Scholar]
- Kaur D.; Sharma V.; Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- Muñoz M. F.; Puebla M.; Figuero X. F. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca2+ signalling. Front. Cell. Neurosci. 2015, 10, 9–59. 10.3389/fncel.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. M.; Girotti A. W. Nitric Oxide Antagonism to Anti-Glioblastoma Photodynamic Therapy: Mitigation by Inhibitors of Nitric Oxide Generation. Cancers 2019, 11, 231. 10.3390/cancers11020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojković S.; Podolski-Renić A.; Dinić J.; Stanković T.; Banković J.; Hadžić S.; Paunovi V.; Isaković A.; Tanić N.; Pešić M. Development of resistance to antiglioma agents in rat C6 cells caused collateral sensitivity to doxorubicin. Exp. Cell Res. 2015, 335, 248–257. 10.1016/j.yexcr.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Dave B.; Gonzalez D. D.; Liu Z.; Li X.; Wong H.; Granados S.; Ezzedine N. E.; Sieglaff D. H.; Ensor J. E.; Miller K. D.; Radovich M.; Etrovic A. K.; Gross S. S.; Elemento O.; Mills G. B.; Gilcrease M. Z.; Chang J. C. Role of RPL39 in Metaplastic Breast Cancer. J. Natl. Cancer Inst 2017, 109, 109. 10.1093/jnci/djw292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy L. C.; Anderson C. T. M.; Chowdhury R.; Trudel L. J.; Wogan G. N. NO supports chemoresistance in melanoma. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 20373–20378. 10.1073/pnas.1218938109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliyaperumal K.; Sharma A. K.; McDonald D. G.; Dhindsa J. S.; Yount C.; Singh A. K.; Won J. S.; Singh I. S-Nitrosoglutathione-mediated STAT3 regulation in efficacy of radiotherapy and cisplatin therapy in head and neck squamous cell carcinoma. Redox Biol. 2015, 6, 41–50. 10.1016/j.redox.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielbik M.; Szulc-Kielbik I.; Klink M. The Potential Role of iNOS in Ovarian Cancer Progression and Chemoresistance. Int. J. Mol. Sci. 2019, 20, 1751. 10.3390/ijms20071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallinan E. A.; Tjoeng F. S.; Fok K. F.; Hagen T. J.; Toth M. V.; Tsymbalov S.; Pitzele B. S. (G.D. Searle and Co.) Preparation of N6-(1-iminoethyl)-L-lysine derivatives useful as nitric oxide synthase inhibitors. US 6143790 A 20001107, 2000.
- Hansen D. W. Jr.; Hallinan E. A.; Hagen T. J.; Kramer S. W.; Metz S.; Peterson K. B.; Spangler D. P.; Toth M. V.; Fok K. F.; Bergmanis A. A.; Webber R. K.; Trivedi M.; Tjoeng F. S.; Pitzele B. S.. Preparation of cyclic amidino agents useful as nitric oxide synthase inhibitors, US 6011028 A 20000104, 2000.
- Hallinan A. E.; Hansen D. W. Jr.; Tsymbalov S.. Preparation of N-tetrazolyl amino acid amides and related compounds as nitric oxide synthase inhibitors. US 5854251 A 19981229, 1998.
- Strub A.; Wolf-Rüdiger H.; Eltze C.; Fuchss M.; Strassner T.; Strand S.; Lehner M. D.; Rainer B. The novel imidazopyridine 2-[2-(4-Methoxy-pyridin-2-yl)-ethyl]-3H- imidazo[4,5-b]pyridine (BYK191023) is a highly selective inhibitor of the inducible nitric-oxide synthase. Mol. Pharmacol. 2006, 69, 328–337. 10.1124/mol.105.017087. [DOI] [PubMed] [Google Scholar]
- Minhas R.; Bansal Y.; Bansal G. Inducible nitric oxide synthase inhibitors: A comprehensive update. Med. Res. Rev. 2020, 40, 823–855. 10.1002/med.21636. [DOI] [PubMed] [Google Scholar]
- Maccallini C.; Gallorini M.; Cataldi A.; Amoroso R. Targeting iNOS As a Valuable Strategy for the Therapy of Glioma. ChemMedChem 2020, 15, 339–344. 10.1002/cmdc.201900580. [DOI] [PubMed] [Google Scholar]
- Garvey E. P.; Oplinger J. A.; Furfine E. S.; Kiff R. J.; Laszlo F.; Whittle B. J.; Knowles R. G. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 1997, 272, 4959–4963. 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- Maccallini C.; Di Matteo M.; Vullo D.; Ammazzalorso A.; Carradori S.; De Filippis B.; Fantacuzzi M.; Giampietro L.; Pandolfi A.; Supuran C. T.; Amoroso R. Indazole, Pyrazole, and Oxazole Derivatives Targeting Nitric Oxide Synthases and Carbonic Anhydrases. ChemMedChem 2016, 11, 1695–1699. 10.1002/cmdc.201600204. [DOI] [PubMed] [Google Scholar]
- Maccallini C.; Montagnani M.; Paciotti R.; Ammazzalorso A.; De Filippis B.; Di Matteo M.; Di Silvestre S.; Fantacuzzi M.; Giampietro L.; Potenza M. A.; Re N.; Pandolfi A.; Amoroso R. Selective acetamidine-based nitric oxide synthase inhibitors: synthesis, docking, and biological studies. ACS Med. Chem. Lett. 2015, 6, 635–640. 10.1021/acsmedchemlett.5b00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantacuzzi M.; Maccallini C.; Di Matteo M.; Ammazzalorso A.; Bruno I.; De Filippis B.; Giampietro L.; Mollica A.; Amoroso R. Screening of NOS activity and selectivity of newly synthesized acetamidines using RP-HPLC. J. Pharm. Biomed. Anal. 2016, 120, 419–424. 10.1016/j.jpba.2015.11.045. [DOI] [PubMed] [Google Scholar]
- Maccallini C.; Di Matteo M.; Gallorini M.; Montagnani M.; Graziani V.; Ammazzalorso A.; Amoia P.; De Filippis B.; Di Silvestre S.; Fantacuzzi M.; Giampietro L.; Potenza M. A.; Re N.; Pandolfi A.; Cataldi A.; Amoroso R. Discovery of N-{3-[(ethanimidoylamino)methyl]benzyl}-l-prolinamidedihydrochloride: A new potent and selective inhibitor of the inducible nitric oxide synthase as a promising agent for the therapy of malignant glioma. Eur. J. Med. Chem. 2018, 25, 53–64. 10.1016/j.ejmech.2018.04.027. [DOI] [PubMed] [Google Scholar]
- Eyler C. E.; Wu Q.; Yan K.; MacSwords J. M.; Chandler-Militello D.; Misuraca K. L.; Lathia J. D.; Forrester M. T.; Lee J.; Stamler J. S.; Goldman S. A.; Bredel M.; McLendon R. E.; Sloan A. E.; Hjelmeland A. B.; Rich J. N. Glioma Stem Cell Proliferation and Tumor Growth Are Promoted by Nitric Oxide Synthase-2. Cell 2011, 146, 53–66. 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello S. S.; Zhu B.; McMonagle-Strucko K.; Pulicicchio C.; Merrill J.; Luo Y.; Shen L.; Wang J.; Adler D.; Natarajan C.. Pharmacokinetic and pharmacodynamic evaluation of inhibitors of inducible nitric oxide synthase (iNOS) in mice. AAPS PharmSci. 2002, 4 ( (S1), ), http://www.aapsj.org/abstracts/AM_2002/AAPS2002-002237.pdf. [Google Scholar]
- Gallorini M.; Maccallini C.; Ammazzalorso A.; Amoia P.; De Filippis B.; Fantacuzzi M.; Giampietro L.; Cataldi A.; Amoroso R. The Selective Acetamidine-Based iNOS Inhibitor CM544 Reduces Glioma Cell Proliferation by Enhancing PARP-1 Cleavage In Vitro. Int. J. Mol. Sci. 2019, 20, 495. 10.3390/ijms20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S.; Terauchi H.; Kawasaki M.; Yano A.; Ido M. Structure–Activity Relationships of 2-Aminothiazole Derivatives as Inducible Nitric Oxide Synthase Inhibitor. Chem. Pharm. Bull. 2004, 52, 634–637. 10.1248/cpb.52.634. [DOI] [PubMed] [Google Scholar]
- Turan-Zitouni G.; Altıntop M. D.; Özdemir A.; Kaplancıklı Z. A.; Çiftçi G. A.; Temel H. E. Synthesis and evaluation of bis-thiazole derivatives as new anticancer agents. Eur. J. Med. Chem. 2016, 107, 288–294. 10.1016/j.ejmech.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Finiuk N. A.; Klyuchivska O.; Ivasechko I.; Hreniukh V.; Ostapiuk Y.; Shalai Y.; Panchuk R.; Matiychuk V.; Obushak M.; Stoika R.; Babsky A. Proapoptotic effects of novel thiazole derivative on human glioma cells. Anti-Cancer Drugs 2019, 30, 27–37. 10.1097/CAD.0000000000000686. [DOI] [PubMed] [Google Scholar]
- Maccallini C.; Patruno A.; Besker N.; Ali J. I.; Ammazzalorso A.; De Filippis B.; Franceschelli S.; Giampietro L.; Pesce M.; Reale M.; Tricca M. L.; Re N.; Felaco M.; Amoroso R. Synthesis, biological evaluation, and docking studies of N-substituted acetamidines as selective inhibitors of inducible nitric oxide synthase. J. Med. Chem. 2009, 52, 1481–1485. 10.1021/jm800846u. [DOI] [PubMed] [Google Scholar]
- Maccallini C.; Di Matteo M.; Ammazzalorso A.; D’Angelo A.; De Filippis B.; Di Silvestre S.; Fantacuzzi M.; Giampietro L.; Pandolfi A.; Amoroso R. Reversed-phase high-performance liquid chromatography method with fluorescence detection to screen nitric oxide synthases inhibitors. J. Sep. Sci. 2014, 37, 1380–1385. 10.1002/jssc.201400059. [DOI] [PubMed] [Google Scholar]
- Sun W.; Zhang W.; Yu J.; Lu Z.; Yu J. Inhibition of Nrf2 might enhance the anti-tumor effect of Temozolomide in glioma cells via inhibition of Ras/Raf/MEK signaling pathway. Int. J. Neurosci. 2020, 1–16. 10.1080/00207454.2020.1766458. [DOI] [PubMed] [Google Scholar]
- Yu D.; Liu Y.; Zhou Y.; Ruiz-Rodado V.; Larion M.; Xu G.; Yang C. Triptolide suppresses IDH1-mutated malignancy via Nrf2-driven glutathione metabolism. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 9964–9972. 10.1073/pnas.1913633117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.