Abstract
Controversy exists around the appropriate types of masks and the situations in which they should be used in community and health care settings for the prevention of SARS-CoV-2 infection. In this article, the American College of Physicians (ACP) provides recommendations based on the best available evidence through 14 April 2020 on the effectiveness of N95 respirators, surgical masks, and cloth masks in reducing transmission of infection. The ACP plans periodic updates of these recommendations on the basis of ongoing surveillance of the literature for 1 year from the initial search date.
Key Question 1
What is the effectiveness of N95 respirators versus surgical masks versus cloth masks for the prevention of coronavirus disease 2019 (COVID-19) in addition to standard precautions (gloves + handwashing) in community settings?
Key Question 2
What is the effectiveness of N95 respirators versus surgical masks versus cloth masks for the prevention of COVID-19 in addition to standard precautions (gowns + gloves + handwashing) in health care settings?
Key Question 3
What is the effectiveness for reuse or extended use of N95 respirators for prevention of COVID-19?
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spreads among persons in close proximity through droplets, although evidence is still emerging regarding potential airborne transmission. Reducing transmission of SARS-CoV-2 infection in health care and community settings is a major priority, especially in the absence of an effective vaccine or treatment. The use of respiratory personal protective equipment (PPE) may decrease the risk for respiratory infection, although controversy exists around the appropriate types of masks and the situations in which they should be used in community and health care settings for the prevention of SARS-CoV-2 infection. The following practice points (Table 1) are intended for clinicians, patients, and the public. Data on SARS-CoV-2 are limited. These practice points are based on the best available evidence on the effectiveness of N95 respirators, surgical masks, and cloth masks in reducing transmission of infection with SARS-CoV-1, Middle East respiratory syndrome coronavirus (MERS-CoV), and influenza-like or other respiratory viruses in community and health care settings. Evidence about reuse or extended use of N95 respirators in health care settings was also considered.
Table 1. Practice Points.
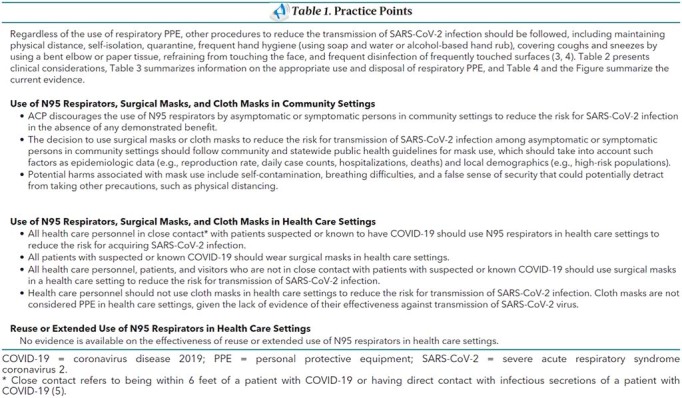
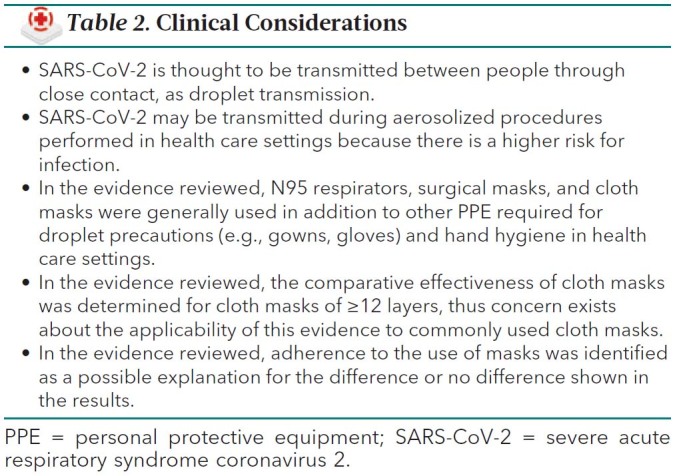
Table 2. Clinical Considerations.
The American College of Physicians (ACP) based these practice points on a rapid, living systematic evidence review funded by the Agency for Healthcare Research and Quality (1, 2). See the Appendix for methods used to develop the practice points. This version of the practice points, based on an evidence review completed on 14 April 2020 with surveillance through 2 June 2020, was approved by the ACP's Executive Committee of Board of Regents on behalf of the Board of Regents on 18 May 2020, and submitted to Annals of Internal Medicine on 13 May 2020. Ongoing surveillance of the literature is planned for 1 year from the initial search date, and the living practice points will be updated alongside the evidence review.
Figure. Evidence description.
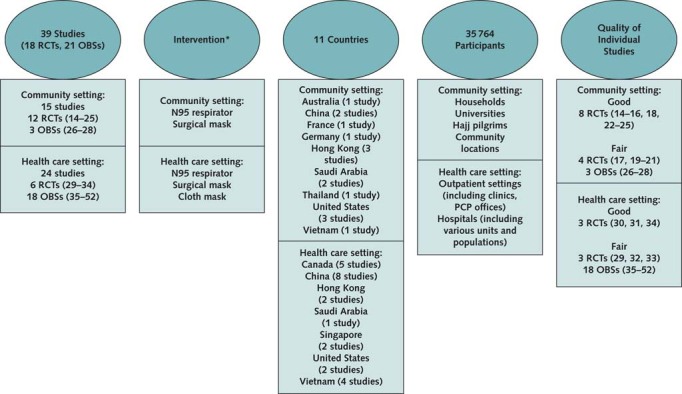
Evidence search and assessment conducted by the Pacific Northwest Evidence-based Practice Center and funded by the Agency for Healthcare Research and Quality (2). Current search for evidence, completed on 14 April 2020 and updated through 2 June 2020, aimed to identify RCTs and OBSs on the use of N95 respirators, surgical masks, and cloth masks to prevent SARS-CoV-2 infection and coronaviruses (SARS-CoV-1, MERS-CoV) infections and RCTs of N95 respirators, surgical masks, and cloth masks to prevent influenza-like (influenza or other respiratory viruses) infections, as well as all studies on the reuse/extended use of N95 respirators. MERS-CoV = Middle East respiratory syndrome coronavirus; OBS = observational study; PCP = primary care physician; PPE = personal protective equipment; RCT = randomized controlled trial; SARS-CoV-2 = severe acute respiratory syndrome 2.
* Different types of respiratory PPE intervention were generally used in addition to additional PPE required for droplet precautions (e.g., gowns, gloves) and hand hygiene in health care settings.
Rationale
What is the effectiveness of N95 respirators, surgical masks, and cloth masks for the prevention of COVID-19 in addition to standard precautions (gloves + handwashing) in community settings?
The goal of using N95 respirators, surgical masks, or cloth masks is to prevent transmission of SARS-CoV-2 infection from asymptomatic or symptomatic infected persons to uninfected persons (source control). Currently, no direct evidence exists for the effectiveness or comparative effectiveness of various types of respirators or masks for preventing SARS-CoV-2 infection in community settings. Low-certainty evidence showed that mask use may reduce the risk for SARS-CoV-1 infection compared with no mask use in the community, but a major limitation of the studies is that they did not specify mask type. Low-certainty indirect evidence also found that N95 respirators may not reduce the risk for noncoronavirus respiratory infections compared with surgical masks or no masks, and moderate-certainty indirect evidence showed that surgical masks probably do not reduce the risk compared with no masks. For surgical masks, there was moderate-certainty evidence of nonserious harms, such as discomfort and difficulty in breathing, compared with no mask use, and low-certainty evidence showed that N95 respirators may not increase discomfort compared with surgical masks. The review identified no eligible studies on the use of cloth masks in community settings.
N95 respirators should not be used in a community setting, given the absence of demonstrated benefit, the possible harm with improper use (that is, the requirement for fit testing), and the global shortage of N95 respirators. Unlike N95 respirators, surgical masks and cloth masks do not require special fitting, making their use more practical if individual fitting is infeasible. Persons should seek guidance from the local community and statewide public health guidelines for mask use in light of the absence of evidence in the community setting to reduce the risk for transmission of SARS-CoV-2 infection. Factors to consider include exposure context (number of people, whether indoors or outdoors, ventilation), epidemiologic data (such as reproduction rate, daily case counts, hospitalizations, and deaths), and local demographics (such as high-risk populations). Individual values and preferences should be taken into account when deciding on the type of mask to use (surgical or cloth mask), because the benefits and harms for surgical versus cloth masks are finely balanced. The use of cloth masks in community settings has been encouraged by the Centers for Disease Control and Prevention (CDC) (4). The World Health Organization (WHO) recommends a risk-based approach for decision makers when recommending use of nonmedical masks, such as cloth masks, in the community setting (6). The WHO notes potential risks associated with mask use, including self-contamination (via improper handling of masks), breathing difficulties, and a false sense of security that could potentially detract from taking other precautions, such as physical distancing (6). Regardless of mask type, clinicians and public health officials should educate the general public about appropriate mask use (Table 3).
Table 3. Appropriate Use and Disposal of N95 Respirators, Surgical Masks, and Cloth Masks.

Table 4. Evidence Summary: What Information Does the Evidence Provide?
Persons at highest risk for SARS-CoV-2 infection are those who are in close contact with persons who have COVID-19 (7, 8). When in close contact with others, persons experiencing symptoms and those in contact with them should wear a surgical mask or cloth mask. A person who interacts with many people (such as flight attendants, restaurant servers, grocery store workers, cab drivers, and others) may benefit from wearing a surgical or cloth mask. The use of masks is not necessary when at home, unless a household member has COVID-19.
What is the effectiveness of N95 respirators, surgical masks, and cloth masks for the prevention of COVID-19 in addition to standard precautions (gowns + gloves + handwashing) in health care settings?
The goal of using respiratory PPE is to reduce exposure and prevent SARS-CoV-2 transmission between health care personnel and patients. Currently, direct evidence on the effectiveness or comparative effectiveness of N95 respirators and surgical masks for preventing SARS-CoV-2 infection in health care settings is insufficient. Given the limited direct evidence, our practice points are based on indirect evidence from studies of SARS-CoV-1, MERS-CoV, influenza or influenza-like infections, and other respiratory infections.
Low-certainty evidence showed that mask use and consistent mask use may reduce the risk for SARS-CoV-1 infection compared with no mask use and inconsistent mask use in health care settings, but studies did not specify mask type. Low-certainty indirect evidence showed that N95 respirators may reduce the risk for SARS-CoV-1 infection compared with surgical masks or no masks. Indirect evidence from studies reporting on the risk for noncoronavirus respiratory infections showed that N95 respirators probably do not reduce the risk for noncoronavirus respiratory infections compared with surgical masks (moderate certainty) and that surgical masks may reduce the risk for clinical respiratory illness, laboratory-confirmed viral infections, and influenza-like illness compared with cloth masks (low certainty). Indirect evidence was insufficient about the effect of N95 respirators or surgical masks compared with cloth masks, and surgical masks and cloth masks compared with no masks, on the risk for SARS-CoV-1 infection. Low-certainty evidence showed that N95 respirators may increase some nonserious harms, such as discomfort, breathing difficulties, and headache, compared with surgical masks and moderate-certainty indirect evidence that those harms probably do not increase with the use of surgical masks compared with cloth masks.
Uncertainty about airborne transmission of SARS-CoV-2 continues (9). Health care workers are at an increased risk for infection, because they are more likely to be in close contact with patients who are confirmed or suspected to have COVID-19. The CDC does not consider cloth masks as PPE in health care settings, given the lack of evidence of their effectiveness against transmission of SARS-CoV-2 (10).
Health care personnel should not be exposed to patients suspected or known to have COVID-19 without proper PPE. It is essential to strictly follow all other infection prevention and control measures (such as hand hygiene, physical distancing, and others) along with appropriate use of other PPE (such as gowns, gloves, and goggles) in health care settings.
What is the effectiveness for reuse or extended use of N95 respirators for prevention of COVID-19?
Extended use is defined as wearing the same N95 respirator without removal between patient encounters (5). Reuse is defined as using the same N95 respirator for several encounters with patients but removing it after each encounter (5). Currently, no evidence is available about the effectiveness of extended use or reuse of N95 respirators in health care settings. However, on the basis of an assessment of nonclinical outcomes (such as measures of filtration, contamination, and mask failure), a previous review comparing extended use and reuse of N95 respirators concluded that extended use of N95 respirators is preferable to reuse of N95 respirators because it involves less touching of the respirator, thus less risk for contact transmission (11).
Supplementary Material
Appendix: Practice Points Development Process
The Scientific Medical Policy Committee (SMPC), in collaboration with staff from ACP's Department of Clinical Policy, developed these practice points on the basis of a rapid systematic evidence review conducted by the Pacific Northwest Evidence-based Practice Center and funded by the Agency for Healthcare Research and Quality (1, 2). The SMPC comprises 11 internal medicine physicians representing various clinical areas of expertise and 1 public (nonclinician) member, and includes members with expertise in epidemiology, healthy policy, and evidence synthesis. In addition to contributing clinical, scientific, and methodological expertise, Clinical Policy staff provided administrative support and liaised between the SMPC, evidence review funding entity and evidence team, and journal. Clinical Policy staff and the SMPC reviewed and prioritized potential topic suggestions from ACP members, SMPC members, and ACP governance. A committee subgroup, including the chair of the SMPC, worked with staff to draft the key questions and lead the development of the practice points. Clinical Policy staff worked with the subgroup and an independent evidence review team to refine the key questions and determine appropriate evidence synthesis methods for each key question. Via conference calls and e-mail, Clinical Policy staff worked with the committee subgroup to draft the practice points on the basis of the results of the rapid systematic evidence review. The full SMPC reviewed and approved the final practice points. Before journal submission, ACP's Executive Committee of the Board of Regents also reviewed and approved the practice points on behalf of the ACP Board of Regents. The evidence review will be continually updated by the evidence review team. American College of Physicians will update the practice points on the basis of the evidence review by using the same process as the Version 1 (described above).
Footnotes
This article was published at Annals.org on 18 June 2020.
* This paper, written by Amir Qaseem, MD, PhD, MHA; Itziar Etxeandia-Ikobaltzeta, PharmD, PhD; Jennifer Yost, RN, PhD; Matthew C. Miller, MD; George M. Abraham, MD, MPH; Adam J. Obley, MD; Mary Ann Forciea, MD; Janet A. Jokela, MD, MPH; and Linda L. Humphrey, MD, MPH, was developed for the Scientific Medical Policy Committee of the American College of Physicians. Individuals who served on the Scientific Medical Policy Committee at the time of its approval were Linda L. Humphrey, MD, MPH (Chair)†; Robert M. Centor, MD (Vice Chair)†; Elie Akl, MD, MPH, PhD‡; Rebecca Andrews, MS, MD†; Thomas A. Bledsoe, MD†; Mary Ann Forciea, MD†; Ray Haeme†§; Janet A. Jokela, MD, MPH†; Devan L. Kansagara, MD, MCR†; Maura Marcucci, MD, MSc†; Matthew C. Miller, MD†; and Adam Jacob Obley, MD†. Approved by the ACP Board of Regents on 18 May 2020.
† Author (participated in discussion and voting).
‡ Nonauthor contributor (participated in discussion but excluded from voting).
§ Nonphysician public representative.
Update Alerts: The authors have specified in the Background section and the Appendix the interval and stop date for updates to this Practice Points article. As Annals receives updates, they will appear in the Comments section of the article on Annals.org. Reader inquiries about updates that are not available at approximately the specified intervals should be submitted as Comments to the article.
References
- 1. Chou R , Dana T , Jungbauer R , et al. Masks for Prevention of COVID-19 in Community and Healthcare Settings: A Living Rapid Review. Rapid Evidence Product. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2015-00009-I). AHRQ publication no. 20-EHC016. Agency for Healthcare Research and Quality; June 2020. doi: 10.23970/AHRQEPCCOVIDMASKS [DOI] [Google Scholar]
- 2. Chou R, Dana T, Jungbauer R, et al. Masks for prevention of respiratory virus infections including SARS-CoV-2 in health care and community settings. A living and rapid review. Ann Intern Med. [Forthcoming]. [DOI] [PMC free article] [PubMed]
- 3. World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance 6 April 2020. Accessed at https://apps.who.int/iris/rest/bitstreams/1274280/retrieve on 29 April 2020.
- 4. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings, especially in areas of significant community-based transmission. Accessed at www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html on 7 May 2020.
- 5. Centers for Disease Control and Prevention. Evaluating and testing persons for coronavirus disease 2019 (COVID-19). Accessed at www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html on 8 May 2020.
- 6. World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance 5 June 2020. Accessed at https://apps.who.int/iris/rest/bitstreams/1279750/retrieve on 9 June 2020.
- 7. World Health Organization. Q&A on coronaviruses (COVID-19). Accessed at www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses on 12 May 2020.
- 8. Centers for Disease Control and Prevention. Quarantine and isolation. Accessed at www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine-isolation.html on 12 May 2020.
- 9. Lewis D . Is the coronavirus airborne? Experts can't agree. Nature. 2020;580:175. [PMID: ] doi: 10.1038/d41586-020-00974-w [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. Accessed at www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html on 7 May 2020.
- 11. Emergency Care Research Institute. Clinical evidence assessment. Safety of extended use and reuse of N95 respirators. Accessed at https://assets.ecri.org/PDF/COVID-19-Resource-Center/COVID-19-Clinical-Care/COVID-ECRI-N95-Respirators-updated-4.pdf on 16 April 2020.
- 12. Centers for Disease Control and Prevention. Using personal protective equipment (PPE). Accessed at www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html on 29 April 2020.
- 13. World Health Organization. Coronavirus disease (COVID-19) advice for the public: when and how to use masks. Accessed at www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks on 8 May 2020.
- 14. Aiello AE , Murray GF , Perez V , et al. Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. J Infect Dis. 2010;201:491-8. [PMID: ] doi: 10.1086/650396 [DOI] [PubMed] [Google Scholar]
- 15. Aiello AE , Perez V , Coulborn RM , et al. Facemasks, hand hygiene, and influenza among young adults: a randomized intervention trial. PLoS One. 2012;7:e29744. [PMID: ] doi: 10.1371/journal.pone.0029744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alfelali M, Haworth EA, Barasheed O, et al. Facemask versus no facemask in preventing viral respiratory infections during Hajj: a cluster randomised open label trial. Accessed at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3349234 on 12 June 2020.
- 17. Barasheed O , Almasri N , Badahdah AM , et al. Hajj Research Team. Pilot randomised controlled trial to test effectiveness of facemasks in preventing influenza-like illness transmission among Australian Hajj pilgrims in 2011. Infect Disord Drug Targets. 2014;14:110-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 18. Canini L , Andréoletti L , Ferrari P , et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS One. 2010;5:e13998. [PMID: ] doi: 10.1371/journal.pone.0013998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cowling BJ , Chan KH , Fang VJ , et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151:437-46 [DOI] [PubMed] [Google Scholar]
- 20. Cowling BJ , Fung RO , Cheng CK , et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS One. 2008;3:e2101. [PMID: ] doi: 10.1371/journal.pone.0002101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larson EL , Ferng YH , Wong-McLoughlin J , et al. Impact of non-pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep. 2010 Mar-Apr;125:178-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacIntyre CR , Cauchemez S , Dwyer DE , et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15:233-41. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacIntyre CR , Zhang Y , Chughtai AA , et al. Cluster randomised controlled trial to examine medical mask use as source control for people with respiratory illness. BMJ Open. 2016;6:e012330. [PMID: ] doi: 10.1136/bmjopen-2016-012330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simmerman JM , Suntarattiwong P , Levy J , et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. Influenza Other Respir Viruses. 2011;5:256-67. [PMID: ] doi: 10.1111/j.1750-2659.2011.00205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suess T , Remschmidt C , Schink SB , et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009-2011. BMC Infect Dis. 2012;12:26. [PMID: ] doi: 10.1186/1471-2334-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lau JT , Lau M , Kim JH , et al. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis. 2004;10:235-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tuan PA , Horby P , Dinh PN , et al. WHO SARS Investigation Team in Vietnam. SARS transmission in Vietnam outside of the health-care setting. Epidemiol Infect. 2007;135:392-401. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J , Xu F , Zhou W , et al. Risk factors for SARS among persons without known contact with SARS patients, Beijing, China. Emerg Infect Dis. 2004;10:210-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chughtai AA , Seale H , Dung TC , et al. Compliance with the use of medical and cloth masks among healthcare workers in vietnam. Ann Occup Hyg. 2016;60:619-30. [PMID: ] doi: 10.1093/annhyg/mew008 [DOI] [PubMed] [Google Scholar]
- 30. MacIntyre CR , Seale H , Dung TC , et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5:e006577. [PMID: ] doi: 10.1136/bmjopen-2014-006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loeb M , Dafoe N , Mahony J , et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-71. [PMID: ] doi: 10.1001/jama.2009.1466 [DOI] [PubMed] [Google Scholar]
- 32. MacIntyre CR , Wang Q , Cauchemez S , et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses. 2011;5:170-9. [PMID: ] doi: 10.1111/j.1750-2659.2011.00198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacIntyre CR , Wang Q , Seale H , et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. 2013;187:960-6. [PMID: ] doi: 10.1164/rccm.201207-1164OC [DOI] [PubMed] [Google Scholar]
- 34. Radonovich LJ Jr , Simberkoff MS , Bessesen MT , et al. ResPECT investigators. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322:824-833. [PMID: ] doi: 10.1001/jama.2019.11645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alraddadi BM , Al-Salmi HS , Jacobs-Slifka K , et al. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerg Infect Dis. 2016;22:1915-1920. [PMID: ] doi: 10.3201/eid2211.160920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scales DC , Green K , Chan AK , et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caputo KM , Byrick R , Chapman MG , et al. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53:122-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 38. Chen WQ , Ling WH , Lu CY , et al. Which preventive measures might protect health care workers from SARS? BMC Public Health. 2009;9:81. [PMID: ] doi: 10.1186/1471-2458-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heinzerling A , Stuckey MJ , Scheuer T , et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:472-476. [PMID: ] doi: 10.15585/mmwr.mm6915e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lau JT , Fung KS , Wong TW , et al. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis. 2004;10:280-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu W , Tang F , Fang L-Q , et al. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Trop Med Int Health. 2009;14 Suppl 1 52-9. [Google Scholar]
- 42. Loeb M , McGeer A , Henry B , et al. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma HJ , Wang HW , Fang LQ , et al. [A case-control study on the risk factors of severe acute respiratory syndromes among health care workers]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:741-4. [PMID: ] [PubMed] [Google Scholar]
- 44. Nishiura H , Kuratsuji T , Quy T , et al. Rapid awareness and transmission of severe acute respiratory syndrome in Hanoi French Hospital, Vietnam. Am J Trop Med Hyg. 2005;73:17-25. [PMID: ] [PubMed] [Google Scholar]
- 45. Nishiyama A , Wakasugi N , Kirikae T , et al. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn J Infect Dis. 2008;61:388-90. [PMID: ] [PubMed] [Google Scholar]
- 46. Pei LY , Gao ZC , Yang Z , et al. Investigation of the influencing factors on severe acute respiratory syndrome among health care workers. Beijing Da Xue Xue Bao Yi Xue Ban. 2006;38:271-5. [PMID: ] [PubMed] [Google Scholar]
- 47. Raboud J , Shigayeva A , McGeer A , et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5:e10717. [PMID: ] doi: 10.1371/journal.pone.0010717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seto WH , Tsang D , Yung RW , et al. Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361:1519-20. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teleman MD , Boudville IC , Heng BH , et al. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol Infect. 2004;132:797-803. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X , Pan Z , Cheng Z . Association between 2019-nCoV transmission and N95 respirator use [Letter]. J Hosp Infect. 2020;105:104-105. [PMID: ] doi: 10.1016/j.jhin.2020.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilder-Smith A , Teleman MD , Heng BH , et al. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11:1142-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yin WW , Gao LD , Lin WS , et al. [Effectiveness of personal protective measures in prevention of nosocomial transmission of severe acute respiratory syndrome]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:18-22. [PMID: ] [PubMed] [Google Scholar]
- 53. Qaseem A , Kansagara D , Lin JS , et al. Clinical Guidelines Committee of the American College of Physicians. The development of clinical guidelines and guidance statements by the Clinical Guidelines Committee of the American College of Physicians: update of methods. Ann Intern Med. 2019;170:863-870. doi: 10.7326/M18-3290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



