Inflammasome Activation Deteriorates Cardiac Function After Myocardial Infarction
Acute myocardial infarction (AMI) is a global leading cause of death, with a prevalence of 3 million worldwide.1,2 Although the acute mortality rate after AMI has been reduced significantly by successful implementation of percutaneous coronary intervention, some patients still experience myocardial remodelling and ultimately heart failure after being discharged from hospital.3 Inflammation is a critical process that mediates tissue healing during the ischemic injury.2 However, mounting evidence suggests that unbalanced inflammation can facilitate adverse myocardial remodelling through the activation of one of the most known innate inflammatory signalling pathways—the nucleotide-binding domain, leucine-rich-repeat family, pyrin-domain-containing 3 (NLRP3) inflammasome.4 The sudden shortage in the oxygen supply during AMI can cause transmural myocardial necrosis, which then leads to the release of damage-associated molecular patterns (DAMPs), such as double-stranded DNA (dsDNA), adenosine triphosphate (ATP), and organelles from the damaged cells. DAMPs are universally considered to be the molecular signal that initiates a cascade of events leading to the activation of NLRP3 inflammasomes in a variety of cell types.4,5 Once the inflammasome is activated, it produces the mature/active caspase-1, a protease that promotes: 1) the release of proinflammatory and profibrotic cytokine interleukin (IL)-1β; and 2) cell death (known as “pyroptosis”) mediated by gasdermin D. Both events progressively worsen cardiac function and perhaps ultimately cause irreversible damage to cardiac muscle. The NLRP3 inflammasome exists
Activation of Neutrophil Inflammasome is Implicated in Acute Myocardial Infarction
In the current issue of the Canadian Journal of Cardiology, work from the Yin lab offers an interesting perspective regarding how the calcium-sensing receptor (CaSR)—mediated activation of neutrophils affects myocardial remodelling after AMI.6 The CaSR, a G protein—coupled receptor, can be activated by increased extracellular Ca2+ levels to promote assembly of the inflammasome in myeloid cells.7 After AMI, the extracellular ionic environment is altered owing to cytolysis. Specifically, the extracellular Ca2+ concentration is increased in the border zone and plasma samples of AMI patients,8 which explains the enhanced activity of CaSR in both peripheral and infiltrated post-AMI neutrophils described in the Ren et al. study.6 By characterizing neutrophils purified from the blood samples of healthy control and AMI patients, Ren et al. found that CaSR expression was transiently increased within the first 24 hours and then gradually declined over the 7 days post AMI. The expression and activity of the NLRP3 inflammasome in neutrophils of AMI patients were simultaneously increased, which subsequently led to increased caspase-1 maturation and IL-1β release. The temporal changes in CaSR protein levels correlated with activation of the NLRP3 inflammasome. To determine whether NLRP3 activation in neutrophils was mediated by CaSR, Ren et al. exposed neutrophils derived from healthy patients to the CaSR agonist calindol, which resulted in dose- and time-dependent activation of the NLRP3 inflammasome. Alternatively, exposure to the CaSR inhibitor calhex-231 resulted in decreased NLRP3 inflammasome activation in neutrophils. Moreover, calhex-231 reduces the already enhanced inflammasome activity of AMI-derived neutrophils in vitro, further confirming that CaSR contributes to the activation of neutrophil NLRP3 inflammasome in AMI patients. In addition to the peripheral neutrophils, Ren et al. demonstrated the direct involvement of CaSR-mediated NLRP3 inflamamsome activation in the myocardium as a consequence of infiltrating neutrophils in a rat model of AMI. Compared with those of the control animals, post-AMI rat hearts exhibited necrotic cardiomyocytes and significant immune cell infiltration (particularly neutrophils) inside the infarct zone, in which the infiltrating neutrophils exhibited enhanced expression of CaSR, NLRP3 inflammasome components, mature caspase-1, and IL-1β. To determine how infiltrating neutrophils affect the function of post-AMI cardiac cells, Ren et al. treated the rat neonatal cardiomyocytes with the culture media of neutrophils prestimulated with either calindol or calbex-231. They found that the medium from neutrophils prestimulated with the CaSR agonist promotes apoptosis and IL-1β secretion in cardiomyocytes, which was absent when the cardiomyocytes were treated with the media from neutrophils prestimulated with the CaSR inhibitor. Meanwhile, the authors also showed that the conditioned media from the CaSR-activated neutrophils stimulates cardiac fibroblasts to transform into myofibroblasts, evidenced by the elevated levels of IL-1β receptor (IL-1R), matrix metalloproteinases (MMP2 and MMP9), alpha smooth muscle actin, and collagen deposition. Fibroblast transformation was suppressed with the conditioned media of neutrophils pretreated with the CaSR inhibitor calbex-231.
Implications and Limitations
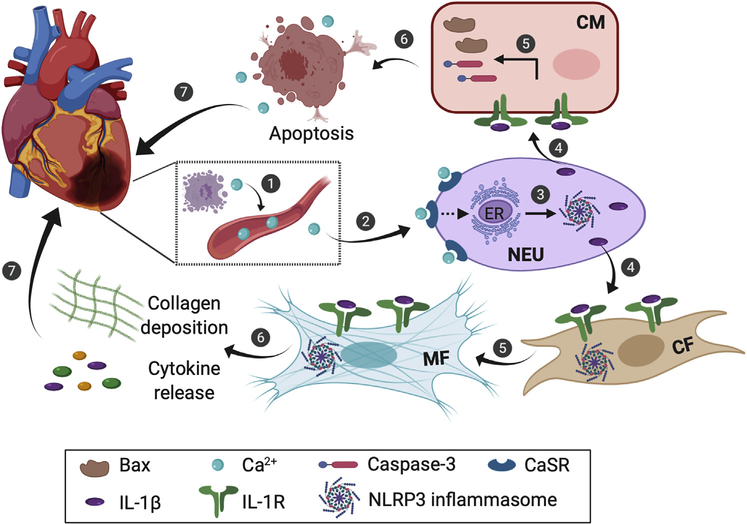
This work by Ren et al. establishes connections between a series of events that could explain myocardial remodelling within the first few days following AMI.6 The sequence of events (Fig. 1) can be described as follows: 1) AMI-induced acute myocyte necrosis elevates extracellular Ca2+ levels, which in turn activates the CaSR on neutrophils; 2) the activated CaSR on neutrophils promotes NLRP3 inflammasome—mediated IL-1β maturation and release; and 3) secreted IL-1β further activates the cardiomyocytes and fibroblasts via IL-1 receptors, which promotes myocyte apoptosis and fibroblast activation, thereby leading to myocardial remodelling. A few follow-up investigations could potentially build on the current work and findings from the Yin lab. First, in this study, Ren et al. found that the CaSR-mediated activation of NLRP3 inflammasome in neutrophils can be attenuated by a phospholipase C (PLC) inhibitor, an inositol-1,4,5-trisphosphate (IP3) receptor antagonist, or an endoplasmic reticulum (ER) Ca2+-ATPase pump inhibitor. This result suggests that CaSR could activate NLRP3 inflammasomes by inducing ER Ca2+ release via the PLC-IP3 system. However, whether ER-mediated Ca2+ release is dependent on CaSR activation in neutrophils remains to be determined. Because a similar observation has been previously reported in macrophages,9 it would be intriguing to explore whether a regulatory axis similar to the CaSR activation—ER Ca2+ release—NLRP3 inflammasome activation axis also exists in other nonimmune cells. Second, Ren et al. showed that targeting CaSR with the use of calbex-231 alleviates some aspects associated with myocardial remodeling in vitro. It would have been informative to quantify scar size and cardiac function in AMI rats treated with calbex-231. Third, inflammation is a dynamic process and usually involves multiple types of immune cells. It would be beneficiai to understand the impact of the early onset of neutrophil activation on other immune cell types to provide a complete picture of inflammasome activity during myocardial remodelling.
Figure 1.
The proposed sequence of events that lead to myocardial remodelling after acute myocardial infarction. The dashed arrow indicates the postulated mechanism. CF, cardiac fibroblast; CaSR, calcium-sensing receptor; CM, cardiomyocyte; ER, endoplasmic reticulum; IL, interleukin; MF, myofibroblast; NEU, neutrophil.
In conclusion, despite the above-mentioned limitations, this work by Ren et al. establishes that CaSR-activated neutrophils behave like a double-edged sword in myocardial remodelling by promoting cell death in cardiomyocytes and fibroblast activation within the injured region. Future studies are warranted to evaluate the therapeutic potential of CaSR antagonists in preclinical models of AMI.
Acknowledgments
Funding Sources
This work was supported by grants from the National Institutes of Health (R01HL136389 and R01HL147108 to N.L.).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Boateng S, Sanborn T. Acute myocardial infarction. Dis Mon 2013;59: 83–96. [DOI] [PubMed] [Google Scholar]
- 2.Westman PC, Lipinski MJ, Luger D, et al. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 2016;67:2050–60. [DOI] [PubMed] [Google Scholar]
- 3.Aso S, Imamura H, Sekiguchi Y, et al. Incidence and mortality of acute myocardial infarction. A population-based study including patients with out-of-hospital cardiac arrest. Int Heart J 2011;52:197–202. [DOI] [PubMed] [Google Scholar]
- 4.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018;15:203–14. [DOI] [PubMed] [Google Scholar]
- 5.Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, et al. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 2018;186:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Z, Yang K, Zhao M, et al. Calcium-sensing receptor on neutrophil promotes myocardial apoptosis and fibrosis after acute myocardial infarction via NLRP3 inflammasome activation. Can J Cardiol 2020;36:813–5. [DOI] [PubMed] [Google Scholar]
- 7.Hendy GN, Canaff L. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin Cell Dev Biol 2016;49:37–43. [DOI] [PubMed] [Google Scholar]
- 8.Speich M, Bousquet B, Nicolas G. Concentrations of magnesium, calcium, potassium, and sodium in human heart muscle after acute myocardial infarction. Clin Chem 1980;26:1662–5. [PubMed] [Google Scholar]
- 9.Lee GS, Subramanian N, Kim AI, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012;492:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]