Abstract
Objective
To investigate whether the Revised Self-Monitoring Scale (RSMS), an informant measure of socioemotional sensitivity, is a potential clinical endpoint for treatment trials for patients with behavioral variant frontotemporal dementia (bvFTD).
Methods
We investigated whether RSMS informant ratings reflected disease severity in 475 participants (71 bvFTD mutation+, 154 bvFTD mutation−, 12 behavioral mild cognitive impairment [MCI] mutation+, 98 asymptomatic mutation+, 140 asymptomatic mutation−). In a subset of 62 patients (20 bvFTD mutation+, 35 bvFTD mutation−, 7 MCI mutation+) who had at least 2 time points of T1-weighted images available on the same 3T scanner, we examined longitudinal changes in RSMS score over time and its correspondence to progressive gray matter atrophy.
Results
RSMS score showed a similar pattern in mutation carriers and noncarriers, with significant drops at each stage of progression from asymptomatic to very mild, mild, moderate, and severe disease (F4,48 = 140.10, p < 0.001) and a significant slope of decline over time in patients with bvFTD (p = 0.004, 95% confidence interval [CI] −1.90 to −0.23). More rapid declines on the RSMS corresponded to faster gray matter atrophy predominantly in the salience network (SN), and RSMS score progression best predicted thalamic volume in very mild and mild disease stages of bvFTD. Higher RSMS score predicted more caregiver burden (p < 0.001, 95% CI −0.30 to −0.11).
Conclusions
The RSMS is sensitive to progression of both socioemotional symptoms and SN atrophy in patients with bvFTD and corresponds directly to caregiver burden. The RSMS may be useful in both neurologic practice and clinical trials aiming to treat behavioral symptoms of patients with bvFTD.
Behavioral variant frontotemporal dementia (bvFTD) belongs to a heterogeneous group of frontotemporal lobar degeneration (FTLD) syndromes that are each characterized by deficits in behavior, language, and motor function. bvFTD selectively targets the salience network (SN)1 and semantic-appraisal network (SAN),2 and drastic alterations in social behavior such as loss of empathy are the hallmark symptoms occurring early in the disease.3,4 There are currently no Food and Drug Administration–approved treatments for bvFTD and other FTLD syndromes, but the first clinical trials targeting the 2 most common FTLD proteinopathies, FTLD-tau and FTLD-TDP,5 are now being conducted. Because the initial symptoms of bvFTD are exclusively behavioral6 and not cognitive and because current clinical trials focus on presymptomatic stages,7 psychometrically valid tests are required to detect the earliest socioemotional changes and to accurately measure symptom and neuroanatomic progression.
The goal of this study was to investigate whether the Revised Self-Monitoring Scale (RSMS),8 an informant-completed measure of socioemotional sensitivity, may be a potential clinical endpoint for treatment trials for patients with bvFTD. We examined whether RSMS score reflects disease severity and whether rate of progression differs between FTLD mutation carriers and noncarriers. In addition, we investigated whether RSMS score declines over time in patients with bvFTD and whether this change corresponds to progressive gray matter atrophy. On the basis of our previous cross-sectional work showing that lower RSMS score is associated with greater atrophy and lower functional connectivity in the SN in bvFTD,9,10 we hypothesized that a drop in RSMS score over time would correspond to progressive atrophy predominantly in regions of the SN.
Methods
Participants
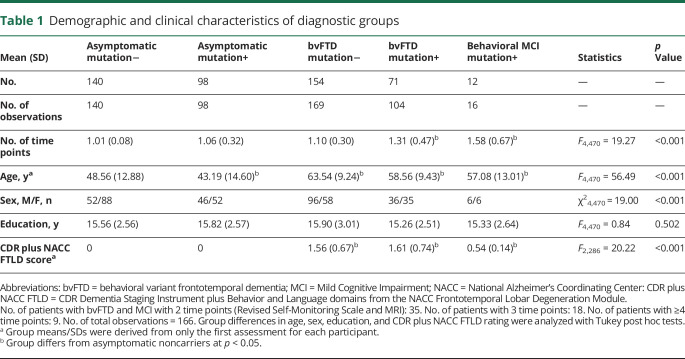
For the initial analysis examining whether RSMS rating reflects disease severity (CDR analysis), 475 participants were included from 3 parent studies: University of California, San Francisco Frontotemporal Dementia Program Project Grant (UCSF FTD PPG) study, as well as the multisite Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) and Advancing Research and Treatment in Frontotemporal Lobar Degeneration (ARTFL) studies (rarediseasesnetwork.org/cms/artfl/) between 2009 and 2019. These included 71 patients diagnosed with bvFTD who carried a mutation in 1 of the 3 main autosomal dominant FTD genes (36 C9orf72, 26 MAPT, 9 GRN), 154 noncarriers with clinical bvFTD,6 12 patients with a diagnosis of behavioral mild cognitive impairment (MCI) who were mutation carriers (5 C9orf72, 3 MAPT, 4 GRN), 98 asymptomatic mutation-positive adults, and 140 asymptomatic mutation-negative adults from families with a known FTLD gene mutation. Patients were diagnosed with behavioral MCI if they had 1 or 2 of the following key symptoms as required for possible bvFTD6: disinhibition, apathy or inertia, loss of sympathy/empathy, ritualistic/compulsive behavior, or hyperorality and appetite changes, and no cognitive domain impaired other than behavior. Patients were diagnosed after comprehensive neurologic, neuroimaging, genetic, and neuropsychological assessments that did not include the RSMS. Each participant had an informant who was a first-degree family member or friend who had known the participant for ≥5 years. Participants were included only if they had at least 1 RSMS informant rating available; administration of this measure was standard for all patients, and attempts were made to collect yearly follow-up RSMS score on all participants regardless of diagnostic status or disease severity. When participants had >1 RSMS time point available, only the first time point within the same disease stage level was included in the first analysis.
For the second set of analyses examining how RSMS score changes longitudinally over the course of disease progression in bvFTD (time analysis) and whether change over time corresponds to progression in gray matter atrophy, a subset of the above sample was analyzed that included only the patients who had at least 2 time points at which a structural imaging scan of sufficient quality was collected on the same 3T scanner. This subsample consisted of 62 patients, including 20 mutation carriers with bvFTD (10 C9orf72, 8 MAPT, 2 GRN),6 35 noncarriers with bvFTD,6 and 7 mutation carriers with a diagnosis of behavioral MCI (2 C9orf72, 3 MAPT, 2 GRN). The average time interval between RSMS score collection and structural imaging was 4.80 ± 3.61 days. We also included a control sample of 53 neurologically and cognitively healthy older adults (age [mean ± SD] 68.73 ± 6.10 years, male/female 25/29) from the Hillblom Network Program. Table 1 shows the demographic and clinical characteristics.
Table 1.
Demographic and clinical characteristics of diagnostic groups
Standard protocol approvals, registrations, and patient consents
The parent studies (UCSF FTD PPG, LEFFTDS, ARTFL) were conducted in accordance with Institutional Review Board approval from each study institution, and all participants and their informants gave their consent to participate and to share data.
Behavioral, functional, and caregiver measures
The RSMS8 is a thoroughly validated 13-item questionnaire that measures sensitivity and responsiveness to subtle emotional expressions during face-to-face interactions. Sample items include “In conversations, the subject is sensitive to even the slightest change in the facial expression of the other person he/she is conversing with,” and “In social situations, the subject has the ability to alter his/her behavior if he/she feels that something else is called for.” The questionnaire has good psychometric characteristics, including internal consistency, retest reliability, and construct validity,11–13 and has previously been used to investigate brain-behavior relationships in both healthy and clinical populations.9,10,14 Informants rated patients on each item on a 6-point Likert scale, ranging from “certainly, always false” to “certainly, always true.”
The CDR Dementia Staging Instrument plus Behavior and Language domains from the National Alzheimer’s Coordinating Center (NACC) FTLD Module (CDR plus NACC FTLD) was included as a proxy of disease severity. This measure is an extension of the standard CDR,15 which assesses functional impairment in 6 domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, personal care) and includes 2 additional domains that are predominantly affected in FTLD: behavior and language.16 The score ranges between 0 (no functional impairment) and 3 (severe functional impairment).
The Zarit Burden Interview17 is a 22-item self-report measure that assesses caregiver burden in different areas, including behavioral symptoms and functional status of the patient, interpersonal relationships, finances, physical health, and social life. The questionnaire was used to test our hypothesis that lower socioemotional sensitivity in patients with bvFTD would be associated with higher caregiver burden.
Behavioral data analysis
CDR analysis: RSMS rating by CDR plus NACC FTLD stage
Group differences on potentially confounding covariates, including age, sex, and education, were analyzed with general linear models in SAS (Proc GLM; SAS Institute Inc, Cary, NC). To investigate whether RSMS score significantly worsened with increasing CDR plus NACC FTLD stage and whether rate of decline differed between carriers and noncarriers, we performed linear mixed-effects (LME) models in SAS (Proc Mixed) with random intercepts and slopes, which accounted for individual differences in baseline RSMS score and rate of progression. The use of LME models also allowed us to use the full sample (n = 475, 527 observations), including individuals who had only 1 time point and those without concurrent MRI. Group (carriers vs noncarriers), CDR plus NACC FTLD score (asymptomatic, very mild, mild, moderate, severe), and the interaction between group and CDR plus NACC FTLD score were included in the model, controlling for age at first assessment and sex. A priori power analysis showed that standard α = 0.05 level tests with 475 participants had power >0.8 to detect small effect sizes of 0.03.
Time analysis: Pattern of longitudinal RSMS score change in bvFTD
The statistical assumptions underlying our second set of analyses required that only patients with bvFTD and MCI who had ≥2 time points of both RSMS and MRI data of sufficient quality and on the same scanner could be included; thus, the smaller set (n = 62, 166 observations) was used. Generalized linear models were performed to assess group differences in age at symptom onset, disease duration since symptom onset, sex, and education. To examine whether RSMS score declined over time, we performed LME models with disease duration, group (carriers vs noncarriers), and the interaction of disease duration by group included as predictors in the model. A priori power analysis showed that standard α = 0.05 level tests with 62 patients had power >0.8 to detect medium effect sizes of 0.23. To determine whether these rates of RSMS score decline differed on the basis of level of disease severity at baseline presentation, we assigned patients to 3 groups based on their baseline CDR plus NACC FTLD score (0.5 = very mild, 1 = mild, 2/3 = moderate and severe). LME models were performed with disease duration, severity group, and the disease duration by group interaction included in the model. Age at symptom onset and sex were entered as covariates of no interest. To model the stability of RSMS score in the comparison group of healthy older adults, we calculated each control's RSMS slope across all time points and derived the group's average.
Context analysis: Relationship between RSMS rating and caregiver burden
To investigate whether RSMS score would correspond with clinically meaningful factors in their living environment, we took the first RSMS rating of each patient with bvFTD (carriers n = 77, noncarriers n = 104) and behavioral MCI (n = 13) who had a valid Zarit score within 90 days of RSMS data collection. A generalized linear model was performed to assess whether Zarit score significantly predicted RSMS score, controlling for age at symptom onset and sex.
Neuroimaging
Participants underwent structural imaging using 3T scanners from 1 of 3 vendors: Siemens (Munich, Germany), Philips Medical Systems (Best, the Netherlands), or General Electric Medical Systems (Chicago, IL). A standard imaging acquisition protocol was used at all centers, managed and reviewed for quality by a core group at the Mayo Clinic (Rochester, MN). A T1-weighted 3D magnetization-prepared rapid gradient echo sequence was used to obtain the T1-weighted images, with parameters as follows: 240 × 256 × 256 matrix, ≈170 slices, and voxel size 1.05 × 1.05 × 1.25 mm3; flip angle, echo time, and repetition time varied by vendor. Preprocessing was performed in SPM12 (fil.ion.ucl.ac.uk/spm/). In brief, the images were visually inspected for artifacts, bias corrected, tissue classified (gray matter, white matter, CSF segments) with unified segmentation,18 and modulated by multiplying the jacobian determinants of the time points with the intra-subject averaged tissues.19 A group template was generated from the averaged within-subject tissue segments with a Large Deformation Diffeomorphic Metric Mapping framework.20 The modulated intrasubject gray and white matter images were normalized to the group template and smoothed with a 10-mm full width at half-maximum gaussian kernel.
Longitudinal voxel-wise and region of interest–based analysis
The voxel-wise longitudinal atrophy trajectories of each subject and the entire group were modeled using a hierarchical Bayesian LME model. In brief, the model rested on 2 hierarchical levels: single-subject trajectory and the group's trajectory. The trajectory was described as a degree D = 1 polynomial, for example, the jth time point of subject i had a gray matter density in 1 voxel yij such as 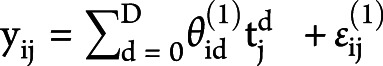 , which was fitted with a design matrix X(1) where tj was the subject's age at the time point j acquisition day, and
, which was fitted with a design matrix X(1) where tj was the subject's age at the time point j acquisition day, and 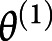 and
and 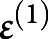 were the first-level vectors of parameters and noise. The complete model was
were the first-level vectors of parameters and noise. The complete model was 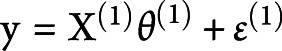 ;
; 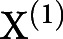 and
and 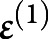 corresponded to the first-level design matrix and noise. The second level was modeled by
corresponded to the first-level design matrix and noise. The second level was modeled by 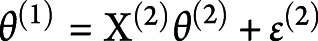 , where
, where 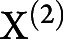 ,
, 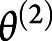 , and
, and 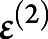 corresponded to the second-level design matrix, parameters, and noise. At each level, the noise distribution was drawn from a centered gaussian:
corresponded to the second-level design matrix, parameters, and noise. At each level, the noise distribution was drawn from a centered gaussian: 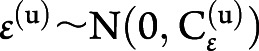 , where
, where 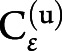 was the hierarchical level u covariance matrix.
was the hierarchical level u covariance matrix.
To test our hypothesis that rate of RSMS score progression in different disease stages would correspond to change in gray matter volume in regions of the SN and SAN, the Desikan brain atlas was used to define bilateral regions of interest (ROIs) in the SN (anterior insula [AI], dorsal anterior cingulate cortex [ACC], thalamus, amygdala, and brainstem) and SAN (temporal pole [TP], orbitofrontal cortex [OFC], subgenual ACC, caudate, and nucleus accumbens).
Brain-behavior analyses
Time analysis: Longitudinal relationship between RSMS rating and brain volume
To examine whether a greater drop in RSMS score would correspond to more rapid volume loss in SN regions, we performed a linear regression analysis in SPM12, investigating whether each patient's slope of change in RSMS score significantly predicted the voxel-wise slope of change in gray matter volume. To investigate whether patients with different levels of disease severity at baseline showed different relationships between change in RSMS score and change in gray matter volume, LME models were performed for each SN and SAN ROI. The ROI volume, severity group (very mild, mild, moderate/severe), and the interaction between ROI and group were entered as predictors, controlling for age at symptom onset, sex, disease duration, and total intracranial volume.
Data availability
Anonymized data will be shared on request from any qualified investigator for the purposes of replicating procedures and results.
Results
Demographic and clinical features
The demographics of the full sample (n = 475) are shown in table 1. The longitudinal analysis was performed in a subset consisting of patients with bvFTD and MCI (n = 62). In the smaller group, mutation carriers had an earlier age at symptom onset, but the groups did not differ in other factors, including disease duration.
RSMS score by CDR plus NACC FTLD stage and its relationship to caregiver burden
CDR analysis
To identify the typical RSMS scores that are predicted to occur at each level of disease severity in patients with bvFTD, we performed an LME model in the full sample of carriers and noncarriers with bvFTD, carriers with behavioral MCI, and asymptomatic carriers and noncarriers. Our results showed a main effect of CDR plus NACC FTLD score (F4,48 = 140.10, p < 0.001) in which RSMS score decreased significantly at each stage as CDR worsened, but no interaction was found between mutation status and CDR plus NACC FTLD score, indicating that mutation status did not influence the timing of RSMS score (figure 1). The same significant main effect was found when the data were reanalyzed including only the first time point for each patient (F4,468 = 137.41, p < 0.001), suggesting that the use of additional time points for some of the patients did not bias the result.
Figure 1. RSMS score reflects disease severity independently of mutation status.
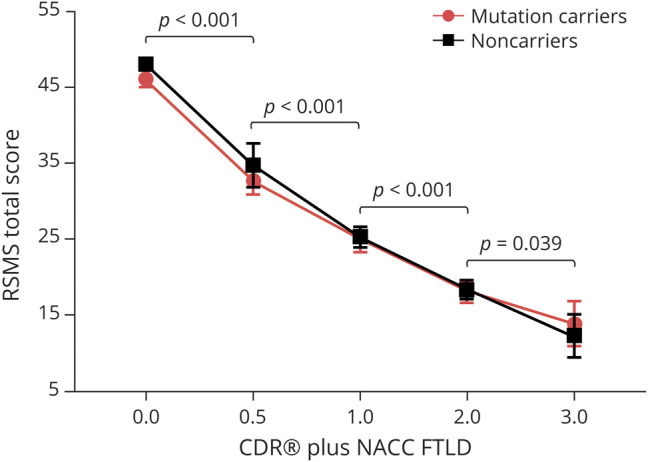
Linear mixed-effects model analysis (n = 475) revealed a significant main effect of CDR Dementia Staging Instrument plus Behavior and Language domains from the National Alzheimer’s Coordinating Center Frontotemporal Lobar Degeneration Module (CDR plus NACC FTLD) (F4,48 = 140.10, p < 0.001), showing that average Revised Self-Monitoring Scale (RSMS) score dropped significantly between asymptomatic (CDR plus NACC FTLD score 0) and very mildly symptomatic (CDR plus NACC FTLD score 0.5) stages (t = 4.99, p < 0.001, estimate 10.58, 95% confidence interval [CI] 6.32–14.85), between very mild (CDR plus NACC FTLD score 0.5) and mild (CDR plus NACC FTLD score 1) stages (t = −5.48, p < 0.001, estimate −12.02, 95% CI −16.43 to −7.61), between mild (CDR plus NACC FTLD score 1) and moderate (CDR plus NACC FTLD score 2) stages (t = 4.46, p < 0.001, estimate 6.50, 95% CI 3.57–9.43), and between moderate and severe stages (t = 2.12, p < 0.039, estimate 4.93, 95% CI 0.25–9.61) in both mutation carriers and noncarriers. Age at first assessment and sex were included as covariates of no interest.
Context analysis
To assess whether lower socioemotional sensitivity would be associated with higher caregiver burden, we examined patients' initial RSMS ratings, controlling for age at symptom onset and sex. As expected, our analysis showed that lower RSMS score was significantly associated with higher Zarit burden score (F1,190 = 17.57, p < 0.001, estimate −0.21, 95% confidence interval [CI] −0.30 to −0.11).
Longitudinal change over time of RSMS score in bvFTD
Time analysis
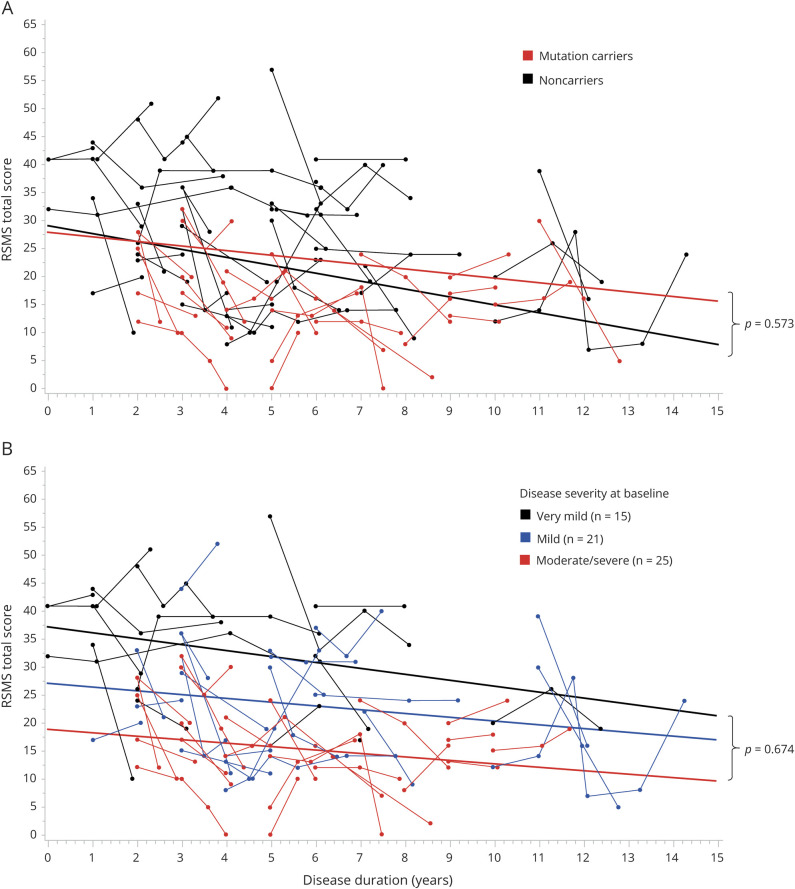
We then examined our subsample of patients with bvFTD and behavioral MCI who had complete longitudinal data (average time interval 0.98 ± 1.12 years) and performed LME model analysis to investigate whether RSMS score would significantly decrease over time. Consistent with the CDR plus NACC FTLD analysis, we found a significant main effect of disease duration (F1,59 = 8.90, p = 0.004, estimate −0.73, 95% CI −1.90 to −0.23; figure 2A) in which RSMS score decreases linearly in patients at a rate of 5 points per year (average RSMS score slope per year −2.13 ± 1.29), but there was no main effect of mutation status, and the interaction between mutation status and disease duration was not significant. To determine whether the small number of patients with >2 time points skewed this result, we performed the same analysis but included only the first 2 time points of each patient (n = 62, 122 observations), and the results remained consistent, including a significant effect of disease duration (F1,59 = 9.88, p = 0.003, estimate −1.26, 95% CI −2.21 to −0.30).
Figure 2. RSMS score shows linear declines over time in behavioral variant frontotemporal dementia.
(A) Linear mixed-effects model analysis in the full longitudinal subsample (n = 62) revealed a significant main effect of disease duration (F1,59 = 8.90, p = 0.004, estimate −0.73, 95% confidence interval −1.90 to −0.23), showing that Revised Self-Monitoring Scale (RSMS) score significantly worsened over time. Rate of RSMS score progression did not differ significantly between mutation carriers and noncarriers. (B) When patients were assigned to 3 groups based on their baseline disease stage (very mild, mild, moderate to severe) measured by the CDR plus National Alzheimer’s Coordinating Center Frontotemporal Lobar Degeneration Module, no significant interaction was found between disease duration and disease stage group, suggesting that the RSMS score progresses at a similar rate in patients who are in different disease stages at baseline. Age at symptom onset and sex were included as covariates of no interest.
For comparison, the average slope of longitudinal change (0.90 ± 0.92) in RSMS score in the healthy control group was flat. We then examined whether patients with different levels of disease severity at baseline showed different slopes of progression on the RSMS. Patients were divided into very mild (CDR plus NACC FTLD score 0.5, average time interval 2.01 ± 1.43 years), mild (CDR plus NACC FTLD score 1, average time interval 1.53 ± 0.94 years), and moderate/severe (CDR plus NACC FTLD score 2/3, average time interval 1.25 ± 0.66 years) disease stage groups at baseline, but no disease duration by disease stage interaction was found (figure 2B).
Longitudinal relationship between RSMS score and gray matter volume in bvFTD
Time analysis
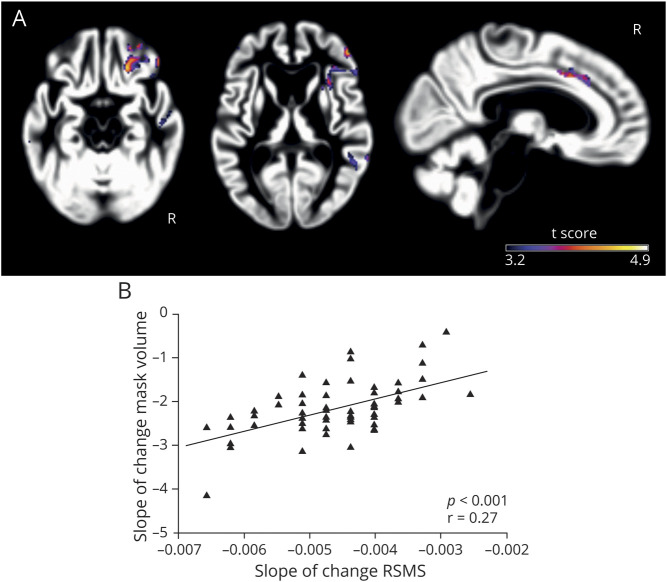
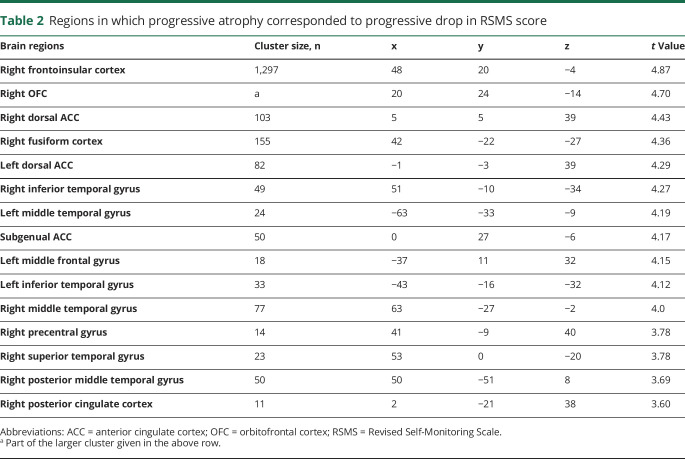
We then investigated whether the drop in RSMS score corresponded to progressive gray matter loss in bvFTD. Our voxel-wise analysis demonstrated that more rapid declines on the RSMS were associated with faster progression of gray matter atrophy in regions of the SN and SAN, including the right AI, dorsal ACC, and OFC (p < 0.001, uncorrected) (figure 3A and table 2). Next, we binarized the uncorrected statistical t map of the RSMS score–gray matter relationship and calculated each patient's average annual slope of change in gray matter volume within that binarized mask. A generalized linear model was performed to assess whether annual gray matter loss predicted annual change in RSMS score, controlling for age at symptom onset, sex, disease duration, and total intracranial volume. The results showed that more rapid yearly worsening on the RSMS was significantly associated with faster gray matter volume loss in these regions (F1,56 = 13.47, p < 0.001, estimate 753.96, 95% CI 342.38–1,165.54) (figure 3B), confirming the presence and direction of the relationship revealed in the voxel-wise analysis.
Figure 3. More rapid decline on the RSMS corresponded to faster progression of salience network atrophy.
(A) Voxel-wise analysis in the full longitudinal subsample (n = 62) showed that a higher drop in Revised Self-Monitoring Scale (RSMS) score was significantly associated with more gray matter volume loss in the right anterior insula, dorsal anterior cingulate cortex, and orbitofrontal cortex (p < 0.001, uncorrected). (B) A general linear model confirmed this result by showing that a higher annual drop in RSMS score significantly predicted (F1,56 = 13.47, p < 0.001, estimate 753.96, 95% confidence interval 342.38–1,165.54) more annual volume loss within the binarized statistical t map derived from the voxel-wise analysis. Age at symptom onset, sex, disease duration, and total intracranial volume were included as covariates of no interest.
Table 2.
Regions in which progressive atrophy corresponded to progressive drop in RSMS score
We next examined whether patients with very mild, mild, and moderate/severe disease severity showed different degrees of correspondence between change in RSMS score and change in any predefined ROI within the SN and SAN. LME models were performed for each ROI, revealing a significant main effect in the thalamus ROI (F1,37 = 9.73, p = 0.004). Although nonsignificant, there was some evidence to suggest a meaningful interaction between thalamus and severity subgroup (F2,37 = 2.89, p = 0.07), showing that RSMS score progression corresponds to thalamic volume primarily in very mild and mild disease stages but to a lesser degree in moderate/severe disease stages. A sensitivity analysis dividing patients by speed of progression and comparing only the highest (n = 12) and lowest (n = 12) speed quartiles yielded the same result, suggesting that both groups had the same linear effect of thalamic volume on RSMS score. These effects lost significance after the exclusion of 12 C9orf72 carriers (30 observations) from the analysis, potentially because of the enrichment of early thalamic atrophy in this group. No significant main effects or interactions were found for the other SN and SAN ROIs.
Discussion
This study shows that both level of disease severity and disease duration in bvFTD are reflected in ratings on the RSMS and that change in RSMS score over time corresponds to progressive gray matter atrophy in mutation carriers and noncarriers with bvFTD. We found that the measure is sensitive to the earliest socioemotional changes occurring during the conversion from the asymptomatic to very mildly symptomatic stage, as well as to later disease progression. Patients with bvFTD who were in different disease stages at baseline showed similar rates of change in RSMS score over time. For an outcome measure in clinical trials, the roughly linear rate of progression across disease severity is a desirable feature. Faster decline on the RSMS predicted greater atrophy, particularly in the SN but also in the SAN. This study highlights that the RSMS is a valid measure to monitor symptoms and corresponding SN progression of patients with bvFTD, both in neurologic practice and in clinical trials aiming to modify socioemotional symptoms of patients with presumed tau- and TDP-related proteinopathies.
Our behavioral analyses revealed 4 main findings. First, our results showed that RSMS score significantly declines with progression across disease stage categories from asymptomatic to very mild, mild, moderate, and severe, and it declines over time regardless of categorical disease stage in patients with bvFTD. Second, we found that the rate of progression did not differ significantly between mutation carriers and noncarriers, which is consistent with previous studies showing that carriers and noncarriers with bvFTD have similar clinical profiles and overlapping atrophy in regions of the SN.22,23 There is currently no evidence that age at disease onset affects the severity of behavioral symptoms in patients with bvFTD, which is in line with our finding that carriers and noncarriers in the longitudinal subsample had different ages at disease onset but showed the same rate of progression. Third, we found that individuals with bvFTD who were in very mild, mild, or moderate to severe disease stage at baseline showed the same slope of linear decline in RSMS score over time. This indicates that the RSMS is a useful clinical test to measure socioemotional progression throughout the disease process in both mutation carriers and noncarriers. A clinical trial of symptom progression in patients with bvFTD requires a measure that is sensitive to the progression of behavioral symptoms at different stages of the disease process.24 Finally, we found that lower RSMS score significantly predicted higher caregiver distress and burden, emphasizing that the RSMS measures a key socioemotional symptom affecting the well-being of caregivers and families. This finding is consistent with previous studies showing that in patients with bvFTD and other neurodegenerative syndromes, both a patient's behavioral and neuropsychiatric symptoms and the quality of the patient-caregiver relationship are associated with caregiver stress.25,26
Our voxel-wise analysis showed that a faster decline in RSMS score is reflected in a greater progression of atrophy in individual regions of the SN, including the AI, dorsal ACC, and thalamus. The AI and dorsal ACC are the hub regions of the SN that work together with subcortical regions, including the thalamus, hypothalamus, amygdala, and periaqueductal gray, to integrate and interpret interoceptive, autonomic signals and to adjust arousal and attention on the basis of perceived relevance.1,27 Specifically, the AI (sensory limbic) is involved in emotional awareness, the dorsal ACC (motor limbic) mediates behavior initiation, and the role of the thalamus is to bind the SN circuit together.1,28 In addition to the SN, we found that decline in socioemotional sensitivity corresponded to progressive atrophy in the OFC and subgenual ACC, which belong to the SAN.2 The SAN has its key node in the right TP, which stores multimodal concepts, including social ones such as emotions, faces, and biographies.29,30 The right TP is intrinsically connected with regions related to reward and hedonics, including the OFC, subgenual ACC, caudate, and nucleus accumbens.2 The interplay between these regions mediates personal evaluations of social semantic concepts, which includes tagging concepts with complex hedonic evaluations and resolving ambiguity about the semantic identity of the concepts based on viscerally experienced valence (this is disgust, not amusement).31 These results also support previous cross-sectional studies showing that gray matter atrophy and functional connectivity changes in the SN and SAN correspond to characteristic bvFTD social symptoms, including loss of empathy32,33 and interpersonal warmth,34,35 as well as disrupted emotion recognition, reactivity, and experience.36–38
Although patients with different disease severities at baseline showed similar rates of RSMS score progression, our ROI analysis showed that thalamic volume was the region that was most consistently predictive of RSMS score change in very mild and mild disease stages of bvFTD and became less predictive in patients with moderate to severe disease severity. One reason for this may be that the thalamic volume loss was so extensive in later disease stages that it reached a plateau so that no brain-behavior relationship was detectable anymore. Another possible explanation is that our linear statistical approach did not adequately model this brain-behavior relationship in later disease stages when brain volume loss may accelerate disproportionately compared to earlier disease stages.39 In addition, the finding that the thalamus plays a critical role for progression of social symptoms in patients with bvFTD supports previous evidence showing that thalamic atrophy causes functional connectivity changes in the SN in C9orf72 carriers with bvFTD40 and is frequently associated with early neuropsychiatric symptoms.41,42 This result is also consistent with our previous work demonstrating that functional connectivity between the right AI and subcortical regions, including the thalamus, predicts socioemotional sensitivity in healthy older adults and in patients with early neurodegenerative disease.9 Because previous studies have shown that thalamic atrophy is closely linked to the C9orf72 mutation,23,43 we performed a post hoc analysis excluding the 12 C9orf72 carriers and found that thalamic volume no longer significantly predicted decline in RSMS score, suggesting that the C9orf72 subtype was a major driver of this finding in our sample. However, because thalamic atrophy is also found in very early stages of patients with sporadic bvFTD44 and in GRN and MAPT carriers,45 we cannot exclude that there may also be a relationship between RSMS score and thalamus across both patients with sporadic bvFTD and those with genetic bvFTD that we were not able to detect because of insufficient power; thus, future studies in larger samples are likely warranted to resolve this issue.
Our previous cross-sectional9 and current longitudinal findings confirm that the RSMS is a valid clinical measure to assess the progression of both symptoms and neural network changes in patients with bvFTD in both neurologic practice and research. We have recently shown that the RSMS shows high interindividual variability and is not susceptible to ceiling or floor effects, both in healthy participants across the lifespan and in patients with neurodegenerative disease,9 psychometric qualities that are required for measures to be used to assess symptom progression in therapeutic trials targeting patients with different disease severities.24 In addition, our previous and current work confirms that the RSMS can be administered quickly as an informant questionnaire to identify patients with bvFTD in the earliest stage of disease and thus could be used to enroll very mildly symptomatic patients in clinical trials on the basis of behavioral phenotype. Moreover, the present results show that the RSMS can measure socioemotional and cortico-subcortical SN progression in both mutation carriers and noncarriers with bvFTD syndrome, including in patients who are in very early disease stage. Finally, this study showed that greater loss of socioemotional sensitivity is associated with higher levels of distress for caregivers and families. Thus, a clinical trial that achieves a delay or remission of the socioemotional symptoms measured by the RSMS may directly affect quality of life for caregivers by reducing their burden.
Our study has some limitations. One limitation was that the number of patients in this study with any of the 3 FTD mutations yielded insufficient power to investigate our longitudinal brain-behavior relationships separately in these subgroups. Another limitation always inherent to longitudinal analyses was the manner in which interindividual variation in patient factors may have influenced our statistical models, including age at symptom onset, disease stage at baseline, rate of progression, and number of and interval between time points. Although we used LME model analyses to account for this statistical noise, we cannot be certain that our models were able to adequately correct for all interindividual variability. Another consideration is that, while our analyses found a significant linear relationship between RSMS score and both disease stage and time, it is possible that, with the increased power afforded by additional data collection in future studies, this linear relationship may actually turn out to be more nuanced, and sigmoid models should be evaluated. Finally, because our behavioral analyses suggest a linear progression of RSMS score, we used linear statistical models to investigate our brain-behavior relationships in all disease stages. However, this statistical approach may not be appropriate to model our brain-behavior relationships in later disease stages because the rate of progression in gray matter atrophy may be different from earlier disease stages.39 It is also possible that baseline atrophy and overall rate of progression differ among carriers and noncarriers, as well as among the 3 different FTD mutations.46,47
The RSMS is a brief but effective informant questionnaire that clinicians and clinical researchers can use to identify the earliest changes in socioemotional behavior in patients with bvFTD and to monitor the progression of both symptoms and damage to vulnerable brain networks throughout the disease. Our findings suggest that this scale measures a valuable clinical endpoint in treatment trials conducted for patients with the predominantly bvFTD syndrome and potentially other neurodegenerative diseases.
Glossary
- ACC
anterior cingulate cortex
- AI
anterior insula
- ARTFL
Advancing Research and Treatment in Frontotemporal Lobar Degeneration
- bvFTD
behavioral variant frontotemporal dementia
- CI
confidence interval
- FTLD
frontotemporal lobar degeneration
- LEFFTDS
Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects
- LME
linear mixed-effects
- MCI
mild cognitive impairment
- NACC
National Alzheimer’s Coordinating Center
- OFC
orbitofrontal cortex
- ROI
region of interest
- RSMS
Revised Self-Monitoring Scale
- SAN
semantic-appraisal network
- SN
salience network
- TP
temporal pole
- UCSF FTD PPG
University of California, San Francisco Frontotemporal Dementia Program Project Grant
Appendix. Authors
Study funding
This study was supported by grants P01AG019724, P50AG023501, U01AG045390, U54NS092089, R01AG029577, 5R01AG032289-08, and K23-AG021606 from the NIH; 2014-A-004-NET from the Larry L. Hillblom Foundation; and P300P1_177667 from the Swiss National Science Foundation.
Disclosure
G. Toller receives research support from the Swiss National Science Foundation. K. Ranasinghe receives research support from the NIH and the Larry L. Hillblom Foundation. Y. Cobigo reports no disclosures. A. Staffaroni receives research support from the NIH and the Larry L. Hillblom Foundation. B. Appleby receives research support from Centers for Disease Control and Prevention and NIH. D. Brushaber reports no disclosures. G. Coppola receives research support from the NIH. B. Dickerson receives research support from the NIH. K. Domoto-Reilly has served as an investigator for clinical trials sponsored by Avid Radiopharmaceuticals, Biogen, and Janssen Pharmaceuticals and has served as Advisory Board consultant for Biogen. She receives research support from the NIH. J. Fields receives research support from the NIH. J. Fong and L. Forsberg report no disclosures. N. Ghoshal has participated or is currently participating in clinical trials of antidementia drugs sponsored by the following companies: Bristol Myers Squibb, Eli Lilly/Avid Radiopharmaceuticals, Janssen Immunotherapy, Novartis, Pfizer, Wyeth, Study of Nasal Insulin to Fight Forgetfulness (SNIFF), and Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) trial. She receives research support from the NIH, Tau Consortium, and Association for Frontotemporal Dementia. N. Graff-Radford has served as an investigator for clinical trials sponsored by Avid Radiopharmaceuticals, Biogen, Janssen Pharmaceuticals. He has served as Advisory Board consultant for Biogen and receives research support from the NIH. M. Grossman receives research support from the NIH, Avid and Piramal. He participates in clinical trials sponsored by Biogen, TauRx, and Alector; serves as a consultant to Bracco and UCB; and serves on the Editorial Board of Neurology. H. Heuer reports no disclosures. G.-Y. Hsiung has served as an investigator for clinical trials sponsored by AstraZeneca, Eli Lilly, and Roche/Genentech. He receives research support from the Canadian Institutes of Health Research and the Alzheimer Society of British Columbia. E. Huey receives research support from the NIH, the Association for Frontotemporal Degeneration, and the Alzheimer's Drug Discovery Foundation. He participates in clinical trials sponsored by the NIH and the Lawson Health Research Institute. D. Irwin receives research support from the NIH, Brightfocus Foundation, and Penn Institute on Aging. K. Kantarci served on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc; served on data monitoring boards of Pfizer and Janssen Alzheimer Immunotherapy; and received research support from Avid Radiopharmaceuticals, Eli Lilly, the Alzheimer's Drug Discovery Foundation, and the NIH. D. Kaufer has served as an investigator for clinical trials sponsored by Abbvie, Axovant, Janssen Research & Development, Navidea Biopharmaceuticals, and TauRx. He has consulted for Abbvie, Axovant, Janssen Research & Development, Takeda/Zinfandel. He serves on the Scientific Advisory Board of the Lewy Body Dementia Association. He receives research support from the NIH, Health Resources and Services Administration, and Bryan Family Foundation. D. Kerwin has served on an Advisory Board for AbbVie and as site principal investigator for studies funded by Roche/Genentech, AbbVie, Avid, Novartis, Eisai, Eli Lilly, and UCSF. D. Knopman serves on the Data Safety Monitoring Board of the DIAN-TU study, is a site PI for clinical trials sponsored by Biogen, Lilly and the University of Southern California, and receives research support from NIH. J. Kornak serves on the Data Safety Monitoring Board of the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU) study; is a site principal investigator for clinical trials sponsored by Biogen, Lilly, and the University of Southern California; and receives research support from the NIH. J. Kramer receives research support from the NIH and serves on an advisory board for Biogen. I. Litvan receives research support from the NIH, Parkinson Study Group, Parkinson Foundation, Michael J. Fox Foundation, AVID Pharmaceuticals, C2N Diagnostics/Abbvie, and Bristol-Myers Squibb. She was a member of the Biogen and Bristol-Myers Squibb Advisory boards and Biotie/Parkinson Study Group Medical Advisory Board and consultant for Toyama Pharmaceuticals. She receives salary from the University of California San Diego and as editor in Frontiers in Neurology. I. Mackenzie receives research support from the Canadian Institutes of Health Research. M. Mendez is supported by NIH research grants and has received research support from Biogen. B. Miller receives research support from the NIH. R. Rademakers receives research support from the NIH and the Bluefield Project to Cure Frontotemporal Dementia. E. Ramos reports no disclosures. K. Rascovsky receives research support from the NIH. E. Roberson receives research support from the NIH, Bluefield Project to Cure Frontotemporal Dementia, Alzheimer's Association, BrightFocus Foundation, Biogen, and Alector and owns intellectual property related to tau. J. Syrjanen reports no disclosures. M. Tartaglia receives research support from the Canadian Institutes of Health Research and NIH and is an investigator on pharmaceutical studies with Biogen, Roche, Eli Lilly, and Boehringer. S. Weintraub receives research support from the NIH. B. Boeve has served as an investigator for clinical trials sponsored by GE Healthcare and Axovant. He receives royalties from the publication Behavioral Neurology of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, and the Little Family Foundation. A. Boxer receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, the Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer's Drug Discovery Foundation, and the Alzheimer's Association. He has served as a consultant for Aeton, Abbvie, Alector, Amgen, Arkuda, Ionis, Iperian, Janssen, Merck, Novartis, Samumed, Toyama, and UCB; he has received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche, and TauRx. H. Rosen has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from the NIH. K. Rankin receives research funding from the NIH, Quest Diagnostics, and the Rainwater Charitable Foundation. Go to Neurology.org/N for full disclosures.
References
- 1.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen HJ, Perry RJ, Murphy J, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain 2002;125:2286–2295. [DOI] [PubMed] [Google Scholar]
- 4.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 5.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol 2012;8:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014;6:228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lennox RD, Wolfe RN. Revision of the self-monitoring scale. J Personal Soc Psychol 1984;46:1349–1364. [DOI] [PubMed] [Google Scholar]
- 9.Toller G, Brown J, Sollberger M, et al. Individual differences in socioemotional sensitivity are an index of salience network function. Cortex 2018;103:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shdo SM, Ranasinghe KG, Gola KA, et al. Deconstructing empathy: neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia 2017;116:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe RN, Lennox RD, Cutler BL. Getting along and getting ahead: empirical support for a theory of protective and acquisitive self-presentation. J Pers Soc Psychol 1986;50:356. [Google Scholar]
- 12.Anderson LR. Test-retest reliability of the Revised Self-Monitoring Scale over a two-year period. Psychol Rep 1991;68:1057–1058. [DOI] [PubMed] [Google Scholar]
- 13.O'Cass A. A psychometric evaluation of a revised version of the Lennox and Wolfe Revised Self-Monitoring Scale. Psychol Marketing 2000;17:397–419. [Google Scholar]
- 14.Hofmann SG. The emotional consequences of social pragmatism: the psychophysiological correlates of self-monitoring. Biol Psychol 2006;73:169–174. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 16.Knopman DS, Weintraub S, Pankratz VS. Language and behavior domains enhance the value of the Clinical Dementia Rating scale. Alzheimers Demen 2011;7:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649–655. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner J, Friston KJ. Unified segmentation. NeuroImage 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler G, Penny WD, Ridgway GR, Ourselin S, Friston KJ. Alzheimer's Disease Neuroimaging Initiative: estimating anatomical trajectories with bayesian mixed-effects modeling. Neuroimage 2015;121:51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashburner J, Friston KJ. Diffeomorphic registration using geodesic shooting and Gauss–Newton optimisation. Neuroimage 2011;55:954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 22.Whitwell JL, Weigand SD, Boeve BF, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain 2012;135:794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sha SJ, Takada LT, Rankin KP, et al. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology 2012;79:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008;131:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mourik JC, Rosso SM, Niermeijer MF, Duivenvoorden HJ, Van Swieten JC, Tibben A. Frontotemporal dementia: behavioral symptoms and caregiver distress. Dement Geriatr Cogn Disord 2004;18:299–306. [DOI] [PubMed] [Google Scholar]
- 26.Mioshi E, Foxe D, Leslie F, et al. The impact of dementia severity on caregiver burden in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Associated Disord 2013;27:68–73. [DOI] [PubMed] [Google Scholar]
- 27.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AD, Craig A. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 29.Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 2007;130:1718–1731. [DOI] [PubMed] [Google Scholar]
- 30.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007;8:976. [DOI] [PubMed] [Google Scholar]
- 31.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005;6:691. [DOI] [PubMed] [Google Scholar]
- 32.Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain 2006;129:2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerami C, Dodich A, Canessa N, et al. Neural correlates of empathic impairment in the behavioral variant of frontotemporal dementia. Alzheimers Demen 2014;10:827–834. [DOI] [PubMed] [Google Scholar]
- 34.Sollberger M, Stanley CM, Wilson SM, et al. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia 2009;47:2812–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toller G, Yang WF, Brown JA, et al. Divergent patterns of loss of interpersonal warmth in frontotemporal dementia syndromes are predicted by altered intrinsic network connectivity. Neuroimage Clin 2019;22:101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturm VE, Sollberger M, Seeley WW, et al. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci 2013;8:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woolley JD, Strobl EV, Sturm VE, et al. Impaired recognition and regulation of disgust is associated with distinct but partially overlapping patterns of decreased gray matter volume in the ventroanterior insula. Biol Psychiatry 2015;78:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumfor F, Irish M, Hodges JR, Piguet O. Discrete neural correlates for the recognition of negative emotions: insights from frontotemporal dementia. PLoS One 2013;8:e67457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitwell JL, Jack CR Jr, Pankratz VS, et al. Rates of brain atrophy over time in autopsy-proven frontotemporal dementia and Alzheimer disease. Neuroimage 2008;39:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SE, Khazenzon AM, Trujillo AJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 2014;137:3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takada L, Sha S, Rankin K, et al. Neuropsychiatric features of C9ORF72 mutation-associated bvFTD and FTD-ALS (IN9-2.003). Neurology 2012;78(suppl):IN9-2.003. [Google Scholar]
- 42.Sellami L, Bocchetta M, Masellis M, et al. Distinct neuroanatomical correlates of neuropsychiatric symptoms in the three main forms of genetic frontotemporal dementia in the GENFI cohort. J Alzheimers Dis 2018;65:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C 9ORF72 mutations. Brain 2012;135:693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 2008;65:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bocchetta M, Gordon E, Cardoso MJ, et al. Thalamic atrophy in frontotemporal dementia: not just a C9orf72 problem. Neuroimage Clin 2018;18:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitwell JL, Boeve BF, Weigand SD, et al. Brain atrophy over time in genetic and sporadic frontotemporal dementia: a study of 198 serial magnetic resonance images. Eur J Neurol 2015;22:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohrer JD, Ridgway GR, Modat M, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage 2010;53:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request from any qualified investigator for the purposes of replicating procedures and results.