Abstract
Intestinal injury is the primary toxicity of radiotherapy for pelvic and abdominal tumors, and it is also one of the common acute complications of radiotherapy. At present, there are no effective drugs to prevent intestinal injury in the clinic. Zingerone is a natural product with radioprotective effects. In this study, a novel compound (thiazolidine hydrochloride, TZC01) was synthesized by structural modification of zingerone. The effects of TZC01 on preventing intestinal injury from radiation were further investigated in this study. C57BL/6N mice were exposed to a lethal dose of abdominal irradiation (ABI) with and without TZC01 treatments. The morphological changes of the intestine and various makers of intestinal crypt cells were investigated. Treatment with TZC01 improved the survival rate of mice exposed to 12 Gy ABI. Moreover, TZC01 protected the intestinal morphology of mice, decreased the apoptotic rate of intestinal crypt cells, maintained cell regeneration and promoted crypt cell proliferation and differentiation. This study suggests that TZC01 has preventive and therapeutic effects on radiation enteritis by promoting the proliferation and differentiation of crypt cells to protect the small intestine from the toxic effects of ionizing radiation. Furthermore, the study of TCZ01 lays a strong foundation for developing novel radioprotectors with multiple properties.
Keywords: intestinal injury, abdominal irradiation, DNA damage, apoptosis
INTRODUCTION
Exposure to radiation produces lesions to the biological system and causes a series of physiological symptoms, which results in many complications. Since the small intestine is one of the organs in which cells proliferate rapidly, it is highly radiosensitive, with the result that radiation injury tends to appear soonest after exposure. Irradiation leads to the well-known symptoms of gastrointestinal (GI) radiation syndrome, for instance, GI hemorrhage, endotoxemia, bacterial infection, anorexia, nausea, vomiting, diarrhea and loss of electrolytes and fluid [1]. People who receive radiotherapy develop gastrointestinal syndrome including radiation enteritis easily and their quality of life is reduced [2]. To date, there is no clinical effective treatment for radiation enteritis. Current research into radiation enteritis mainly focuses on reducing the risk of the syndrome, and the widely used radiation protectant is amifostine [3–5]. Amifostine is a cytoprotective agent approved for protection during radiotherapy. It may cause adverse effects, such as hypotension, vomiting and allergic reactions [6]. As a result, amifostine is unattractive in preventing radiation-induced intestinal injury. With the wide range use of radiotherapy in cancer treatment, there is an urgent need to develop highly effective radioprotective agents with fewer adverse effects for radiation enteritis.
Ginger, is the rhizome of zingiber officinale roscoe. It has a long history in traditional medicine for the treatment of various diseases. Recent studies have shown that ginger and its extract possess a radioprotective effect [7, 8]. Zingerone is an essential oil extracted from ginger that has various biological functions, including antioxidant, anti-inflammatory and antiapoptosis properties [9–12]. Zingerone is known as a potent free-radical scavenger with protective properties against reactive oxygen species (ROS)-mediated DNA damage by scavenging and degrading free radicals and ROS [13, 14]. Zingerone was also found to attenuate cellular senescence and dampens production of pro-inflammatory cytokines induced by UVB irradiation [15]. However, zingerone is insoluble in water and ethanol was used as cosolvent when administered orally [16]. Cysteamine has been known as a radioprotector for decades. In patients treated with ionizing radiations, the symptoms of radiation sickness are often eliminated when cysteamine is adminstered intravenously in a 200 mg dose [17]. Brown hypothesized that cysteamine may limit the propagation of DNA damage and increase the fidelity of DNA repair to inhibit proliferaton and thereby protect the cells [18]. However, this exogenous thiol compound can generate an oxidant stress, which makes cysteamine cytotoxic [19]. Moreover, a near-toxic dose is needed to achieve adequate protection [20].
In this study, for the first time, we developed a new radioprotector by incorporating zingerone and cysteamine together so as to achieve a higher radioprotective effect and optimize their fundamental physicochemical properties. The newly synthesized compound was converted into its hydrochloride (thiazolidine hydrochloride, TZC01) to obtain higher water solubility. The protective effects of TZC01 on ionzing radiation-induced intestinal damage in mouse models exposed to 12 Gy abdominal irradiation (ABI) was evaluated. Our data showed that TZC01 could improve the survival rate of mice and reduce the radiation-induced intestinal damage.
MATERIALS AND METHODS
Animals and ethics approval and consent to participate
Male C57BL/6J mice (8-10 weeks) were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). Animals were bred in the certified animal facility in the Institute of Radiation Medicine (IRM) of the Chinese Academy of Medical Sciences (CAMS).
All experimental procedures were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of IRM-CAMS (Permit Number 20180310). The animals were cared for in accordance with the dictates of the National Animal Welfare Law of China.
Synthesis of TZC01
To a suspension of cysteamine hydrochloride (3.25 g, 28.6 mmol) and triethylamine (2.9 g, 28.6 mmol) in toluene was added zingerone (5 g, 25.7 mmol) and p-toluenesulfonic acid monohydrate (0.245 g, 1.3 mmol) while stirring. The reaction mixture was refluxed for 6 h with a Dean-Stark water trap. The reaction mixture was cooled, washed with 0.1 M sodium hydroxide solution and the aqueous phase was extracted with ethyl acetate (50 mL × 2). The combined toluene and ethyl acetate phase was washed with brine and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to give the crude product which was purified by column chromatography elution with hexane and ethyl acetate to obtain the thiazolidine (4.47 g, 68.5%). Thiazolidine was reacted with a solution of hydrogen chloride in methanol to afford thiazolidine hydrochloride (5.11 g, 100%). MS (ESI): m/z calcd for C13H19NO2S [M+H]+: 254.1, found: 254.2. 1H NMR (400 MHz, DMSO) δ 10.32 (br s, 2H), 8.76 (br s, 1H), 6.84 (d, J = 2 Hz, 1H), 6.70 (d, J = 8.0 Hz, 1H), 6.63 (dd, J = 8.0 Hz, 2 Hz, 1H), 3.76 (s, 3H), 3.72-3.58 (m, 2H), 3.33-3.21 (m, 2H), 2.72-2.58 (m, 1H), 2.63-2.53 (m, 1H), 2.33-2.13 (m, 2H), 1.74 (s, 3H). 13C NMR (100 MHz, DMSO) δ 147.9, 145.2, 131.7, 120.8, 115.8, 113.0, 75.3, 56.0, 46.9, 42.6, 40.5, 40.3, 40.1, 39.9, 39.7, 39.5, 39.3, 31.3, 29.3, 25.9.
Irradiation and treatment
Irradiation was performed using a X source housed in an Exposure Instrument Biological X-ray radiometer (Rad Source, Buford, Georgia, USA) at a dose-rate of 1.2 Gy per min. We first carried out two mouse survival rate experiments. Mice were randomly divided into five groups (n=10), including Control, Solvent, TZC01 (50 mg/kg), TZC01 (100 mg/kg) and TZC01 (200 mg/kg). All four groups except the control group were exposed to 12 Gy ABI. The Control group were treated similarly to the irradiation group but without exposure to IR. Mice were randomly divided into five groups (n=10), including Control, Solvent, TZC01 (100 mg/kg), cysteamine (100 mg/kg) and zingerone (100 mg/kg). All four groups except the Control group were exposed to 12 Gy ABI. Control group were treated similarly to the irradiation group but without exposure to IR.
In the remaining experiments, the mice were randomly divided into three groups (n=5): (a) Control; (b) Solvent; (c) TZC01. Groups Solvent and TZC01 received 12 Gy ABI. The TZC01 group was intragastrically administered with a dose of 100 mg/kg 1 hour before the irradiation, and was continuously administered for 2 days after irradiation. Mice of groups Control and Solvent were treated with solvent similarly to the procedure described for the treatment group.
Histological analysis
Three days after ABI, mice were sacrificed and paraffin sections were prepared from small intestine tissue and then stained with hematoxylin–eosin (H&E) and analyzed under a microscope. The number of intestinal crypts were counted using ImageJ 1.37 software to assess the effect of TZC01 on the apoptosis of irradiated mice intestinal crypt cells.
Immunohistochemistry analysis
Mice were sacrificed after 3 days of ABI and small intestine tissues were fixed in 10% neutral formalin. The duodenum, jejunum and ileum of appropriate size were selected and embedded in wax. They were cut into 4 μm wax pieces and attached to the glass slide and dried in a dryer. After antigen retrieval, nonspecific sites were blocked with goat serum for 15 min at room temperature. Sections were incubated with primary antibody overnight at 4°C, including antibodies against Lgr5 (Abcam, Cambridge, MA, USA), villi (Abcam), lysozyme (Abcam) and Ki67 (Novus, Littleton,CO, USA). After thoroughly washing with PBS, the secondary antibody was added and incubated at room temperature for 1 h at 37°C, and positive cells were detected by a DAB (3, 3’-Diaminobenzidine tetrahydrochloride hydrate) kit (Sigma-Aldrich).
Immunofluorescence analysis
The steps before antigen retrieval are the same as described for immunohistochemistry. The tissues were washed thoroughly with PBS and goat serum was added for 1 h at room temperature. Sections were incubated with primary antibody overnight at 4°C, including antibodies against γH2AX (BD biosciences, NJ, USA) and caspase-3 (CST, MA, USA). After thorough washing with PBS, the slices were incubated with a secondary antibody mixed with DAPI (4’, 6-diamidino-2-phenylindole) in a 37°C incubator for 40 min in the dark. The slices were sealed and observed under a laser scanning confocal microscope.
Statistical analysis
All analyses were performed using GraphPad Prism6 software, and differences were considered significant at P < 0.05. Log-rank test was used to analyze the significant difference of survival rate among groups and Tukey’s test was used to analyze other data.
RESULTS
Synthesis and characterization of TZC01
In this study, we designed and synthesized a novel compound, TZC01, to contain properties of zingerone and cysteamine. As shown in Fig. 1, zingerone reacted with cysteamine in toluene under reflux to give thiazolidine. Then, thiazolidine was treated with a solution of HCl in MeOH to produce TZC01. The total yield of TZC01 was 68.5%. The structure of the synthesized compound was characterized by nuclear magnetic resonance (NMR) and electrospray ionization mass spectrometer (ESI-MS) (see Supplementary Fig. S1, Fig. S2 and Fig. S3, available as Supplementary material online). The results showed that the new compound was successfully prepared with a facile synthetic approach.
Fig.1.
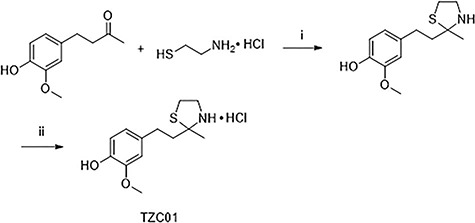
Synthesis of TZC01. i, triethylamine, p-toluenesulfonic acid monohydrate, toluene, reflux; ii, HCl/MeOH, 0°C.
TZC01 improves the survival rate of mice after ABI
To determine the protective effect of TZC01 on mice exposed to radiation, we first observed the survival rate of mice after 12 Gy ABI (Fig. 2). The mice were treated with TZC01 in three dosages (50 mg/kg, 100 mg/kg, 200 mg/kg). We found that the best effect was obtained when the dose of TZC01 was 100 mg/kg. The solvent-administered group began to die on the fourth day after 12 Gy ABI. The mortality rate was 80%, and the average survival time was 4 days. The average survival times of the of zingerone-administration or cysteamine-administration groups were 4.5 days. For the TZC01-administration group, the average survival time was 5.5 days. This result suggested that TZC01 had not only a certain protective effect on radiation-induced intestinal damage, but also presented an improved protective effect compared with zingerone or cysteamine. It is worth noting that TZC01-administrated mice die soon after X-irradiation because the irradiation dose and dose-rate were too high to be tolerated by mice.
Fig. 2.
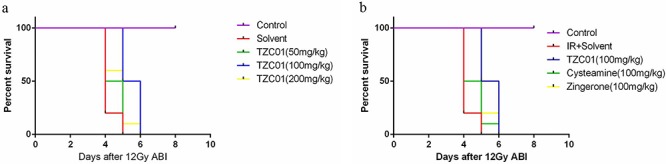
Survival curves after 12 Gy of abdominal irradiation. (a) The effect of different doses of TZC01 on survival rate in mice (P < 0.05, n = 10 per group). (b) The effect of the same dose of TZC01, zingerone and cysteamine on survival rate in mice (P < 0.05, n = 10 per group). P values were calculated by the log-rank test.
TZC01 protects against radiation-induced gastrointestinal injury
In order to test whether TZC01 has a radiation protection effect, we evaluated the morphological changes in the small intestine of mice. The villus length and crypt density of the small intestine were significantly reduced in the Solvent group compared with the TZC01 group at 3 days after 12 Gy ABI (Fig. 3a and c). It is gratifying that the mice treated with TZC01 have significantly improved intestinal villus length and crypt biology. Compared with the Solvent group, the number of crypts in the TZC01 group was increased by 41.7% (P < 0.05), and the villus length of the small intestine increased by 16.39% on average (P < 0.01) (Fig. 3b and d). The results show that TZC01 has a protective effect on the damage to intestinal morphology.
Fig. 3.

(a) H&E-stained small intestine cross-sectional structure. (b) Histogram showing the number of crypts. (c) Immunohistochemistry images showing the expression of Villi. (d) Histogram demonstrating villus length in intestinal section from the Control group, Solvent-treated group and TZC01-treated group. The results are represented as mean ± SD, n = 5 mice per group. *P<0.05, **P<0.01, using Tukey’s test.
Lgr5 is a well-established adult intestinal stem cell marker [21]. The regenerating rate of the intestinal lining is due to the population of stem cells at the base of the intestinal crypt. Thus, Lgr5 levels represent radiation-induced intestinal regeneration. Three days after administration of 12 Gy ABI to mice, the number of Lgr5+ intestinal stem cells in mice treated with TZC01 was significantly higher than that in mice treated with solvent (Fig. 4a and b). Ki67 is a nuclear antigen present in proliferating cells and is one of the most widely used proliferating cell markers [22]. Therefore, ki67 levels also represent radiation-induced intestinal proliferation. The results show the number of Ki67-positive cells in the TZC01 group was significantly higher than that of solvent group after ABI (Fig. 4c and d). Lysozyme is a protein secreted by Paneth cells [23]. Paneth cells residing at the base of the small intestinal crypts contribute to the mucosal intestinal first-line defense by secreting granules filled with antimicrobial polypeptides including lysozyme [24]. We found a significant improvement in the number of lysozyme− positive cells in the TZC01 group compared with the solvent group (Fig. 4e and f). These data show that TZC01 can effectively improve the proliferation and differentiation of small-intestine crypt cells.
Fig. 4.
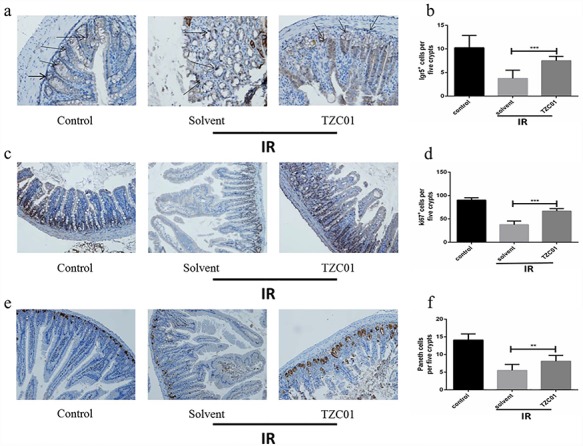
Radiation protection of TZC01. The small intestinal sections were analyzed by immunohistochemistry. (a) Photomicrograph of Lgr5 immunostaining section of Control, Solvent and TZC01 groups. (b) Histogram showing Lgr5-positive cells quantified in five crypts per section. (c) Immunostaining images showing quantitative analysis of Ki67 expression of intestinal crypts. (d) Histogram demonstrating Ki67-positive cells counted in five crypts per section. (e) Representative immunohistochemistry images for lysozyme-stained sections of small intestine. (f) Histogram showing the number of paneth cells per five crypts. The results are represented as mean ± SD, n = 5 mice per group. **P<0.01. ***P<0.005, using Tukey’s test.
Phosphorylation of H2AX to form γH2AX is a hallmark of DNA double-strand breaks [25]. As shown in Fig. 5, the expression of γ-H2AX in the small intestine of the Solvent group was increased 88.8% compared with the Control group. Compared with the Solvent group, the expression level of γH2AX protein in crypt of the TZC01 group was decreased by 77.6%, and the difference was significant (P<0.005). The results indicate that TZC01 can reduce radiation-induced DNA damage. This data showed that TZC01 could attenuate DNA damage of the small intestine after 12 Gy ABI.
Fig. 5.
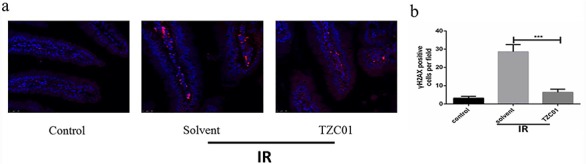
TZC01 attenuates DNA damage of mice after ABI. The small intestines of control mice, solvent-treated mice and TZC01-treated mice were obtained at 3 days after 12 Gy ABI. (a) Representative immunofluorescence images for the expression of γH2AX in the small intestine (red, γH2AX; blue, DAPI). (b) Histogram demonstrating quantitative analysis of γH2AX-positive cells per view field. The results are represented as mean ± SD, n = 5 mice per group. ***P<0.005, using Tukey’s test.
Caspase-3 is the most important terminal cleavage enzyme in the process of apoptosis [26]. Thus, we assessed the caspase-3 levels of intestinal tissues by DAPI and caspase-8 staining (Fig. 6a and b). The expression level of caspase-3 can reflect the apoptosis of cells. Compared with the Solvent group, the expression level of caspase-3 in the TZC01 administration group was reduced by 75.5% (P<0.005). These data demonstrate that TZC01 treatment can reduce apoptosis and protect against intestinal damage caused by radiation.
Fig. 6.
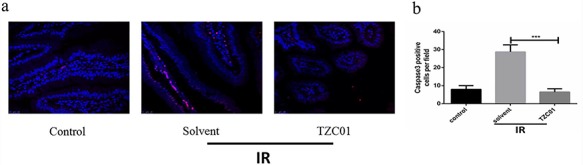
TZC01 reduces apoptosis in the small intestine after ABI. (a) Representative DAPI and caspase-3 staining images of the small intestine (red, caspase3; blue, DAPI). (b) Histogram showing quantitative analysis of caspase3-positive cells per field of view. The results are represented as mean ± SD, n = 5 mice per group. ***P<0.005, using Tukey’s test.
DISCUSSION
Abdominal local radiotherapy is a common treatment for abdominal and pelvic tumors. Although a precise radiation can be applied to tumor sites, other surrounding normal tissues, especially intestine, may be affected and damaged. In addition, the irradiation of individuals in a nuclear accident and patients with localized radiotherapy, for conditions such as leukemia, can cause damages to the digestive tract, especially the intestines. Radiation-induced intestinal damage is an important cause of acute gastrointestinal symptoms and death in patients. Ionizing radiation can cause abnormal changes in intestinal crypt, resulting in inhibition of epithelial cell proliferation, increasing intestinal stem cell apoptosis, blocking the regeneration of villus epithelium, destroying the integrity of villus epithelial structure, inducing intestinal barrier dysfunction, and even causing bacteremia. In severe cases, the individual will die [23]. To date, there is no effective clinical radioprotector to prevent intestinal injury to combine with radiotherapy for cancer patients. It is crucial to develop protective agents against radiation damage of gastrointestinal cells [27]. In the present study, TZC01 enhanced the survival rates of mice after exposure to 12 Gy ABI, indicating that TZC01 protects against radiation-induced intestinal injury.
Lgr5 is an intestinal stem cell marker, we visualized stem cells and studied their behavior and differentiation in this case. We found that after 12 Gy of ABI, the number of Lgr5-positive stem cells in the TZC01 administration group was significantly higher than that in the Solvent group. As shown in Fig. 4, other differentiated cells such as Paneth cells were increased after ABI, indicating that TZC01 can protect against intestinal injury caused by radiation in the small intestine by promoting the proliferation and differentiation of intestinal stem cells. The increased expression of Ki67, a proliferative marker in the small intestine, in the TZC01-treated group may indicate the recovery of intestinal damage after IR. The epithelium of the small intestine contains crypts and villi. Intestinal stem cells are located at the bottom of the crypt and are responsible for maintaining intestinal epithelial homeostasis and regeneration after injury [28, 29]. Our results show that the structural integrity of the small intestine villi is improved after irradiation with 12 Gy in TZC01-treated mice. These results indicate that TZC01 may have protective effects on intestinal damage caused by radiation.
The main cytotoxic effects of ionizing radiation are related to various DNA damages including double-strand breaks. Double-strand breaks can lead to chromosomal aberrations, which can lead to lesions such as most biological damage including cell death [30]. In this study, we found that the expression level of γH2AX was lower in the TZC01 group than in the solvent group. Caspase-3 is an apoptotic executor, which was used as an indicator of intestinal cell apoptosis in our study [31, 32]. Caspase-3 gene expression in γ-radiation-induced cardiac apoptosis was significantly downregulated by zingerone in previous reports [33]. In this study, apoptosis was reduced in the TZC01 group compared with the Solvent group. The expression of caspase-3 in the small intestines of the TZC01 group was also decreased compared with the Solvent group. These results indicate that TZC01 has an inhibitory effect on radiation-induced apoptosis of small intestinal cells.
Both zingerone and cysteamine have been widely studied as radioprotectors. Zingerone has anti-oxidant, anti-inflammatory and anti-apoptosis properties. The poor water solubility of zingerone is the main problem for its development as a new radioprotective agent. Cysteamine may increase the fidelity of DNA repair to protect the cells. However, cysteamine causes oxidant stress, leading to cytotoxity. For the first time, we synthesized a new radioprotector by incorporating both zingerone and cysteamine. We hoped to combine the two different radioprotectors in TZC01 to produce a compound with improved physicochemical properties. Our research indicates that TZC01 may play a protective role in radiation-induced intestinal damage by promoting proliferation and differentiation of intestinal crypt stem cells, inhibiting apoptosis and reducing DNA damage. This study provides a strong basis for the development of a novel radioprotective agent by incorporating multiple radioprotectors.
Supplementary Material
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This study was supported by the National Natural Science Foundation of China (No. 81573094), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-3-022 and 2017-I2M-3-019).
REFERENCES
- 1. Monti P, Wysocki J, Van Der Meeren A et al. The contribution of radiation-induced injury to the gastrointestinal tract in the development of multi-organ dysfunction syndrome or failure. Br J Radiol 2005;Supplement_27:89–94. [Google Scholar]
- 2. Gami B, Harrington K, Blake P et al. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2003;18:987–94. [DOI] [PubMed] [Google Scholar]
- 3. Vidal A, De La Cuerda C, Luis Escat J et al. Chronic radiation enteritis after ovarian cancer: From home parenteral nutrition to oral diet. Clin Nutr 2006;25:701–4. [DOI] [PubMed] [Google Scholar]
- 4. He J-Y, Wang W-Z, Qi H-Z et al. Use of recombinant lactobacillus sakei for the prevention and treatment of radiation-induced enteritis. Med Hypotheses 2018;119:37–40. [DOI] [PubMed] [Google Scholar]
- 5. Grabenbauer GG, Holger G. Management of radiation and chemotherapy related acute toxicity in gastrointestinal cancer. Best Pract Res Clin Gastroenterol 2016;30:655–64. [DOI] [PubMed] [Google Scholar]
- 6. Rades D, Fehlauer F, Bajrovic A et al. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol 2004;70:261–4. [DOI] [PubMed] [Google Scholar]
- 7. Ji K, Fang L, Zhao H et al. Ginger oleoresin alleviated gamma-ray irradiation-induced reactive oxygen species via the Nrf2 protective response in human Mesenchymal stem cells. Oxidative Med Cell Longev 2017;2017: 1480294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saberi H, Keshavarzi B, Shirpoor A et al. Rescue effects of ginger extract on dose dependent radiation-induced histological and biochemical changes in the kidneys of male Wistar rats. Biomed Pharmacother 2017;94:569–76. [DOI] [PubMed] [Google Scholar]
- 9. Alibakhshi T, Khodayar MJ, Khorsandi L et al. Protective effects of zingerone on oxidative stress and inflammation in cisplatin-induced rat nephrotoxicity. Biomed Pharmacother 2018;105:225–32. [DOI] [PubMed] [Google Scholar]
- 10. Shin S-G, Kim J-Y, Chung H-Y et al. Zingerone as an antioxidant against peroxynitrite. J Agric Food Chem 2005;53:7617–22. [DOI] [PubMed] [Google Scholar]
- 11. Kaygusuzoglu E, Caglayan C, Kandemir FM et al. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomed Pharmacother 2018;102:517–30. [DOI] [PubMed] [Google Scholar]
- 12. Mani V, Arivalagan S, Siddique AI et al. Antioxidant and anti-inflammatory role of zingerone in ethanol-induced hepatotoxicity. Mol Cell Biochem 2016;421:169–81. [DOI] [PubMed] [Google Scholar]
- 13. Rajan I, Narayanan N, Rabindran R et al. Zingerone protects against stannous chloride-induced and hydrogen peroxide-induced oxidative DNA damage in vitro. Biol Trace Elem Res 2013;155:455–9. [DOI] [PubMed] [Google Scholar]
- 14. Rao BN, Archana PR, Aithal BK et al. Protective effect of zingerone, a dietary compound against radiation induced genetic damage and apoptosis in human lymphocytes. Eur J Pharmacol 2011;657:59–66. [DOI] [PubMed] [Google Scholar]
- 15. Lee J, Oh S-W, Shin S-W et al. Zingerone protects keratinocyte stem cells from UVB-induced damage. Chem-Biol Interact 2018;279:27–33. [DOI] [PubMed] [Google Scholar]
- 16. Rao BN, Rao BSS, Aithal BK et al. Radiomodifying and anticlastogenic effect of Zingerone on Swiss albino mice exposed to whole body gamma radiation. Mutat Res Genet Toxicol Environ Mutagen 2009;677:33–41. [DOI] [PubMed] [Google Scholar]
- 17. Bacq ZM, Dechamps G, Fischer P et al. Protection against x-rays and therapy of radiation sickness with beta-mercaptoethylamine. Science 1953;117:633–6. [DOI] [PubMed] [Google Scholar]
- 18. Brown PE. Mechanism of action of Aminothiol Radioprotectors. Nature 1967;213:363–4. [DOI] [PubMed] [Google Scholar]
- 19. Jeitner TM, Lawrence DA. Mechanisms for the cytotoxicity of cysteamine. Toxicol Sci 2001;63:57–64. [DOI] [PubMed] [Google Scholar]
- 20. Mönig H, Messerschmidt O, Streffer C. Chemical Radioprotection in Mammals and in Man In: Scherer E, Streffer C, Trott K-R (eds). Radiation Exposure and Occupational Risks. Berlin, Heidelberg: Springer Berlin Heidelberg, 1990, 97–143. [Google Scholar]
- 21. Carroll TD, Newton IP, Chen Y et al. Lgr5(+) intestinal stem cells reside in an unlicensed G1 phase. J Cell Biol 2018;217:1667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gong W, Guo M, Han Z et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis 2016;7:e2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bel S, Pendse M, Wang Y et al. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science 2017;357:1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinez Rodriguez NR, Eloi MD, Huynh A et al. Expansion of Paneth cell population in response to enteric salmonella enterica serovar Typhimurium infection. Infect Immun 2012;80:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weyemi U, Paul BD, Snowman AM et al. Histone H2AX deficiency causes neurobehavioral deficits and impaired redox homeostasis. Nat Commun 2018;9:1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu C, Zhang J, Chen Y, et al. A trigger model of apoptosis induced by tumor necrosis factor signaling. BMC Syst Biol 2011; 5Suppl 1: S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res 2004;161:123–36. [DOI] [PubMed] [Google Scholar]
- 28. Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: The mucosal governor. Int J Exp Pathol 1997;78:219–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Booth C, Potten CS. Gut instincts: Thoughts on intestinal epithelial stem cells. J Clin Invest 2000;105:1493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carabajal E, Massari N, Croci M et al. Radioprotective potential of histamine on rat small intestine and uterus. Eur J Histochem 2012;56:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong Y, Cheng Y, Hou Q et al. The protective effect of new compound XH-103 on radiation-induced GI syndrome. Oxidative Med Cell Longev 2018;2018:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kantara C, Moya SM, Houchen CW et al. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest 2015;95:1222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soliman AF, Anees LM, Ibrahim DM. Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn-Schmiedebergs Arch Pharmacol 2018;391:819–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


