Abstract
Objectives
To investigate remodeling of hippocampal cholinergic inputs after noise exposure and determine the relevance of these changes to tinnitus.
Methods
To assess the effects of noise exposure on the hippocampus, guinea pigs were exposed to unilateral noise for two hours and 2 weeks later, immunohistochemistry was performed on hippocampal sections to examine vesicular acetylcholine transporter (VAChT) expression. To evaluate whether the changes in VAChT were relevant to tinnitus, another group of animals was exposed to the same noise band twice to induce tinnitus, which was assessed using gap-prepulse Inhibition of the acoustic startle (GPIAS) 12 weeks after the first noise exposure, followed by immunohistochemistry.
Results
Acoustic Brainstem Response (ABR) thresholds were elevated immediately after noise exposure for all experimental animals but returned to baseline levels several days after noise exposure. ABR wave I amplitude-intensity functions did not show any changes after 2 or 12 weeks of recovery compared to baseline levels. In animals assessed two-weeks following noise-exposure, hippocampal VAChT puncta density decreased on both sides of the brain by 20%−60% in exposed animals. By 12 weeks following the initial noise exposure, changes in VAChT puncta density largely recovered to baseline levels in exposed animals that did not develop tinnitus, but remained diminished in animals that developed tinnitus. These tinnitus-specific changes were particularly prominent in hippocampal synapse-rich layers of the dentate gyrus and areas CA3 and CA1, and VAChT density in these regions negatively correlated with tinnitus severity.
Conclusions
The robust changes in VAChT labeling in the hippocampus two weeks after noise exposure suggest involvement of this circuitry in auditory processing. After chronic tinnitus induction, tinnitus-specific changes occurred in synapse-rich layers of the hippocampus, suggesting that synaptic processing in the hippocampus may play an important role in the pathophysiology of tinnitus.
Keywords: vesicular acetylcholine transporter (VAChT), auditory, limbic system, memory, neuroplasticity
1. INTRODUCTION
Tinnitus, the phantom perception of sound in the absence of external acoustic stimuli, affects millions of people around the world (Martinez, Wallenhorst, McFerran, & Hall, 2015; Shargorodsky, Curhan, & Farwell, 2010). While some habituate to the persistent noise, many tinnitus sufferers experience depression (Bhatt, Bhattacharyya, & Lin, 2017; House et al., 2017) and emotional distress (Riedl et al., 2015), which leads to a significant decrement in quality of life. Therefore, there is a pressing need to unveil the mechanisms of tinnitus, making the way for effective cures. Tinnitus generation is multifactorial. Stress, sleep, hearing loss, gender and age are all associated with tinnitus (Kim et al., 2015; Park et al., 2014). Patients with tinnitus often report a history of acoustic overexposure (Schmuzigert, Fostiropoulos, & Probst, 2006), and noise exposure is widely used as a method of tinnitus induction in animal models (Berger et al., 2018; Marks et al., 2018; Wu, Martel, & Shore, 2016).
A large body of tinnitus-related research has focused on auditory sensory pathways, including cochlear nucleus (Koehler & Shore, 2013; Stefanescu, Koehler, & Shore, 2015; Wu et al., 2016), inferior colliculus (Bauer, Turner, Caspary, Myers, & Brozoski, 2008; Smit et al., 2016; F. Wang et al., 2013), medial geniculate body (Kalappa, Brozoski, Turner, & Caspary, 2014; Sametsky, Turner, Larsen, Ling, & Caspary, 2015), and auditory cortex (Basura, Koehler, & Shore, 2015; Geven, de Kleine, Willemsen, & van Dijk, 2014; Llano, Turner, & Caspary, 2012). However, accumulating evidence suggests that non-auditory systems (Landgrebe et al., 2009; Marks et al., 2018; Ouyang et al., 2017; Vanneste & De Ridder, 2012; Vanneste, Plazier, van der Loo, Van de Heyning, & De Ridder, 2011; Zhang, Luo, Pace, Li, & Liu, 2016) might also play a role in tinnitus. Upregulation of somatosensory inputs to cochlear nucleus in compensation for reduced auditory innervation after cochlear damage is related to altered neural plasticity in cochlear nucleus, which is thought to be an underlying mechanism of tinnitus (Koehler & Shore, 2013; Marks et al., 2018; Wu et al., 2016). The hippocampus, a brain region implicated in learning and memory as well as mood (Mineur et al., 2013; Ramirez et al., 2013; S. H. Wang, Finnie, Hardt, & Nader, 2012), provides a dense input to auditory cortex (Cenquizca & Swanson, 2007) and receives auditory input from auditory association cortices directly or indirectly via the parahippocampal cortex, or via other forebrain pathways including medial frontal cortex, insula or amygdala ((Kraus & Canlon, 2012; Mohedano-Moriano et al., 2007; Munoz-Lopez, Mohedano-Moriano, & Insausti, 2010). The hippocampus has been suggested as a potential site involved in tinnitus (Goble, Moller, & Thompson, 2009; Landgrebe et al., 2009; Ueyama et al., 2013; Vanneste, Faber, Langguth, & De Ridder, 2016). For example, resting-state functional MRI demonstrated that bilateral hippocampal activity is positively correlated with tinnitus loudness in patients (Ueyama et al., 2013). Furthermore, sound exposure alters previously stable responses of hippocampal place cells (Goble et al., 2009), and acoustic trauma can impair hippocampal-dependent learning, (Zheng, Hamilton, Begum, Smith, & Darlington, 2011), all suggesting a potential involvement of the hippocampus in auditory processing.
Sensory information reaches the hippocampus via the entorhinal cortex, which is the upstream gate of the so-called “trisynaptic circuit” (Brankack & Buzsaki, 1986; Deadwyler, West, & Robinson, 1981; Witter et al., 2000). Neurons in the superficial layers of entorhinal cortex (EC) project to granule cells in the dentate gyrus (DG), which, in turn, send out mossy fibers to CA3 pyramidal neurons. Schaffer collateral fibers from CA3 pyramidal neurons densely innervate the apical dendrites of CA1 pyramidal neurons in stratum radiatum. Acetylcholine is an essential neuromodulator for regulating synaptic plasticity in the hippocampus (Al-Onaizi et al., 2017). The cholinergic inputs to the hippocampus originate primarily from the medial septum and diagonal band of Broca in the basal forebrain (Frotscher & Leranth, 1985; Mesulam, Mufson, Levey, & Wainer, 1983; Woolf, 1991). Damage to the cholinergic system in the basal forebrain is accompanied by memory and cognitive impairment (Laursen, Mork, Plath, Kristiansen, & Bastlund, 2013; Turnbull, Boskovic, & Coulson, 2018) and increased risk for Alzheimer’s disease (Grothe et al., 2010; Teipel et al., 2014). Especially relevant to the current study, is the finding that cholinergic activity in the hippocampus changes after noise exposures (Azman, Zakaria, Abdul Aziz, & Othman, 2016; Lai, 1987; Lai, Carino, & Wen, 1989; Sembulingam, Sembulingam, & Namasivayam, 2005) and stress induction (Mark, Rada, & Shors, 1996), raising the question of whether cholinergic innervation in the hippocampus is persistently affected by noise exposure and/or associated with tinnitus.
Here, we examine changes in immunohistochemical labeling of the vesicular acetylcholine transporter (VAChT) to investigate cholinergic innervation in the guinea pig hippocampus after noise exposure and determine the relevance of these changes to tinnitus. Surprisingly, we observed significant downregulation of cholinergic input density in numerous hippocampal sub-regions, including the DG, CA3, and CA1 areas on both sides of the brain two weeks following unilateral sound overexposure. To explore the time course of these changes and their relevance to tinnitus, we exposed a second group of animals to the same noise overexposure twice four weeks apart, a paradigm that induces tinnitus (Koehler & Shore, 2013; Wu et al., 2016) in a subset of animals. Twelve weeks after the first noise exposure, we found that VAChT density recovery was dependent on whether the animals exhibited tinnitus. Whereas animals that were noise exposed but resistant to tinnitus exhibited near complete recovery of VAChT density in every hippocampal sub-region examined, animals that developed tinnitus exhibited a persistence of diminished VAChT density in synapse-rich layers of the DG, area CA3 and CA1. Moreover, among the animals that developed tinnitus, the severity of tinnitus was negatively correlated with the degree of VAChT density recovery in multiple hippocampal regions. Collectively, our results identify a novel association between cholinergic input remodeling in the hippocampus and the development of noise-induced tinnitus.
2. METHODS
2.1. Animals
Pigmented guinea pigs (n=19) of either sex were obtained from Elm Hill Labs at two to three weeks of age. Animals were housed two per cage at constant temperature and humidity under a 12-hour light/dark cycle. Water and food were given ad libitum. All animal procedures were performed in accordance with protocols established by the National Institutes of Health and approved by the University Committee on Use and Care of Animals at the University of Michigan.
2.2. Experimental design and noise exposures
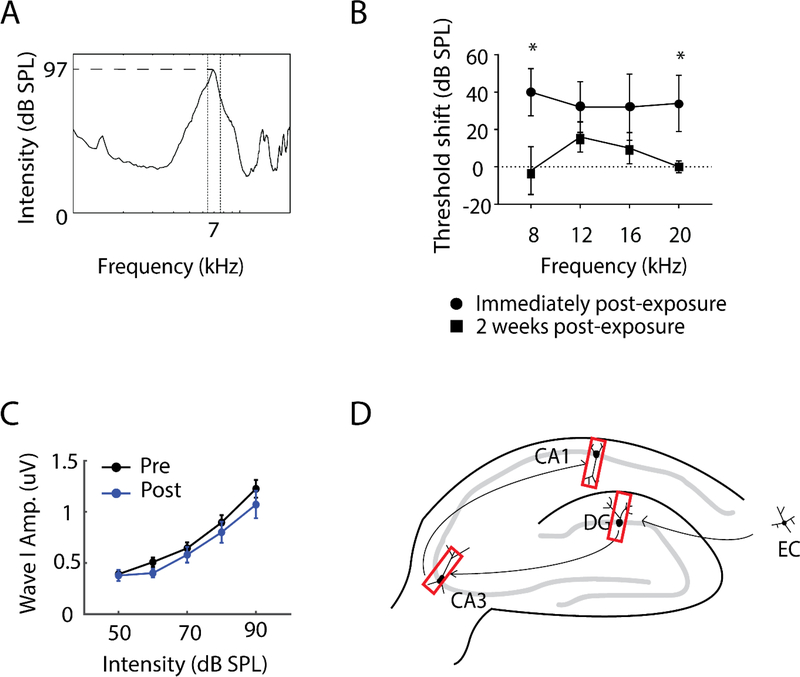
The method of noise exposure was previously described (Marks et al., 2018; Stefanescu et al., 2015; Wu et al., 2016). First, to investigate the effects of noise exposure on the hippocampus, guinea pigs were anesthetized with ketamine/xylazine (40 mg/kg ketamine; 10 mg/kg xylazine) and placed in a double-walled soundproof booth. Three guinea pigs served as sham-exposed controls in which they were anesthetized but not noise exposed. Five guinea pigs were exposed via unilateral microphone inserts which produced a 7 kHz centered noise band at 97 dB sound pressure level (SPL) for two hours (Figure 1A). This level and duration of noise exposure was chosen as it was previously shown to produce only temporary threshold shifts and no suprathreshold ABR Wave 1 amplitude deficits in guinea pigs (Marks et al, 2018, Wu et al, 2016). Auditory brainstem responses (ABRs) were recorded at 8, 12, 16, and 20 kHz, before and after each noise exposure to assess shifts in hearing thresholds (Figure 1B) and wave I amplitude-intensity functions (Figure 1C). ABRs were also assessed at least one week after each noise exposure to determine the extent to which hearing thresholds had recovered. Two weeks following noise exposure, animals were sacrificed and brains were collected as described below in Tissue preparation.
Figure 1. Experimental procedures of two-weeks post-noise-exposure animals.
(A) Features of the noise band to which experimental animals were unilaterally exposed for two hours.
(B) Ipsilateral Acoustic Brainstem Response (ABR) thresholds of noise-exposed animals(n=5) at 8, 12, 16, 20 kHz immediately following noise exposure, and following a two-week recovery period. ABR thresholds recovered to baseline levels at 8 and 20 kHz and to near baseline levels at 12 and 16 kHz within two weeks. *p<0.05.
(C) ABR wave I amplitude-intensity functions for noise-exposed animals prior to and two weeks following noise exposure. No differences were apparent two weeks post-exposure compared to baseline levels (pre-exposure).
(D) Schematic diagram of hippocampal circuit with red rectangles depicting where images in the dentate gyrus (DG), area CA3 and CA1 were taken for immunohistochemistry. EC, entorhinal cortex.
To explore whether the effects seen in the two-weeks post-noise exposure related to tinnitus, another group of nineteen animals were exposed using the same noise exposure paradigm as in the 2 week group (n=13)/sham(n=6) twice in sessions conducted four weeks apart. Tinnitus was assessed using gap-prepulse-inhibition of acoustic startle reflex (GPIAS) (Berger, Coomber, Shackleton, Palmer, & Wallace, 2013; Turner et al., 2006) for four weeks before to establish a baseline and again eight weeks following the last noise exposure.
2.3. Tinnitus assessment
GPIAS in guinea pigs was performed as previously described (Wu et al., 2016). Sound attenuating chambers were used inside of sound proof booths. The internal walls of each chamber was lined with sound dampening material to prevent sound reflections and reverberations (Dehmel, Eisinger, & Shore, 2012; Lauer, Behrens, & Klump, 2017). A constant background carrier (band limited at 8–10, 12–14, 16–18, and 20–30kHz) was presented at 65dB SPL. Pinna reflex startle responses elicited by broadband noise pulses (20ms) at 95dB SPL were quantified by video tracking (Point Gray Research). Startle reflexes were inhibited by a 50ms silent gap or 75dB SPL pre-pulse embedded in the band-limited carrier (8–10, 12–14, 16–18, and 20–30 kHz, corresponding to the carrier) 100ms before the startle pulse. Pinna tips were marked with non-toxic, water-soluble green paint, manually applied by trained investigators. Green pixels were identified using a custom-written k-nearest-neighbor classifier algorithm (Mathworks MATLAB knnsearch) (Altman, 1992; Friedman, 1977). Frames where green points constituted less than 0.01% of pixels were excluded, as this indicated the animal’s ears were not located in the frame. Pinna locations were identified by clustering green pixels and computing the centroids of a two-dimensional Gaussian mixture model (McLachlan & Chang, 2004). The Euclidean distance between (Xear(t), Year (t)) points was computed over the trial duration. Startle amplitudes were computed by fitting the Euclidean distance to a Gaussian-windowed sine-wave cycle and computed as the resultant amplitude parameter.
A normalized startle inhibition ratio (R) was computed by dividing the mean startle amplitude for the gap (or pre-pulse) trials by those for the no-gap trials. Tinnitus index was used to quantify the difference in R values between post-exposure and pre-exposure, as shown by the equation above. xpost is the mean of post-exposure R value. μpre and σpre are the mean and standard deviation (SD) of pre-exposure R value, which was the behavioral baseline. Baseline data were collected twice weekly for four weeks before the first noise exposure. Eight weeks after the second noise exposure, GPIAS data collection was completed and post-exposure R values for each animal were calculated. An animal was presumed to have tinnitus if the post-exposure mean R value for gap inhibition was significantly greater than the baseline value (α = 0.05). A larger positive index indicates a higher degree of impaired gap detection (“worse tinnitus”) after noise exposure.
2.4. Tissue preparation
Animals were euthanized and transcardially perfused with 100 mL 0.1M phosphate buffered saline (PBS; pH 7.3–7.4), followed by 400 mL paraformaldehyde (PFA; 4%) in PBS. Brains were collected and post-fixed in 4% PFA overnight at 4°C. The following day, brains were washed in PBS before being transferred to a 30% sucrose solution in PBS for four to five days at 4°C for dehydration. When sunken, brains were transferred to a 1:1 mix of 30% sucrose and Tissue Tek (Sakura, Finetek) solution overnight at 4°C. Brains were rapidly frozen using dry ice and stored at −20°C or −80°C. Five series of 30 μm coronal hippocampal sections were collected using a cryostat (Leica, CM 3050S), mounted on glass slides, air dried for 24 hours, and stored at −20°.
2.5. Immunohistochemistry
Slides were removed from −20°C and thawed at room temperature for one hour. Brain sections were rehydrated in 0.1M phosphate buffered saline (PBS; pH 7.3–7.4), 10 min*3 times, to optimize morphological details. Subsequently, sections were incubated in blocking solution containing 1% normal goat serum (Jackson ImmunoResearch Labs, Cat# 005–000-121, RRID: AB_2336990), and 0.1% Triton-X 100 (MP Biomedicals, Cat# 807423) in PBS for 30min, to limit non-specific binding. Sections were then incubated with primary antibody, rabbit anti-VAChT antibody (Synaptic Systems, Cat# 139 103, RRID: AB_887864), 1:200 diluted in blocking solution, for 24 hours. The next day, all sections were incubated with secondary antibody (Alexa Fluor 555-conjugated goat anti-rabbit, Molecular Probes Cat# A-21429, RRID: AB_141761) diluted 1:500 in blocking solution for two hours after thorough rinsing (10min*3 times) in PBS to remove unbound primary antibody. Counterstaining was done with DAPI (Thermo Fisher Scientific, Cat# D1306, RRID: AB_2629482) 1:1000 diluted in blocking solution applied together with the secondary antibody. After the incubation, another rinsing (10min*3 times) was performed to remove excess secondary antibody. Slides were mounted with Fluoromount-G (Southern Biotech, Cat# 0100–01). To ensure specificity of the secondary antibody, negative controls were done in sections only treated with secondary (and not primary) antibody. All procedures were performed at room temperature. All matched groups were processed in parallel.
2.6. Image Processing
Image processing was performed as previously described (Zeng, Yang, Shreve, Bledsoe, & Shore, 2012). Images were acquired using a fluorescent microscope (Leica, DMLB, Type 020–519.011) equipped with the appropriate filters for Alexa Fluor 555, with images captured using Qcapture Pro7 software. All parameters used for image acquisition were determined in preliminary experiments to optimize the dynamic range of signal intensities and to minimize background fluorescence. Once determined, all parameters were kept consistent for all imaging sessions. Images were taken from three hippocampal sub-regions – Dentate Gyrus (DG), area CA3, and area CA1 (Figure 1D). We subdivided the molecular layer of the DG into proximal and distal regions, the former being adjacent to the granule cell layer and covering roughly 2/3 of the thickness of the molecular layer. In CA1, we subdivided the stratum radiatum into proximal and distal layers, each covering half of the width of the layer. All images for processing were taken at 400X magnification.
2.7. Quantification and statistics
Quantification was performed blind as to whether the tissue was from control or noise-exposed, tinnitus or non-tinnitus animals. Images were analyzed with ImageJ (version 1.50i, National Institutes of Health, USA, RRID:SCR_003070). First, RGB images were converted into single channels, and only the red channel corresponding to the Alexa Fluor 555 signal was used for subsequent processing. Then, the contrast was enhanced and background was subtracted with consistent parameters. Subsequently, an auto threshold was applied followed by a watershed paradigm which separated overlapping puncta. Puncta counts were divided by image area, to yield puncta density. Means and standard errors of the mean (SEM) were calculated for the VAChT puncta density. Statistical analysis was done with MATLAB (The MathWorks, RRID: SCR_001622). One-way analysis of variance (ANOVA) or two-way ANOVA followed by Tukey-Kramer post-hoc correction for multiple comparisons were used to identify significant differences (P ≤ 0.05).
3. RESULTS
3.1. VAChT labeling in hippocampus was decreased two weeks after noise exposure
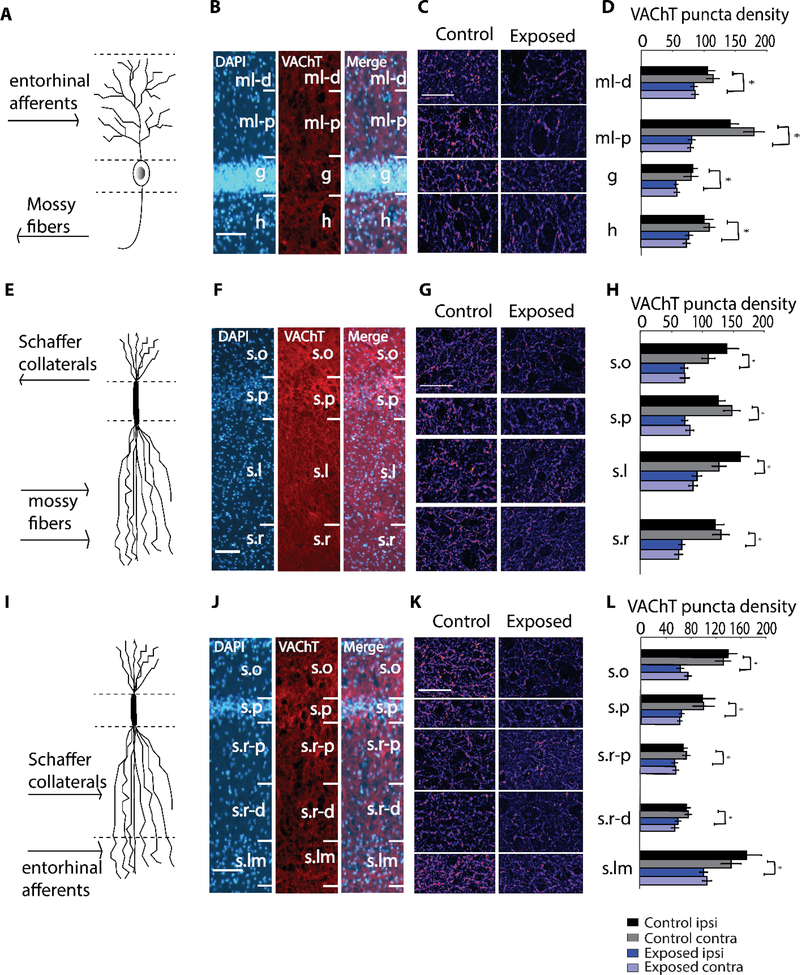
To determine whether altered cholinergic innervation of the hippocampus accompanies noise exposure, we used immunohistochemical detection of VAChT to identify cholinergic terminals in hippocampal sub-regions. Exposed animals received a unilateral 7 kHz-centered noise band (97 dB SPL) for two hours under anesthesia, whereas control animals underwent anesthesia but were not noise exposed (Figure 1A). ABR thresholds of noise-exposed animals (n=5) on the ipsilateral side were elevated at 8, 12, 16, 20 kHz immediately following noise exposure and recovered to baseline levels at 8 and 20 kHz and to near baseline levels at 12 and 16 kHz within two weeks (Figure 1B). Using this noise exposure paradigm, ABR thresholds of control animals and contralateral sides of exposed animals do not exhibit any changes in previous studies (Marks et al., 2018; Wu et al., 2016). ABR wave I amplitude-intensity functions for exposed animals two weeks post-exposure were not significantly different from baseline levels (Figure 1C; Repeated Measures ANOVA, p=0.408, df=2, F=0.741). Despite the relatively mild noise trauma, causing only temporary threshold shifts, there were significant decreases in VAChT expression throughout the hippocampus on both sides two weeks following the noise exposure (Figure 2). In the dentate gyrus (DG; see Figure 2A–D), the most striking decrease in VAChT puncta density was seen in the proximal molecular layer (F(1,86)=75.73, p=1.99*10−13, 43.21%), but the hilus (F(1,86)=14.01, p=0.0003, 23.99%), granule cell layer (F(1,86)=16.54, p=0.0001, 32.38%), and distal molecular layer (F(1,86)=10.82, p=0.0015, 20.37%) also showed significant reductions in VAChT density on both sides. In hippocampal area CA3 (Figure 2E–H), noise exposed animals exhibited similar significant reductions in VAChT expression in all four discernable layers on both sides - stratum oriens (F(1,85)=26.13, p=1.94*10−6, 49.35%), pyramidale (F(1,85)=51.97, p=2.15*10−10, 43.41%), lucidum (F(1,86)=33.43, p=1.16*10−7, 43.18%) and radiatum (F(1,94)=44.21, p=1.92*10−9, 48.48%). In area CA1 (Figure 2I–L), the most striking decrease in VAChT density in noise-exposed animals was seen in stratum oriens (F(1,86)=60.93, p=1.31*10−11, 54.64%) and stratum lacunosum-moleculare (F(1,86)=17.89, p=5.81*10−5, 40.31%) on both sides. Stratum pyramidale (F(1,85)=12.27, p=0.0007, 34.13%), proximal (F(1,86)=7.89, p=0.0061, 19.56%) and distal (F(1,86)=9.39, p=0.0029, 17.38%) stratum radiatum showed moderate, yet significant, reductions in VAChT density on both sides. Importantly, these changes in hippocampal VAChT expression appeared bilaterally in noise-exposed animals, despite the unilateral nature of noise-exposure. Nearly identical noise-induced changes in VAChT expression were observed on the ipsilateral and contralateral sides of the hippocampus. These results demonstrate robust changes in cholinergic innervation of the hippocampus following mild noise trauma.
Figure 2. Robust decreases in VAChT puncta density in dentate gyrus (DG), area CA3, and area CA1 two weeks following noise exposure.
(A) Schematic granule cell, depicting organization of inputs corresponding to the layers in (B), which are images of VAChT labeling in DG at 100X magnification. (C) Representative images at 400X magnification in DG with layers corresponding to (D), which depicts mean (±SEM) VAChT puncta density (per 104 μm2) in the indicated layers.
(E) Schematic pyramidal neuron, depicting organization of inputs corresponding to the layers in (F), which are images of VAChT labeling in area CA3 at 100X magnification. (G) Representative images at 400X magnification in area CA3 with layers corresponding to (H), which depicts mean (±SEM) VAChT puncta density (per 104 μm2) in the indicated layers.
(I) Schematic pyramidal neuron, depicting organization of inputs corresponding to the layers in (J), which are images of VAChT labeling in area CA1 at 100X magnification. (K) Representative images at 400X magnification in area CA1 with layers corresponding to (L), which depicts mean (±SEM) VAChT puncta density (per 104 μm2) in the indicated layers.
In (B), (F), (J), scale bar is 100 μm. In (C), (G), (K), scale bar is 50 μm.
Abbreviations: ml-d, distal region of molecular layer; ml-p, proximal region of molecular layer; g, granule cell layer; h, hilus; s.o, stratum oriens; s.p, stratum pyramidale; s.l, stratum lucidum; s.r, stratum radiatum; s.r-p, proximal region of stratum radiatum; s.r-d, distal region of stratum radiatum; s.lm, stratum lacunosum-moleculare. *p < 0.05
3.2. Chronic effects of noise exposure: induction of tinnitus in a subset of animals
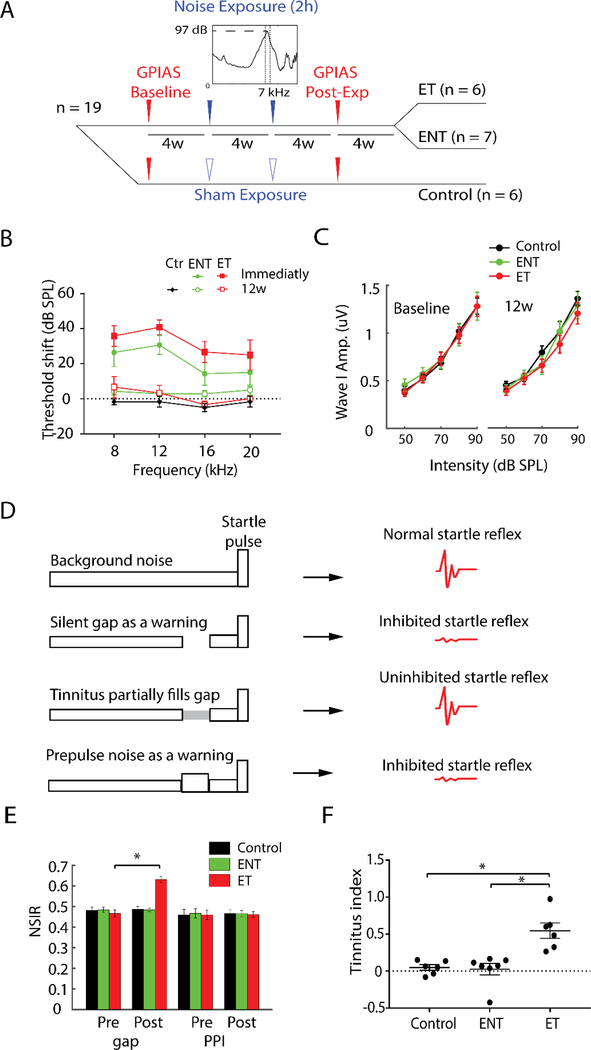
Given the significant changes in VAChT density two weeks following noise exposure, we next asked how persistent these changes are and whether they are associated with tinnitus. We thus exposed a second cohort of animals to the same noise stimulus on two successive occasions four weeks apart, as this paradigm has previously been used to successfully induce tinnitus in guinea pigs (Marks et al., 2018; Wu et al., 2016). Following the second noise exposure, animals were allowed to recover for eight additional weeks (12 weeks relative to the initial noise trauma; see Figure 3A). As the noise exposure used typically induces tinnitus in roughly 50% of experimental animals, chronically-exposed animals were divided into three groups: sham exposed controls (n=6), noise exposed animals that exhibit no signs of tinnitus (ENT, n=7), and exposed animals that exhibit signs of tinnitus (ET, n=6). ABR thresholds of noise-exposed animals (n=13) ipsilateral to the noise exposure were elevated immediately following noise exposure but recovered to normal sensitivity levels within 12 weeks (Figure 3B). In addition, ABR wave I amplitude-intensity functions, which are affected by cochlear-synaptopathy, were not significantly different for ET and ENT animals, pre- or post-noise exposure (Figure 3C), suggesting the tinnitus phenotype is not expressed in the cochlea. Neither ENT nor ET animals showed any supra-threshold deficits, which means these animals did not have any observable hidden hearing loss. Tinnitus was assessed using GPIAS as previously described (Basura et al., 2015; Wu et al., 2016) (Figure 3D) and the behavioral test results of this second cohort of animals have been partially published (Marks et al., 2018; Wu et al., 2016). When the silent gap in background noise was replaced by a pre-pulse noise, none of the noise-exposed animals showed altered pre-pulse inhibition (PPI) ratios (Figure 3E), indicating that the animals’ inhibited responses to gap trials were not due to hearing impairment, temporal processing anomalies or anomalous startle behavior. The baseline startle reflexivity, which is the startle amplitude for no-gap/no-prepulse condition, was unaltered post-exposure in control, ENT and ET animals in this study, which is a consistent finding with our preparation (Dehmel et al., 2012; Koehler & Shore, 2013; Marks et al., 2018; Wu et al., 2016). Despite the complete recovery of hearing thresholds (as assessed via ABR thresholds), roughly half (6/13) of the noise exposed animals developed tinnitus as assessed by GPIAS. The tinnitus index was significantly higher (F(2,16)=13.87, p=0.0003) in ET animals compared to sham-exposed control animals or ENT animals (Figure 3F).
Figure 3. Repeated noise exposure induces tinnitus in a subset of experimental animals.
(A) Timeline of the experimental procedures of the chronically-exposed group. Nineteen animals were grouped into sham controls (n=6) and noise-exposed animals(n=13). GPIAS was used as tinnitus assessment and baseline thereof was acquired for four weeks pre-noise exposure. Animals were exposed to the same noise band/sham for two hours twice in sessions conducted four weeks apart, and then assessed for tinnitus eight weeks following the first noise exposure. ABR measurements were performed before and after each noise exposure and GPIAS. Noise-exposed animals were divided into two groups according to GPIAS assessment: noise exposed animals that exhibit no signs of tinnitus (ENT, n=7), and exposed animals that exhibit tinnitus (ET, n=6).
(B) Mean (±SEM) ABR thresholds of animals with tinnitus (ET) and without tinnitus (ENT). ABR thresholds on the ipsilateral side were elevated immediately following noise exposure in both groups, but recovered to baseline levels at 8, 12, 16, 20 kHz 12 weeks after the first noise exposure.
(C) Mean (±SEM) ABR wave I amplitude-intensity functions for ENT and ET animals pre- (baseline) and post-noise exposure (12w) were not significantly different, suggesting no underlying cochlear synaptopathy in both ENT and ET animals after the noise exposure.
(D) Rationale of GPIAS (adapted from Turner et al., 2006). Row 1: Normal animals respond with a robust startle to the presentation of a startle pulse (20ms, 95 dB) embedded in a continuous background sound (65 dB). Row 2: When a silent gap (50ms) is introduced in the background sound, normal animals use the gap to predict the incoming startle pulse and respond with decreased startle amplitude. Row 3: Animals with tinnitus fail to detect the gap due to their tinnitus percept and respond with an uninhibited startle to the pulse presentation. Row 4: The gap is replaced with a prepulse noise (75dB). Both normal hearing and tinnitus animals respond with decreased startle amplitude due to alarm effects of the prepulse noise. Animals with hearing loss fail to detect the prepulse noise and thus respond with an uninhibited startle to the pulse presentation. This assessment tells whether animals’ inhibited responses to gap trials are due to hearing impairment.
(E) Mean (±SEM) normalized startle inhibition ratio (NSIR) was the ratio of the startle amplitudes for the gap (or prepulse inhibition, PPI) trials and those for the no-gap trials. NSIR for gap trials was significantly higher post-exposure(Post) relative to baseline levels (Pre) for ET animals, but not for ENT or control animals. All animals exhibited stable responses to PPI trials both pre and post noise exposure.
(F) Tinnitus indices of animals with tinnitus (ET) were significantly higher than those of controls and no-tinnitus animals (ENT).
3.2.1. Tinnitus-expressing animals exhibit incomplete recovery of VAChT density in the Dentate Gyrus
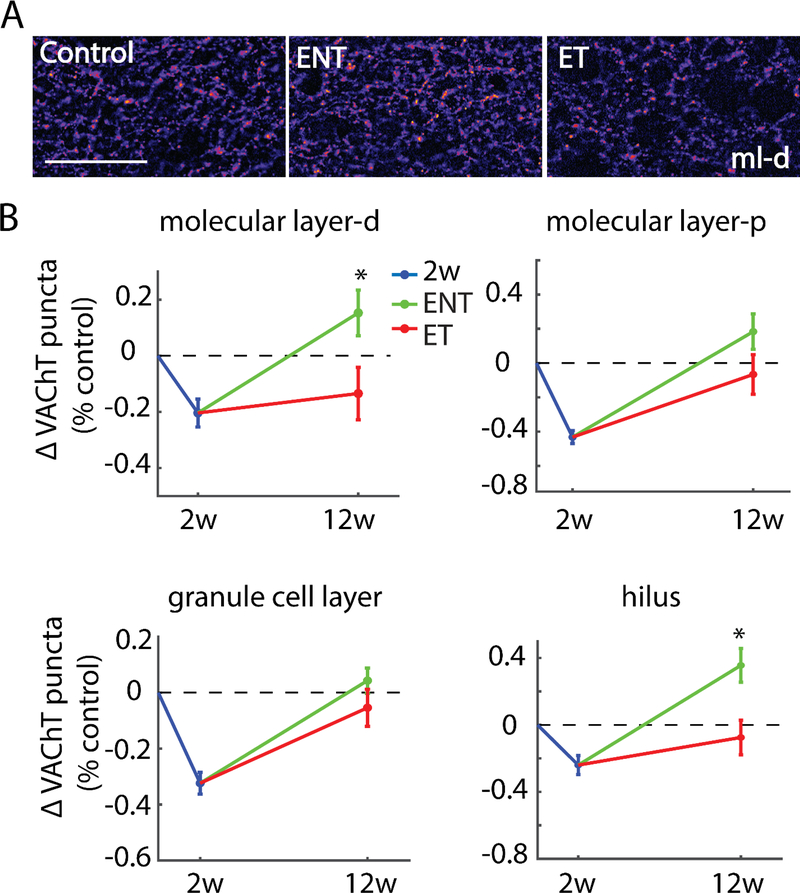
Overall, the robust decreases in hippocampal VAChT density evident two weeks after noise exposure were largely recovered to control levels 12 weeks after noise damage. However, animals that developed noise-induced tinnitus (ET) exhibited a significant alteration in this recovery relative to noise-exposed animals that did not develop tinnitus (ENT). Similar to the two-week post-exposure group, nearly identical noise-induced changes in VAChT expression were observed in the ipsilateral and contralateral sides of the hippocampus for the tinnitus induction group. So in subsequent analyses, we show VAChT expression in pooled ipsilateral and contralateral hippocampal sub regions. In the distal regions of the molecular layer of the DG, ENT and ET animals showed significant differences (F(1,154)=5.45,p=0.0195) in VAChT recovery, with animals developing tinnitus (ET) exhibiting incomplete recovery, while those resistant to tinnitus (ENT) exhibiting a nominal increase in VAChT density relative to paired controls. Likewise, ET and ENT animals exhibited significant differences in VAChT recovery in the hilus (F(1,154)=8.78, p=0.0030), with those animals resistant to tinnitus exhibiting a clear increase in VAChT density relative to both controls and ET animals. By contrast, ENT and ET animals each displayed similar recovery of VAChT density in the granule cell layer (F(1,154)=1.53, p=0.2155) and in proximal regions of the molecular layer (F(1, 152)=2.6, p=0.065; see Figure 4).
Figure 4. Tinnitus animals exhibit diminished recovery of VAChT labeling relative to no-tinnitus animals in the dentate gyrus(DG).
(A) Representative images from distal molecular layer at 400X magnification; Scale bar = 50 μm. (B) Mean (±SEM) change of VAChT density (normalized to respective control) in the DG in two-weeks and 12-weeks post-noise-exposure animals. Distal molecular layer (ml-d) and hilus (h) showed significantly higher VAChT labeling in no-tinnitus animals (ENT) than in tinnitus animals (ET), whereas granule cell layer (g) and proximal molecular layer (ml-p) showed similar VAChT labeling in ENT and ET animals; *p < 0.05.
3.2.2. Tinnitus-expressing animals demonstrate persistent decreases in VAChT density in synapse-rich layers of areas CA3 and CA1
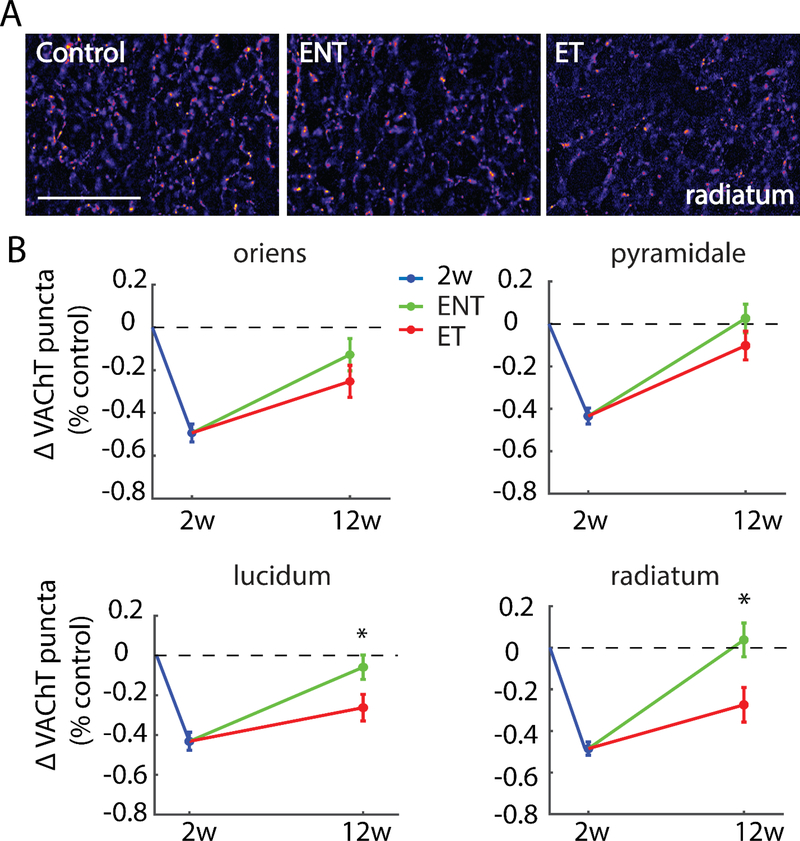
In synapse rich layers of CA3 - stratum lucidum (F(1,144)=5.08, p=0.0242) and stratum radiatum (F(1,139)=7.21, p=0.0072) - the recovery of VAChT density exhibited a striking association with tinnitus: Whereas VAChT density in ENT animals recovered completely to pre-noise-exposure levels, VAChT density remained significantly lower in animals that developed tinnitus (ET). In contrast, ET and ENT animals exhibited similar levels of VAChT recovery in stratum oriens (F(1,145)=1.37, p=0.2419) and pyramidale (F(1,143)=1.81, p=0.1789; see Figure 5). These results thus suggest a relationship between incomplete recovery of noise-induced plasticity of cholinergic innervation in CA3 and increased susceptibility to develop tinnitus.
Figure 5. Tinnitus animals exhibit persistent decreases in VAChT labeling relative to no-tinnitus animals in synapse-rich areas of hippocampal area CA3.
(A) Representative images from stratum radiatum at 400x magnification; Scale bar = 50 μm. (B) Mean (±SEM) change of VAChT density (normalized to respective control) in area CA3 in two-weeks and 12-weeks post-noise-exposure animals. Strata lucidum (s.l) and radiatum (s.r) showed significantly lower VAChT labeling in tinnitus animasl (ET) than in no-tinnitus animals (ENT), whereas strata oriens (s.o) and pyramidale (s.p) showed similar VAChT labeling in ENT and ET animals; *p < 0.05.
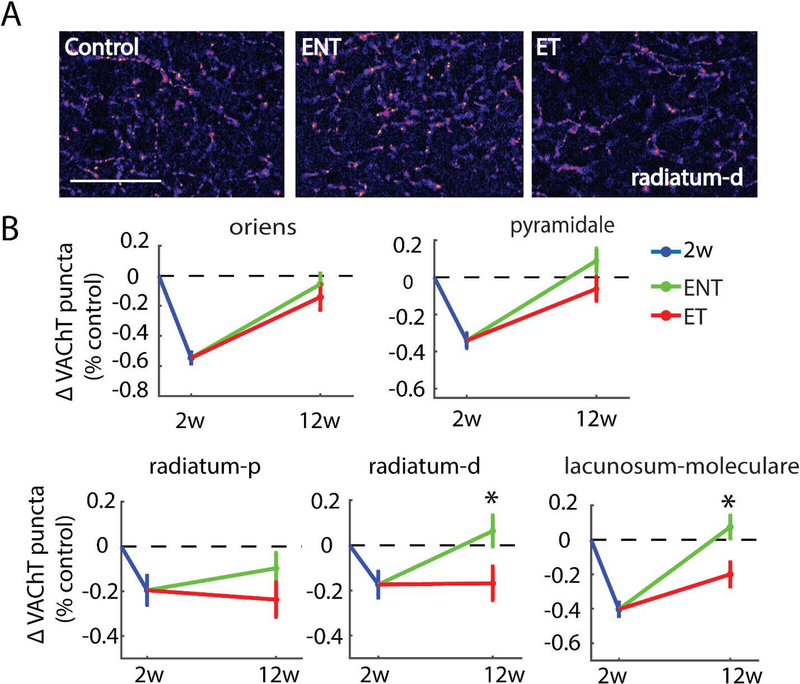
Tinnitus and no tinnitus animals exhibited striking differences in recovery of VAChT density in stratum radiatum and stratum lacunosum moleculare in CA1. In both regions, animals resistant to tinnitus demonstrated more robust recovery of VAChT labeling, while the animals that developed tinnitus exhibited incomplete recovery. Significant differences in VAChT density were found between ET and ENT animals in distal stratum radiatum (F(1,154)=5.27, p=0.0217) and stratum lanunosum moleculare (F(1,154)=8.38, p=0.0038) at the 12 week time point. Although a similar trend was evident in the proximal region of stratum radiatum, differences between ET and ENT animals did not reach statistical significance. As we observed two weeks following noise exposure, no significant differences for any hippocampal sub-region were seen between ipsilateral and contralateral sides 12 weeks after noise exposure.
Similar to what we observed in area CA3, VAChT density in stratum oriens and pyramidale in area CA1 recovered completely at 12 weeks in noise-exposed animals regardless of whether they developed tinnitus. Despite exhibiting the most striking precipitous decrease in VAChT two weeks following noise exposure, VAChT density in both no-tinnitus (F(1,110)=15.71, p=0.0001) and tinnitus (F(1,98)=8.82, p=0.0038) animals in stratum oriens was back near control levels 12 weeks following noise exposure (Figure 6). A similar pattern of results in tinnitus and no-tinnitus animals was observed in stratum pyramidale (F(1,154)=2.64, p=0.1043) of area CA1.
Figure 6. Tinnitus animals exhibit persistent decreases in VAChT labeling relative to no-tinnitus animals in synapse-rich areas of hippocampal area CA1.
(A) Representative images from distal half of stratum radiatum at 400X magnification; Scale bar = 50 μm. (B) Mean (±SEM) change of VAChT density (normalized to respective control) in area CA1 in two-weeks and 12-weeks post-noise-exposure animals. Distal half of stratum radiatum(s.r-d) and stratum lacunosum-moleculare (s.lm) showed significantly lower VAChT labeling in tinnitus animals (ET) than in no-tinnitus animals (ENT), whereas strata oriens (s.o) and pyramidale (s.p) showed similar VAChT labeling in ENT and ET animals; *p < 0.05.
3.2.3. Correlation between tinnitus index and VAChT puncta density
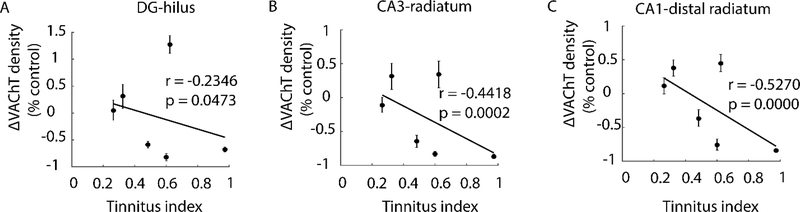
Given that tinnitus-expressing animals, as a group, exhibited significantly reduced VAChT expression in several hippocampal sub-regions relative to tinnitus-resistant animals, we next asked the extent to which the decreased VAChT density was associated with the intensity of tinnitus. As shown in Figure 7, the severity of tinnitus (as indicated by increasing values of tinnitus index) was significantly correlated with decreases in VAChT puncta density in DG hilus (r=−0.2346, p=0.0473), CA3 stratum radiatum (r=−0.4418, p=0.0002), and CA1 distal stratum radiatum (r=−0.5270, p=0.0000).
Figure 7. Tinnitus severity correlates with VAChT puncta density changes in tinnitus animals.
Scatterplots of VAChT density (normalized to control) vs tinnitus index in the hilus of DG (A), stratum radiatum of CA3 (B), and in distal stratum radiatum of CA1 (C). Severity of tinnitus is indicated by increasing values of tinnitus index; each data point represents an individual animal. Shown in inset are Pearson Correlation Coefficients (r) and accompanying p value. VAChT density in all 3 areas correlate with tinnitus severity, though the association is stronger in synapse-rich layers of CA3 and CA1.
Taken together, our results demonstrate that VAChT expression in several hippocampal regions recovers differently in tinnitus and no-tinnitus animals 12 weeks after noise exposure (Figure 8). Our findings further suggest that tinnitus susceptibility may be influenced by the degree to which initial changes in VAChT expression evident two weeks following noise exposure persist over time.
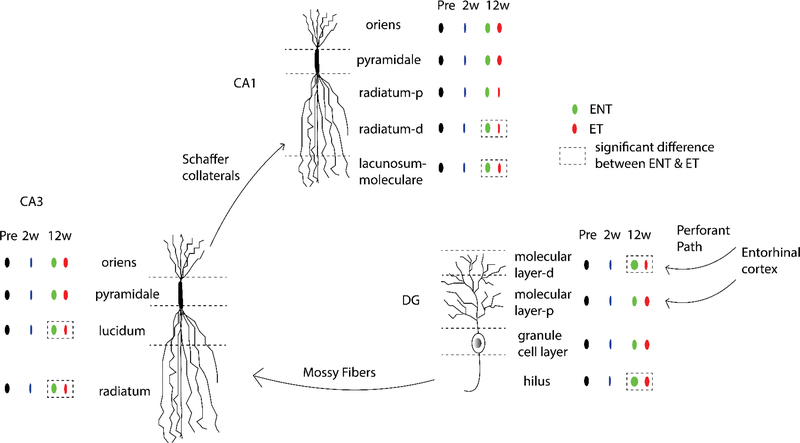
Figure 8. Summary of changes in hippocampus after noise exposure and tinnitus.
VAChT labeling decreased in all three hippocampal sub-regions two weeks after noise exposure, suggesting short-term alterations in cholinergic neurotransmission after noise exposure. Twelve weeks after noise exposure, VAChT labeling remained low in animals that exhibited signs of tinnitus (ET), but recovered in exposed animals that exhibited no signs of tinnitus (ENT). Animals with tinnitus showed persistent disruption of VAChT in synapse-rich layers of hippocampus that receive inputs from upstream stages in the “trisynaptic circuit”, including distal region of molecular layer and hilus in the DG, strata lucidum and radiatum in area CA3, distal region of stratum radiatum and stratum lacunosum-moleculare in area CA1. This pattern of results raises the possibility that synaptic processing in the hippocampus plays an important role in the physiopathology of tinnitus.
4. DISCUSSION
The hippocampus has been implicated as potentially playing a role in tinnitus (Goble et al., 2009; Ueyama et al., 2013; Vanneste & De Ridder, 2012). Here, we investigated the remodeling of cholinergic inputs to the hippocampus after noise exposure and determined the relevance of these changes to tinnitus. We found that VAChT labeling decreased across CA1, CA3, and DG areas in the hippocampus two weeks after noise exposure. Twelve weeks later, animals with and without tinnitus showed differential patterns of recovery, suggesting involvement of hippocampal cholinergic signaling in the pathophysiology of tinnitus.
4.1. Acute downregulation of hippocampal cholinergic innervation after noise exposure
To investigate the effects of noise exposure on the hippocampus, animals were exposed to noise that produced unilateral temporary threshold shifts and followed for two weeks. We found 20%−60% decreases in VAChT labeling in DG, CA3, and CA1 areas of the hippocampus. These results suggest short-term alterations in cholinergic transmission after temporary threshold shift (TTS) noise exposure, consistent with previous studies (Azman et al., 2016; Lai, 1987; Lai & Carino, 1990, 1992; Sembulingam et al., 2005). This pattern of results is also consistent with findings that acoustic trauma impairs animals’ spatial performance (Zheng et al., 2011) and learning (Di & Qin, 2018), which are mediated by cholinergic transmission in the hippocampus. We observed decreases in cholinergic input following exposure to noise of 97 dB SPL, but the effects of noises of different intensities on central cholinergic activity were biphasic in a previous study (Lai, 1987). Exposure to noise of 70 dB SPL increased choline uptake in the hippocampus, but exposure to noise of 100 dB SPL showed the opposite effect, consistent with our results. It is possible that cholinergic remodeling following noise exposure is driven by stress, as the cholinergic system is also affected by exposure to other types of stress, and the effects are biphasic(Finkelstein, Koffler, Rabey, & Gilad, 1985; Gilad, Rabey, & Shenkman, 1983; Lai, Zabawska, & Horita, 1986). Acute stress induced higher levels of choline uptake, whereas longer adaptive stress induced lower levels of choline uptake (Finkelstein et al., 1985; Katz, 1982; Katz & Baldrighi, 1982; Mark et al., 1996; Roth & Katz, 1979), suggesting plasticity in the cholinergic system in response to stress.
There is a wealth of anatomical connections indirectly linking the hippocampus to sensory cortices, including the entorhinal cortex (EC), which conveys auditory and other sensory information to the hippocampus (Burwell & Amaral, 1998; Insausti, Amaral, & Cowan, 1987). Sensory gating is the processes of filtering out unnecessary stimuli from a complex environment, thereby preventing an overload of irrelevant information in higher cortical centers of the brain. The gating of hippocampal responses to auditory stimuli utilizes branches from the lemniscal auditory pathway at the level of the lateral lemniscus, which ascend to the hippocampus via the brainstem reticular formation (Bickford, Luntz-Leybman, & Freedman, 1993). These pathways are mediated by nicotinic receptors in the hippocampus. Furthermore, neurons in the medial pontine reticular formation have efferent projections to the basal forebrain, which in turn sends massive cholinergic projections to the hippocampus (Luntz-Leybman, Bickford, & Freedman, 1992).
Noise exposure can trigger the release of stress hormones (Green, Jones, Sun, & Neitzel, 2015; Pouryaghoub, Mehrdad, & Valipouri, 2016) and can result in severe effects on health such as diabetes, cardiovascular diseases, immune-suppression, and disturbed hormone balance (Ising & Kruppa, 2004; Spreng, 2000). Acetylcholine is an essential neuromodulator playing a key role in regulating neural activity in the hippocampus(Al-Onaizi et al., 2017), and the content of hippocampal acetylcholine can be affected by noise exposure (Lai, 1987) and stress (Mark et al., 1996). Cholinergic inputs to the hippocampus originate primarily in the basal forebrain, an area receiving dense inputs from subcortical stress-related brain regions (Hu, Jin, He, Xu, & Hu, 2016). In the present study, the decreases in VAChT labeling in the hippocampus indicate less cholinergic input from the basal forebrain, presumably also reflect an altered neurotransmission in the basal forebrain. Therefore, one interpretation of the downregulation of cholinergic inputs to the hippocampus after noise exposure is that the stress system in the brain was activated by noise exposure and delivered information to the basal forebrain, which in turn affected the cholinergic inputs to the hippocampus.
Another interesting finding in the present study is that no differences between ipsilateral and contralateral sides of hippocampal VAChT labeling were seen despite the unilateral noise exposure, which is consistent with previous studies (Ueyama et al., 2013; Vanneste & De Ridder, 2012; Vanneste et al., 2016; Vanneste et al., 2011) and may reflect information exchange between the left and right sides of the brain.
4.2. Tinnitus but not no-tinnitus animals showed lower cholinergic innervation than controls twelve weeks after noise exposure
In the present study, we used gap-prepulse inhibition of the acoustic startle reflex to assess the presence of tinnitus. The acoustic startle reflex is a reflex to a loud acoustic stimulus in animals (Koch, 1999). A pre-stimulus can inhibit the reflex – here a gap in a continuous background noise as a pre-stimulus. If the pre-stimulus is not perceived, the reflex amplitude remains unchanged. A phantom perception of sound might make the gap less salient and thereby reduce the inhibition of the acoustic startle reflex. Whether tinnitus perception “fills in” the gap (Fournier & Hebert, 2013) or interferes with temporally resolving the gap (Hickox & Liberman, 2014), or both, remains to be explored further. Acoustic startle circuits are complex, therefore, GPIAS should be performed with caution to ensure the inhibition of startle reflex reflects tinnitus (Lauer et al., 2017). One important concern regarding GPIAS assessments of tinnitus is that hearing loss will affect GPIAS outcome as would tinnitus, so it is essential to rule out hearing loss as a contributing factor. Our paradigm uses carefully titrated noise exposure that only causes unilateral temporary threshold shifts (Figure 3B). Animals’ hearing thresholds recovered to baseline levels by two weeks after each noise exposure. Furthermore, there were no supra-threshold deficits in the ABR wave I amplitude-intensity functions for the animals with (ET) and without tinnitus (ENT) (Figure 3C), which means these animals did not have any observable hidden hearing loss using the tests commonly used to identify hidden hearing loss in animal (Kujawa & Liberman, 2015). Since there were no significant differences between the ET and the ENT animals for ABR threshold shifts or wave I amplitude-intensity functions, hidden hearing loss cannot account for the differences in their gap inhibition scores in this study.
In addition to the trisynaptic path, CA1 and CA3 pyramidal neurons also receive a direct glutamatergic projection from EC through the temporoammonic or perforant path in stratum lacunosum-moleculare (SLM). To explore whether the effects seen in the two-week post-exposure animals were relevant to tinnitus, another group of animals was exposed to the same noise band twice to induce tinnitus. Since it takes time for tinnitus development, those animals were followed for twelve weeks instead of two weeks and were assessed for the presence of tinnitus with GPIAS measures. In synapse–rich layers of the hippocampus, including the hilus and distal molecular layer in DG, stratum lucidum and radiatum in CA3, as well as stratum radiatum and lacunosum-moleculare in CA1, tinnitus animals showed lower VAChT labeling levels than the controls, like those in the two-week post-exposure animals. By contrast, noise-exposed animals resistant to tinnitus showed similar VAChT labeling levels to controls, suggesting recovery of cholinergic innervation in those tinnitus-resistant animals. The layers exhibiting recovery in the no-tinnitus include the layers through which the “trisynaptic circuit” and temporoammonic path run, indicating that cholinergic- information- processing in these circuits is potentially involved in the pathophysiology of tinnitus.
While most types of hippocampal plasticity rely on long-lasting changes in glutamatergic signaling, the cholinergic system can modulate through interactions with glutamatergic and GABAergic systems. Acetylcholine signals through two classes of receptors: metabotropic muscarinic receptors (mAChRs) and ionotropic nicotinic receptors (nAChRs) (Picciotto, Caldarone, King, & Zachariou, 2000; Wess, 2003). Muscarinic and nicotinic receptors are localized at both pre- and post-synaptic sites(Picciotto, Higley, & Mineur, 2012). Presynaptic mAChRs are largely inhibitory and act a s inhibitory autoreceptors on cholinergic terminals. Post-synaptic mAChRs can be either inhibitory or excitatory (Picciotto et al., 2012). Cholinergic signals shape nervous system function by activating inhibitory interneurons or excitatory principal neurons, but given that cholinergic receptors can be either inhibitory or excitatory, the overall effect of cholinergic signaling is complex. Acetylcholine can modulate hippocampal output to entorhinal cortex by activating GABAergic oriens lacunosum moleculare (OLM) interneurons (Haam, Zhou, Cui, & Yakel, 2018) and can modulate granule cell excitability by innervating glutamatergic mossy cells in dentate hilus (Sun, Grieco, Holmes, & Xu, 2017). To the extent that these targets are similarly regulated by noise exposure is an important question to be addressed by future studies.
Tinnitus is thought to be the result of altered neural plasticity in the central nervous system beginning at the brainstem (Basura et al., 2015; Koehler & Shore, 2013; Marks et al., 2018; Stefanescu et al., 2015). Upregulation of somatosensory inputs to cochlear nucleus in compensation for reduced auditory innervation after cochlear damage is related to altered stimulus-timing-dependent plasticity (STDP) of cochlear nucleus fusiform cells and manipulating STDP by inducing LTD reduces tinnitus in both guinea pigs and humans (Marks et al., 2018). The hippocampus also responds to somatosensory stimuli via projections from layer II of entorhinal cortex (Bellistri, Aguilar, Brotons-Mas, Foffani, & de la Prida, 2013; Pereira et al., 2007). In the present study, tinnitus was associated with incomplete recovery of cholinergic innervation following noise exposure. Because interactions between cholinergic, glutamatergic, and GABAergic systems affect neural plasticity, it is likely that tinnitus is further associated with altered hippocampal synaptic plasticity or excitability, a possibility to be tested in future studies.
4.3. Limitations
There are some limitations in this study. First, though the results here provide evidence on the involvement of cholinergic signaling in the hippocampus in tinnitus, the causal relationship is not clear. Future studies could address the question better by taking advantage of cholinergic agents or transgenic animals to see if these alter the development and/or maintenance of tinnitus. Second, it is widely accepted that noise exposure can lead to hippocampus-dependent impairments, including cognition and memory impairment (Dong et al., 2016; Liu et al., 2016), so future studies would benefit from the inclusion of behavioral tests of hippocampal function.
5. CONCLUSIONS
In conclusion, this is a novel study addressing the remodeling of cholinergic innervation to the hippocampus in tinnitus animals. Our results demonstrate robust neural circuitry changes in the hippocampus two weeks after noise exposure, which suggest involvement of this circuitry in auditory processing. After chronic tinnitus induction, tinnitus-specific changes occurred in synapse-rich layers of DG, CA3, and CA1 areas in the hippocampus. This pattern of changes raises the possibility that cholinergic synaptic processing in the hippocampus plays an important role in the pathophysiology of tinnitus.
Acknowledgements
This research was funded by NIH R01-DC004825(SES), NIH P30-DC005188(SES), R01-NS097498(MAS). LZ was supported by Central South University Xiangya Medical School. The authors thank Cindy Carruthers, Christian Althaus and Christopher Chung for technical assistance. All authors declare no competing interests.
References
- Al-Onaizi MA, Parfitt GM, Kolisnyk B, Law CS, Guzman MS, Barros DM, … Prado VF (2017). Regulation of Cognitive Processing by Hippocampal Cholinergic Tone. Cereb Cortex, 27(2), 1615–1628. doi: 10.1093/cercor/bhv349 [DOI] [PubMed] [Google Scholar]
- Altman NS (1992). An introduction to kernel and nearest-neighbor nonparametric regression. The American Statistician, 46(3), 10. doi: 10.1080/00031305.1992.10475879 [DOI] [Google Scholar]
- Azman KF, Zakaria R, Abdul Aziz CB, & Othman Z (2016). Tualang Honey Attenuates Noise Stress-Induced Memory Deficits in Aged Rats. Oxid Med Cell Longev, 2016, 1549158. doi: 10.1155/2016/1549158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Koehler SD, & Shore SE (2015). Bimodal stimulus timing-dependent plasticity in primary auditory cortex is altered after noise exposure with and without tinnitus. J Neurophysiol, 114(6), 3064–3075. doi: 10.1152/jn.00319.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, & Brozoski TJ (2008). Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res, 86(11), 2564–2578. doi: 10.1002/jnr.21699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellistri E, Aguilar J, Brotons-Mas JR, Foffani G, & de la Prida LM (2013). Basic properties of somatosensory-evoked responses in the dorsal hippocampus of the rat. J Physiol, 591(10), 2667–2686. doi: 10.1113/jphysiol.2013.251892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JI, Coomber B, Shackleton TM, Palmer AR, & Wallace MN (2013). A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods, 213(2), 188–195. doi: 10.1016/j.jneumeth.2012.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JI, Owen W, Wilson CA, Hockley A, Coomber B, Palmer AR, & Wallace MN (2018). Gap-induced reductions of evoked potentials in the auditory cortex: A possible objective marker for the presence of tinnitus in animals. Brain Res, 1679, 101–108. doi: 10.1016/j.brainres.2017.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt JM, Bhattacharyya N, & Lin HW (2017). Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope, 127(2), 466–469. doi: 10.1002/lary.26107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford PC, Luntz-Leybman V, & Freedman R (1993). Auditory sensory gating in the rat hippocampus: modulation by brainstem activity. Brain Res, 607(1–2), 33–38. [DOI] [PubMed] [Google Scholar]
- Brankack J, & Buzsaki G (1986). Hippocampal responses evoked by tooth pulp and acoustic stimulation: depth profiles and effect of behavior. Brain Res, 378(2), 303–314. [DOI] [PubMed] [Google Scholar]
- Burwell RD, & Amaral DG (1998). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol, 398(2), 179–205. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, & Swanson LW (2007). Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev, 56(1), 1–26. doi: 10.1016/j.brainresrev.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, West MO, & Robinson JH (1981). Entorhinal and septal inputs differentially control sensory-evoked responses in the rat dentate gyrus. Science, 211(4487), 1181–1183. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, & Shore SE (2012). Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci, 6, 42. doi: 10.3389/fnsys.2012.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di GQ, & Qin ZQ (2018). Influences of combined traffic noise on the ability of learning and memory in mice. Noise Health, 20(92), 9–15. doi: 10.4103/nah.NAH_27_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhou Y, Chu X, Chen S, Chen L, Yang B, … Li W. (2016). Dental noise exposed mice display depressive-like phenotypes. Mol Brain, 9(1), 50. doi: 10.1186/s13041-016-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein Y, Koffler B, Rabey JM, & Gilad GM (1985). Dynamics of cholinergic synaptic mechanisms in rat hippocampus after stress. Brain Res, 343(2), 314–319. [DOI] [PubMed] [Google Scholar]
- Fournier P, & Hebert S (2013). Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear Res, 295, 16–23. doi: 10.1016/j.heares.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Friedman JH, Bentely Jon L., Finkel Raphael A. (1977). An algorithm for finding best matches in logarithmic expected time. ACM Transactions on Mathematical Software, 3(3), 209–226. doi: 10.1145/355744.355745 [DOI] [Google Scholar]
- Frotscher M, & Leranth C (1985). Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol, 239(2), 237–246. doi: 10.1002/cne.902390210 [DOI] [PubMed] [Google Scholar]
- Geven LI, de Kleine E, Willemsen AT, & van Dijk P (2014). Asymmetry in primary auditory cortex activity in tinnitus patients and controls. Neuroscience, 256, 117–125. doi: 10.1016/j.neuroscience.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Gilad GM, Rabey JM, & Shenkman L (1983). Strain-dependent and stress-induced changes in rat hippocampal cholinergic system. Brain Res, 267(1), 171–174. [DOI] [PubMed] [Google Scholar]
- Goble TJ, Moller AR, & Thompson LT (2009). Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear Res, 253(1–2), 52–59. doi: 10.1016/j.heares.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Green A, Jones AD, Sun K, & Neitzel RL (2015). The Association between Noise, Cortisol and Heart Rate in a Small-Scale Gold Mining Community-A Pilot Study. Int J Environ Res Public Health, 12(8), 9952–9966. doi: 10.3390/ijerph120809952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Zaborszky L, Atienza M, Gil-Neciga E, Rodriguez-Romero R, Teipel SJ, … Cantero JL (2010). Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer’s disease. Cereb Cortex, 20(7), 1685–1695. doi: 10.1093/cercor/bhp232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haam J, Zhou J, Cui G, & Yakel JL (2018). Septal cholinergic neurons gate hippocampal output to entorhinal cortex via oriens lacunosum moleculare interneurons. Proc Natl Acad Sci U S A, 115(8), E1886–E1895. doi: 10.1073/pnas.1712538115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, & Liberman MC (2014). Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol, 111(3), 552–564. doi: 10.1152/jn.00184.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House L, Bishop CE, Spankovich C, Su D, Valle K, & Schweinfurth J (2017). Tinnitus and its risk factors in african americans: The Jackson Heart Study. Laryngoscope. doi: 10.1002/lary.26964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Jin S, He X, Xu F, & Hu J (2016). Whole-Brain Monosynaptic Afferent Inputs to Basal Forebrain Cholinergic System. Front Neuroanat, 10, 98. doi: 10.3389/fnana.2016.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, & Cowan WM (1987). The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol, 264(3), 356–395. doi: 10.1002/cne.902640306 [DOI] [PubMed] [Google Scholar]
- Ising H, & Kruppa B (2004). Health effects caused by noise: evidence in the literature from the past 25 years. Noise Health, 6(22), 5–13. [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG, & Caspary DM (2014). Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol, 592(22), 5065–5078. doi: 10.1113/jphysiol.2014.278572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RJ (1982). Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav, 16(6), 965–968. [DOI] [PubMed] [Google Scholar]
- Katz RJ, & Baldrighi G (1982). A further parametric study of imipramine in an animal model of depression. Pharmacol Biochem Behav, 16(6), 969–972. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee HJ, An SY, Sim S, Park B, Kim SW, … Choi HG (2015). Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One, 10(5), e0127578. doi: 10.1371/journal.pone.0127578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M (1999). The neurobiology of startle. Prog Neurobiol, 59(2), 107–128. [DOI] [PubMed] [Google Scholar]
- Koehler SD, & Shore SE (2013). Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci, 33(50), 19647–19656. doi: 10.1523/JNEUROSCI.2788-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, & Canlon B (2012). Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res, 288(1–2), 34–46. doi: 10.1016/j.heares.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Kujawa SG, & Liberman MC (2015). Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res, 330(Pt B), 191–199. doi: 10.1016/j.heares.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H (1987). Acute exposure to noise affects sodium-dependent high-affinity choline uptake in the central nervous system of the rat. Pharmacol Biochem Behav, 28(2), 147–151. [DOI] [PubMed] [Google Scholar]
- Lai H, & Carino MA (1990). Effects of noise on high-affinity choline uptake in the frontal cortex and hippocampus of the rat are blocked by intracerebroventricular injection of corticotropin-releasing factor antagonist. Brain Res, 527(2), 354–358. [DOI] [PubMed] [Google Scholar]
- Lai H, & Carino MA (1992). Opioid receptor subtypes mediating the noise-induced decreases in high-affinity choline uptake in the rat brain. Pharmacol Biochem Behav, 42(3), 553–558. [DOI] [PubMed] [Google Scholar]
- Lai H, Carino MA, & Wen YF (1989). Repeated noise exposure affects muscarinic cholinergic receptors in the rat brain. Brain Res, 488(1–2), 361–364. [DOI] [PubMed] [Google Scholar]
- Lai H, Zabawska J, & Horita A (1986). Sodium-dependent, high-affinity choline uptake in hippocampus and frontal cortex of the rat affected by acute restraint stress. Brain Res, 372(2), 366–369. [DOI] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, … Hajak G (2009). Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage, 46(1), 213–218. doi: 10.1016/j.neuroimage.2009.01.069 [DOI] [PubMed] [Google Scholar]
- Lauer AM, Behrens D, & Klump G (2017). Acoustic startle modification as a tool for evaluating auditory function of the mouse: Progress, pitfalls, and potential. Neurosci Biobehav Rev, 77, 194–208. doi: 10.1016/j.neubiorev.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen B, Mork A, Plath N, Kristiansen U, & Bastlund JF (2013). Cholinergic degeneration is associated with increased plaque deposition and cognitive impairment in APPswe/PS1dE9 mice. Behav Brain Res, 240, 146–152. doi: 10.1016/j.bbr.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Liu L, Shen P, He T, Chang Y, Shi L, Tao S, … Wang J (2016). Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice. Sci Rep, 6, 20374. doi: 10.1038/srep20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Turner J, & Caspary DM (2012). Diminished cortical inhibition in an aging mouse model of chronic tinnitus. J Neurosci, 32(46), 16141–16148. doi: 10.1523/JNEUROSCI.2499-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, & Freedman R (1992). Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res, 587(1), 130–136. [DOI] [PubMed] [Google Scholar]
- Mark GP, Rada PV, & Shors TJ (1996). Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience, 74(3), 767–774. [DOI] [PubMed] [Google Scholar]
- Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, & Shore SE (2018). Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med, 10(422). doi: 10.1126/scitranslmed.aal3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Wallenhorst C, McFerran D, & Hall DA (2015). Incidence rates of clinically significant tinnitus: 10-year trend from a cohort study in England. Ear Hear, 36(3), e69–75. doi: 10.1097/AUD.0000000000000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan GJ, & Chang SU (2004). Mixture modelling for cluster analysis. Stat Methods Med Res, 13(5), 347–361. doi: 10.1191/0962280204sm372ra [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, & Wainer BH (1983). Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol, 214(2), 170–197. doi: 10.1002/cne.902140206 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, & Picciotto MR (2013). Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A, 110(9), 3573–3578. doi: 10.1073/pnas.1219731110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Arroyo-Jimenez MM, Artacho-Perula E, Insausti AM, Marcos P, … Insausti R (2007). Topographical and laminar distribution of cortical input to the monkey entorhinal cortex. J Anat, 211(2), 250–260. doi: 10.1111/j.1469-7580.2007.00764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Lopez MM, Mohedano-Moriano A, & Insausti R (2010). Anatomical pathways for auditory memory in primates. Front Neuroanat, 4, 129. doi: 10.3389/fnana.2010.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Pace E, Lepczyk L, Kaufman M, Zhang J, Perrine SA, & Zhang J (2017). Blast-Induced Tinnitus and Elevated Central Auditory and Limbic Activity in Rats: A Manganese-Enhanced MRI and Behavioral Study. Sci Rep, 7(1), 4852. doi: 10.1038/s41598-017-04941-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Lee SH, Koo JW, Park HY, Lee KY, Choi YS, … Cho YS (2014). Prevalence and associated factors of tinnitus: data from the Korean National Health and Nutrition Examination Survey 2009–2011. J Epidemiol, 24(5), 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A, Ribeiro S, Wiest M, Moore LC, Pantoja J, Lin SC, & Nicolelis MA (2007). Processing of tactile information by the hippocampus. Proc Natl Acad Sci U S A, 104(46), 18286–18291. doi: 10.1073/pnas.0708611104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, & Zachariou V (2000). Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology, 22(5), 451–465. doi: 10.1016/S0893-133X(99)00146-3 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, & Mineur YS (2012). Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron, 76(1), 116–129. doi: 10.1016/j.neuron.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouryaghoub G, Mehrdad R, & Valipouri A (2016). Effect of Acute Noise Exposure on Salivary Cortisol: A Randomized Controlled Trial. Acta Med Iran, 54(10), 657–661. [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, … Tonegawa S (2013). Creating a false memory in the hippocampus. Science, 341(6144), 387–391. doi: 10.1126/science.1239073 [DOI] [PubMed] [Google Scholar]
- Riedl D, Rumpold G, Schmidt A, Zorowka PG, Bliem HR, & Moschen R (2015). The influence of tinnitus acceptance on the quality of life and psychological distress in patients with chronic tinnitus. Noise Health, 17(78), 374–381. doi: 10.4103/1463-1741.165068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KA, & Katz RJ (1979). Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neurosci Biobehav Rev, 3(4), 247–263. [DOI] [PubMed] [Google Scholar]
- Sametsky EA, Turner JG, Larsen D, Ling L, & Caspary DM (2015). Enhanced GABAA-Mediated Tonic Inhibition in Auditory Thalamus of Rats with Behavioral Evidence of Tinnitus. J Neurosci, 35(25), 9369–9380. doi: 10.1523/JNEUROSCI.5054-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuzigert N, Fostiropoulos K, & Probst R (2006). Long-term assessment of auditory changes resulting from a single noise exposure associated with non-occupational activities. Int J Audiol, 45(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Sembulingam K, Sembulingam P, & Namasivayam A (2005). Effect of Ocimum sanctum Linn on the changes in central cholinergic system induced by acute noise stress. J Ethnopharmacol, 96(3), 477–482. doi: 10.1016/j.jep.2004.09.047 [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, & Farwell WR (2010). Prevalence and characteristics of tinnitus among US adults. Am J Med, 123(8), 711–718. doi: 10.1016/j.amjmed.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Smit JV, Janssen ML, van Zwieten G, Jahanshahi A, Temel Y, & Stokroos RJ (2016). Deep brain stimulation of the inferior colliculus in the rodent suppresses tinnitus. Brain Res, 1650, 118–124. doi: 10.1016/j.brainres.2016.08.046 [DOI] [PubMed] [Google Scholar]
- Spreng M (2000). Possible health effects of noise induced cortisol increase. Noise Health, 2(7), 59–64. [PubMed] [Google Scholar]
- Stefanescu RA, Koehler SD, & Shore SE (2015). Stimulus-timing-dependent modifications of rate-level functions in animals with and without tinnitus. J Neurophysiol, 113(3), 956–970. doi: 10.1152/jn.00457.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Grieco SF, Holmes TC, & Xu X (2017). Local and Long-Range Circuit Connections to Hilar Mossy Cells in the Dentate Gyrus. eNeuro, 4(2). doi: 10.1523/ENEURO.0097-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S, Heinsen H, Amaro E Jr., Grinberg LT, Krause B, Grothe M, & Alzheimer’s Disease Neuroimaging, I. (2014). Cholinergic basal forebrain atrophy predicts amyloid burden in Alzheimer’s disease. Neurobiol Aging, 35(3), 482–491. doi: 10.1016/j.neurobiolaging.2013.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull MT, Boskovic Z, & Coulson EJ (2018). Acute Down-regulation of BDNF Signaling Does Not Replicate Exacerbated Amyloid-beta Levels and Cognitive Impairment Induced by Cholinergic Basal Forebrain Lesion. Front Mol Neurosci, 11, 51. doi: 10.3389/fnmol.2018.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, & Caspary DM (2006). Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci, 120(1), 188–195. doi: 10.1037/0735-7044.120.1.188 [DOI] [PubMed] [Google Scholar]
- Ueyama T, Donishi T, Ukai S, Ikeda Y, Hotomi M, Yamanaka N, … Kaneoke Y (2013). Brain regions responsible for tinnitus distress and loudness: a resting-state FMRI study. PLoS One, 8(6), e67778. doi: 10.1371/journal.pone.0067778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, & De Ridder D (2012). The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front Syst Neurosci, 6, 31. doi: 10.3389/fnsys.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Faber M, Langguth B, & De Ridder D (2016). The neural correlates of cognitive dysfunction in phantom sounds. Brain Res, 1642, 170–179. doi: 10.1016/j.brainres.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Plazier M, van der Loo E, Van de Heyning P, & De Ridder D (2011). The difference between uni- and bilateral auditory phantom percept. Clin Neurophysiol, 122(3), 578–587. doi: 10.1016/j.clinph.2010.07.022 [DOI] [PubMed] [Google Scholar]
- Wang F, Zuo L, Hong B, Han D, Range EM, Zhao L, … Liu, L. (2013). Tonotopic reorganization and spontaneous firing in inferior colliculus during both short and long recovery periods after noise overexposure. J Biomed Sci, 20, 91. doi: 10.1186/1423-0127-20-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Finnie PS, Hardt O, & Nader K (2012). Dorsal hippocampus is necessary for novel learning but sufficient for subsequent similar learning. Hippocampus, 22(11), 2157–2170. doi: 10.1002/hipo.22036 [DOI] [PubMed] [Google Scholar]
- Wess J (2003). Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci, 24(8), 414–420. doi: 10.1016/S0165-6147(03)00195-0 [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, … Lopes da Silva FH (2000). Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus, 10(4), 398–410. doi: [DOI] [PubMed] [Google Scholar]
- Woolf NJ (1991). Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol, 37(6), 475–524. [DOI] [PubMed] [Google Scholar]
- Wu C, Martel DT, & Shore SE (2016). Increased Synchrony and Bursting of Dorsal Cochlear Nucleus Fusiform Cells Correlate with Tinnitus. J Neurosci, 36(6), 2068–2073. doi: 10.1523/JNEUROSCI.3960-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, & Shore S (2012). Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci, 32(45), 15791–15801. doi: 10.1523/JNEUROSCI.2598-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Luo H, Pace E, Li L, & Liu B (2016). Psychophysical and neural correlates of noised-induced tinnitus in animals: Intra- and inter-auditory and non-auditory brain structure studies. Hear Res, 334, 7–19. doi: 10.1016/j.heares.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Hamilton E, Begum S, Smith PF, & Darlington CL (2011). The effects of acoustic trauma that can cause tinnitus on spatial performance in rats. Neuroscience, 186, 48–56. doi: 10.1016/j.neuroscience.2011.04.052 [DOI] [PubMed] [Google Scholar]